Abstract
Objective
To systematically evaluate the efficacy and safety of combination regimens containing daratumumab in patients with multiple myeloma (MM).
Methods
A systematic search of publications listed on electronic databases (PubMed®, The Cochrane Library, Science Direct and Web of Science) between inception and 13 November 2020 was conducted to find randomized controlled trials (RCTs) that included patients with MM that were treated with combination regimens containing daratumumab.
Results
A total of seven RCTs were included (n = 4268 patients). Meta-analysis showed that compared with the control group, the group containing daratumumab showed a significantly better overall response rate and a complete response or better. Daratumumab improved efficacy in both standard-risk and cytogenetically high-risk patients with MM. The prevalence of neutropenia (≥grade 3) and pneumonia was significantly higher in the daratumumab group compared with the control group.
Conclusion
The available evidence demonstrated that the clinical application of combination regimens containing daratumumab improved the efficacy in patients with MM and had acceptable safety.
Keywords: Daratumumab, multiple myeloma, CD38, meta-analysis, systematic review
Introduction
Multiple myeloma (MM) is a malignant disease that is characterized by the abnormal proliferation of clonal plasma cells, which remains incurable.1 With the clinical application of various new drugs, the survival and prognosis of patients with MM has been significantly improved, but most patients with MM still face the risk of recurrence.1 Therefore, it is very important to introduce more effective drugs into the treatment strategy for patients with MM. The cluster of differentiation (CD)38 antigen is highly expressed on the surface of myeloma cells, but at low levels on the surface of some normal cells, so it can be used as a target for MM therapy.2 Daratumumab is a human immunoglobulin (Ig)G1-κ CD38 antibody, which exerts its antitumour effects through a variety of mechanisms, such as complement-dependent cytotoxicity, antibody-dependent phagocytosis and direct induction of apoptosis.2,3 Research has found that daratumumab showed good efficacy in patients with MM,4 but there has been a lack of systematic evaluation of the efficacy and safety of daratumumab in patients with MM. Therefore, this current study used direct meta-analysis and network meta-analysis methods to analyse the efficacy and safety of combination regimens containing daratumumab in the treatment of patients with MM in order to provide reference for the selection of clinical MM treatment.
Materials and methods
Literature search
This study was performed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-Analysis (PRISMA) guideline.5 This meta-analysis has been registered on INPLASY (registration no. 202150092). A systematic search of publications listed on electronic databases (PubMed®, The Cochrane Library, Science Direct and Web of Science) between inception and 13 November 2020 was conducted to find randomized controlled trials (RCTs) that included patients with MM that were treated with combination regimens containing daratumumab. The following medical terms were used in the search: (1) daratumumab; (2) Darzalex; (3) 1 OR 2; (4) multiple myeloma; (5) myeloma; (6) 4 OR 5; (7) 3 AND 6. The search was limited to publications in English.
Study selection
Studies were eligible for inclusion if they met the following criteria: (i) RCTs of MM patients treated with combination regimens containing daratumumab; (ii) including the main outcome indicators (overall response rate; complete response or better). The exclusion criteria were as follows: (i) not an RCT; (ii) lack of main outcome indicators; (iii) duplicate record; (iv) irrelevant literature. Two authors (Y.W. and Y.L.) independently screened the literature, extracted and cross-checked the data. If there were differences between the two reviewers, they were resolved through discussion or consultation with a third party (Y.C.). When screening the literature, each reviewer first read the title, and after excluding the obviously irrelevant literature, further read the abstract and the full text to determine whether to include it or not in the meta-analysis. If necessary, missing information was obtained directly from the original author by email or telephone.
Quality assessment
The quality of the included literature was evaluated using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2; Cochrane Collaboration, Oxford, UK). The risk of study bias was independently evaluated by two researchers (Y.W. and Y.L.) and the results were cross-checked. If there were differences, they will be resolved through discussion or negotiation with a third party (Y.C.).
Data extraction
Two of the authors (Y.W. and Y.L.) independently extracted the data from the included RCTs. The data were obtained from published reports. The extracted data included the following: (i) overall response rate (ORR); (ii) complete response (CR); (iii) CR or better (≥CR); (iv) CR in patients with high-risk cytogenetics; (v) neutropenia (≥3 grade); (vi) thrombocytopenia (≥3 grade); (vii) pneumonia; (viii) the incidence of second primary malignancy.
Statistical analyses
RevMan software (version 5.4; Cochrane Collaboration, Oxford, UK) was used for the meta-analyses. The odds ratio (OR) was used as the effect analysis statistic of the two classified variables and its 95% confidence interval (CI) was provided. Heterogeneity among the results was analysed using χ2-test and the heterogeneity was quantitatively judged using I2 statistics. If there was no statistical heterogeneity among the results of each study, a fixed-effect model was used for the meta-analysis. If there was statistical heterogeneity among the results, the source of heterogeneity was further analysed. After excluding the obvious clinical heterogeneity, a random-effect model was used for the meta-analysis. The obvious clinical heterogeneity was overcome using subgroup analysis, sensitivity analysis or only descriptive analysis. A funnel plot was drawn by the RevMan software (version 5.4) to test for publication bias. Based on the Bayesian hierarchical model, a network meta-analysis of the outcome index was carried out using Addis Software (version 1.16.6; Addis Software, Addis Ababa, Ethiopia). The first iteration was set to 50000. The convergence of the network meta-analysis was tested by the potential scale reduction parameter (PSRF). If the PSRF was closer to 1, the better the convergence efficiency was, and the higher the credibility of the model analysis conclusion was. The concordance model was used in the analysis of the network meta-analysis was. A rank probability ranking chart was used to show the ranking of the outcome indicators of each intervention. A P-value < 0.05 was considered statistically significant.
Results
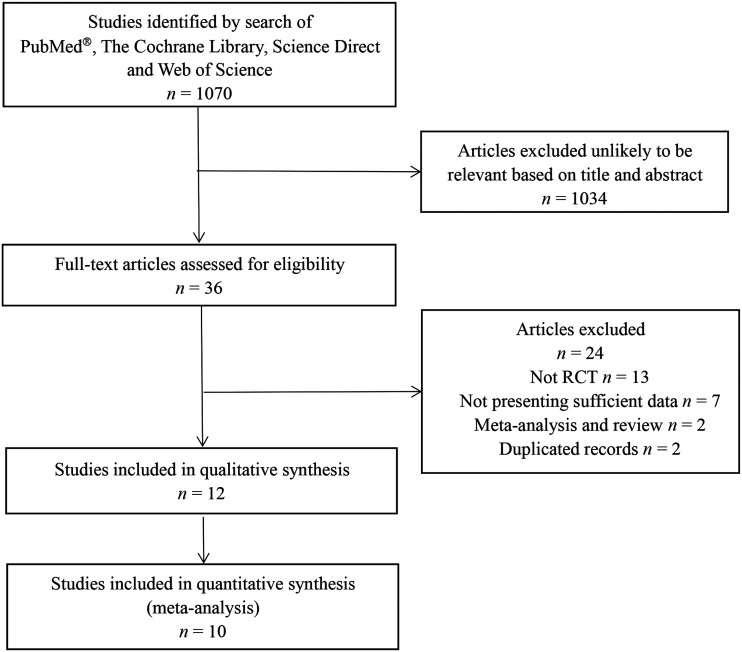
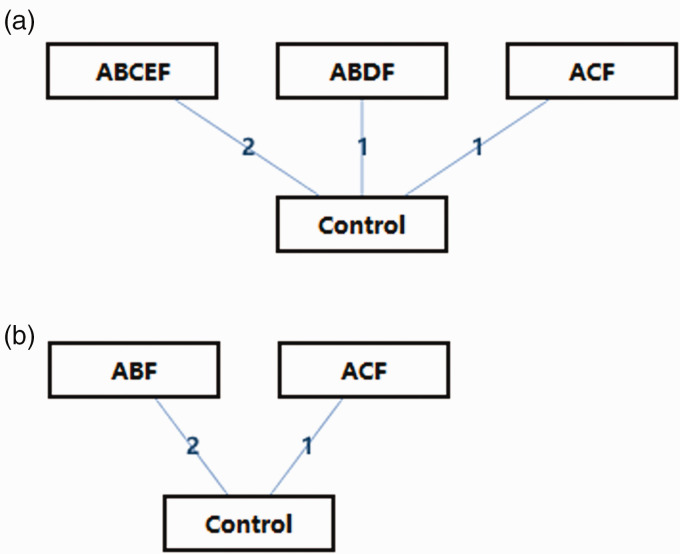
The initial database search identified 1070 studies. After applying the inclusion and exclusion criteria, seven RCTs (described in 10 articles) with 4268 patients were included in the meta-analysis (Figure 1).6–15 The remaining studies were excluded largely because they were duplicates or unlikely to be relevant based on the title and abstract. The studies were assessed for and found to have high quality (Figure 2). The major characteristics of each of the seven studies are shown in Table 1. The network diagram of the interventions included in the studies is shown in Figure 3.
Figure 1.
Flow diagram of eligible studies showing the number of citations identified, retrieved and included in the meta-analysis of the efficacy and safety of daratumumab in the treatment of multiple myeloma.
Figure 2.
Risk of bias in the studies included in the meta-analysis of the efficacy and safety of daratumumab in the treatment of multiple myeloma.
Table 1.
Major characteristics of the seven studies included in the meta-analysis of the efficacy and safety of daratumumab in the treatment of multiple myeloma.6–12
| Author | Study name | Registration number | Number of patients | Disease indication | Regimens | Median age, years | Dose of daratumumab, mg |
|---|---|---|---|---|---|---|---|
| Mateos et al. 20206 | ALCYONE | NCT02195479 | 350:356 | NDMM | D-VMP versus VMP | 71 | 16 |
| Dimopoulos et al. 20207 | CANDOR | NCT03158688 | 312:154 | RRMM | KdD versus Kd | – | 8/16 |
| Moreau et al. 20198 | CASSIOPEIA | NCT02541383 | 543:542 | NDMM | D-VTd versus VTd | 58 | 16 |
| Mateos et al. 20209 | CASTOR | NCT02136134 | 251:247 | RRMM | DVd versus Vd | 64 | 16 |
| Voorhees et al. 202010 | GRIFFIN | NCT02874742 | 104:103 | NDMM | D-RVd versus RVd | 60 | 16 |
| Facon et al. 201911 | MAIA | NCT02252172 | 368:369 | NDMM | DRd versus Rd | 73 | 16 |
| Dimopoulos et al. 201612 | POLLUX | NCT02076009 | 286:283 | RRMM | DRd versus Rd | 65 | 16 |
NDMM, newly diagnosed multiple myeloma; D-VMP, daratumumab + bortezomib + melphalan + prednisone; VMP, bortezomib+ melphalan+ prednisone; RRMM, relapsed/refractory multiple myeloma; KdD, carfilzomib + dexamethasone + daratumumab; Kd, carfilzomib + dexamethasone; D-VTd, daratumumab + bortezomib + thalidomide + dexamethasone; VTd, bortezomib + thalidomide + dexamethasone; DVd, daratumumab + bortezomib+ dexamethasone; Vd, bortezomib + dexamethasone; D-RVd, daratumumab + lenalidomide+ bortezomib+ dexamethasone; RVd, lenalidomide + bortezomib + dexamethasone; DRd, daratumumab + lenalidomide + dexamethasone; Rd, lenalidomide + dexamethasone.
Figure 3.
Network diagram of different interventions: (a) newly diagnosed multiple myeloma; (b) relapsed/refractory multiple myeloma. A, daratumumab; B, proteasome inhibitor; C, immunomodulatory drug; D, melphalan; E, autologous stem cell transplantation; F, glucocorticoids.
The ORR and ≥CR were reported in seven RCTs.6–12 The results of a random-effect model meta-analysis showed that the ORR in the daratumumab group was significantly higher than that in the control group (OR 2.71, 95% CI 1.94, 3.77, P < 0.00001) (Figure 4A). The results of a random-effect model meta-analysis showed that the rate of ≥CR in the daratumumab group was significantly higher than that in the control group (OR 2.54, 95% CI 2.01, 3.22, P < 0.00001) (Figure 4B). Three RCTs reported ORR in patients with high-risk cytogenetics.6,13,14 High-risk cytogenetics was defined as having at least one of the following abnormalities: t(4; 14), t(14; 16), del(17p). The results of a fixed-effect model meta-analysis showed that the ORR of high-risk cytogenetic patients with MM in the daratumumab group was significantly higher than that in the control group (OR 3.23, 95% CI 1.69, 6.17, P = 0.0004) (Figure 4C).
Figure 4.
Meta-analysis of the efficacy of daratumumab compared with control groups (without daratumumab) in the treatment of multiple myeloma: (A) comparison of overall response rate (ORR) between the two groups; (B) comparison of the rate of complete response or better (≥CR) between the two groups; (C) comparison of ORR in patients with high-risk cytogenetics between the two groups.
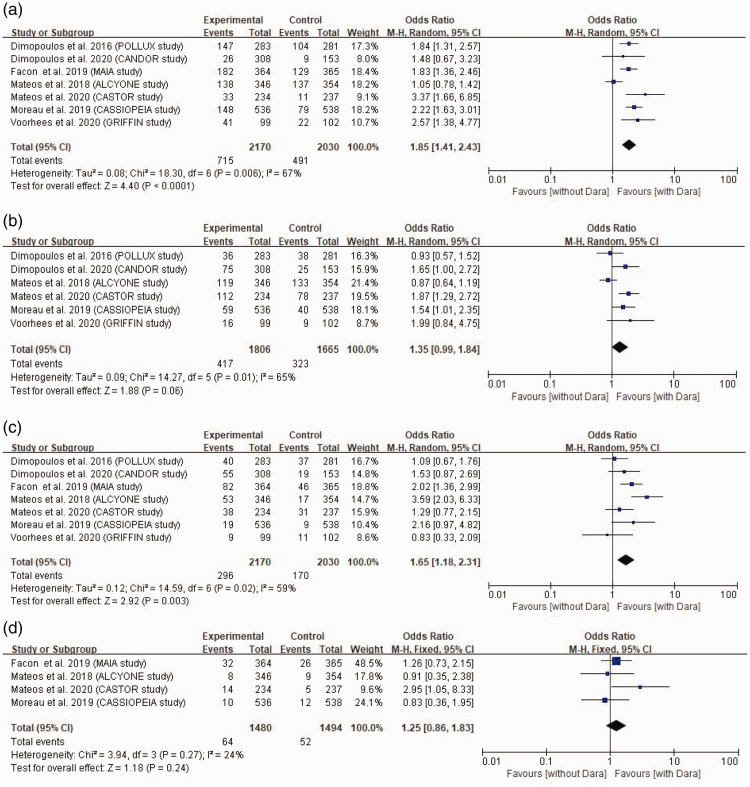
The prevalence of neutropenia (≥grade 3), thrombocytopenia (≥grade 3), pneumonia and second primary malignancy in the daratumumab and control groups were analysed. The incidence of neutropenia (≥grade 3) was reported in all seven RCTs.7–12,15 The results of a random-effect model meta-analysis showed that the incidence of neutropenia (≥grade 3) in the daratumumab group was significantly higher than that in the control group (OR 1.85, 95% CI 1.41, 2.43, P < 0.0001) (Figure 5A). Six RCTs reported the incidence of thrombocytopenia (≥grade 3).7–10,12,15 The results of a random-effect model meta-analysis showed that there was no significant difference in the incidence of thrombocytopenia (≥grade 3) in the daratumumab group and the control group (OR 1.35, 95% CI 0.99, 1.84) (Figure 5B). The incidence of pneumonia was reported in all seven RCTs.7–12,15 The results of a random-effect model meta-analysis showed that the incidence of pneumonia in the daratumumab group was significantly higher than that in the control group (OR 1.65, 95% CI 1.18, 2.31, P = 0.003) (Figure 5C). Four RCTs reported the incidence of second primary malignancy.8,9,11,15 The results of a fixed-effect model meta-analysis showed that there was no significant difference in the incidence of second primary malignancy between the daratumumab group and the control group (OR 1.25, 95% CI 0.86, 1.83) (Figure 5D).
Figure 5.
Meta-analysis of the adverse effects of daratumumab compared with control groups (without daratumumab) in the treatment of multiple myeloma: (A) comparison of the incidence of neutropenia (≥grade 3) between the two groups; (B) comparison of the incidence of thrombocytopenia (≥grade 3) between the two groups; (C) comparison of the incidence of pneumonia between the two groups; (D) comparison of the incidence of second primary malignancies between the two groups.
In patients with newly diagnosed multiple myeloma (NDMM), in terms of ORR, the ORR of daratumumab + proteasome inhibitor (PI) + melphalan + glucocorticoids (GC) (relative risk [RR] 3.54, 95% CI 0.30, 42.02), daratumumab +immunomodulatory drug (IMiD) + GC (RR 3.00, 95% CI 0.26, 33.73) and daratumumab +PI + IMiD + autologous stem cell transplantation (ASCT) + GC (RR 2.55, 95% CI 0.51, 21.81) was higher than that of the control group. In terms of ≥CR, the rate of ≥CR of daratumumab + IMiD + GC (RR 2.74, 95% CI 0.95, 8.19), daratumumab +PI + melphalan + GC (RR 2.49, 95% CI 0.81, 7.47) and daratumumab + PI +IMiD + ASCT + GC (RR 1.70, 95% CI 0.76, 3.70) was higher than that of the control group.
In patients with relapsed/refractory multiple myeloma (RRMM), in terms of ORR, the ORR of daratumumab + PI + GC (RR 2.39, 95% CI 0.75, 7.20) and daratumumab +IMiD + GC (RR 3.98, 95% CI 0.78, 20.31) were higher than that of the control group. Compared with other groups, daratumumab + IMiD + GC had the highest ORR (Figure 6A). In terms of ≥CR, the rates of ≥CR of daratumumab +PI + GC (RR 3.77, 95% CI 1.32, 10.72) and daratumumab + IMiD + GC (RR 3.21, 95% CI 0.80, 13.22) were higher than that of the control group. Compared with other groups, daratumumab + PI + GC had the highest ≥CR rate (Figure 6B).
Figure 6.
The rankogram of the efficacy of each intervention in terms of overall response rate (ORR) (A) and complete response or better (≥ CR) (B) in patients with relapsed/refractory multiple myeloma. A, daratumumab; B, proteasome inhibitor; C, immunomodulatory drug; D, melphalan; E, autologous stem cell transplantation; F, glucocorticoids. The colour version of this figure is available at: http://imr.sagepub.com.
A sensitivity analysis was conducted by excluding individual studies one by one and it was found that after excluding the CASSIOPEIA study,8 the meta-analysis of ORR and ≥CR showed a significant decrease in heterogeneity (I2 < 50%), while after excluding the ALCYONE study,6 the meta-analysis of neutropenia (≥3 grade), thrombocytopenia (≥3 grade) and pneumonia showed a significant decrease in heterogeneity (I2 < 50%). The possible reason is that the sample size of these two studies was large and accounted for a large weight in the combined analysis, while the results of the other studies were different to those of these two studies. After re-reading the two studies, the two authors (Y.W. and Y.L.) agreed that the two publications were of high quality and had a low risk of bias; and that the results of the meta-analysis did not change direction after excluding the literature. Taking the results of the two studies into consideration, the conclusions of the two studies were incorporated into this meta-analysis.
A funnel plot of all outcome indicators was drawn to test for publication bias (data not shown). The results showed that the left and right distribution of each outcome index was basically symmetrical, suggesting that the possibility of publication bias was small.
Discussion
As a consequence of the development and use of new drugs, the survival and prognosis of patients with MM has been greatly improved.16 The number of new treatment regimens has gradually increased, so there are now more treatment options for patients with MM.16 In order to enable patients to achieve the maximum benefit from treatment, it is necessary to summarize and analyse the existing clinical data and experience. Daratumumab exerts its anti-tumour effects through several mechanisms.2,17 It has been shown to be effective in many clinical studies.18,19 The purpose of this current meta-analysis was to systematically evaluate the efficacy and safety of daratumumab in patients with MM in order to provide a reference for clinical drug selection.
The results of this current meta-analysis showed that compared with the control group, the combined daratumumab group had better rates of ORR and ≥CR, indicating that the introduction of daratumumab not only increased the remission rate of patients, but also helped patients to achieve deeper remission, and then improve the survival prognosis. In addition, the results of the meta-analysis of ORR in high-risk cytogenetic patients also showed that daratumumab increased the rate of ORR and ≥CR, indicating that the application of daratumumab should not be limited to patients with MM without high-risk cytogenetics. A previous meta-analysis on the efficacy of daratumumab in the treatment of patients with MM with high-risk cytogenetics demonstrated that daratumumab improved progression-free survival (PFS) in both high-risk cytogenetic NDMM and RRMM patients.20
Furthermore, the current network meta-analysis of the efficacy of combination regimens containing daratumumab in the treatment of NDMM and RRMM showed that combination regimens containing daratumumab showed better ORR and ≥CR rates for NDMM and RRMM than those in the control group. As for NDMM, considering the differences in their enrolment (e.g. there were differences in age and complications between transplant-eligible patients and transplant-ineligible patients), it was not possible to rank the efficacy of each intervention. Among the patients with RRMM, ORR was better in the daratumumab + IMiD + GC group; and the ≥CR rate was better in daratumumab +PI + GC group. In a previous systematic review,21 the efficacy of 16 treatments for MM was analysed and it was found that the combination of daratumumab, lenalidomide and dexamethasone was the best for prolonging PFS in patients with RRMM. However, due to the inconsistent follow-up time of each study included in this current meta-analysis, and the different time-points used to evaluate the efficacy, the results of the order of efficacy should be interpreted carefully. Overall, this current meta-analysis has demonstrated that after combination treatment with daratumumab, the rates of ORR and ≥ CR in patients with NDMM and RRMM were increased, indicating a better therapeutic effect.
In terms of safety, this current meta-analysis showed that the prevalence of neutropenia (≥grade 3) and thrombocytopenia (≥grade 3) in the daratumumab group was higher than that in the control group, which may be related to prolonged drug exposure.2,22 It is important to note that in patients receiving daratumumab combination therapy, neutropenia should be closely monitored for infection.23 For patients with thrombocytopenia, it is recommended to monitor blood cells dynamically and consider blood transfusion or growth-promoting factor treatment if necessary.9 In addition, pneumonia is a common non-haematological adverse reaction in some clinical studies.11,15 Therefore, this current meta-analysis also analysed the effect of daratumumab on the incidence of pneumonia and it demonstrated that the prevalence of pneumonia in the combined daratumumab group was higher than that in the control group. Severe pneumonia can lead to forced withdrawal or death of patients, so when using daratumumab, physicians should be vigilant in order to detect pneumonia in a timely manner so that they can give treatment as soon as possible. Although the incidence of secondary primary tumour is low,8,9,11,15 it has a great impact on the survival and prognosis of patients, so this current meta-analysis also analysed the effect of daratumumab on the occurrence of secondary primary tumours. This current meta-analysis showed that daratumumab did not increase the incidence of secondary primary tumours. In summary, daratumumab will cause some adverse reactions in patients, but most are mild and controllable, and its safety is acceptable.23
Although the publications included in this current meta-analysis were all high-quality RCTs, so the results provide a certain reference value, the study had some limitations: (i) the number of RCTs included was relatively small, so the conclusions drawn may be biased; (ii) ORR, one of the outcome indicators included in this study, is a subjective evaluation determined by physicians, so the introduction of bias to the results is inevitable; (iii) the follow-up time of the included studies varied and the outcome index might change with the extension of the follow-up time; (iv) some unpublished literature with negative results might lead to publication bias. Therefore, the conclusions of this current meta-analysis should be interpreted with caution. Further large-scale, high-quality studies are needed to confirm the efficacy and safety of daratumumab in patients with MM.
In conclusion, this current meta-analysis demonstrated that the combination of daratumumab improves the efficacy of patients, regardless of standard risk or high-risk patients. And Dara has acceptable security. We believe that Dara is a promising drug for the treatment of multiple myeloma.
Footnotes
Author contributions: Yin Wang was responsible for the analysis of relevant materials and the writing of this article. Yanqing Li was responsible for the data collection and assembly. Ye Chai designed, instructed and revised this article. All authors agree with the content of the article and are responsible for the authenticity of the content. All authors approved the typescript.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Yin Wang https://orcid.org/0000-0002-5269-6818
References
- 1.Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers 2017; 3: 17046. DOI: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- 2.van de Donk NWCJ, Richardson PG, Malavasi F.CD38 antibodies in multiple myeloma: back to the future. Blood 2018; 131: 13–29. DOI: 10.1182/blood-2017-06-740944. [DOI] [PubMed] [Google Scholar]
- 3.Shah UA, Mailankody S.Emerging immunotherapies in multiple myeloma. BMJ 2020; 370: m3176. DOI: 10.1136/bmj.m3176. [DOI] [PubMed] [Google Scholar]
- 4.Sekine L, Ziegelmann PK, Manica D, et al. Upfront treatment for newly diagnosed transplant-ineligible multiple myeloma patients: A systematic review and network meta-analysis of 14,533 patients over 29 randomized clinical trials. Crit Rev Oncol Hematol 2019; 143: 102–116. DOI: 10.1016/j.critrevonc.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372: n160. DOI: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet 2020; 395: 132–141. DOI: 10.1016/s0140-6736(19)32956-3. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020; 396: 186–197. DOI: 10.1016/S0140-6736(20)30734-0. [DOI] [PubMed] [Google Scholar]
- 8.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019; 394: 29–38. DOI: 10.1016/s0140-6736(19)31240-1. [DOI] [PubMed] [Google Scholar]
- 9.Mateos MV, Sonneveld P, Hungria V, et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin Lymphoma Myeloma Leuk 2020; 20: 509–518. DOI: 10.1016/j.clml.2019.09.623. [DOI] [PubMed] [Google Scholar]
- 10.Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood 2020; 136: 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Facon T, Kumar S, Plesner T, et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N Engl J Med 2019; 380: 2104–2115. DOI: 10.1056/NEJMoa1817249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med 2016; 375: 1319–1331. DOI: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, San-Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of POLLUX. Haematologica 2018; 103: 2088–2096. DOI: 10.3324/haematol.2018.194282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: updated analysis of CASTOR. Haematologica 2018; 103: 2079–2087. DOI: 10.3324/haematol.2018.194118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N Engl J Med 2018; 378: 518–528. DOI: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 16.van de Donk NWCJ, Pawlyn C, Yong KL.Multiple myeloma. Lancet 2021; 397: 410–427. DOI: 10.1016/S0140-6736(21)00135-5. [DOI] [PubMed] [Google Scholar]
- 17.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016; 128: 384–394. DOI: 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nooka AK, Kaufman JL, Hofmeister CC, et al. Daratumumab in multiple myeloma. Cancer 2019; 125: 2364–2382. DOI: 10.1002/cncr.32065. [DOI] [PubMed] [Google Scholar]
- 19.Touzeau C, Moreau P.Daratumumab for the treatment of multiple myeloma. Expert Opin Biol Ther 2017; 17: 887–893. DOI: 10.1080/14712598.2017.1322578. [DOI] [PubMed] [Google Scholar]
- 20.Giri S, Grimshaw A, Bal S, et al. Evaluation of Daratumumab for the Treatment of Multiple Myeloma in Patients With High-risk Cytogenetic Factors A Systematic Review and Meta-analysis. JAMA Oncol 2020; 6: 1759–1765. DOI: 10.1001/jamaoncol.2020.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Beurden-Tan CHY, Franken MG, Blommestein HM, et al. Systematic Literature Review and Network Meta-Analysis of Treatment Outcomes in Relapsed and/or Refractory Multiple Myeloma. J Clin Oncol 2017; 35: 1312–1319. DOI: 10.1200/JCO.2016.71.1663. [DOI] [PubMed] [Google Scholar]
- 22.Richter J, Thibaud S.Anti-body building: The exercise of advancing immune based myeloma therapies. Blood Rev 2021; 48: 100789. DOI: 0.1016/j.blre.2020.100789. [DOI] [PubMed] [Google Scholar]
- 23.Dima D, Dower J, Comenzo RL, et al. Evaluating Daratumumab in the Treatment of Multiple Myeloma: Safety, Efficacy and Place in Therapy. Cancer Manag Res 2020; 12: 7891–7903. DOI: 10.2147/CMAR.S212526. [DOI] [PMC free article] [PubMed] [Google Scholar]