SUMMARY
Cardiopulmonary resuscitation (CPR) is an emergency lifesaving endeavor, performed in either the hospital or outpatient settings, that significantly improves outcomes and survival rates when performed in a timely fashion. As with any other medical procedure, CPR can bear potential risks not only for the patient but also for the rescuer. Among those risks, transmission of an infectious agent has been one of the most compelling triggers of reluctance to perform CPR among providers. The concern for transmission of an infection from the resuscitated subject may impede prompt initiation and implementation of CPR, compromising survival rates and neurological outcomes of the patients. Infections during CPR can be potentially acquired through airborne, droplet, contact, or hematogenous transmission. However, only a few cases of infection transmission have been actually reported globally. In this review, we present the available epidemiological findings on transmission of different pathogens during CPR and data on reluctance of health care workers to perform CPR. We also outline the levels of personal protective equipment and other protective measures according to potential infectious hazards that providers are potentially exposed to during CPR and summarize current guidelines on protection of CPR providers from international societies and stakeholders.
KEYWORDS: infection, airborne, blood-borne, SARS-CoV-2, COVID-19, cardiopulmonary, resuscitation, transmission
INTRODUCTION
Cardiopulmonary resuscitation (CPR) is an emergency lifesaving endeavor performed to increase survival rates following cardiac or respiratory arrest through high-quality external chest compressions and (usually) invasive or noninvasive ventilation. It is performed either in the outpatient setting or in the hospital, with Emergency Departments (EDs) representing the most frequent places wherein hospital CPR is provided. It is estimated that about 100,000 resuscitations are performed annually in the United States by health care workers (HCWs) and non-HCW bystanders (1).
Although it is a lifesaving process, the transmission of infections during CPR remains an existing hazard, especially during the ongoing COVID-19 pandemic (2). During CPR, providers are prone to pathogen exposure through airborne, droplet, contact, or hematogenous transmission, raising public concerns on safety issues, especially during outbreaks, such as the recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (3, 4). The risk of transmission during mouth-to-mouth ventilation causes anxiety, uncertainty, and fear among CPR providers, leading to substantial barriers for the provision of CPR and even reluctance to perform it (5). Widespread concerns of infection transmission among HCWs lead to ethical challenges on balancing between possible risks for the providers and the potential benefit for the patient from this lifesaving process.
Infection prevention and control measures are imperative for the safety of patients, HCWs and the community in total, and the appropriate use of personal protective equipment (PPE) is an effective way to reduce infection transmission (6, 7). Suboptimal adherence to basic infection control measures by HCWs could result in widespread in-hospital outbreaks (3, 4).
The aim of this review is to outline the risk of infection transmission during CPR and to summarize the current guidelines on the proposed infection control measures by the person performing the process. Moreover, we will investigate current data on willingness or reluctance of HCWs to perform CPR.
MODES OF INFECTION TRANSMISSION DURING CPR
There are various potential ways of contracting communicable diseases when providing CPR. Modes of transmission can be either direct or indirect. Direct transmission occurs through contact with infectious materials, including skin, secretions, blood, and body fluids, as well as through large droplet (diameter > 5 μm) spread. Indirect modes consist of the transfer of pathogens by suspended air particles (airborne transmission of droplet nuclei, <5 μm) (8). Table 1 shows potential pathogens categorized by mode of transmission and examples of procedures undertaken during CPR that can contribute to transmission.
TABLE 1.
Pathogens categorized by mode of transmission and examples of procedures undertaken during CPR that can be transmitted
| Mode of transmission | Pathogensa | Examples of condition(s) leading to transmission during CPR |
|---|---|---|
| Direct transmission | ||
| Contact with blood (blood-borne) | HIV*, HBV*, HCV*, Ebola | Needlestick injury during cannulation and blood sampling |
| Contact with body fluids | Ebola, CCHF virus, Neisseria meningitidis, HSV, Norovirus, HAV, Clostridium difficile, other gastrointestinal pathogens (Salmonella, Shigella) | Contact with pleural fluid during insertion of ICD, contact with saliva during mouth-to-mouth ventilation, contact with feces |
| Contact with skin | VZV, HSV, HPV*, Staphylococcus aureus* and Streptococcus pyogenes* (from impetigo lesions) | Chest compressions without gloves, mouth-to-mouth ventilation |
| Contact with contaminated surfaces | Influenza, Clostridium difficile (spores), SARS-CoV-2(?), CMV | Unprotected handling of equipment |
| Droplet transmission (>5-μm droplet diam) | SARS-CoV-2, MERS-CoV, influenza, CMV | Intubation, suctioning of secretion, administration of nebulized drugs |
| Indirect transmission | ||
| Airborne transmission | Measles, VZV, Mycobacterium tuberculosis, influenza, CMV | Unprotected |
*, requires skin breach. Abbreviations: CCHF virus, Crimean-Congo hemorrhagic fever virus; CMV, cytomegalovirus; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSV, herpes simplex virus; ICD, intercostal chest drain; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VZV, varicella-zoster virus.
EPIDEMIOLOGY AND RISK OF INFECTION TRANSMISSION DURING CPR
In the era of emerging and re-emerging communicable diseases, infection transmission to HCWs may occur during any CPR related procedure, including intravenous cannulation, mouth-to-mouth resuscitation, noninvasive and invasive ventilation, insertion of intercostal chest or pericardial drain. In theory, unprotected rescuers may be exposed to numerous pathogens during CPR, either through direct contact with the patient’s blood or other body fluids or through the production of aerosols (Table 2). Outside the context of highly transmissible pathogens such as measles, influenza, or SARS-CoV-2, the risk of transmission remains extremely low, especially when CPR providers adhere tightly to the proper use of PPE. Specifically for mouth-to-mouth ventilation, only 15 isolated cases of infection transmission have been reported, since its first medical use in 1744 (9, 10).
TABLE 2.
Selection of pathogens that can be possibly transmitted during CPRa
| Pathogen | High-risk exposure | PEP | Preventative measures for HCWs |
|---|---|---|---|
| Viruses | |||
| Crimean-Congo hemorrhagic fever virus | Unprotected contact with infectious fomites, blood and body fluids including parenteral exposures to infectious blood or body fluids | Ribavirin (uncertain evidence for its efficacy) | PPE, hand hygiene, proper cleaning and disposal of medical equipment |
| Cytomegalovirus | Droplets, aerosol or direct contact, contact with contaminated surfaces or body fluids | NA | Hand hygiene, avoid oral contact, sharing objects that have contact with saliva, particularly when you are pregnant or immunocompromised |
| Ebola virus | Direct contact with infected blood or body fluids | Isolation | PPE, hand hygiene, proper cleaning and disposal of medical equipment, rVSVΔG-ZEBOV-GP Ebola vaccine (Ervebo) (approved by the FDA for adults at potential risk of exposure to Ebola – including HCWs) |
| Hepatitis B virus | Percutaneous, mucosal, or nonintact skin exposure to infectious blood or body fluids | HBIG alone or in combination with HepB vaccination within 24 h | HepB vaccination, HBIG adjunct to HepB vaccination |
| Hepatitis C virus | Parenteral exposures to infectious blood or body fluids that contain blood | Baseline and follow-up testing, immunoglobulin not effective, no data for use on antivirals | Personal protective equipment |
| Herpes simplex virus 1 | Oral-to-oral contact, oral-to-genital contact | Acyclovir soon after mouth-to-mouth ventilation | Avoid oral contact, sharing objects that have contact with saliva |
| Human immunodeficiency virus | Needlestick injury involving infected blood, fluid splashes to mucus membranes extremely rare | Tenofovir, emtricitabine, and raltegravir within 72 h | Standard precautions (gloves, googles, hand hygiene, safety devices to prevent needlestick) |
| Influenza virus (seasonal, avian) | Direct contact with infectious droplets (and during aerosol producing procedures) | Oseltamivir, inhaled zanamivir (zanamivir is not recommended for people with underlying respiratory disease) | Hand hygiene, PPE, airborne isolation precautions |
| Measles | Direct contact with infectious droplets or by airborne spread | MMR vaccine within 72 h or immunoglobulin within 6 days of exposure | MMR vaccine, airborne isolation precautions |
| MERS-CoV | Through respiratory secretions/airborne spread | NA | Hand hygiene, PPE, airborne isolation precautions |
| SARS-CoV-2 | Exposure of eyes, nose, or mouth to material potentially containing SARS-CoV-2, particularly if present in a room for an aerosol-generating procedure (prolonged [>15-min] close contact, or independently of time if aerosol-generating procedure) | NA | Hand hygiene, PPE, airborne isolation precautions |
| Varicella-zoster virus | Droplets, aerosol, or direct contact | Varicella vaccine within 5 days from exposure; pregnant women, neonates, or immunocompromised persons: varicella-zoster immune globulin | Varicella vaccine |
| Bacteria | |||
| Mycobacterium tuberculosis | Airborne transmission from patients with TB disease of the lungs or throat | If tuberculin skin test is ≥5 mm or the interferon assay is positive, isoniazid plus vitamin B6 for 9 mo | Isoniazid, rifapentine, rifampin (various combinations and duration), BGC vaccination |
| Neisseria meningitidis | Close contact with respiratory and throat secretions | Ciprofloxacin or azithromycin or ceftriaxone or rifampin as early as possible | Meningococcal vaccine |
| Salmonella spp. | Fecal-oral route | NA | Hand hygiene |
| Shigella spp. | Fecal-oral transmission (foodborne and waterborne infection) | Isolation | Hand hygiene |
| Syphilis | Sexually transmitted (genital-fecal-oral), mother to infant, blood transfusion, direct contact with syphilis ulcer | Single dose of benzathine penicillin G (2.4 million units) | Contact of patient with gloves |
The most important pathogens potentially transmitted during CPR, definition of high-risk exposure, and recommended postexposure prophylaxis (PEP) for each pathogen are included. Abbreviations: CMV, cytomegalovirus; HBIG, hepatitis B immune globulin; HCWs, health care workers; HepB, hepatitis B vaccination; MERS-CoV, Middle East respiratory syndrome coronavirus; MMR, measles, mumps, rubella; NA, not applicable; PEP, postexposure prophylaxis; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Viral Infections
Influenza.
HCWs are at high risk for both exposure and nosocomial transmission of seasonal influenza through fomites, large droplets, and aerosols generated during aerosol-producing procedures (11). In fact, this risk was quantified in a systematic review and meta-analysis by Kuster et al., who found that “incidence rates of unvaccinated as well as vaccinated individuals with serologically proven, combined symptomatic and asymptomatic influenza is higher among HCWs than non-HCWs” (12). Although no epidemiological data are available regarding transmission during CPR specifically, rescuers are at higher risk during CPR since many aerosol-producing procedures are performed. In contrast to other possibly transmitted respiratory pathogens during resuscitation, seasonal influenza can be effectively prevented with annual vaccination. Therefore, the World Health Organization (WHO) highlights the importance of immunization for influenza among HCWs (13).
The highly pathogenic avian influenza H5N1 strain emerged initially in China and subsequently spread in Europe and Africa; this strain causes outbreaks, involving several hundred human cases and resulting in high death rates (14). Similarly, other avian influenza strains, like the H7N9 strain, are responsible for outbreaks among humans and for epidemics in poultry (14). Since the primary source of infection is contact with infected poultry rather than human-to-human transmission, little is known regarding the risk of transmission of the highly pathogenic avian influenza H5N1 strain to HCWs, especially during CPR. However, in a study involving 60 HCWs who cared for two patients with H5N1 with limited PPE and infection control measures, found no transmission of the virus, suggesting inefficient human-to-human H5N1 transmission (15).
SARS-CoV-2, SARS-CoV-1, and MERS-CoV.
Since the emergence of SARS-CoV-2 pandemic, more than 160 million cases and 3.3 million COVID-19-attributable deaths have been reported globally as of 13 May 2021 (16). Understandably, the pressure generated by the continuously rising cases is immense. Thus, front-line HCWs are at high-risk for contracting SARS-CoV-2 infection, since this virus is very contagious; the basic reproduction number for SARS-CoV-2 without social distancing has been estimated between 2.0 and 4.0 (17–19). SARS-CoV-2 is transmitted primarily through close contact with droplets produced by an infected person or through aerosol-producing procedures (20). Moreover, transmission through contact of contaminated objects (fomite transmission) is possible and remains under investigation (20).
As the pandemic evolves, data accumulate on the actual disease burden among HCWs. In Wuhan, China, SARS-CoV-2 infections among HCWs accounted for 29% (21), although countrywide the reported percentage in HCWs was as low as 4.4% until 24 February 2020 (22). In Italy, SARS-CoV-2 infections in HCWs accounted for approximately 10% of all COVID-19 cases until April 2020, although this rate was double in March 2020 (22, 23). Moreover, a prospective cohort study in Spain during the first wave, documented a COVID-19 incidence of 11.1% among 1,191 HCWs irrespective of different occupational exposure levels (24). Regarding the actual risk of SARS-CoV-2 transmission during CPR, data are limited. According to a study during the first wave (January to April 2020) in the United States, the emergency medical service (EMS) responded to 1,067 outpatient cardiac arrest calls of which 478 (44.8%) were treated by EMS; suspected or laboratory confirmed cases of COVID-19 accounted for 3.7% of patients on EMS arrival and 6.5% of EMS-treated cases (25). After 26 February 2020, 5% of outpatient cardiac arrests in homes and 11% of outpatient cardiac arrests in nursing homes involved patients with COVID-19 (25). Although the authors did not report COVID-19 infection among EMS CPR providers, they assumed that the risk of contracting COVID-19 infection during hands-only CPR performance without PPE is 1 bystander per 100 treated cases (based on a 10% prevalence of COVID-19 and a 10% risk of transmission during unprotected CPR) (25). Moreover, the prevalence of symptomatic COVID-19 infection during unprotected exposure has been reported to be lower than 5% among exposed HCWs in the United States (26). Thus, even during the following waves of COVID-19 pandemic, occupational exposure is not by any means negligible, but a high index of suspicion, preparedness, and legitimate protection for possible cases can definitely eliminate the risk of transmission. This may also change with more transmissible virus variants like those recently reported (27).
In 2003, an outbreak by SARS-CoV-1 was reported, causing high morbidity and mortality rates. The mode of transmission of this virus is similar to that of SARS-CoV-2. Only one report has been published to investigate the transmission of SARS-CoV-1 during CPR (28).This study describes the apparent transmission of SARS-CoV-1 from a patient to HCWs during CPR, despite wearing suitable PPE. It is worth noting that there were no breaches in droplet protection equipment, the exposure times were short, the patient was unresponsive, and the intubation procedure was performed quickly. The authors of that report concluded that the possible source of exposure was the aerosolization of SARS-CoV-1 caused by the ventilation of the patient with a bag-valve mask, without a bacterial/viral filter, before the intubation (28).
Middle East respiratory syndrome coronavirus (MERS-CoV) is the most fatal virus among the three emerged betacoronaviruses since 2002 (SARS-CoV-1, SARS-CoV-2, and MERS-CoV), with a case fatality rate of 35% (29). Human-to-human transmission is not the primary way of transmission, as it requires close contact; thus, the majority of human-to-human transmission cases have been attributed to occupational exposure within health care settings and HCWs with inadequate or inappropriate PPE donning are at high-risk for acquiring the infection (29, 30). Nonetheless, there are no epidemiological data regarding the risk of transmission during resuscitation to date.
Other respiratory viruses.
To date, there no reports of transmission of other respiratory viruses, such as respiratory syncytial virus, human rhinovirus, and human metapneumovirus, during CPR.
Measles.
Clusters of unvaccinated or incompletely vaccinated people, including HCW and non-HCW CPR providers, are susceptible to acquire measles from infected patients, leading to outbreaks, as seen in many countries during the last few years (31, 32). Measles is one of the world’s most contagious diseases, with a basic reproduction number of about 12 to 18 (33). It is transmitted through droplets or via breathing contaminated air or touching contaminated surfaces as it remains active and contagious in the air and on infected surfaces for up to 2 h (34). Nonimmune CPR providers are at high risk for contracting measles. Although no data are available for transmissions during CPR, sporadic clusters of HCWs infected in hospital settings have been reported (35, 36). In particular, in the Netherlands, measles occurred in six twice-vaccinated HCWs, despite two having adequate preexposure neutralizing antibodies. None of these cases had severe measles, and none had onward transmission (35). In another outbreak in China, measles occurred in 19 HCWs due to the low vaccination coverage, low knowledge about measles, delay in reporting the cases, and the absence of appropriate management (36). Therefore, educating the HCWs about measles and improving the two-dose MMR (measles, mumps, rubella) vaccination coverage could prevent transmission and reduce these outbreaks.
Human immunodeficiency virus, hepatitis B, and hepatitis C.
Contracting human immunodeficiency virus (HIV) and other blood-borne viruses such as hepatitis B virus (HBV) and hepatitis C virus (HCV), remains a serious concern for many CPR providers, leading to hesitancy to perform resuscitation in patients with a “high-risk” epidemiological profile (37, 38). Large-scale, tailored, evidence-based efforts have been made by international stakeholders, such as the WHO and the Centers for Disease Control and Prevention (CDC), to inform the public and educate HCWs on the possible ways of HIV and hepatitis virus transmission (37, 39, 40). Nonetheless, there are still many people and, among them many HCWs, who incorrectly believe that HIV can be easily contracted via contact with patients’ saliva or skin. It is now evident that HIV infection cannot be transmitted through exposure to saliva (2). Neither saliva exchange during CPR nor inoculation of a mucous membrane or a skin breach with saliva from people living with HIV has been implicated in viral transmission (41, 42). According to the guidelines issued by the U.S. Public Health service in 2013, feces, nasal secretions, saliva, sputum, sweat, tears, urine, and vomitus are not considered infectious unless they are visibly bloody (43). With respect to occupational exposure, the estimated risk of HIV transmission via contact with infectious bodily fluids (blood, semen, breast milk, and vaginal secretions) is as low as 0.3% and 0.63% for skin puncture and direct contact with mucous membranes, respectively (44, 45). Evidently, the risk of HIV transmission during CPR has been estimated to be from one in 1 million events (when HIV prevalence is about 30%) to 1 in 1 billion events when prevalence is 0.1 to 1% (46, 47). Importantly, the risk of contracting HIV is practically zero after exposure to infective body fluids from people living with HIV/AIDS who are compliant with their antiretroviral treatment and have undetectable viral load (the so-called “undetectable equals untransmittable” consensus statement, U = U) (48).
Mouth-to-mouth ventilation remains controversial for HBV, as hepatitis B surface antigen (HBsAg) has been detected in the saliva of 76% and 81% of patients with severe hepatitis and chronic hepatitis B, respectively (10, 49). Nonetheless, HBV viral load in saliva remains 1,000 to 10,000 times lower than blood, thus making the potential for transmission rather low (10). In contrast, both HIV and HBV viruses can be contracted via direct contact of a wound, oral mucosa, or cornea with blood (or blood products) from an infected person (10), which underscores the importance of the strict adherence to proper PPE utilization during CPR, as well as the significance of immunization of high-risk professionals against HBV. According to the WHO, “the risk from a single needlestick or cut exposure to HBV-infected blood ranges from 6 to 30% and depends on the hepatitis B e antigen (HBeAg) status of the source individual” in immune-susceptible people (50). However, the risk of transmission is low due to universal vaccination of HCWs (50).
HCV transmission requires blood-to-blood contact; thus, the potential of contracting HCV through mouth-to-mouth ventilation is extremely low. The calculated risk for HCV transmission through contact of rescuer's nonintact oral mucosa with patient's blood is as low as 1:250,000 (when the estimated population seroprevalence for anti-HCV antibody is 1.25% and the possibility of rescuer’s mucosal breach is 50%) (10). There is no documented transmission from exposure of providers with intact skin and/or mucosa to blood from an HCV-infected person (10). Moreover, the risk of contracting HCV though needle stick injury from an HCV-positive person has been estimated to be as low as 1.8% (50, 51). Thankfully, HCV infection can be treated with highly effective direct-acting antivirals in cases of high-risk exposure. Currently, no prophylaxis is indicated after high-risk incidents (52) (Table 1).
Herpes simplex virus.
The majority of adolescents and adults are seropositive for herpes simplex virus 1 (HSV-1), and the virus can be isolated in saliva of 2 to 10% of adults without clinical evidence of infection (10). Moreover, HSV-1 is capable of surviving for up to 88 h in dry gauze (10). Therefore, there is a potential transmission risk of HSV-1 to seronegative providers during mouth-to-mouth ventilation. Since the infection is initially asymptomatic in the majority of cases (>99%), it is difficult to estimate the incidence of transmission during CPR (10), although isolated cases have been reported (53). More specifically, two female HCWs contracted herpes labialis (the first one) and ocular herpes (the second one) after participating in the same CPR training course, suggesting that precautions against the transmission of HSV-1 should be taken during CPR (53).
Varicella-zoster virus.
Varicella-zoster virus (VZV) is highly contagious and is transmitted by direct contact, inhalation of aerosols from vesicular fluid of acute varicella or zoster skin lesions, and possibly through infected respiratory secretions that also may be aerosolized (54). As such, HCWs and CPR providers who are either unvaccinated or naturally nonimmune are at high risk for developing chickenpox; however, breakthrough varicella can occur in a vaccinated person, which is also contagious (54).
Cytomegalovirus.
Cytomegalovirus (CMV) is transmitted through saliva and respiratory secretions or through contact with contaminated surfaces and body fluids, such as urine; there is a risk of acquiring CMV during CPR that may lead to severe disease in pregnant and immunocompromised HCWs (55). It has been estimated that the mean annual seroconversion rate among HCWs is 3%, which is significantly lower than the seroconversion rate of day care workers (14%) and higher than that of people living in the community (1.6%) (2). Hence, the risk of CMV infection among HCWs remains low; for example, a CMV-seronegative pediatrician who performed mouth-to-mouth resuscitation to a newborn with congenital CMV infection did not acquire the infection despite ingesting the newborn’s infected secretions (56). Moreover, a meta-analysis revealed that pediatric HCWs are not at high risk for contracting CMV infection (57). Hence, the risk for contracting CMV infection through CPR is low and can be further minimized by handwashing and the use of universal contact precautions.
Epstein-Barr virus.
Epstein-Barr virus (EBV) is an omnipresent human herpesvirus; most people will get infected at some point during their lives (58). EBV is transmitted through contact with body fluids and—most importantly—saliva or contact with contaminated objects (such as a toothbrush recently used by an actively infected person) (58). Obviously, EBV can be transmitted to HCWs during CPR, especially during mouth-to-mouth ventilation; however, no confirmed cases have been reported in the literature to date.
Ebola virus and Crimean-Congo hemorrhagic fever virus.
The feasibility and ethical challenges of performing CPR to Ebola patients remain controversial for many countries where epidemics are ongoing (47, 59). Person-to-person transmission of Ebola virus occurs through direct contact with organs, all body fluids, and surfaces or materials contaminated with these fluids of infected patients (60). Ebola virus disease has a substantially high case-fatality rate that ranges from 25 to 90%, and many HCWs caring for Ebola patients have lost their lives (47, 60). Moreover, it has been estimated by the WHO that HCWs are 21 to 32 times more likely to be infected with Ebola (61). Adherence to well-organized standard operating procedures, strict contact measures, appropriate education on proper donning of PPE, and performing CPR when it is not medically futile might eliminate the reluctance of HCWs to resuscitate Ebola patients (47). In addition to strict adherence to contact measures, a new vaccine for the Zaire ebolavirus strain was recently approved by the U.S. Food and Drug Administration (FDA); the Ebola vaccine—commercially known as “Ervebo”—is based on a replication-competent, live, attenuated recombinant vesicular stomatitis virus (VSV) that contains the gene of Ebola virus glycoprotein instead of the VSV glycoprotein (rVSVΔG-ZEBOV-GP vaccine) (62). The FDA approved its administration in adults (≥18 years old) that are at potential risk for Ebola virus exposure, including HCWs at designated Ebola treatment centers and the personnel of biosafety-level 4 laboratories (62).
The Crimean-Congo hemorrhagic fever (CCHF) is a vector-borne zoonotic disease that is transmitted to humans through arthropods (ticks) (63). Although human-to-human transmission is quite rare, outbreaks among HCWs have been reported in areas of endemicity (Table 2); a series from Turkey reported nine cases of HCWs (including one fatal case) who contracted the disease through direct unprotected contact with infectious body fluids and blood (including unprotected handling of contaminated devices and equipment) and/or needlestick injury (64). Among these cases, a nurse was infected through aspiration of bloody secretions during intubation of an infected neonate (64). Another case series from Pakistan reported 2 HCWs who acquired CCHF from an infected patient; among them, one HCW died 13 days after the contact with the index case, while the other one was successfully treated with ribavirin (65). Although human-to-human transmission has been reported among HCWs, there are no known cases of CCHF transmission during CPR to date.
Bacterial and Mycobacterial Infections
Tuberculosis.
Tuberculosis is an airborne disease caused in the majority of cases by Mycobacterium tuberculosis or, less commonly, by other members of Mycobacterium tuberculosis complex that can be transmitted through droplets produced by infected patients; these droplets may remain in the air for several hours, depending on the environment (66). It is estimated that in 2019, 10 million people were infected with tuberculosis worldwide (67). Interestingly, inhalation of a small quantity of mycobacteria is sufficient to cause tuberculosis. Therefore, high-level PPE for airborne communicable diseases should be applied by HCWs when rescuing a person with active pulmonary or upper airway tuberculosis (68). However, only one case of tuberculosis transmission has been reported in a CPR provider: an HCW who developed primary cutaneous tuberculosis, that was transmitted by a patient with active tuberculosis of the right upper lobe during CPR (9, 69).
Neisseria species.
Meningococcal disease causes outbreaks and epidemics, especially among children and young adults; transmission requires prolonged and close contact with droplets from either asymptomatic carriers (usually children below the age of 5 years, adolescents, and young adults) or patients with symptomatic infection (70). Therefore, CPR, and especially mouth-to-mouth ventilation, can be associated with Neisseria meningitidis transmission. Although it is estimated that mouth-to-mouth resuscitation can result in nasopharynx colonization in 33% of the rescuers, adults can be immune to N. meningitidis, due to development of cross-immunity to other microorganisms (10, 71). High-risk occupational exposures performed without the use of surgical masks, such as mouth-to-mouth resuscitation, intubation, aspiration of respiratory secretions and fundoscopy examination of secretions should receive postexposure prophylaxis (Table 2) (70). In total, four cases of N. meningitidis transmission during rescue breathing have been reported in the literature (2). Finally, one case of Neisseria gonorrhoeae transmission has been reported in a doctor performing neonatal resuscitation, after ingesting infected secretions of the neonate (72).
Streptococcus and Staphylococcus spp.
Streptococcus and Staphylococcus species are ubiquitous pathogens that can be easily transmitted from human to human, although rarely this leads to development of clinical infection. Only two cases of transmission during CPR have been reported: one case of a fireman who contracted Streptococcus pyogenes through a skin abrasion contaminated with secretions from a 3-year-old boy during bag-valve-mask ventilation, who subsequently developed cellulitis and septic shock, and a case of transmission of Panton-Valentine leukocidin-producing Staphylococcus aureus to a physician during the resuscitation of an infant with fatal pneumonia, who exhibited multiple furuncles of the fingers and the face (73, 74). These two cases highlight the importance of the appropriate donning of PPE by the HCWs, when performing CPR.
Gastrointestinal pathogens.
Isolated cases of transmission of gastrointestinal pathogens have been reported during mouth-to-mouth ventilation, including a case of Shigella sonnei contracted during CPR of an infant with gastroenteritis and a case of Salmonella enterica serovar Infantis transmitted from an adult patient with gastroenteritis (2, 75, 76). Helicobacter pylori transmission by vomit has also been reported in a doctor who performed mouth-to-mouth resuscitation (2, 77).
Other pathogens.
While we could not identify relevant reports, any pathogen can be potentially transmitted during CPR. The potential for infection relates to several factors, including the burden of the infection in the affected individual, the route of exposure, the burden of the exposure of the CPR performing person, as well as their respective health status. As an example, Creutzfeldt-Jakob disease is a transmittable, rare, fatal prion-induced neurocognitive disorder (78). Few cases of occupational exposure have been described to date (79). However, transmission of prions requires contact with infected cerebrospinal fluid and/or brain tissue, and in theory, with blood although no blood-borne transmissions have been reported (78). Thus, transmission through the CPR process is unlikely. Evidently, Creutzfeldt-Jakob disease cannot be transmitted through the air or through touching or most other forms of casual contact (78).
WILLINGNESS OF HEALTH CARE WORKERS TO PERFORM CPR WHEN INFECTION IS SUSPECTED
The reluctance of lay people and medical personnel to perform CPR and especially mouth-to-mouth ventilation in hospital and community settings has been documented in several studies (38, 80–83) (Table 3). This hesitancy arises from a remarkably high level of fear of contracting infectious diseases, mainly HIV infection, during CPR; unfortunately, these concerns remain an unsurpassed obstacle despite the continuous reassurance that CPR-acquired infections are extremely rare (2). On the other hand, any delay in commencing ventilation and chest compressions will compromise both survival rates and neurologic outcomes of the patients, as supported by a considerable body of data (38).
TABLE 3.
Willingness of HCWs to perform CPR and mouth-to-mouth resuscitationa
| Reference | Yr/country | No. of participants | Willingness of HCWs to perform CPR and mouth-to-mouth resuscitation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Strangers | Children | Trauma | HIV or high-risk persons for HIV | Elderly | Infection | With barrier device | |||
| Hew et al. (38) | 1997/USA | 104 HCWs (74% EMTs, 26% paramedics) | EMTs, 43%; paramedics, 0% | EMTs, 77%; paramedics, 63% | EMTs, 25%; paramedics, 0% | Homosexuals: EMTS, 33%; paramedics, 0% | EMTs, 40%; paramedics: 4% | (–) | 98% |
| Horowitz et al. (5) | 1997/USA | 379 HCWs (20% physicians, 63% nurses, 17% paramedics) | 35% | 57% | (–) | HIV-negative adults, 62–65%; HIV-negative children, 75% | (–) | (–) | (–) |
| Melanson et al. (86) | 2000/USA | 342 HCWs (92% paramedics, 8% nurses) | ≤5% | 52% | (–) | 0% | (–) | (–) | HIV, 47%; other, >60% |
| Locke et al. (80) | 1995/USA | 975 participants (20% HCWs, 80% lay persons) | MTM, 15%; compression-only, 68% | (–) | (–) | (–) | (–) | (–) | (–) |
| Boucek et al. (85) | 2009/USA | 560 HCWs (26% physicians, 29% nurses, 13% EMTs and paramedics, 13% medical students, 6% nursing students, 13% other HCWs) | 38% | Own child, 96%; neighbor’s child, 78.5% | (–) | HIV, 16%; drug overdose, 6% | 46% | Ebola, 0.1%; TB, 6%; herpes, 4%; rabies, 6%; URI, 7%; antibiotic-resistant pneumonia, 19% | (–) |
| Ornato et al. (84) | 1990/USA | 1,794 BLS instructors (87% HCWs [8% physicians, 50% nurses, 9% allied HCWs, 20% EMTs and paramedics]) | Male, 18–29%; female, 54% | 97% | (–) | HIV, 40%; drug overdose, 10%; hemophiliac, 35% | (–) | (–) | (–) |
| Brenner et al. (83) | 1993/USA | 585 HCWs (74% physicians, 26% nurses) | Physicians, 57%; nurses, 20% | Physicians, 84%; nurses, 78% | Physicians, 55%; nurses, 38% | Homosexuals: physicians, 17%; nurses, 9% | Physicians, 57%; nurses, 35% | (–) | (–) |
| Brenner et al. (81) | 1994/USA | 74 HCWs (100% physicians) | 45% | (–) | 26% | Homosexuals, 7% | 39% | (–) | (–) |
| Brenner et al. (82) | 1996/USA | 140 HCWs (100% physicians) | Inpatient, 43–45%; outpatient, 50–54% | Outpatient, 86–99% | Inpatient, 12–16%; outpatient, 33–36% | HIV: inpatient, 7–14%; outpatient, 21–34% | Inpatient, 29–39%; outpatient, 26–64% | (–) | (–) |
High-risk persons for HIV: homosexual, drug overdose, and hemophiliac. Abbreviations: HCWs, health care workers; CPR, cardiopulmonary resuscitation; BLS, basic life support; MTM, mouth-to-mouth resuscitation; EMTs, emergency medical technicians; URI, upper respiratory infection; HIV, human immunodeficiency virus. (–), no data.
In 1990s, shortly after the onset of the AIDS pandemic, 40% of basic life support (BLS) instructors hesitated to provide mouth-to-mouth ventilation in patients with epidemiological risk factors for HIV infection (84). Only 10% of the instructors would not be reluctant to perform CPR on a person with a heroin overdose, while 97% would readily perform CPR on a drowning child (84). The unwillingness of BLS trainers to perform CPR under these circumstances may not only lead to loss of their credibility but might also serve as a wrong example to other people to avoid performing CPR in certain instances.
A series of studies, have demonstrated an unwillingness of HCWs to perform CPR and, in particular, mouth-to-mouth ventilation (38, 80–83). More specifically, only 5% of licensed vocational nurses, 10% of registered nurses, 16% of attending physicians, and 21% of resident physicians were willing to perform CPR on a man living in a homosexual neighborhood, whereas a considerably higher percentage of the same HCWs groups would provide CPR on a child who collapses (82%, 75%, 81%, and 99% of licensed vocational nurses, registered nurses, attending physicians, and resident physicians, respectively) (83). Moreover, only 16% of HCWs would provide CPR on a stranger, but 74% of them would do it on a friend or a relative (80). In addition, a study reported that 57% of HCWs would refuse to perform mouth-to-mouth ventilation on a man living in a homosexual neighborhood and more surprisingly, only 32% of the medical staff and none of the paramedics would provide CPR to the same patient. Similarly, 23% of medical staff and 37% of paramedics would also refuse to carry out mouth-to-mouth ventilation on a child as well (38). In another study, less than half of HCWs surveyed were willing to do mouth-to-mouth resuscitation to an unknown adult, but if the patient was known to them, such as a neighbor or a relative, 84 to 94% of them would unrestrictedly provide CPR (5). On the other hand, only one-third of HCWs would do CPR if the HIV status was unknown, while two-thirds would do so if the patient was HIV negative. Nonetheless, these percentages were higher when the patient was a child (57% and 79%, respectively) (5). Conclusively, this survey showed that HCWs’ eagerness to provide mouth-to-mouth resuscitation is profoundly influenced by the level of familiarity with the patients and their ages, as well as their HIV status or possibility of being exposed to HIV (5).
More recently, a study indicated that HIV infection is not the only condition whereby rescuers hesitate to provide mouth-to-mouth resuscitation. Indeed, other conditions may include visible evidence of substance abuse, visible body fluids and other infections, such as herpes labialis, tuberculosis and Ebola (85). The age and the level of experience tend to reduce the propensity to engage in mouth-to-mouth resuscitation, while parental propensity to ventilate one’s own child was undeniably stronger than any other motivator (85). To our knowledge, there are no data regarding HCWs willingness to provide CPR on patients with SARS-CoV-2.
Less than 5% of HCWs would perform mouth-to-mouth ventilation without a barrier, excluding cases involving pediatric drowning patients (86). The availability of barrier devices decreased substantially this reluctance, although only 25% of HCWs carry such devices on a regular basis (86). Therefore, the widespread availability of such devices could result in increased readiness to do CPR, that will invariably raise the numbers of resuscitated patients with cardiac and/or respiratory arrest (86). Finally, all HCWs and potential CPR providers should be educated about the low risk of contracting HIV infection through CPR in order to reduce the refusal rates and the reluctance to perform mouth-to-mouth resuscitation.
CURRENT GUIDELINES ON PROTECTION OF CPR PROVIDERS
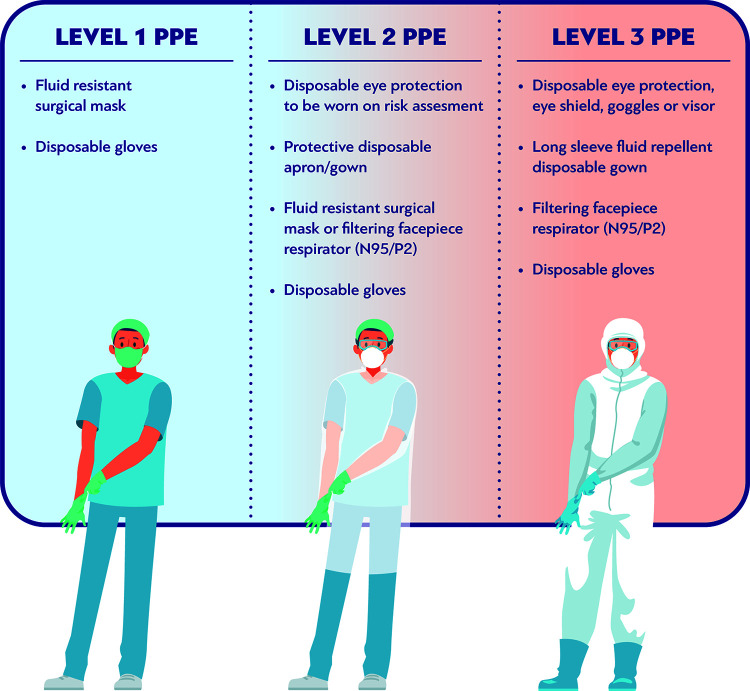
The level of PPE recommended during CPR depends on the risk of infection transmission via droplet, contact or airborne spread. There are two main levels of PPE: the “level 2” or droplet PPE, which consists of gloves, gown or apron, fluid-resistant surgical mask, and eye and face protection and the “level 3” or airborne PPE, which consists of gloves, long-sleeved gown, either filtering face piece 2 or 3 (FFP2/FFP3) or N95 or N99 mask respirator, and eye and face protection (Fig. 1). The level 2 PPE reduces the risk of transmission of droplet-induced infections, while the airborne PPE is recommended for HCWs who are involved in aerosol-generating procedures, such as intubation, when an airborne disease is suspected or known (87).
FIG 1.
Levels of personal protective equipment (PPE).
Guidelines on prevention of respiratory infections or infections transmitted via respiratory tract during resuscitation have been recently published by many international societies due to the COVID-19 pandemic in order to protect both HCWs and patients, minimize aerosolization, and reduce potential delays in performing CPR (Fig. 2). Several controlled trials comparing N95 respirators with surgical masks have been reported, but most of them revealed no statistically significant difference, suggesting that the surgical mask was sufficiently protective for influenza virus (88–90). In contrast, one study showed that N95 respirators do not provide adequate protection against respiratory infections during chest compression (91). Therefore, all current guidelines recommend the use of N95 respirators during resuscitation procedures along with face shields covering the entire face and, preferably, equipped with FFP3 class filters (Fig. 2) (92, 93).
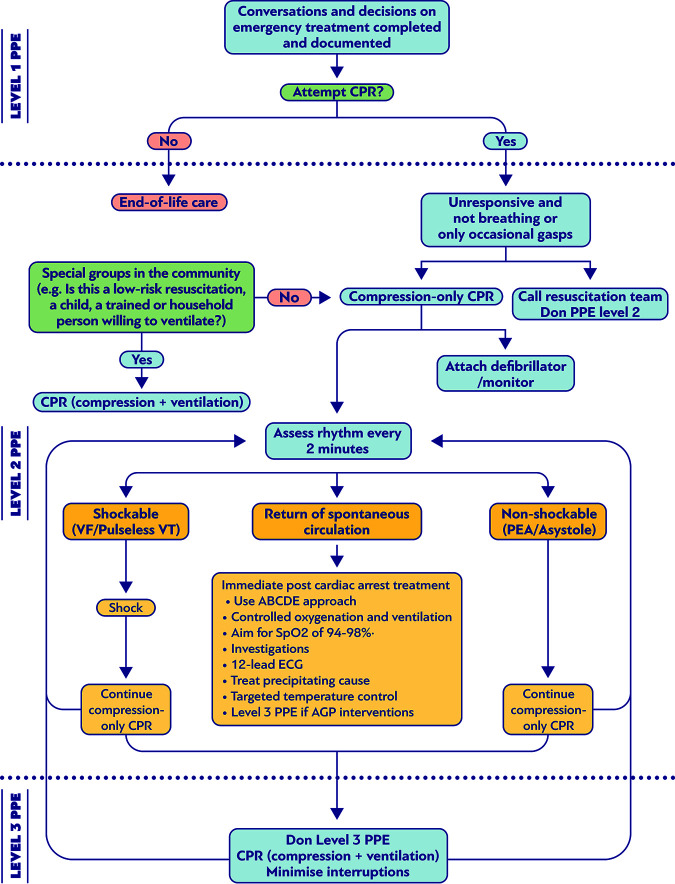
FIG 2.
Suggested algorithm for cardiopulmonary resuscitation (CPR) and personal protective equipment (PPE) in patients with airborne or droplet transmitted infection.
Chest compressions and CPR have the potential to generate aerosols according to the recommendations issued by the International Liaison Committee on Resuscitation (ILCOR) (94). Consequently, the ILCOR proposes a compression-only resuscitation approach and a public-access defibrillation (where applicable) for lay bystanders; mouth-to-mouth ventilation is suggested only if the provider is willing and able to do so, and in case of a child or a household member, especially during the COVID-19 pandemic. For infants and children, the bystander rescuers are usually family members or persons that are responsible for their care. The risk of infection through ventilation is greatly outweighed by improved outcome in this population, who are commonly in asphyxial cardiopulmonary arrest (94). Moreover, the ILCOR suggests the use of PPE by HCWs during CPR procedures and the consideration of defibrillation before PPE donning, where the provider assesses the benefits may exceed the risks (94).
The guidelines published by the European Resuscitation Council (ERC) suggest the following measures for outpatient CPR performed by non-HCW bystanders: chest compressions and defibrillation with an automated external defibrillator (AED) or chest compressions, active abdominal compression-decompression instrument, and defibrillation with an AED (87, 95). Regarding CPR provided by HCWs, the use of the highest-level airborne precaution PPE is recommended for all procedures. Endotracheal intubation is advised to be performed under the guidance of visual laryngoscope or under sedative state. Furthermore, the use of mechanical cardiopulmonary resuscitation, as well as the limitation of the personnel in the room, is encouraged (87, 95).
The American Heart Association (AHA) recently published guidance on CPR in COVID-19 patients (96). They propose the hands-only CPR by non-HCW bystanders, covering the mouth of the patient with a face mask or cloth, while in case of a child, the AHA suggests additionally mouth-to-mouth ventilation, if willing and able to do so (96). Also, when CPR is performed in the hospital, HCWs should perform endotracheal intubation under the guidance of visual laryngoscope, while manual ventilation with a supraglottic airway and a bag-mask device with a high-efficiency particulate air (HEPA) filter should be considered as an alternative means of ventilation; moreover, the AHA suggests to leave the patient on mechanical ventilation with a HEPA filter, where possible (96).
A consensus statement by the Australasian College for Emergency Medicine recommends compression-only CPR, covering the face of the patient and defibrillation, wearing level 2 PPE (gloves, eye protection, and surgical mask or N95 if available), while further resuscitation should be performed wearing level 3 or airborne PPE (97). Furthermore, the Public Health England (PHE) and the Health Protection Scotland (HPS) guidelines state that chest compressions and defibrillation can be started with level 2 PPE and, if another aerosol-generating procedure is required, level 3 PPE should be donned (97–99).
The guidelines of the Australasian College for Emergency Medicine, the PHE, and the HPS are in contrast to those published by the AHA, the ILCOR, the ERC, the Royal College of Physicians of the United Kingdom (UK), the American College of Chest Physicians and the Resuscitation Council (UK), and the Hellenic Society of Cardiology, which recommend full airborne PPE throughout resuscitation (87, 94–96, 100, 101). A review by Brown et al. concluded that since there is insufficient evidence to suggest that chest compressions are not an aerosol-generating procedure, level 3 PPE should be worn during CPR in patients with suspected COVID-19 (102).
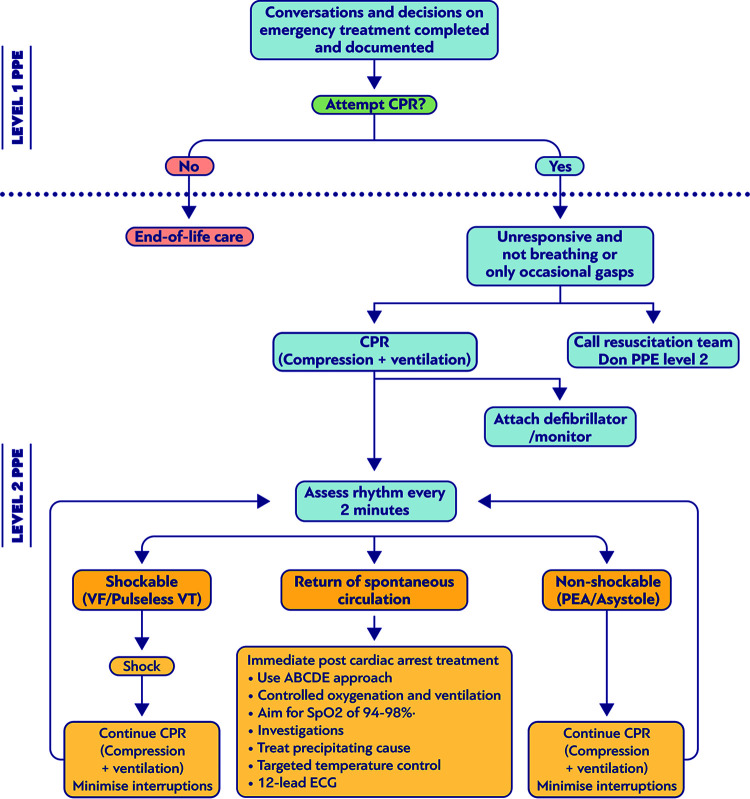
Finally, the CDC issued recommendations in 1988 regarding the transmission of other severe infections, such as HIV and HBV infections, during CPR, stating that every patient should be considered HIV or HBV positive and suggested the use of gloves and handwashing after exposure to saliva (103) (Fig. 3). Moreover, it was proposed to use disposable devices, pocket masks and bag-valve masks to prevent direct mouth-to-mouth contact (1, 104–107).
FIG 3.
Suggested algorithm for cardiopulmonary resuscitation (CPR) and personal protective equipment (PPE) in patients with bloodborne infection or infection transmitted through contact with skin or other body fluids.
OTHER PROTECTIVE MEASURES PROPOSED FOR CPR PROVIDERS
In order to minimize the risk of respiratory infections during CPR, it is proposed to perform endotracheal intubation as quickly as possible and all aerosol-generating procedures should be done in an airborne infection isolation room (92). Although CPR in prone position has been suggested, it still remains controversial. The AHA guidelines recommend the avoidance of turning the patients supine if advanced airway is in place due to the risk of circuit disconnection and aerosolization; moreover, the AHA proposes the placement of defibrillator pads in the anterior-posterior position and the chest compression to be performed with the provider’s hands placed over the 7th to the 10th thoracic vertebral bodies (96). The efficacy of CPR and ventilation in prone position is not completely known, but it has been suggested for critically ill adults with COVID-19 (108).
Regarding ventilation during CPR, ventilation through a supraglottic device that limits the exposure to patient-generated droplets is recommended, while AHA proposes ventilation with the tight seal bag-mask device or an oxygen high flow facial mask (109). Furthermore, it has been demonstrated that loose-fitting powered air-purifying respirators during CPR procedures leads to high degree of protection of HCWs against infectious pathogens and reduction of transmission of respiratory infections (110).
Although automated chest compression devices have been proposed to be used during CPR, when these devices are not available it is recommended to reduce the duration of CPR cycles from the current 2-min to 1-min cycle, since this approach increases significantly the quality of chest compressions performed by rescuers wearing an airborne PPE (111). Recently, the utilization of drones for PPE or automated external defibrillator delivery for communication of safety instructions has been proposed as a potential strategy to encourage, instruct, and reassure rescuers who perform CPR during the pandemic (112). Finally, the use of a plastic blanket as a quick and alternative PPE has been suggested in order to offer extra protection to the lifeguards during on-boat resuscitation without any delay of the CPR initiation (113).
Despite the fear of infection transmission during CPR, data show a suboptimal compliance with basic infection control measures among HCWs, including the use of PPE, due to the lack of knowledge and familiarization with self-protective behaviors during resuscitation (114). Continuing educational programs for HCWs on how to minimize the risk of exposure to infections during CPR, by decreasing the number of aerosol-generating procedures, using a small team, and considering early termination of resuscitative efforts, as well as how to use PPE properly, will definitely reduce concerns about infection transmission and unnecessary delays in commencing the resuscitation (115).
CONCLUSIONS
CPR is a selfless lifesaving endeavor that saves thousands of lives each year globally. As with any other medical procedure, CPR can bear potential risks—not only for the patient but also for the rescuers. Among these risks, the transmission of infections has been one of the most compelling triggers of reluctance and fear to perform CPR among providers. Although the risk is not ignorable, only a few cases of infection transmission to rescuers have been attributed to CPR. Thus, strict adherence to proper self-protective measures is a potent and highly effective way of prevention of infection transmission.
Biographies

Paraskevi C. Fragkou is an Internal Medicine Consultant and Academic Fellow in Infectious Diseases (ID) at Attikon University Hospital, National and Kapodistrian University of Athens (NKUA), Greece. She graduated from the Medical School of the University of Patras, Greece (top ranked student), and completed her M.Sc. degree in Internal Medicine with distinction at the University of Edinburgh, UK. She is undertaking a Ph.D. in ID at the Medical School of Athens, NKUA, and her research is supported by a European Union cofunded Ph.D. excellence scholarship program from the State Scholarships Foundation of Greece (IKY). She completed her specialty training in Internal Medicine in the UK and Greece. She is an ERC and RCUK certified ALS Instructor. ID and, especially viral infections in critical care settings, is her main interest. To date, she has been involved in multiple laboratory and clinical research projects in ID and host immune responses both in the UK and in Greece, and she has >30 publications in international peer-reviewed journals.

Dimitra Dimopoulou is a Consultant Pediatrician in the Second Department of Pediatrics of the National and Kapodistrian University of Athens, Medical School, at Children’s Hospital of Athens “Panagiotis and Aglaia Kyriakou.” She graduated from Medical School of Heraklion, Crete, Greece, and then was a research fellow in the same University, where she completed her Ph.D. (2015), receiving two Excellence Scholarships during the first and second years of the Ph.D. preparation. She specialized in Pediatrics at the University of Athens Medical School (Attikon University General Hospital of Athens), and from 2018 to 2020 she was registrar of Pediatrics in the Third Department of Pediatrics (Attikon University General Hospital of Athens). Since 2020, she has been a clinical fellow of Pediatric Infectious Diseases at Children’s Hospital “Panagiotis and Aglaia Kyriakou.” Her fields of interest include pediatric infectious diseases. To date, she has 46 publications in international peer review journals.

George Latsios, M.D. (1999), Ph.D. (honors) (2003), is at Athens Medical School, National and Kapodistrian University of Athens, Athens, Greece. He specialized in Cardiology at Athens Medical School, GHA “Hippokration,” Athens, Greece (2007). His subspecialty was Interventional Cardiology at Athens Medical School, GHA “Hippokration,” Athens, Greece (and member of European Association of PCI) (2008), and Heart Center “Helios” Siegburg-Bonn, Germany (2010). From 2012 to 2021, he was a Consultant at the Interventional Cardiology and Coronary Care Unit (deputy in-charge), in the First University Department of Cardiology, GHA “Hippokration,” Athens, Greece. He was President (President-Elect) of WG CPR of the Hellenic Cardiological Society from 2014 to 2018. The process of Cardiopulmonary Resuscitation (CPR), in both its basic (BLS) and advanced (ALS) forms, is the primary way to increase survival of cardiac arrest victims. However, its utilization still remains suboptimal, while the recent fear of infectious diseases posed an additional concern. During the last decade, Dr. Latsios has tried, as a member of a team of enthusiastic colleagues, to increase CPR awareness, to educate civilians (BLS) and medical personnel (ALS), and to add scientific data and published documents to this topic.

Panagiotis Koudounis received his Degree in Medicine at University of Ioannina, Ioannina, Greece, in 2009 and his M.Sc. in Intensive Care Units and Cardiology at the First University Department of Cardiology, Hippokration Hospital Athens, Greece, in 2017. In 2018, he specialized in Cardiology at Red Cross Hospital, Athens, Greece, and in 2019 he became a Fellow in Electrophysiology (grant awarded by Hellenic Society of Cardiology) at Red Cross Hospital, Athens, Greece. From 2019 to 2021, he was a Consultant in Cardiology with interest in Invasive Electrophysiology and Devices, Red Cross Hospital, Athens, Greece, and he is currently a Consultant Cardiologist with interest in Devices and Ιnvasive Electrophysiology at Athens Medical Group, Greece. He successfully passed the European Examination in General Cardiology and the Greek board examination in Cardiology in 2018 (ranked first of 40 of his peers) and is a fully authorized Cardiologist in Greece. In October 2020, he became a fully certified EHRA Cardiac Device Specialist. He became an ALS instructor in 2015 (ERC and Hellenic Society of Cardiology certified), and since then he has been intensively involved in teaching ALS/BLS courses. His main goal is to educate the community and increase awareness regarding cardiac arrest in Greece.

Andreas Synetos is a Consultant Interventional Cardiologist in the First Department of Cardiology of the National and Kapodistrian University of Athens, at “Hippokration” General Hospital of Athens, Greece. He specialized in Cardiology in the University of Athens Medical School and was a research fellow at Emory University in Atlanta, Georgia, USA. In 2017, he became a fellow of the European Society of Cardiology, and in 2018 he became a Fellow of the American College of Cardiology. His fields of interest include coronary artery disease, especially complex interventional procedures such as chronic total occlusions, and structural heart procedures, such as transcatheter aortic valve replacement. Since 2019, he has served as the Chairman of the Working Group of Cardiopulmonary Resuscitation and Intensive care of the Hellenic Society of Cardiology. He is an organizer and Director of Basic Life Support seminars and an Instructor and Provider of Advance Life support Seminars. He has >150 publications in international peer-reviewed journals.

Anastasia Dimopoulou is a Consultant Pediatric Surgeon in the Department of Pediatric Surgery of the National and Kapodistrian University of Athens, Medical School, at Attikon University General Hospital of Athens. She graduated from Medical School of Heraklion, Crete, Greece, and then completed her M.Sc. program at the National and Kapodistrian University of Athens. Afterwards, she received her Ph.D. degree at the same university. She specialized in Pediatric Surgery in the Children’s Hospital of Athens “Agia Sofia.” From 2016 to 2017, she was registrar of Pediatric Surgery in the Department of Pediatric Surgery in the Children’s Hospital of Athens “Agia Sofia.” Since 2017, she has been a Consultant of Pediatric Surgery in the Attikon University General Hospital of Athens. Her fields of interest include surgical site infections, minimal invasive pediatric surgery, and pediatric trauma. To date, she has 30 publications in international peer review journals.

Konstantinos Tsioufis is a Professor of Cardiology in the National and Kapodistrian University of Athens, and Chief of the First Cardiology Clinic, Hippokration Hospital, Athens, Greece. He was trained in Interventional Cardiology and Hypertension at the University of Athens, Greece, and later at the Veterans Affairs Medical Center, Georgetown University, Washington, DC. He has published widely on hypertensive disease, cardiorenal disease, atherosclerotic cardiovascular disease, and interventional cardiology, and he is involved in major clinical trials regarding novel interventional therapies of hypertension and particularly renal denervation. He has >470 publications in peer-reviewed Journals (H index > 62). He is coeditor of the book Interventional Therapies of Secondary and Essential Hypertension and has contributed to >25 chapters in books. He is a member of the editorial boards of major cardiology journals and a reviewer for several cardiology, hypertension, and critical care journals. He is immediate past-President of ESH (European Society of Hypertension) and immediate past-President of the Hellenic Society of Cardiology. His academic Interests are Hypertension and Interventional Cardiology.

Vassiliki Papaevangelou is a trained pediatrician with a special interest in pediatric infectious diseases. After completing her M.D. degree at the National and Kapodistrian University of Athens (NKUA), she moved to the United States for her pediatric residency and PID fellowship at the NYU Medical Centre, USA. Since she returned to Athens Greece (1996), she has been a full-time clinical pediatrician in tertiary teaching pediatric departments, initially as an attending physician at Agia Sophia Children's Hospital and, later (2003), as a faculty member of NKUA at the Aglaia Kyriakou Children Hospital. She became a full Professor of Pediatric Infectious Diseases in 2014. In September 2013 she was appointed Chairman of NKUA's Third Department of Pediatrics, which is located at Attikon University Hospital. Over the past 25 years, she has been actively involved in multiple research projects involving the area of Pediatric Infectious Diseases.

Sotirios Tsiodras is a Professor of Medicine and Infectious Diseases at the National and Kapodistrian University of Athens (NKUA) Medical School and the Chief Scientific Advisor for the COVID-19 pandemic for the Hellenic CDC and the Greek Government. He completed postgraduate training in Internal Medicine and Infectious Diseases at Harvard Medical School in the United States. He also holds a master’s degree in the Medical Sciences from Harvard Medical School and was a recipient of the Clinical Investigator Training Program award and the subsequent fellowship conducted by Harvard Medical School and the MIT Division of Health Sciences and Technology, both in Boston, MA. He has a Ph.D. in Medicine from the NKUA Medical School. He has been a member of the European CDC Advisory Forum since 2009 and an IDSA fellow; he additionally serves as a member of the board of the ESCMID Study Group for Respiratory Viruses. He has published >280 peer-reviewed papers and is currently doing clinical work and research in internal medicine and infectious diseases. He currently serves on the European Council scientific advisor team for COVID response.
REFERENCES
- 1.Michael AD, Forrester JS. 1992. Mouth-to-mouth ventilation: the dying art. Am J Emerg Med 10:156–161. 10.1016/0735-6757(92)90051-X. [DOI] [PubMed] [Google Scholar]
- 2.Mejicano GC, Maki DG. 1998. Infections acquired during cardiopulmonary resuscitation: estimating the risk and defining strategies for prevention. Ann Intern Med 129:813–828. 10.7326/0003-4819-129-10-199811150-00014. [DOI] [PubMed] [Google Scholar]
- 3.Chan-Yeung M. 2004. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health 10:421–427. 10.1179/oeh.2004.10.4.421. [DOI] [PubMed] [Google Scholar]
- 4.Lau JTF, Fung KS, Wong TW, Kim JH, Wong E, Chung S, Ho D, Chan LY, Lui SF, Cheng A. 2004. SARS transmission among hospital workers in Hong Kong. Emerg Infect Dis 10:280–286. 10.3201/eid1002.030534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horowitz BZ, Matheny L. 1997. Health care professionals’ willingness to do mouth-to-mouth resuscitation. West J Med 167:392–397. [PMC free article] [PubMed] [Google Scholar]
- 6.Honda H, Iwata K. 2016. Personal protective equipment and improving compliance among healthcare workers in high-risk settings. Curr Opin Infect Dis 29:400–406. 10.1097/QCO.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 7.Liang SY, Riethman M, Fox J. 2018. Infection prevention for the emergency department: out of reach or standard of care? Emerg Med Clin North Am 36:873–887. 10.1016/j.emc.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2012. Principles of epidemiology in public health practice, lesson 1, section 10. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 9.Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, O’Neil BJ, Peberdy MA, Rittenberger JC, Rodriguez AJ, Sawyer KN, Berg KM, Adult Basic and Advanced Life Support Writing Group. 2020. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 142:S366–S468. 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 10.Arend CF. 2000. Transmission of infectious diseases through mouth-to-mouth ventilation: evidence-based or emotion-based medicine? Arq Bras Cardiol 74:86–97. [PubMed] [Google Scholar]
- 11.Rule AM, Apau O, Ahrenholz SH, Brueck SE, Lindsley WG, de Perio MA, Noti JD, Shaffer RE, Rothman R, Grigorovitch A, Noorbakhsh B, Beezhold DH, Yorio PL, Perl TM, Fisher EM. 2018. Healthcare personnel exposure in an emergency department during influenza season. PLoS One 13:e0203223. 10.1371/journal.pone.0203223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuster SP, Shah PS, Coleman BL, Lam PP, Tong A, Wormsbecker A, McGeer A. 2011. Incidence of influenza in healthy adults and healthcare workers: a systematic review and meta-analysis. PLoS One 6:e26239. 10.1371/journal.pone.0026239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. 2019. How to implement seasonal influenza vaccination of health workers. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 14.World Health Organization. 2020. Influenza (avian and other zoonotic). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 15.Schultsz C, Dong VC, Chau NVV, Le NTH, Lim W, Thanh TT, Dolecek C, De Jong MD, Hien TT, Farrar J. 2005. Avian influenza H5N1 and healthcare workers. Emerg Infect Dis 11:1158–1159. 10.3201/eid1107.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. 2020. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 17.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382:1199–1207. 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2021. COVID-19 pandemic planning scenarios. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 19.Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, Pastore y Piontti A, Mu K, Rossi L, Sun K, Viboud C, Xiong X, Yu H, Elizabeth Halloran M, Longini IM, Vespignani A. 2020. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 368:395–400. 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 2020. Transmission of SARS-CoV-2: implications for infection prevention precautions. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 21.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323:1061–1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Wei X, Wang Z. 2020. Protecting healthcare workers from SARS-CoV-2 and other infections. Epidemiol Infect 148:e217. 10.1017/S0950268820002198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Istituto Superiore di Sanità. 2020. COVID-19 integrated surveillance: key national data. EpiCentro, Portal of Epidemiology for Public Health, Italy.
- 24.Suárez-García I, Martínez de Aramayona López MJ, Sáez Vicente A, Lobo Abascal P. 2020. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect 106:357–363. 10.1016/j.jhin.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werner N, Nickenig G, Sinning JM. 2018. Complex PCI procedures: challenges for the interventional cardiologist. Clin Res Cardiol 107:64–73. 10.1007/s00392-018-1316-1. [DOI] [PubMed] [Google Scholar]
- 26.Heinzerling A, Stuckey MJ, Scheuer T, Xu K, Perkins KM, Resseger H, Magill S, Verani JR, Jain S, Acosta M, Epson E. 2020. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient—Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep 69:472–476. 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, Pearson CAB, Russell TW, Tully DC, Washburne AD, Wenseleers T, Gimma A, Waites W, Wong KLM, van Zandvoort K, Silverman JD, Diaz-Ordaz K, Keogh R, Eggo RM, Funk S, Jit M, Atkins KE, Edmunds WJ, CMMID COVID-19 Working Group. 2021. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 372:eabg3055. 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christian MD, Loutfy M, McDonald LC, Martinez KF, Ofner M, Wong T, Wallington T, Gold WL, Mederski B, Green K, Low DE, SARS Investigation Team. 2004. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis 10:287–293. 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. 2018. Middle East respiratory syndrome coronavirus (MERS-CoV). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 30.Maltezou HC, Tsiodras S. 2014. Middle East respiratory syndrome coronavirus: implications for health care facilities. Am J Infect Control 42:1261–1265. 10.1016/j.ajic.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fragkou PC, Thomas K, Sympardi S, Liatsos GD, Pirounaki M, Sambatakou H, Marantos T, Karofylakis E, Dourakis SP, Tsiodras S, Kavvatha D. 2020. Clinical characteristics and outcomes of measles outbreak in adults: a multicenter retrospective observational study of 93 hospitalized adults in Greece. J Clin Virol 131:104606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. 2020. Worldwide measles deaths climb 50% from 2016 to 2019 claiming over 207 500 lives in 2019. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33.Guerra FM, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS. 2017. The basic reproduction number (R0) of measles: a systematic review. Lancet Infect Dis 17:e420–e428. 10.1016/S1473-3099(17)30307-9. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 2019. Measles. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 35.Hahné SJM, Lochlainn LMN, Van Burgel ND, Kerkhof J, Sane J, Yap KB, Van Binnendijk RS. 2016. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis 214:1980–1986. 10.1093/infdis/jiw480. [DOI] [PubMed] [Google Scholar]
- 36.Jia H, Ma C, Lu M, Fu J, Rodewald LE, Su Q, Wang H, Hao L. 2018. Transmission of measles among healthcare workers in hospital W, Xinjiang Autonomous Region, China, 2016. BMC Infect Dis 18. 10.1186/s12879-018-2950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. 2020. Ways HIV is not transmitted | HIV transmission | HIV basics | HIV/AIDS. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 38.Hew P, Brenner B, Kaufman J. 1997. Reluctance of paramedics and emergency medical technicians to perform mouth-to-mouth resuscitation. J Emerg Med 15:279–284. 10.1016/s0736-4679(97)00006-1. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. 2020. HIV/AIDS. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 40.World Health Organization. 2019. Hepatitis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 41.Sande MA. 1986. Transmission of AIDS. N Engl J Med 314:380–382. 10.1056/NEJM198602063140609. [DOI] [PubMed] [Google Scholar]
- 42.Emergency Cardiac Care Committee, Heart and Stroke Foundation of Canada. 1990. Disease transmission and cardiopulmonary resuscitation. CMAJ 143:1007–1008. [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhar DT, Henderson DK, Struble KA, Heneine W, Thomas V, Cheever LW, Gomaa A, Panlilio AL, US Public Health Service Working Group. 2013. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol 34:875–892. 10.1086/672271. [DOI] [PubMed] [Google Scholar]
- 44.Ippolito G, Puro V, De Carli G. 1993. The risk of occupational human immunodeficiency virus infection in health care workers: Italian multicenter study. Arch Intern Med 153:1451–1458. 10.1001/archinte.1993.00410120035005. [DOI] [PubMed] [Google Scholar]
- 45.Bell DM. 1997. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med 102:9–15. 10.1016/s0002-9343(97)89441-7. [DOI] [PubMed] [Google Scholar]
- 46.Bierens JJLM, Berden HJJM. 1996. Basic-CPR and AIDS: are volunteer life-savers prepared for a storm? Resuscitation 32:185–191. 10.1016/0300-9572(96)00943-4. [DOI] [PubMed] [Google Scholar]
- 47.Torabi-Parizi P, Davey RT, Suffredini AF, Chertow DS. 2015. Ethical and practical considerations in providing critical care to patients with Ebola virus disease. Chest 147:1460–1466. 10.1378/chest.15-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lancet. 2017. U=U taking off in 2017. Lancet HIV 4:e475. 10.1016/S2352-3018(17)30183-2. [DOI] [PubMed] [Google Scholar]
- 49.Villarejos VM, Visoná KA, Gutiérrez A, Rodríguez A. 1974. Role of saliva, urine and feces in the transmission of type B hepatitis. N Engl J Med 291:1375–1378. 10.1056/NEJM197412262912602. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. 2003. Exposure to blood: what healthcare personnel need to know. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 51.Wilkins T, Malcolm J, Raina D, Schade R. 2010. Hepatitis C: diagnosis and treatment. Am Fam Physician 81:1351–1357. [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. 2020. 2020 guidance for healthcare personnel exposed to HCV. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 53.Mannis MJ, Wendel RT. 1984. Transmission of herpes simplex during CPR training. Ann Ophthalmol 16:64–66. [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention. 2018. Chickenpox for healthcare professionals: varicella. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 55.Adler SP, Baggett J, Wilson M, Lawrence L, McVoy M. 1986. Molecular epidemiology of cytomegalovirus in a nursery: lack of evidence for nosocomial transmission. J Pediatr 108:117–123. 10.1016/S0022-3476(86)80785-5. [DOI] [PubMed] [Google Scholar]
- 56.Demmler GJ, Petrella R, Brady MT. 1986. Cytomegalovirus and neonatal resuscitation. Pediatr Infect Dis 5:605. 10.1097/00006454-198609000-00032. [DOI] [PubMed] [Google Scholar]
- 57.Flowers RH, Torner JC, Farr BM. 1988. Primary cytomegalovirus infection in pediatric nurses: a meta-analysis. Infect Control Hosp Epidemiol 9:491–496. 10.1086/645758. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. 2020. About Epstein-Barr virus (EBV). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 59.Ulrich CM, Grady C. 2015. Cardiopulmonary resuscitation for Ebola patients: ethical considerations. Nurs Outlook 63:16–18. 10.1016/j.outlook.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization. 2020. Ebola virus disease. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 61.World Health Organization. 2020. Ebola health worker infections. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 62.Centers for Disease Control and Prevention. 2021. Ebola vaccine: information about Ervebo® | Clinicians | Ebola (Ebola virus disease). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 63.Centers for Disease Control and Prevention. 2013. Transmission | Crimean-Congo hemorrhagic fever (CCHF). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 64.Celikbas AK, Dokuzoğuz B, Baykam N, Gok SE, Eroğlu MN, Midilli K, Zeller H, Ergonul O. 2014. Crimean-Congo hemorrhagic fever among health care workers, Turkey. Emerg Infect Dis 20:477–479. 10.3201/eid2003.131353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Athar MN, Baqai HZ, Ahmad M, Khalid MA, Bashir N, Ahmad AM, Balouch AH, Bashir K. 2003. Short report: Crimean-Congo hemorrhagic fever outbreak in Rawalpindi, Pakistan, February 2002. Am J Trop Med Hyg 69:284–287. 10.4269/ajtmh.2003.69.284. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. 2020. Transmission and pathogenesis of tuberculosis. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 67.World Health Organization. 2020. Tuberculosis. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 68.Centers for Disease Control and Prevention. 2019. Healthcare setting: TB. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 69.Heilman KM, Muschenheim C. 1965. Primary cutaneous tuberculosis resulting from mouth-to-mouth respiration. N Engl J Med 273:1035–1036. 10.1056/NEJM196511042731908. [DOI] [PubMed] [Google Scholar]
- 70.Batista RS, Gomes AP, Dutra Gazineo JL, Balbino Miguel PS, Santana LA, Oliveira L, Geller M. 2017. Meningococcal disease, a clinical and epidemiological review. Asian Pac J Trop Med 10:1019–1029. 10.1016/j.apjtm.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 71.Ministerio da Saude. 2005. Guia de vigilância epidemiológica. Biblioteca Virtual em Saúde, Brazil.
- 72.Ballard JL, Musial MJ, Myers MG. 1986. Hazards of delivery room resuscitation using oral methods of endotracheal suctioning. Pediatr Infect Dis 5:198–200. 10.1097/00006454-198603000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Valenzuela TD, Hooton TM, Kaplan EL, Schlievert P. 1991. Transmission of “toxic strep” syndrome from an infected child to a firefighter during CPR. Ann Emerg Med 20:90–92. 10.1016/S0196-0644(05)81129-1. [DOI] [PubMed] [Google Scholar]
- 74.Chalumeau M, Bidet P, Lina G, Mokhtari M, André MC, Gendrel D, Bingen E, Raymond J. 2005. Transmission of Panton-Valentine leukocidin-producing Staphylococcus aureus to a physician during resuscitation of a child. Clin Infect Dis 41:e29–e30. 10.1086/431762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Todd MA, Bell JS. 1980. Shigellosis from cardiopulmonary resuscitation. JAMA 243:331. 10.1001/jama.1980.03300300013009. [DOI] [PubMed] [Google Scholar]
- 76.Ahmad F, Senadhira DCA, Charters J, Acquilla S. 1990. Transmission of Salmonella via mouth-to-mouth resuscitation. Lancet 335:787–788. 10.1016/0140-6736(90)90898-F. [DOI] [PubMed] [Google Scholar]
- 77.Fanconi S, Gehri M, Benkebil F, Nydegger A, Cachat F, Zeier G, Pastore YD, Diezi M. 2007. Pediatry. Rev Med Suisse 3:155–160. [PubMed] [Google Scholar]
- 78.National Institute of Neurological Disorders and Stroke. 2020. Creutzfeldt-Jakob disease fact sheet. National Institute of Neurological Disorders and Stroke, Bethesda, MD. [Google Scholar]
- 79.Berger JR, David NJ. 1993. Creutzfeldt-Jakob disease in a physician: a review of the disorder in health care workers. Neurology 43:205–206. 10.1212/wnl.43.1_part_1.205. [DOI] [PubMed] [Google Scholar]
- 80.Locke CJ, Berg RA, Sanders AB, Davis MF, Milander MM, Kern KB, Ewy GA. 1995. Bystander cardiopulmonary resuscitation: concerns about mouth-to-mouth contact. Arch Intern Med 155:938–943. 10.1001/archinte.155.9.938. [DOI] [PubMed] [Google Scholar]
- 81.Brenner B, Stark B, Kauffman J. 1994. The reluctance of house staff to perform mouth-to-mouth resuscitation in the inpatient setting: what are the considerations? Resuscitation 28:185–193. 10.1016/0300-9572(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 82.Brenner B, Kauffman J, Sachter JJ. 1996. Comparison of the reluctance of house staff of metropolitan and suburban hospitals to perform mouth-to-mouth resuscitation. Resuscitation 32:5–12. 10.1016/0300-9572(96)00966-5. [DOI] [PubMed] [Google Scholar]
- 83.Brenner BE, Kauffman J. 1993. Reluctance of internists and medical nurses to perform mouth-to-mouth resuscitation. Arch Intern Med 153:1763–1769. 10.1001/archinte.1993.00410150041004. [DOI] [PubMed] [Google Scholar]
- 84.Ornato JP, Hallagan LF, McMahan SB, Peeples EH, Rostafinski AG. 1990. Attitudes of BCLS instructors about mouth-to-mouth resuscitation during the AIDS epidemic. Ann Emerg Med 19:151–156. 10.1016/S0196-0644(05)81800-1. [DOI] [PubMed] [Google Scholar]
- 85.Boucek CD, Phrampus P, Lutz J, Dongilli T, Bircher NG. 2009. Willingness to perform mouth-to-mouth ventilation by health care providers: a survey. Resuscitation 80:849–853. 10.1016/j.resuscitation.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 86.Melanson SW, O’Gara K. 2000. EMS provider reluctance to perform mouth-to-mouth resuscitation. Prehospital Emerg Care 4:48–52. 10.1080/10903120090941641. [DOI] [PubMed] [Google Scholar]
- 87.Nolan JP, Monsieurs KG, Bossaert L, Böttiger BW, Greif R, Lott C, Madar J, Olasveengen TM, Roehr CC, Semeraro F, Soar J, Van de Voorde P, Zideman DA, Perkins GD, European Resuscitation Council COVID-Guideline Writing Groups. 2020. European Resuscitation Council COVID-19 guidelines executive summary. Resuscitation 153:45–55. 10.1016/j.resuscitation.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.MacIntyre CR, Wang Q, Seale H, Yang P, Shi W, Gao Z, Rahman B, Zhang Y, Wang X, Newall AT, Heywood A, Dwyer DE. 2013. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med 187:960–966. 10.1164/rccm.201207-1164OC. [DOI] [PubMed] [Google Scholar]
- 89.Loeb M, Dafoe N, Mahony J, John M, Sarabia A, Glavin V, Webby R, Smieja M, Earn DJD, Chong S, Webb A, Walter SD. 2009. Surgical mask versus N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA 302:1865–1871. 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 90.Seto WH, Cowling BJ, Lam HS, Ching PTY, To ML, Pittet D. 2011. Clinical and nonclinical health care workers faced a similar risk of acquiring 2009 pandemic H1N1 infection. Clin Infect Dis 53:280–283. 10.1093/cid/cir375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hwang SY, Yoon H, Yoon A, Kim T, Lee G, Jung KY, Park JH, Shin TG, Cha WC, Sim MS, Kim S. 2020. N95 filtering facepiece respirators do not reliably afford respiratory protection during chest compression: a simulation study. Am J Emerg Med 38:12–17. 10.1016/j.ajem.2019.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruetzler K, Smereka J, Ludwin K, Drozd A, Szarpak L. 2020. Respiratory protection among healthcare workers during cardiopulmonary resuscitation in COVID-19 patients. Am J Emerg Med 39:233. 10.1016/j.ajem.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smereka J, Ruetzler K, Szarpak L, Filipiak KJ, Jaguszewski M. 2020. Role of mask/respirator protection against SARS-CoV-2. Anesth Analg 131:E33–E34. 10.1213/ANE.0000000000004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Perkins GD, Morley PT, Nolan JP, Soar J, Berg K, Olasveengen T, Wyckoff M, Greif R, Singletary N, Castren M, de Caen A, Wang T, Escalante R, Merchant RM, Hazinski M, Kloeck D, Heriot G, Couper K, Neumar R. 2020. International Liaison Committee on Resuscitation: COVID-19 consensus on science, treatment recommendations, and task force insights. Resuscitation 151:145–147. 10.1016/j.resuscitation.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Song W, Liu Y, Ouyang Y, Chen W, Li M, Xianyu S, Yi S. 2020. Recommendations on cardiopulmonary resuscitation strategy and procedure for novel coronavirus pneumonia. Resuscitation 152:52–55. 10.1016/j.resuscitation.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Edelson DP, Sasson C, Chan PS, Atkins DL, Aziz K, Becker LB, Berg RA, Bradley SM, Brooks SC, Cheng A, Escobedo M, Flores GE, Girotra S, Hsu A, Kamath-Rayne BD, Lee HC, Lehotsky RE, Mancini ME, Merchant RM, Nadkarni VM, Panchal AR, Peberdy MAR, Raymond TT, Walsh B, Wang DS, Zelop CM, Topjian AA, American Heart Association ECC Interim COVID Guidance Authors. 2020. Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19: from the Emergency Cardiovascular Care Committee and get with the guidelines-resuscitation adult and pediatric task forces of the American Heart Association. Circulation 141:E933–E943. 10.1161/CIRCULATIONAHA.120.047463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Craig S, Cubitt M, Jaison A, Troupakis S, Hood N, Fong C, Bilgrami A, Leman P, Ascencio-Lane JC, Nagaraj G, Bonning J, Blecher G, Mitchell R, Burkett E, McCarthy SM, Rojek AM, Hansen K, Psihogios H, Allely P, Judkins S, Foong LH, Bernard S, Cameron PA. 2020. Management of adult cardiac arrest in the COVID-19 era: consensus statement from the Australasian College for Emergency Medicine. Med J Aust 213:126–133. 10.5694/mja2.50699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.gov.uk*. 2020. COVID-19: personal protective equipment use for non-aerosol generating procedures. Government of the United Kingdom, London, United Kingdom. [Google Scholar]
- 99.Health Protection of Scotland. 2020. Review of national and international guidance on infection prevention and control measures for personal protective equipment (PPE) and aerosol generating procedures (AGPs) for COVID-19. Health Protection of Scotland, Glasgow, Scotland. [Google Scholar]
- 100.Resuscitation Council, UK. 2020. Updated RCUK statement on PHE PPE guidance: 28 April 2020. Resuscitation Council, UK, London, United Kingdom. [Google Scholar]
- 101.Royal College of Physicians. 2020. CPR, personal protective equipment, and COVID-19. Royal College of Physicians, London, United Kingdom. [Google Scholar]
- 102.Brown E, Chan LM. 2020. Should chest compressions be considered an aerosol-generating procedure? A literature review in response to recent guidelines on personal protective equipment for patients with suspected COVID-19. Clin Med (Lond) 20:E154–E159. 10.7861/clinmed.2020-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention. 1988. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in health-care settings. MMWR Morb Mortal Wkly Rep 37:387–388. [PubMed] [Google Scholar]
- 104.Eickhoff TC, Axnick KJ, Brimhall D. 1984. A hospitalwide approach to AIDS. Recommendations of the advisory committee on infections within hospitals American Hospital Association. Infect Control 5:242–248. [DOI] [PubMed] [Google Scholar]
- 105.Conte JE, Hadley WK, Sande M. 1983. Infection-control guidelines for patients with the acquired immunodeficiency syndrome (AIDS). N Engl J Med 309:740–744. 10.1056/nejm198309223091228. [DOI] [PubMed] [Google Scholar]
- 106.Anonymous. 1989. Guidelines for prevention of transmission of human immunodeficiency virus and hepatitis B virus to health-care and public-safety workers. Lab Med 20:783–797. 10.1093/labmed/20.11.783. [DOI] [PubMed] [Google Scholar]
- 107.American College of Emergency Physicians. 1988. AIDS: statement of principles and interim recommendations for emergency department personnel and prehospital care providers. Ann Emerg Med 17:1249–1251. 10.1016/s0196-0644(88)80080-5. [DOI] [PubMed] [Google Scholar]
- 108.Mędrzycka-Dąbrowska W, Lewandowska K, Ślęzak D, Dąbrowski S. 2020. Prone ventilation of critically ill adults with COVID-19: how to perform CPR in cardiac arrest? Crit Care 24:258. 10.1186/s13054-020-02970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scapigliati A, Gullì A, Semeraro F, Ristagno G, Arlotta G, Bevilacqua F, Barelli A. 2020. How to ventilate during CPR in time of COVID-19? Resuscitation 151:148–149. 10.1016/j.resuscitation.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park SH, Hwang SY, Lee G, Park JE, Kim T, Shin TG, Sim MS, Jo IJ, Kim S, Yoon H. 2021. Are loose-fitting powered air-purifying respirators safe during chest compression? A simulation study. Am J Emerg Med 44:235–240. 10.1016/j.ajem.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malysz M, Dabrowski M, Böttiger BW, Smereka J, Kulak K, Szarpak A, Jaguszewski M, Filipiak KJ, Ladny JR, Ruetzler K, Szarpak L. 2020. Resuscitation of the patient with suspected/confirmed COVID-19 when wearing personal protective equipment: a randomized multicenter crossover simulation trial. Cardiol J 27:497–506. 10.5603/CJ.a2020.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van Veelen MJ, Kaufmann M, Brugger H, Strapazzon G. 2020. Drone delivery of AED’s and personal protective equipment in the era of SARS-CoV-2. Resuscitation 152:1–2. 10.1016/j.resuscitation.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barcala-Furelos R, Abelairas-Gómez C, Alonso-Calvete A, Cano-Noguera F, Carballo-Fazanes A, Martínez-Isasi S, Rodríguez-Núñez A. 2021. Safe on-boat resuscitation by lifeguards in COVID-19 era: a pilot study comparing three sets of personal protective equipment. Prehosp Disaster Med 36:163–169. 10.1017/S1049023X2100011X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiang WC, Wang HC, Chen SY, Chen LM, Yao YC, Wu GHM, Ko PCI, Yang CW, Tsai MT, Hsai CC, Su CP, Chen SC, Ma MHM. 2008. Lack of compliance with basic infection control measures during cardiopulmonary resuscitation: are we ready for another epidemic? Resuscitation 77:356–362. 10.1016/j.resuscitation.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heaton HA, Luke A, Sztajnkrycer MD, Clements CM, De Moraes AG, Raukar NP. 2020. Best practices in managing cardiac arrest in the emergency department during the COVID-19 pandemic. Mayo Clin Proc 95:2704–2708. 10.1016/j.mayocp.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]