SUMMARY
Prion diseases are a group of fatal, infectious neurodegenerative disorders affecting various species of mammals, including humans. The infectious agent in these diseases, termed prion, is composed exclusively of a misfolded protein that can spread and multiply in the absence of genetic materials. In this article, we provide an overview of the mechanisms of prion replication, interindividual transmission, and dissemination in communities. In particular, we review the potential role of the natural environment in prion transmission, including the mechanisms and pathways for prion entry and accumulation in the environment as well as its roles in prion mutation, adaptation, evolution, and transmission. We also discuss the transmission of prion diseases through medical practices, scientific research, and use of biological products. Detailed knowledge of these aspects is crucial to limit the spreading of existing prion diseases as well as to prevent the emergence of new diseases with possible catastrophic consequences for public health.
INTRODUCTION
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs), are a group of uniformly fatal neurodegenerative disorders affecting humans and various species of mammals, including sheep, goats, mink, cervids, cattle, felines, and ungulates (1). Prion diseases have several etiologies, manifesting as sporadic, acquired, and familial forms. Despite this apparent diversity, prion diseases are unified by their underlying mechanism in which the accumulation of the pathological form of the prion protein (PrPSc) leads to neurodegeneration in the form of spongiform encephalopathy, neuronal loss, synaptic dysfunction, and brain inflammation (2). PrPSc forms by autocatalytic conversion of the host’s normal prion protein (PrPC) (3).
The history of prion diseases can be traced back to the early 18th century when farmers observed a peculiar disease affecting Merino sheep in which animals exhibited an altered gait, excessive licking behavior, and, most curiously, incessant itching that drove them to compulsively scrape their bodies against fence posts (4). This bizarre disease was fittingly named “scrapie” and, although unknown at the time, would become the first recorded account of a TSE. The first description of human prion disease was not until 1920, when German neurologists described a neuropathology now known as Creutzfeldt-Jakob disease (CJD) (5, 6), followed by Kuru in 1957 (7, 8). Two years later, researchers noted the similarities among scrapie, CJD, and Kuru, hypothesizing for the first time that these degenerative diseases might be caused by a similar, albeit unknown, pathogen (8, 9). Over the next decade, chronic wasting disease (CWD) was identified in farmed mule deer and later in free-ranging mule deer and white-tail deer (4, 10).
The first evidence of the infectious nature of scrapie was described as early as the 1930s, when scientists successfully transmitted the disease to healthy animals by inoculation of brain homogenate from affected sheep and goats (11). Kuru and CJD were later transmitted to primates (12). Despite the growing evidence of its infectious nature, the identity of the disease-causing agent remained elusive. Perhaps blinded by the discovery that nucleic acids encode genetic information, most believed a “slow virus” was responsible for prion disease (13, 14). Nonetheless, emerging evidence began to implicate a novel type of infectious agent. In 1967, it was first proposed that the infectious agent could be a self-replicating protein and not a virus (15). That same year, Griffith proposed several thermodynamic models explaining how a protein could replicate independently of nucleic acids and instead propagate via the transmission of its corrupted folding to a natively folded host protein (16). The notion that PrPSc itself is an infectious agent forms the basis of what is known as the “protein-only” or “prion” hypothesis. Stanley Prusiner provided perhaps the most compelling evidence for the protein-only hypothesis in 1982, when he discovered that the scrapie-causative agent consisted of a partially protease-resistant protein and, notably, infectivity was impervious to procedures known to modify or destroy nucleic acids (17). Prusiner coined the term “prion” for this novel proteinaceous infective particle (17).
While initially considered radical, the evidence for the protein-only hypothesis continually mounted over the next decades to become almost universally accepted (3). It can be argued that protein-based infectious agents satisfy the postulates set out by Robert Koch in 1876 for determining a causative relationship between a microbe and disease (Table 1). The pathological PrPSc isoform can be readily purified from TSE-affected brain tissue (18, 19). Prions can be kept growing indefinitely in vitro in a cell-free system via the use of the protein misfolding cyclic amplification (PMCA) technology (20). PMCA can be used to generate large amounts of new PrPSc from a minute amount of “seed,” analogous to bacteria multiplying in pure culture albeit mechanistically distinct. Castilla et al. further showed that after sufficient PMCA rounds to dilute out the initial inoculum, the in-vivo-generated PrPSc can be inoculated back into animals and perfectly recapitulate the pathological, histological, and biochemical characteristics of the initial inoculum (20), thus satisfying Koch’s final postulates (21). Later, it was shown that infectious prions can be generated using purified components using recombinant prion protein produced in bacteria (22, 23).
TABLE 1.
Main characteristics of prions as infectious agents in comparison with those of traditional infectious microorganisms
| Characteristic | Prions | Conventional infectious agent (virus or bacteria) |
|---|---|---|
| Composition | Solely protein | DNA or RNA, mostly covered with protein coat |
| Genetic material | Absent | Present |
| Self-replication | Yes, transforms cellular protein into misfolded variant | Yes, generation of new microorganisms |
| Infectivity | Yes, infectious | Yes, infectious |
| Incubation period | Long asymptomatic incubation period | Depends, but mostly short |
| Spread through infected organism | Possible | Possible |
| Immune response and inflammation | Atypical | Typical |
| Resistance to common decontaminant | Very resistant | Susceptible |
Remarkably, prions have even been shown to exceed the mandates set out by Koch and have been shown to fulfil some characteristics more often associated with complex microorganisms such as bacteria or viruses (Table 1). Despite being composed of the same amino acid sequence, prions have been shown to exist in multiple strains that can present markedly distinct phenotypes as defined by differences in clinical symptoms, neuropathological lesion profile, incubation period, and localization of PrPSc accumulation in the brain as well as the biochemical properties of PrPSc (24–28). Also akin to higher-order infectious organisms is the ability of the prion agent to infect some species but not others. This perplexing characteristic known as the “species barrier” has been postulated as one of the primary mechanisms driving the aforementioned strain diversity (29–31). As PrPSc is transmitted from one host to another, differences in prion protein sequence can lead to the generation of novel conformations that may manifest as different strains and, more alarmingly, have different interspecies transmission capabilities (32, 33).
Interspecies transmission of prion disease remains one of the greatest risks in relation to human public health. Undisputable evidence demonstrates that the consumption of bovine spongiform encephalopathy (BSE)-contaminated material resulted in the outbreak of a novel prion disease in humans termed variant Creutzfeldt-Jakob disease (vCJD) (34, 35), which has taken the lives of 228 people, mostly in Europe and especially in the United Kingdom. Despite no reported cases of vCJD since 2016, the threat of BSE to humans looms large in the form of atypical types of BSE (36) and transmission through intermediate species that cannot only pass the disease along to humans but also transform strain characteristics (37–39). The recent emergence and exponential spread of CWD has further reignited fears of potential transmission to humans.
Intricately tied to the rise of BSE and subsequent surge of vCJD was the spike in iatrogenic CJD (iCJD) in the mid to late 1990s caused primarily by the transplantation of CJD-contaminated dura mater grafts and cadaveric human growth hormone (40). While the number of iCJD cases has decreased steadily since its peak, intermittent clusters of cases such as those linked to blood transfusions (41) serve to remind us that the risks are never truly gone. Moreover, as we expand our biological toolkit and develop novel therapeutic products derived from living organisms, we continue to put ourselves at risk of iatrogenic infections of prion diseases.
While the perils of interspecies transmission and iatrogenic infection have received abundant attention, the mechanisms responsible for horizontal transmission through environmental materials or medical tools and procedures remain incompletely understood. The asymptomatic incubation periods of prion diseases can last months, years, and even decades before clinical signs are observed (42, 43), providing ample opportunity for silent prion carriers to transmit the disease. This, coupled with the extraordinary resilience of prions (44), allows them to persist and accumulate in association with diverse materials. In this article, we will overview the mechanisms implicated in prion transmission, with particular emphasis on the role of the environment and medical practices in prion dissemination.
MECHANISMS AND ROUTES OF PRION TRANSMISSION
Since the first depiction of prions as a proteinaceous infectious agent, much thought has been given to the mechanism by which a single protein can replicate and induce disease. Multiple theories have been proposed to explain the molecular basis of prion replication (45–47). The most widely accepted theory proposes that infectious PrPSc exists as β-sheet-rich, insoluble amyloid-like fibrils or smaller soluble oligomers. These aggregates serve as a “seed” or template for the nucleation event in which PrPC is misfolded and added to the growing polymers (48–50). The exponential rate of PrPSc accumulation can be mathematically explained by factoring in the growth of the aggregates at the expense of the growth of normal PrPC and the propensity of the fibrils to fragment and break, in essence multiplying the number of available growing fibril ends to which PrPC can be added (51, 52). The unique physiochemical method of prion replication has since been applied to understand the propagation of peptides and proteins involved in other neurodegenerative diseases (53). Indeed, some of the most prevalent neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), frontal-temporal dementia (FTD), and Lewy-body dementia (DLB), are caused by the accumulation of β-sheet-rich aggregates formed by a seeding/nucleation mechanism (53). Compelling research has shown in cellular and animal models of these diseases that misfolded protein aggregates can self-propagate as prions in these diseases, but whether a transmissible origin for these diseases indeed occurs in “real life” is presently unknown (53).
Prion diseases in humans are typically categorized as familial, sporadic, or infectious based on their etiology (1). Familial or genetic prion diseases are caused by autosomal dominant mutations in the prion protein gene (PRNP) and include familial Creutzfeldt-Jakob disease (fCJD), Gerstmann-Sträussler-Scheinker (GSS) disease, and fatal familial insomnia (FFI) (54, 55). Most identified mutations leading to disease are missense, but octapeptide repeat insertions, deletions, and nonsense mutations have also been described (54, 55). In the familial forms of prion disease, the assumption is that the mutations destabilize protein folding, increasing its propensity to form the misfolded toxic form (54, 55). Sporadic TSEs are characterized by their spontaneous origin, and currently it is unclear how disease is initiated. It has been hypothesized that sporadic disease is the result of PRNP somatic mutations or a stochastic change in PrP structure, although it is often difficult to completely exclude the possibility of acquired infection (56–58). Infectious transmission of prion disease in humans is rare and includes vCJD acquired by exposure to BSE (34, 35), iatrogenic CJD (iCJD) due to exposure to medical treatments with infected materials (59), and Kuru caused by cannibalistic ritual practices (4, 9).
The capacity of prions to cross the species barrier and generate novel strains leaves us theoretically exposed to prions coming from distant species. For instance, classical scrapie has long been considered a nonzoonotic disease in terms of transmission to humans; however, both sheep and goats develop a disease pathologically similar to scrapie when challenged with BSE (60). When transgenic (Tg) mice expressing human PrP were challenged with sheep BSE, they developed a disease pathologically similar to vCJD (38, 39, 61). Moreover, BSE passaged through sheep was more efficient than cattle BSE at converting human PrP in Tg mice (38, 39), suggesting increased infectivity after passage through an intermediate species. While there have been no known natural cases of BSE in sheep, it is impossible to exclude the possibility that BSE-contaminated materials may have already afflicted sheep. At this point in time, it is not yet possible to accurately predict which intermediate species may allow for the genesis of new, more potent zoonotic strains that are capable of inducing disease when passed into humans. For these reasons, it is imperative to closely monitor and manage animal prion diseases and, just as importantly, the buildup of prions in the environment.
In animals, ingestion or close contact with contaminated material is the most relevant route of infection. In CWD, the only naturally occurring prion disease in wild animals, infection can be transmitted horizontally or vertically. Vertical transmission, also termed in utero transmission, has been confirmed in several TSEs, including those of cattle (62), sheep (63), and Reeves’ muntjac deer (64). Surprisingly, it was found that 80% of fetuses from CWD-positive muntjac dams were infected with CWD, demonstrating plausible in utero prion infection (64). Since these experimental studies, CWD transmission from doe to fawn has also been demonstrated to contribute to the CWD epidemic in free-ranging elk in Colorado (65).
Horizontal transmission is likely the foremost factor contributing to the rapid spread of CWD in North America (66). It is often classified as direct or indirect based on the route of transmission. Direct horizontal transmission is the result of close contact between uninfected cervids and CWD-infected animals (67, 68). Studies have shown that in animals sharing the same location, direct horizontal transmission is remarkably efficient, with an incidence of 89% in herds of captive mule deer in which the possibility of vertical transmission was excluded (69). Indirect horizontal transmission is the result of exposure to a prion-contaminated environment, typically by decomposed carcasses or excreta (68–70) (Fig. 1). In most cases, it likely involves the oral ingestion of contaminated water, foliage, and/or soil. The efficiency of indirect horizontal transmission has also been demonstrated experimentally in animals exposed to a contaminated environment. In a study simulating indirect transmission by housing naive mule deer with decomposed skeletal remains of a CWD-affected deer that had died >2 years earlier, researchers found a relatively high rate of infection (25%) (71). Furthermore, when housed in excreta-contaminated paddocks, 11% became infected (71).
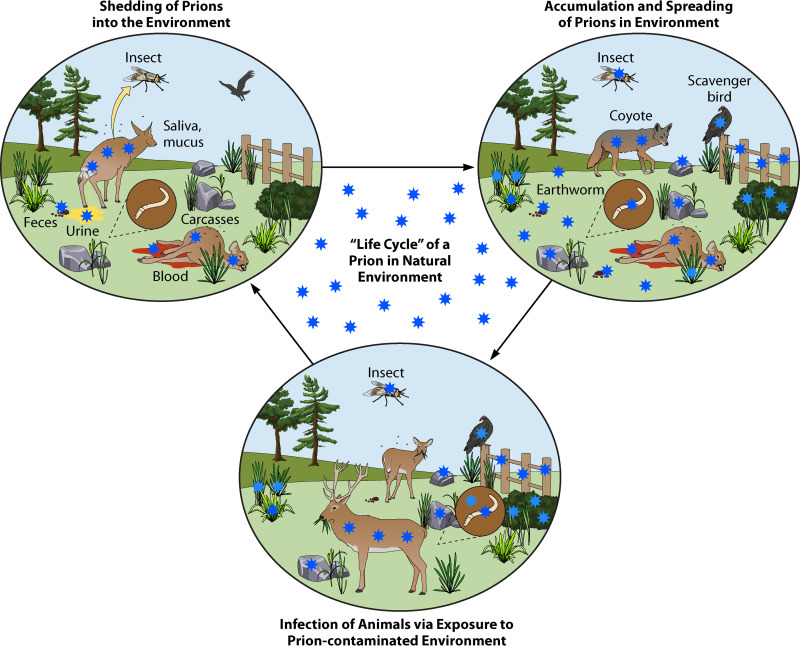
FIG 1.
Schematic representation of the life cycle of a prion in the natural ecosystem. Natural dissemination of prions within a natural environment can be achieved by infected animals excreting infectious prions (blood, saliva, mucous, urine, and feces) or by deposition from infected carcasses of dead animals. Over time, gradual accumulation of infectious prions can be found spread throughout the immediate environment bound to solid surfaces (wood, rock, plants, and soil) and can be further disseminated to broader spatial locations through other organisms (insects, worms, scavenger birds, and mammals). Within the highly prion-contaminated environment, reuptake and eventual infection can occur via consumption or contact with contaminated surfaces or materials.
The high propensity of prions to contaminate the environment or medical instruments is due in large part to their structural stability. Prions are astoundingly resistant to degradation, and conventional sterilization methods developed to eliminate bacteria and viruses have proven to be largely insufficient in eradicating prion infectivity (44). Indeed, iatrogenic transmission of the disease has been observed despite cleaning of tools with formaldehyde (70, 72) and even after cleaning with benzene followed by formaldehyde and 70% alcohol disinfection (73). As prions rely only on their misfolded conformation to transmit infectivity, decontamination must involve protein denaturation and the disruption of the tertiary structure of PrPSc. Today, incineration, steam sterilization, or a combination of sodium hydroxide (NaOH)/sodium hypochlorite (NaClO) and high temperature autoclaving are considered the most effective means of prion decontamination (74). The application of corrosive agents to large areas of the environment is perhaps less feasible, but recent studies have shown treatment with 2 N NaOH effectively inhibited CWD replication in farm soil (75).
PRION EXPOSURE THROUGH THE NATURAL ENVIRONMENT
Pathways for Prion Entry into the Environment
By far, the most likely route of transmission in naturally acquired animal TSEs is through oral ingestion, after which, prions are able to effectively cross the intestinal barrier and enter lymphoid tissues associated with the gut (76–78). After passing to lymphoreticular tissues, prions can enter the enteric nervous system and then make their way to the central nervous system, including the brain (79–81). Following replication in lymphoid tissues (82), there is significant dissemination of prions throughout the periphery and the expelling of prions through the excretion of saliva and bodily wastes. In CWD, the shedding of infectious prions in the excreta of already infected individuals is considered the most significant source of environmental prion contamination (68, 71, 83) (Fig. 1). Indeed, prions or prion seeding activity has been detected in feces, urine, and saliva of both symptomatic and asymptomatic infected deer (68–70).
The distribution of prions through the environment via urine and fecal matter is suspected to play a major role in the high efficiency of horizontal transmission of CWD (84). Due to the long incubation periods characteristic of prion diseases, there is prolonged opportunity for prion shedding and entry into the environment. Evidence shows that CWD prions could be detected by a Tg mouse bioassay in CWD-infected mule deer feces approximately 6 months before the appearance of clinical symptoms (85). When irradiated fecal homogenates from infected mule deer (4 months postinfection [p.i.]) were inoculated into Tg mice expressing cervid PrP, 14/15 samples transmitted disease similarly to inoculation with brain homogenate. Not surprisingly, prion titers and attack rates were much lower (29% on average) for animals inoculated with fecal homogenates than for those inoculated with homogenates from brain (85). Nevertheless, multiple exposures to contaminated feces are likely in both captive and free-ranging deer and could increase the overall probability of infection (86). More-sensitive tests to detect infectious prions, including protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC), confirmed the presence of prions in urine and feces during the course of CWD (87–90). These highly sensitive detection techniques facilitate the estimation of infectious PrPSc that is deposited into the environment by CWD-infected animals. Tamgüney and colleagues estimated that over the course of the disease, an infected deer may shed nearly the same amount of prions in feces as that which accumulates in its brain at terminal disease (85). Pulford et al. estimated the concentration of PrPSc in feces of naturally exposed free-ranging elk at 100 to 5,000 pg PrPSc/g of feces (89). While the amount of daily fecal excrement produced fluctuates significantly between cervid species and diets as well as the season (91), it is estimated that cervids produce between 400 and 3,000 g of feces per day (92). This would equate to between 0.04 and 15 μg of PrPSc excreted in feces per day from a single infected animal. Over the course of a year, an individual cervid may shed almost 5.5 mg of infectious PrPSc into the environment. In an area in which CWD is endemic, such as the state of Colorado, it has been estimated that as much as 50% of the state’s approximately 420,000 free-ranging white-tailed deer (93) have been infected with CWD (94). An infected population this size could deposit >3 g of PrPSc into the environment every single day. This in turn means that >1 kg of CWD PrPSc every year enters the Colorado environment through feces alone, effectively turning this habitat into a reservoir of infectious PrPSc.
Similarly to that for feces, urine is expected to be a significant route of prion entry into the environment (Fig. 1). Initially, detection in urine was hindered by the sensitivity of traditional procedures such as Western blotting, but the advent of amplification techniques has allowed for the detection of prions in urine (68, 69, 91, 95–97). Prions have also been detected in the urine of CWD-infected animals as early as 6 months post-oral inoculation of deer (68). Using RT-QuIC, prion seeding activity was detected in the urine of roughly 25% of CWD-infected deer and was estimated to be roughly equivalent to that of a 10−6 to 10−7 dilution of 10% (wt/vol) of CWD brain homogenate (68). Analogous to that for feces, the titer of prions in urine is relatively low compared to that in brain material or other bodily fluids such as blood and saliva. Nonetheless, the sheer volume of urine excreted daily makes up for its lack in potency. An adult deer can produce approximately 1 liter of urine per day, enough to provide a lethal dose of prions to 100 cervidized mice (68). Notably, prions have also been detected in the urine of patients with vCJD (97). The ability to reliably detect subclinical levels of prions in a readily accessible bodily fluid is a major milestone for the field, as it opens new avenues for antemortem diagnosis.
Aside from blood, saliva is likely the bodily fluid with the highest prion content (83, 94). As in feces and urine, prions have been detected in saliva during the asymptomatic course of the disease (68, 83, 98, 99). Due to their diet, herbivores produce extraordinary amounts of saliva each day. Sheep can produce as much as 17 liters per day (100), while cattle can produce >50 liters daily (101). While it is likely that some saliva is left behind during grazing, the majority of saliva is swallowed to aid in digestion. The prevalent thought is that swallowed salivary prions may contribute to the infection of lymphoid and epithelial tissues of the gastrointestinal tract and possibly contribute to peripheral replication (82, 102). As they are swallowed, it is plausible that salivary prions are eventually excreted through feces and, in this manner, contribute further to the horizontal transmission of CWD and scrapie.
Even upon their death, prion-infected animals continue to contribute to the contamination of the environment. As their carcasses decompose, prions leech from their bodies into the environment (Fig. 1). Miller and colleagues found that decomposed skeletal remains from a CWD-infected mule deer remained infectious 2 years later, suggesting that such carcasses remain an important foci for infection (71). Prions have been detected in the skeletal muscle (103) and blood of symptomatic and asymptomatic animals and humans (104–109). Furthermore, the carcasses of large prey species such as cervids are often consumed by predators and scavenging species. Indeed, recent publications have demonstrated that prions remain intact after passage through the digestive systems of crows (110) and coyotes (111). It is possible that these secondary species are aiding the spread of prion diseases such as CWD and scrapie (Fig. 2).
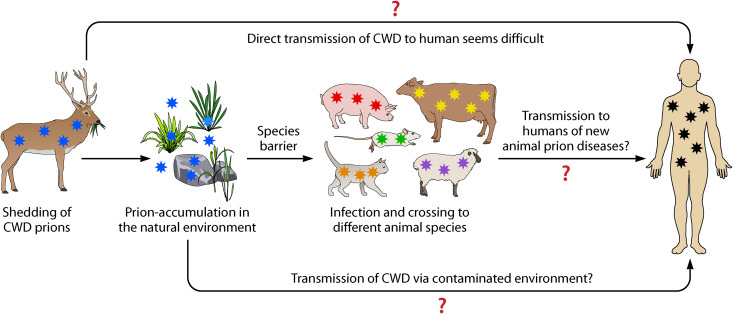
FIG 2.
Effect of the natural environment and interspecies transmission in the zoonotic potential of animal prions. The direct transmission of prion disease from animals to humans is likely difficult, except in the case of BSE. Shedding of animal prions to diverse materials within the natural environment may contribute to prion concentration and perhaps mutation, which may increase their potential for human infection. Transmission of the disease to other animal species may facilitate the replication of novel prion conformational strains with altered zoonotic potential. All these processes increase the risk for human infection with novel forms of animal prions.
Accumulation of Prions in the Environment
While scientists have long suspected prions are excreted and secreted into the environment (69), what exactly happens after their entry has been harder to explicate. It does appear that prions are able to persist in the environment for prolonged periods of time (71, 112, 113). In the perhaps most illustrative example, the scrapie agent was found to persist in the environment, remaining bioavailable and infectious for >16 years (114). It has been hypothesized that prions may interact with select components of the environment, leading to changes in their infectivity, strain properties, and potentially even the ability to cross the species barrier (115, 116) (Fig. 1 and 2).
Soil is considered the component most likely to come into contact with prions shed into environment via feces, urine, carcasses, or other means. Several lines of evidence have come to suggest that soil may play the largest role in indirectly facilitating the transmission of scrapie and, more recently, CWD. Scrapie has been shown to remain infectious after being buried in soil for 3 years (112). Similarly, BSE infectivity was shown to persist 5 years after burial in both sandy and clay soil and, most interestingly, displayed no significant loss of infectivity over this time (117). Other groups have shown that certain soil qualities may actually increase the infectivity titer. Johnson and colleagues demonstrated that prions bound to montmorillonite, a mineral found in clay soil, were not only bioavailable and orally infectious but also 680 times more infectious experimentally than unbound PrPSc (118). Likewise, Walter et al. reported a positive correlation between the clay content of soil within the habitat of individual free-ranging deer and their likelihood of CWD infection (119). Critically, widespread CWD contamination has been detected in natural mineral licks (soils rich in salts and minerals) frequented by deer in Wisconsin, illustrating that such prion accumulation in soil occurs in natural settings as well (120). Conversely, other soils and organic compounds have been linked with a prolonged incubation period and appear to negatively affect prion infectivity (121). Moreover, it has been demonstrated that prions bound to soil might be affected by natural events such as cycles of wetting and drying or freezing and thawing, which may alter infectivity (117), strain properties, and ability to cross species barriers (115). These findings make increasingly evident that the relationship between soil and prion transmissibility is more complex and multidimensional than initially anticipated.
The persistence of prions in soil, as well as the fact that most animals affected by prion diseases are herbivores, lends to the likelihood that grass, plants, and other foliage may too play a role in the seemingly uninhibited spread of CWD (Fig. 1). Our group was one of the first to investigate the relationship between grass plants and prion transmission (90). Our data demonstrated that grass plant roots and leaves effectively bound PrPSc from CWD-infected brain, urine, or feces. Prions could be detected in living plants as far as 7 weeks after treatment, demonstrating their capacity to persist following deposition (90). Moreover, we showed that prions bound to soil can be taken up by plants and transported to aerial parts of the plant. While additional studies are needed to further elucidate the extent of their exact contribution, it seems probable that plants may play a role in the dissemination of prion disease in nature (Fig. 1). Collectively, these discoveries suggest that environmental accumulation and the interaction of prions with the environment may not only play a dynamic role in facilitating disease transmission but also play a critical part in modifying characteristics such as infectivity titer, strain properties, and, most alarmingly, potential to cross the species barrier (Fig. 1 and 2).
Disease Transmission by Environmental Materials
In the United States, CWD was limited to a small range of northern Colorado and southern Wyoming as recently as the late 1990s. As of January 2021, CWD has been reported in 339 counties within 26 states of continental United States as well as three provinces in Canada and the European nations of Norway, Sweden, and Finland in addition to imported cases documented in South Korea (122). This profound expansion has undoubtedly been aided by the propensity of prions to enter the environment, persist, accumulate, and finally infect new animals (Fig. 1). In this revolving ever-amplifying cycle, the environment serves as a vector, facilitating the transmission of prion disease from one organism to the next.
Several studies reported the aptitude of soil to transmit prion disease in this manner (118, 123). Soil contaminated with prions was found to induce disease in hamsters when orally consumed 29 months later (123). Many ruminant species, including cervids, are known to regularly consume soil (124, 125), thus illustrating a plausible natural pathway of reinfection. Consistently, oral consumption of plants contaminated with infected brain homogenate was sufficient to induce disease in reporter animals (90). Furthermore, other natural environmental materials, including rocks and wood, have also been reported to bind PrPSc and preserve the ability to serve as a template for the PrPC to PrPSc conversion in vitro and infectivity in vivo (126). Also, human-made components of the environment, including polypropylene, glass, cement, and stainless steel as well as aluminum, have been shown to efficiently bind prions, which remain capable to infect animals (126). Strikingly, we also previously demonstrated that simply cohousing hamsters with contaminated materials was sufficient to induce disease (126). Our data suggest that even minimal indirect contact with the contaminated surface (e.g., rubbing/brushing of the coat against a surface) may be sufficient to transfer prions from the contaminated surface to the animal and eventually lead to infection (126). The potential role of environmental materials as vectors merits further exploration, as it is highly likely that wild and, even more so, captive populations of deer and sheep readily come in contact with materials such as wood, steel, and cement.
Of additional concern is the possibility that prions might be able to disseminate throughout the environment itself. The notion that prions may spread from one element of the environment to another is particularly worrisome, as this could drastically increase possible points of infection, infinitely complicating decontamination strategies and theoretically leading to the migration of prions to sites distant from the initial point of dissemination. Indeed, our data showed that prions can be mobilized from contaminated environmental surfaces to the cage bedding of reporter animals and effectively infect them (126). Other groups have reported limited lateral and vertical movement of infectivity when infected carcasses were buried in soil, suggesting soil contamination is limited to the site of deposition (117). Despite this, the same group found that rainwater filtered through such contaminated soils was capable of eluting prions from the soil and could potentially carry them to distant sites or even lead to the contamination of groundwater (117). Indeed, CWD prions have been detected in water (albeit below infectious levels) from a water treatment facility located in a CWD area of endemicity, suggesting transmission through the environment via water (127). Moreover, animals living in the contaminated environment may help to disperse prions across the environment (Fig. 1), even though some of these animals may not be able to replicate prions but just act as carriers of infectious materials. In a yet unpublished study, we found that earthworms can take up, bind, retain, and disperse infectious prions in the soil, which maintain infectious properties (S. Pritzkow and C. Soto, unpublished observations). More studies regarding the possibility of various environmental components transmitting disease to animals and throughout the environment itself are critical to understanding and managing the rapid spread of CWD.
PRION EXPOSURE THROUGH THE ARTIFICIAL ENVIRONMENT
Prion Transmission through Medical Practices
Whereas animal prion diseases such as CWD and scrapie appear to have significant transmission via the natural environment, human prion diseases have been transmitted by more artificial means (Fig. 3). Iatrogenic CJD (iCJD) refers to the unintentional transfer of prions via exposure to contaminated materials during medical procedures (41). Philip Duffy and colleagues described one of the first cases of iatrogenic transmission in which the cornea from a recently deceased donor was transplanted to a 55-year-old female (128). Symptoms characteristic of CJD appeared 18 months after the transplant and death only 8 months thereafter (128). Just 3 years later, Christoph Bernoulli recognized that cortical electrical probes had likely transmitted prions from a woman presenting with signs of dementia to two younger individuals when the same instruments were used months later (73). All three patients were later diagnosed with CJD (129). After multiple benzene cleanings, repeated sterilization in ethanol and formaldehyde vapor, and the passing of 2 years’ time, the very same electrodes were surgically implanted in a chimpanzee. In spite of all disinfection attempts, the animal developed neurological symptoms after 18 months and, upon sacrifice 7 weeks later, contained the spongiform degeneration and vacuolation characteristic of prion diseases (129). In the years since, the capacity of surgical materials such as stainless steel to both bind and later release animal and human prions has been demonstrated many times over (70, 72, 126, 130).
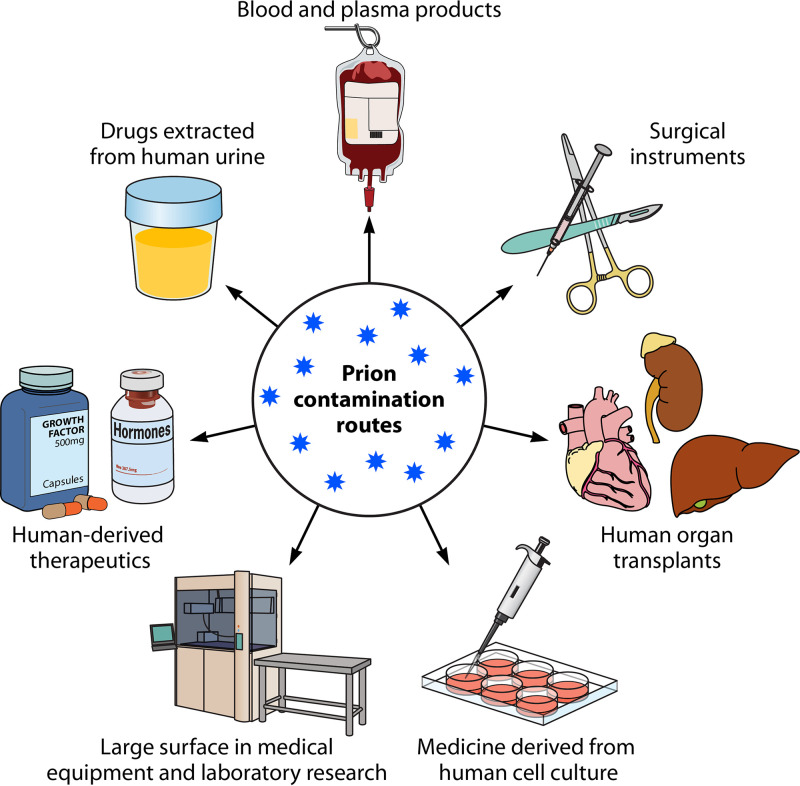
FIG 3.
Potential routes for prion contamination in medical or laboratory research settings. Within a medical or laboratory setting, humans can be exposed to prions via an array of contamination routes, including (i) blood- and plasma-derived products, (ii) surgical equipment, particularly, those made of metals provide excellent surfaces for the binding and retention of prions, (iii) transplants with organs contaminated by prions, (iv) biological drugs derived from prion-contaminated cell lines, (v) large surfaces in medical or research facilities where extensive handling of brain tissue or infectious prion samples may have occurred, (vi) drugs and biological products purified from humans tissues, or (vii) from human biologicals, such as urine.
The number of iCJD cases directly attributable to neurosurgical procedures is relatively few. There are only 4 confirmed cases related to neurosurgical instruments and the aforementioned 2 cases related to electroencephalogram probes (40, 59). Nonetheless, the possibility of prion transmission remains a realistic concern for patients and physicians alike (Fig. 3). Despite the development of guidelines for the proper decontamination of nondisposable surgical instruments (131), these procedures are often not followed if CJD is not suspected, due the corrosive effect NaOH and sodium hypochlorite (bleach) can have on costly surgical tools (74). Nonetheless, presymptomatic and even symptomatic CJD patients may undergo neurosurgical procedures prior to their diagnosis. Even after such surgical interventions, CJD may not be readily diagnosed or even suspected for an extended period of time (132). In the meantime, the now-contaminated instruments are then likely to be suboptimally disinfected by conventional methods and returned to circulation to be reused on other patients, inadvertently exposing them to prions and potentially transmitting the disease. Indeed, over a 15-year period, 19 such cases of suspected exposure to CJD-contaminated instruments were reported to the CDC (132). Alarmingly, in the 58% of these cases, the specific surgical instruments could not be identified or traced down, making it impossible to reliably determine the number of potential exposures or even inform those affected (132).
Far more numerous are cases of CJD iatrogenic transmission related to dura mater grafts transplanted from cadavers with undiagnosed CJD. Of the 228 confirmed cases of dura mater graft-associated CJD (dCJD) (40), >60% occurred in Japan (133). This strikingly elevated incidence of dCJD was first reported in 1997, and by 2008, the vast majority of these cases had been linked to a single manufacturer and brand (Lyodura) of dural grafts (134) commonly utilized in Japan. As a result of this tragic outbreak, the use of cadaveric dura mater grafts has been discontinued. Nonetheless, cadaveric tissue donation (skin, corneas, tendons, and bone) as well as organ donation (heart, lungs, kidney, and pancreas) is becoming more accepted and widespread, unquestionably benefitting the lives of many people each year. However, the risk of iatrogenic CJD transmission remains, especially in time-sensitive cases where tissues and/or organs are transplanted prior to complete autopsy (Fig. 3). Importantly, a recent study showed that prion infectivity was detected in a wide variety of peripheral tissues in both vCJD and sCJD cases (135).
Prion Transmission through Scientific Research
The contribution of scientific research toward the understanding of prion diseases cannot be overstated. Nonetheless, the infectious nature of prions and their resistance to decontamination makes their study in laboratory or hospital settings inherent to certain risks, including that of transmission (Fig. 3). The Biosafety in Microbiological and Biomedical Laboratories (BMBL) manual (6th edition) established by the CDC advises that in the laboratory setting, prions from human tissues and human prions propagated in animals can be manipulated at biosafety level 2 (BSL2) or higher. Due to concerns about BSE prions infecting humans and cattle, certain circumstances may call for the use of BSL3 facilities and/or practices (136). Research on animal prions, in which transmission to humans is considered low risk, is conducted in biosafety level 2 (BSL2) laboratories. These facilities and the safe handling guidelines function to provide a secure contained space where authorized personnel can safely handle contaminated samples in a manner that minimizes both the risk to themselves and the risk of disseminating prions into the environment (137). Research personnel are advised to wear preferably disposable personal protective equipment, such as a splash shield, face mask, hair and shoe covers, gloves, and a gown (136).
Even with these protective measures, accidental exposures are still possible (Fig. 3). Just recently, Brandel and colleagues reported what is likely the first case of accidental prion transmission due to scientific research (138). The patient, a technician in a prion research laboratory, was handling murine brains experimentally infected with an adapted form of BSE. While manipulating these samples, the patient punctured her thumb with a contaminated instrument (138). Neurological symptoms appeared 7.5 years later and the patient died roughly a year and half later, upon which the diagnosis of CJD was confirmed (138). While this case is the first report of transmission through scientific research, it seems incontestable that those working hands-on with prions on a daily basis are more likely to come into contact with prions and thus more likely to accidentally become infected. This unfortunate case study serves to remind the entire field of the inherent risks of studying infectious agents and, in particular, the unambiguously fatal nature of prion disease. Just as importantly, it underscores the need to reexamine safety protocols and guidelines for laboratory research of prion agents.
Prion Transmission by Human/Animal-Derived Products
The greatest threat of iatrogenic transmission of prion disease likely comes not from medical procedures or from accidental occupational exposures but from the use of contaminated human- or animal-derived products (Fig. 3). Transmission from human-derived products is perhaps best exemplified by the 244 growth hormone-associated cases of iCJD that occurred primarily in France (n = 120), the United Kingdom (n = 77), and the United States (n = 32) (139). In between the 1960s and 1980s, growth hormone purified from cadaver pituitaries, including tissue coming from an individual infected by CJD, was used to treat patients suffering from a variety of hormone deficiency-related ailments (140, 141). Hormone treatments such as these typically commence at a young age, and tragically, children as young as 10 years of age were diagnosed with iCJD (142). Conversely, incubation times of up to 40 years have also been reported (143). Another 4 cases of iCJD have been ascribed to contaminated pituitary gonadotropins also pooled from cadavers and administered to women as a fertility treatment (40, 144).
In more recent years, evidence has begun to suggest that blood transfusions may be another potential route of infection (Fig. 3). While no such cases of sCJD have been confirmed, there are 4 suspected cases in which CJD presented after the transfusion of blood from a donor who later went on to develop vCJD (40, 145–147). According to the Red Cross, 13.6 million units of whole blood and red blood cells are donated each year by an estimated 6.8 million people. The sheer number of people who give blood, a lack of mandated prion testing, and the long presymptomatic period of CJD give rise to the plausibility that more cases can occur in the future. While the era of BSE and vCJD appears to be fortunately ending, the possibility of transmission by blood transfusion of sporadic CJD, which affects approximately one in one million people every year (148), would be of greater concern. There is no evidence, however, implicating that blood transfusions have spread the sporadic form of the disease (41). More studies are ongoing to ascertain why the sporadic form does not appear to transmit via blood like the variant form.
As in blood, prions have been detected in urine of symptomatic vCJD patients (97) and both the presymptomatic and symptomatic phases of animal prion diseases (68, 96, 149). This suggests the possibility that products derived from human urine, if sourced from presymptomatic CJD patients, may be capable of transmitting prion disease (Fig. 3). Indeed, the same aforementioned gonadotropins that were initially sourced from cadavers were later derived from the urine of postmenopausal women and administered as a fertility treatment (150). These extracted hormones have been reported to be of dubious purity (151). Indeed, the presence of cellular prion protein has been reported in such purified fertility products ready for human injection (152), suggesting that the misfolded infectious form could too bypass the purification process. No cases of prion disease transmission due to infertility injectables have been reported, and as recombinant hormone preparations have largely replaced urine derived ones (150), the chances of such transmission continue to diminish.
The progressive decrease in cases of vCJD can be directly attributed to the decline of its causative agent, BSE. The BSE epidemic that peaked in the early 1990s is estimated to have caused 195,000 diagnosed cases in cattle (153) in the United Kingdom alone. The total number of infected cows and how many entered the food chain is difficult to estimate. Nonetheless, changes to cattle feed, eradication programs, and surveillance have essentially eradicated BSE. In 2017, only 4 cases were reported globally (153), and only 6 have ever been reported in the United States (154). As such, the risk of consuming BSE-contaminated beef is exceedingly rare. Despite this, products derived from or containing bovine material are plentiful, and although unproven, some of them might be potential sources of infection. Cattle by-products include certain vaccines, various medications and other medical products (i.e., heparin), certain hormones, including insulin and some thyroid hormones, and even the gelatin (155) coating capsules consumed by millions of people. While the likelihood is remote (156), it is plausible that some of these products could become contaminated with BSE prions and lead to a resurgence in vCJD in the future. Nevertheless, it has been reported that the gelatin manufacturing process appears to inactivate BSE prions (157, 158).
Finally, biological products, including stem cells, antibodies, vaccines, and cell-derived products, have been increasingly used for treatment. It is theoretically possible that biological products may contain and even increase the presence of prions (Fig. 3). We recently reported that the PMCA technology might be very useful to screen biological products (159). Indeed, we showed that the technique is capable of detecting a single infected cell among a pool of one million noninfected cells.
RISKS FOR HUMAN HEALTH
The risks for human health expand beyond just those associated with variant and iatrogenic CJD. Perhaps the utmost threat, and distressingly, the most underestimated one, is the likely progressive accumulation of CWD in the environment. Possibly luring us into an unwarranted sense of security is evidence of a strong species barrier suggested by a series of landmark experiments using humanized mouse models (160–163).
Nonetheless, not all the evidence has been so easy to interpret. Transmission experiments in nonhuman primates have suggested that the species barrier protecting humans from CWD may not be as ironclad as initially suspected. Squirrel monkeys inoculated intracerebrally and, more relevantly, orally with CWD were later found to develop subtle outward signs of wasting disease and, more tellingly, spongiform degeneration in the brain (164, 165). Curiously, similar studies found that cynomolgus macaques displayed no evidence of disease when inoculated by either route (164–166). It is not yet known why CWD transmission appears possible in squirrel monkeys but not in macaques, which are closer human relatives genetically. Adding yet another layer of mystery and anticipation are unpublished reports coming from another group in which macaques do appear susceptible to CWD infection, most pertinently after orally consuming CWD-contaminated meat (167). What these studies really demonstrate in terms of CWD transmission to humans is subject to various interpretations and speculation. It is clear that more studies using relevant models such as nonhuman primates are needed to better elucidate the risk CWD poses to humans.
In the early days of the BSE epidemic, the UK government assured its citizens that BSE was a rare disease of cattle alone and posed no risk to humans (168, 169). At its height, >1,000 cattle BSE cases were confirmed each week (170), and since then, vCJD, likely caused by exposure to cow material contaminated with BSE prions, has taken >200 human lives (171). Just like the BSE epidemic, the consumption of CWD-contaminated venison is probably the most likely way for CWD to transmit to humans, if such transmission is indeed possible. The CDC recommends against consuming deer or elk meat from CWD-positive animals (172). Despite this, the popularity of deer hunting as well as venison consumption (173) make it likely that large quantities of CWD-contaminated meat are unwittingly consumed each year in the United States. Indeed, some reports of CWD consumption have already surfaced (174). Fortunately, follow-up studies of these potentially affected individuals have not yet revealed any transmission of prion disease (174), although more time is needed to make a conclusive determination.
What distinguishes the current CWD epidemic from previous outbreaks is the substantial role of the environment in propagating the disease. Never before have we seen the environment and its components act as a vector, accumulating prions only to further the infection of yet more animals. As CWD continues to spread, the buildup of prions in the environment will only continue, facilitating the probability of human contact with the disease but also that of other species. The interspecies transmission to other species, particularly those animals used for human consumption (i.e., porcine) can not only augment human exposure but also provide a suitable setting for the emergence of new strains that may more readily cross the cervid-human species barrier (Fig. 2). Several animal species have already been shown to be sensitive to CWD prion infection (172, 175–177). CWD transmission to those other species may serve as a biological reservoir for infectious prions and also for the emergence of new animal prion disease with different zoonotic potential.
The widespread distribution of prions in the natural environment and their aforementioned persistence make it difficult to envision management strategies and, even more challenging, to implement them. Unfortunately, a complete halt to the spread of CWD is likely impossible. We can, however, strive to control the spread of CWD and reduce the role of the environment in its dissemination. The keys to limiting the horizontal transmission of CWD by environmental materials include (i) limiting environmental exposure, (ii) targeted removal of prions from the environment, and (iii) monitoring for potential environmental contamination.
Limiting PrPSc accumulation in the environment is the first and most difficult step in limiting environmentally propagated transmission. Steps to limit prion deposition include controlling the prevalence of CWD in populations of cervids and the proper disposal of CWD-infected carcasses. Several lines of evidence suggest that culling strategies have a positive impact on CWD prevalence in free-ranging cervids (178–181) and consequently limit the amount of CWD prions deposited into the environment. As discussed earlier, evidence has identified carcasses as potential foci for infection (71) that can remain in the environment for a prolonged length of time (71, 117). The International Association of Fish and Wildlife Agency recommends the disposal of CWD-contaminated carcasses in approved lined landfills, which can help prevent the accumulation of CWD in the environment (182). While the large-scale disinfection of the environment is not realistic or feasible, it is possible to selectively decontaminate areas where CWD deposition is probable and thus eliminate possible hot spots of infectivity. Sodium hydroxide (75), sodium hypochlorite, and enzymatic treatments (183) have shown promise for soil decontamination in experimental settings (Fig. 4). The potential side effects of such treatments still need to be explored. The final step in reducing the role of environmental transmission is to implement strategies for the targeted monitoring of possible foci of infection, namely, areas where deer are known to gather. Newly developed assays show promise in detection from a wide array of environmental samples and materials (90, 120, 126) and may be used to monitor the spread and accumulation of CWD in the environment as well as to identify possible transmission hot spots (Fig. 4).
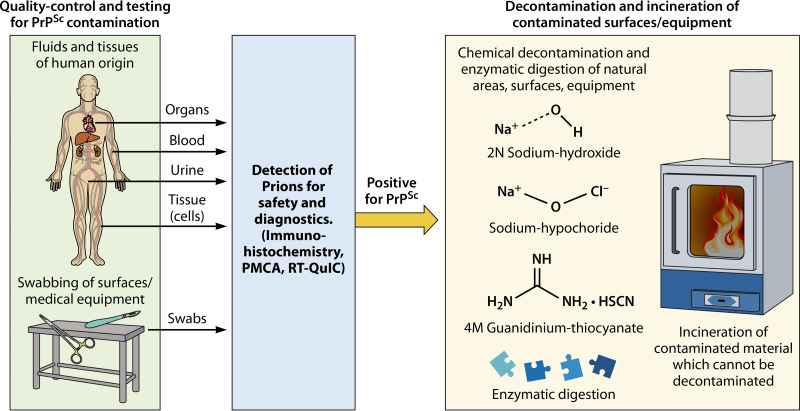
FIG 4.
Strategies for prion biosafety and potential methods of decontamination. Strategies to mitigate the spread of prions in clinically relevant settings are vital to disease prevention. Quality control and testing of potential prion contamination in biological samples (organs, tissues, cells, and fluids) or the sampling from relevant surgical/medical equipment can be most effectively analyzed via highly sensitive molecular diagnostic lab methods, such as PMCA or RT-QuIC. These methodologies may unveil subdetectable quantities of infectious prions which may be overlooked by conventional lab methods (Western blotting and immunohistology). Once a sample has been deemed positive for prion contamination, cleaning and elimination strategies can be used depending on the nature of the contaminated objects. It is possible to attempt the decontamination of the materials with harsh chemicals or enzymatic digestion. In the case of materials which cannot be so facilely decontaminated, incineration is the suggested option to completely remove the infectious agent.
TESTS TO DETECT PRIONS IN ENVIRONMENTAL AND BIOLOGICAL SAMPLES
Postmortem analysis of the brain for spongiform degeneration and PrPSc deposition by immunohistochemistry is the only way to definitively diagnosis CWD or any other prion disease. Despite this, the development of several highly sensitive assays, including protein misfolding cyclic amplification (PMCA) (184) and real-time quaking-induced conversion (RT-QuIC) (185), have shown significant progress toward the development of a definitive antemortem test (Fig. 4). By providing either brain-derived or recombinant prion protein as a substrate, these assays are able to amplify a minute amount of seed, essentially taking advantage of the natural propensity of PrPSc to serve as a template for the misfolding of the normal prion protein.
The ability of these assays to detect miniscule amounts of infectious protein has made them invaluable tools for the screening and detection of both biological and environmental samples. Perhaps the most significant advantage conferred is the ability to screen for prion infection antemortem from easily accessible bodily fluids (Fig. 4). The detection of CWD from cervid blood samples at the asymptomatic and symptomatic stages of the disease has already been demonstrated (107) and was also shown from the blood of symptomatic cases of vCJD (105). Importantly, similar detection capacities have been demonstrated in urine (68, 90, 96, 97), which can be obtained even less invasively than blood. Saliva (68, 98) and nasal brushings (186) have also shown the capacity to be used as screening fluids. Improvements to these technologies over the years have progressively increased the sensitivity of these assays and widened the pool of fluids and tissues which can be used to detect prion infection. Indeed, CWD prions have recently been detected in samples as diverse as cervid third eyelids (187) and semen (188), highlighting not only the value of these detection assays but also the extensive peripheral spread and deposition of prions.
Due to their remarkable amplification capacities, PMCA and RT-QuIC have demonstrated value in detection from a variety of environmental samples and surfaces. Detection of such dilute quantities from a variety of materials was previously thought impossible due to the restrictions of conventional techniques such as Western blotting. Prions have since been detected in unwieldy environmental samples such as soil (120, 123), grass plants (90), and even water (127). The prion detection of various human-made and natural materials contaminated with prions has also been successfully achieved (127, 131). Collectively, these techniques have greatly furthered our understanding of prion diseases and their methods of replication. PMCA and RT-QuIC seem poised for approval for the accurate screening of alive and even asymptomatic animals and are likely to soon represent a new gold standard in prion disease testing and screening as well as to help in monitoring and management strategies of prion diseases in the future (Fig. 4).
CONCLUSIONS AND PERSPECTIVES
The unconventional nature of prions as a protein-only infectious agent determines that many of the rules that apply to conventional infectious agents are not valid for prions. Thus, a detailed understanding of the mechanisms of prion replication, transmission, dissemination, and elimination is needed to combat these dangerous agents. The potential of prions to produce fatal diseases that can be transmitted across species barriers and their capacity to mutate, adapt, and evolve, as well as their persistence and extreme resistance to decontamination, make prions a high risk for animals and humans. Prions can be released into the natural environment where they can persist, accumulate, and remain infectious for long periods of time. They can also be spread through medical practices, scientific research, and the use of biological products.
Future research in prion diseases need to address some of the pressing pending questions in the field, including the elucidation of the natural strain diversity of animal and human prions, the mechanism of prion transmission and the role of accessory components (e.g., environmental materials), the cellular and molecular mechanisms responsible for strain variability and interspecies transmission, the atomic resolution structure of PrPSc, the development and utilization of methodologies for highly sensitive detection of prions, and the design of efficient therapeutic strategies for these devastating diseases.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the National Institutes of Health (P01AI077774 to C.S.).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
C.S. is an inventor on several patents related to the PMCA technology and is currently Founder, Chief Scientific Officer, and member of the Board of Directors of Amprion Inc., a biotech company focusing on the commercial utilization of PMCA for prion diagnosis. S.P. also has a conflict of interest related to the PMCA technology and Amprion. Finally, The University of Texas System has licensed intellectual property to Amprion.
Biographies

Sandra Pritzkow is currently Assistant Professor in the Department of Neurology at the University of Texas Health Science Center in Houston. She completed her Ph.D. in 2011 at the Robert Koch Institute in Berlin, Germany. Between 2012 and 2016 she was a postdoctoral fellow in Dr. Claudio Soto’s lab at the University of Texas Health Science Center in Houston. In 2017, she was promoted to Assistant Professor and started to develop her own research group. Dr. Pritzkow’s main interest is translational research on neurodegenerative diseases associated with the misfolding and aggregation of proteins, mostly working in Alzheimer’s, Parkinson’s, and prion diseases.

Damian Gorski received his bachelor of science in Biological Sciences from Marquette University in Milwaukee, WI. In November 2017, he joined the Mitchell Center for Alzheimer’s Disease and Related Disorders at the University of Texas Health Science Center at Houston directed by Dr. Claudio Soto as a Research Assistant under the supervision of Dr. Sandra Pritzkow. Damian’s interests include the interplay between protein misfolding and neuroinflammation as it occurs in proteinopathies. Damian is part of the 2021 incoming class at the University of Texas Health Science Center Graduate School of Biomedical Sciences.

Frank Ramirez completed his undergraduate studies in May 2018 at the University of Texas Rio Grande Valley, Texas. Since then, he has been working full time as a Research Assistant in the Mitchell Center for Alzheimer’s Disease and Related Disorders under the supervision of Dr. Sandra Pritzkow, in the Protein Misfolding Disorders laboratory headed by Dr. Claudio Soto. His work during the past 3 years involves optimizing the prion protein misfolding cyclic amplification (PMCA) assay to detect human prions in biological fluids and cells and to further elucidate the molecular underpinnings of the infectious agent responsible for prion diseases. Upon completion of his time as a Research Assistant, Frank hopes to further continue his journey pursuing higher educational studies.

Claudio Soto is Professor of Neurology and Director of the Mitchell Center for Alzheimer’s Disease and Related Brain Disorders at The University of Texas Health Science Center at Houston, McGovern Medical School. Dr. Soto has been working in the field of neurodegenerative diseases, in particular, in Alzheimer’s, Parkinson’s, and prion diseases, for the past 28 years and has made several important discoveries both in the basic science understanding of the diseases and in the translation of this knowledge into novel strategies for treatment and early diagnosis. He invented and developed protein misfolding cyclic amplification (PMCA, also known as RT-QuIC) technology for ultrasensitive detection of misfolded proteins and the beta-sheet breaker approach to produce therapeutic compounds for various protein misfolding disorders. Dr. Soto has published more than 190 peer review publications, which have been cited more than 25,000 times (H index 81).
REFERENCES
- 1.Collinge J. 2001. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24:519–550. 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 2.Soto C, Satani N. 2011. The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol Med 17:14–24. 10.1016/j.molmed.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto C. 2011. Prion hypothesis: the end of the controversy? Trends Biochem Sci 36:151–158. 10.1016/j.tibs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zabel MD, Reid C. 2015. A brief history of prions. Pathog Dis 73:ftv087. 10.1093/femspd/ftv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creutzfeldt HG. 1920. Über eine eigenartige herdförmige Erkrankung des Zentralnervensystems (Vorläufige mitteilung). Z Gesamte Neurol Psy 57:1–18. 10.1007/BF02866081. [DOI] [Google Scholar]
- 6.Jakob A. 1921. Uber eigenartige Erkrankungen des Zentralnervensystems mit bemerkenswertem anatomischen Befunde. Z Gesamte Neurol Psy 64:147–228. [Google Scholar]
- 7.Gajdusek DC, Zigas V. 1957. Degenerative disease of the central nervous system in New Guinea. N Engl J Med 257:974–978. 10.1056/NEJM195711142572005. [DOI] [PubMed] [Google Scholar]
- 8.Hadlow WJ. 1959. Scrapie and kuru. Lancet 274:289–290. 10.1016/S0140-6736(59)92081-1. [DOI] [Google Scholar]
- 9.Gajdusek DC, Zigas V. 1959. Kuru. Clinical, pathological and epidemiological study of an acute progressive degenerative disease of the central nervous system among natives of the Eastern Highlands of New Guinea. Am J Med 26:442–469. 10.1016/0002-9343(59)90251-7. [DOI] [PubMed] [Google Scholar]
- 10.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis 16:89–90. 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 11.Cuille J, Chelle PL. 1939. Experimental transmission of trembling to the goat. C R Seances Acad Sci 208:1058–1160. [Google Scholar]
- 12.Gibbs CJ, Gajdusek DC, Asher DM, Alpers MP, Beck E, Daniel PM, Matthews WB. 1968. Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 161:388–389. 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- 13.Cuillé J. 1938. Investigations of scrapie in sheep. Vet Med 34:417–418. [Google Scholar]
- 14.Gajdusek DC. 1967. Slow-virus infections of the nervous system. N Engl J Med 276:392–400. 10.1056/NEJM196702162760708. [DOI] [PubMed] [Google Scholar]
- 15.Pattison IH, Jones KM. 1967. The possible nature of the transmissible agent of scrapie. Vet Rec 80:2–9. 10.1136/vr.80.1.2. [DOI] [PubMed] [Google Scholar]
- 16.Griffith JS. 1967. Nature of the scrapie agent: self-replication and scrapie. Nature 215:1043–1044. 10.1038/2151043a0. [DOI] [PubMed] [Google Scholar]
- 17.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 18.Raymond GJ, Chabry J. 2004. Purification of the pathological isoform of prion protein (PrPSc or PrPres) from transmissible spongiform encephalopathy-affected brain tissue, p 16–26. In Techniques in prion research. Birkhäuser, Basel, Switzerland. [Google Scholar]
- 19.Polymenidou M, Verghese-Nikolakaki S, Groschup M, Chaplin MJ, Stack MJ, Plaitakis A, Sklaviadis T. 2002. A short purification process for quantitative isolation of PrPSc from naturally occurring and experimental transmissible spongiform encephalopathies. BMC Infect Dis 2:23. 10.1186/1471-2334-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castilla J, Saá P, Hetz C, Soto C. 2005. In vitro generation of infectious scrapie prions. Cell 121:195–206. 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Zou WQ, Gambetti P. 2005. From microbes to prions: the final proof of the prion hypothesis. Cell 121:155–157. 10.1016/j.cell.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim J-I, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R, Race B, Qing L, Gambetti P, Caughey B, Surewicz WK. 2010. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285:14083–14087. 10.1074/jbc.C110.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Wang X, Yuan CG, Ma J. 2010. Generating a prion with bacterially expressed recombinant prion protein. Science 327:1132–1135. 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayers JI, Schutt CR, Shikiya RA, Aguzzi A, Kincaid AE, Bartz JC, Mabbott NA. 2011. The strain-encoded relationship between PrP Sc replication, stability and processing in neurons is predictive of the incubation period of disease. PLoS Pathog 7:e1001317. 10.1371/journal.ppat.1001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM. 2000. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J Virol 74:5542–5547. 10.1128/jvi.74.12.5542-5547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bessen RA, Marsh RF. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J Gen Virol 73:329–334. 10.1099/0022-1317-73-2-329. [DOI] [PubMed] [Google Scholar]
- 27.DeArmond SJ, Yang SL, Lee A, Bowler R, Taraboulos A, Groth D, Prusiner SB. 1993. Three scrapie prion isolates exhibit different accumulation patterns of the prion protein scrapie isoform. Proc Natl Acad Sci U S A 90:6449–6453. 10.1073/pnas.90.14.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecker R, Taraboulos A, Scott M, Pan KM, Yang SL, Torchia M, Jendroska K, DeArmond SJ, Prusiner SB. 1992. Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev 6:1213–1228. 10.1101/gad.6.7.1213. [DOI] [PubMed] [Google Scholar]
- 29.Bruce ME, Boyle A, Cousens S, McConnell I, Foster J, Goldman W, Fraser H. 2002. Strain characterization of natural sheep scrapie and comparison with BSE. J Gen Virol 83:695–704. 10.1099/0022-1317-83-3-695. [DOI] [PubMed] [Google Scholar]
- 30.Chernoff YO. 2004. Do amyloids remember their origin? New insights into the prion species barrier. Mol Cell 14:147–148. 10.1016/s1097-2765(04)00208-4. [DOI] [PubMed] [Google Scholar]
- 31.Hill AF, Collinge J. 2004. Prion strains and species barriers. Contrib Microbiol 11:33–39. 10.1159/000077061. [DOI] [PubMed] [Google Scholar]
- 32.Jones EM, Surewicz WK. 2005. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell 121:63–72. 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton DR, DeArmond SJ, Prusiner SB, Scott MR. 2002. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34:921–932. 10.1016/s0896-6273(02)00726-2. [DOI] [PubMed] [Google Scholar]
- 34.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. 1997. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501. 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 35.Hill AF, Desbruslais M, Joiner S, Sidle KCL, Gowland I, Collinge J, Doey LJ, Lantos P. 1997. The same prion strain causes vCJD and BSE. Nature 389:448–450. 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 36.Kong Q, Zheng M, Casalone C, Qing L, Huang S, Chakraborty B, Wang P, Chen F, Cali I, Corona C, Martucci F, Iulini B, Acutis P, Wang L, Liang J, Wang M, Li X, Monaco S, Zanusso G, Zou W-Q, Caramelli M, Gambetti P. 2008. Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 82:3697–3701. 10.1128/JVI.02561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marín-Moreno A, Huor A, Espinosa JC, Douet JY, Aguilar-Calvo P, Aron N, Píquer J, Lugan S, Lorenzo P, Tillier C, Cassard H, Andreoletti O, Torres JM. 2020. Radical change in zoonotic abilities of atypical BSE prion strains as evidenced by crossing of sheep species barrier in transgenic mice. Emerg Infect Dis 26:1130–1139. 10.3201/eid2606.181790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padilla D, Bé Ringue V, Carlos Espinosa J, Andreoletti O, Jaumain E, Reine F, Herzog L, Gutierrez-Adan A, Pintado B, Laude H, Torres JM. 2011. Sheep and goat BSE propagate more efficiently than cattle BSE in human PrP transgenic mice. PLoS Pathog 7:e1001319. 10.1371/journal.ppat.1001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plinston C, Hart P, Chong A, Hunter N, Foster J, Piccardo P, Manson JC, Barron RM. 2011. Increased susceptibility of human-PrP transgenic mice to bovine spongiform encephalopathy infection following passage in sheep. J Virol 85:1174–1181. 10.1128/JVI.01578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown P, Brandel JP, Sato T, Nakamura Y, MacKenzie J, Will RG, Ladogana A, Pocchiari M, Leschek EW, Schonberger LB. 2012. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg Infect Dis 18:901–907. 10.3201/eid1806.120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Urwin PJM, Mackenzie JM, Llewelyn CA, Will RG, Hewitt PE. 2016. Creutzfeldt-Jakob disease and blood transfusion: updated results of the UK Transfusion Medicine Epidemiology Review study. Vox Sang 110:310–316. 10.1111/vox.12371. [DOI] [PubMed] [Google Scholar]
- 42.Collinge J, Whitfield J, McKintosh E, Beck J, Mead S, Thomas DJ, Alpers MP. 2006. Kuru in the 21st century-an acquired human prion disease with very long incubation periods. Lancet 367:2068–2074. 10.1016/S0140-6736(06)68930-7. [DOI] [PubMed] [Google Scholar]
- 43.Valleron AJ, Boelle PY, Will R, Cesbron JY. 2001. Estimation of epidemic size and incubation time based on age characteristics of vCJD in the United Kingdom. Science 294:1726–1728. 10.1126/science.1066838. [DOI] [PubMed] [Google Scholar]
- 44.Dickinson AG, Taylor DM. 1978. Resistance of scrapie agent to decontamination. N Engl J Med 299:1413–1414. 10.1056/NEJM197812212992512. [DOI] [PubMed] [Google Scholar]
- 45.Lansbury PT. 1994. Mechanism of scrapie replication. Science 265:1510. 10.1126/science.8079159. [DOI] [PubMed] [Google Scholar]
- 46.Cohen FE, Pan KM, Huang Z, Baldwin M, Fletterick RJ, Prusiner SB. 1994. Structural clues to prion replication. Science 264:530–531. 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- 47.Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB. 1996. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082. 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 48.Bamborough P, Wille H, Telling GC, Yehiely F, Prusiner SB, Cohen FE. 1996. Prion protein structure and scrapie replication: theoretical, spectroscopic, and genetic investigations. Cold Spring Harbor Symp Quant Biol 61:495–509. [PubMed] [Google Scholar]
- 49.Bolton DC, Rudelli RD, Currie JR, Bendheim PE. 1991. Copurification of Sp33-37 and scrapie agent from hamster brain prior to detectable histopathology and clinical disease. J Gen Virol 72:2905–2913. 10.1099/0022-1317-72-12-2905. [DOI] [PubMed] [Google Scholar]
- 50.Soto C, Estrada L, Castilla J. 2006. Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci 31:150–155. 10.1016/j.tibs.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Knowles TPJ, Waudby CA, Devlin GL, Cohen SIA, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. 2009. An analytical solution to the kinetics of breakable filament assembly. Science 326:1533–1537. 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
- 52.Masel J, Jansen VAA, Nowak MA. 1999. Quantifying the kinetic parameters of prion replication. Biophys Chem 77:139–152. 10.1016/S0301-4622(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 53.Soto C. 2012. Transmissible proteins: expanding the prion heresy. Cell 149:968–977. 10.1016/j.cell.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiesa R, Restelli E, Comerio L, Del Gallo F, Imeri L. 2016. Transgenic mice recapitulate the phenotypic heterogeneity of genetic prion diseases without developing prion infectivity: role of intracellular PrP retention in neurotoxicity. Prion 10:93–102. 10.1080/19336896.2016.1139276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takada LT, Kim MO, Metcalf S, Gala II, Geschwind MD. 2018. Prion disease. Handb Clin Neurol 148:441–464. 10.1016/B978-0-444-64076-5.00029-6. [DOI] [PubMed] [Google Scholar]
- 56.Di Fede G, Catania M, Atzori C, Moda F, Pasquali C, Indaco A, Grisoli M, Zuffi M, Guaita MC, Testi R, Taraglio S, Sessa M, Gusmaroli G, Spinelli M, Salzano G, Legname G, Tarletti R, Godi L, Pocchiari M, Tagliavini F, Imperiale D, Giaccone G. 2019. Clinical and neuropathological phenotype associated with the novel V189I mutation in the prion protein gene. Acta Neuropathol Commun 7:1. 10.1186/s40478-018-0656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi A, Parchi P, Yamada M, Mohri S, Kitamoto T. 2016. Neuropathological and biochemical criteria to identify acquired Creutzfeldt-Jakob disease among presumed sporadic cases. Neuropathology 36:305–310. 10.1111/neup.12270. [DOI] [PubMed] [Google Scholar]
- 58.Zerr I, Parchi P. 2018. Sporadic Creutzfeldt–Jakob disease. Handb Clin Neurol 153:155–174. 10.1016/B978-0-444-63945-5.00009-X. [DOI] [PubMed] [Google Scholar]
- 59.Bonda DJ, Manjila S, Mehndiratta P, Khan F, Miller BR, Onwuzulike K, Puoti G, Cohen ML, Schonberger LB, Cali I. 2016. Human prion diseases: surgical lessons learned from iatrogenic prion transmission. Neurosurg Focus 41:E10. 10.3171/2016.5.FOCUS15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Foster JD, Hope J, Fraser H. 1993. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet Rec 133:339–341. 10.1136/vr.133.14.339. [DOI] [PubMed] [Google Scholar]
- 61.Joiner S, Asante EA, Linehan JM, Brock L, Brandner S, Bellworthy SJ, Simmons MM, Hope J, Collinge J, Wadsworth JDF. 2018. Experimental sheep BSE prions generate the vCJD phenotype when serially passaged in transgenic mice expressing human prion protein. J Neurol Sci 386:4–11. 10.1016/j.jns.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Braun U, Tschuor A, Hässig M, Franitza S, Berli E, El Gedaily A, Franscini N, Matthey U, Zahn R. 2009. Untersuchung von BSE-nachkommen auf proteaseresistentes prion protein (PrP res) im blut. Schweiz Arch Tierheilkd 151:433–436. (In German.) 10.1024/0036-7281.151.9.433. [DOI] [PubMed] [Google Scholar]
- 63.Foster JD, Goldmann W, Hunter N. 2013. Evidence in sheep for pre-natal transmission of scrapie to lambs from infected mothers. PLoS One 8:e79433. 10.1371/journal.pone.0079433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, Hayes-Klug J, Anderson K, Stewart P, Goldmann W, Hoover EA, Mathiason CK. 2013. Mother to offspring transmission of chronic wasting disease in Reeves’ Muntjac deer. PLoS One 8:e71844. 10.1371/journal.pone.0071844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, Wyckoff CA, Goldmann W, Zabel MM, Wild MA, Hoover EA, Mathiason CK. 2015. In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. J Gen Virol 96:3444–3455. 10.1099/jgv.0.000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jo Moore S, Kunkle R, West Greenlee MH, Nicholson E, Richt J, Hamir A, Ray Waters W, Greenlee J. 2016. Horizontal transmission of chronic wasting disease in Reindeer. Emerg Infect Dis 22:2142–2145. 10.3201/eid2212.160635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. 2011. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol 85:6309–6318. 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. 10.1128/JVI.01118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller MW, Williams ES. 2003. Horizontal prion transmission in mule deer. Nature 425:35–36. 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 70.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. 1999. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med 5:240–243. 10.1007/BF03402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miller MW, Williams ES, Hobbs NT, Wolfe LL. 2004. Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006. 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flechsig E, Hegyi I, Enari M, Schwarz P, Collinge J, Weissmann C. 2001. Transmission of scrapie by steel-surface-bound prions. Mol Med 7:679–684. 10.1007/BF03401958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernoulli C, Siegfried J, Baumgartner G, Regli F, Rabinowicz T, Gajdusek DC, Gibbs CJ. 1977. Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet 309:478–479. 10.1016/S0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- 74.McDonnell G, Burke P. 2003. The challenge of prion decontamination. Clin Infect Dis 36:1152–1154. 10.1086/374668. [DOI] [PubMed] [Google Scholar]
- 75.Sohn HJ, Park KJ, Roh IS, Kim HJ, Park HC, Kang HE. 2019. Sodium hydroxide treatment effectively inhibits PrPCWD replication in farm soil. Prion 13:137–140. 10.1080/19336896.2019.1617623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gough KC, Maddison BC. 2010. Prion transmission: prion excretion and occurrence in the environment. Prion 4:275–282. 10.4161/pri.4.4.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeffrey M, González L, Espenes A, Press CM, Martin S, Chaplin M, Davis L, Landsverk T, MacAldowie C, Eaton S, McGovern G. 2006. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol 209:4–14. 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 78.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover EA. 1999. Oral transmission and early lymphoid tropism of chronic wasting PrP disease PrPres in mule deer fawns (in mule deer fawns (Odocoileus hemionus). J Gen Virol 80:2757–2764. 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 79.Beekes M, Baldauf E, Diringer H. 1996. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J Gen Virol 77:1925–1934. 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- 80.Beekes M, McBride PA. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci Lett 278:181–184. 10.1016/s0304-3940(99)00934-9. [DOI] [PubMed] [Google Scholar]
- 81.Donaldson DS, Else KJ, Mabbott NA. 2015. The gut-associated lymphoid tissues in the small intestine, not the large intestine, play a major role in oral prion disease pathogenesis. J Virol 89:9532–9547. 10.1128/JVI.01544-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fox KA, Jewell JE, Williams ES, Miller MW. 2006. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol 87:3451–3461. 10.1099/vir.0.81999-0. [DOI] [PubMed] [Google Scholar]
- 83.Denkers ND, Hoover CE, Davenport KA, Henderson DM, McNulty EE, Nalls AV, Mathiason CK, Hoover EA. 2020. Very low oral exposure to prions of brain or saliva origin can transmit chronic wasting disease. PLoS One 15:e0237410. 10.1371/journal.pone.0237410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller MW, Thompson Hobbs N, Tavener SJ. 2006. Dynamics of prion disease transmission in mule deer. Ecol Appl 16:2208–2214. 10.1890/1051-0761(2006)016[2208:DOPDTI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 85.Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, Palmer C, Lemus A, Dearmond SJ, Prusiner SB. 2009. Asymptomatic deer excrete infectious prions in faeces. Nature 461:529–532. 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Diringer H, Roehmel J, Beekes M. 1998. Effect of repeated oral infection of hamsters with scrapie. J Gen Virol 79:609–612. 10.1099/0022-1317-79-3-609. [DOI] [PubMed] [Google Scholar]
- 87.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S. 2016. Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS One 11:e0166187. 10.1371/journal.pone.0166187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henderson DM, Tennant JM, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. 2017. Detection of chronic wasting disease prion seeding activity in deer and elk feces by real-time quaking-induced conversion. J Gen Virol 98:1953–1962. 10.1099/jgv.0.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pulford B, Spraker TR, Christy Wyckoff A, Meyerett C, Bender H, Ferguson A, Wyatt B, Lockwood K, Powers J, Telling GC, Wild MA, Zabel MD. 2012. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J Wildl Dis 48:425–434. 10.7589/0090-3558-48.2.425. [DOI] [PubMed] [Google Scholar]
- 90.Pritzkow S, Morales R, Moda F, Khan U, Telling GC, Hoover E, Soto C. 2015. Grass plants bind, retain, uptake, and transport infectious prions. Cell Rep 11:1168–1175. 10.1016/j.celrep.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rogers LL. 1987. Seasonal changes in defecation rates of free-ranging white-tailed deer. J Wildl Manage 51:330–333. 10.2307/3801011. [DOI] [Google Scholar]
- 92.Iii WJA, Alldredge AW. 1980. Seasonal estimates of masses of mule deer fecal pellets and pellet groups. J Wildl Manage 44:750. 10.2307/3808034. [DOI] [Google Scholar]
- 93.Colorado Parks and Wildlife. 2020. Deer 2019 Post Hunt Population & Sex Ratio Estimates. https://cpw.state.co.us/Documents/Hunting/BigGame/Statistics/Deer/2019DeerPopulationEstimates.pdf.
- 94.Rivera NA, Brandt AL, Novakofski JE, Mateus-Pinilla NE. 2019. Chronic wasting disease in cervids: prevalence, impact and management strategies. Vet Med (Auckl) 10:123–139. 10.2147/VMRR.S197404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. 2009. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 4:e7990. 10.1371/journal.pone.0007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.John TR, Schätzl HM, Gilch S. 2013. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion 7:253–258. 10.4161/pri.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moda F, Gambetti P, Notari S, Concha-Marambio L, Catania M, Park KW, Maderna E, Suardi S, Haïk S, Brandel JP, Ironside J, Knight R, Tagliavini F, Soto C. 2014. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N Engl J Med 371:530–539. 10.1056/NEJMoa1404401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davenport KA, Mosher BA, Brost BM, Henderson DM, Denkers ND, Nalls AV, McNulty E, Mathiason CK, Hoover EA. 2018. Assessment of chronic wasting disease prion shedding in deer saliva with occupancy modeling. J Clin Microbiol 56:e01243-17. 10.1128/JCM.01243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8:e74377. 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robbins CT, Spalinger DE, van Hoven W. 1995. Adaptation of ruminants to browse and grass diets: are anatomical-based browser-grazer interpretations valid? Oecologia 103:208–213. 10.1007/BF00329082. [DOI] [PubMed] [Google Scholar]
- 101.Maekawa M, Beauchemin KA, Christensen DA. 2002. Chewing activity, saliva production, and ruminal pH of primiparous and multiparous lactating dairy cows. J Dairy Sci 85:1176–1182. 10.3168/jds.S0022-0302(02)74180-5. [DOI] [PubMed] [Google Scholar]
- 102.Tamgüney G, Richt JA, Hamir AN, Greenlee JJ, Miller MW, Wolfe LL, Sirochman TM, Young AJ, Glidden DV, Johnson NL, Giles K, DeArmond SJ, Prusiner SB. 2012. Salivary prions in sheep and deer. Prion 6:52–61. 10.4161/pri.6.1.16984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Angers RC, Browning SR, Seward TS, Sigurdson CJ, Miller MW, Hoover EA, Telling GC. 2006. Prions in skeletal muscles of deer with chronic wasting disease. Science 311:1117. 10.1126/science.1122864. [DOI] [PubMed] [Google Scholar]
- 104.Concha-Marambio L, Chacon AM, Soto C. 2020. Preclinical detection of prions in the blood of nonhuman primates infected with variant Creutzfeldt-Jakob disease. Emerg Infect Dis 26:34–43. 10.3201/eid2601.181423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Concha-Marambio L, Pritzkow S, Moda F, Tagliavini F, Ironside JW, Schulz PE, Soto C. 2016. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci Transl Med 8:370ra183. 10.1126/scitranslmed.aaf6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dassanayake RP, Truscott TC, Zhuang D, Schneider DA, Madsen-Bouterse SA, Young AJ, Stanton JB, Davis WC, O'Rourke KI. 2015. Classical natural ovine scrapie prions detected in practical volumes of blood by lamb and transgenic mouse bioassays. J Vet Sci 16:179–186. 10.4142/jvs.2015.16.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kramm C, Pritzkow S, Lyon A, Nichols T, Morales R, Soto C. 2017. Detection of prions in blood of cervids at the asymptomatic stage of chronic wasting disease. Sci Rep 7:17241. 10.1038/s41598-017-17090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Slota JA, Medina SJ, Klassen M, Gorski D, Mesa CM, Robertson C, Mitchell G, Coulthart MB, Pritzkow S, Soto C, Booth SA. 2019. Identification of circulating microRNA signatures as potential biomarkers in the serum of elk infected with chronic wasting disease. Sci Rep 9:19705. 10.1038/s41598-019-56249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thorne L, Terry LA. 2008. In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J Gen Virol 89:3177–3184. 10.1099/vir.0.2008/004226-0. [DOI] [PubMed] [Google Scholar]
- 110.VerCauteren KC, Pilon JL, Nash PB, Phillips GE, Fischer JW. 2012. Prion remains infectious after passage through digestive system of american crows (Corvus brachyrhynchos). PLoS One 7:e45774. 10.1371/journal.pone.0045774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nichols TA, Fischer JW, Spraker TR, Kong Q, VerCauteren KC. 2015. CWD prions remain infectious after passage through the digestive system of coyotes (Canis latrans). Prion 9:367–375. 10.1080/19336896.2015.1086061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown P, Gajdusek DC. 1991. Survival of scrapie virus after 3 years’ interment. Lancet 337:269–270. 10.1016/0140-6736(91)90873-n. [DOI] [PubMed] [Google Scholar]
- 113.Woolhouse MEJ, Stringer SM, Matthews L, Hunter N, Anderson RM. 1998. Epidemiology and control of scrapie within a sheep flock. Proc Biol Sci 265:1205–1210. 10.1098/rspb.1998.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Georgsson G, Sigurdarson S, Brown P. 2006. Infectious agent of sheep scrapie may persist in the environment for at least 16 years. J Gen Virol 87:3737–3740. 10.1099/vir.0.82011-0. [DOI] [PubMed] [Google Scholar]
- 115.Holec SAM, Yuan Q, Bartz JC. 2019. Alteration of prion strain emergence by nonhost factors. mSphere 4:e00630-19. 10.1128/mSphere.00630-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan Q, Eckland T, Telling G, Bartz J, Bartelt-Hunt S. 2015. Mitigation of prion infectivity and conversion capacity by a simulated natural process—repeated cycles of drying and wetting. PLoS Pathog 11:e1004638. 10.1371/journal.ppat.1004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Somerville RA, Fernie K, Smith A, Bishop K, Maddison BC, Gough KC, Hunter N. 2019. BSE infectivity survives burial for five years with only limited spread. Arch Virol 164:1135–1145. 10.1007/s00705-019-04154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM. 2007. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog 3:e93. 10.1371/journal.ppat.0030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.David Walter W, Walsh DP, Farnsworth ML, Winkelman DL, Miller MW. 2011. Soil clay content underlies prion infection odds. Nat Commun 2:200. 10.1038/ncomms1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Plummer IH, Johnson CJ, Chesney AR, Pedersen JA, Samuel MD. 2018. Mineral licks as environmental reservoirs of chronic wasting disease prions. PLoS One 13:e0196745. 10.1371/journal.pone.0196745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kuznetsova A, Cullingham C, McKenzie D, Aiken JM. 2018. Soil humic acids degrade CWD prions and reduce infectivity. PLoS Pathog 14:e1007414. 10.1371/journal.ppat.1007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Centers for Disease Control and Prevention. 2021. Chronic wasting disease. Occurrence. https://www.cdc.gov/prions/cwd/occurrence.html.
- 123.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, Terytze K. 2007. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS One 2:e435. 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beyer WN, Connor EE, Gerould S. 1994. Estimates of soil ingestion by wildlife. J Wildl Manag 58:375–382. 10.2307/3809405. [DOI] [Google Scholar]
- 125.Weeks HP, Kirkpatrick CM. 1976. Adaptations of white-tailed deer to naturally occurring sodium deficiencies. J Wildl Manage 40:610. 10.2307/3800555. [DOI] [Google Scholar]
- 126.Pritzkow S, Morales R, Lyon A, Concha-Marambio L, Urayama A, Soto C. 2018. Efficient prion disease transmission through common environmental materials. J Biol Chem 293:3363–3373. 10.1074/jbc.M117.810747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, Gertig K, Hoover EA, Jewell JE, Telling GC, Zabel MD. 2009. Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 3:171–183. 10.4161/pri.3.3.9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duffy P, Wolf J, Collins G, Devoe AG, Streeten B, Cowen D. 1974. Possible person-to-person transmission of Creutzfeldt-Jakob disease. N Engl J Med 290:692–693. [PubMed] [Google Scholar]
- 129.Gibbs CJ, Jr, Asher DM, Kobrine A, Amyx HL, Sulima MP, Gajdusek DC. 1994. Transmission of Creutzfeldt-Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery. J Neurol Neurosurg Psychiatry 57:757–758. 10.1136/jnnp.57.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mori T, Atarashi R, Furukawa K, Takatsuki H, Satoh K, Sano K, Nakagaki T, Ishibashi D, Ichimiya K, Hamada M, Nakayama T, Nishida N. 2016. A direct assessment of human prion adhered to steel wire using real-time quaking-induced conversion. Sci Rep 6:24993–24998. 10.1038/srep24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Belay ED, Schonberger LB, Brown P, Priola SA, Chesebro B, Will RG, Asher DM. 2010. Disinfection and sterilization of prion-contaminated medical instruments. Infect Control Hosp Epidemiol 31:1304–1306. 10.1086/657579. [DOI] [PubMed] [Google Scholar]
- 132.Belay ED, Blase J, Sehulster LM, Maddox RA, Schonberger LB. 2013. Management of neurosurgical instruments and patients exposed to Creutzfeldt-Jakob disease. Infect Control Hosp Epidemiol 34:1272–1280. 10.1086/673986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ae R, Hamaguchi T, Nakamura Y, Yamada M, Tsukamoto T, Mizusawa H, Belay ED, Schonberger LB. 2018. Update: dura mater graft–associated Creutzfeldt-Jakob disease — Japan, 1975–2017. MMWR Morb Mortal Wkly Rep 67:274–278. 10.15585/mmwr.mm6709a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Centers for Disease Control and Prevention. 2008. Update: Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts–Japan, 1978–2008. MMWR Morb Mortal Wkly Rep 57:1152–1154. [PubMed] [Google Scholar]
- 135.Douet J-Y, Huor A, Cassard H, Lugan S, Aron N, Arnold M, Vilette D, Torres J-M, Ironside JW, Andreoletti O. 2021. Wide distribution of prion infectivity in the peripheral tissues of vCJD and sCJD patients. Acta Neuropathol 141:383–397. 10.1007/s00401-021-02270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Centers for Disease Control and Prevention. 2009. Biosafety in microbiological and biomedical laboratories, 6th ed. CDC, Atlanta, GA. [Google Scholar]
- 137.WHO/CDS/CSR/APH. 2000. WHO infection control guidelines for transmissible spongiform encephalopathies. WHO, Geneva, Switzerland. [Google Scholar]
- 138.Brandel JP, Vlaicu MB, Culeux A, Belondrade M, Bougard D, Grznarova K, Denouel A, Plu I, Bouaziz-Amar E, Seilhean D, Levasseur M, Haïk S. 2020. Variant Creutzfeldt-Jakob disease diagnosed 7.5 years after occupational exposure. N Engl J Med 383:83–85. 10.1056/NEJMc2000687. [DOI] [PubMed] [Google Scholar]
- 139.Peckeu L, Brandel JP, Welaratne A, Costagliola D, Haïk S. 2018. Susceptibility to Creutzfeldt-Jakob disease after human growth hormone treatment in France. Neurology 91:e724–e731. 10.1212/WNL.0000000000006028. [DOI] [PubMed] [Google Scholar]
- 140.Brown P, Gajdusek DC, Gibbs CJ, Asher DM. 1985. Potential epidemic of Creutzfeldt–Jakob disease from human growth hormone therapy. N Engl J Med 313:728–731. 10.1056/NEJM198509193131205. [DOI] [PubMed] [Google Scholar]
- 141.Powell-Jackson J, Kennedy P, Whitcombe EM, Weller RO, Preece MA, Newsom-Davis J. 1985. Creutzfeldt-Jakob Disease after administration of human growth hormone. Lancet 326:244–246. 10.1016/S0140-6736(85)90292-2. [DOI] [PubMed] [Google Scholar]
- 142.Billette de Villemeur T, Gourmelen M, Beauvais P, Rodriguez D, Vaudour J, Deslys JP, Dormont D, Richard P, Richardet JM. 1992. Creutzfeldt-Jakob disease in 4 children treated with growth hormone. Rev Neurol (Paris) 148:328–334. [PubMed] [Google Scholar]
- 143.Rudge P, Jaunmuktane Z, Adlard P, Bjurstrom N, Caine D, Lowe J, Norsworthy P, Hummerich H, Druyeh R, Wadsworth JDF, Brandner S, Hyare H, Mead S, Collinge J. 2015. Iatrogenic CJD due to pituitary-derived growth hormone with genetically determined incubation times of up to 40 years. Brain 138:3386–3399. 10.1093/brain/awv235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dumble LJ, Klein RD. 1992. Creutzfeldt-Jakob legacy for Australian women treated with human pituitary gonadotropins. Lancet 340:847–848. 10.1016/0140-6736(92)92720-z. [DOI] [PubMed] [Google Scholar]
- 145.Llewelyn CA, Hewitt PE, Knight RSG, Amar K, Cousins SJM, Mackenzie J, Will RG. 2004. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet 363:417–421. 10.1016/S0140-6736(04)15486-X. [DOI] [PubMed] [Google Scholar]
- 146.Peden AH, Head MW, Ritchie DL, Bell PJE, Ironside PJW. 2004. Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 364:527–529. 10.1016/S0140-6736(04)16811-6. [DOI] [PubMed] [Google Scholar]
- 147.Seed CR, Hewitt PE, Dodd RY, Houston F, Cervenakova L. 2018. Creutzfeldt-Jakob disease and blood transfusion safety. Vox Sang 113:220–231. 10.1111/vox.12631. [DOI] [PubMed] [Google Scholar]
- 148.Centers for Disease Control and Prevention. 2018. Occurrence and transmission. Creutzfeldt-Jakob Disease, classic (CJD). CDC, Atlanta, GA. [Google Scholar]
- 149.Gonzalez-Romero D, Barria MA, Leon P, Morales R, Soto C. 2008. Detection of infectious prions in urine. FEBS Lett 582:3161–3166. 10.1016/j.febslet.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lunenfeld B, Bilger W, Longobardi S, Alam V, D’Hooghe T, Sunkara SK. 2019. The development of gonadotropins for clinical use in the treatment of infertility. Front Endocrinol (Lausanne) 10:429. 10.3389/fendo.2019.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuwabara Y, Mine K, Katayama A, Inagawa T, Akira S, Takeshita T. 2009. Proteomic analyses of recombinant human follicle-stimulating hormone and urinary-derived gonadotropin preparations. J Reprod Med 54:459–466. [PubMed] [Google Scholar]
- 152.van Dorsselaer A, Carapito C, Delalande F, Schaeffer-Reiss C, Thierse D, Diemer H, McNair DS, Krewski D, Cashman NR. 2011. Detection of prion protein in urine-derived injectable fertility products by a targeted proteomic approach. PLoS One 6:e17815. 10.1371/journal.pone.0017815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Casalone C, Hope J. 2018. Atypical and classic bovine spongiform encephalopathy. Handb Clin Neurol 153:121–134. 10.1016/B978-0-444-63945-5.00007-6. [DOI] [PubMed] [Google Scholar]
- 154.Richt JA, Kunkle RA, Alt D, Nicholson EM, Hamir AN, Czub S, Kluge J, Davis AJ, Hall SM. 2007. Identification and characterization of two bovine spongiform encephalopathy cases diagnosed in the United States. J Vet Diagn Invest 19:142–154. 10.1177/104063870701900202. [DOI] [PubMed] [Google Scholar]
- 155.Mora L, Toldrá‐Reig F, Prates JAM, Toldrá F. 2019. Cattle byproducts, p 43–55. In Simpson BK, Aryee AN, Toldrá F. (ed), Byproducts from agriculture and fisheries. Wiley and Sons, Hoboken, NJ. [Google Scholar]
- 156.Federal Drug Administration. 2016. FDA announces final rule on bovine spongiform encephalopathy. FDA, Silver Spring, MD. [Google Scholar]
- 157.Grobben AH, Steele PJ, Somerville RA, Taylor DM. 2004. Inactivation of the bovine-spongiform-encephalopathy (BSE) agent by the acid and alkaline processes used in the manufacture of bone gelatine. Biotechnol Appl Biochem 39:329–338. 10.1042/BA20030149. [DOI] [PubMed] [Google Scholar]
- 158.Grobben AH, Steele PJ, Somerville RA, Taylor DM, Schreuder BEC. 2005. Inactivation of the BSE agent by the heat and pressure process for manufacturing gelatine. Vet Rec 157:277–281. 10.1136/vr.157.10.277. [DOI] [PubMed] [Google Scholar]
- 159.Lyon A, Mays CE, Borriello F, Telling GC, Soto C, Pritzkow S. 2019. Application of pMCA to screen for prion infection in a human cell line used to produce biological therapeutics. Sci Rep 9:4847. 10.1038/s41598-019-41055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kong Q, Huang S, Zou W, Vanegas D, Wang M, Wu D, Yuan J, Zheng M, Bai H, Deng H, Chen K, Jenny AL, O'Rourke K, Belay ED, Schonberger LB, Petersen RB, Sy M-S, Chen SG, Gambetti P. 2005. Chronic wasting disease of elk: transmissibility to humans examined by transgenic mouse models. J Neurosci 25:7944–7949. 10.1523/JNEUROSCI.2467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tamgüney G, Giles K, Bouzamondo-Bernstein E, Bosque PJ, Miller MW, Safar J, DeArmond SJ, Prusiner SB. 2006. Transmission of elk and deer prions to transgenic mice. J Virol 80:9104–9114. 10.1128/JVI.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sandberg MK, Al-Doujaily H, Sigurdson CJ, Glatzel M, O'Malley C, Powell C, Asante EA, Linehan JM, Brandner S, Wadsworth JDF, Collinge J. 2010. Chronic wasting disease prions are not transmissible to transgenic mice overexpressing human prion protein. J Gen Virol 91:2651–2657. 10.1099/vir.0.024380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wilson R, Plinston C, Hunter N, Casalone C, Corona C, Tagliavini F, Suardi S, Ruggerone M, Moda F, Graziano S, Sbriccoli M, Cardone F, Pocchiari M, Ingrosso L, Baron T, Richt J, Andreoletti O, Simmons M, Lockey R, Manson JC, Barron RM. 2012. Chronic wasting disease and atypical forms of bovine spongiform encephalopathy and scrapie are not transmissible to mice expressing wild-type levels of human prion protein. J Gen Virol 93:1624–1629. 10.1099/vir.0.042507-0. [DOI] [PubMed] [Google Scholar]
- 164.Race B, Meade-White KD, Miller MW, Barbian KD, Rubenstein R, LaFauci G, Cervenakova L, Favara C, Gardner D, Long D, Parnell M, Striebel J, Priola SA, Ward A, Williams ES, Race R, Chesebro B. 2009. Susceptibilities of nonhuman primates to chronic wasting disease. Emerg Infect Dis 15:1366–1376. 10.3201/eid1509.090253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Race B, Meade-White KD, Phillips K, Striebel J, Race R, Chesebro B. 2014. Chronic wasting disease agents in nonhuman primates. Emerg Infect Dis 20:833–837. 10.3201/eid2005.130778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Race B, Williams K, Orrú CD, Hughson AG, Lubke L, Chesebro B. 2018. Lack of transmission of chronic wasting disease to cynomolgus macaques. J Virol 92:e00550-18. 10.1128/JVI.00550-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bichell RE. 2019. Bent out of shape: could a mysterious animal epidemic become the next mad cow? KUNC, Greeley CO. [Google Scholar]
- 168.Abbasi K. 2000. BSE inquiry plays down errors. BMJ 321:1097. 10.1136/bmj.321.7269.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jones KE. 2001. BSE, risk and the communication of uncertainty: a review of Lord Phillips’ report from the BSE inquiry (UK). Can J Sociol 26:655–666. 10.2307/3341496. [DOI] [PubMed] [Google Scholar]
- 170.Centers for Disease Control and Prevention. 2018. BSE in North America. BSE (bovine spongiform encephalopathy). Prion diseases. CDC, Atlanta, GA. [Google Scholar]
- 171.Diack AB, Head MW, McCutcheon S, Boyle A, Knight R, Ironside JW, Manson JC, Will RG. 2014. Variant CJD: 18 years of research and surveillance. Prion 8:286–295. 10.4161/pri.29237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Centers for Disease Control and Prevention. 2018. Prevention. Chronic wasting disease (CWD). Prion diseases. CDC, Atlanta, GA. [Google Scholar]
- 173.Abrams JY, Maddox RA, Harvey AR, Schonberger LB, Belay ED. 2011. Travel History, Hunting, and Venison Consumption Related to Prion Disease Exposure, 2006–2007 FoodNet Population Survey. J Am Diet Assoc 111:858–863. 10.1016/j.jada.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 174.Olszowy KM, Lavelle J, Rachfal K, Hempstead S, Drouin K, Darcy JM, Reiber C, Garruto RM. 2014. Six-year follow-up of a point-source exposure to CWD contaminated venison in an Upstate New York community: risk behaviours and health outcomes 2005–2011. Public Health 128:860–868. 10.1016/j.puhe.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 175.Heisey DM, Mickelsen NA, Schneider JR, Johnson CJ, Johnson CJ, Langenberg JA, Bochsler PN, Keane DP, Barr DJ. 2010. Chronic Wasting Disease (CWD) Susceptibility of Several North American Rodents That Are Sympatric with Cervid CWD Epidemics. J Virol 84:210–215. 10.1128/JVI.00560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mathiason CK, Nalls AV, Seelig DM, Kraft SL, Carnes K, Anderson KR, Hayes-Klug J, Hoover EA. 2013. Susceptibility of Domestic Cats to Chronic Wasting Disease. J Virol 87:1947–1956. 10.1128/JVI.02592-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Moore SJ, Smith JD, Richt JA, Greenlee JJ. 2019. Raccoons accumulate PrP Sc after intracranial inoculation of the agents of chronic wasting disease or transmissible mink encephalopathy but not atypical scrapie. J Vet Diagn Invest 31:200–209. 10.1177/1040638718825290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Manjerovic MB, Green ML, Mateus-Pinilla N, Novakofski J. 2014. The importance of localized culling in stabilizing chronic wasting disease prevalence in white-tailed deer populations. Prev Vet Med 113:139–145. 10.1016/j.prevetmed.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 179.Mateus-Pinilla N, Weng HY, Ruiz MO, Shelton P, Novakofski J. 2013. Evaluation of a wild white-tailed deer population management program for controlling chronic wasting disease in Illinois, 2003–2008. Prev Vet Med 110:541–548. 10.1016/j.prevetmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 180.Uehlinger FD, Johnston AC, Bollinger TK, Waldner CL. 2016. Systematic review of management strategies to control chronic wasting disease in wild deer populations in North America. BMC Vet Res 12:173. 10.1186/s12917-016-0804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wasserberg G, Osnas EE, Rolley RE, Samuel MD. 2009. Host culling as an adaptive management tool for chronic wasting disease in white-tailed deer: a modelling study. J Appl Ecol 46:457–466. 10.1111/j.1365-2664.2008.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.International Association of Fish and Wildlife Agency. 2006. Transport and Disposal of Hunter-killed Cervid Carcasses: Recommendations to Wildlife Agencies to Reduce Chronic Wasting Disease Risks. https://www.wildlifedepartment.com/sites/default/files/CWD%20Flyer.pdf
- 183.Saunders SE, Bartz JC, Vercauteren KC, Bartelt-Hunt SL. 2011. An enzymatic treatment of soil-bound prions effectively inhibits replication. Appl Environ Microbiol 77:4313–4317. 10.1128/AEM.00421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saborio GP, Permanne B, Soto C. 2001. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810–813. 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 185.Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, Priola SA, Caughey B. 2007. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods 4:645–650. 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- 186.Debaun MR, Casella JF. 2014. A Test for Creutzfeldt–Jakob Disease Using Nasal Brushings. N Engl J Med 371:1842–1843. [DOI] [PubMed] [Google Scholar]
- 187.Cooper SK, Hoover CE, Henderson DM, Haley NJ, Mathiason CK, Hoover EA. 2019. Detection of CWD in cervids by RT-QuIC assay of third eyelids. PLoS One 14:e0221654. 10.1371/journal.pone.0221654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kramm C, Gomez-Gutierrez R, Soto C, Telling G, Nichols T, Morales R. 2019. In vitro detection of chronic wasting disease (CWD) prions in semen and reproductive tissues of white tailed deer bucks (Odocoileus virginianus). PLoS One 14:e0226560. 10.1371/journal.pone.0226560. [DOI] [PMC free article] [PubMed] [Google Scholar]