Abstract
Various stresses activate the c-Jun N-terminal kinase (JNK), which is involved in the regulation of many aspects of cellular physiology, including apoptosis. Here we demonstrate that in contrast to UV irradiation, heat shock causes little or no stimulation of the JNK-activating kinase SEK1, while knocking out the SEK1 gene completely blocks heat-induced JNK activation. Therefore, we tested whether heat shock activates JNK via inhibition of JNK dephosphorylation. The rate of JNK dephosphorylation in unstimulated cells was high, and exposure to UV irradiation, osmotic shock, interleukin-1, or anisomycin did not affect this process. Conversely, exposure of cells to heat shock and other protein-damaging conditions, including ethanol, arsenite, and oxidative stress, strongly reduced the rate of JNK dephosphorylation. Under these conditions, we did not observe any effects on dephosphorylation of the homologous p38 kinase, suggesting that suppression of dephosphorylation is specific to JNK. Together, these data indicate that activation of JNK by protein-damaging treatments is mediated primarily by inhibition of a JNK phosphatase(s). Elevation of cellular levels of the major heat shock protein Hsp72 inhibited a repression of JNK dephosphorylation by these stressful treatments, which explains recent reports of the suppression of JNK activation by Hsp72.
Exposure of eukaryotic cells to various stresses stimulates the stress-activated kinases JNK (c-Jun N-terminal kinase) and p38 (for a review, see reference 22). JNK activates the transcription factor AP-1, thus regulating cell proliferation, immune responses, inflammation, and programmed cell death, or apoptosis (6, 19, 22, 35). UV irradiation, osmotic stress, as well as certain cytokines and mitogens activate JNK via a signal transduction pathway which involves small GTP-binding proteins (7, 29) and a cascade of protein kinases. This kinase cascade includes MEKKs (30) followed by the dual-specificity kinases SEK1 (MKK4) (39, 44) and MKK7 (41), both of which phosphorylate JNK at the vicinal threonine and tyrosine residues, thus activating this kinase (23).
Heat shock, ethanol, oxidative stress, and certain other stresses, on the other hand, activate JNK through a pathway which has not yet been established. Furthermore, recent data indicate that heat shock and UV irradiation activate JNK via distinct pathways (1). Consistent with this notion, the yeast Spc1 kinase, a homolog of p38 and JNK, is activated by heat shock through a pathway different from that used by other stresses (38, 40). This pathway was reported to involve inhibition of Spc1 dephosphorylation by its phosphatase Pyp1 (although this report has recently been disputed [40]). Phosphatases were also implicated in activation of JNK in mammalian cells. For example, the protein-damaging agent arsenite was demonstrated to activate JNK through specific inhibition of a constitutively active JNK phosphatase (2).
Certain stresses including heat shock and ethanol not only activate JNK and p38 but also induce synthesis of heat shock proteins. Heat shock proteins repair damaged proteins and protect cells from exposure to stressful conditions (see reference 9 for a review). Recently a member of the family of 70-kDa heat shock proteins, Hsp72, was shown to prevent a programmed cell death in response to certain stresses (31). We have demonstrated that this antiapoptotic effect of Hsp72 is due to suppression of activation of JNK (12, 43, 43a). Furthermore, Hsp72 suppresses UV-induced JNK activation probably downstream of SEK1 in the JNK-signaling cascade (43a), which suggests that a JNK phosphatase could be a target for regulation by Hsp72. It is noteworthy that total cellular phosphatase activity is reportedly increased in cells which overexpress Hsp72 (8, 24). Moreover, members of the Hsp70 family can directly associate with some phosphatases in cells (36).
In the present work, we investigated the mechanism of JNK activation by heat shock, ethanol, and certain other stresses and the role of JNK dephosphorylation in this activation. We also investigated the involvement of Hsp72 in the regulation of JNK dephosphorylation.
MATERIALS AND METHODS
Antibodies.
In this study, we used antibodies against active JNK, phospho-JNK active (pTPpY; Promega, Madison, Wis.), JNK1 (C-17; Santa Cruz Biotechnology, Santa Cruz, Calif.), active p38 kinase, phospho-p38 (Tyr182), and phospho-SEK1 (Thr223) (New England Biolabs, Beverly, Mass.), and Hsp72 (SPA-810; StressGen, Victoria, British Columbia, Canada). A working dilution of 1:1,000 was used for all antibodies except the anti-phospho-JNK antibody (1:5,000).
After immunoblotting analysis, secondary antibodies conjugated with peroxidase were visualized with enhanced chemiluminescence substrates (Amersham, Arlington Heights, Ill.), and resulting films were quantified by densitometry.
Cell lines and stresses.
The H9c2 (2-1) rat myogenic cell line was grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. Mice embryonic stem (ES) cells were grown in DMEM supplemented with 15% fetal bovine serum, nonessential amino acids, 0.1 mM 2-mercaptoethanol, and 50 ng of mouse leukemia inhibitory factor (R&D Systems Inc., Minneapolis, Minn.) per ml. All cells were grown at 37°C in an atmosphere of 5% CO2 to 60 to 85% confluence.
To activate stress kinases, cells were subjected to one of the following stimuli: osmotic stress (0.7 M NaCl), exposure to anisomycin (10 μg/ml), exposure to interleukin-1 (IL-1; 20 ng/ml), exposure to 150 μM sodium arsenite, heat shock (20 min at 45°C, unless stated otherwise), exposure to 8% ethanol for 1 h, exposure to 1 mM menadione sodium bisulfite for 30 min, or UV irradiation (400 J/m2, unless stated otherwise; performed in a UV Stratalinker 1800 [Stratagene, La Jolla, Calif.]). The time of exposure to each stress is indicated in figure legends.
Adenovirus-based expression of Hsp72.
A recombinant adenovirus vector expressing Hsp72 (AdTR5-DC/HSP70-GFP) was constructed by cloning a dicistronic transcription unit encoding human Hsp72 and the Aequorea victoria green fluorescent protein gene, separated by the encephalomyocarditis virus internal ribosome entry site from pTR-DC/HSP70-GFP (31, 32), into an adenovirus transfer vector. Expression of this transcription unit is controlled by the tetracycline-regulated transactivator protein tTA (15), which we expressed from the separate recombinant adenovirus (AdCMV/tTA [26]). Recombinant adenoviruses were generated by standard techniques as detailed by Jani et al. (18). Inoculation of cells with 3 × 107 PFU of each virus per 35-mm-diameter dish was sufficient to infect almost 100% of cells. This was confirmed each time by observation under a fluorescence microscope of a fraction of the cells expressing the green fluorescent protein. Twenty-four hours after infection, the medium was changed and cells were left for another 12 h. Thirty-six hours after infection, the cells accumulated large amounts of Hsp72, as judged by immunoblotting of cytosol extracts (not shown).
Inhibition of JNK activation.
For ATP depletion, cells were washed twice with phosphate-buffered saline (PBS) preheated to 37°C and then left for the indicated times in PBS supplemented with 5 μM rotenone and 10 mM 2-deoxyglucose. Alternatively, staurosporine was added to cells in DMEM to 4 μM. To inhibit JNK activation in cells after their exposure to ethanol, staurosporine was added in DMEM supplemented with 10% fetal bovine serum. Inhibitor of JNK pathway CEP-1347 (KT7515; kindly provided by Cephalon, Inc. [West Chester, Pa.]) (25) was added to cells in the medium without fetal bovine serum to 2 μM.
Measurement of ATP content.
Cellular ATP content was measured by the luciferin-luciferase method, using an ATP bioluminescent somatic cell assay kit (Sigma). Bioluminescence was determined with a scintillation counter (37). The cytosolic ATP content was normalized to total cytosolic protein.
Measurement of phosphorylation of purified SEK1 and JNK1.
Recombinant JNK2, SEK1, and a constitutively active deletion form of MEKK1 (ΔN terminus) (kindly provided by J. Kyriakis and J. Avruch) were expressed in Escherichia coli as glutathione S-transferase (GST) fusion polypeptides and isolated by using a glutathione-Sepharose affinity column according to the Pharmacia protocol. MEKK1 (1 μg), SEK1 (2 μg), and JNK2 (2 μg) were mixed in 14 μl of buffer containing 25 mM HEPES (pH 7.4), 10 mM MgCl2, 2 mM dithiothreitol, and 20 μM ATP. Then 10 μCi of [γ-32P]ATP was added, and the mixture was incubated at 30°C for 10 min. The reaction was stopped by addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Staurosporine was added to the reaction immediately before addition of labeled ATP.
Plasmid minipreps were done with Turbomix (Scientific Industries).
Preparation of cytosol extracts and analysis of JNK activity.
Cells were washed twice with PBS (unless they were depleted of ATP and hence already in PBS), aspirated, and lysed in 200 μl of lysis buffer (40 mM HEPES [pH 7.5], 50 mM KCl, 1% Triton X-100, 2 mM dithiothreitol, 1 mM Na3VO4, 50 mM β-glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 5 μg each of leupeptin, pepstatin A, and aprotinin per ml) per 35-mm-diameter dish. The lysates were clarified by centrifugation in a microcentrifuge at 15,000 rpm for 5 min. Total protein concentration was measured in the supernatants, after which they were diluted with lysis buffer to equalize the protein concentration among all samples. All procedures were performed at 4°C.
To measure JNK activity, 5 μl of extract was added to a reaction mixture (20 μl, final volume) containing (final concentration) 40 mM HEPES (pH 7.5), 1 mM Na3VO4, 25 mM β-glycerophosphate, 10 mM MgCl2, 20 μM ATP, 15 μCi of [γ-32P]ATP, and 40 ng of recombinant c-Jun–GST. The reaction was allowed to proceed for 25 min at 30°C and then stopped by addition of 10 μl of SDS-PAGE sample buffer. Samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, and analyzed on a Molecular Imager. Membranes were subsequently immunoblotted with an anti-JNK1 antibody to verify equivalent protein loading.
RESULTS
Heat shock activates JNK in H9c2 cells without an increase in SEK1 activity.
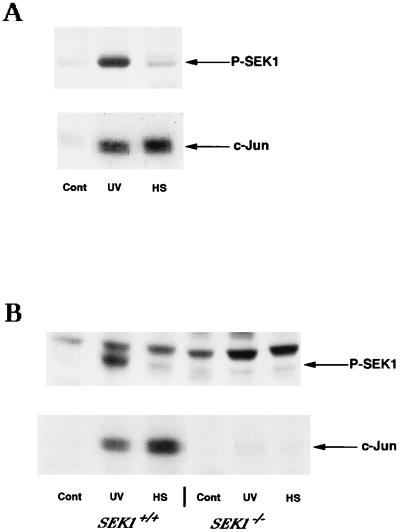
As shown previously with several cell lines, the majority of stresses activate JNK through SEK1 (39, 46). Accordingly, UV irradiation (400 J/m2) of H9c2 cells led to a severalfold increase in SEK1 activity (as revealed by an antibody which specifically recognizes the active form of this kinase) and subsequent dramatic JNK activation (Fig. 1A). Surprisingly, heat shock (20 min at 45°C) of H9c2 cells, which activated JNK to a similar level, caused little if any activation of SEK1 above basal levels (Fig. 1A). These data taken together indicated that JNK activation by heat shock is not dependent on SEK1 activation. This conclusion was in apparent contradiction with the previously reported observation that SEK1 is essential for heat-induced JNK activation. Indeed, inhibition of SEK1 activity by expression of its dominant-negative mutant in various cell lines or by gene knockout in either mouse ES cells or fibroblasts blocked activation of JNK by heat shock almost completely (13, 28, 34, 45). To clarify the contradiction, we used mouse ES cells, in which SEK1 is known to be essential for the heat-induced JNK activation (13, 34), to test whether this kinase is activated by heat shock. Similar to previously reported results, there was an almost complete lack of JNK activation in response to heat shock (44°C, 20 min) and profound reduction of JNK activation after UV irradiation in SEK1−/− ES cells in comparison with the isogenic SEK1+/+ ES cells (Fig. 1B).
FIG. 1.
Effects of UV irradiation and heat shock on phosphorylation of SEK1. (A) Activation of SEK1 and JNK in H9c2 cells. Top, immunoblot of cytosol extracts probed with anti-phospho-SEK1 antibody; bottom, JNK activity in the same extracts, as measured with c-Jun as a substrate (see Materials and Methods). Cont, unstressed cells; UV, cells exposed to UV irradiation (400 J/m2) followed by a 15-min rest; HS, cells exposed to heat shock at 45°C for 20 min. (B) Activation of SEK1 and JNK in ES cells. Top, immunoblot of cytosol extracts probed with anti-phospho-SEK1 antibody; bottom, JNK activity in the same extracts. Wild-type and SEK1 double-knockout ES cells were exposed to heat shock (HS) at 44°C for 20 min or UV irradiation (UV; 400 J/m2) followed by a 20-min rest.
These results indicate that SEK1 is essential for the heat-induced JNK activation and significantly contributes to UV-induced JNK activation. On the other hand, effects of heat shock and UV on SEK1 phosphorylation (and hence activation) in SEK1+/+ cells differed dramatically. While UV irradiation caused strong SEK1 phosphorylation, the effect of heat shock (20 min at 43 or 44°C) on SEK1 phosphorylation was negligible (Fig. 1B). More severe heat shock (20 min at 45°C), which led to JNK activation threefold higher than that after UV irradiation, caused slight SEK1 phosphorylation (not shown), possibly because of the feedback activation by JNK. The likely explanation for these data is that heat shock activates JNK via a distinct pathway which requires only basal activity of SEK1. We suggested that heat shock can inhibit JNK dephosphorylation, leading to an accumulation of phosphorylated JNK which has been activated by SEK1. If SEK1 activity is abolished, inhibition of a JNK phosphatase(s) would not result in an increased level of JNK phosphorylation.
Development of experimental approaches to observe in vivo dephosphorylation of JNK.
To test the hypothesis that the activity of a JNK phosphatase(s) is affected by heat shock, we developed an assay to compare the rate of JNK dephosphorylation in cells under various stressful conditions. The level of JNK phosphorylation in cells is determined by two processes: its phosphorylation by upstream kinases and its dephosphorylation by a putative phosphatase(s). The most straightforward approach to assess the rate of JNK dephosphorylation is to activate JNK by different stresses and then to block its further activation by upstream kinases and follow the decrease of the levels of phosphorylated JNK. Under these conditions, the phosphatase activity would become the only factor which controls the level of JNK phosphorylation. Unfortunately, there is no well-characterized specific inhibitor of JNK activation. Chase of 32P-pulse-labeled JNK with unlabeled phosphate (16) was too slow to measure rapid JNK dephosphorylation. Therefore, in this study we inhibited JNK activation by one of three independent methods: (i) addition of the protein kinase inhibitor staurosporine, which was successfully used to analyze dephosphorylation of Stat1 (17); (ii) rapid depletion of ATP, a substrate for all protein kinases; or (iii) addition of CEP-1347, a compound designed by Cephalon to specifically inhibit JNK activation by various stimuli (25).
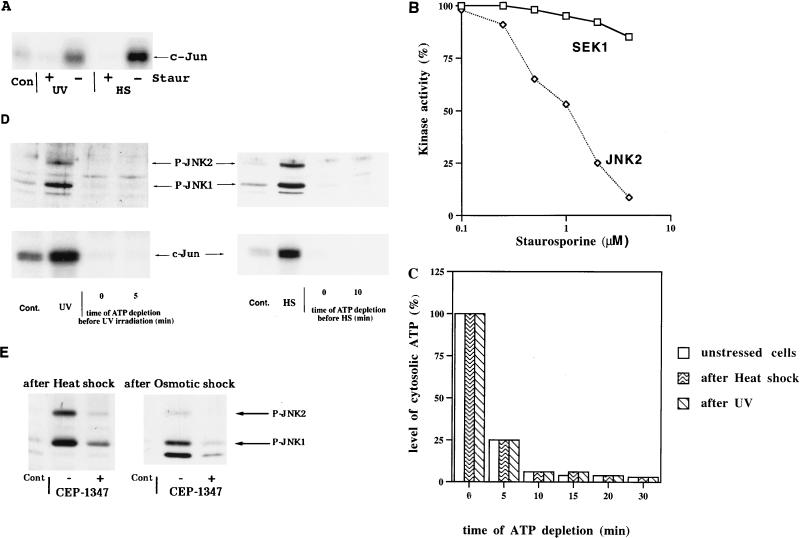
To test the efficiency of staurosporine in the inhibition of JNK activation, we pretreated cells with this inhibitor (see Materials and Methods) and then exposed the cells to either UV irradiation or heat shock. A 5-min preincubation with staurosporine appeared to be sufficient to block completely heat shock (45°C for 20 min)- and UV irradiation (400 J/m2)-induced (Fig. 2A) as well as ethanol-induced (not shown) JNK activation. This was demonstrated by immunoblotting with an antibody which recognizes only the phosphorylated forms of JNK1 and JNK2 and by a more sensitive assay of JNK activity that uses recombinant c-Jun as a substrate (both methods reflect JNK activity and in this study provided similar results when used in parallel). It is important that staurosporine did not reduce activation of SEK1 by UV significantly, as detected by immunoblotting with anti-phospho-SEK1 antibody (not shown). These data suggest that in the JNK signaling pathway, SEK1 is the target for staurosporine inhibition. To test this possibility directly, we assessed effects of staurosporine on activities of SEK1 and MEKK1 by using purified kinases. Recombinant SEK1, JNK2, and a constitutively active deletion mutant of MEKK1 (ΔN terminus) were incubated with [γ-32P]ATP. In this reaction, MEKK1 phosphorylates SEK1, which becomes active and, in turn, phosphorylates JNK2. Addition of staurosporine had little effect on phosphorylation of SEK1 but dramatically inhibited phosphorylation of JNK2 (Fig. 2B). These data indicate that staurosporine could be used as an inhibitor of SEK1-dependent activation of JNK.
FIG. 2.
Methods of inhibition of JNK activation by stresses. (A) Staurosporine blocks JNK activation after heat shock and UV irradiation. H9c2 cells were incubated for 5 min in the presence (+) or absence (−) of 4 μM staurosporine (Staur) in serum-free DMEM for 5 min and then exposed to either heat shock (HS) or UV irradiation (UV) as described for Fig. 1A. JNK activity was measured in cytosolic extracts. Con, unstressed cells. (B) Staurosporine inhibits activity of SEK1 in vitro. Isolated recombinant MEKK1 (ΔN terminus), SEK1, and JNK2 were incubated with [γ-32P]ATP for 10 min in the presence or absence of staurosporine. Proteins were separated by SDS-PAGE, and incorporation of 32P into SEK1 and JNK2 was measured. (C) Decrease in cytosolic ATP levels in the ATP depletion experiments. ATP concentration was measured in cytosolic extracts prepared from either unstressed cells or cells exposed to stresses collected at the indicated times after addition of rotenone and 2-deoxyglucose (see Materials and Methods). The histogram represents data of a typical experiment which was repeated three times. Samples for these measurements were taken from the experiment described in the legend to Fig. 3A. (D) ATP depletion inhibits activation of JNK after heat shock and UV irradiation. Cells either in regular medium or incubated in PBS with rotenone and 2-deoxyglucose for the indicated time periods were exposed to either UV (400 J/m2) followed by a 15-min rest or to heat shock (45°C for 20 min). Cont., unstressed cells. Top, immunoblot of cytosol extracts probed with anti-phospho-JNK antibody; bottom, JNK activity measured in the same extracts. (E) CEP-1347 inhibits JNK activation by heat shock and osmotic shock. Cells were transferred to serum-free DMEM with or without 2 μM CEP-1347 and 5 min later were exposed to either heat shock (45°C for 20 min) or osmotic shock (0.7 M NaCl for 15 min). Cytosol extracts were subjected to immunoblotting with anti-phospho-JNK antibody.
To rapidly and completely block JNK activation by all stressful treatments, we also developed a method based on ATP depletion (see Materials and Methods). Cells were transferred into PBS, and synthesis of ATP was blocked by rotenone, an inhibitor of the respiratory chain, and 2-deoxyglucose, a competitive inhibitor of glycolysis. This treatment led to a 10-fold decrease in cytosolic ATP concentration in less than 10 min (Fig. 2C). The choice of H9c2 cardiomyocytes for this investigation was determined by the rapid drop of ATP concentration in these cells after addition of rotenone and 2-deoxyglucose. In cells exposed to stresses, initial ATP concentrations and the rates of ATP depletion were similar to those in untreated cells (Fig. 2C).
The sharp decrease in ATP levels rapidly blocked JNK activation de novo. Indeed, JNK was activated in cells exposed to UV within 15 min after the irradiation, but in ATP-depleted cells we did not observe any UV-induced JNK activation even if cells were irradiated immediately after starting the depletion procedure (Fig. 2D). Similarly, ATP depletion completely blocked JNK activation by heat shock within minutes (Fig. 2D). These experiments indicated that both staurosporine and ATP depletion can be used to study JNK dephosphorylation.
Similarly, addition of CEP-1347 rapidly (within 5 min) and strongly (by about 80%) inhibited activation of JNK by heat shock, osmotic stress, and some other stimuli (but less than 40% by UV irradiation) in H9c2 cells (Fig. 2E). Therefore, we used CEP-1347 to evaluate the effects of heat shock and osmotic stress on rates of JNK dephosphorylation.
Heat shock inhibits JNK dephosphorylation.
The described methods of inhibition of JNK-activating kinases allowed us to follow the rate of JNK dephosphorylation. Dephosphorylation of JNK in unstressed cells (starting from a basal level) following ATP depletion (see Fig. 4) or addition of staurosporine or CEP-1347 (not shown) was very rapid. ATP depletion also led to a remarkably fast dephosphorylation of UV-activated JNK. In UV-irradiated cells, the level of JNK activity increased severalfold within 20 min and then decreased slowly during the next hour (Fig. 3B). However, when such cells at the peak of JNK activity were subjected to ATP depletion, JNK activity plunged below the basal (in unstressed cells) levels in less than 15 min (Fig. 3A and B). Treatment of cells with staurosporine 15 min after UV irradiation also led to a rapid decrease in JNK activity. In fact, in 4 min after addition of staurosporine, JNK activity decreased by about 80%, and in 8 min its activity was at the basal level (Fig. 3B).
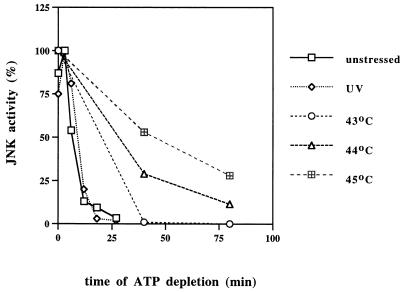
FIG. 4.
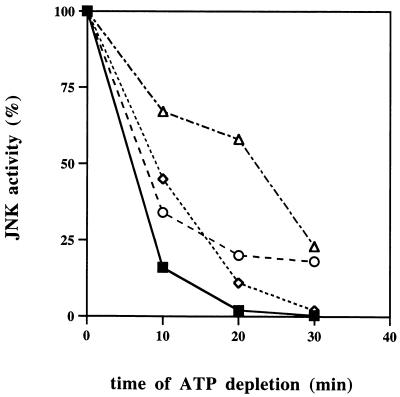
Extent of inhibition of JNK dephosphorylation correlates with severity of heat shock. After activation of JNK by UV irradiation (1000 J/m2) or heat shock (at indicated temperatures for 20 min), ATP depletion was started and samples were collected during the time course. JNK activity was measured in cytosolic extracts, and membranes were quantified with a Molecular Imager.
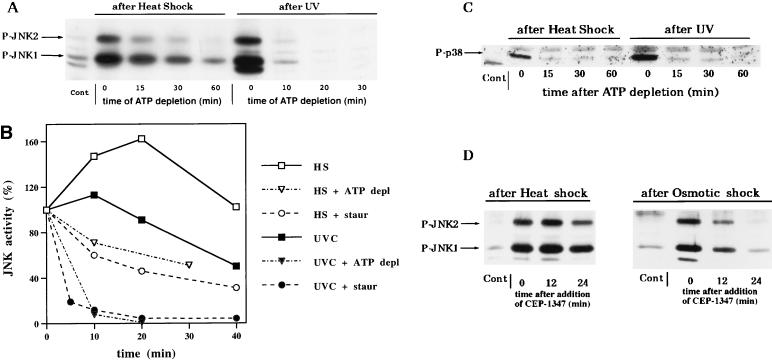
FIG. 3.
Heat shock delays JNK dephosphorylation. (A) Comparison of dephosphorylation of JNK activated by heat shock and UV in ATP-depleted cells. Cells were exposed to UV (400 J/m2) followed by a 15-min rest or to heat shock (45°C for 20 min), then rotenone and 2-deoxyglucose were added, and samples were collected at the indicated time periods after the beginning of ATP depletion. Cytosolic extracts were probed with the anti-phospho-JNK antibody on an immunoblot. Cont., unstressed cells. ATP levels in these samples are presented in Fig. 2C. (B) Comparison of the rates of JNK dephosphorylation in ATP-depleted (ATP depl) and staurosporine (Staur)-inhibited cells. JNK was activated by either heat shock (HS) or UV irradiation (UVC) as described for Fig. 3A. The plot represents data of a typical experiment which was repeated three times. (C) Heat shock does not affect dephosphorylation of p38 kinase. The samples used for panel A were immunoblotted with the anti-phospho-p38 antibody. Cont., unstressed cells. (D) Comparison of the rates of JNK dephosphorylation in CEP-1347-inhibited cells. Cells were exposed to osmotic shock (0.7 M NaCl for 15 min) or heat shock (45°C for 20 min), and samples were collected at the indicated time periods after the addition of CEP-1347 to 2 μM in serum-free DMEM. Cytosolic extracts were immunoblotted with the anti-phospho-JNK antibody.
In contrast, dephosphorylation of heat-induced JNK was much slower. Upon heat shock (45°C), the level of JNK activity in cells increased steadily during 20 min and continued to rise for 20 more min after a shift of the temperature back to 37°C (Fig. 3B). ATP depletion or addition of staurosporine rapidly blocked the raise of JNK activity in such cells, allowing JNK to undergo dephosphorylation. As opposed to the swift JNK dephosphorylation in unstressed or UV-irradiated cells, the rate of its dephosphorylation in heat-shocked cells was slow, since less than 50% of JNK was inactivated in 30 min following ATP depletion or addition of staurosporine (Fig. 3A and B). Similar rates of JNK dephosphorylation in cells after heat shock (less than 50% in 30 min) were observed when JNK phosphorylation was blocked with CEP-1347 (Fig. 3D). On the other hand, if CEP-1347 was added to cells exposed to osmotic stress, JNK was dephosphorylated much faster than in heat-shocked cells (Fig. 3D). The suppressive effect of heat shock on JNK dephosphorylation is emphasized by the fact that in the absence of the inhibitors, JNK activity decayed more slowly in heat-shocked cells than in UV-irradiated cells (Fig. 3B).
It is important that the effect of heat shock on JNK dephosphorylation was very specific, since we did not observe any suppression of dephosphorylation of the homologous stress kinase p38 under these conditions (Fig. 3C).
We next tested if the extent of inhibition of JNK dephosphorylation correlates with the severity of heat shock. Indeed, in cells stressed for 20 min at temperatures ranging from 43 to 45°C, the degree of inhibition of JNK dephosphorylation increased with an increase of temperature and was highest at 45°C (Fig. 4). It is noteworthy that an increase of the energy of UV irradiation from 400 J/m2 to the extremes of more than 3,000 J/m2 caused no significant decrease in the rate of JNK inactivation (not shown). This finding indicates that in contrast to heat shock, UV does not affect JNK dephosphorylation even at doses which cause 100% cell death.
We also found that rapid dephosphorylation of UV-induced JNK can be delayed by various protein phosphatase inhibitors. Sodium vanadate, an inhibitor of tyrosine phosphatases (14), significantly decreased the rate of dephosphorylation of JNK activated by UV (Fig. 5). JNK inactivation was also reduced by calyculin A, an inhibitor of serine-threonine phosphatases (4) (Fig. 5). The inhibitory effects of these agents were additive (Fig. 5). Interestingly, dephosphorylation of the JNK2 isoform was affected by both inhibitors slightly more than that of JNK1 (not shown).
FIG. 5.
Dephosphorylation of JNK is delayed by phosphatase inhibitors. Cells were exposed to UV (400 J/m2), 15 min later washed with PBS, and then not treated (■) or incubated for 5 min with either 1 mM sodium vanadate (○) or 100 nM calyculin A (◊) or both (▵). Then rotenone and deoxyglucose were added to start ATP depletion. The plot represents data from a typical experiment which was repeated three times.
Various stresses differ in the ability to affect JNK dephosphorylation.
To determine how unique heat shock is in its ability to suppress JNK dephosphorylation, we compared the dephosphorylation rates in cells exposed to different kinds of stresses. To inhibit JNK-activating kinases in these experiments, we also used either ATP depletion or staurosporine as described above. In the presence of the inhibitors, phosphatase activity becomes the sole factor which determines the level of JNK phosphorylation. Therefore, under these conditions the rate of decay of phosphorylated JNK is not dependent on the duration of activity of upstream kinases, which varies for different stresses.
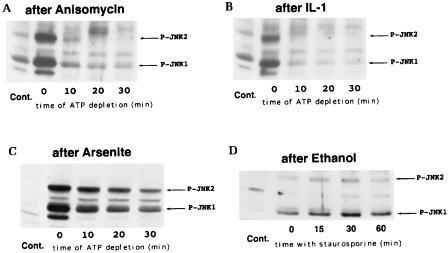
We found that there is a group of stresses which activate JNK without causing any detectable delay in JNK dephosphorylation. Indeed, in cells treated with either IL-1 or anisomycin (Fig. 6A and B) or exposed to either osmotic stress (0.7 M NaCl or 0.4 M sorbitol for 15 min) or the phorbol ester analog phorbol dibutyrate (1 μM for 7 min) (not shown), the rates of JNK dephosphorylation were similar to those in UV-irradiated or unstimulated cells.
FIG. 6.
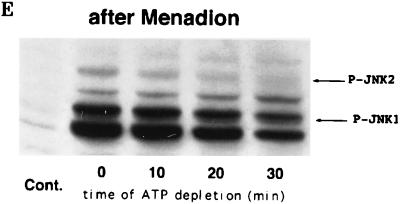
Effects of different stresses on the rate of JNK dephosphorylation. JNK was activated by treatment of H9c2 cells with the indicated agents, and JNK dephosphorylation was assayed by the ATP depletion method (or with staurosporine in the case of ethanol). Immunoblots of cytosol extracts were probed with the anti-phospho-JNK antibody. Cont., unstressed cells. (A) Cells were treated for 15 min with anisomycin (10 μg/ml); (B) cells were treated for 15 min with IL-1 (20 ng/ml); (C) cells were exposed to 150 μM arsenite for 30 min; (D) cells were exposed to 8% ethanol for 1 h, and then staurosporine was added instead of ATP being depleted; (E) cells were exposed to 1 mM menadione sodium bisulfite for 30 min.
On the other hand, we identified several stresses which reduced the rate of JNK dephosphorylation. Exposure of cells to sodium arsenite or ethanol activated JNK and led to a delay in its dephosphorylation (Fig. 6C and D). Similarly, when JNK was activated by menadione sodium bisulfite (menadione), which causes oxidative stress through generation of reactive oxygen species (5), the rate of JNK1 dephosphorylation markedly decreased. As seen in the Fig. 6E, exposure of cells to menadione preferentially activated the JNK1 isoform, most likely because menadione has a specific inhibitory effect on phosphorylation of the JNK2 isoform (27a). Dephosphorylation of p38 kinase was not affected by any stress tested in this study except exposure of cells to menadione (not shown). Taken together, these data strongly suggest that there are two types of stresses, both of which activate JNK but which differ in the ability to inhibit JNK dephosphorylation.
As shown above, heat shock activates JNK without stimulation of its immediate upstream kinase, SEK1 (Fig. 1). Do other stresses which inhibit JNK dephosphorylation activate SEK1? To answer this question, we exposed cells to various JNK-activating stimuli and analyzed cytosolic extracts for SEK1 phosphorylation. As seen in Table 1, most stresses which inhibit JNK dephosphorylation did not activate SEK1, while most of the stresses which have no effect on JNK dephosphorylation stimulated SEK1. There were two exceptions from this correlation: IL-1, which reportedly activates JNK through another kinase, MKK7 (10), and arsenite, which activates JNK by both stimulation of SEK1 and suppression of JNK dephosphorylation. These observations imply that inhibition of JNK dephosphorylation plays a major role in JNK activation by a group of stresses including heat shock and ethanol.
TABLE 1.
Responses of cells to various stressesa
| Stress | Effect
|
|
|---|---|---|
| JNK phosphatase inhibition | Phospho-SEK1 | |
| UV | − | + |
| Osmotic shock | − | + |
| Anisomycin | − | + |
| IL-1 | − | − |
| Arsenite | + | + |
| Heat shock | + | − |
| Ethanol | + | − |
| Menadione | + | − |
Cytosolic extracts of cells subjected to different stresses (UV irradiation [400 J/m2], osmotic shock [0.7 M NaCl for 20 min], anisomycin [10 μg/ml for 15 min], IL-1 [20 ng/ml for 15 min], arsenite [150 μM for 30 min], heat shock [20 min at 45°C], ethanol [8% ethanol for 1 h], and menadione [1 mM menadione sodium bisulfite for 30 min]) were immunoblotted with an antibody which recognizes the phosphorylated form of SEK1. JNK was activated in all samples (not shown). −, no significant effect; +, profound effect.
Hsp72 suppresses stress-induced inhibition of JNK dephosphorylation.
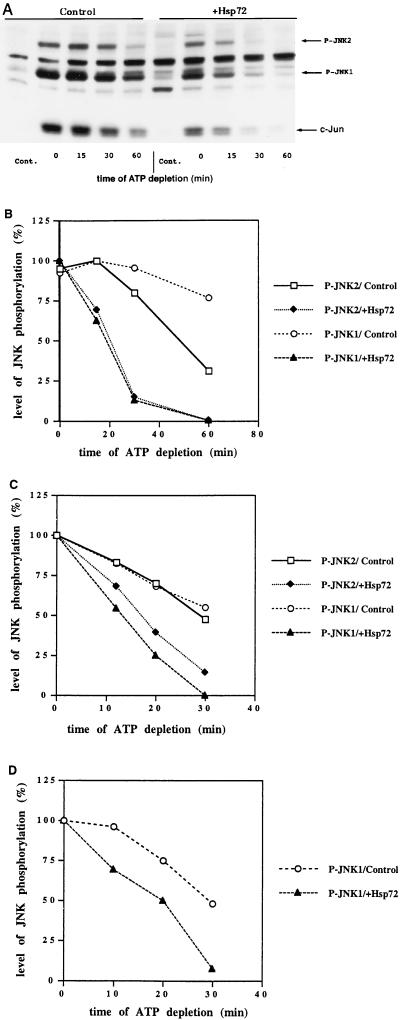
Recently, Hsp72 was shown to suppress activation of JNK, thus protecting cells from heat-induced apoptosis (12, 31). We suggested that since heat shock activates JNK through inhibition of a JNK phosphatase(s), Hsp72 might reduce such inhibition, thus preventing JNK activation. To test this hypothesis, we expressed Hsp72 by using an adenovirus-based expression system (see Materials and Methods). H9c2 cells were infected with two adenoviruses, one with the Hsp72 gene under a tetracycline-regulated promoter and another expressing the transactivator protein. In these cells Hsp72 accumulated within 36 h to a high level (not shown). Cells infected with a double dose of one type of virus did not express Hsp72 and were used as a control. As expected, in cells with elevated levels of Hsp72, heat shock activated JNK to a lesser degree, and the inhibitory effect of heat shock on JNK dephosphorylation was significantly less than in control cells (Fig. 7A and B).
FIG. 7.
Hsp72 reverses inhibition of JNK phosphatase after heat shock and other stresses. Cells were infected with adenoviruses as described in Materials and Methods. Thirty-six hours after infection, cells were subjected to a stress followed by ATP depletion. Samples were collected at the indicated times after the start of ATP depletion. (A) Cells were exposed to heat shock (45°C for 20 min). Shown is an immunoblot of cytosolic extracts probed with the anti-phospho-JNK antibody. Cont., unstressed cells. (B to D) Plots of the data from the immunoblots of cytosolic extracts probed with the anti-phospho-JNK antibody and quantified on a Molecular Imager. (B) Cells were exposed to heat shock at 45°C for 20 min; (C) cells were exposed to 150 μM arsenite for 30 min; (D) cells were exposed to 1 mM menadione sodium bisulfite for 30 min.
An increase in the cellular concentration of Hsp72 also suppressed the inhibition of JNK dephosphorylation by exposure to arsenite, menadione (Fig. 7C and D), or ethanol (not shown). Taken together, these data indicate that Hsp72 suppresses activation of JNK by certain stresses through a suppression of their inhibitory effects on JNK dephosphorylation.
DISCUSSION
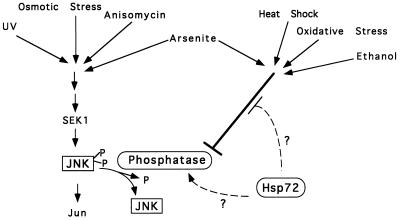
We have addressed in this report two major questions: the mechanism of JNK activation by heat shock and the nature of suppression of JNK activation by Hsp72. In the course of this study, we developed methods which allowed us to monitor JNK dephosphorylation in vivo. We established that stresses differ in their influences on JNK dephosphorylation. Treatment of cells with IL-1, phorbol dibutyrate, and anisomycin as well as UV irradiation and osmotic stress activated JNK with no inhibition of its dephosphorylation. In contrast, stresses in another group, including heat shock and exposure to arsenite, ethanol, and menadione, caused JNK activation through inhibition of its dephosphorylation. The effect of heat shock on JNK dephosphorylation was not unique for H9c2 cells, being also observed in human IMR90 fibroblasts (not shown) and Rat1 cells (12a). The regulation of JNK activity by heat shock and other stresses could be related to the mechanism suggested for the activation of the yeast stress kinase Spc1 (38). While osmotic stress activates Spc1 through stimulation of its upstream kinases, heat shock has been reported to activate Spc1 via blocking the Pyp1 phosphatase, which keeps the basal activity of this kinase at low levels. In mammalian cells, activation of JNK by arsenite has also been shown to involve inhibition of JNK dephosphorylation (2).
Our working model is that the activity of JNK in unstressed cells results from a basal activity of a kinase cascade(s) balanced by JNK dephosphorylation. Cellular stresses which inhibit JNK dephosphorylation would ultimately shift this balance toward activation of JNK (Fig. 8). Accordingly, for activation of JNK by such stresses, stimulation of an upstream kinase cascade is not required but its basal activity is essential. The source of such JNK phosphorylation activity appears to be the basal activity of SEK1, because SEK1 is not activated by heat shock over its basal level, while deletion of the SEK1 gene completely blocks heat-induced JNK activation (Fig. 1B) (13, 34).
FIG. 8.
Model of the pathways of JNK activation.
Do the stresses which act through inhibition of JNK dephosphorylation have something in common which distinguishes them from other stresses? Unlike stresses which do not affect JNK dephosphorylation, heat shock, ethanol, arsenite, and menadione cause protein damage. Indeed, heat shock leads to protein denaturation, ethanol reduces the fidelity of translation, and arsenite modifies thiol groups, while menadione causes oxidation of amino acid residues in polypeptides. These protein-damaging effects could be critical for inhibition of a JNK phosphatase(s) (see below).
Recently we found that activation of JNK by heat shock and ethanol could be suppressed by elevated levels of Hsp72 (12, 31). Now we have clarified the mechanism of this suppression. We demonstrate that an increase in the cellular levels of Hsp72 reverses the inhibition of JNK dephosphorylation caused by protein-damaging stresses. There are two possible mechanisms of heat shock-induced inhibition of a JNK phosphatase(s) which is relieved by Hsp72. The first is that a JNK phosphatase(s) or its regulator is damaged and inactivated upon heat shock (unlike p38 phosphatase), and Hsp72, as a chaperone, prevents such damage. The second model is that Hsp72 can associate with a JNK phosphatase(s) (or its regulator) and control its activity. According to this model, protein-damaging stresses do not affect a JNK phosphatase(s) directly. The inactivation results from the accumulation of damaged polypeptides, which sequester Hsp72, thus preventing its association with a JNK phosphatase(s). Such a mechanism resembles the accepted model for the induction of synthesis of heat shock proteins. It appears that damaged proteins, accumulated in a cell after stresses, sequester members of Hsp70 family. This, in turn, causes the release of the heat shock transcription factor from its complex with constitutively expressed Hsp72 homolog Hsc73, resulting in activation of the heat shock factor (42). Sequestering free Hsc73 by damaged proteins also causes dissociation of this heat shock protein from its complex with a heme-regulated eIF-2α kinase, leading to the kinase activation (27). Similarly, under normal conditions Hsp72 could associate with a JNK phosphatase(s) (or its regulator) and be subsequently sequestered by damaged proteins upon heat shock, leading to a loss of the phosphatase(s) activity.
We have previously demonstrated that Hsp72 also suppresses JNK activation by stimuli which do not cause significant protein damage and act through the JNK-activating kinase cascade. In this cascade, Hsp72 regulates a step downstream of MEKK1, and possibly downstream of SEK1 (43a). Since SEK1 directly phosphorylates JNK, Hsp72 can either inhibit JNK phosphorylation by SEK1 or accelerate JNK dephosphorylation. The latter possibility seems more likely because a JNK dephosphorylation is regulated by Hsp72 in cells exposed to protein-damaging stresses.
A close JNK homolog, p38 kinase, could also be activated by heat shock and other protein-damaging stresses, and its activation could be suppressed by Hsp72 (12). However, to our surprise, none of these stresses except menadione caused any detectable delay in dephosphorylation of p38 (Fig. 3C and not shown). Hence, protein-damaging stresses seem to activate JNK and p38 kinases by distinct mechanisms, and Hsp72 suppresses their activation in different fashions.
Defining an Hsp72-regulated JNK phosphatase(s) would be important for elucidation of the mechanisms of JNK activation and is the subject for future studies. At present, mostly dual-specificity phosphatases have been implicated in the dephosphorylation of stress-activated kinases, although single-specificity phosphatases were also reported to be involved (for reviews, see references 20 and 21). Some dual-specificity phosphatases are highly specific to JNK (3, 11, 33); however, with few exceptions such phosphatases are known to be stress inducible. Apparently, the major phosphatase(s) involved in JNK dephosphorylation is constitutive, since its activity was observed in unstressed cells. Also, our data may suggest that there is more than one phosphatase involved in JNK dephosphorylation. In fact, this process was inhibited both by vanadate, the tyrosine and dual-specificity phosphatase inhibitor, and by calyculin A, the inhibitor of serine-threonine phosphatases PP1 and PP2A. These inhibitors apparently affected different activities, since their effects were additive (Fig. 5).
In summary, a putative JNK phosphatase appears to be a crucial element in cellular responses to protein-damaging stresses. These findings could be critical for understanding progression of a variety of diseases in which protein damage and aggregation play a role. For example, accumulation and aggregation of abnormal polypeptides upon Huntington’s disease or prion disease may trigger neuronal apoptosis via this pathway.
ACKNOWLEDGMENTS
This study was supported by an RO1 grant from the NIH and a New Investigator Award from the Medical Foundation to M.Y.S. and by a BBRI scholarship to J.Y.
We thank Sophia Ritz-Volloch for help with experiments, J. Kyriakis and J. Avruch for providing plasmids, Sula Ganiatsas for preparation of SEK1−/− cells, and V. Volloch, A. Toker, and J. Badwey for helpful discussions.
REFERENCES
- 1.Adler V, Shaffer A, Kim J, Dolan L, Ronai Z. UV irradiation and heat shock mediate JNK activation via alternate pathways. J Biol Chem. 1995;270:26071–26077. doi: 10.1074/jbc.270.44.26071. [DOI] [PubMed] [Google Scholar]
- 2.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 3.Chu Y, Solski P A, Khosravi-Far R, Der C J, Kelly K. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem. 1996;271:6497–6501. doi: 10.1074/jbc.271.11.6497. [DOI] [PubMed] [Google Scholar]
- 4.Cohen P, Holmes C F, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 5.Comporti M. Three models of free radical-induced cell injury. Chem Biol Interact. 1989;72:1–56. doi: 10.1016/0009-2797(89)90016-1. [DOI] [PubMed] [Google Scholar]
- 6.Cosulich S, Clarke P. Apoptosis: does stress kill? Curr Biol. 1996;6:1586–1588. doi: 10.1016/s0960-9822(02)70779-3. [DOI] [PubMed] [Google Scholar]
- 7.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 8.Ding X Z, Tsokos G C, Kiiang J G. Overexpression of Hsp-70 inhibits the phosphorylation of HSF1 by activating protein phosphatase and inhibiting protein kinase C activity. FASEB J. 1998;12:451–459. doi: 10.1096/fasebj.12.6.451. [DOI] [PubMed] [Google Scholar]
- 9.Feige U, Morimoto R, Yahara I, Polla B. Stress-inducible cellular responses. Basel, Switzerland: Birkhauser Verlag; 1996. [Google Scholar]
- 10.Finch A, Holland P, Cooper J, Saklatvala J, Kracht M. Selective activation of JNK/SAPK by interleukin-1 in rabbit liver is mediated by MKK7. FEBS Lett. 1997;418:144–148. doi: 10.1016/s0014-5793(97)01364-1. [DOI] [PubMed] [Google Scholar]
- 11.Franklin C C, Kraft A S. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 12.Gabai V L, Meriin A B, Mosser D D, Caron A W, Rits S, Shifrin V I, Sherman M Y. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- 12a.Gabai, V. Submitted for publication.
- 13.Ganiatsas S, Kwee L, Fujiwara Y, Perkins A, Ikeda T, Labow M A, Zon L I. SEK1 deficiency reveals mitogen-activated protein cascade crossregulation and leads to abnormal hepatogenesis. Proc Natl Acad Sci USA. 1998;95:6881–6886. doi: 10.1073/pnas.95.12.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon J A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- 15.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy G R, Cairns J, Ng S B, Tan Y H. Inactivation of redox-sensitive protein phosphatase during the early events of tumor necrosis factor/interleukin-1 signal transduction. J Biol Chem. 1993;268:2141–2148. [PubMed] [Google Scholar]
- 17.Haspel R L, Salditt-Georgieff M, Darniell J E J. The rapid inactivation of nuclear tyrosine phosphorylated Stat1 depends upon a protein tyrosine phosphatase. EMBO J. 1996;15:6262–6268. [PMC free article] [PubMed] [Google Scholar]
- 18.Jani A, Lochmuller H, Acsadi G, Simoneau M, Huard J, Garnier A, Karpati G, Massie B. Generation, validation, and large scale production of adenoviral recombinants with large size inserts such as a 6.3 kb human dystrophin cDNA. J Virol Methods. 1997;64:111–124. doi: 10.1016/s0166-0934(96)02138-6. [DOI] [PubMed] [Google Scholar]
- 19.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. Philos Trans R Soc London Ser B. 1996;351:127–134. doi: 10.1098/rstb.1996.0008. [DOI] [PubMed] [Google Scholar]
- 20.Keyse S M. An emerging family of dual specificity MAP kinase phosphatases. Biochim Biophys Acta. 1995;1265:152–160. doi: 10.1016/0167-4889(94)00211-v. [DOI] [PubMed] [Google Scholar]
- 21.Keyse S M. Protein phosphatases and the regulation of MAP kinase activity. Semin Cell Dev Biol. 1998;9:143–152. doi: 10.1006/scdb.1997.0219. [DOI] [PubMed] [Google Scholar]
- 22.Kyriakis J M, Avruch J. Sounding the alarm; protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 23.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 24.Liossis S-N C, Ding X Z, Kiang J G, Tsokos G C. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J Immunol. 1997;158:5668–5675. [PubMed] [Google Scholar]
- 25.Maroney A C, Glickman M A, Basma A N, et al. Motoneuron apoptosis is blocked by CEP-1347 (KT 7515), a novel inhibitor of the JNK signaling pathway. J Neurosci. 1998;18:104–111. doi: 10.1523/JNEUROSCI.18-01-00104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massie B, Couture F, Lamoureux L, Mosser D D, Guilbault C, Jolicoeur P, Belanger F, Langelier Y. Inducible overexpression of a toxic protein by an adenovirus vector with a tetracycline-regulatable expression cassette. J Virol. 1998;72:2289–2296. doi: 10.1128/jvi.72.3.2289-2296.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matts R L, Hurst R, Xu Z. Denatured proteins inhibit translation in hemin-supplemented rabbit reticulocyte lysate by inducing the activation of the heme-regulated eIF-2 alpha kinase. Biochemistry. 1993;32:7323–7328. doi: 10.1021/bi00080a001. [DOI] [PubMed] [Google Scholar]
- 27a.Meriin, A. Unpublished observation.
- 28.Meriin A, Gabai V, Yaglom J, Shifrin V, Sherman M. Proteasome inhibitors activate stress-kinases and induce Hsp72: diverse effects on apoptosis. J Biol Chem. 1998;273:6373–6379. doi: 10.1074/jbc.273.11.6373. [DOI] [PubMed] [Google Scholar]
- 29.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 30.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 31.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosser D D, Caron A W, Bourget L, Jolicoeur P, Massie B. Use of a dicistronic expression cassette encoding the green fluorescent protein for the screening and selection of cells expressing inducible gene products. BioTechniques. 1997;22:150–161. doi: 10.2144/97221rr02. [DOI] [PubMed] [Google Scholar]
- 33.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 34.Nishina H, Fischer K D, Radvanyi L, Shahinian A, Hakem R, Rubie E A, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 35.Pena L A, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997;53:615–621. doi: 10.1016/s0006-2952(96)00834-9. [DOI] [PubMed] [Google Scholar]
- 36.Polanowska-Grabowska R, Simon C G, Jr, Falchetto R, Shabanowitz J, Hunt D F, Gear A R L. Platelet adhesion to collagen under flow causes dissociation of a phosphoprotein complex of heat-shock proteins and protein phosphatase 1. Blood. 1997;90:1516–1526. [PubMed] [Google Scholar]
- 37.Rieger D. Batch analysis of the ATP content of bovine sperm, oocytes, and early embryos using a scintillation couter to measure the chemiluminescence produced by the luceferin-luciferase reaction. Anal Biochem. 1997;246:67–70. doi: 10.1006/abio.1996.9978. [DOI] [PubMed] [Google Scholar]
- 38.Samejima I, Mackie S, Fantes P A. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 40.Shiozaki K, Shiozaki M, Russel P. Heat stress activates fission yeast Sps1/StyI MAPK by a MEKKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voellmy R. Sensing stress and responding to stress. In: Feige U, Morimoto I, Yahara I, Polla B, editors. Stress-inducible cellular responses. Vol. 77. Basel, Switzerland: Birkhauser Verlag; 1996. pp. 121–37. [Google Scholar]
- 43.Volloch V, Mosser D D, Masie B, Sherman M Y. Reduced thermotolerance in aged cells results from loss of the Hsp72-mediated control of JNK signalling pathway. Cell Stress Chaperones. 1998;3:265–271. [PMC free article] [PubMed] [Google Scholar]
- 43a.Yaglom, J., et al. Submitted for publication.
- 44.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 45.Zanke B W, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 46.Zanke B W, Rubie E A, Winnett E, Chan J, Randall S, Parsons M, Boudreau K, McInnis M, Yan M, Templeton D J, Woodgett J R. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]