Abstract
Background
Recent studies have reported associations between, human bocavirus (HBoV), and respiratory tract diseases in children. However, there is limited information on the epidemiology of HBoV in infants. This prospective study investigated the prevalence and clinical characteristics of HBoV infection in infants with acute lower respiratory tract infection (ALRTI) in eastern China.
Methods
Nasopharyngeal aspirates and throat swab samples were collected from infants with ALRTI and age-matched healthy infants between January 2016 and December 2019. HBoV was identified by polymerase chain reaction. Laboratory data and clinical characteristics were analyzed.
Results
Of 2510 infants, 145 tested positive for HBoV. The highest prevalence of HBoV was detected during the winter. Co-infection was frequently observed during this period of high viral transmission. There were no HBoV-positive infants in the control group. Clinical signs and symptoms included cough, wheezing, fever, nasal discharge, vomiting, diarrhea, hypoxemia, and tachypnea. Co-infections included: Streptococcus pneumoniae, Staphylococcus aureus, Mycoplasma pneumoniae, Chlamydophila pneumoniae, respiratory syncytial virus, and adenovirus.
Conclusions
HBoV was frequently detected in infants with ALRTI in China. The prevalence of HBoV was highest in winter. Co-infection was common, especially in infants requiring intensive care unit admission. Comprehensive clinical evaluation may facilitate optimal treatment.
Keywords: Human bocavirus, infant, acute lower respiratory tract infection, co-infection, prevalence, eastern China
Introduction
Acute lower respiratory tract infection (ALRTI) is the leading cause of illness and death in infants worldwide,1 especially in developing countries.2 However, most respiratory tract infections remain undiagnosed.3 Previous studies have detected human bocavirus (HBoV) in human nasopharyngeal aspirates (NPAs) from children with respiratory tract infections. Comprehensive sequencing and phylogenetic analyses revealed that HBoV belongs to the Parvoviridae family.4 Recent reports have demonstrated a strong correlation between HBoV and upper and lower respiratory tract infection,5 asthma exacerbation,6 bronchiolitis,7 and acute otitis media.8 The results of these studies have been contradictory as they focused entirely on the viral pathogens and disregarded the characteristics and clinical features of the study population, resulting in high variation in prevalence estimates (0.6%,9 19%,10 and 24%11). Infants younger than 2 years of age are thought to be at high risk of HBoV infection because they are no longer protected by maternal antibodies.12,13 Infants younger than 1 year of age have even higher risk of respiratory tract infections, but little is known regarding the incidence of HBoV infection in this age group. To fill this HBoV research gap, we conducted a 3-year prospective study in infants younger than 1 year hospitalized with ALRTIs.
Materials and methods
This was a prospective study. The study population comprised infants of Chinese Han nationality who were hospitalized with ALRTIs at Ningbo Women and Children’s Hospital between January 2016 and December 2020. We also collected samples from a control group of age-matched healthy infants from the health examination clinic service (sample size estimation: p = 0.03, ε = 0.1, α = 0.05, Z1-α/2 = 1.96, N = 1794). The study protocol was approved by the Ethics Committee of Ningbo Women and Children’s Hospital with approval number 2019-ky-041. Signed informed consent was obtained from the parents of each infant. We collected data on the epidemiological, clinical, and laboratory characteristics of HBoV-positive infants during the study period. We collected NPAs and throat swab samples from both HBoV-positive and control infants. The study followed the STROBE-NI guidelines. Specimens were transported to the virology laboratory within 24 hours and stored at −80°C. Additionally, the presence of respiratory syncytial virus, adenovirus, rhinovirus, and influenza virus A was assessed by polymerase chain reaction. Serum samples were obtained to measure levels of antibody against Mycoplasma pneumoniae and Chlamydia pneumoniae by ELISA. The ELISA kit was from Shanghai Sangon Bioengineering (Shanghai, China). We recorded biochemical data and clinical findings for all hospitalized infants.
Samples from both groups were tested for HBoV using a reverse transcription polymerase chain reaction14 kit and two primer sets specific for the NP1and NS1 genes4 (Nippon Chemi-Con, Tokyo, Japan). The QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used to extract DNA from samples following the manufacturer’s protocol. The first set of primers (F: 5′-GAGCTCTGTAAGTACTATTAC-3′ and R: 5′-CTCTGTGTTGACTGAATACAG-3′) targeted a 354-bp fragment in the NP1 gene, while the second set of primers (F: 5ʹ-TATGGCCAAGGCAATCGTCCAAG-3ʹ and R: 5ʹ-GCCGCGTGAACATGAGAAACAGA-3ʹ) targeted a 271-bp fragment in the NS1 gene. The primers were synthesized by the Shanghai Sangon Bioengineering. The amplification conditions were: 94°C pre-denaturation for 4 minutes; 35 cycles of 94°C denaturation for 1 minute, 55°C annealing for 1 minute, and 72°C extension for 1 minute; 72°C extension for 10 minutes. Following amplification, 5 µL of the reaction was analyzed by agarose gel electrophoresis. Samples that showed amplification using both primer sets were considered positive for HBoV.
Bacterial specimens were collected on the day of admission. Deep sputum was obtained by negative pressure before antibiotics were administered. After the infant was admitted to hospital, oropharyngeal and nasopharyngeal secretions were cleaned, and then a disposable sterile sputum suction tube was fully inserted into the subglottic airway to extract sputum from the deep trachea. Sputum was cultured on M2H agar with various antimicrobial susceptibility disks (Oxoid, Basingstoke, UK). The isolation and identification of strains was carried out according to conventional methods and in strict accordance with the operating instructions of the VITEK232 automatic microbial analyzer (bioMérieux, Marcy-l'Étoile, France).
Parametric data were described as means ± standard deviations. All analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA). The chi square test and Student’s t-test were used to assess differences between groups. Values of p < 0.05 were considered statistically significant. The Research Registry Number was: researchregistry6791.
Results
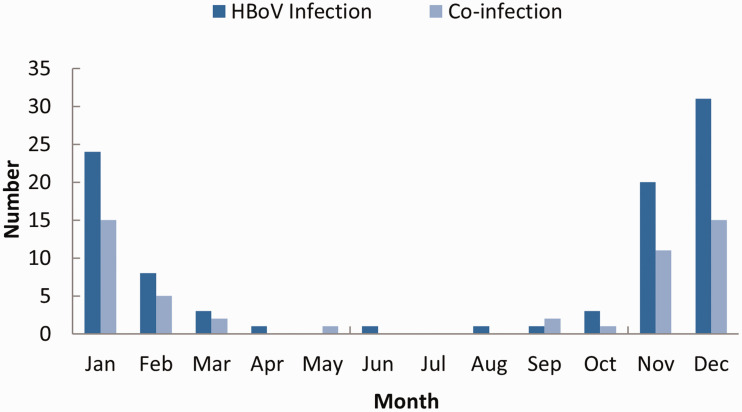
A total of 2510 infants hospitalized with ALRTIs between January 2016 to December 2020 as well as 1255 age-matched healthy infants were enrolled in the study. There were no significant differences in age or sex between the infants with ALRTIs (age 8.9 ± 2.1 months, male:female ratio 1.67:1) and the control group (age 7.4 ± 1.7 months, male:female ratio 1.73:1). Approximately 20 samples were obtained each month. HBoV DNA was detected in 145 (5.78%) of 2510 NPA samples. The median age of HBoV-positive infants was 8.4 months (range: 3 to 11 months); 89 (61.4%) were male and 56 (38.6%) were female. The male:female ratio was 1.6:1. HBoV-positive NPA samples were detected in every season, with the highest prevalence observed during the winter months. No samples from healthy control infants were HBoV-positive (Figure 1).
Figure 1.
Number of HBoV-positive infants by month.
In HBoV-positive infants, cough (130 infants, 89.7%), wheezing (92 infants, 62.1%), and fever (76 infants, 52.4%) were frequent symptoms. Abnormal chest radiography findings were present in all HBoV-positive infants, while 95 infants had pneumonia, 50 had acute bronchitis or bronchiolitis as shown by X-ray examination, 18 had focal pulmonary atelectasis, and 13 had localized pulmonary emphysema. A total of 39 infants had pO2 < 94% (all of whom were admitted to the intensive care unit [ICU]; among then, 11 infant had pO2 < 90%. In 14 infants, HBoV was complicated by acute heart failure and in 12 infants, HBoV was complicated by respiratory failure. Septicemia was diagnosed and Staphylococcus aureus isolated from one infant with ALRTI. Tables 1 and 2 summarize the clinical presentation of the infants.
Table 1.
Clinical presentation of infants with HBoV infection.
| Symptom | No. of infants with HBoV infection (%) | Mean duration of presentation (days) | p-value |
|---|---|---|---|
| Cough | 130 (89.7) | 10.3 | 0.36* |
| Wheezing | 92 (62.1) | 4.8 | 0.72* |
| Fever | 76 (52.4) | 5.1 | 0.11* |
| Nasal discharge | 38 (26.2) | 3.3 | ND |
| Vomiting | 11 (7.6) | 2 | ND |
| Diarrhea | 26 (17.9) | 1 | ND |
| Hypoxemia | 28 (19.3) | 1.5 | ND |
| Tachypnea | 58 (40.0) | 1 | ND |
*Cough, wheezing, and fever showed no differences between infants with HBoV infection alone or infants with co-infection.
ND, not done.
Table 2.
Complications in infants with HBoV infection.
| Complication | No. of infants with HBoV infection (%) |
|---|---|
| Acute heart failure | 14 (16.7) |
| Acute respiratory failure | 18 (21.4) |
| Respiratory acidosis | 16 (19.0) |
| Septicemia | 11 (13.1) |
| Mild dehydration | 10 (11.9) |
| Virulence erythra | 15 (17.9) |
Co-infection was frequently detected in winter. M. pneumoniae and respiratory syncytial virus were the most common pathogens responsible for co-infection. Three infants with severe ALRTI had two or more co-infections (Table 3). Presence of multiple co-infections was associated with ICU admission (p < 0.05) (Table 4).
Table 3.
Pathogens responsible for co-infections in infants with HBoV infection.
| Pathogen | No. of infants with HBoV infection (%) |
|---|---|
| Streptococcus pneumoniae | 16 (13.33) |
| Staphylococcus aureus | 11 (6.67) |
| Mycoplasma pneumoniae | 28 (33.33) |
| Chlamydophila pneumoniae | 7 (13.33) |
| Respiratory syncytial virus | 21 (20.00) |
| Adenovirus | 10 (13.33) |
| Total | 93 (100) |
Table 4.
Frequency of co-infection in infants with HBoV infection in the pediatric ward and the ICU.
| HBov mono-infection | Co-infection | χ2 | p-value | |
|---|---|---|---|---|
| Pediatric ward | 43 | 61 | 4.809 | 0.028 |
| ICU | 9 | 32 |
Abbreviations: ICU, intensive care unit.
Discussion
Here, we studied the epidemiological characteristics of infants with ALRTI in eastern China. The prevalence of HBoV was 5.58%, consistent with recent studies conducted in eastern China and southeast Asia15–20 but lower than prevalence estimates from Singapore21 and Japan.12 Differences among these estimates could be attributable to sample size.
Studies of the prevalence of HBoV infection in Asia showed that prevalence maxima were observed in different seasons across different regions,12,16–19,22,23 with no distinctive seasonal pattern.20 Our data revealed that although HBoV could be detected throughout the year, a higher HBoV prevalence was observed in the winter months (November, December, January). In HBoV-positive infants, co-infection was frequently observed and mainly occurred during periods of high viral transmission. We also observed a few cases of HBoV co-infection in pediatric patients with M. pneumoniae and C. pneumoniae. The etiological characteristics of co-infection require further investigation.
In this study, bronchitis, bronchiolitis, and pneumonia were frequently diagnosed in infants with cough, wheezing, and fever. Other symptoms included nasal discharge, vomiting, diarrhea, and tachypnea. Infants with severe infection had complicated dysphoria, respiratory exacerbation, and hypoxemia, and some developed respiratory acidosis, respiratory failure, and acute heart failure. Pathogens involved in co-infection included S. pneumoniae, S. aureus, M. pneumoniae, C. pneumoniae, respiratory syncytial virus, and adenovirus. Co-infection was observed in all infants with severe infection, suggesting a correlation with clinical severity. Our findings suggested that respiratory tract co-infection was a potential risk factor for HBoV-positive infants. However, HBoV was probably not an independent etiological factor in infants with severe ALRTI, as was previously reported in Switzerland.24 Although most cases of HBoV infection are mild, some may be severe. In our study, some HBoV-positive infants developed sepsis, heart failure, and respiratory failure. Previous studies have reported other severe complications of co-infection with HBoV and other viruses such as pneumothorax, acute otitis media, encephalitis, and pneumomediastinum. Our results were consistent with those of previous studies carried out worldwide. Chia-Yunn et al.25 found that HBoV infection peaked during winter and spring. Aykut et al.26 found that in Turkey, children with coinfection had higher risks of ICU admission compared with those with HBoV mono-infection. In another study carried out in Turkey, co-infections by HBoV and other viruses were frequently detected.27 A study in Finland showed that HBoV persisted in the respiratory tract for long periods, increasing the chance of co-detection with other viruses.28 Emanuels et al. also found a high prevalence of HBoV and other pathogens in Nepal.29 Nada et al. found that in Kuwait, the incidence of HBoV infection was highest among children <1 year old. Co-infection was detected in 56.8% of patients.30 The prevalence of HBoV is greatly affected by geographical location. In Italy, Cangiano et al. reported a prevalence of 1.8% over 10 consecutive years,31 while Sobkowiak et al.32 found a HBOV prevalence of 27% in wheezy children under age 2 years in Poland. In Portugal, Huguenin reported a HBoV prevalence of 37.1%.33
There were several limitations to our study. The number of HBoV positive infants was relatively small, and differences in the clinical manifestations of infection need to be further studied. The symptoms of infants were described by their parents and might not have been completely objective. The study was conducted in eastern China, and data from hospitals in other geographical areas are needed to extend our results. Further studies are required to determine the role of HBoV infection in different regions and in children of different ages. Moreover, there were no HBoV-positive infants in the control group. Further studies are needed to explore HBoV epidemiological characteristics in healthy individuals.
In conclusion, this study found a high HBoV prevalence during winter among infants in eastern China. Our data suggest that the clinical severity of disease in hospitalized infants with ALRTI might not be independently associated with HBoV infection. However, co-infection might be a risk factor for severe clinical infection.. Further studies need to be conducted to explore the clinical epidemiology of HBoV infection.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was funded by the Zhejiang Province Medicine and Health Technology Project (2020KY882).
ORCID iD: Huiqing Xu https://orcid.org/0000-0002-6259-1315
References
- 1.Bourgeois FT, Valim C, Wei JC, et al. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics 2006; 118: e1–e8. doi: 10.1542/peds.2005-2248. [DOI] [PubMed] [Google Scholar]
- 2.Marangu D, Zar HJ.Childhood pneumonia in low-and-middle-income countries: An update. Paediatr Respir Rev 2019; 32: 3–9. doi: 10.1016/j.prrv.2019.06.001. Epub 2019 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma X, Conrad T, Alchikh M, et al. Can we distinguish respiratory viral infections based on clinical features? A prospective pediatric cohort compared to systematic literature review. Rev Med Virol 2018; 28: e1997. doi: 10.1002/rmv.1997. Epub 2018 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allander T, Tammi MT, Eriksson M, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples [published correction appears in Proc Natl Acad Sci USA. 2005; 102(43): 15712]. Proc Natl Acad Sci USA 2005; 102: 12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrarca L, Nenna R, Frassanito A, et al. Human bocavirus in children hospitalized for acute respiratory tract infection in Rome. World J Pediatr 2020; 16: 293–298. doi: 10.1007/s12519-019-00324-5. Epub 2019 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Rosal T, García-García ML, Calvo C, et al. Recurrent wheezing and asthma after bocavirus bronchiolitis. Allergol Immunopathol (Madr) 2016; 44: 410–414. doi: 10.1016/j.aller.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child 2010; 95: 35–41. doi: 10.1136/adc.2008.153361. [DOI] [PubMed] [Google Scholar]
- 8.Rezes S, Söderlund-Venermo M, Roivainen M, et al. Human bocavirus and rhino-enteroviruses in childhood otitis media with effusion. J Clin Virol 2009; 46: 234–237. doi: 10.1016/j.jcv.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miron D, Srugo I, Kra-Oz Z, et al. Sole pathogen in acute bronchiolitis: Is there a role for other organisms apart from respiratory syncytial virus? Pediatr Infect Dis J 2010; 29: e7–e10. doi: 10.1097/INF.0b013e3181c2a212. [DOI] [PubMed] [Google Scholar]
- 10.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007; 44: 904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreno CM, Solís YO, O'Ryan MG.Human bocavirus: Studies in the literature and in Chile. Rev Chilena Infectol 2009; 26: 504–510. doi: 10.4067/S0716-10182009000700003. [PubMed] [Google Scholar]
- 12.Moriyama Y, Hamada H, Okada M, et al. Distinctive clinical features of human bocavirus in children younger than 2 years. Eur J Pediatr 2010; 169: 1087–1092. doi: 10.1007/s00431-010-1183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedolla-Barajas M, Montero H, Morales-Romero J, et al. Prevalence of respiratory viruses in wheezing children not older than 24 months of age. Gac Med Mex 2017; 153: 329–334. [PubMed] [Google Scholar]
- 14.Pierangeli A, Gentile M, Di Marco P, et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol 2007; 79: 463–468. doi: 10.1002/jmv.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau SK, Yip CC, Que TL, et al. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis 2007; 196: 986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chieochansin T, Samransamruajkit R, Chutinimitkul S, et al. Human bocavirus (HBoV) in Thailand: Clinical manifestations in a hospitalized pediatric patient and molecular virus characterization. J Infect 2008; 56: 137–142. doi: 10.1016/j.jinf.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JH, Chiu SC, Lin YC, et al. Clinical and genetic analysis of human bocavirus in children with lower respiratory tract infection in Taiwan. J Clin Virol 2009; 44: 219–224. doi: 10.1016/j.jcv.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun JK, Lee JH, Kim HS, et al. Establishing a surveillance network for severe lower respiratory tract infections in Korean infants and young children. Eur J Clin Microbiol Infect Dis 2009; 28: 841–844. DOI: 10.1007/s 10096 -009-0701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng M, Zhu QR, Wang XH, et al. Human bocavirus in children with respiratory tract infection in Shanghai: A retrospective study. World J Pediatr 2010; 6: 65–70. DOI: 10.1007/s12519-010-0009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharaj P, Sullender WM, Kabra SK, et al. Human bocavirus infection in children with acute respiratory tract infection in India. J Med Virol 2010; 82: 812–816. DOI: 10.1002/jmv. 21637 [DOI] [PubMed] [Google Scholar]
- 21.Tan BH, Lim EA, Seah SG, et al. The incidence of human bocavirus infection among children admitted to hospital in Singapore. J Med Virol 2009; 81: 82–89. DOI: 10.1002/jmv.21361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu XW, Duan ZJ, Qi ZY, et al. Human bocavirus infection, People’s Republic of China. Emerg Infect Dis 2007; 13: 165–168. doi: 10.3201/eid1301.060824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang LL, Tang LY, Xie ZD, et al. Human bocavirus in children suffering from acute lower respiratory tract infection in Beijing Children's Hospital. Chin Med J (Engl) 2008; 121: 1607–1610. [PubMed] [Google Scholar]
- 24.Regamey N, Frey U, Deffernez C, et al. Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J 2007; 26: 177–179. doi: 10.1097/01.inf.0000250623.43107.bc. [DOI] [PubMed] [Google Scholar]
- 25.Chuang CY, Kao CL, Huang LM, et al. Human bocavirus as an important cause of respiratory tract infection in Taiwanese children. J Microbiol Immunol Infect 2011; 44: 323–327. doi: 10.1016/j.jmii.2011.01.036 [DOI] [PubMed] [Google Scholar]
- 26.Eşki A, Öztürk GK, Çiçek C, et al. Is viral coinfection a risk factor for severe lower respiratory tract infection? A retrospective observational study. Pediatr Pulmonol 2021. doi: 10.1002/ppul.25422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakir A, Karabulut N, Alacam S, et al. Investigation of human bocavirus in pediatric patients with respiratory tract infection. J Infect Dev Ctries 2020; 14: 1191–1196. doi: 10.3855/jidc.12553 [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Perdomo MF, Mattola S, et al. Persistence of human bocavirus 1 in tonsillar germinal centers and antibody-dependent enhancement of infection. mBio 2021; 12: e03132-20. doi: 10.1128/mBio.03132-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emanuels A, Hawes SE, Newman KL, et al. Respiratory viral coinfection in a birth cohort of infants in rural Nepal. Influenza Other Respir Viruses 2020; 14: 739–746. doi: 10.1111/irv.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madi NM, Al-Adwani A.Human bocavirus (HBoV) in Kuwait: Molecular epidemiology and clinical outcome of the virus among patients with respiratory diseases. J Med Microbiol 2020; 69: 1005–1012. doi: 10.1099/jmm.0.001219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cangiano G, Nenna R, Frassanito A, et al. Bronchiolitis: Analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol 2016; 51: 1330–1335. doi: 10.1002/ppul.23476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobkowiak P, Mikoś M, Bręborowicz A, et al. Human bocavirus and metapneumovirus in acute wheezing in children – Is there a link with atopy? Clin Respir J 2020; 14: 1201–1207. doi: 10.1111/crj.13261 [DOI] [PubMed] [Google Scholar]
- 33.Huguenin A, Moutte L, Renois F, et al. Broad respiratory virus detection in infants hospitalized for bronchiolitis by use of a multiplex RT-PCR DNA microarray system. J Med Virol 2012; 84: 979–985. doi: 10.1002/jmv.23272 [DOI] [PMC free article] [PubMed] [Google Scholar]