Abstract
Objective In China, polyene phosphatidylcholine (PPC) is widely used to treat alanine aminotransferase (ALT) elevation associated with various liver diseases. Here, we assessed the efficacy and safety of PPC in treating drug-induced liver injury (DILI).
Methods Data from a multicenter retrospective cohort study (DILI-R) were analyzed to compare PPC and magnesium isoglycyrrhizinate (MgIG) for treatment of DILI. We used the Roussel Uclaf causality assessment method (RUCAM) to evaluate patients with DILI. Patients with RUCAM scores ≥6 were included in the study, while those with RUCAM scores <6 were further evaluated by a panel of hepatologists. The primary outcome was the proportion of patients with ALT normalization at discharge. Propensity score matching was used to identify 183 matched pairs of patients (366 patients in total) from 25,927 patients with DILI.
Results Among the DILI patients, 64 of 183 (34.97%) achieved normal ALT levels after treatment in both the PPC and the MgIG groups.
Conclusion There were no significant differences in safety biomarkers including serum creatinine, blood urea nitrogen, white blood cells, platelets, hemoglobin, and albumin between patients treated with PPC or MgIG. The safety and efficacy of these two agents for treatment of DILI were comparable.
Keywords: Drug-induced liver injury, polyene phosphatidylcholine, propensity score matching, magnesium isoglycyrrhizinate, Roussel Uclaf causality assessment method, alanine aminotransferase
Introduction
Drug hepatotoxicity, also known as drug-induced liver injury (DILI), is a major cause of drug withdrawal from the pharmaceutical market and failure of investigational new drugs during development. Very few regimens have been approved for treatment of DILI. However, in clinical practice, liver-protective drugs are widely used in China and some other countries.
Polyene phosphatidylcholine (PPC) is extracted from soy and is rich in polyunsaturated fatty acids including linoleic acid, linolenic acid, and oleic acid. Previous studies have suggested that PPC exerts hepatoprotective effects through multiple mechanisms including anti-inflammation, antioxidant, and immunoregulatory functions.1,2
In China, PPC is widely used to treat alanine aminotransferase (ALT) elevation associated with various liver diseases such as steatohepatitis and DILI.3–5 However, limited evidence supports the application of PPC in patients with DILI. Thus, we investigated the effects of PPC in treating DILI using data from a nationwide retrospective cohort study of patients with DILI. The efficacy and safety of PPC were compared with those of magnesium isoglycyrrhizinate (MgIG), which has received approval from the Chinese Food and Drug Administration for treatment of acute DILI.
Methods
Data from a 3-year retrospective multicentric study (DILI-R) were analyzed to compare the efficacy and safety of PPC and MgIG for treatment of DILI.
Study population
As described in our previous report,6 a 3-year (2012–2014) retrospective study of hospitalized patients was conducted involving 308 centers in China (trial registration number: NCT02407964). All patients whose diagnosis at discharge was DILI were further evaluated using the Roussel Uclaf causality assessment method (RUCAM).7–9 Patients with RUCAM scores ≥6 were enrolled in the study directly. The medical records of patients with RUCAM scores <6 were further reviewed by a panel of three hepatologists with DILI expertise (consistent with the expert opinion method of causality assessment). The expert panel evaluated these patients based on the RUCAM criteria. Patients judged by at least two of the three hepatologists as probable DILI were enrolled in the study. A total of 25,927 patients classified as probable DILI and enrolled in the DILI-R study.
In the current study, patients with DILI participating in the DILI-R study who received only PPC injections (brand name: Tianxing) or only magnesium isoglycyrrhizinate (MgIG) injections (brand name: Ganmei) for DILI therapy were identified. All patient details were deidentified. The study was approved by the Renji Hospital Ethics Committee, Shanghai Jiaotong University School of Medicine (approval number [2015]040K). Because this was an analysis of existing deidentified data, the requirement for informed consent was waived. The reporting of this study conforms to the STROBE statement.10
Study design
The study was designed to compare the efficacy and safety of PPC and MgIG for treatment of DILI. Both drugs were administered according to their labels. PPC was administered intravenously at a daily dose of 5 to 10 mL (10–20 mL for serious DILI or 30–40 mL for critical DILI). MgIG was administered intravenously at a daily dose of 20 mL or 40 mL. A 1:1 propensity score matching (PSM) was applied to ensure even distribution of confounders in the PPC group and the MgIG group.
Data collection
The following parameters were collected for all enrolled patients: (1) demographic information; (2) disease history and alcohol consumption history; (3) information regarding drugs that may have caused liver injury, including time of symptom onset after starting the drug and the time of recovery after stopping the drug; (4) symptoms and signs, including time of occurrence, time of disappearance, and detailed records of symptoms at discharge; (5) serum biochemical parameters before and during DILI, including levels of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin (TBil), direct bilirubin (DBil), albumin (ALB), and creatinine (Cr), as well as prothrombin time and the international normalized ratio; and (6) examinations to exclude other causes of liver injury. The Hepatox website (www.hepatox.org), a Chinese nationwide DILI research network resource, was used as the data collection platform for the DILI-R study.
Study endpoint
The primary endpoint was the proportion of patients with serum ALT normalization at discharge. The secondary endpoint was the time required for ALT and AST normalization.
Statistical analysis
A propensity score for each patient was calculated using multivariable logistic regression. The covariates included in the analysis were sex, age, baseline ALT level, baseline TBiL level, liver disease history, acute or chronic liver damage, and suspected drug category. We used the caliper matching algorithm to match patients treated with PPC or MgIG 1:1 without replacement (i.e., a single patient could not be selected multiple times).11
Values were given as medians and interquartile ranges or as percentages where appropriate. Inter-group differences were assessed using either the Mann–Whitney U test or the Kruskal–Wallis test. Categorical variables were analyzed using the χ2 test, the Cochran–Mantel–Haenszel χ2 test, or Fisher’s exact test as appropriate. Two-sided 95% confidence levels (CIs) were calculated. To assess the efficacy of treatment, the proportions of patients with ALT normalization were compared between the two groups were compared using an overall Chi‐square test. Statistical tests were interpreted at a two-sided significance level of 5%.
Noninferiority of the treatment group compared with the control group was assessed via the rate of ALT normalization. The noninferiority margin of the ALT normalization rate was 15%. The one-sided 97.5% CI for the difference in ALT normalization rate was set, and a value of p < 0.025 was considered statistically significant. If the 97.5% CI fell within the noninferiority range, PPC was considered noninferior to MgIG. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
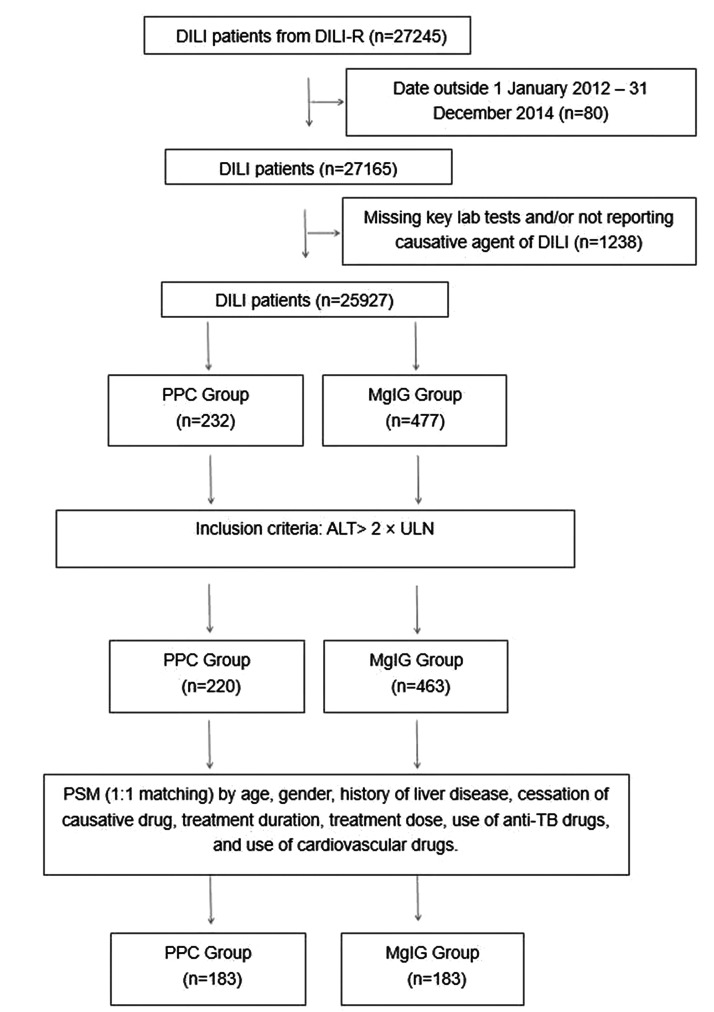
This study enrolled 232 patients with DILI treated with PPC and 477 patients with DILI treated with MgIG. After excluding patients whose ALT levels were not tested at discharge, 220 patients treated with PPC and 463 patients treated with MgIG remained. The final analysis included 183 matched pairs of patients with DILI (366 patients in total). The study flow diagram is shown in Figure 1.
Figure 1.
Study flow diagram.
DILI, drug induced liver injury; PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate; ALT, alanine aminotransferase; PSM, propensity score matching; ULN, upper limit of normal; TB, tuberculosis.
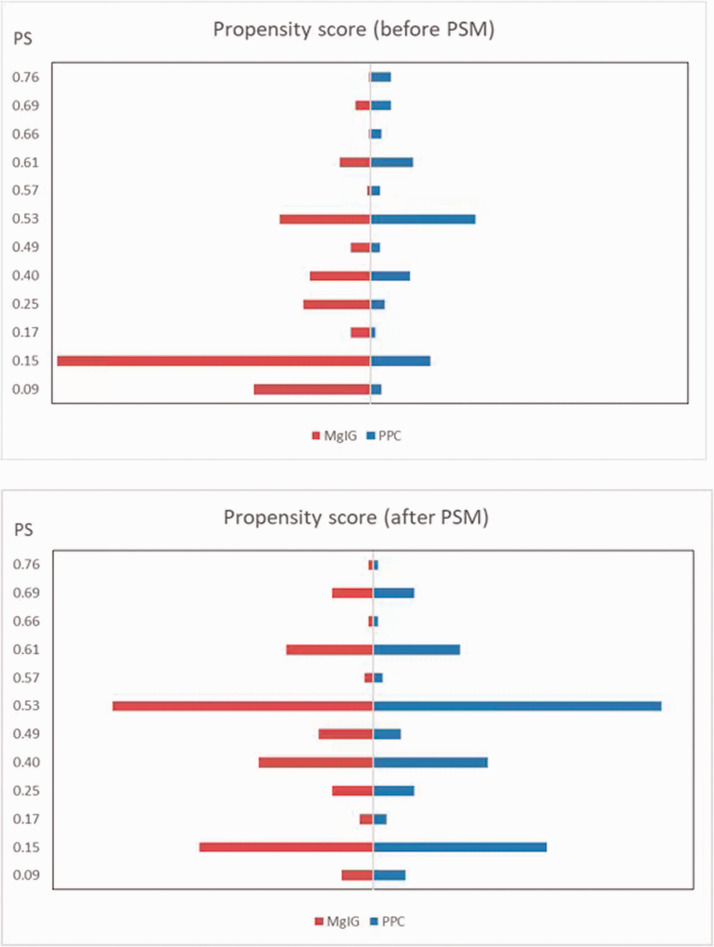
PSM was used to identify 183 well-matched pairs of patients (366 patients in total), one of whom received PPC and the other of whom received MgIG, from 25,927 patients with DILI (Figure 2). The baseline characteristics of the two groups before and after PSM, including demographics, are summarized in Table 1.
Figure 2.
Characteristics of patients before and after PSM.
PSM, propensity score matching; PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate.
Table 1.
Patient characteristics before and after propensity score matching.
| Before PSM |
After PSM |
|||||
|---|---|---|---|---|---|---|
| Variables | PPC groupN=220 | MgIG groupN=463 | p | PPC groupN=183 | MgIG groupN=183 | p |
| Age (years), n (missing) | 215 (5) | 459 (4) | 0.1650 | 178 (5) | 181 (2) | 0.1391 |
| Mean ± SD | 45.77 ± 19.52 | 48.09 ± 16.99 | 45.52 ± 19.38 | 48.51 ± 17.41 | ||
| Median | 47.00 | 49.00 | 47.00 | 50.00 | ||
| IQR | 32.00–58.00 | 37.00–60.00 | 33.00–58.00 | 36.00–61.00 | ||
| Range | 1.00–91.00 | 3.00–84.00 | 1.00–91.00 | 3.00–84.00 | ||
| Sex, n (missing) | 210 (10) | 455 (8) | 0.4929 | 174 (9) | 179 (4) | 0.6897 |
| Female | 96 (45.71%) | 221 (48.57%) | 77 (44.25%) | 83 (46.37%) | ||
| Male | 114 (54.29%) | 234 (51.43%) | 97 (55.75%) | 96 (53.63%) | ||
| Age | 215 (5) | 459 (4) | 0.8444 | 178(5) | 181(2) | 0.8237 |
| <65 years | 180 (83.72%) | 387 (84.31%) | 151 (84.83%) | 152 (83.98%) | ||
| >65 years | 35 (16.28%) | 72 (15.69%) | 27 (15.17%) | 29 (16.02%) | ||
| Liver disease history, n (missing) | 220 (0) | 463 (0) | 0.0049 | 183 (0) | 183 (0) | 1.0000 |
| Yes | 51 (23.18%) | 67 (14.47%) | 25 (13.66%) | 25 (13.66%) | ||
| No | 169 (76.82%) | 396 (85.53%) | 158 (86.34%) | 158 (86.34%) | ||
| Causative drug stopped, n (missing) | 220 (0) | 463 (0) | 0.1530 | 183 (0) | 183 (0) | 0.4728 |
| Yes | 54 (24.55%) | 138 (29.81%) | 44 (24.04%) | 50 (27.32%) | ||
| No | 166 (75.45%) | 325 (70.19%) | 139 (75.96%) | 133 (72.68%) | ||
| Treatment course, n (missing) | 218 (2) | 457 (6) | 0.0612 | 181 (2) | 180 (3) | 0.1615 |
| <14 days | 166 (76.15%) | 376 (82.28%) | 138 (76.24%) | 148 (82.22%) | ||
| >14 days | 52 (23.85%) | 81 (17.72%) | 43 (23.76%) | 32 (17.78%) | ||
| Anti-TB causative drug, n (missing) | 220 (0) | 463 (0) | 0.2015 | 183 (0) | 183 (0) | 0.5773 |
| Yes | 30 (13.64%) | 81 (17.49%) | 29 (15.85%) | 33 (18.03%) | ||
| No | 190 (86.36%) | 382 (82.51%) | 154 (84.15%) | 150 (81.97%) | ||
| Cardiovascular causative drug, n (missing) | 220 (0) | 463 (0) | 0.0131 | 183 (0) | 183 (0) | 0.1755 |
| Yes | 24 (10.91%) | 26 (5.62%) | 18 (9.84%) | 11 (6.01%) | ||
| No | 196 (89.09%) | 437 (94.38%) | 165 (90.16%) | 172 (93.99%) | ||
| ALT: interval from first to last test (days), n (missing) | 206 (14) | 436 (27) | 0.1426 | 171 (12) | 165 (18) | 0.2649 |
| Mean ± SD | 16.01 ± 29.82 | 10.63 ± 9.25 | 17.20 ± 32.33 | 10.56 ± 7.67 | ||
| Median | 8.50 | 8.00 | 9.00 | 8.00 | ||
| IQR | 5.00–17.00 | 5.00–14.00 | 6.00–17.00 | 5.00–13.00 | ||
| Range | 1.00–262.00 | 1.00–68.00 | 1.00–262.00 | 1.00–40.00 | ||
| ALT (IU/L), n (missing) | 220 (0) | 463 (0) | 0.5395 | 183 (0) | 183 (0) | 0.2556 |
| Mean ± SD | 243.75 ± 287.94 | 286.52 ± 470.90 | 254.21 ± 309.18 | 316.62 ± 600.44 | ||
| Median | 137.50 | 145.00 | 138.00 | 153.00 | ||
| IQR | 89.90–263.20 | 90.00–289.10 | 86.40–264.00 | 95.00–289.10 | ||
| Range | 36.00–1620.60 | 37.00–6238.00 | 36.00–1620.60 | 37.00–6238.00 | ||
| AST (IU/L), n (missing) | 210 (10) | 454 (9) | 0.3543 | 174 (9) | 179 (4) | 0.6034 |
| Mean ± SD | 214.16 ± 288.79 | 194.20 ± 305.45 | 219.98 ± 302.63 | 207.69 ± 336.57 | ||
| Median | 103.50 | 98.00 | 103.00 | 98.00 | ||
| IQR | 58.60–226.00 | 58.50–201.00 | 60.00–228.00 | 58.00–215.50 | ||
| Range | 24.70–1581.00 | 5.00–3093.50 | 25.00–1581.00 | 22.00–3093.50 | ||
| ALP (IU/L), n (missing) | 153 (67) | 357 (106) | 0.3456 | 125 (58) | 127 (56) | 0.9140 |
| Mean ± SD | 147.50 ± 145.56 | 143.69 ± 278.37 | 138.04 ± 130.89 | 168.88 ± 435.75 | ||
| Median | 108.00 | 100.00 | 108.00 | 105.00 | ||
| IQR | 70.00–171.60 | 71.00–150.00 | 71.00–160.00 | 69.90–154.00 | ||
| Range | 31.00–1174.20 | 12.20–4886.00 | 31.00–1174.20 | 12.20–4886.00 | ||
| γ-GT (IU/L), n (missing) | 172 (48) | 393 (70) | 0.2011 | 141 (42) | 140 (43) | 0.1871 |
| Mean ± SD | 172.22 ± 203.65 | 144.31 ± 174.20 | 168.96 ± 205.46 | 140.80 ± 178.51 | ||
| Median | 97.78 | 89.00 | 94.00 | 84.00 | ||
| IQR | 49.50–221.25 | 47.00–170.00 | 49.00–212.00 | 40.00–149.00 | ||
| Range | 5.00–1281.00 | 8.00–1290.00 | 5.00–1281.00 | 10.00–1290.00 | ||
| TBil (µmol/L), n (missing) | 184 (36) | 399 (64) | 0.3814 | 151 (32) | 150 (33) | 0.3376 |
| Mean ± SD | 56.74 ± 108.20 | 41.36 ± 79.08 | 58.80 ± 112.47 | 39.45 ± 68.94 | ||
| Median | 15.23 | 14.70 | 14.80 | 13.30 | ||
| IQR | 9.90–35.50 | 9.40–29.70 | 10.00–35.60 | 9.10–32.40 | ||
| Range | 2.30–578.30 | 1.70–574.20 | 3.60–578.30 | 1.70–537.00 | ||
| Acute DILI, n (missing) | 220 (0) | 463 (0) | 0.9052 | 183 (0) | 183 (0) | 0.1579 |
| Yes | 210 (95.45%) | 441 (95.25%) | 174 (95.08%) | 179 (97.81%) | ||
| No | 10 (4.55%) | 22 (4.75%) | 9 (4.92%) | 4 (2.19%) | ||
| RUCAM score, n (missing) | 220 (0) | 463 (0) | 0.2609 | 183 (0) | 183 (0) | 0.1136 |
| R≥5 | 70 (31.82%) | 153 (33.05%) | 60 (32.79%) | 61 (33.33%) | ||
| R≤2 | 51 (23.18%) | 86 (18.57%) | 42 (22.95%) | 25 (13.66%) | ||
| 2<R<5 | 71 (32.27%) | 177 (38.23%) | 56 (30.60%) | 64 (34.97%) | ||
| NA | 28 (12.73%) | 47 (10.15%) | 25 (13.66%) | 33 (18.03%) | ||
| TCM causative drug, n (missing) | 220 (0) | 463 (0) | 0.1026 | 183 (0) | 183 (0) | 0.1390 |
| Yes | 61 (27.73%) | 102 (22.03%) | 49 (26.78%) | 37 (20.22%) | ||
| No | 159 (72.27%) | 361 (77.97%) | 134 (73.22%) | 146 (79.78%) | ||
| Antitumor and immunomodulatory causative drug, n (missing) | 220 (0) | 463 (0) | 0.0131 | 183 (0) | 183 (0) | 0.1755 |
| Yes | 24 (10.91%) | 26 (5.62%) | 18 (9.84%) | 11 (6.01%) | ||
| No | 196 (89.09%) | 437 (94.38%) | 165 (90.16%) | 172 (93.99%) | ||
PSM, propensity score matching; PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate; SD, standard deviation; IQR, interquartile range; TB, tuberculosis; ALT, alanine aminotransferase; IU, international unit; AST, asparagine aminotransferase; ALP, alkaline phosphatase; γ-GT, gamma-glutamyl transpeptidase; TBil, total bilirubin; DILI, drug induced liver injury; RUCAM, Roussel Uclaf causality assessment method; NA, not applicable; TCM, traditional Chinese medicine.
Efficacy
Primary endpoint
We first compared the efficacy of PPC and MgIG in treating DILI as measured via ALT levels at discharge (Table 2). Sixty-four of 183 (34.97%) DILI patients in both the PPC and MgIG groups achieved normal ALT levels at discharge. Thus, there was no significant difference between the two groups.
Table 2.
Efficacy assessment via ALT levels.
| Assessment | PPC group | MgIG group | p |
|---|---|---|---|
| Overall ALT normalization | |||
| N (missing) | 183 (0) | 183 (0) | 1.0000 |
| Yes | 64 (34.97%) | 64 (34.97%) | |
| No | 119 (65.03%) | 119 (65.03%) | |
| Time to normalization (days) | |||
| N (missing) | 61 (3) | 60 (4) | 0.2246 |
| Mean ± SD | 6.75 ± 6.17 | 8.15 ± 6.78 | |
| Median | 5.00 | 7.50 | |
| IQR | 2.00–9.00 | 2.00–11.00 | |
| Range | 1.00–27.00 | 1.00–33.00 |
ALT, alanine aminotransferase; PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate; SD, standard deviation; IQR, interquartile range.
Secondary endpoint
Similar lengths of time were required for ALT normalization among patients with DILI treated with PPC and MgIG (median 5 days vs. 7.5 days, respectively). There was no statistically significant difference between the two groups.
We next compared the efficacy of PPC and MgIG in treating DILI as measured via AST levels at discharge (Table 3). Seventy-five of 183 (40.98%) patients in the PPC group achieved normal AST levels after treatment, while 89 of 183 (48.63%) patients in the MgIG group achieved normal AST levels after treatment. There was no significant difference between the two groups.
Table 3.
Efficacy Assessment via AST levels.
| Assessment | PPC group | MgIG group | p |
|---|---|---|---|
| Overall ALT normalization | 0.1411 | ||
| N (missing) | 183 (0) | 183 (0) | |
| Yes | 75 (40.98%) | 89 (48.63%) | |
| No | 108 (59.02%) | 94 (51.37%) | |
| Time to normalization (days) | 0.4996 | ||
| N (missing) | 55 (20) | 61 (28) | |
| Mean ± SD | 7.51 ± 5.69 | 7.95 ± 5.68 | |
| Median | 7.00 | 7.00 | |
| IQR | 3.00–9.00 | 5.00–9.00 | |
| Range | 1.00–9.00 | 1.00–33.00 |
AST, asparagine aminotransferase; PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate; SD, standard deviation; IQR, interquartile range.
Analyses of efficacy stratified by sex, age, ALT level, and cessation of the suspected causative drug also showed no significant difference between the two groups (Table 4).
Table 4.
Frequency of ALT normalization by stratified variables.
| Variables | PPC group | MgIG group | p |
|---|---|---|---|
| Sex: male, n (missing) | 97 (0) | 96 (0) | 0.0538 |
| Yes | 39 (40.21%) | 26 (27.08%) | |
| No | 58 (59.79%) | 70 (72.92%) | |
| Sex: female, n (missing) | 77 (0) | 83 (0) | 0.0810 |
| Yes | 24 (31.17%) | 37 (44.58%) | |
| No | 53 (68.83%) | 46 (55.42%) | |
| Age<65 years, n (missing) | 151 (0) | 152 (0) | 0.4458 |
| Yes | 57 (37.75%) | 51 (33.55%) | |
| No | 94 (62.25%) | 101 (66.45%) | |
| Age>65 years, n (missing) | 27 (0) | 29 (0) | 0.0630 |
| Yes | 5 (18.52%) | 12 (41.38%) | |
| No | 22 (81.48%) | 17 (58.62%) | |
| ALT>3ULN, n (missing) | 96 (0) | 120 (0) | 0.3190 |
| Yes | 20 (20.83%) | 32 (26.67%) | |
| No | 76 (79.17%) | 88 (73.33%) | |
| ALT<3ULN, n (missing) | 87 (0) | 63 (0) | 0.9789 |
| Yes | 44 (50.57%) | 32 (50.79%) | |
| No | 43 (49.43%) | 31 (49.21%) | |
| Stop causative drug, n (missing) | 44 (0) | 50 (0) | 0.3082 |
| Yes | 14 (31.82%) | 21 (42.00%) | |
| No | 30 (68.18%) | 29 (58.00%) | |
| Continue causative drug, n (missing) | 139 (0) | 133 (0) | 0.5269 |
| Yes | 50 (35.97%) | 43 (32.33%) | |
| No | 89 (64.03%) | 90 (67.67%) | |
ALT, alanine aminotransferase; PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate; ULN, upper limit of normal.
Safety assessment
Complete blood counts and biochemistry profiles including serum Cr, blood urea nitrogen, white blood cells, platelets, hemoglobin, and ALB were compared between the two groups (Table 5). There was no significant difference in any safety parameter between the two groups.
Table 5.
Safety assessment of PPC and MgIG for treatment of DILI.
| Variables | PPC groupN = 183 | MgIG groupN = 183 | p |
|---|---|---|---|
| Outcome after treatment, n (missing) | 183 (0) | 183 (0) | >0.05 |
| Cured and fully relieved | 162 (88.52%) | 153 (83.61%) | |
| Worse | 6 (3.28%) | 4 (2.19%) | |
| Death | 2 (1.09%) | 1 (0.55%) | |
| Unchanged | 4 (2.19%) | 4 (2.19%) | |
| Unknown | 9 (4.92%) | 21 (11.48%) | |
| Cr change (normal → abnormal), n (missing) | 79 (0) | 117 (0) | 0.1913 |
| Yes | 5 (6.33%) | 3 (2.56%) | |
| No | 74 (93.67%) | 114 (97.44%) | |
| Cr change (abnormal → worse), n (missing) | 27 (0) | 6 (0) | 0.4916 |
| Yes | 2 (7.41%) | 0 (0.00%) | |
| No | 25 (92.59%) | 6 (100.00%) | |
| Cr change (normal → abnormal & abnormal → worse), n (missing) | 106 (0) | 123 (0) | 0.1241 |
| Yes | 7 (6.60%) | 3 (2.44%) | |
| No | 99 (93.40%) | 120 (97.56%) | |
| BUN change (normal → abnormal), n (missing) | 89 (0) | 96 (0) | 0.5393 |
| Yes | 3 (3.37%) | 5 (5.21%) | |
| No | 86 (96.63%) | 91 (94.79%) | |
| BUN change (abnormal → worse), n (missing) | 14 (0) | 20 (0) | 0.3475 |
| Yes | 2 (14.29%) | 1 (5.00%) | |
| No | 12 (85.71%) | 19 (95.00%) | |
| BUN change (normal → abnormal & abnormal → worse), n (missing) | 103 (0) | 116 (0) | 0.9143 |
| Yes | 5 (4.85%) | 6 (5.17%) | |
| No | 98 (95.15%) | 110 (94.83%) | |
| WBC change (normal → abnormal), n (missing) | 130 (0) | 125 (0) | 0.9233 |
| Yes | 11 (8.46%) | 11 (8.80%) | |
| No | 119 (91.54%) | 114 (91.20%) | |
| WBC change (abnormal → worse), n (missing) | 50 (0) | 47 (0) | 0.9272 |
| Yes | 4 (8.00%) | 4 (8.51%) | |
| No | 46 (92.00%) | 43 (91.49%) | |
| WBC change (normal → abnormal & abnormal → worse), n (missing) | 180 (0) | 172 (0) | 0.8964 |
| Yes | 15 (8.33%) | 15 (8.72%) | |
| No | 165 (91.67%) | 157 (91.28%) | |
| Hb change (normal → abnormal), n (missing) | 117 (0) | 113 (0) | 0.5868 |
| Yes | 14 (11.97%) | 11 (9.73%) | |
| No | 103 (88.03%) | 102 (90.27%) | |
| Hb change (abnormal → worse), n (missing) | 63 (0) | 56 (0) | 0.1303 |
| Yes | 0 (0.00%) | 2 (3.57%) | |
| No | 63 (100.00%) | 54 (96.43%) | |
| Hb change (normal → abnormal & abnormal → worse), n (missing) | 180 (0) | 169 (0) | 0.9762 |
| Yes | 14 (7.78%) | 13 (7.69%) | |
| No | 166 (92.22%) | 156 (92.31%) | |
| PLT change (normal → abnormal), n (missing) | 137 (0) | 122 (0) | 0.7986 |
| Yes | 9 (6.57%) | 9 (7.38%) | |
| No | 128 (93.43%) | 113 (92.62%) | |
| PLT change (abnormal → worse), n (missing) | 42 (0) | 47 (0) | 0.0623 |
| Yes | 3 (7.14%) | 0 (0.00%) | |
| No | 39 (92.86%) | 47 (100.00%) | |
| PLT change (normal → abnormal & abnormal → worse), n (missing) | 179 (0) | 169 (0) | 0.5894 |
| Yes | 12 (6.70%) | 9 (5.33%) | |
| No | 167 (93.30%) | 160 (94.67%) | |
| ALB change (normal → abnormal), n (missing) | 91 (0) | 100 (0) | 0.6105 |
| Yes | 8 (8.79%) | 11 (11.00%) | |
| No | 83 (91.21%) | 89 (89.00%) | |
| ALB change (abnormal → worse), n (missing) | 41 (0) | 35 (0) | |
| Yes | 41 (100.00%) | 35 (100.00%) | |
| ALB change (normal → abnormal & abnormal → worse), n (missing) | 132 (0) | 135 (0) | 0.5071 |
| Yes | 8 (6.06%) | 11 (8.15%) | |
| No | 124 (93.94%) | 124 (91.85%) |
PPC, polyene phosphatidylcholine; MgIG, magnesium isoglycyrrhizinate; DILI, drug induced liver injury; Cr, creatinine; BUN, blood urea nitrogen; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; ALB, albumin.
Discussion
DILI can result from both idiosyncratic and intrinsic mechanisms. Little is known with certainty regarding the mechanisms of idiosyncratic DILI. However, there is growing evidence that idiosyncratic DILI is primarily immune-mediated and is caused by reactive metabolites. It is imperative that upon the development of DILI, the causative drug should be discontinued, especially in the presence of elevated transaminases and/or jaundice. Stopping the causative medication is clearly the most important treatment for patients with DILI. However, this may put patients at risk of primary disease progression. Some medical interventions, including N-acetylcysteine and corticosteroids, have shown clinical benefit in selected patients according to some clinical studies.12 In this study, ALT normalization and time to ALT normalization were used to assess the efficacy of DILI therapy.
There has been substantial interest in drug treatment of DILI. In addition to N-acetylcysteine and corticosteroids, liver-protective drugs such as MgIG and PPC are commonly used in some countries to counter the hepatotoxicity of antitumor and antituberculosis drugs. MgIG is the magnesium salt of the saponin isoglycyrrhizinate, a derivative of glycyrrhizic acid with anti-inflammatory, antioxidant, and hepatoprotective properties.12–14 MgIG has been shown to reduce ALT and AST levels,15 and may prevent or ameliorate hepatotoxicity through scavenging of free radicals. PPC, also referred to as phosphatidylcholine, is a nontoxic phospholipid enriched in polyunsaturated fatty acids that serves as a resource for biomembranes. PPC has been shown to increase membrane function and integrity. PPC has anti-inflammation, antioxidant, and immunoregulatory functions.16
While discovery of new agents, mechanisms, and risk factors involved in DILI is ongoing, advances in the treatment of acute DILI have been slower. A few years ago, MgIG was approved by the Chinese Food and Drug Administration as a safe and effective treatment for patients with acute DILI. This approval provides an opportunity for comparing PPC and other liver-protection agents with MgIG for treatment of patients with DILI. In this study, PPC and MgIG were comparable in both efficacy and safety.
Randomized controlled trials are viewed as the most rigorous tools available to study medical interventions. A propensity score is defined as the probability of each individual study patient being assigned to a group of interest for comparison purposes. Propensity score adjustment is a method of ensuring an even distribution of confounders between groups, thereby increasing inter-group comparability. Propensity score analysis is, therefore, increasingly applied in observational studies.11 In retrospective studies like this one, on should make every effort to recapitulate the rigor and strength of randomized controlled trials. However, observational studies may have inherent indication biases, and the tools available to address such biases must be considered. Specifically, we used PSM in this study. This tool allowed us to group subjects according to their propensity to be assigned to a particular treatment group and thus to account for indication bias.
Conclusion
In this study, two agents used for treatment of DILI (PPC and MgIG) were comparable in both efficacy and safety.
Acknowledgment
We thank the curators of the Hepatox website (www.hepatox.org), the Chinese nationwide DILI registry, for providing the DILI-R study data for this analysis.
Footnotes
Authors' contributions: Yimin Mao, Qingling Xu, Jing Li, and Jianzhong Zhang were responsible for study design and drafting and critical revision of the manuscript. Yunsong Qian, Jing Zhang, Liangming Liu, Wei Zhong, Yongfeng Wang, Jieting Tang, Minde Zeng, and Xian Han were responsible for data collection and analysis. Xiaohong Lei was responsible for critical revision of the manuscript.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding statement: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Major Project of National Twelfth Five-Year Plan (2012ZX09303-001), the Major Project of National Thirteenth Five-Year Plan (2017ZX09304016), the National Natural Science Foundation of China (NSFC 81670524 and 81970513), the Project of Shanghai Shenkang Hospital Development Center (16CR2009A), and the Project of Clinical Research Center, Shanghai Jiao Tong University School of Medicine (DLY201607).
Data availability: The data used to support the findings of this study were derived from a multicenter retrospective cohort study (DILI-R).
ORCID iD: Yimin Mao https://orcid.org/0000-0002-2928-3425
References
- 1.Xiang Z, Chen YP, Ma KF, et al. The role of ursodeoxycholic acid in non-alcoholic steatohepatitis: A systematic review. BMC Gastroenterol. 2013; 13: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duan RL, Sun X, Liu J, et al. Mixed micelles loaded with silybin-polyene phosphatidylcholine complex improve drug solubility. Acta Pharmacol Sin. 2011; 32: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan XF, Deng YQ, Ye L, et al. Effect of Xuezhikang capsule on serum tumor necrosis factor-alpha and interleukin-6 in patients with nonalcoholic fatty liver disease and hyperlipidemia. Chin J Integr Med. 2010; 16:119–123. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Zhang XJ, Lan Y, et al. Treatment of non-alcoholic fatty liver disease by Qianggan capsule. Chin J Integr Med. 2010; 16:23–27. [DOI] [PubMed] [Google Scholar]
- 5.Cao M, Li X, Zhang B, et al. The effect of polyene phosphatidyl choline intervention on nonalcoholic steatohepatitis and related mechanism. Am J Transl Res. 2016; 8: 2325–2330. [PMC free article] [PubMed] [Google Scholar]
- 6.Shen T, Liu Y, Shang J, et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology .2019; 156: 2230–2241.e11. [DOI] [PubMed] [Google Scholar]
- 7.Danan G, Benichou C.Causality assessment of adverse reactions to drugs I. A novel method based on the conclusions of international consensus meetings: Application to drug induced liver injuries. J Clin Epidemiol. 1993; 46: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 8.Danan G, Teschke R.RUCAM in drug and herb induced liver injury: The update. Int J Mol Sci. 2016; 17: 14–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teschke R.Idiosyncratic DILI: Analysis of 46,266 cases assessed for causality by RUCAM and published from 2014 to early 2019. Front Pharmacol. 2019; 10: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 11.Guo S and Fraser MW. Propensity score analysis: Statistical methods and applications. 2nd ed. Thousand Oaks, CA: Sage Publications, 2015.
- 12.Katarey D, Verma S.Drug-induced liver injury. Clin Med (Lond). 2016; 16(Suppl 6): s104–s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie C, Li X, Wu J, et al. Anti‐inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation. 2015; 38: 1639–1648. [DOI] [PubMed] [Google Scholar]
- 14.Yan Y, Mo Y, Zhang D.Magnesium isoglycyrrhizinate prevention of chemotherapy‐induced liver damage during initial treatment of patients with gastrointestinal tumors. Zhonghua Gan Zang Bing Za Zhi. 2015; 23: 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wang Z, Gao M, et al. Efficacy and safety of magnesium isoglycyrrhizinate injection in patients with acute drug-induced liver injury: A phase II trial. Liver Int. 2019; 39: 2102–2111. [DOI] [PubMed] [Google Scholar]
- 16.Pan W, Hao WT, Xu HW, et al. Polyene phosphatidylcholine inhibited the inflammatory response in LPS-stimulated macrophages and ameliorated the adjuvant-induced rat arthritis. Am J Transl Res. 2017; 9: 4206–4216. [PMC free article] [PubMed] [Google Scholar]