SUMMARY
Microsporidia are obligate intracellular pathogens identified ∼150 years ago as the cause of pébrine, an economically important infection in silkworms. There are about 220 genera and 1,700 species of microsporidia, which are classified based on their ultrastructural features, developmental cycle, host-parasite relationship, and molecular analysis. Phylogenetic analysis suggests that microsporidia are related to the fungi, being grouped with the Cryptomycota as a basal branch or sister group to the fungi. Microsporidia can be transmitted by food and water and are likely zoonotic, as they parasitize a wide range of invertebrate and vertebrate hosts. Infection in humans occurs in both immunocompetent and immunodeficient hosts, e.g., in patients with organ transplantation, patients with advanced human immunodeficiency virus (HIV) infection, and patients receiving immune modulatory therapy such as anti-tumor necrosis factor alpha antibody. Clusters of infections due to latent infection in transplanted organs have also been demonstrated. Gastrointestinal infection is the most common manifestation; however, microsporidia can infect virtually any organ system, and infection has resulted in keratitis, myositis, cholecystitis, sinusitis, and encephalitis. Both albendazole and fumagillin have efficacy for the treatment of various species of microsporidia; however, albendazole has limited efficacy for the treatment of Enterocytozoon bieneusi. In addition, immune restoration can lead to resolution of infection. While the prevalence rate of microsporidiosis in patients with AIDS has fallen in the United States, due to the widespread use of combination antiretroviral therapy (cART), infection continues to occur throughout the world and is still seen in the United States in the setting of cART if a low CD4 count persists.
KEYWORDS: microsporidia, therapy, diagnostics, prevention, Enterocytozoon, Encephalitozoon, Anncaliia, Vittaforma, AIDS, albendazole, fumagillin
INTRODUCTION
Microsporidia are a diverse group of unicellular obligate intracellular parasites that were previously believed to be “primitive” early branching protozoa but are now understood to belong to the fungi as either a basal branch or a sister group (1). Phylogenetic studies indicate that the microsporidia should be placed with the Cryptomycota as a basal branch of the fungal kingdom (2). The Cryptomycota have been identified by environmental microbiome studies, and while poorly described at the biological level, they are closely related to the microsporidia (3). Cryptomycota that grow as intranuclear parasites of amoeba have been discovered, and these parasites bear strong morphological similarities to microsporidia (4, 5). These and other microsporidia-like organisms, such as Nucleophaga terricola, Mitosporidium daphniae, and Paramicrosporidium, demonstrate that additional environmental sampling is required to completely ascertain the relationship of fungi and microsporidia and to provide a better understanding of the Cryptomycota and the origin of microsporidia.
The class or order Microsporidia was elevated to the phylum Microspora by Sprague in 1977 (5). In 1998, Sprague and Becnel suggested that the term Microsporidia instead be used for the phylum name (6). Microsporidia produce distinctive unicellular spores that are environmentally resistant (7). The shape and size of these spores vary with the species, but most microsporidia infecting humans have ovoid spores that range from 1 to 4 μm. A defining feature of all microsporidia is the presence of an extrusion apparatus which consists of a polar tube attached to the inside of the anterior end of the spore by an anchoring disk. Depending on the microsporidian species, the polar tube forms 4 to approximately 30 coils around the sporoplasm in the spore. When exposed to specific environmental conditions, such as passage through the host's gastrointestinal tract, germination of the spore occurs. During germination, the polar tube rapidly everts, forming a hollow tube acting as a conduit to deliver the sporoplasm to the host cell. The mechanism by which the polar tube interacts with the host cell membrane is an area of active investigation. If a spore is phagocytosed by a host cell, germination can also occur, enabling the polar tube to pierce the phagocytic vacuole and resulting in the sporoplasm being delivered into the host cell cytoplasm or into an adjacent host cell.
Microsporidia have many typical eukaryotic features and contain an intracytoplasmic membrane system, vesicular Golgi complex, a nucleus with a nuclear envelope, and chromosome separation on mitotic spindles, as well as a mitochondrial remnant organelle called a mitosome (8, 9). Microsporidia have several characteristics that are unusual for eukaryotic organisms. They possess prokaryotic-size ribosomes (10) which lack a 5.8S ribosome subunit; however, in their 23S subunit, there is sequence homologous to the 5.8S region (11). Their genome size varies from 2.18 to 51.35 Mbp, with 2,000 to 5,000 protein coding genes (Table 1) (12, 13). The genomic size of the Encephalitozoonidae is less than 2.5 Mbp, which places them among the smallest eukaryotic nuclear genomes identified to date. In this compact Encephalitozoonidae genome, the gene density is high, almost no introns exist, and the encoded proteins are significantly shorter than the orthologous proteins seen in other eukaryotes such as Saccharomyces cerevisiae (14, 15).
TABLE 1.
Microsporidial genomesa
| Organism | Strain | Genome size (Mbp) | No. of protein coding genes | No. of nonprotein coding genesb | No. of pseudogenes | No. of chromosomes |
|---|---|---|---|---|---|---|
| Amphiamblys sp. | WSBS2006 | 5.62 | 3,695 | 48 | 5 | |
| Anncaliia algerae | PRA109 | 17.53 | 5,330 | 85 | 0 | |
| Anncaliia algerae | PRA339 | 12.16 | 3,661 | 63 | 0 | |
| Anncaliia algerae | Undeen | 13.82 | NA | NA | NA | |
| Edhazardia aedis | USNM 41457 | 51.35 | 4,262 | 72 | 2 | |
| Encephalitozoon cuniculi | EC1 | 2.24 | 1,898 | 51 | 0 | 12 |
| Encephalitozoon cuniculi | EC2 | 2.24 | 1,902 | 51 | 0 | 16 |
| Encephalitozoon cuniculi | EC3 | 2.24 | 1,898 | 51 | 2 | 15 |
| Encephalitozoon cuniculi | Ecun III-L | 2.29 | 1,896 | 51 | 11 | 15 |
| Encephalitozoon cuniculi | GB-M1 | 2.5 | 2,126 | 33 | 0 | 11 |
| Encephalitozoon hellem | ATCC 50504 | 2.25 | 2,006 | 78 | 0 | 12 |
| Encephalitozoon hellem | Swiss | 2.18 | 1,864 | 51 | 0 | 13 |
| Encephalitozoon intestinalis | ATCC 50506 | 2.22 | 2,011 | 72 | 0 | 11 |
| Encephalitozoon romaleae | SJ-2008 | 2.19 | 1,883 | 51 | 1 | 13 |
| Enterocytozoon bieneusi | H348 | 3.86 | 3,806 | 173 | 3 | |
| Enterocytozoon hepatopenaei | TH1 | 3.25 | 2,581 | 45 | 1 | |
| Enterospora canceri | GB1 | 3.1 | 2,225 | 47 | 9 | |
| Hamiltosporidium tvaerminnensis | OER-3-3 | 13.27 | NA | NA | NA | |
| Hepatospora eriocheir | canceri | 4.83 | 3,117 | 59 | 7 | |
| Hepatospora eriocheir | GB1 | 4.57 | 2,770 | 54 | 26 | |
| Mitosporidium daphniae | UGP3 | 5.64 | 3,428 | 98 | 0 | |
| Nematocida ausubeli | ERTm2 | 4.7 | 2,831 | 61 | 0 | |
| Nematocida ausubeli | ERTm6 | 4.27 | 2,544 | 111 | 0 | |
| Nematocida displodere | JUm2807 | 3.05 | 2,324 | 46 | 0 | |
| Nematocida parisii | ERTm1 | 4.07 | 2,724 | 63 | 0 | |
| Nematocida parisii | ERTm3 | 4.15 | 2,788 | 62 | 0 | |
| Nosema bombycis | CQ1 | 15.69 | 4,643 | 175 | 12 | |
| Nosema ceranae | BRL01 | 7.86 | 2,678 | 64 | 554 | |
| Nosema ceranae | PA08_1199 | 5.69 | 3,265 | 37 | 19 | |
| Ordospora colligata | OC4 | 2.29 | 1,879 | 59 | 0 | |
| Pseudoloma neurophilia | MK1 | 5.25 | 3,676 | 31 | 0 | |
| Spraguea lophii | 42_110 | 4.98 | 2,596 | 53 | 44 | |
| Trachipleistophora hominis | unknown | 8.5 | 3,253 | 41 | 0 | |
| Vavraia culicis subsp. floridensis | floridensis | 6.12 | 2,875 | 102 | 0 | |
| Vittaforma corneae | ATCC 50505 | 3.12 | 2,340 | 101 | 0 |
Data were obtained from MicrosporidiaDB (https://microsporidiadb.org/micro/). NA, not available.
Noncoding genes: rRNA, tRNA, etc.
Analysis suggests that microsporidia may have experienced an early bottleneck in evolution with a massive gene loss resulting in a core set of conserved genes, and this core set has been complemented by expansion of transporters that facilitate the obligate intracellular life cycle of these pathogens (16). Further expansion from this core set of genes has been driven by the acquisition of noncoding DNA (such as transposable elements, etc.), e.g., 9% of the 51.35-Mbp Edhazardia aedis genome and 90% of the 2.24-Mbp Encephalitozoon cuniculi genome code for proteins (17, 18). Genome data for the microsporidia are available online at MicrosporidiaDB.org (https://microsporidiadb.org/micro/) (19), which is part of the VEuPath database (https://veupathdb.org/veupathdb/) website (Table 1). This website includes the genomes of the human-pathogenic microsporidia Encephalitozoon cuniculi (15) (all three strains [20]), Encephalitozoon hellem (21), Encephalitozoon intestinalis (22), Enterocytozoon bieneusi (23, 24), Anncaliia algerae, and Vittaforma corneae, as well as microsporidia found in other organisms (Table 1).
Microsporidia are classically classified by their ultrastructural features, which include the number of coils of their polar tube, the size, shape, and morphology of their spores, the features of their developmental life cycle, and their host-parasite relationship. Molecular phylogenetic data, such as small subunit rRNA sequences, provides a complementary set of data for assigning microsporidia to specific genera as well as distinguishing morphologically similar microsporidia at the species level (25, 26). Examination of molecular data demonstrates the polyphyletic nature of the microsporidia and the problems with current classification systems that use single character states for higher taxonomic groupings. Overviews of the ultrastructural and structural characteristics, life cycle differences, and history of the microsporidian taxa can be found in the work of Tuzet (27), Sprague et al. (5), Larsson (28), Issi (29), Weiser (30), and Sprague et al. (31). Microsporidia are currently separated into three main groups: (i) the “primitive” microsporidia (Metchnikovellidae), which are hyperparasites of gregarines found in annelids and which have a rudimentary polar filament and lack a polaroplast in their spores; (ii) the Burkeidae, Chytridopsidae, and Hesseidsae, which have a short polar filament and minimal development of a polaroplast and endospore; and (iii) the “higher” microsporidia that possess a well-developed polar filament, a polaroplast, and a posterior vacuole. Variations between the current microsporidian classification systems focus primarily on the various characteristics used to divide the “higher” microsporidia into subgroups.
These pathogenic protists were first recognized over 150 years ago with the identification of Nosema bombycis by Nageli in 1857 as the cause of the disease pébrine in silkworms, an economically important insect (13) (Fig. 1). In 2008, Franzen (32) published an excellent overview of the history of research on microsporidia, starting with the discovery of N. bombycis. Microsporidia cause diseases in a wide range of hosts from invertebrates to vertebrates, including commercially important insects (e.g., silkworms and honeybees), fish, cattle, rabbits, rodents, dogs, and primates. Microsporidia have been developed for use as biologic control agents for several insect pests (13).
FIG 1.
Timeline of investigations on microsporidia.
The majority of these pathogenic organisms infect their host's gastrointestinal tract; however, there are documented microsporidian infections of almost all organ systems (13, 33). Rare reports of human infection were noted as early as 1959 (34), when microsporidia were identified in a child with encephalitis. In the 1980s, microsporidia were identified as opportunistic pathogens in patients with severe immune deficiency due to HIV infection, and they are now recognized to occur in other immunodeficient patients as well as in immunocompetent hosts (35, 36). Infection with these pathogens can produce a wide spectrum of clinical diseases, with chronic diarrhea being the most common presentation in patients with immune deficiency. Since the advent of combination antiretroviral therapy (cART), the incidence of microsporidiosis has declined in the HIV-infected population; however, this infection continues to occur in patients who have low CD4 counts despite cART (37–40). Besides gastrointestinal tract involvement, there are many examples in the literature of microsporidian infections causing encephalitis, keratitis, sinusitis, and myositis, as well as disseminated disease (13, 33). The phylum Microsporidia contains at least 1,700 species distributed into approximately 220 genera, of which the following have been demonstrated in human disease (13) (Table 2): Nosema (Nosema corneum, reclassified as Vittaforma corneae [41]; Nosema algerae, reclassified initially as Brachiola algerae [42] and then Anncaliia algerae [43]), Enterocytozoon (44), Septata (45) (reclassified as Encephalitozoon [46]), Encephalitozoon, Pleistophora, Trachipleistophora (47, 48), Brachiola (42), Anncaliia (43), Tubulinosema (49, 50), Endoreticulatus (51), and Microsporidium (13).
TABLE 2.
Microsporidia infecting humans
| Microsporidium species | Clinical manifestation(s) | Zoonotic reservoir(s) | In vitro culture (cell lines) | Reference(s) |
|---|---|---|---|---|
| Anncaliia algerae | Skin infection, keratoconjunctivitis, myositis | Insects (mosquitoes) | E6, HLF, RK13, sf9, GFSK-S1, GFB3C-W1, ZEB2J | 182, 350, 351, 355, 456 |
| Anncaliia (Nosema) connori | Disseminated infection | Unknown | Not reported | 358, 457 |
| Anncaliia vesicularum | Myositis | Unknown | Not reported | 42 |
| Encephalitozoon cuniculi | Keratoconjunctivitis, sinusitis, bronchitis, nephritis, peritonitis, hepatitis, diarrhea, prostatitis, nephritis, cellulitis, disseminated infection, encephalitis | Mammals (rabbits, rodents, dogs, primates) | RCP, E6, HLF, MDCK, MRC-5, RK13, HFF, L929, Vero, SIRC, HeLa | 95–97, 99, 169, 170, 458–463 |
| Encephalitozoon hellem | Keratoconjunctivitis, sinusitis, bronchitis, nephritis, cystitis, prostatitis, urethritis, pneumonitis, disseminated infection | Psittacine birds (parrots, lovebirds, parakeets), other birds (ostrich, hummingbirds, finches) | MDCK, E6, HLF, MRC-5, RK13, FBF, HFF, HT-29, Caco2, Vero, SIRC, HeLa | 95, 97, 104, 107, 111, 114, 155, 462, 464, 465 |
| Encephalitozoon intestinalis | Diarrhea, intestinal perforation, cholangitis, keratoconjunctivitis, sinusitis, bronchitis, nephritis | Mammals (donkeys, dogs, pigs, cows, goats, primates) | E6, HLF, MDCK, HEL, MDM, RK13, I047, HT-29, Caco-2, HFF, Vero, SIRC, HeLa | 97, 116, 118, 120, 462 |
| Enterocytozoon bieneusi | Chronic diarrhea, wasting syndrome, cholangitis, rhinitis, bronchitis | Mammals (pigs, primates, cows, dogs, cats), birds (chickens, waterfowl) | E6, HLF, HT-29, Caco-2 | 102, 113, 122, 393, 466, 467 |
| Pleistophora ronneafiei | Myositis | Unknown | Not reported | 369, 375 |
| Pleistophora sp. | Myositis | Fish | Not reported | 368, 373 |
| Trachipleistophora anthropopthera | Disseminated infection, including encephalitis | Unknown | Fibroblasts (derived from mouse brain) | 48, 92, 371, 372 |
| Trachipleistophora hominis | Myositis, keratoconjunctivitis, sinusitis | Unknown | MDCK, RK13, COS-1, L6-C10, MM | 47, 92, 367, 469 |
| Tubulinosema acridophagus | Myositis, disseminated infection | Insects (Drosophila melanogaster, grasshoppers) | MRC-5, Vero | 49, 50, 92 |
| Vittaforma corneae (Nosema corneum) | Keratoconjunctivitis, urinary tract infection, disseminated infection | Unknown | MDCK, MRC-5, XEN, RK13, THP-1, E6, Vero, SIRC, HeLa, HLF | 41, 93, 100, 378, 462, 468, 470–472 |
| Nosema ocularum | Keratoconjunctivitis | Unknown | Not reported | 377, 473 |
| Endoreticulatus sp. | Myositis | Insects (Lepidopteran) | Not reported | 51 |
| Microsporidium africanum | Corneal ulcer | Unknown | Not reported | 56, 474 |
| Microsporidium ceylonensis | Corneal ulcer | Unknown | Not reported | 56, 475 |
Morphology of the Microsporidian Spore
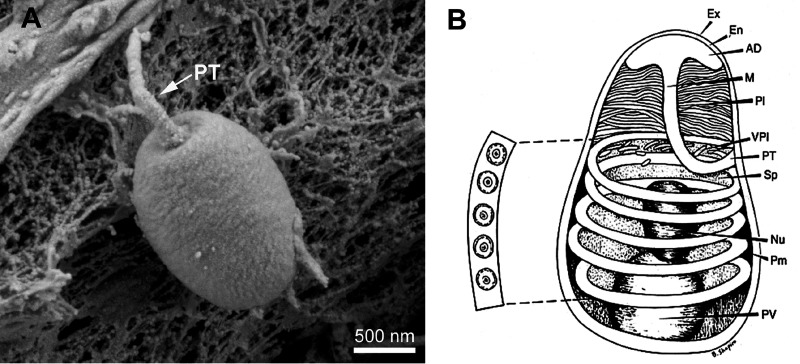
Microsporidia form distinctive spores (Fig. 2) which are the infectious stage of their life cycle and the only stage that can survive outside of the host cell due to their environmental resistance. The majority of microsporidian infections in humans are thought to be a consequence of the ingestion of these spores in either food or water (52). The spore structure is characteristic of the phylum, and the shape and size of microsporidia vary depending on the species (53, 54). The spores, depending on the species, range from 1 to 12 μm in size, and most spores are either oval or pyriform (5, 55, 56). The general structure of the spore was first observed in 1894 by Thélohan (57). Since those initial observations, studies have defined the internal structure of these spores, including the anchoring disk, polar tube, spore coat, polaroplast, nucleus, and posterior vacuole (58, 59).
FIG 2.
Structure of the microsporidian spore. (A) Scanning electron microscropy (SEM) image of a microsporidian spore. (B) Microsporidian spore diagram. Microsporidian spores range in size from 1 to 10 μm. The spore coat is thinner at the anterior end of the spore and consists of an electron-lucent endospore (En), an electron-dense exospore (Ex), and the plasma membrane (Pm). The sporoplasm (Sp) contains ribosomes, the posterior vacuole (PV), and a single nucleus (Nu). The anchoring disc (AD) at the anterior end of the spore is the site of attachment of the polar tube. It should be noted that the polar tube is often called the polar filament when it is within the spore prior to germination. The anterior or straight region of the polar tube that connects to the anchoring disc is called the manubroid (M), and the posterior region of the tube coils around the sporoplasm. The number of coils and their arrangement (i.e., single row or multiple rows) is used in microsporidian taxonomic classification. The lamellar polaroplast (Pl) and vesicular polaroplast (VPl) surround the manubroid region of the polar tube. The insert depicts the 5 polar tube coils in this figure in a cross section illustrating that the polar tube within the spore (i.e., polar filament) has several layers of different electron densities by electron microscopy. (Reproduced from reference 68 with permission.)
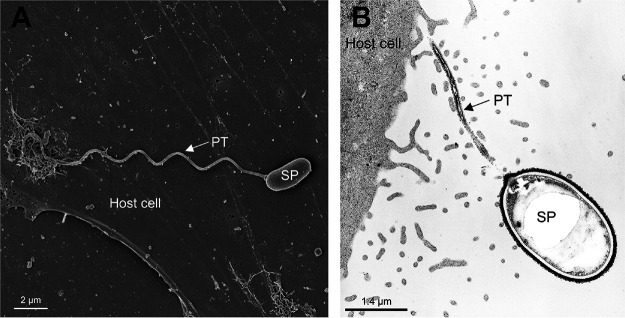
The spore coat is composed of three layers, an electron-dense, proteinaceous exospore, an electron-lucent endospore, and an inner membrane or plasmalemma (60). The endospore, composed mainly of chitin, functions as the connection of the exospore and plasma membrane (54, 61). Several proteins found in the spore coat possess adhesion domains that are probably involved in the interaction of microsporidian spores with either the cell surface or mucus of the gastrointestinal tract before germination (62). The anchoring disk is situated at the anterior pole of the spore, where the endospore is thinnest in the anterior region (61, 63). In addition, the anchoring disc is where the polar tube attaches to the spore wall and is released during spore germination (62). The polar tube was first identified more than 125 years ago by Thélohan and is the defining characteristic of the phylum (57). Prior to germination, the polar tube is coiled around the nucleus in the spore (the number of coils depends on the specific microsporidian species) (60, 64, 65). During germination, the polar tube is extruded from the anchoring disk of the spore and forms a hollow tube that acts as a bridge between the microsporidian spore and host cell, allowing transfer of the sporoplasm and nucleus into the host cell cytoplasm, resulting in host cell infection (Fig. 3) (60, 66, 67).
FIG 3.
Germinated microsporidian spores. SEM (A) and transmission electron microscopy (TEM) (B) images of a germinated spore during infection. Upon exposure to suitable environmental conditions, the polar tube (PT) rapidly discharges out of the spore (SP) and then interacts with the host cell membrane, serving as a conduit for the nucleus and sporoplasm passage into the host cell.
Germination of a spore with extrusion of the polar tube is dependent on environmental conditions and is species specific, probably reflecting microsporidian adaptation to their host and external environment (reviewed by Keohane and Weiss [68]). Conditions that promote spore discharge include various anions and cations, hydrogen peroxide, pH shifts, dehydration followed by rehydration, mucin, polyanions, the calcium ionophore A23187, and UV irradiation. Inhibitors of spore discharge include calcium channel antagonists, low salt concentrations, calmodulin inhibitors, itraconazole, magnesium chloride, ammonium chloride, sodium fluoride, cytochalasin D, demecolcine, and UV light. The exact mechanisms that initiate polar tube extrusion are still poorly understood. It has been suggested that germination is due to increased intrasporal osmotic pressure that results in an influx of water into the spore, which is accompanied by swelling of the polaroplast and posterior vacuole before spore discharge. For Ancaliia (Nosema) algerae, it has been theorized that the increase in osmotic pressure is due to activation, causing trehalose to come into contact with trehalase (69, 70). Another study suggested that calcium displacement from the polaroplast membrane results in polaroplast swelling (71, 72).
The Microsporidian Life Cycle
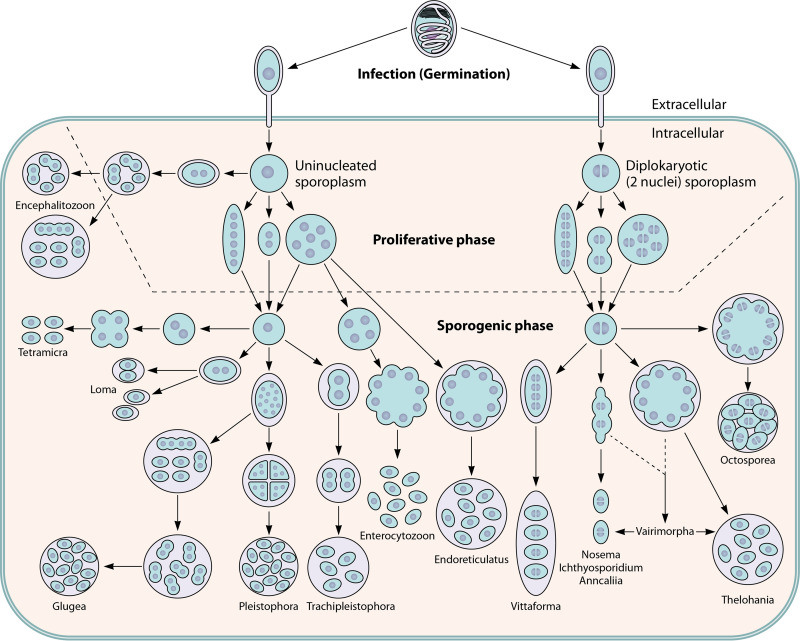
The majority of species of microsporidia have simple single-host life cycles; however, a few species have complex life cycles with multiple hosts and can form morphologically distinct spores in different hosts (e.g., Amblyospora sp. in mosquitoes) (73, 74). The life cycle pattern for microsporidia can be divided into three stages (Fig. 4): the infective or environmental stage, the proliferative stage, and the sporogonic or spore-forming stage. During development inside of the host cell, microsporidia demonstrate different interfacial relationships depending on the genus of microsporidia (75, 76). These host-parasite interfacial relationships of microsporidia have been divided into four categories (Table 3), which are thought to represent important physiological/nutritional differences among the various microsporidia (77–79).
FIG 4.
Developmental life cycles of the microsporidia. Spores germinate and the polar tube is extruded when they are exposed to specific environmental conditions, which vary depending on the species of microsporidia. Upon germination, the polar tube is extruded and interacts with the host cell membrane, allowing invasion (see Fig. 6 for a model of this interaction). The sporoplasm traverses the polar tube, is introduced into the host cell cytoplasm, and begins to proliferate. The morphology of the organism during the proliferative phase (which is indicated by the region above the dotted line) is used in classification systems for defining the various microsporidian genera. Relationships of the various microsporidia with their host cells during the proliferative phase are described in Table 3. On the left side of the figure, the sporoplasm is uninucleate and the microsporidian genera that are defined during the development of spores, i.e., the sporogenic phase (which is indicated by the region below the dotted line), are all uninucleate. On the right side of the figure, the sporoplasm is diplokaryotic, and all of the microsporidian genera defined during spore development have diplokaryotic developmental patterns. Regardless of whether they are uninucleate or diplokaryotic, there are three basic types of developmental forms. The first developmental pathway, as illustrated by Anncaliia spp., involves cell division by binary fission that immediately follows karyokinesis. The second type of pathway, as illustrated by some Nosema spp., involves multiple fission of elongated moniliform multinucleate cells. The third type of developmental pathway, as illustrated by Endoreticulatus spp., involves the division by plasmotomy of rounded plasmodial multinucleate cells. During the proliferative phase, cells can have one to several division cycles. Many microsporidia are either in direct contact with the host cell cytoplasm or closely abutted to the host endoplasmic reticulum; however, other microsporidia are found within a parasitophorous vacuole (e.g., Encephalitozoon spp. and probably Tetramicra spp.) or are separated from the host cell cytoplasm by a thick layer of their own secretions (e.g., Pleistophora spp.). In the sporogonic phase, this thick layer of microsporidian secretions becomes the sporophorous vesicle. During sporogony, several of the microsporidian genera, such as Enterocytozoon, Nosema, Anncaliia, Ichthyosporidium, and Tetramicra, maintain direct contact with the host cell cytoplasm. A sporophorous vesicle, illustrated by a circle around the developing sporogonial stage, is formed by the other microsporidian genera. During the developmental cycle of Thelohania and the Thelohania-like part of the Vairimorpha cycle, it should be noted that the diplokarya separate and continue their development as cells with isolated nuclei. (Adapted from reference 453 with permission.)
TABLE 3.
Interfacial relationships of the microsporidia
| Type of contact | Interfacial relationship |
|---|---|
| Type I. Direct contact | Direct contact of microsporidian plasmalemma with the host cell cytoplasm. This is seen in Enterocytozoon bieneusi, Anncaliia, and Nosema. |
| Type II. Indirect contact by host-produced isolation | Subtype A. Microsporidia are in a parasitophorous vacuole (PV)-host-formed single membrane that surrounds the developing organism. The PV is seen in the proliferative and sporogonic phases of development, but the relationship of the developing microsporidia changes during development. Early in development, the organisms have a close association with the PV membrane until the development of a thickened sporont plasmalemma, when they appear loose within the PV. This type of development is seen in Encephalitozoon hellem and Encephalitozoon cuniculi. |
| Subtype B. Throughout development, the microsporidia are surrounded by the host endoplasmic reticulum (ER) that appears as a double membrane surrounding the developing microsporidian cells. In the proliferative phase, these host ER double membranes closely follow the dividing microsporidian plasmalemma and no vacuole is seen. During sporogony, a double-membraned PV is formed by the host ER, which surrounds the cluster of organisms formed in sporogony. This type of development is seen in Endoreticulatus and Vittaforma. | |
| Type III. Indirect contact by parasite-produced isolation | Subtype A. During development, microsporidia secrete an envelope that surrounds the developing organisms. In sporogony, this envelope becomes a sporophorous vesicle (SPOV) as the microsporidian plasmalemma thickens and pulls away from the secreted envelope. This type of development is seen in Pleistophora. |
| Subtype B. During early development, the microsporidia are in direct contact with the host cell cytoplasm. As development proceeds, a membrane formed by the microsporidia isolates sporonts from contact with the host cell cytoplasm. This type of development is seen in Vairimorpha. | |
| Type IV. Indirect contact by host- and parasite-produced isolation | Subtype A. During merogony, the host ER closely associates with the microsporidian plasmalemma. As development proceeds, the developing microsporidia form an interfacial envelope by producing “blisters” that arise from the plasmalemma, resulting in a SPOV, which can contain tubules, being formed in sporogony. This type of development is seen in Glugea and Loma. |
| Subtype B. During development, both the host and parasite contribute to the formation of a thick interfacial envelope surrounding the developing organisms. This type of development is seen in Trachipleistophora. | |
| Subtype C. During development, a PV formed by the host surrounds the organisms. Within the PV, microsporidian-secreted material surrounds each organism. This type of development is seen in Encephalitozoon intestinalis. |
The infective stage, i.e., the spores, are the only stage that can survive outside of the host cell. When the spores meet an appropriate environmental condition, germination occurs and the sporoplasm is transported into the host cell via the hollow polar tube (68, 80). As long as the sporoplasm enters the host cell, it will initiate a reproduction cycle, which terminates with the production of mature spores. Merogony follows, during which the injected sporoplasm develops into meronts (the proliferative stage), which multiply, depending on the species, by binary fission or multiple fission. While most microsporidia develop within the host cell cytoplasm, some genera of microsporidia will infect the nucleoplasm of the host cell or develop in both the cytoplasm and nucleus (81–84). There are two distinct phases in the development of microsporidia inside of host cells: the proliferative (merogony) stage and the sporogonic stage (Fig. 4 and 5). The merogony stage is responsible for the massive increase in number of infecting organisms (61). At the end of merogony, an electron-dense material is formed on the external face of the merozoite plasma membrane, which is the sign of the switch from merogony to sporogony (85). Sporonts give rise to sporoblasts, which go on to form mature spores without additional multiplication. In most species, the dense material on the plasma membrane in sporogony will become the primordium of the exospore layer of the spore wall (86). The polar tube and Golgi complex will also develop in the sporoplasm of the sporoblast (54, 87, 88). Even though the sporoblast plasma membrane is covered by a continuous layer of dense material, this layer can also form tubular or fibrillar expansions that protrude from the surface of the sporoblast into the parasitophorous vacuole (86). Once a host cell becomes distended with mature spores, the cell ruptures, releasing mature spores into the environment and completing the life cycle. The combination of merogony and sporogony results in a large number of spores being produced from a single infection and underlies the huge reproductive potential of these organisms.
FIG 5.
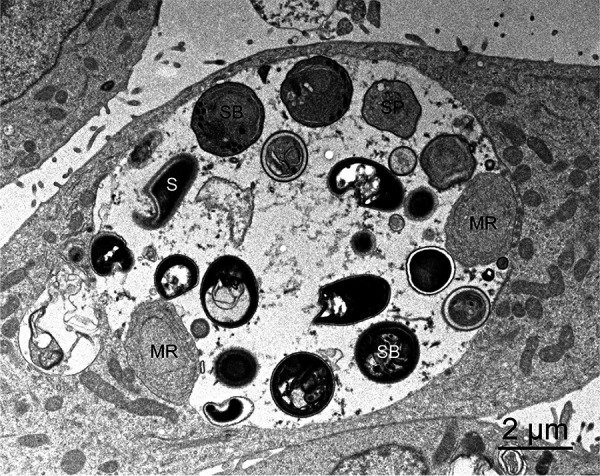
Transmission electron microscopy of an Encephalitozoon hellem parasitophorous vacuole. Meronts (MR) can be seen adhering to edge of the vacuole, whereas sporonts (SP), sporoblasts (SB), and mature spores (S) detach from the vacuole membrane and move to the center of the vacuole.
IN VITRO CULTIVATION OF MICROSPORIDIA INFECTING HUMANS
Early studies on the in vitro culture of microsporidia focused on economically important species such as Nosema bombycis (a pathogen of silkworms), Nosema apis (a pathogen of honeybees), Ameson michaelis (a pathogen of blue crabs), Brachiola (Nosema) algerae (a pathogen of mosquitoes), and Vavraia culiceis (a pathogen of mosquitoes) (6, 89, 90). The first successful in vitro cultivation of microsporidia was conducted by Trager in 1934 by inoculating silkworm ovarian tube lining cells with the insect parasite Nosema bombycis (91). Encephalitozoon cuniculi was the first microsporidian of mammalian origin cultured in vitro (89). In order to prevent the growth of bacteria and fungi during the primary isolation of microsporidia and establishment of in vitro cultures, a combination of antibiotics and antifungal agents is usually utilized (61, 89–91).
Since microsporidia were found to be able to infect humans, there have been 10 genera and 17 species of microsporidia reported as etiologic in human infections. Several of these human-infecting microsporidia have been stably cultured in various cell lines (Table 2) (90, 92). The first successful in vitro cultivation of a human microsporidium was Vittaforma corneae, which was isolated from the corneal biopsy specimen of an immunocompetent patient (93). The corneal samples were either minced or digested by trypsin and collagenase and then inoculated into different cell lines, such as SIRC cells, MDCK cells, or rabbit embryo fibroblasts, and infection could be seen after 30 days postinoculation. More than 13 isolates of Encephalitozoon cuniculi, 30 isolates of Encephalitozoon hellem, and 30 isolates of Encephalitozoon intestinalis have been reported to be successfully cultured (94–121). These parasites were isolated from various tissue or biological samples, including corneal scrapings, conjunctival biopsy specimens, urine, sputum, bronchoalveolar lavage fluid, nasal mucosal biopsy specimens, duodenal biopsy specimens, and stool. Enterocytozoon bieneusi is the most frequently identified microsporidian that can cause the diarrhea in AIDS patients. A continuous in vitro culture system for Enterocytozoon bieneusi has not been established (116, 119). Short-term culture of Enterocytozoon bieneusi has been achieved in E6 and HLF cells, but those cultivation systems could not produce significant numbers of spores (119, 122).
EPIDEMIOLOGY
Prevalence in Humans
Microsporidia infect a wide range of animals, including humans, and spores are shed into the environment, increasing the probability for human exposure. Microsporidian spores are often found in surface water, and human-pathogenic microsporidia have been found in municipal water supplies (123), hospital effluent (124), tertiary sewage effluent, and groundwater (125, 126). Human infections due to microsporidia have been identified from all continents except Antarctica, and stool samples from Africa, Asia, Europe, Australia, North America, Central America, and South America have all been shown to contain microsporidia (13, 127).
Before the AIDS pandemic, microsporidia were rarely identified in humans (56, 128). The first authenticated record of a human infected with microsporidia was reported in 1959, when an Encephalitozoon sp. was detected in Japan in a boy with convulsions (34). Prior to this, there was a report of a case in 1927 in a newborn baby attributed to “Encephalitozoon chagasi” (129), but it is not clear that that organism was correctly identified. Another early case of microsporidiosis was reported in 1937 in a 4-week-old child who presented with fatal encephalomyelitis and chorioretinitis (130).
The prevalence data for microsporidiosis in human populations, before the era of AIDS, relied mainly upon serology based on detecting antibodies to Encephalitozoon cuniculi. The worldwide seroprevalence rates that were reported ranged from 0% to 42% (9, 56, 131–133). Singh et al. (134) found positive titers in 8.7% of healthy adults in England (n = 69), 18.5% of Malaysians with filariasis (n = 70), 42% of Nigerians with tuberculosis (n = 89), and 36% of Ghanaians with malaria (n = 92). In another study, while no nontravelers (n = 48) were seropositive, 12% of travelers (n = 115) returning from the tropics were seropositive (135). In another study, 33% of HIV-positive men (n = 30) were seropositive and of them 100% had traveled to the tropics (132). Antibodies to Encephalitozoon intestinalis were seen in 5% of pregnant French women and 8% of Dutch blood donors (136). In a study of HIV-positive Czech patients, 5.3% were seropositive to Encephalitozoon cuniculi and 1.3% to Encephalitozoon hellem (137). In a study in Slovakia, 5.1% of slaughterhouse workers were seropositive to Encephalitozoonidae (138). Taken as a group, these various serologic studies suggest that exposure to microsporidia is common and that this exposure often results in an asymptomatic infection.
Increased cases of microsporidian infections were reported in humans with the expansion of the AIDS pandemic. The majority of these were due to Enterocytozoon bieneusi or Encephalitozoon intestinalis (Table 2). Reported prevalence rates in the studies conducted in HIV-infected patients before 1998 and the widespread use of combination antiretroviral therapy (cART) demonstrated a prevalence of gastrointestinal microsporidiosis that varied between 2% and 70%, depending on the symptoms of the population studied and the diagnostic techniques utilized (13, 33, 139). When these early studies were combined, the overall prevalence of microsporidial infection in patients was estimated to be 15% (133, 140–142), and in the setting of chronic diarrhea and advanced AIDS, the prevalence of Enterocytozoon bieneusi infection was estimated to be 30%. A more recent meta-analysis in 2018 of 131 studies examining microsporidia, cryptosporidium, and isospora infections in HIV-infected individuals found a pooled prevalence of 11.8% (confidence interval [CI], 10.1% to 13.4%) for human gastrointestinal microsporidiosis using a random effects model (139). A lower prevalence of infection was found in high-income countries and higher prevalence in low-income countries, especially in sub-Saharan Africa (139). In a 10-year study, the prevalence of microsporidia was significantly higher in samples from HIV-positive patients (13.9%) than in samples from HIV- negative patients (8.5%) (143), and the percentage of children that were microsporidia positive (18.8%) was significantly higher than that of adults (10.2%) (143). A study in Mexico in 2019 found that 67% of HIV patients with diarrhea had microsporidiosis. A study in Argentina in 2019 found that 12.6% of HIV patients with chronic diarrhea had microsporidiosis by endoscopy and stool examination (144). A study in Malawi in 2018 in HIV-positive children found a baseline prevalence of microsporidiosis of 37% before starting cART and that this prevalence dropped after cART was started (145). While it is clear from several studies that cART can hasten the resolution of microsporidiosis, the literature contains many reports that microsporidia can still be a problem despite cART, especially if the CD4 count remains low (37–40). In France, a 2012 study found microsporidia in 8% of HIV patients on cART with a low CD4 count who had diarrhea (37). A study in China in 2017 demonstrated an 11.7% prevalence of microsporidia in HIV-positive patients, and half of these patients were on cART (146). A study from Cameroon in 2016 demonstrated a prevalence of microsporidia of 24.5% in HIV patients on cART (147).
Microsporidiosis has been reported in patients without HIV, including travelers to tropical countries (3.3% to 10%), children with and without diarrhea (1.7% to 17.4%), and the elderly (17.2%) (133, 148–151). A study of school children in Malaysia demonstrated a rate of 27% (143, 152). Both Enterocytozoon bieneusi and Encephalitozoon intestinalis infections have been reported in travelers to and residents of tropical countries (26, 153–156). It has also been observed that latent infection with shedding of spores is common in immunocompetent humans (157). In immunocompetent hosts, while the majority of reported cases of microsporidiosis have manifested as self-limited diarrhea, there are also many reports from Asia of ocular infections with Vittaforma corneae that presented as keratoconjunctivitis (158–160). The incidence of microsporidia in healthy individuals indicates that there is high exposure to microsporidia in the environment (151, 161).
Risk Factors
Microsporidia are considered opportunistic pathogens, and the major risk factor, especially in HIV infection, is depressed cell-mediated immunity (162, 163), with individuals with a CD4 T-lymphocyte count below 50 to 100 cells/mm3 having a high susceptibility to microsporidiosis (164). Other immunosuppressed patients are also at risk, i.e., cases have occurred in patients on chemotherapy, in organ transplant recipients, and in bone marrow graft recipients (165–175). Enterocytozoon bieneusi infections have occurred in patients who have had liver, heart-lung, or kidney transplantation; Encephalitozoon sp. infections have occurred in patients who have had bone marrow, liver, kidney, or pancreas transplantation; Tubulinosema acridophagus has occurred in a patient who had a bone marrow transplantation; and Anncaliia algerae has occurred in a patient who had a lung transplantation (49, 168, 169, 171, 176–183). Chronic bilateral keratoconjunctivitis due to Encephalitozoon sp. has occurred in a patient taking prednisone (20 mg/day) (184).
There have been several cases of the transmission of microsporidiosis due to transplanted organs (185, 186). Three patients were infected by transplanted organs (kidney, kidney, and bilateral lungs) from an Encephalitozoon cuniculi-seropositive donor, and kidney biopsy led to the correct diagnosis in all three cases. The onset of symptoms (fever, renal dysfunction, and encephalopathy) was 7 to 10 weeks posttransplantation, and death in one patient was directly related to failure of the transplanted kidney. The other kidney recipient received 6 months of albendazole therapy and remained healthy afterward (185). In a second cluster, which also involved three patients infected by transplanted organs (kidney, liver, heart/kidney), the index case presented with neurological symptoms, including headache and encephalitis (186). Kidney and urine specimens demonstrated Encephalitozoon cuniculi in these patients, and infection responded to albendazole (186).
The consumption of untreated water has been a risk factor for infection among travelers and residents, contact lens use is a strongly associated risk factor in microsporidian keratoconjunctivitis, and both young and advanced ages have been identified as risk factors for infection (148, 154, 187–189). Water contact has been found to be an independent risk factor for microsporidiosis in some studies (190, 191) but not in others (192, 193). Outbreaks of Vittaforma corneae infection have been associated with hot springs exposure (194) and exposure to soil (195, 196). Encephalitozoon cuniculi spores in water remain viable for 6 days (197).
Modes of Transmission
Microsporidia as an obligate intracellular parasite can infect a wide range of hosts, including all major animal groups and humans; however, the manner of transmission and source of human infection have not been fully elucidated (34, 133, 198). In humans, microsporidiosis is most likely a zoonotic infection. Species of microsporidia that infect humans have been found in wild animals, domestic pets, and food-producing farm animals (140). Transmission routes for infection include ingestion of contaminated food and water, fecal-oral contamination, inhalation of contaminated aerosols, inoculation into open wounds, inoculation into the eye, and sexual transmission (7, 52, 133, 199, 200). Encephalitozoon cuniculi was found to be transmissible via rectal infection in rabbits (201). Encephalitozoon hellem has been demonstrated in respiratory mucosa, the prostate, and the urogenital tract of patients, indicating that respiratory and sexual transmission are possible in humans (202, 203). While congenital transmission of Encephalitozoon cuniculi has not been seen in humans, it has been seen in other mammals, including mice, horses, rabbits, dogs, foxes, alpaca, and squirrel monkeys (204).
Zoonotic Transmission
Many species of microsporidia that can infect humans are also able to infect a wide range of animals, supporting that zoonotic transmission likely occurs. Direct evidence of zoonotic transmission was reported in a child who was infected with Encephalitozoon cuniculi after being exposed to an infected litter of puppies and in a 2-year-old child who was infected with Enterocytozoon bieneusi after being exposed to infected guinea pigs (205, 206). The species that infect humans, particularly Enterocytozoon bieneusi and Encephalitozoon spp., have been reported frequently from a wide range of mammalian hosts, including dogs, pigs, donkeys, cats, goats, rabbits, and cattle (26, 133, 141, 199, 207–210). Molecular epidemiologic studies, based on genotyping of polymorphisms in the ribosomal internal transcriber spacer (ITS) nucleotide sequence, have enabled the identification of Enterocytozoon bieneusi isolates that are unique to specific animals or humans as well as isolates seen in both humans and animals (12–14, 26). Additional molecular epidemiologic studies on Enterocytozoon bieneusi have been performed using multilocus sequence typing (MLST) based on microsatellites, and this technique supports the ITS data (13). Variations in the ITS region also define isolates of Encephalitozoon cuniculi that correlate with their original mammalian hosts and additionally can be used to distinguish isolates of Encephalitozoon hellem (12, 13, 97).
Studies performed on urban pigeons revealed that there is no barrier to microsporidian transmission between park pigeons and humans for Encephalitozoon intestinalis and Encephalitozoon hellem, suggesting the potential zoonotic transmission of microsporidia between birds and humans (211). Visiting urban parks has been identified as a high-risk factor for microsporidiosis in children and the elderly (211). Encephalitozoon cuniculi is often identified in rabbits and dogs (97, 212). The Encephalitozoonidae are found in numerous species of mammals and birds, and in several reports, the onset of human infection was associated with exposure to fowl, budgerigars, lovebirds, or livestock (198, 200, 213–215). Tubulinosema and Anncaliia are pathogens of insects. Nosema and Vittaforma infections are thought to be due to the inoculation of environmental spores of insect pathogens into the cornea (198). Even though zoonotic transmission has not been proven for all species of microsporidia, there appears to be a risk for infection when there is close contact with infected wild, domesticated, and pet animals and also following exposure to water, food, or aerosols contaminated by microsporidian spores (199, 214, 216, 217).
Waterborne Transmission
Waterborne transmission is an important risk factor for intestinal diseases, causing significant morbidity and mortality worldwide (123, 218). Several characteristics of microsporidian spores indicate a high probability that waterborne transmission is important for microsporidiosis. Microsporidian spores have been demonstrated to survive for extended periods of time in water, the small size of spores make filtering processes less efficient, and the oral infectious dose is relatively low (219). Microsporidian spores are released from feces, urine, and animal carcasses of infected animals and can be dispersed into water sources, contaminating potable and recreational water (220, 221). The presence of microsporidia seen in human infections, e.g., Enterocytozoon bieneusi, Encephalitozoon intestinalis, Vittaforma corneae, and Pleistophora sp., has been confirmed in tertiary sewage effluent, surface water, and groundwater (123, 125, 222, 223). Encephalitozoon hellem and Encephalitozoon intestinalis have both been demonstrated to be present in surface waters contaminated by waterfowl feces (224).
Foodborne Transmission
Microsporidia are pathogens of concern for foodborne transmission due to the globalization of food supply, faster transportation of food, modifications in food consumption patterns, and increased consumer travel (225, 226). Transmission of microsporidiosis may occur due to the global food chain industry involving consumption of crustaceans (e.g., clams, lobster, shrimp) and fish (215, 218). Eating raw or insufficiently cooked meat or unwashed vegetables and fruits has been associated with microsporidiosis. People who consumed raw vegetables had a 2-fold increase in infection with microsporidia compared to those who consumed cooked vegetables (150). In a study conducted in agricultural markets in the Central Valley of Cost Rica, microsporidia were detected in cilantro, parsley, lettuce, blackberries, and strawberries (227). A study in 2018 demonstrated the presence of Enterocytozoon bieneusi and Encephalitozoon spp. in vegetable samples from farms that were irrigated with contaminated water resources (228). Eating of undercooked meat has also been associated with microsporidian infection in HIV-infected individuals (133, 191). Encephalitozoon cuniculi has been identified in fermented meat products that are consumed without cooking (229).
Vertical and Horizontal Transmission
Vertical transmission of microsporidia has been observed in insects, nematodes, fish, crustaceans, rabbits, and carnivores (204, 230–234). Vertical transmission of microsporidia can induce a sex ratio distortion, which makes these the only eukaryotic parasites known to change the sex of their hosts (235, 236). Vertical transmission in humans has not been observed (204, 237–239).
Horizontal transmission is usually characterized by a high parasite burden and accompanying pathogenicity (233). As noted previously, the probable routes of horizontal transmission of microsporidia include ingestion of contaminated food or water, fecal-oral transmission, and inhalation of contaminated aerosols (7, 127, 200, 239). Oral transmission of spores by means of contaminated food and water is probably the major pathway for horizontal transmission (198, 240, 241). An early serologic study of homosexual men in Sweden indicating an increased prevalence of antibodies to Encephalitozoon cuniculi in this group suggested that oral-anal contact or anal penetration during sex may contribute to horizontal transmission, and these were subsequently identified as risk factors for intestinal microsporidiosis (132, 191, 242).
HOST-PARASITE INTERACTIONS
Microsporidia possess one of the most fascinating parasite invasion mechanisms, with their environmentally resistant spore and unique polar tube invasion apparatus (243). The polar tube’s function is to transmit infection by penetrating the host cell, acting as the conduit for the parasite sporoplasm and nucleus from the spore into a new host cell (13, 66, 243, 244). Due to the size of the spores (1 to 10 μm), diameter of the polar tube (0.1 to 0.2 μm), and rapidity of both polar tube discharge and sporoplasm passage (<2 s), study of this process has been difficult (59, 245, 246). To this end, despite being described over 100 years ago (57), both invasion and the polar tube are poorly characterized. We do not understand how this structure forms during development, how it functions during eversion, or how the polar tube interacts with the host cell to permit penetration of the sporoplasm and nucleus into the host cell (243). Furthermore, we do not understand the full complement of proteins that make up this structure.
Spore Wall Proteins and Host Cell Interaction
There are three layers in the microsporidian spore wall, a proteinaceous exospore, an electron-lucent endospore, and an underlying plasma membrane (Fig. 2) (54, 56, 78). Chitin is a component of the endospore wall (247) and fibrillar system of the exospore wall. To this end, a chitin deacetylase has been identified as a spore wall protein (SWP) (248, 249). It is believed that several of the proteins in the spore wall, especially in the exospore, participate in binding to the host cell or triggering signaling during invasion (62, 250, 251).
SWP1, a glycine- and serine-rich protein, is developmentally regulated and is present in the exospore wall (67, 86, 252). SWP2, a 150-kDa spore wall glycoprotein, is found in mature spores of Encephalitozoon intestinalis (253). A third spore wall protein (SWP3/Enp2) was identified by proteomic studies and is found in all microsporidia (254, 255). A fourth spore wall protein localized in the exospore wall (SWP4/Enp1) appears to be involved in the adhesion of spores to various carbohydrates (62, 251, 255). Other putative SWPs have been identified in Nosema bombycis (256–260) but have not been confirmed to occur in other microsporidia. Several of these various SWPs are modified by posttranslational glycosylation involving mannosylation (261–263). These modifications are probably important for spore adherence to mucin or host cells during passage of spores in the gastrointestinal tract, facilitating invasion; in support of this concept, exogenous glycosaminoglycans decreased adherence of spores to host cells (62, 251, 255). Using proteomic techniques, SWP5 from Encephalitozoon cuniculi (EcSWP5) and Encephalitozoon hellem (EhSWP5) (264) were also identified. Antibody to EcSWP5 stained parasites that were undergoing cytokinesis but did not stain mature spores, indicating that SWP5 is developmentally regulated (264).
Several SWPs identified from Antonospora locustae, Nosema bombycis, and Encephalitozoon spp. are able to interact with host cells through their ability to bind to host cell surface sulfated glycosaminoglycans (GAGs) as well as heparin-binding motifs (HBM) (62, 251, 253, 257, 265, 266). Encephalitozoon cuniculi EnP1 was the first spore wall protein reported to bind host cell GAGs (Fig. 6) (62). Six spore wall proteins of Nosema bombycis (NbSWP7, NbSWP9, NbSWP11, NbSWP12, NbSWP16, and NbSWP26) have been shown to have heparin binding domains and have been demonstrated to be important for host cell binding and invasion (265, 267–269). The spore wall protein EhSWP1 of Enterocytozoon hepatopenaei also contains a heparin binding domain and can bind to GAGs of host cells during invasion (270). In addition to binding to GAGs, Nosema bombycis spore wall protein NbSWP26 interacts with Bombyx mori turtle-like protein (BmTLP), which is a receptor involved in antigen presentation to the immune system (271). Incubation of host cells with recombinant alpha 5 beta 1 and alpha 3 beta 1 human integrin proteins inhibited host cell infection and spore adherence, indicating that integrins on the host cell surface are involved in invasion and spore adhesion (272). Genomic analysis of Encephalitozoon intestinalis indicates that numerous proteins in this organism possess integrin binding motifs that could participate in interactions of the spore wall and host cell surface integrins (272).
FIG 6.
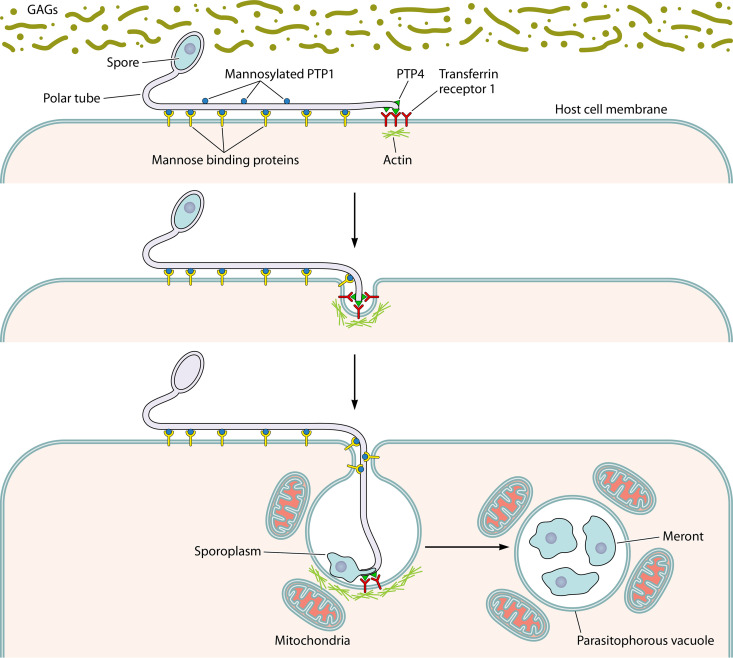
Model of microsporidian (Encephalitozoon) host cell invasion. The spore wall contains spore wall proteins (SWPs) that can interact with glycosaminoglycans (GAGs) and other substances in the mucin layer (green) of the gastrointestinal track. These interactions are probably involved in germination. As the polar tube germinates, the polar tube adheres to the host surface by interactions of polar tube protein 1 (PTP1) with host cell surface mannose binding proteins (MBP). This allows the polar tube to form an invasion synapse by pushing into the host cell membrane. In the formation of the invasion synapse, interactions of PTP1 (and possibly PTP4) with the host cell membrane result in the establishment of a protected microenvironment for the extruded microsporidian sporoplasm which excludes the external environment. Within the invasion synapse, epitopes of polar tube protein 4 (PTP4) that are exposed at the tip of the polar tube interact with transferrin receptor 1 (TfR1), and possibly other host cell-interacting proteins (HCIPs), at the host cell plasma membrane, triggering signaling events. During the final steps of invasion, these various interactions lead to the formation of the invasion vacuole, which can include clathrin-mediated endocytosis as well as the involvement of host cell actin. The sporoplasm (and meront) possesses surface proteins, such as sporoplasm protein 1 (SSP1), which interact with various host cell surface proteins, tethering the sporoplasm to the plasma membrane during invasion and facilitating development of the invasion vacuole. At this early stage of infection, host mitochondria are already located around the invasion vacuole. As the vacuole completes its internalization, the sporoplasm becomes a meront and starts replicating. The meront surface interacts with the invasion vacuole membrane, forming electron-dense membrane structures that allow meront SSP1 to interact with voltage-dependent anion selective channels (VDAC) located on the outer membrane of the mitochondria. The interaction of SSP1 and VDAC appears to play a crucial role in association of host cell mitochondria with the invasion vacuole, facilitating energy acquisition from the host cell by the replicating meronts.
Polar Tube Proteins and Host Cell Interaction
Microsporidia possess a unique, highly specialized invasion apparatus termed the polar tube (Fig. 3). During infection, the polar tube is rapidly extruded from the spore under appropriate environmental stimulation and transports the nucleus and sporoplasm into the host cell (59, 245, 246). There are six polar tube proteins (PTP1 to PTP6) that have been identified from microsporidia, and several of these PTPs are involved in host cell interactions (Fig. 6) (243, 273). PTP1 is a key component of the polar tube (274–276) and is a heavily mannosylated protein with a substantial number of O-linked mannosylation modification sites (262). Mannose pretreatment of host cells decreased Encephalitozoon hellem infection, consistent with an interaction between the glycoepitopes of PTP1 and host cell mannose binding molecules such as dectin-2, mannose binding lectin (MBL), and C-type lectin receptors (262, 277). Proteomic and immunologic approaches resulted in the identification of PTP2 and PTP3 (276, 278). PTP1, PTP2, and PTP3 can be purified as a complex using cross-linking agents (278, 279). Yeast two-hybrid methods confirmed an interaction of PTP1, PTP2, and PTP3 and allowed mapping of PTP1 subdomain interactions (279). Interestingly, PTP1 interacts with itself and other PTPs at both its C- and N-terminal subdomains, but its central repetitive core does not interact with other PTPs (279), consistent with the hypothesis that the PTP1 central region is not involved in protein-protein interactions that are needed to form the polar tube. A unique epitope of Encephalitozoon hellem PTP4 was found to specifically localize to the tip of the polar tube and was shown to bind to the host transferrin receptor 1 (TfR1), which is one of the key receptor proteins in the clathrin-mediated endocytosis pathway (280). Blocking this interaction with recombinant proteins or antibodies specific to either protein reduced host cell invasion, indicating that PTP4 and TfR1 are crucial for microsporidian invasion (280). PTP4 and PTP5 homologs are present in the genomes of other microsporidia such as Anncaliia algerae, Encephalitozoon intestinalis, and Encephalitozoon cuniculi. Like the ptp1 and ptp2 genes (276) that occur in close proximity to each other in the genome, the ptp4 and ptp5 genes are also found in close proximity to each other within the genome, suggesting that they may have been linked in either evolution or expression (13, 60). More recently, a sixth polar tube protein (PTP6) was identified from the insect microsporidian Nosema bombycis. NbPTP6 is rich in histidine and serine and has multiple glycosylation sites (273). This protein has also been identified by proteomic studies of the composition of the polar tube in Encephalitozoon hellem (L. M. Weiss, unpublished data). NbPTP6 has been shown to bind to the host cell surface, suggesting a potential role in the process of polar tube interaction with host cell (273).
Sporoplasm Proteins and Host Cell Interaction
It had been postulated that the polar tube functions like a hypodermic needle in penetration of the host cell (13, 66, 243); however, electron micrographs of the polar tube interaction with its host cell demonstrated that an invagination occurs at the site of this interaction (13, 243). If spores are germinated in medium and the sporoplasm emerges from the polar tube tip, it is very delicate and swells and breaks, suggesting that survival of the emerging sporoplasm probably requires a protected environment (13). It is thought that the polar tube invaginates the host cell membrane, forming a microenvironment (the invasion synapse) in which final penetration occurs (Fig. 6). During infection, the sporoplasm is discharged from the spore through the polar tube into this invasion synapse, which is formed by the polar tube and host cell plasma membrane (243). An ultrastructural study of Anncaliia algerae demonstrated that the extracellular discharged sporoplasm tightly abutted the host plasmalemma and appeared to be in the process of being incorporated into the host cytoplasm by phagocytosis and/or endocytosis (281).
A sporoplasm surface protein (SSP1) was identified from Encephalitozoon hellem, and EhSSP1 was shown to bind to the host cell surface at the site where the polar tube invaginated the host cell membrane during invasion (282). Upon invasion, the sporoplasm enters the proliferative phase of the microsporidian life cycle, which is marked by extensive multiplication via merogony (243, 283). Host cell voltage-dependent anion channels (VDAC1, VDAC2, and VDAC3) interact with EhSSP1 and EhSSP1 colocalized with host mitochondria and the microsporidian parasitophorous vacuoles in infected cells. (282). Electron microscopy demonstrated that the outer mitochondrial membrane interacted with meronts and the parasitophorous vacuole membrane, that mitochondria clustered around meronts, and that VDACs were concentrated at the interface of the mitochondria and parasites (282). RNA interference (RNAi) knockdown of VDAC1, VDAC2, and VDAC3 in host cells resulted in a significant decrease in the number and size of the parasitophorous vacuoles and a decrease in mitochondrial parasitophorous vacuole association (282). Microsporidia lack mitochondria and are reliant on the host for ATP generation, suggesting that the interaction between the microsporidian intracellular sporoplasm and the parasitophorous vacuole is crucial for the energy transportation from the host (8, 284, 285). The interaction of EhSSP1 with VDAC probably plays an important part in energy acquisition by microsporidia via its role in the association of mitochondria with the parasitophorous vacuole (Fig. 6).
An ATP-binding cassette (ABC) transporter subfamily protein NoboABCG1.1 identified in Nosema bombycis was found on the sporoplasm, meront, and mature spore. This ABC transporter has been shown to be important for microsporidian nutrient acquisition from its host cells (286). The family of nucleotide transporters (NTTs) which localize to the surface of the sporoplasm membrane of Encephalitozoon cuniculi and Trachipleistophora hominis has been demonstrated to transport ATP from the host cytoplasm (287). The major facilitator superfamily protein, which localizes to the proliferative meronts of microsporidia, can also uptake ATP and GTP (288). In Encephalitozoon spp., host mitochondria are in direct contact with the parasitophorous vacuole, which is thought to facilitate the energy transport into meronts (289, 290).
IMMUNOLOGY
There are limited data for humans on the immune response to microsporidiosis. A significant humoral response clearly occurs during infection, and antibodies are formed that react with both the spore wall and polar tube. Immune defects in cell-mediated immunity are associated with microsporidiosis, as demonstrated by the prevalence of this infection in patients with AIDS or transplantation. Encephalitozoon cuniculi infection in mammals results in chronic infection with persistently high antibody titers and ongoing inflammation, as demonstrated by the occurrence of persistent encephalitis and chronic renal disease in rabbits. Latent infection occurs, allowing relapse, and albendazole treatment cannot eliminate latency in immunodeficient mice (291, 292). The protective immune response to microsporidia is dependent on cell-mediated immunity. Adoptive transfer of sensitized syngeneic T-enriched spleen cells protects athymic or SCID mice against lethal Encephalitozoon cuniculi infection but not naive lymphocytes or hyperimmune serum (293, 294). Studies using macrophages have demonstrated that reactive nitrogen and oxygen species or iron sequestration can contribute to the ability of these cells to control Encephalitozoon cuniculi infection (295). Maternal antibodies protect newborn rabbits from infection with Encephalitozoon cuniculi during the first 2 weeks of life (296). IgM antibodies against PTP1 in normal human serum may play a role in preventing infection with E. cuniculi (297). These data indicate that antibodies almost certainly have a role in limiting infection in the host, although they are not sufficient by themselves to prevent mortality or cure infection. Studies with both Encephalitozoon intestinalis and Encephalitozoon cuniculi have shown that gamma interferon (IFN-γ) knockout mice cannot clear infection (298). Treatment of mice infected with Encephalitozoon cuniculi with neutralizing antibody to IFN-γ or interleukin 12 (IL-12) results in increased mortality (299). In p40 knockout mice, which are unable to produce IL-12 and have impaired CD8+ cell function, Encephalitozoon cuniculi infection results in death (300, 301).
The role of individual T-cell subtypes in disseminated microsporidiosis following intraperitoneal infection has been examined using murine models (301). The cytotoxic T-cell response is a key factor in the immune response to Encephalitozoon cuniculi. Mice deficient in CD8+ cells succumb to infection, but mice deficient in CD4+ cells are similar to wild-type mice. The protective effect of CD8+ T cells is mediated by their ability to produce cytokines and to reduce microsporidian tissue burden. Studies have demonstrated that the protection provided by CD8+ T cells is through the perforin pathway, and mice lacking perforin die when infected with Encephalitozoon cuniculi (301). The route of infection is important in cases where immune cells are involved in a protective response. For example, if mice are infected orally with microsporidia, both the CD4+ and CD8+ T cells are involved in the protective immune response generated by the gastrointestinal tract (302, 303). The CD8+ αβ population is a critical cell subset for protective immunity (304). IFN-γ production by dendritic cells is important for priming the gut intraepithelial lymphocyte response following oral infection with Encephalitozoon cuniculi (304). Dendritic cells play a key role in the immune response to microsporidia (305) by facilitating T-cell responses through recognition of the pattern recognition receptors expressed by these pathogens (e.g., TLR4 [306] and TLR2 [307]). Observations indicate that aging is a risk factor for microsporidiosis; consistent with this observation, plasmacytoid dendritic cells in older mice infected with Encephalitozoon cuniculi have been shown to downregulate CD8+ T-cell responses by inhibiting the maturation of normal dendritic cells (308).
CLINICAL MANIFESTATIONS IN HUMAN INFECTIONS
Microsporidia can cause localized or disseminated infection in humans (309). The microsporidian genera associated with human infection and related clinical manifestations are summarized in Table 2. Human microsporidiosis represents a significant emerging opportunistic disease: once thought to be restricted to immunocompromised patients, infections in immunocompetent individuals are also known to occur (161, 310). The clinical manifestations of microsporidiosis are diverse, varying according to the mode of transmission, the causal species, and the underlying host immune status and response (Fig. 7 and 8) (1, 309). Enterocytozoon bieneusi and Encephalitozoon intestinalis are the most commonly detected microsporidia in human infection, and chronic diarrhea is the most frequent clinical syndrome. Although most reported cases of microsporidiosis involve diarrhea, the gamut of diseases caused by these organisms includes myositis, kidney and urogenital infection, cholangitis, ascites, sinusitis, hepatitis, keratoconjunctivitis, and disseminated infection, as well as asymptomatic infection (1, 7, 13, 162, 311) (Fig. 7).
FIG 7.
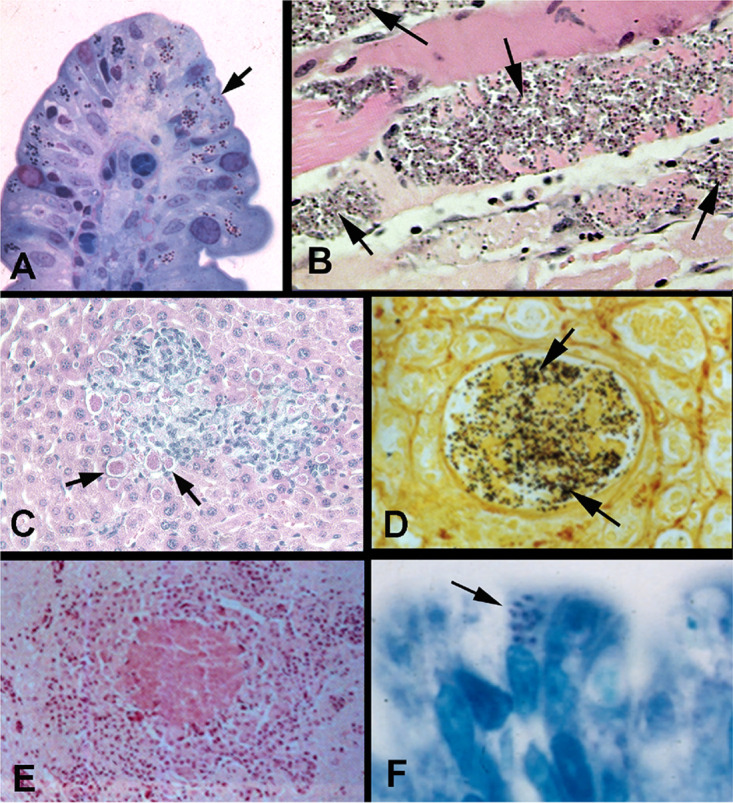
Light microscopy of biopsy specimens from patients with microsporidiosis. (A) Methylene blue-azure II-fuchsin stain of an intestinal biopsy specimen from a patient with Encephalitozoon intestinalis (arrow) infection. (Reproduced from reference 454 with permission.) (B) Hematoxylin and eosin stain of muscle tissue from a patient with rheumatoid arthritis treated with antibody to TNF-α demonstrating Anncaliia algerae (arrow) myositis. (C) Tissue chromotrope stain of liver biopsy specimen from an immunodeficient mouse infected with Encephalitozoon cuniculi (arrows). (D) Steiner stain of a renal biopsy specimen demonstrating Encephalitozoon hellem (black spores) within the lumen of the renal tubule. (Reproduced from reference 386 with permission.) (E) Hematoxylin and eosin stain of brain tissue from a rabbit with torticollis, demonstrating a microgranuloma with central necrosis due to Encephalitozoon cuniculi infection. No spores are seen in this image. (Reproduced from reference 386 with permission.) (F) Light micrograph of intestinal villus biopsy specimen (plastic section) stained with methylene blue-azure II-fuchsin from a patient with AIDS and Enterocytozoon bieneusi infection. The arrow points to microsporidia within cells. These spores were present only on the apical epithelial surface; no spores were present on the basal surface or in the lamina propria. (Reproduced from reference 454 with permission.)
FIG 8.
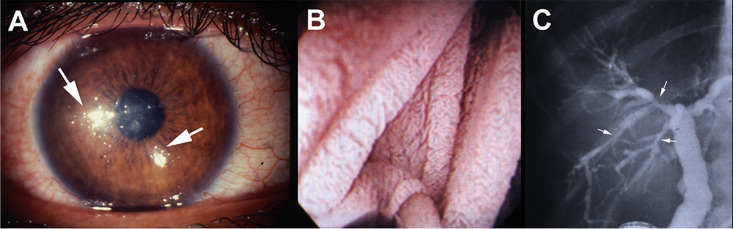
Clinical images from patients with microsporidiosis. (A) Encephalitozoon hellem keratoconjunctivitis demonstrating punctate corneal lesions (white arrows). (B) Endoscopy of jejunal mucosa of a patient with gastrointestinal microsporidiosis due to Enterocytozoon bieneusi demonstrating fusion of the villi. (Reproduced from reference 454 with permission.) (C) Endoscopic retrograde cholangiogram (ERCP) of a patient with HIV infection (AIDS) and sclerosing cholangitis due to Enterocytozoon bieneusi infection. The ERCP demonstrates diffuse dilations of the common bile duct with irregular walls, plus areas of narrowing and dilation (arrows) of the intrahepatic bile ducts. (Reproduced from reference 454 with permission.)
Enterocytozoon bieneusi
In 1985, the first case of Enterocytozoon bieneusi infection was reported in a Haitian AIDS patient with diarrhea and wasting (44). Enterocytozoon bieneusi infection in non-HIV-infected individuals was first reported in travelers to the tropics, and the microsporidium-associated diarrhea in those immunocompetent patients tended to be self-limiting (154). Enterocytozoon bieneusi is the most commonly seen microsporidium causing human infection, being etiologic in 90% of cases of chronic diarrhea in AIDS patients (33, 161). Infection does not produce active enteritis or ulceration, although it does result in various degrees of crypt hyperplasia and villous blunting (Fig. 8B). Enterocytozoon bieneusi is found at the apical surface of small intestinal enterocytes, biliary tract epithelial cells, and pancreatic epithelial cells. Spores are essentially not seen on the basal surface of these cells or in the lamina propria (1, 33, 128, 219, 312) (Fig. 7F and 9A). Infection with Enterocytozoon bieneusi usually presents with chronic diarrhea (which can last for years [313]), anorexia, weight loss, and bloating without associated fever. Infection frequently occurs in AIDS patients with CD4+ counts lower than 50 cells/μl. On a daily basis, 3 to 10 bowel movements which are loose to watery and do not contain blood or fecal leukocytes occur (314). Malabsorption is usually evident, is often worsened by food ingestion, and can result in weight loss and a wasting syndrome. This pathogen can also invade the cholangioepithelium (315), leading to sclerosing cholangitis (316). When this occurs, imaging studies such as endoscopic retrograde cholangiopancreatography (ERCP), computerized axial tomography, abdominal ultrasonography, or endoscopic ultrasonography can demonstrate irregularities of the bile duct wall (Fig. 8C), dilated biliary ducts, and gallbladder abnormalities, such as distention, thickening, or the presence of sludge. Encephalitozoon intestinalis can also cause biliary infection (316). Reports of systemic dissemination of Enterocytozoon bieneusi are rare; respiratory involvement associated with chronic diarrhea, wheezing, dyspnea, persistent cough, and chest radiographs showing interstitial infiltrates have been reported (317, 318).
FIG 9.
Transmission electron micrographs of biopsy specimens from patients with microsporidiosis. (A) Duodenal epithelium from a patient with AIDS and Enterocytozoon bieneusi infection demonstrating proliferating stages (P) and late sporogonial plasmodia (Sp). The arrow points to a sloughing enterocyte containing mature spores. (Reproduced from reference 386 with permission.) (B) Intestinal biopsy specimen from a patient with AIDS and Encephalitozoon intestinalis infection demonstrating spores within vacuoles containing a fibrillar matrix. (Reproduced from reference 386 with permission.) (C) Enterocytozoon bieneusi spore demonstrating the characteristic finding of two rows of three cross sections of the polar tube (indicated by an arrow). (Reproduced from reference 386 with permission.) (D) Muscle biopsy specimen from a patient with rheumatoid arthritis on an anti-TNF-α antibody who presented with myositis. Proliferative forms are seen in the biopsy specimen with a characteristic diplokaryotic nucleus (N). (E) Conjunctival biopsy specimen from a patient with keratoconjunctivitis due to Encephalitozoon hellem demonstrating characteristic spores (arrowheads) with a single row of six cross sections of the polar tube.
Encephalitozoonidae
The Encephalitozoon species, including Encephalitozoon intestinalis, Encephalitozoon hellem, and Encephalitozoon cuniculi, are widely distributed in mammals and have been identified in both immunocompetent and immunocompromised humans (128, 310). These microsporidia have the capacity to disseminate widely in their hosts and can involve almost any organ system during infection (Fig. 7A, C, D, and E) (7, 13, 33, 319). The Encephalitozoonidae have been described as etiologic in cases of sinusitis, bronchiolitis, nephritis, keratitis, peritonitis, hepatitis, fulminant hepatic failure, gastroenteritis, cerebritis, cystitis, urethritis, prostatitis, nodular skin lesions, and disseminated infection (7, 13, 33, 99, 320–327). Infection is most commonly seen in immunocompromised patients (e.g., patients with HIV infection, especially in patients with CD4 counts of <50/mm3, transplant recipients, and those receiving immunomodulatory therapy) (49, 169–171, 219, 328–330). Interstitial nephritis due to microsporidiosis, similar to what has been described in rabbits, occurs in AIDS patients and has also been seen in patients with renal transplants (169, 171, 178, 331). Encephalitozoon species have also been identified in immunocompetent travelers with a symptom of self-limiting diarrhea (154).
About 10% of cases of diarrhea and wasting in immunodeficient patients with AIDS has been due to Encephalitozoon intestinalis (116) (Fig. 7A and 9B). Infection can result in areas of bowel necrosis and a presentation similar to that of an acute abdomen (322) with perforation of the intestine and peritonitis (332). This pathogen has also been reported to cause cholangitis (333), keratoconjunctivitis, osteomyelitis of the mandible (334), renal failure, upper respiratory infection, and disseminated infection (118, 335).
Encephalitozoon cuniculi infection has been reported to cause hepatitis (336), peritonitis (323), hepatic failure (320), renal insufficiency, disseminated disease with fever (324), intractable cough (94), and endocarditis (337) (Fig. 7C). Encephalitozoon cuniculi has been found in the periprosthetic tissue of several patients in Poland who presented with hip implant loosening, requiring hip replacement, despite an absence of symptoms associated with infection (338). There are many reports of disseminated infections in transplant recipients. The diagnosis is often delayed, perhaps because of the lack of specificity of symptoms and a low index of suspicion. Granulomatous encephalitis due to this pathogen has been reported in mammals (rabbits) since 1922, and infection in humans has been reported to cause encephalitis and seizures in AIDS patients (99, 324) (Fig. 7E). Encephalitozoon infection was reported in a 3-year-old boy with seizures and hepatomegaly, as confirmed by the presence of IgG and IgM antibodies that reacted with Encephalitozoon cuniculi (339). Infection with Encephalitozoon sp. with recurrent fever has also been reported in a 9-year-old Japanese boy with spastic convulsions, headache, and vomiting (34). Encephalitozoon cuniculi and Streptococcus intermedius were isolated together from a brain abscess in a patient with diabetes (340).
Encephalitozoon hellem infections have been reported in cases of keratoconjunctivitis, nephritis, pneumonia, bronchitis, and disseminated disease with renal failure (112, 203, 341). Encephalitozoon hellem can also infect the nasal epithelium, causing sinusitis in AIDS patients (342). The majority of reported ocular infections presenting as punctate keratoconjunctivitis are caused by Encephalitozoonidae (160, 343–345), and of these, the majority are due to Encephalitozoon hellem, including three cases originally classified as Encephalitozoon cuniculi (346, 347) (Fig. 8A and 9E). The few reported cases not due to Encephalitozoon hellem were caused by Encephalitozoon sp. or Encephalitozoon intestinalis. Infection presents as bilateral coarse punctate epithelial keratopathy limited to the superficial epithelium of the cornea and conjunctival inflammation. Clinically, infection may be bilateral or unilateral and can present with redness, foreign body sensation, photophobia, excessive tearing, blurred vision, and changes in visual acuity (13, 347). Disseminated disease is often present in patients with microsporidian keratoconjunctivitis; therefore, microsporidian spores can be found in urine samples in patients with this ocular infection.
Anncaliia spp.
There are three species in the Anncaliia genus that have been reported in humans: Anncaliia algerae, Anncaliia connori, and Anncaliia vesicularum (Table 2). Anncaliia algerae was first known as Nosema algerae and then as Brachiola algerae before being moved to the genus Anncaliia (43). Anncaliia algerae was originally recognized as a pathogen of insects but has now been seen in human infections (Fig. 7B and 9D) (348–350). Infection with Anncaliia algerae has been reported to cause severe myositis in patients getting immunosuppressive medication for solid-organ transplantation and for rheumatoid arthritis and in a patient with graft-versus-host disease after hemopoietic stem cell transplantation (182, 350–354). Anncaliia algerae has also been reported to cause skin abscesses and infection of the false vocal cord in patients receiving chemotherapy for hematologic malignancies as well as myositis and keratitis in a man with no significant medical history (182, 350–354). Anncaliia algerae infection of the cornea has also been reported (355, 356), and this infection has been associated with endophthalmitis (356). Anncaliia vesicularum (previously called Brachiola vesicularum) has been reported to cause myositis in a patient with AIDS, and Anncaliia connori (previously called Nosema connori) was reported in a case of disseminated infection in an infant who had thymic dysplasia and malabsorption syndrome (42, 357, 358).
Vittaforma corneae
Vittaforma corneae was first described as Nosema corneum in a stromal biopsy specimen from a patient without HIV, and it is now recognized to be responsible for keratoconjunctivitis in healthy persons (with over 300 cases being reported associated with water or soil exposure) and HIV-infected patients (41, 100, 359–363). An outbreak of Vittaforma corneae has been associated with exposure to soil during a rugby tournament in Singapore (159, 196). Vittaforma corneae has also been seen in the urine in a patient with AIDS, indicating that it is capable of dissemination in immunocompromised hosts (100). This pathogen has also been reported as a cause of enteritis in both HIV-positive and HIV-negative patients from Portugal (364). Stromal keratitis due to microsporidia occurs less often than keratoconjunctivitis, is typically identified in immunocompetent individuals, and has been caused by infection with Vittaforma corneae, Nosema ocularum, and Trachipleistophora species (365). A case of urinary tract infection with prostatitis due to Vittaforma corneae has been reported in a patient with AIDS (366).
Other Species of Microsporidia
The genus Trachipleistophora was established for a microsporidium isolated from the skeletal muscle of an AIDS patient, and subsequently, more cases of myositis caused by Trachipleistophora spp. have been reported (47, 367–370). Disseminated infection due to Trachipleistophora hominis has been described in several patients with AIDS (47), and infection has been reported to cause myositis, keratoconjunctivitis, and sinusitis. Trachipleistophora anthropopthera infection has presented with myositis, encephalitis, and keratoconjunctivitis (48, 371, 372). Pleistophora sp. and Pleistophora ronneafiei infections presenting as myositis have occurred in patients with AIDS (368, 373–375). The presentation of Pleistophora myositis included myalgias, elevated serum creatine phosphokinase (CPK) and aldolase levels, weakness, and abnormal electromyograms consistent with an inflammatory myopathy. Pleistophora spp. have also been reported as a cause of myositis in patients who are HIV negative (368, 373). There are several cases of Tubulinosema acridophagus being reported to cause myositis and disseminated disease in immunosuppressed patients (49, 50, 376). Endoreticulatus spp. were reported from a patient with myositis with difficulty swallowing, muscle pain, and necrotizing granulomatous inflammation in the muscle tissue (1). Microsporidium ceylonensis and Microsporidium africanum were identified from an 11-year-old Tamil boy and a woman from Botswana, respectively, causing corneal ulcers and deep corneal stroma infection (the genus Microsporidium is used for unclassified microsporidia) (349). Nosema ocularum (377), Nosema corneum (now V. corneae) and Trachipleistophora hominis have been described as the causes of corneal stromal microsporidiosis in humans (41, 92, 362, 377, 378).
DIAGNOSIS
During the past 20 years, with the recognition of microsporidia as opportunistic pathogens, the diagnostic procedures for their detection has dramatically improved (379). With these improved techniques, there has been increasing recognition of microsporidial infection in immunocompetent humans (151, 161, 343, 351). Species-level identification of microsporidia requires ultrastructural examination (e.g., electron microscopy) or molecular techniques (e.g., a species-specific PCR assay). Serologic tests for microsporidiosis have been developed for research purposes and have been used to estimate prevalence of infection in epidemiologic studies. Serologic tests, however, are not useful for the diagnosis of active microsporidiosis. Isolation of microsporidia using tissue culture has not been useful for routine clinical diagnosis; however, it should be pursued if a novel microsporidium is identified in a clinical sample.
Light Microscopy
Examination of stool specimens by light microscopy is the standard technique for diagnosing gastrointestinal microsporidiosis. Since renal involvement with shedding of spores in the urine is common in microsporidia that disseminate, urine specimens should be obtained whenever the diagnosis of microsporidiosis is being considered. This has therapeutic implications, as microsporidia that disseminate (e.g., Encephalitozoonidae) are sensitive to albendazole, whereas those that do not disseminate (e.g., Enterocytozoon bieneusi) are resistant.
There are several methods available for the detection of microsporidia (219, 380) (Fig. 10). Examination of body fluids (e.g., urine, respiratory specimens, duodenal aspirates, cerebrospinal fluid) or histologic examination of biopsy specimens by light microscopy is one of the most common ways for the diagnosis of microsporidial infection, but this method cannot confirm the species of microsporidia. In contrast to stool, it is easier to identify microsporidian spores in body fluids because of the absence of bacteria and debris that could be confused with microsporidian spores. Demonstration of microsporidia by light microscopy is accomplished by the use of stains that produce differential contrast between the spores of microsporidia and the cells and debris in clinical samples. As spores are 1 to 3 μm in size magnification, a ×60 to ×100 objective is required for their visualization. The proteinaceous exospore and the chitinous endospore of microsporidia make it possible to stain spores by different methods, such as Giemsa, Gram, and trichrome stains and fluorescent dyes (i.e., Uvitex 2B and calcofluor white). Giemsa and Gram stains, however, while useful in histology, are not useful for stool examination, as these methods cannot distinguish between microsporidia and other similarly sized elements present in stool (381). Chromotrope 2R stains and fluorescent stains have markedly improved detection of microsporidia in body fluids and stool samples (219) (Fig. 10). The chromotrope 2R staining technique described by Weber et al. is based on a modification of routine trichrome staining using a 10-fold-higher concentration of chromotrope 2R and a longer staining time (382) (Fig. 10A, B, and E). Modifications of this staining method, which are preferred by some laboratories, have been described by Ryan and coworkers (383) (using aniline blue in place of fast green) and by Kokoskin and colleagues (384) (using a higher temperature). Spores stained by chromotrope 2R, against a green (Weber stain) or blue (Ryan stain) background, appear as ovoid, light pink structures that are usually 1 to 3 μm in size, and these spores display a belt-like stripe girding them diagonally and equatorially (382) (Fig. 10B). The chromotrope 2R stains are time-consuming procedures, and the hot Gram-chromotrope technique which stains microsporidian spores dark violet in a much shorter period time is preferred by some laboratories (385) (Fig. 10D). Fluorescent stains that bind to chitin in the spore wall of microsporidia, such as calcofluor white M2R, Uvitex 2B, Fungi-Fluor, Fungiqual A, Cellufluor, and other chemofluorescent optical brighteners, have also been useful for visualizing microsporidian spores in various clinical samples (386) (Fig. 10C). These stains are easy and quick, but these stains also stain fungi and other fecal elements (387). Microsporidia can be distinguished from yeast cells by their size and lack of budding. Both chromotrope 2R and chemifluorescent brightening stains have demonstrated excellent sensitivity (100%) and specificity (100% for chromotrope 2R and 90 to 100% for chemifluorescent stains) (387–389). The limit of detecting microsporidia by these techniques appears to be 50,000 organisms/ml. Microsporidian spores in food can give a false-positive result; however, despite the fact that microsporidia are common in the environment, this has not been a significant problem in diagnosis. Neither the chromotrope nor chemifluorescent stain provide microsporidian species-specific information.
FIG 10.
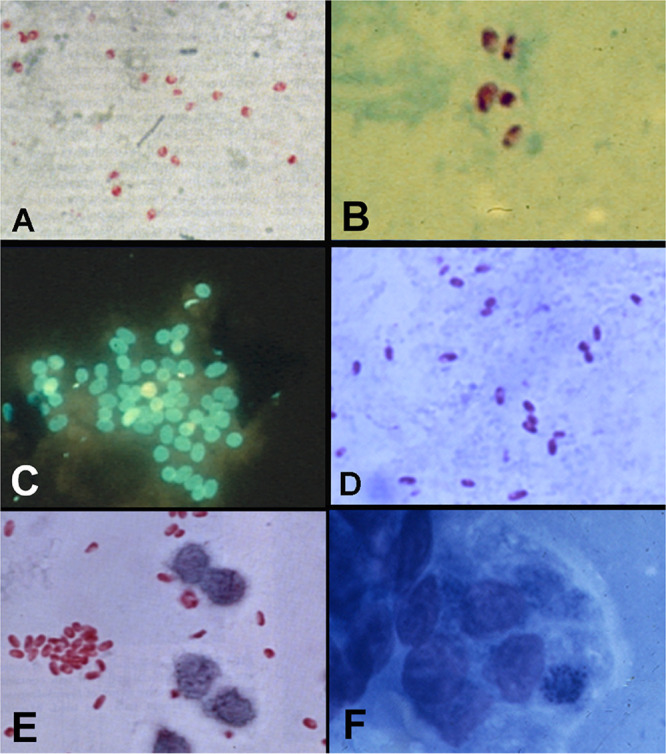
Demonstration of microsporidia in stool, urine, and other clinical specimens. (A and B) Chromotrope stains of stool specimens from patients with AIDS and diarrhea demonstrating spores of Enterocytozoon bieneusi measuring 0.7 to 1.0 by 1.1 to 1.6 μm. Spores have a pink to reddish hue with this stain. Stained spores can have a safety pin appearance as well. (Reproduced from reference 386 with permission.) (C) Conjunctival scraping from a patient with Encephalitozoon hellem keratoconjunctivitis stained with a chemifluorescent brightening agent (that stains chitin) demonstrating microsporidian spores. (D) Stool specimen from a patient with AIDS and Encephalitozoon intestinalis infection stained with a quick hot Gram-chromotrope stain demonstrating spores of 1.0 to 1.2 by 2.0 to 2.5 μm. The spores have a violet hue with this stain. (Reproduced from reference 455 with permission.) (E) Chromotrope stain of urine sediment from a patient with HIV infection who had a disseminated Encephalitozoon cuniculi infection demonstrating pink- to red-colored spores that are 1.0 to 1.5 by 2.0 to 3.0 μm. (Courtesy of Elizabeth Didier; reproduced with permission.) (F) Methylene blue-azure II-fuchsin stain of a touch preparation of an intestinal biopsy specimen (obtained by endoscopy) from a patient with intestinal Enterocytozoon bieneusi infection demonstrating intracellular spores.
Histologically, microsporidian spores can be identified in routine fixed tissue by using several standard stains, including a modified tissue chromotrope 2R, tissue Gram stain (Brown-Hopps or Brown-Brenn), or Luna stain (390) (Fig. 7). If microsporidia are suspected, then a sample of the biopsy or autopsy material should also be put into electron microscopy fixative, as the definitive classification of a microsporidian species depends on ultrastructural information. Since microsporidian infections often involve mucosa or epithelium, cytological preparations are especially useful for diagnosis. In cases of microsporidian keratitis, microscopic examination material obtained by gently rubbing the conjunctiva and cornea with a tissue swab will demonstrate microsporidia within the epithelial cells (Fig. 10C). Monoclonal antibodies to Encephalitozoon hellem (121) Encephalitozoon intestinalis (380), and Enterocytozoon bieneusi (391, 392) have been developed and used for immunofluorescence or other immunostaining techniques to detect microsporidia in tissue sections, stool, and other body fluids. Commercial kits for the detection of microsporidia in environmental samples that utilize antibodies to Encephalitozoonidae and Enterocytozoon bieneusi are available.
Electron Microscopy
Electron microscopy is the “gold standard” for diagnostic confirmation and species identification of microsporidia (386). While mature spores seen in body fluids or stool samples can provide important ultrastructural data for species identification, definitive identification requires examination of the developmental life cycle seen in tissue samples (219) (Fig. 9). In general, it is not used for routine diagnosis, as electron microscopy is less sensitive than routine microscopy or molecular techniques, requires specialized equipment, is very labor-intensive, and is time-consuming (393). Molecular techniques can also be used to identify the exact species of microsporidia that are known to cause human infections and are much more sensitive for diagnosis (394). Electron microscopy is, however, very important when a novel microsporidium is suspected as causing an infection, as along with sequencing the rRNA gene of such organisms, the ultrastructural data provided by electron microscopy enables classification of such novel microsporidia.
Molecular Biology-Based Techniques
The successfully sequencing of various microsporidial genomes has led to the application of molecular biology-based techniques for the diagnosis of microsporidial infections in humans (219). Weiss and Vossbrinck (25) and Ghosh and Weiss (395) have reviewed molecular diagnostic tests for microsporidiosis. The detection of microsporidia using molecular-based methods such as PCR and in situ hybridization provides sensitive and specific methods for species identification (219, 396). PCR-based tests using single and nested primer pairs of the small subunit rRNA (SSU rRNA), large subunit rRNA (LSU rRNA), and the intergenic spacer region have been described for the detection of different species of microsporidia in humans (219, 397). PCR-based tests have been used with both tissue biopsy specimens and body fluids, including stool samples (219, 395, 398–400). Nucleic acids are isolated from clinical specimens by mechanical disruption of the microsporidial spores in clinical specimens combined with the use of lyticase and/or chitinase to increase the efficiency of DNA extraction (219, 401). PCR-based methods have been shown to be able to detect 100 to 1,000 spores/ml in clinical samples (402, 403). In a study comparing real-time PCR and traditional microscopy for three species of microsporidia, the real-time PCR method was found to be three times more likely to detect the presence of spores in samples (402). In situ hybridization using rRNA probes has also been used to identify microsporidia to the species level in clinical samples and can be useful in examining tissue specimens (396, 404, 405). However, in situ hybridization is not widely used for routine clinical diagnosis, as it is less sensitive than PCR, is time-consuming, and requires significant expertise to perform (395).
THERAPY
Microsporidiosis is often seen in immunocompromised hosts, especially in patients with advanced AIDS and CD4+ cell counts lower than 50/μl. Improved immune function can result in a clinical response in patients with gastrointestinal microsporidiosis leading to elimination of the organism and normalization of the intestinal architecture (406–410) (Table 4). Based on these observations, the institution of effective cART is important as part of the primary treatment of microsporidiosis in the setting of AIDS. Immune reconstitution syndrome (IRIS), while reported, has not generally been a problem in the setting of microsporidiosis (411). Interestingly, relapse of microsporidiosis can occur if there is a decline in immune function and falling CD4+ counts, suggesting that latent infection is occurring. To this end, latent infection has been observed in immunodeficient mice, where albendazole treatment has not been able to eliminate microsporidia and relapse is seen. Costa and Weiss (412) have reviewed the drugs used for the treatment of microsporidiosis in humans and animals.
TABLE 4.
Therapy of microsporidiosis
| Organism | Therapy | Dosagea |
|---|---|---|
| All microsporidia | Immune reconstitution (cART in HIV with a goal of a CD4 count greater than 100 cells/μl) is associated with resolution of symptoms of enteric microsporidiosis. Severe dehydration, malnutrition, and wasting syndrome should be managed by fluid support and nutritional supplementation. Diarrhea can be controlled by antimotility agents as needed. |
|
| Enterocytozoon bieneusi | No current effective commercial therapy. Fumagillinb (oral form has been effective in a clinical trial and is available on expanded access). Alternatives: Nitazoxanide at 1,000 mg BID with food for 60 days has shown efficacy in anecdotal cases. Albendazole has some limited efficacy; however, the sequence of Enterocytozoon bieneusi contains amino acids associated with resistance to this class of drugs. In general, medications have also been less effective in patients with low CD4 counts. |
20 mg TID (i.e., 60 mg/day) |
| Encephalitozoonidae (systemic infections); E. cuniculi, E. hellem, and E. intestinalis | Albendazole | 400 mg BID |
| Encephalitozoonidae keratoconjunctivitis | Fumagillin solution (70 μg/ml)c. Patients may also need albendazole if systemic infection is present. |
2 drops every 2 h for 4 days and then 2 drops 4 times a day |
| Vittaforma corneae keratoconjunctivitis | No defined therapy. Fumagillin solution has had activity. Topical fluoroquinolones and itraconazole have been used in case reports. The sequence of V. cornea tubulin contains amino acids associated with resistance to albendazole and related agents in this class of drugs. |
|
| Trachipleistophora hominis | Albendazole | 400 mg BID |
| Anncaliia spp. | Albendazole In a reported case of Anncaliia vesicularum, itraconazole (400 mg BID) was added to albendazole |
400 mg BID |
| Tubulinosema spp. | No defined therapy. There was no response to albendazole in published cases. Fumagillin is a reasonable therapeutic choice. | |
| Enoreticulatus spp. | Albendazole | 400 mg BID |
The duration of treatment for microsporidiosis has not been established. Relapse of infection has occurred on stopping therapy. Patients should be maintained on treatment for at least 4 weeks. If immunosuppression persists (e.g., CD4 count less than 200 cells/μl), then consideration should be given to continuing therapy. TID, three times a day; BID, twice a day.
Fumagillin is available from Sanofi in France as Flisint 20-mg capsules and has been obtained for patients in the United States (contact https://app.smartsheet.com/b/form/4dea2fbff8c449f095ce5f335b9c7881).
Topical fumagillin solution must made by a compounding pharmacy, as it is not commercially available in the United States. This solution can be made by using fumagillin bicylohexylammonium (Fumidil B) at 3 mg/ml in sterile saline (fumagillin, 70 μg/ml).
Based on studies in vitro and in vivo in animal models as well as clinical data on microsporidiosis in humans, albendazole (which inhibits β-tubulin) and fumagillin (which inhibits methionine aminopeptidase type 2) have the most consistent activity against the microsporidia that cause human infections (118, 312, 335, 357, 413–418) (Table 4). Albendazole is highly active in vitro against Encephalitozoon hellem, Encephalitozoon cuniculi, and Encephalitozoon intestinalis (357, 419), their β-tubulins demonstrate an amino acid sequence that is known to be associated with sensitivity to benzimidazoles (420), and numerous reports demonstrate the efficacy of 2 to 4 weeks of albendazole at 400 mg twice daily for these infections (118, 335, 417, 418). Other benzimidazole derivatives can also inhibit the growth of Encephalitozoonidae in vitro (421). The sequences of both Enterocytozoon (422) and Vittaforma β-tubulin contain amino acid residues associated with albendazole resistance consistent with the observation that albendazole is less effective for the treatment of Enterocytozoon bieneusi infection (423). Albendazole has been effective in patients with microsporidiosis due to Trachipleistophora hominis and in patients with infections due to Anncaliia spp. (42, 47, 350, 354).
Fumagillin has been used for the treatment of microsporidiosis in various types of fish as well as the treatment of honeybees infected with Nosema apis (412, 424). Microsporidia, unlike most eukaryotes, are dependent on methionine aminopeptidase type 2, as they lack aminopeptidase type 1. Fumagillin and its semisynthetic analogues have in vitro and in vivo activity against the Encephalitozoonidae and Vittaforma corneae and have been used to treat ocular microsporidiosis (312, 357, 413–416). Fumagillin (20 mg three times a day) has been effective for the treatment of Enterocytozoon bieneusi gastrointestinal infections in patients with AIDS and in patients with various organ transplants (413, 424–427). The main limiting toxicity of fumagillin has been reversible thrombocytopenia.
There are several other medications that have been reported as treatments for microsporidiosis. Atovaquone has shown variable results against microsporidia (350, 414, 428). Furazolidone has been shown to have transient efficacy for Enterocytozoon bieneusi (429). Itraconazole has been used in the treatment of microsporidial stromal keratitis, sinusitis, and keratoconjunctivitis in both immunocompetent and immunocompromised patients (72, 430–432). While some early case reports suggested that metronidazole was effective for Enterocytozoon bieneusi, the majority of data have shown that this drug is not effective (71, 312, 433). Nitazoxanide has been effective in a few cases of Enterocytozoon bieneusi infection in patients with AIDS (434–436), and unpublished anecdotal experience exists for its successful use in the treatment of this infection in transplant patients. Thalidomide, an anti-tumor necrosis factor alpha (TNF-α) drug, has been effective in decreasing diarrhea in 50% of patients with gastrointestinal microsporidiosis, but on biopsy, the organisms were still present in responding patients (437).
Several potential new therapeutic approaches for the treatment of microsporidiosis have been published. Novel synthetic polyamines have been shown to be effective in the treatment of experimental microsporidiosis (438). The process of spore germination is calcium dependent; to this end, calmodulin inhibitors (trifluoperazine and chlorpromazine) and calcium antagonists (verapamil and lanthanum) both prevented spore germination in Spraguea lophii (439). Nifedipine has been demonstrated to inhibit the spore germination of Encephalitozoon intestinalis and Encephalitozoon hellem (71, 72). The endospore of microsporidia contains mainly chitin and proteins, which is important for extracellular survival and infection of microsporidia (419). Chitin synthesis inhibitors such as polyoxins, nikkomycins, and lufenuron have shown some efficacy against microsporidia (440–442). A topoisomerase IV subunit (part C) was identified in the Vittaforma corneae genome, and fluoroquinolones have been shown in vitro to inhibit both Vittaforma corneae and Encephalitozoon intestinalis (443). Triosephosphate isomerase (TIM) plays a crucial role in glycolysis of Encephalitozoon intestinalis, and its inhibition by three different sulfhydryl reagents {MTSES [methanethiosulfonate ethylsulfonate], MMTS [methyl methanethiosulfonate], and DTNB [5,5′-dithiobis-(2-nitrobenzoic acid)]} inhibited microsporidian energy use (444).
PREVENTION
The presence of infective spores in bodily fluids indicates that safety measures when handling body fluids in clinical settings and personal hygiene measures should help prevent infection. Common-sense approaches include drinking boiled water, washing hands, eating thoroughly cooked meat, and carefully washing vegetables and fruits before ingestion (127, 133, 140, 200, 445). Spores can be killed in the environment by exposure to 70% ethanol, phenolic derivatives, formaldehyde, 1% hydrogen peroxide, sodium hydroxide, chloramine, or amphoteric surfactants for 30 min at 22°C and autoclaving at 120°C for 10 min or boiling for 5 min (197, 446). No prophylactic agents that prevent microsporidiosis have been identified. Patients have developed microsporidiosis while on trimethoprim-sulfamethoxazole prophylaxis (447), and infection has been reported in patients receiving dapsone, atovaquone, azithromycin, itraconazole, or pyrimethamine. The most effective preventative measure is restoration of immune function in immunocompromised hosts. Based on the epidemiology of these infections, screening close contacts of index cases for infection and/or carriage is rational.
CONCLUSIONS
The first clearly defined case of human infection with microsporidia was a 9-year-old Japanese boy with disseminated Encephalitozoon infection who suffered from recurrent fever, vomiting, headache, and spastic convulsions in 1959 (34, 448). Microsporidiosis was rarely reported until the advent of the AIDS epidemic and the subsequent identification of Enterocytozoon bieneusi gastrointestinal infections in patients with AIDS, chronic diarrhea, and wasting syndrome (44, 449). Since that initial description, microsporidia have been recognized as opportunistic pathogens in patients with various type of immune suppression as well as in immunocompetent hosts (136, 151, 161, 450–452). There are about 10 genera and 17 species of microsporidia associated with human infection, and infections have occurred in almost all organ systems. There are data to suggest that latent infection can occur in humans, and the importance of this during immune suppression remains to be elucidated. Observations indicate that environmental exposure to microsporidia is high and that asymptomatic infection is common. Many of the microsporidia that are human pathogens are probably zoonotic infections that are either water- or foodborne. More research is needed on the natural reservoirs and environmental sources of microsporidia as well as improved therapeutic agents and prevention strategies to avoid transmission. Furthermore, additional studies are needed to more fully understand the molecular pathogenesis of microsporidiosis.
ACKNOWLEDGMENTS
This work was supported by grant no. R01 AI124753 (L.M.W.) and R01 AI132614 (L.M.W.), the National Natural Science Foundation of China (grant no. 32000106) (B.H.), and the QILU Young Scholars Program of Shandong University (grant no. 21510082063092) (B.H.).
We thank David Schwartz, Peter M. Takvorian, Imtiaz Khan, Rainer Weber, Max A. Spycher, Donald P. Kotler, Jan M. Orenstein, and Elizabeth Didier for sharing photographs over the years.
Biographies

Bing Han, Ph.D., completed his thesis project in Dr. Louis Weiss’ laboratory in the Pathology Department of the Albert Einstein College of Medicine, New York, NY, as a joint student funded by the Chinese Scholarship Council. In 2017, he obtained his Ph.D. from Southwest University, Chongqing, China. He subsequently did a postdoctoral research fellowship in the laboratory of Dr. Weiss, examining the interaction of the polar tube with the host cell during invasion. Dr. Han is currently a Full Professor at the School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, China. His research interests include the molecular mechanism(s) of microsporidian host cell invasion and the interaction of host mitochondria with the microsporidian sporoplasm.

Guoqing Pan, Ph.D., is a Full Professor of the State Key Laboratory of Silkworm Genome Biology, Chongqing Key Laboratory of Microsporidia Infection and Control, Southwest University, Chongqing, China. He earned his Ph.D. at Southwest University, Chongqing, China, in 2009. Dr. Pan was trained at the University of California San Diego as a visiting scholar during 2013-2014. Dr. Pan’s current research interests include the genomic biology of Nosema bombycis, a microsporidian pathogen of silkworms, the interaction of microsporidia with host cells, and the evolution of intestinal microorganisms in insects and their interaction with their hosts.

Louis M. Weiss, M.D., M.P.H., is Professor of Pathology (Parasitology and Tropical Medicine) and Professor of Medicine (Infectious Diseases), at the Albert Einstein College of Medicine, Bronx, NY. In 1982, he earned his M.D. from the Johns Hopkins University School of Medicine, Baltimore, MD, and his M.P.H. from the Johns Hopkins University School of Public Health. He then completed a residency in internal medicine at the University of Chicago, followed by an Infectious Diseases Fellowship at Montefiore Medical Center-Albert Einstein College of Medicine. Dr. Weiss’ interest in these pathogens started with the recognition of microsporidia as opportunistic pathogens in patients with AIDS. His research on microsporidia over the last 25 years has included studies on diagnostic approaches, microsporidian taxonomy, drug development for the treatment of microsporidiosis, the structure and composition of the polar tube, and the interactions of polar tube proteins with host cell proteins.
REFERENCES
- 1.Weiss LM. 2020. Microsporidiosis, p 825–831. In Ryan ET, Hill DR, Solomon T, Aronson N, Endy TP (ed), Hunter's tropical medicine and emerging infectious diseases. Elsevier, Amsterdam, Netherlands. [Google Scholar]
- 2.James TY, Pelin A, Bonen L, Ahrendt S, Sain D, Corradi N, Stajich JE. 2013. Shared signatures of parasitism and phylogenomics unite Cryptomycota and microsporidia. Curr Biol 23:1548–1553. 10.1016/j.cub.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 3.Jones MD, Forn I, Gadelha C, Egan MJ, Bass D, Massana R, Richards TA. 2011. Discovery of novel intermediate forms redefines the fungal tree of life. Nature 474:200–203. 10.1038/nature09984. [DOI] [PubMed] [Google Scholar]
- 4.Corsaro D, Michel R, Walochnik J, Venditti D, Muller KD, Hauroder B, Wylezich C. 2016. Molecular identification of Nucleophaga terricolae sp. nov. (Rozellomycota), and new insights on the origin of the Microsporidia. Parasitol Res 115:3003–3011. 10.1007/s00436-016-5055-9. [DOI] [PubMed] [Google Scholar]
- 5.Sprague V. 1977. Systematics of the microsporidia, p 1–510. In Bulla LA, Cheng TC (ed), Comparative pathobiology, vol 2. Plenum Press, New York, NY. [Google Scholar]
- 6.Sprague V, Becnel JJ. 1998. Note on the name-author-date combination for the taxon MICROSPORIDIES Balbiani, 1882, when ranked as a phylum. J Invertebr Pathol 71:91–94. 10.1006/jipa.1997.4702. [DOI] [PubMed] [Google Scholar]
- 7.Weber R, Deplazes P, Schwartz D. 2000. Diagnosis and clinical aspects of human microsporidiosis. Contrib Microbiol 6:166–192. 10.1159/000060360. [DOI] [PubMed] [Google Scholar]
- 8.Williams BAP, Hirt RP, Lucocq JM, Embley TM. 2002. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature 418:865–869. 10.1038/nature00949. [DOI] [PubMed] [Google Scholar]
- 9.Hollister WS, Canning EU. 1987. An enzyme-linked immunosorbent assay (ELISA) for detection of antibodies to Encephalitozoon cuniculi and its use in determination of infections in man. Parasitology 94:209–219. 10.1017/S0031182000053890. [DOI] [PubMed] [Google Scholar]
- 10.Curgy JJ, Vavra J, Vivares C. 1980. Presence of ribosomal RNAs with prokaryotic properties in Microsporidia, eukaryotic organisms. Biol Cell 38:49–52. [Google Scholar]
- 11.Vossbrinck CR, Woese CR. 1986. Eukaryotic ribosomes that lack a 5.8S RNA. Nature 320:287–288. 10.1038/320287a0. [DOI] [PubMed] [Google Scholar]
- 12.Weiss LM, Vossbrinck CR. 1999. Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the microsporidia, p 129–171. In Wittner M, Weiss LM (ed), The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 13.Weiss LM, Bechnel JJ. 2014. Microsporidia: pathogens of opportunity. Wiley Blackwell, Oxford, United Kingdom. [Google Scholar]
- 14.Weiss LM. 2000. Molecular phylogeny and diagnostic approaches to microsporidia. Contrib Microbiol 6:209–235. 10.1159/000060362. [DOI] [PubMed] [Google Scholar]
- 15.Katinka MD, Duprat S, Cornillot E, Metenier G, Thomarat F, Prensier G, Barbe V, Peyretaillade E, Brottier P, Wincker P, Delbac F, El Alaoui H, Peyret P, Saurin W, Gouy M, Weissenbach J, Vivares CP. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450–453. 10.1038/35106579. [DOI] [PubMed] [Google Scholar]
- 16.Nakjang S, Williams TA, Heinz E, Watson AK, Foster PG, Sendra KM, Heaps SE, Hirt RP, Martin Embley T. 2013. Reduction and expansion in microsporidian genome evolution: new insights from comparative genomics. Genome Biol Evol 5:2285–2303. 10.1093/gbe/evt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadi L, Reinke AW. 2020. Evolution of microsporidia: an extremely successful group of eukaryotic intracellular parasites. PLoS Pathog 16:e1008276. 10.1371/journal.ppat.1008276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams BA, Lee RC, Becnel JJ, Weiss LM, Fast NM, Keeling PJ. 2008. Genome sequence surveys of Brachiola algerae and Edhazardia aedis reveal microsporidia with low gene densities. BMC Genomics 9:200. 10.1186/1471-2164-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aurrecoechea C, Barreto A, Brestelli J, Brunk BP, Caler EV, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Iodice J, Kissinger JC, Kraemer ET, Li W, Nayak V, Pennington C, Pinney DF, Pitts B, Roos DS, Srinivasamoorthy G, Stoeckert CJ, Jr, Treatman C, Wang H. 2011. AmoebaDB and MicrosporidiaDB: functional genomic resources for Amoebozoa and Microsporidia species. Nucleic Acids Res 39:D612–D619. 10.1093/nar/gkq1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pombert JF, Xu J, Smith DR, Heiman D, Young S, Cuomo CA, Weiss LM, Keeling PJ. 2013. Complete genome sequences from three genetically distinct strains reveal high intraspecies genetic diversity in the microsporidian Encephalitozoon cuniculi. Eukaryot Cell 12:503–511. 10.1128/EC.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pombert JF, Selman M, Burki F, Bardell FT, Farinelli L, Solter LF, Whitman DW, Weiss LM, Corradi N, Keeling PJ. 2012. Gain and loss of multiple functionally related, horizontally transferred genes in the reduced genomes of two microsporidian parasites. Proc Natl Acad Sci U S A 109:12638–12643. 10.1073/pnas.1205020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corradi N, Pombert JF, Farinelli L, Didier ES, Keeling PJ. 2010. The complete sequence of the smallest known nuclear genome from the microsporidian Encephalitozoon intestinalis. Nat Commun 1:77. 10.1038/ncomms1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keeling PJ, Corradi N, Morrison HG, Haag KL, Ebert D, Weiss LM, Akiyoshi DE, Tzipori S. 2010. The reduced genome of the parasitic microsporidian Enterocytozoon bieneusi lacks genes for core carbon metabolism. Genome Biol Evol 2:304–309. 10.1093/gbe/evq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akiyoshi DE, Morrison HG, Lei S, Feng X, Zhang Q, Corradi N, Mayanja H, Tumwine JK, Keeling PJ, Weiss LM, Tzipori S. 2009. Genomic survey of the non-cultivatable opportunistic human pathogen, Enterocytozoon bieneusi. PLoS Pathog 5:e1000261. 10.1371/journal.ppat.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss LM, Vossbrinck CR. 1998. Microsporidiosis: molecular and diagnostic aspects. Adv Parasitol 40:351–395. 10.1016/s0065-308x(08)60127-x. [DOI] [PubMed] [Google Scholar]
- 26.Matos O, Lobo ML, Xiao L. 2012. Epidemiology of Enterocytozoon bieneusi infection in humans. J Parasitol Res 2012:981424–981424. 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuzet O, Maurand J, Fize JA, Michel R, Fenwick B. 1971. Proposition d'un nouveau cadre systematique pour les genres de Microsporidies. C R Hebd Seances Acad Sci 272:1268–1271. [Google Scholar]
- 28.Larsson JIR. 1988. Identification of microsporidian genera—a guide with comments on the taxonomy. Arch Protistenkd 136:1–37. 10.1016/S0003-9365(88)80032-0. [DOI] [Google Scholar]
- 29.Issi IV. 1986. Microsporidia as a phylum of parasitic protozoa. Protozoology 10:6–135. (In Russian.) [Google Scholar]
- 30.Weiser J. 1977. A proposal of the basis for microsporidian taxonomy. Proc Int Congr Protozool 5:267. [Google Scholar]
- 31.Sprague V, Becnel JJ, Hazard EI. 1992. Taxonomy of phylum Microspora. Crit Rev Microbiol 18:285–395. 10.3109/10408419209113519. [DOI] [PubMed] [Google Scholar]
- 32.Franzen C. 2008. Microsporidia: a review of 150 years of research. Open Parasitol J 2:1–34. 10.2174/1874421400802010001. [DOI] [Google Scholar]
- 33.Weber R, Bryan RT, Schwartz DA, Owen RL. 1994. Human microsporidial infections. Clin Microbiol Rev 7:426–461. 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsubayashi H, Koide T, Mikata T, Hagiwara S. 1959. A case of Encephalitozoon-like body infection in man. Arch Pathol Lab Med 67:181–185. [PubMed] [Google Scholar]
- 35.Steinhaus EA, Martignoni ME. 1970. Abridged glossary of terms used in invertebrate pathology, 2nd ed. USDA, Pacific Northwest Forest and Range Experiment Station, Portland, OR. [Google Scholar]
- 36.Fine PE. 1975. Vectors and vertical transmission: an epidemiologic perspective. Ann N Y Acad Sci 266:173–194. 10.1111/j.1749-6632.1975.tb35099.x. [DOI] [PubMed] [Google Scholar]
- 37.Pavie J, Menotti J, Porcher R, Donay JL, Gallien S, Sarfati C, Derouin F, Molina JM. 2012. Prevalence of opportunistic intestinal parasitic infections among HIV-infected patients with low CD4 cells counts in France in the combination antiretroviral therapy era. Int J Infect Dis 16:e677-9. 10.1016/j.ijid.2012.05.1022. [DOI] [PubMed] [Google Scholar]
- 38.Logan C, Beadsworth MB, Beeching NJ. 2016. HIV and diarrhoea: what is new? Curr Opin Infect Dis 29:486–494. 10.1097/QCO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 39.Greigert V, Pfaff AW, Abou-Bacar A, Candolfi E, Brunet J. 2018. Intestinal microsporidiosis in Strasbourg from 2014 to 2016: emergence of an Enterocytozoon bieneusi genotype of Asian origin. Emerg Microbes Infect 7:97. 10.1038/s41426-018-0099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Zhang H, Zhao X, Zhang L, Zhang G, Guo M, Liu L, Feng Y, Xiao L. 2013. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J Clin Microbiol 51:557–563. 10.1128/JCM.02758-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silveira H, Canning EU. 1995. Vittaforma corneae n. comb. for the human microsporidium Nosema corneum Shadduck, Meccoli, Davis & Font, 1990, based on its ultrastructure in the liver of experimentally infected athymic mice. J Eukaryot Microbiol 42:158–165. 10.1111/j.1550-7408.1995.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 42.Call A, Takvorian PM, Lewin S, Rendel M, Sian CS, Wittner M, Tanowitz HB, Keohane E, Weiss LM. 1998. Brachiola vesicularum, n. g., n. sp., a new microsporidium associated with AIDS and myositis. J Eukaryot Microbiol 45:240–251. 10.1111/j.1550-7408.1998.tb04532.x. [DOI] [PubMed] [Google Scholar]
- 43.Franzen C, Nassonova ES, Scholmerich J, Issi IV. 2006. Transfer of the members of the genus Brachiola (microsporidia) to the genus Anncaliia based on ultrastructural and molecular data. J Eukaryot Microbiol 53:26–35. 10.1111/j.1550-7408.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 44.Desportes I, Charpentier YL, Galian A, Bernard F, Cochand-Priollet B, Lavergne A, Ravisse P, Modigliani R. 1985. Occurrence of a new microsporidian: Enterocytozoon bieneusi n. g., n. sp., in the enterocytes of a human patient with AIDS. J Protozool 32:250–245. 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 45.Cali A, Kotler DP, Orenstein JM. 1993. Septata intestinalis n. g., n. sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol 40:101–112. 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 46.Hartskeerl RA, Van Gool T, Schuitema AR, Didier ES, Terpstra WJ. 1995. Genetic and immunological characterization of the microsporidian Septata intestinalis Cali, Kotler and Orenstein, 1993: reclassification to Encephalitozoon intestinalis. Parasitology 110:277–285. 10.1017/S0031182000080860. [DOI] [PubMed] [Google Scholar]
- 47.Field AS, Marriott DJ, Milliken ST, Brew BJ, Canning EU, Kench JG, Darveniza P, Harkness JL. 1996. Myositis associated with a newly described microsporidian, Trachipleistophora hominis, in a patient with AIDS. J Clin Microbiol 34:2803–2811. 10.1128/JCM.34.11.2803-2811.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yachnis AT, Berg J, Martinez-Salazar A, Bender BS, Diaz L, Rojiani AM, Eskin TA, Orenstein JM. 1996. Disseminated microsporidiosis especially infecting the brain, heart, and kidneys. Report of a newly recognized pansporoblastic species in two symptomatic AIDS patients. Am J Clin Pathol 106:535–543. 10.1093/ajcp/106.4.535. [DOI] [PubMed] [Google Scholar]
- 49.Meissner EG, Bennett JE, Qvarnstrom Y, da Silva A, Chu EY, Tsokos M, Gea-Banacloche J. 2012. Disseminated microsporidiosis in an immunosuppressed patient. Emerg Infect Dis 18:1155–1158. 10.3201/eid1807.120047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choudhary MM, Metcalfe MG, Arrambide K, Bern C, Visvesvara GS, Pieniazek NJ, Bandea RD, Deleon-Carnes M, Adem P, Choudhary MM, Zaki SR, Saeed MU. 2011. Tubulinosema sp. microsporidian myositis in immunosuppressed patient. Emerg Infect Dis 17:1727–1730. 10.3201/eid1709.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suankratay C, Thiansukhon E, Nilaratanakul V, Putaporntip C, Jongwutiwes S. 2012. Disseminated infection caused by novel species of Microsporidium, Thailand. Emerg Infect Dis 18:302–304. 10.3201/eid1802.111319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fayer R, Santin-Duran M. 2014. Epidemiology of microsporidia in human infections, p 135–164. In Weiss LM, Becnel JJ (ed), Microsporidia: pathogens of opportunity. Wiley Blackwell, Oxford, United Kingdom. 10.1002/9781118395264.ch3. [DOI] [Google Scholar]
- 53.Kent ML, Elliott DG, Groff JM, Hedrick RP. 1989. Loma salmonae (Protozoa: Microspora) infections in seawater reared coho salmon Oncorhynchus kisutch. Aquaculture 80:211–222. 10.1016/0044-8486(89)90169-5. [DOI] [Google Scholar]
- 54.Vavra J. 1976. Structure of the microsporidia, p 1–85. In Bulla LA, Cheng TC (ed), Comparative pathobiology, vol 1. Plenum Press, New York, NY. [Google Scholar]
- 55.Olson RE, Tiekotter KL, Reno PW. 1994. Nadelspora canceri n. g., n. sp., an unusual microsporidian parasite of the Dungeness crab, Cancer magister. J Eukaryot Microbiol 41:349–359. 10.1111/j.1550-7408.1994.tb06089.x. [DOI] [Google Scholar]
- 56.Canning EU, Lom J, Dykova I. 1987. The microsporidia of vertebrates. J Basic Microbiol 27:252–252. [Google Scholar]
- 57.Thélohan P. 1894. Sur la presence d’une capsule a filament dans les spores des microsporidies. C R Hebd Seances Acad Sci 118:1425–1427. [Google Scholar]
- 58.Keeling PJ, Fast NM. 2002. Microsporidia: biology and evolution of highly reduced intracellular parasites. Annu Rev Microbiol 56:93–116. 10.1146/annurev.micro.56.012302.160854. [DOI] [PubMed] [Google Scholar]
- 59.Jaroenlak P, Cammer M, Davydov A, Sall J, Usmani M, Liang F-X, Ekiert DC, Bhabha G. 2020. 3-Dimensional organization and dynamics of the microsporidian polar tube invasion machinery. PLoS Pathog 16:e1008738. 10.1371/journal.ppat.1008738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han B, Weiss LM. 2017. Microsporidia: obligate intracellular pathogens within the fungal kingdom. Microbiol Spec 5:10.1128/microbiolspec.FUNK-0018-2016. 10.1128/microbiolspec.FUNK-0018-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bigliardi E, Sacchi L. 2001. Cell biology and invasion of the microsporidia. Microbes Infect 3:373–379. 10.1016/s1286-4579(01)01393-4. [DOI] [PubMed] [Google Scholar]
- 62.Southern TR, Jolly CE, Lester ME, Hayman JR. 2007. EnP1, a microsporidian spore wall protein that enables spores to adhere to and infect host cells in vitro. Eukaryot Cell 6:1354–1362. 10.1128/EC.00113-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bigliardi E. 2001. Microsporidia, enigmatic parasites. Ital J Zool 68:263–271. 10.1080/11250000109356418. [DOI] [Google Scholar]
- 64.Kudo R. 1920. On the structure of some microsporidian spores. J Parasitol 6:178–182. 10.2307/3270841. [DOI] [Google Scholar]
- 65.Lom J. 1972. On the structure of the extruded microsporidian polar filament. Z Parasitenkd 38:200–213. 10.1007/BF00329598. [DOI] [Google Scholar]
- 66.Xu Y, Weiss LM. 2005. The microsporidian polar tube: a highly specialised invasion organelle. Int J Parasitol 35:941–953. 10.1016/j.ijpara.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delbac F, Polonais V. 2008. The microsporidian polar tube and its role in invasion. Subcell Biochem 47:208–220. 10.1007/978-0-387-78267-6_17. [DOI] [PubMed] [Google Scholar]
- 68.Keohane E, Weiss LM. 1999. The structure, function, and composition of the microsporidian polar tube, p 196–224. In Wittner M, Weiss LM (ed), The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 69.Undeen AH, Frixione E. 1990. The role of osmotic pressure in the germination of Nosema algerae spores. J Protozool 37:561–567. 10.1111/j.1550-7408.1990.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 70.Undeen AH, Vander Meer RK. 1999. Microsporidian intrasporal sugars and their role in germination. J Invertebr Pathol 73:294–302. 10.1006/jipa.1998.4834. [DOI] [PubMed] [Google Scholar]
- 71.He Q, Leitch GJ, Visvesvara GS, Wallace S. 1996. Effects of nifedipine, metronidazole, and nitric oxide donors on spore germination and cell culture infection of the microsporidia Encephalitozoon hellem and Encephalitozoon intestinalis. Antimicrob Agents Chemother 40:179–185. 10.1128/AAC.40.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leitch GJ, He Q, Wallace S, Visvesvara GS. 1993. Inhibition of the spore polar filament extrusion of the microsporidium, Encephalitozoon hellem, isolated from an AIDS patient. J Eukaryot Microbiol 40:711–717. 10.1111/j.1550-7408.1993.tb04463.x. [DOI] [PubMed] [Google Scholar]
- 73.Andreadis TG. 1985. Experimental transmission of a microsporidian pathogen from mosquitoes to an alternate copepod host. Proc Natl Acad Sci U S A 82:5574–5577. 10.1073/pnas.82.16.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sweeney AW, Hazard EI, Graham MF. 1985. Intermediate host for an Amblyospora sp. (microspora) infecting the mosquito, Culex annulirostris. J Invertebr Pathol 46:98–102. 10.1016/0022-2011(85)90133-8. [DOI] [PubMed] [Google Scholar]
- 75.Cali A, Becnel J, Takvorian P. 2017. Microsporidia, p 1559–1618. In Archibald J, Simpson A, Slamovits C (ed), Handbook of the protists. Springer, Cham, Switzerland. 10.1007/978-3-319-28149-0_27. [DOI] [Google Scholar]
- 76.Cali A, Neafie RC, Takvorian PM. 2011. Microsporidiosis, p 61–76. In Meyers WM, Firpo A, Wear DJ (ed), Topics on the pathology of protozoan and invasive arthropod diseases, 3rd ed. Armed Forces Institute of Pathology, Washington, DC. [Google Scholar]
- 77.Weidner E. 1975. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd 47:1–9. 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]
- 78.Cali A, Owen RL. 1988. Microsporidiosis. In Balows A, Hausler WJ, Ohashi M, Turano A, Lennete EH (ed), Laboratory diagnosis of infectious diseases. Springer, New York, NY. [Google Scholar]
- 79.Overstreet RM, Weidner E. 1974. Differentiation of microsporidian spore-tails in Inodosporus spraguei Gen. et Sp. N. Z Parasitenkd 44:169–186. 10.1007/BF00328760. [DOI] [PubMed] [Google Scholar]
- 80.Weidner E. 1976. The microsporidian spore invasion tube. The ultrastructure, isolation, and characterization of the protein comprising the tube. J Cell Biol 71:23–34. 10.1083/jcb.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Docker MF, Kent ML, Hervio DML, Khattra JS, Weiss LM, Cali A, Devlin RH. 1997. Ribosomal DNA sequence of Nucleospora salmonis Hedrick, Groff and Baxa, 1991 (Microsporea: Enterocytozoonidae): implications for phylogeny and nomenclature. J Eukaryot Microbiol 44:55–60. 10.1111/j.1550-7408.1997.tb05692.x. [DOI] [PubMed] [Google Scholar]
- 82.Stentiford G, Bateman K, Longshaw M, Feist S. 2007. Enterospora canceri n. gen., n. sp., intranuclear within the hepatopancreatocytes of the European edible crab Cancer pagurus. Dis Aquat Org 75:61–72. 10.3354/dao075061. [DOI] [PubMed] [Google Scholar]
- 83.Freeman MA, Sommerville C. 2009. Desmozoon lepeophtherii n. gen., n. sp., (Microsporidia: Enterocytozoonidae) infecting the salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae). Parasit Vectors 2:58–58. 10.1186/1756-3305-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palenzuela O, Redondo MJ, Cali A, Takvorian PM, Alonso-Naveiro M, Alvarez-Pellitero P, Sitja-Bobadilla A. 2014. A new intranuclear microsporidium, Enterospora nucleophila n. sp., causing an emaciative syndrome in a piscine host (Sparus aurata), prompts the redescription of the family Enterocytozoonidae. Int J Parasitol 44:189–203. 10.1016/j.ijpara.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Fries I, Paxton RJ, Tengö J, Slemenda SB, da Silva AJ, Pieniazek NJ. 1999. Morphological and molecular characterization of Antonospora scoticae n. gen., n. sp. (Protozoa, Microsporidia) a parasite of the communal bee, Andrena scotica Perkins, 1916 (Hymenoptera, Andrenidae). Eur J Protist 35:183–193. 10.1016/S0932-4739(99)80036-4. [DOI] [Google Scholar]
- 86.Bohne W, Ferguson DJ, Kohler K, Gross U. 2000. Developmental expression of a tandemly repeated, glycine-and serine-rich spore wall protein in the microsporidian pathogen Encephalitozoon cuniculi. Infect Immun 68:2268–2275. 10.1128/IAI.68.4.2268-2275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang D, Pan L, Chen Z, Du H, Luo B, Luo J, Pan G. 2018. The roles of microsporidia spore wall proteins in the spore wall formation and polar tube anchorage to spore wall during development and infection processes. Exp Parasitol 187:93–100. 10.1016/j.exppara.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Beznoussenko GV, Dolgikh VV, Seliverstova EV, Semenov PB, Tokarev YS, Trucco A, Micaroni M, Di Giandomenico D, Auinger P, Senderskiy IV, Skarlato SO, Snigirevskaya ES, Komissarchik YY, Pavelka M, De Matteis MA, Luini A, Sokolova YY, Mironov AA. 2007. Analogs of the Golgi complex in microsporidia: structure and avesicular mechanisms of function. J Cell Sci 120:1288–1298. 10.1242/jcs.03402. [DOI] [PubMed] [Google Scholar]
- 89.Jaronski ST. 1984. Microsporida in cell culture. Adv Cell Culture 3:183–229. 10.1016/B978-0-12-007903-2.50011-8. [DOI] [Google Scholar]
- 90.Visvesvara GS. 2002. In vitro cultivation of microsporidia of clinical importance. Clin Microbiol Rev 15:401–413. 10.1128/cmr.15.3.401-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trager W. 1937. The hatching of spores of Nosema bombycis Nägeli and the partial development of the organism in tissue cultures. J Parasitol 23:226–227. 10.2307/3272075. [DOI] [Google Scholar]
- 92.Juarez S, Putaporntip C, Jongwutiwes S, Ichinose A, Yanagi T, Kanbara H. 2005. In vitro cultivation and electron microscopy characterization of Trachipleistophora anthropophthera isolated from the cornea of an AIDS patient. J Eukaryot Microbiol 52:179–190. 10.1111/j.1550-7408.2005.00024.x. [DOI] [PubMed] [Google Scholar]
- 93.Shadduck JA, Meccoli RA, Davis R, Font RL. 1990. Isolation of a microsporidian from a human patient. J Infect Dis 162:773–776. 10.1093/infdis/162.3.773. [DOI] [PubMed] [Google Scholar]
- 94.De Groote MA, Visvesvara G, Wilson ML, Pieniazek NJ, Slemenda SB, daSilva AJ, Leitch GJ, Bryan RT, Reves R. 1995. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis 171:1375–1378. 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 95.del Aguila C, Moura H, Fenoy S, Navajas R, Lopez-Velez R, Li L, Xiao L, Leitch GJ, da Silva A, Pieniazek NJ, Lal AA, Visvesvara GS. 2001. In vitro culture, ultrastructure, antigenic, and molecular characterization of Encephalitozoon cuniculi isolated from urine and sputum samples from a Spanish patient with AIDS. J Clin Microbiol 39:1105–1108. 10.1128/JCM.39.3.1105-1108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollister WS, Canning EU, Colbourn NI, Aarons EJ. 1995. Encephalitozoon cuniculi isolated from the urine of an AIDS patient, which differs from canine and murine isolates. J Eukaryot Microbiol 42:367–372. 10.1111/j.1550-7408.1995.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 97.Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R. 1996. Immunologic and molecular characteristics of encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis 22:557–559. 10.1093/clinids/22.3.557. [DOI] [PubMed] [Google Scholar]
- 98.Mathis A, Michel M, Kuster H, Müller C, Weber R, Deplazes P. 1997. Two Encephalitozoon cuniculi strains of human origin are infectious to rabbits. Parasitology 114:29–35. 10.1017/S0031182096008177. [DOI] [PubMed] [Google Scholar]
- 99.Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Luthy R. 1997. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection [see comments. N Engl J Med 336:474–478. 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- 100.Deplazes P, Mathis A, van Saanen M, Iten A, Keller R, Tanner I, Glauser MP, Weber R, Canning EU. 1998. Dual microsporidial infection due to Vittaforma corneae and Encephalitozoon hellem in a patient with AIDS. Clin Infect Dis 27:1521–1524. 10.1086/515023. [DOI] [PubMed] [Google Scholar]
- 101.Rossi P, Rosa GL, Ludovisi A, Tamburrini A, Morales MAG, Pozio E. 1998. Identification of a human isolate of Encephalitozoon cuniculi type I from Italy. Int J Parasitol 28:1361–1366. 10.1016/S0020-7519(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 102.Visvesvara GS, Leitch GJ, Wallace S, Seaba C, Erdman D, Ewing EP, Jr. 1996. Adenovirus masquerading as microsporidia. J Parasitol 82:316–319. 10.2307/3284168. [DOI] [PubMed] [Google Scholar]
- 103.Didier ES, Didier PJ, Friedberg DN, Stenson SM, Orenstein JM, Yee RW, Tio FO, Davis RM, Vossbrinck C, Millichamp N. 1991. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis 163:617–621. 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- 104.Didier ES, Rogers LB, Orenstein JM, Baker MD, Vossbrinck CR, Van Gool T, Hartskeerl R, Soave R, Beaudet LM. 1996. Characterization of Encephalitozoon (Septata) intestinalis isolates cultured from nasal mucosa and bronchoalveolar lavage fluids of two AIDS patients. J Eukaryot Microbiol 43:34–43. 10.1111/j.1550-7408.1996.tb02470.x. [DOI] [PubMed] [Google Scholar]
- 105.Didier ES, Visvesvara GS, Baker MD, Rogers LB, Bertucci DC, Groote MAD, Vossbrinck CR. 1996. A microsporidian isolated from an AIDS patient corresponds to Encephalitozoon cuniculi III, originally isolated from domestic dogs. J Clin Microbiol 34:2835–2837. 10.1128/JCM.34.11.2835-2837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Visvesvara GS, Leitch GJ, Moura H, Wallace S, Weber R, Bryan RT. 1991. Culture, electron microscopy, and immunoblot studies on a microsporidian parasite isolated from the urine of a patient with AIDS. J Protozool 38:105S–111S. [PubMed] [Google Scholar]
- 107.Hollister WS, Canning EU, Colbourn NI, Curry A, Lacey CJN. 1993. Characterization of Encephalitozoon hellem (Microspora) isolated from the nasal mucosa of a patient with AIDS. Parasitology 107:351–358. 10.1017/S003118200006769X. [DOI] [PubMed] [Google Scholar]
- 108.Ws H, Eu C, Ni C. 1993. A species of Encephalitozoon isolated from an AIDS patient: criteria for species differentiation. Folia Parasitol 40:293–295. [PubMed] [Google Scholar]
- 109.Scaglia M, Sacchi L, Gatti S, Bernuzzi AM, Polver PP, Piacentini I, Concia E, Croppo GP, da Silva AJ, Pieniazek NJ. 1994. Isolation and identification of Encephalitozoon hellem from an Italian AIDS patient with disseminated microsporidiosis. APMIS 102:817–827. 10.1111/j.1699-0463.1994.tb05240.x. [DOI] [PubMed] [Google Scholar]
- 110.Scaglia M, Sacchi L, Croppo GP, da Silva A, Gatti S, Corona S, Orani A, Bernuzzi AM, Pieniazek NJ, Slemenda SB, Wallace S, Visvesvara GS. 1997. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J Infection 34:119–126. 10.1016/S0163-4453(97)92414-2. [DOI] [PubMed] [Google Scholar]
- 111.Scaglia M, Gatti S, Sacchi L, Corona S, Chichino G, Bernuzzi AM, Barbarini G, Croppo GP, Silva AJD, Pieniazek NJ, Visvesvara GS. 1998. Asymptomatic respiratory tract microsporidiosis due to Encephalitozoon hellem in three patients with AIDS. Clin Infect Dis 26:174–176. 10.1086/516264. [DOI] [PubMed] [Google Scholar]
- 112.Visvesvara GS, Leitch GJ, da Silva AJ, Croppo GP, Moura H, Wallace S, Slemenda SB, Schwartz DA, Moss D, Bryan RT. 1994. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol 32:2760–2768. 10.1128/JCM.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Visvesvara GS, da Silva AJ, Croppo GP, Pieniazek NJ, Leitch GJ, Ferguson D, de Moura H, Wallace S, Slemenda SB, Tyrrell I. 1995. In vitro culture and serologic and molecular identification of Septata intestinalis isolated from urine of a patient with AIDS. J Clin Microbiol 33:930–936. 10.1128/JCM.33.4.930-936.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gatti S, Sacchi L, Novati S, Cornona S, Bernuzzp AM, Moura H, Pieniazek NJ, Visvervara GS, Scaglia M. 1997. Extraintestinal microsporidiosis in AIDS patients: clinical features and advanced protocols for diagnosis and characterization of the isolates. J Eukaryot Microbiol 44:79S. 10.1111/j.1550-7408.1997.tb05793.x. [DOI] [PubMed] [Google Scholar]
- 115.Peman J, Bornay-Llinares FJ, Acosta BA, López-Aldeguer J, Figueras M, Peset V, Gobernado M, Meseguer Y, Visvesvara G. 1997. First report of a case of Encephalitozoon spp. microsporidiosis in a Spanish AIDS patient. Rev Ibér Parasitol 57:131–134. [Google Scholar]
- 116.van Gool T, Canning EU, Gilis H, van den Bergh Weerman MA, Eeftinck Schattenkerk JK, Dankert J. 1994. Septata intestinalis frequently isolated from stool of AIDS patients with a new cultivation method. Parasitology 109:281–289. 10.1017/S0031182000078318. [DOI] [PubMed] [Google Scholar]
- 117.Doultree JC, Maerz AL, Ryan NJ, Baird RW, Wright E, Crowe SM, Marshall JA. 1995. In vitro growth of the microsporidian Septata intestinalis from an AIDS patient with disseminated illness. J Clin Microbiol 33:463–470. 10.1128/JCM.33.2.463-470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Molina J-M, Oksenhendler E, Beauvais B, Sarfati C, Jaccard A, Derouin F, Modaï J. 1995. Disseminated microsporidiosis due to Septata intestinalis in patients with AIDS: clinical features and response to albendazole therapy. J Infect Dis 171:245–249. 10.1093/infdis/171.1.245. [DOI] [PubMed] [Google Scholar]
- 119.Visvesvara GS, Leitch GJ, Pieniazek NJ, Silva AJD, Wallace S, Slemenda SB, Weber R, Schwartz DA, Gorelkin L, Wilcox CM, Bryan RT. 1995. Short-term in vitro culture and molecular analysis of the microsporidian, Enterocytozoon bieneusi. J Eukaryot Microbiol 42:506–510. 10.1111/j.1550-7408.1995.tb05896.x. [DOI] [PubMed] [Google Scholar]
- 120.Croppo GP, Croppo GP, Moura H, Da Silva AJ, Leitch GJ, Moss DM, Wallace S, Slemenda SB, Pieniazek NJ, Visvesvara GS. 1998. Ultrastructure, immunofluorescence, Western blot, and PCR analysis of eight isolates of Encephalitozoon (Septata) intestinalis established in culture from sputum and urine samples and duodenal aspirates of five patients with AIDS. J Clin Microbiol 36:1201–1208. 10.1128/JCM.36.5.1201-1208.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Croppo GP, Visvesvara GS, Leitch GJ, Wallace S, Schwartz DA. 1998. Identification of the microsporidian Encephalitozoon hellem using immunoglobulin G monoclonal antibodies. Arch Pathol Lab Med 122:182–186. [PubMed] [Google Scholar]
- 122.Desportes-Livage I, Chilmonczyk S, Hedrick R, Ombrouck C, Monge D, Maiga I, Gentilini M. 1996. Comparative development of two microsporidian species: Enterocytozoon bieneusi and Enterocytozoon salmonis, reported in AIDS patients and salmonid fish, respectively. J Eukaryot Microbiol 43:49–60. 10.1111/j.1550-7408.1996.tb02472.x. [DOI] [PubMed] [Google Scholar]
- 123.Izquierdo F, Castro Hermida JA, Fenoy S, Mezo M, González-Warleta M, del Aguila C. 2011. Detection of microsporidia in drinking water, wastewater and recreational rivers. Water Res 45:4837–4843. 10.1016/j.watres.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 124.Jiang W, Roellig DM, Li N, Wang L, Guo Y, Feng Y, Xiao L. 2020. Contribution of hospitals to the occurrence of enteric protists in urban wastewater. Parasitol Res 119:3033–3040. 10.1007/s00436-020-06834-w. [DOI] [PubMed] [Google Scholar]
- 125.Dowd SE, Gerba CP, Pepper IL. 1998. Confirmation of the human pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol 64:3332–3335. 10.1128/AEM.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dowd SE, John D, Eliopolus J, Gerba CP, Naranjo J, Klein R, Lopez B, de Mejia M, Mendoza CE, Pepper IL. 2003. Confirmed detection of Cyclospora cayetanesis, Encephalitozoon intestinalis and Cryptosporidium parvum in water used for drinking. J Water Health 1:117–123. 10.2166/wh.2003.0014. [DOI] [PubMed] [Google Scholar]
- 127.Bryan RT, Schwartz DA. 1999. Epidemiology of microsporidiosis, p 502–516. In Wittner M, Weiss LM (ed), The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 128.Kotler DP, Orenstein JM. 1998. Clinical syndromes associated with microsporidiosis. Adv Parasitol 40:321–349. 10.1016/s0065-308x(08)60126-8. [DOI] [PubMed] [Google Scholar]
- 129.Torres CM. 1927. A new disease parasite of man, Encephalitozoon chagasi, found in muscle and nervous tissue. C R Seances Soc Biol 97:1798–1799. [Google Scholar]
- 130.Wolf A. 1937. Granulomatous encephalomyelits due to an encephalitozoon (encephalitozoic encephalomyelitis). A new protozoan disease of man. Bull Neurol Inst NY 6:306–371. [Google Scholar]
- 131.Hollister W, Canning E, Willcox A. 1991. Evidence for widespread occurrence of antibodies to Encephalitozoon cuniculi (Microspora) in man provided by ELISA and other serological tests. Parasitology 102:33–43. 10.1017/S0031182000060315. [DOI] [PubMed] [Google Scholar]
- 132.Bergquist R, Morfeldt-Mansson L, Pehrson PO, Petrini B, Wasserman J. 1984. Antibody against Encephalitozoon cuniculi in Swedish homosexual men. Scand J Infect Dis 16:389–391. 10.3109/00365548409073966. [DOI] [PubMed] [Google Scholar]
- 133.Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. 2004. Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol 126:145–166. 10.1016/j.vetpar.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 134.Singh M, Kane GJ, Mackinlay L, Quaki I, Yap EH, Ho BC, Ho LC, Lim KC. 1982. Detection of antibodies to Nosema cuniculi (Protozoa: Microscoporidia) in human and animal sera by the indirect fluorescent antibody technique. Southeast Asian J Trop Med Public Health 13:110–113. [PubMed] [Google Scholar]
- 135.WHO Parasitic Diseases Surveillance. 1983. Antibody to Encephalitozoon cuniculi in man. WHO Wkly Epidemiol Rec 58:30–32. [Google Scholar]
- 136.van Gool T, Vetter JC, Weinmayr B, Van Dam A, Derouin F, Dankert J. 1997. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis 175:1020–1024. 10.1086/513963. [DOI] [PubMed] [Google Scholar]
- 137.Pospisilova Z, Ditrich O, Stankova M, Kodym P. 1997. Parasitic opportunistic infections in Czech HIV-infected patients—a prospective study. Cent Eur J Public Health 5:208–213. [PubMed] [Google Scholar]
- 138.Cislakova L, Prokopcakova H, Stef'kovic M, Halanova M. 1997. Encephalitozoon cuniculi—clinical and epidemiologic significance. Results of a preliminary serologic study in humans. Epidemiol Mikrobiol Imunol 46:30–33. (In Slovak.) [PubMed] [Google Scholar]
- 139.Wang ZD, Liu Q, Liu HH, Li S, Zhang L, Zhao YK, Zhu XQ. 2018. Prevalence of Cryptosporidium, Microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasit Vectors 11:28. 10.1186/s13071-017-2558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Didier ES. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop 94:61–76. 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 141.Didier ES, Weiss LM. 2006. Microsporidiosis: current status. Curr Opin Infect Dis 19:485–492. 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chacin-Bonilla L, Panunzio AP, Monsalve-Castillo FM, Parra-Cepeda IE, Martinez R. 2006. Microsporidiosis in Venezuela: prevalence of intestinal microsporidiosis and its contribution to diarrhea in a group of human immunodeficiency virus-infected patients from Zulia State. Am J Trop Med Hyg 74:482–486. 10.4269/ajtmh.2006.74.482. [DOI] [PubMed] [Google Scholar]
- 143.Lobo ML, Xiao L, Antunes F, Matos O. 2012. Microsporidia as emerging pathogens and the implication for public health: a 10-year study on HIV-positive and -negative patients. Int J Parasitol 42:197–205. 10.1016/j.ijpara.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 144.Velasquez JN, di Risio C, Etchart C, Chertcoff AV, Astudillo OG, Carnevale S. 2019. Multimethodological approach to gastrointestinal microsporidiosis in HIV-infected patients. Acta Parasitol 64:658–669. 10.2478/s11686-019-00095-z. [DOI] [PubMed] [Google Scholar]
- 145.Huibers MHW, Moons P, Maseko N, Gushu MB, Iwajomo OH, Heyderman RS, Boele van Hensbroek M, Brienen EA, van Lieshout L, Calis JCJ. 2018. Multiplex real-time PCR detection of intestinal protozoa in HIV-infected children in Malawi: Enterocytozoon bieneusi is common and associated with gastrointestinal complaints and may delay BMI (nutritional status) recovery. Pediatr Infect Dis J 37:910–915. 10.1097/INF.0000000000001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu H, Jiang Z, Yuan Z, Yin J, Wang Z, Yu B, Zhou D, Shen Y, Cao J. 2017. Infection by and genotype characteristics of Enterocytozoon bieneusi in HIV/AIDS patients from Guangxi Zhuang autonomous region, China. BMC Infect Dis 17:684. 10.1186/s12879-017-2787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nsagha DS, Njunda AL, Assob NJC, Ayima CW, Tanue EA, Kibu OD, Kwenti TE. 2015. Intestinal parasitic infections in relation to CD4(+) T cell counts and diarrhea in HIV/AIDS patients with or without antiretroviral therapy in Cameroon. BMC Infect Dis 16:9. 10.1186/s12879-016-1337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lores B, López-Miragaya I, Arias C, Fenoy S, Torres J, del Aguila C. 2002. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus-negative patients from Vigo, Spain. Clin Infect Dis 34:918–921. 10.1086/339205. [DOI] [PubMed] [Google Scholar]
- 149.Aoun K, Barbouche R, Bouratbine A, Béjaoui M, Dellagi K, Ben Ismail R. 1997. Intestinal microsporidia infections in children with primary immunologic deficiencies. Parasite 4:386–387. (In French.) [PubMed] [Google Scholar]
- 150.Shahrul Anuar T, M Al-Mekhlafi H, Md Salleh F, Moktar N. 2013. New insights of microsporidial infection among asymptomatic aboriginal population in Malaysia. PLoS One 8:e71870. 10.1371/journal.pone.0071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mumcuoglu I, Cetin F, Dogruman Al F, Oguz I, Aksu N. 2016. Prevalence of microsporidia in healthy individuals and immunocompetent patients with acute and chronic diarrhea. Infect Dis 48:133–137. 10.3109/23744235.2015.1094572. [DOI] [PubMed] [Google Scholar]
- 152.Anuar TS, Bakar NH, Al-Mekhlafi HM, Moktar N, Osman E. 2016. Prevalence and risk factors for asymptomatic intestinal microsporidiosis among aboriginal school children in Pahang, Malaysia. Southeast Asian J Trop Med Public Health 47:441–449. [PubMed] [Google Scholar]
- 153.Wanke CA, DeGirolami P, Federman M. 1996. Enterocytozoon bieneusi infection and diarrheal disease in patients who were not infected with human immunodeficiency virus: case report and review. Clin Infect Dis 23:816–818. 10.1093/clinids/23.4.816. [DOI] [PubMed] [Google Scholar]
- 154.Wichro E, Hoelzl D, Krause R, Bertha G, Reinthaler F, Wenisch C. 2005. Microsporidiosis in travel-associated chronic diarrhea in immune-competent patients. Am J Trop Med Hyg 73:285–287. 10.4269/ajtmh.2005.73.285. [DOI] [PubMed] [Google Scholar]
- 155.Raynaud L, Delbac F, Broussolle V, Rabodonirina M, Girault V, Wallon M, Cozon G, Vivares CP, Peyron F. 1998. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J Clin Microbiol 36:37–40. 10.1128/JCM.36.1.37-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cotte L, Rabodonirina M, Chapuis F, Bailly F, Bissuel F, Raynal C, Gelas P, Persat F, Piens MA, Trepo C. 1999. Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J Infect Dis 180:2003–2008. 10.1086/315112. [DOI] [PubMed] [Google Scholar]
- 157.Sak B, Kvac M, Kucerova Z, Kvetonova D, Sakova K. 2011. Latent microsporidial infection in immunocompetent individuals—a longitudinal study. PLoS Negl Trop Dis 5:e1162. 10.1371/journal.pntd.0001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Das S, Sharma S, Sahu SK, Nayak SS, Kar S. 2012. Diagnosis, clinical features and treatment outcome of microsporidial keratoconjunctivitis. Br J Ophthalmol 96:793–795. 10.1136/bjophthalmol-2011-301227. [DOI] [PubMed] [Google Scholar]
- 159.Loh RS, Chan CM, Ti SE, Lim L, Chan KS, Tan DT. 2009. Emerging prevalence of microsporidial keratitis in Singapore: epidemiology, clinical features, and management. Ophthalmology 116:2348–2353. 10.1016/j.ophtha.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 160.Sharma S, Das S, Joseph J, Vemuganti GK, Murthy S. 2011. Microsporidial keratitis: need for increased awareness. Surv Ophthalmol 56:1–22. 10.1016/j.survophthal.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 161.Sak B, Brady D, Pelikánová M, Květoňová D, Rost M, Kostka M, Tolarová V, Hůzová Z, Kváč M. 2011. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol 49:1064–1070. 10.1128/JCM.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sarfati C, Liguory O, Derouin F. 2001. Microsporidiosis. Presse Med 30:143–147. (In French.) [PubMed] [Google Scholar]
- 163.Hennequin C, Pialoux G, Bouree P, Dupont B. 1992. Microsporidiosis and acquired immunodeficiency syndrome (AIDS). Med Mal Infect 22:487–492. 10.1016/S0399-077X(05)80284-7. [DOI] [Google Scholar]
- 164.Halánová M, Valenčáková A, Jarčuška P, Halán M, Danišová O, Babinská I, Dedinská K, Čisláková L. 2019. Screening of opportunistic Encephalitozoon intestinalis and Enterocytozoon bieneusi in immunocompromised patients in Slovakia. Cent Eur J Pub Health 27:330–334. 10.21101/cejph.a5407. [DOI] [PubMed] [Google Scholar]
- 165.Hamamcı B, Çetinkaya Ü, Berk V, Kaynar L, Kuk S, Yazar S. 2015. Prevalence of Encephalitozoon intestinalis and Enterocytozoon bieneusi in cancer patients under chemotherapy. Mikrobiyol Bul 49:105–113. 10.5578/mb.8787. (In Turkish). [DOI] [PubMed] [Google Scholar]
- 166.Chandramathi S, Suresh K, Anita ZB, Kuppusamy UR. 2012. Infections of Blastocystis hominis and microsporidia in cancer patients: are they opportunistic? Trans R Soc Trop Med Hyg 106:267–269. 10.1016/j.trstmh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 167.Lono AR, Kumar S, Chye TT. 2008. Incidence of microsporidia in cancer patients. J Gastrointest Cancer 39:124–129. 10.1007/s12029-009-9065-z. [DOI] [PubMed] [Google Scholar]
- 168.Sing A, Tybus K, Heesemann J, Mathis A. 2001. Molecular diagnosis of an Enterocytozoon bieneusi human genotype C infection in a moderately immunosuppressed human immunodeficiency virus seronegative liver-transplant recipient with severe chronic diarrhea. J Clin Microbiol 39:2371–2372. 10.1128/jcm.39.6.2371-2372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohindra AR, Lee MW, Visvesvara G, Moura H, Parasuraman R, Leitch GJ, Xiao L, Yee J, del Busto R. 2002. Disseminated microsporidiosis in a renal transplant recipient. Transpl Infect Dis 4:102–107. 10.1034/j.1399-3062.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 170.Nagpal A, Pritt BS, Lorenz EC, Amer H, Nasr SH, Cornell LD, Iqbal S, Wilhelm MP. 2013. Disseminated microsporidiosis in a renal transplant recipient: case report and review of the literature. Transpl Infect Dis 15:526–532. 10.1111/tid.12119. [DOI] [PubMed] [Google Scholar]
- 171.George B, Coates T, Mcdonald S, Russ G, Cherian S, Nolan J, Brealey J. 2012. Disseminated microsporidiosis with Encephalitozoon species in a renal transplant recipient. Nephrology 17:5–8. 10.1111/j.1440-1797.2012.01580.x. [DOI] [PubMed] [Google Scholar]
- 172.Doshi N, Thet Z, Han T, Martin J. 2019. A case of intestinal microsporidiosis in a renal transplant recipient. J Med Cases 10:229–233. 10.14740/jmc3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brown M, Longano A, Dendle C, Polkinghorne KR, Kanellis J. 2018. Confirmed microsporidial graft infection in a HIV-negative renal transplant recipient: a case report and review of the literature. Transpl Infect Dis 20:e12888. 10.1111/tid.12888. [DOI] [PubMed] [Google Scholar]
- 174.Kicia M, Wesolowska M, Kopacz Z, Jakuszko K, Sak B, Květonová D, Krajewska M, Kváč M. 2016. Prevalence and molecular characteristics of urinary and intestinal microsporidia infections in renal transplant recipients. Clin Microbiol Infect 22:462.e5-9. 10.1016/j.cmi.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 175.Shah S, Jacob SS, Mani R, Parameswaran A, Kumar S, Annigeria RA, Mahesh R, Uppluluri R. 2020. Renal microsporidiosis in pediatric bone marrow transplant recipients: a case series. Turk Patoloji Derg 36:68–72. 10.5146/tjpath.2017.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rabodonirina M, Bertocchi M, Desportes-Livage I, Cotte L, Levrey H, Piens MA, Monneret G, Celard M, Mornex JF, Mojon M. 1996. Enterocytozoon bieneusi as a cause of chronic diarrhea in a heart-lung transplant recipient who was seronegative for human immunodeficiency virus. Clin Infect Dis 23:114–117. 10.1093/clinids/23.1.114. [DOI] [PubMed] [Google Scholar]
- 177.Sax PE, Rich JD, Pieciak WS, Trnka YM. 1995. Intestinal microsporidiosis occurring in a liver transplant recipient. Transplantation 60:617–618. 10.1097/00007890-199509270-00018. [DOI] [PubMed] [Google Scholar]
- 178.Gumbo T, Hobbs RE, Carlyn C, Hall G, Isada CM. 1999. Microsporidia infection in transplant patients. Transplantation 67:482–484. 10.1097/00007890-199902150-00024. [DOI] [PubMed] [Google Scholar]
- 179.Kelkar R, Sastry PS, Kulkarni SS, Saikia TK, Parikh PM, Advani SH. 1997. Pulmonary microsporidial infection in a patient with CML undergoing allogeneic marrow transplant. Bone Marrow Transplant 19:179–182. 10.1038/sj.bmt.1700536. [DOI] [PubMed] [Google Scholar]
- 180.Mahmood MN, Keohane ME, Burd EM. 2003. Pathologic quiz case: a 45-year-old renal transplant recipient with persistent fever. Arch Pathol Lab Med 127:e224-6. 10.5858/2003-127-e224-PQCAYO. [DOI] [PubMed] [Google Scholar]
- 181.Metge S, Van Nhieu JT, Dahmane D, Grimbert P, Foulet F, Sarfati C, Bretagne S. 2000. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur J Clin Microbiol Infect Dis 19:221–223. 10.1007/s100960050463. [DOI] [PubMed] [Google Scholar]
- 182.Field A, Paik J, Stark D, Qiu M, Morey A, Plit M, Canning E, Glanville A. 2012. Myositis due to the microsporidian Anncaliia (Brachiola) algerae in a lung transplant recipient. Transpl Infect Dis 14:169–176. 10.1111/j.1399-3062.2012.00724.x. [DOI] [PubMed] [Google Scholar]
- 183.Lanternier F, Boutboul D, Menotti J, Chandesris MO, Sarfati C, Mamzer Bruneel MF, Calmus Y, Mechai F, Viard JP, Lecuit M, Bougnoux ME, Lortholary O. 2009. Microsporidiosis in solid organ transplant recipients: two Enterocytozoon bieneusi cases and review. Transpl Infect Dis 11:83–88. 10.1111/j.1399-3062.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 184.Silverstein BE, Cunningham ETJ, Margolis TP, Cevallos V, Wong IG. 1997. Microsporidial keratoconjunctivitis in a patient without human immunodeficiency virus infection. Am J Ophthalmol 124:395–396. 10.1016/S0002-9394(14)70833-5. [DOI] [PubMed] [Google Scholar]
- 185.Hocevar SN, Paddock CD, Spak CW, Rosenblatt R, Diaz-Luna H, Castillo I, Luna S, Friedman GC, Antony S, Stoddard RA, Tiller RV, Peterson T, Blau DM, Sriram RR, da Silva A, de Almeida M, Benedict T, Goldsmith CS, Zaki SR, Visvesvara GS, Kuehnert MJ, Microsporidia Transplant Transmission Investigation Team . 2014. Microsporidiosis acquired through solid organ transplantation: a public health investigation. Ann Intern Med 160:213–220. 10.7326/M13-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Smith RM, Muehlenbachs A, Schaenmann J, Baxi S, Koo S, Blau D, Chin-Hong P, Thorner AR, Kuehnert MJ, Wheeler K, Liakos A, Jackson JW, Benedict T, da Silva AJ, Ritter JM, Rollin D, Metcalfe M, Goldsmith CS, Visvesvara GS, Basavaraju SV, Zaki S. 2017. Three cases of neurologic syndrome caused by donor-derived microsporidiosis. Emerg Infect Dis 23:387–395. 10.3201/eid2303.161580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Goodgame R. 2003. Emerging causes of traveler's diarrhea: Cryptosporidium, Cyclospora, Isospora, and Microsporidia. Curr Infect Dis Rep 5:66–73. 10.1007/s11908-003-0067-x. [DOI] [PubMed] [Google Scholar]
- 188.Tu EY, Joslin CE. 2012. Microsporidia and Acanthamoeba: the role of emerging corneal pathogens. Eye 26:222–227. 10.1038/eye.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Theng J, Chan C, Ling ML, Tan D. 2001. Microsporidial keratoconjunctivitis in a healthy contact lens wearer without human immunodeficiency virus infection. Ophthalmology 108:976–978. 10.1016/S0161-6420(01)00542-5. [DOI] [PubMed] [Google Scholar]
- 190.Enriquez FJ, Taren D, Cruz-Lopez A, Muramoto M, Palting JD, Cruz P. 1998. Prevalence of intestinal encephalitozoonosis in Mexico. Clin Infect Dis 26:1227–1229. 10.1086/520278. [DOI] [PubMed] [Google Scholar]
- 191.Hutin YJ, Sombardier MN, Liguory O, Sarfati C, Derouin F, Modai J, Molina JM. 1998. Risk factors for intestinal microsporidiosis in patients with human immunodeficiency virus infection: a case-control study. J Infect Dis 178:904–907. 10.1086/515353. [DOI] [PubMed] [Google Scholar]
- 192.Conteas CN, Berlin OG, Lariviere MJ, Pandhumas SS, Speck CE, Porschen R, Nakaya T. 1998. Examination of the prevalence and seasonal variation of intestinal microsporidiosis in the stools of persons with chronic diarrhea and human immunodeficiency virus infection. Am J Trop Med Hyg 58:559–561. 10.4269/ajtmh.1998.58.559. [DOI] [PubMed] [Google Scholar]
- 193.Wuhib T, Silva TMJ, Newman RD, Garcia LS, Pereira MLD, Chaves CS, Wahlquist SP, Bryan RT, Guerrant RL, Sousa A.dQ, de Queiroz TRBS, Sears CL. 1994. Cryptosporidial and microsporidial infections in human immunodeficiency virus-infected patients in northeastern Brazil. J Infect Dis 170:494–497. 10.1093/infdis/170.2.494. [DOI] [PubMed] [Google Scholar]
- 194.Fan NW, Wu CC, Chen TL, Yu WK, Chen CP, Lee SM, Lin PY. 2012. Microsporidial keratitis in patients with hot springs exposure. J Clin Microbiol 50:414–418. 10.1128/JCM.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chan KS, Koh TH. 2015. Microsporidian eye infection from outdoor recreational activities. J Clin Microbiol 53:753–753. 10.1128/JCM.03192-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tan J, Lee P, Lai Y, Hishamuddin P, Tay J, Tan AL, Chan KS, Lin R, Tan D, Cutter J, Goh KT. 2013. Microsporidial keratoconjunctivitis after rugby tournament, Singapore. Emerg Infect Dis 19:1484–1486. 10.3201/eid1909.121464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Waller T. 1979. Sensitivity of Encephalitozoon cuniculi to various temperatures, disinfectants and drugs. Lab Anim 13:227–230. 10.1258/002367779780937753. [DOI] [PubMed] [Google Scholar]
- 198.Mathis A, Weber R, Deplazes P. 2005. Zoonotic potential of the microsporidia. Clin Microbiol Rev 18:423–445. 10.1128/CMR.18.3.423-445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Anane S, Attouchi H. 2010. Microsporidiosis: epidemiology, clinical data and therapy. Gastroenterol Clin Biol 34:450–464. 10.1016/j.gcb.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 200.Deplazes P, Mathis A, Weber R. 2000. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib Microbiol 6:236–260. 10.1159/000060363. [DOI] [PubMed] [Google Scholar]
- 201.Fuentealba IC, Mahoney NT, Shadduck JA, Harvill J, Wicher V, Wicher K. 1992. Hepatic lesions in rabbits infected with Encephalitozoon cuniculi administered per rectum. Vet Pathol 29:536–540. 10.1177/030098589202900608. [DOI] [PubMed] [Google Scholar]
- 202.Schwartz DA, Visvesvara G, Weber R, Bryan RT. 1994. Male genital tract microsporidiosis and AIDS: prostatic abscess due to Encephalitozoon hellem. J Eukaryot Microbiol 41:61S. [PubMed] [Google Scholar]
- 203.Schwartz DA, Bryan RT, Hewan-Lowe KO, Weber R, Visvesvara GS, Cali A, Angritt P. 1992. Disseminated microsporidiosis (Encephalitozoon hellem) and acquired immunodeficiency syndrome. Autopsy evidence for respiratory acquisition. Arch Pathol Lab Med 116:660–668. [PubMed] [Google Scholar]
- 204.Hunt RD, King NW, Foster HL. 1972. Encephalitozoonosis: evidence for vertical transmission. J Infect Dis 126:212–214. 10.1093/infdis/126.2.212. [DOI] [PubMed] [Google Scholar]
- 205.McInnes EF, Stewart CG. 1991. The pathology of subclinical infection of Encephalitozoon cuniculi in canine dams producing pups with overt encephalitozoonosis. J S Afr Vet Assoc 62:51–54. 10.4102/jsava.v62i2.1590. [DOI] [PubMed] [Google Scholar]
- 206.Cama VA, Pearson J, Cabrera L, Pacheco L, Gilman R, Meyer S, Ortega Y, Xiao L. 2007. Transmission of Enterocytozoon bieneusi between a child and guinea pigs. J Clin Microbiol 45:2708–2710. 10.1128/JCM.00725-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Karim MR, Dong H, Yu F, Jian F, Zhang L, Wang R, Zhang S, Rume FI, Ning C, Xiao L. 2014. Genetic diversity in Enterocytozoon bieneusi isolates from dogs and cats in China: host specificity and public health implications. J Clin Microbiol 52:3297–3302. 10.1128/JCM.01352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lores B, Del Aguila C, Arias C. 2002. Enterocytozoon bieneusi (microsporidia) in faecal samples from domestic animals from Galicia, Spain. Mem Inst Oswaldo Cruz 97:941–945. 10.1590/s0074-02762002000700003. [DOI] [PubMed] [Google Scholar]
- 209.Bornay-Llinares FJ, da Silva AJ, Moura H, Schwartz DA, Visvesvara GS, Pieniazek NJ, Cruz-López A, Hernández-Jaúregui P, Guerrero J, Enriquez FJ. 1998. Immunologic, microscopic, and molecular evidence of Encephalitozoon intestinalis (Septata intestinalis) infection in mammals other than humans. J Infect Dis 178:820–826. 10.1086/515356. [DOI] [PubMed] [Google Scholar]
- 210.Valencáková A, Balent P, Húska M, Novotný F, Luptáková L. 2006. First report on Encephalitozoon intestinalis infection of swine in Europe. Acta Vet Hung 54:407–411. 10.1556/AVet.54.2006.3.11. [DOI] [PubMed] [Google Scholar]
- 211.Haro M, Izquierdo F, Henriques-Gil N, Andrés I, Alonso F, Fenoy S, del Águila C. 2005. First detection and genotyping of human-associated microsporidia in pigeons from urban parks. Appl Environ Microbiol 71:3153. 10.1128/AEM.71.6.3153-3157.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Snowden K, Logan K, Didier E. 1999. Encephalitozoon cuniculi strain III is a cause of encephalitozoonosis in both humans and dogs. J Infect Dis 180:2086–2088. 10.1086/315154. [DOI] [PubMed] [Google Scholar]
- 213.Black SS, Steinohrt LA, Bertucci DC, Rogers LB, Didier ES. 1997. Encephalitozoon hellem in budgerigars (Melopsittacus undulatus). Vet Pathol 34:189–198. 10.1177/030098589703400303. [DOI] [PubMed] [Google Scholar]
- 214.Santín M, Fayer R. 2011. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res Vet Sci 90:363–371. 10.1016/j.rvsc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 215.Stentiford GD, Becnel J, Weiss LM, Keeling PJ, Didier ES, Williams BP, Bjornson S, Kent ML, Freeman MA, Brown MJF, Troemel ER, Roesel K, Sokolova Y, Snowden KF, Solter L. 2016. Microsporidia—emergent pathogens in the global food chain. Trends Parasitol 32:336–348. 10.1016/j.pt.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Askari Z, Mirjalali H, Mohebali M, Zarei Z, Shojaei S, Rezaeian T, Rezaeian M. 2015. Molecular detection and identification of zoonotic microsporidia spore in fecal samples of some animals with close-contact to human. Iran J Parasitol 10:381–388. [PMC free article] [PubMed] [Google Scholar]
- 217.Vahedi SM, Jamshidi S, Shayan P, Bokaie S, Ashrafi Tamai I, Javanmard E, Mirjalali H. 2020. Intestinal microsporidia infection among cat owners and non-pet owners in Iran: a case-control study. Parasitol Res 119:1903–1913. 10.1007/s00436-020-06690-8. [DOI] [PubMed] [Google Scholar]
- 218.Cissé G. 2019. Food-borne and water-borne diseases under climate change in low- and middle-income countries: further efforts needed for reducing environmental health exposure risks. Acta Trop 194:181–188. 10.1016/j.actatropica.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Franzen C, Müller A. 1999. Molecular techniques for detection, species differentiation, and phylogenetic analysis of microsporidia. Clin Microbiol Rev 12:243–285. 10.1128/CMR.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Paterson RRM, Lima N. 2015. Molecular biology of food and water borne mycotoxigenic and mycotic fungi. CRC Press, Boca Raton, FL. [Google Scholar]
- 221.Moss JA, Snyder RA. 2017. Biofilms for monitoring presence of microsporidia in environmental water. J Eukaryot Microbiol 64:533–538. 10.1111/jeu.12390. [DOI] [PubMed] [Google Scholar]
- 222.Fournier S, Liguory O, Santillana-Hayat M, Guillot E, Sarfati C, Dumoutier N, Molina J-M, Derouin F. 2000. Detection of microsporidia in surface water: a one-year follow-up study. FEMS Immunol Med Microbiol 29:95–100. 10.1111/j.1574-695X.2000.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 223.Chen J-S, Hsu B-M, Tsai H-C, Chen Y-P, Huang T-Y, Li K-Y, Ji D-D, Lee H-S. 2018. Molecular surveillance of Vittaforma-like microsporidia by a small-volume procedure in drinking water source in Taiwan: evidence for diverse and emergent pathogens. Environ Sci Pollut Res Int 25:18823–18837. 10.1007/s11356-018-2081-4. [DOI] [PubMed] [Google Scholar]
- 224.Slodkowicz-Kowalska A, Graczyk TK, Tamang L, Jedrzejewski S, Nowosad A, Zduniak P, Solarczyk P, Girouard AS, Majewska AC. 2006. Microsporidian species known to infect humans are present in aquatic birds: implications for transmission via water? Appl Environ Microbiol 72:4540–4544. 10.1128/AEM.02503-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Slifko TR, Smith HV, Rose JB. 2000. Emerging parasite zoonoses associated with water and food. Int J Parasitol 30:1379–1393. 10.1016/S0020-7519(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 226.Orlandi PA, Chu D-MT, Bier JW, Jackson GJ. 2002. Parasites and the food supply. Food Technol 56:72–79. [Google Scholar]
- 227.Calvo M, Carazo M, Arias ML, Chaves C, Monge R, Chinchilla M. 2004. Prevalence of Cyclospora sp., Cryptosporidium sp., microsporidia and fecal coliform determination in fresh fruit and vegetables consumed in Costa Rica. Archiv Latinoam Nutr 54:428–432. (In Spanish.) [PubMed] [Google Scholar]
- 228.Javanmard E, Mirjalali H, Niyyati M, Jalilzadeh E, Seyed Tabaei SJ, Asadzadeh Aghdaei H, Nazemalhosseini-Mojarad E, Zali MR. 2018. Molecular and phylogenetic evidences of dispersion of human-infecting microsporidia to vegetable farms via irrigation with treated wastewater: one-year follow up. Int J Hyg Environ Health 221:642–651. 10.1016/j.ijheh.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 229.Sak B, Vecková T, Brdíčková K, Smetana P, Hlásková L, Kicia M, Holubová N, McEvoy J, Kváč M. 2019. Experimental Encephalitozoon cuniculi infection acquired from fermented meat products. Foodborne Pathog Dis 16:394–398. 10.1089/fpd.2018.2569. [DOI] [PubMed] [Google Scholar]
- 230.Vu-Khac H, Nguyen Thi Thanh T, Thu G, Le CH, Nguyen VD. 2018. Vertical transmission and early diagnosis of the microsporidian Enterocytozoon hepatonaei in whiteleg shrimp Penaeus vannamei. J Pure Appl Microbiol 12:1125–1131. 10.22207/JPAM.12.3.11. [DOI] [Google Scholar]
- 231.Goertz D, Solter LF, Linde A. 2007. Horizontal and vertical transmission of a Nosema sp. (Microsporidia) from Lymantria dispar (L.) (Lepidoptera: Lymantriidae). J Invertebr Pathol 95:9–16. 10.1016/j.jip.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 232.Terry RS, Smith JE, Bouchon D, Rigaud T, Duncanson P, Sharpe RG, Dunn AM. 1999. Ultrastructural characterisation and molecular taxonomic identification of Nosema granulosis n. sp., a transovarially transmitted feminising (TTF) microsporidium. J Eukaryot Microbiol 46:492–499. 10.1111/j.1550-7408.1999.tb06066.x. [DOI] [PubMed] [Google Scholar]
- 233.Dunn AM, Terry RS, Smith JE. 2001. Transovarial transmission in the microsporidia. Adv Parasitol 48:57–100. 10.1016/s0065-308x(01)48005-5. [DOI] [PubMed] [Google Scholar]
- 234.Stentiford GD, Feist SW, Stone DM, Bateman KS, Dunn AM. 2013. Microsporidia: diverse, dynamic, and emergent pathogens in aquatic systems. Trends Parasitol 29:567–578. 10.1016/j.pt.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 235.Bulnheim HP, Vávra J. 1968. Infection by the microsporidian Octosporea effeminans sp. n., and its sex determining influence in the amphipod Gammarus duebeni. J Parasitol 54:241–248. 10.2307/3276928. [DOI] [PubMed] [Google Scholar]
- 236.Williams BAP. 2009. Unique physiology of host-parasite interactions in microsporidia infections. Cell Microbiol 11:1551–1560. 10.1111/j.1462-5822.2009.01362.x. [DOI] [PubMed] [Google Scholar]
- 237.Didier ES, Snowden KF, Shadduck JA. 1998. Biology of microsporidian species infecting mammals. Adv Parasitol 40:283–320. 10.1016/S0065-308X(08)60125-6. [DOI] [PubMed] [Google Scholar]
- 238.Snowden KF, Didier ES, Orenstein JM, Shadduck JA. 1998. Animal models of human microsporidial infections. Lab Anim Sci 48:589–592. [PubMed] [Google Scholar]
- 239.Mota P, Rauch CA, Edberg SC. 2000. Microsporidia and Cyclospora: epidemiology and assessment of risk from the environment. Crit Rev Microbiol 26:69–90. 10.1080/10408410091154192. [DOI] [PubMed] [Google Scholar]
- 240.Poley JD, Sutherland BJG, Fast MD, Koop BF, Jones SRM. 2017. Effects of the vertically transmitted microsporidian Facilispora margolisi and the parasiticide emamectin benzoate on salmon lice (Lepeophtheirus salmonis). BMC Genomics 18:630. 10.1186/s12864-017-4040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Work TM. 2019. Ecology of invertebrate diseases. J Wildl Dis 55:742–744. 10.7589/0090-3558-55.3.742. [DOI] [Google Scholar]
- 242.Birthistle K, Moore P, Hay P. 1996. Microsporidia: a new sexually transmissable cause of urethritis. Genitourin Med 72:445. 10.1136/sti.72.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Han B, Takvorian PM, Weiss LM. 2020. Invasion of host cells by microsporidia. Front Microbiol 11:172. 10.3389/fmicb.2020.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wittner M, Weiss LM (ed). 1999. The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 245.Frixione E, Ruiz L, Santillán M, de Vargas LV, Tejero JM, Undeen AH. 1992. Dynamics of polar filament discharge and sporoplasm expulsion by microsporidian spores. Cell Motil Cytoskeleton 22:38–50. 10.1002/cm.970220105. [DOI] [Google Scholar]
- 246.Weidner E. 1972. Ultrastructural study of microsporidian invasion into cells. Z Parasitenkd 40:227–242. 10.1007/BF00329623. [DOI] [PubMed] [Google Scholar]
- 247.Erickson B, Blanquet R. 1969. The occurrence of chitin in the spore wall of Glugea weissenbergi. J Invertebr Pathol 14:358–364. 10.1016/0022-2011(69)90162-1. [DOI] [PubMed] [Google Scholar]
- 248.Brosson D, Kuhn L, Prensier G, Vivares CP, Texier C. 2005. The putative chitin deacetylase of Encephalitozoon cuniculi: a surface protein implicated in microsporidian spore-wall formation. FEMS Microbiol Lett 247:81–90. 10.1016/j.femsle.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 249.Urch JE, Hurtado-Guerrero R, Brosson D, Liu Z, Eijsink VG, Texier C, van Aalten DM. 2009. Structural and functional characterization of a putative polysaccharide deacetylase of the human parasite Encephalitozoon cuniculi. Protein Sci 18:1197–1209. 10.1002/pro.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Takvorian PM, Han B, Cali A, Rice WJ, Gunther L, Macaluso F, Weiss LM. 2020. An ultrastructural study of the extruded polar tube of Anncaliia algerae (Microsporidia). J Eukaryot Microbiol 67:28–44. 10.1111/jeu.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hayman JR, Southern TR, Nash TE. 2005. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect Immun 73:841–848. 10.1128/IAI.73.2.841-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Dolgikh VV, Semenov PB. 2003. The spore wall and polar tube proteins of the microsporidian Nosema grylli: the major spore wall protein is released before spore extrusion. Tsitologiia 45:324–329. [PubMed] [Google Scholar]
- 253.Hayman JR, Hayes SF, Amon J, Nash TE. 2001. Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect Immun 69:7057–7066. 10.1128/IAI.69.11.7057-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Xu Y, Takvorian P, Cali A, Wang F, Zhang H, Orr G, Weiss LM. 2006. Identification of a new spore wall protein from Encephalitozoon cuniculi. Infect Immun 74:239–247. 10.1128/IAI.74.1.239-247.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Peuvel-Fanget I, Polonais V, Brosson D, Texier C, Kuhn L, Peyret P, Vivares C, Delbac F. 2006. EnP1 and EnP2, two proteins associated with the Encephalitozoon cuniculi endospore, the chitin-rich inner layer of the microsporidian spore wall. Int J Parasitol 36:309–318. 10.1016/j.ijpara.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 256.Cai S, Lu X, Qiu H, Li M, Feng Z. 2011. Identification of a Nosema bombycis (Microsporidia) spore wall protein corresponding to spore phagocytosis. Parasitology 138:1102–1109. 10.1017/S0031182011000801. [DOI] [PubMed] [Google Scholar]
- 257.Wu Z, Li Y, Pan G, Zhou Z, Xiang Z. 2009. SWP25, a novel protein associated with the Nosema bombycis endospore. J Eukaryot Microbiol 56:113–118. 10.1111/j.1550-7408.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- 258.Ambrosioni J, van Delden C, Krause KH, Bouchuiguir-Wafa C, Nagy M, Passweg J, Chalandon Y. 2009. Invasive microsporidiosis in allogeneic haematopoietic SCT recipients. Bone Marrow Transpl 45:1249–1251. 10.1038/bmt.2009.315. [DOI] [PubMed] [Google Scholar]
- 259.Wu Z, Li Y, Pan G, Tan X, Hu J, Zhou Z, Xiang Z. 2008. Proteomic analysis of spore wall proteins and identification of two spore wall proteins from Nosema bombycis (Microsporidia). Proteomics 8:2447–2461. 10.1002/pmic.200700584. [DOI] [PubMed] [Google Scholar]
- 260.Wang JY, Chambon C, Lu CD, Huang KW, Vivares CP, Texier C. 2007. A proteomic-based approach for the characterization of some major structural proteins involved in host-parasite relationships from the silkworm parasite Nosema bombycis (Microsporidia). Proteomics 7:1461–1472. 10.1002/pmic.200600825. [DOI] [PubMed] [Google Scholar]
- 261.Bouzahzah B, Weiss LM. 2010. Glycosylation of the major polar tube protein of Encephalitozoon cuniculi. Parasitol Res 107:761–764. 10.1007/s00436-010-1950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xu Y, Takvorian PM, Cali A, Orr G, Weiss LM. 2004. Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans. Infect Immun 72:6341–6350. 10.1128/IAI.72.11.6341-6350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dolgikh VV, Semenov PB, Beznusenko GV. 2007. Protein glycosylation in the spores of the microsporidia Paranosema (Antonospora) grylli. Tsitologiia 49:607–613. (In Russian.) [PubMed] [Google Scholar]
- 264.Ghosh K, Nieves E, Keeling P, Pombert JF, Henrich PP, Cali A, Weiss LM. 2011. Branching network of proteinaceous filaments within the parasitophorous vacuole of Encephalitozoon cuniculi and Encephalitozoon hellem. Infect Immun 79:1374–1385. 10.1128/IAI.01152-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Li Y, Wu Z, Pan G, He W, Zhang R, Hu J, Zhou Z. 2009. Identification of a novel spore wall protein (SWP26) from microsporidia Nosema bombycis. Int J Parasitol 39:391–398. 10.1016/j.ijpara.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 266.Chen L, Li R, You Y, Zhang K, Zhang L. 2017. A novel spore wall protein from Antonospora locustae (Microsporidia: Nosematidae) contributes to sporulation. J Eukaryot Microbiol 64:779–791. 10.1111/jeu.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Yang D, Pan G, Dang X, Shi Y, Li C, Peng P, Luo B, Bian M, Song Y, Ma C. 2015. Interaction and assembly of two novel proteins in the spore wall of the microsporidian species Nosema bombycis and their roles in adherence to and infection of host cells. Infect Immun 83:1715–1731. 10.1128/IAI.03155-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yang D, Dang X, Peng P, Long M, Ma C, Qin JJG, Wu H, Liu T, Zhou X, Pan G. 2014. NbHSWP11, a microsporidia Nosema bombycis protein, localizing in the spore wall and membranes, reduces spore adherence to host cell BME. J Parasitol 100:623–633. 10.1645/13-286.1. [DOI] [PubMed] [Google Scholar]
- 269.Wang Y, Dang X, Ma Q, Liu F, Pan G, Li T, Zhou Z. 2015. Characterization of a novel spore wall protein NbSWP16 with proline-rich tandem repeats from Nosema bombycis (microsporidia). Parasitology 142:534–542. 10.1017/S0031182014001565. [DOI] [PubMed] [Google Scholar]
- 270.Jaroenlak P, Boakye DW, Vanichviriyakit R, Williams BA, Sritunyalucksana K, Itsathitphaisarn O. 2018. Identification, characterization and heparin binding capacity of a spore-wall, virulence protein from the shrimp microsporidian, Enterocytozoon hepatopenaei (EHP). Parasite Vectors 11:177. 10.1186/s13071-018-2758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Barclay AN. 2003. Membrane proteins with immunoglobulin-like domains—a master superfamily of interaction molecules. Sem Immunol 15:215–223. 10.1016/S1044-5323(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 272.Leonard CA, Hayman JR. 2017. Role of host cell integrins in the microsporidium Encephalitozoon intestinalis adherence and infection in vitro. FEMS Microbiol Lett 364. 10.1093/femsle/fnx169. [DOI] [PubMed] [Google Scholar]
- 273.Lv Q, Wang L, Fan Y, Meng X, Liu K, Zhou B, Chen J, Pan G, Long M, Zhou Z. 2020. Identification and characterization a novel polar tube protein (NbPTP6) from the microsporidian Nosema bombycis. Parasit Vectors 13:475. 10.1186/s13071-020-04348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. 1996. Purification and characterization of a microsporidian polar tube protein. Mol Biochem Parasitol 79:255–259. 10.1016/0166-6851(96)02666-7. [DOI] [PubMed] [Google Scholar]
- 275.Delbac F, Duffieux F, David D, Metenier G, Vivares CP. 1998. Immunocytochemical identification of spore proteins in two microsporidia, with emphasis on extrusion apparatus. J Eukaryot Microbiol 45:224–231. 10.1111/j.1550-7408.1998.tb04529.x. [DOI] [PubMed] [Google Scholar]
- 276.Delbac F, Peuvel I, Metenier G, Peyretaillade E, Vivares CP. 2001. Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect Immun 69:1016–1024. 10.1128/IAI.69.2.1016-1024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xu Y, Takvorian P, Cali A, Weiss LM. 2003. Lectin binding of the major polar tube protein (PTP1) and its role in invasion. J Eukaryot Microbiol 50:600–601. 10.1111/j.1550-7408.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 278.Peuvel I, Peyret P, Metenier G, Vivares CP, Delbac F. 2002. The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol Biochem Parasitol 122:69–80. 10.1016/s0166-6851(02)00073-7. [DOI] [PubMed] [Google Scholar]
- 279.Bouzahzah B, Nagajyothi F, Ghosh K, Takvorian PM, Cali A, Tanowitz HB, Weiss LM. 2010. Interactions of Encephalitozoon cuniculi polar tube proteins. Infect Immun 78:2745–2753. 10.1128/IAI.01205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Han B, Polonais V, Sugi T, Yakubu R, Takvorian PM, Cali A, Maier K, Long M, Levy M, Tanowitz HB, Pan G, Delbac F, Zhou Z, Weiss LM. 2017. The role of microsporidian polar tube protein 4 (PTP4) in host cell infection. PLoS Pathog 13:e1006341. 10.1371/journal.ppat.1006341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Takvorian PM, Weiss LM, Cali A. 2005. The early events of Brachiola algerae (Microsporidia) infection: spore germination, sporoplasm structure, and development within host cells. Folia Parasitol (Praha) 52:118–129. 10.14411/fp.2005.015. [DOI] [PubMed] [Google Scholar]
- 282.Han B, Ma Y, Tu V, Tomita T, Mayoral J, Williams T, Horta A, Huang H, Weiss LM. 2019. Microsporidia interact with host cell mitochondria via voltage-dependent anion channels using sporoplasm surface protein 1. mBio 10:e01944-19. 10.1128/mBio.01944-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cali A, Takvorian PM. 2014. Developmental morphology and life cycles of the microsporidia, p 71–133. In Weiss LM, Becnel JJ (ed) Microsporidia: pathogens of opportunity. Wiley Press, Hoboken, NJ. 10.1002/9781118395264.ch2. [DOI] [Google Scholar]
- 284.Goldberg AV, Molik S, Tsaousis AD, Neumann K, Kuhnke G, Delbac F, Vivares CP, Hirt RP, Lill R, Embley TM. 2008. Localization and functionality of microsporidian iron-sulphur cluster assembly proteins. Nature 452:624–628. 10.1038/nature06606. [DOI] [PubMed] [Google Scholar]
- 285.Tamim El Jarkass H, Reinke AW. 2020. The ins and outs of host-microsporidia interactions during invasion, proliferation and exit. Cell Microbiol 22:e13247. 10.1111/cmi.13247. [DOI] [PubMed] [Google Scholar]
- 286.He Q, Vossbrinck CR, Yang Q, Meng X-Z, Luo J, Pan G-Q, Zhou Z-Y, Li T. 2019. Evolutionary and functional studies on microsporidian ATP-binding cassettes: insights into the adaptation of microsporidia to obligated intracellular parasitism. Infect Genet Evol 68:136–144. 10.1016/j.meegid.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 287.Tsaousis AD, Kunji ER, Goldberg AV, Lucocq JM, Hirt RP, Embley TM. 2008. A novel route for ATP acquisition by the remnant mitochondria of Encephalitozoon cuniculi. Nature 453:553–556. 10.1038/nature06903. [DOI] [PubMed] [Google Scholar]
- 288.Major P, Sendra KM, Dean P, Williams TA, Watson AK, Thwaites DT, Embley TM, Hirt RP. 2019. A new family of cell surface located purine transporters in microsporidia and related fungal endoparasites. Elife 8:e47037. 10.7554/eLife.47037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hacker C, Howell M, Bhella D, Lucocq J. 2014. Strategies for maximizing ATP supply in the microsporidian Encephalitozoon cuniculi: direct binding of mitochondria to the parasitophorous vacuole and clustering of the mitochondrial porin VDAC. Cell Microbiol 16:565–579. 10.1111/cmi.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Scanlon M, Leitch GJ, Visvesvara GS, Shaw AP. 2004. Relationship between the host cell mitochondria and the parasitophorous vacuole in cells infected with Encephalitozoon microsporidia. J Eukaryot Microbiol 51:81–87. 10.1111/j.1550-7408.2004.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 291.Kotkova M, Sak B, Kvetonova D, Kvac M. 2013. Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One 8:e60941. 10.1371/journal.pone.0060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sak B, Kotkova M, Hlaskova L, Kvac M. 2017. Limited effect of adaptive immune response to control encephalitozoonosis. Parasite Immunol 39:e12496. 10.1111/pim.12496. [DOI] [PubMed] [Google Scholar]
- 293.Hermanek J, Koudela B, Kucerova Z, Ditrich O, Travnicek J. 1993. Prophylactic and therapeutic immune reconstitution of SCID mice infected with Encephalitozoon cuniculi. Folia Parasitol (Praha) 40:287–291. [PubMed] [Google Scholar]
- 294.Schmidt EC, Shadduck JA. 1984. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol 133:2712–2719. [PubMed] [Google Scholar]
- 295.Didier ES, Bowers LC, Martin AD, Kuroda MJ, Khan IA, Didier PJ. 2010. Reactive nitrogen and oxygen species, and iron sequestration contribute to macrophage-mediated control of Encephalitozoon cuniculi (Phylum Microsporidia) infection in vitro and in vivo. Microbes Infect 12:1244–1251. 10.1016/j.micinf.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bywater JE, Kellett BS. 1979. Humoral immune response to natural infection with Encephalitozoon cuniculi in rabbits. Lab Anim 13:293–297. 10.1258/002367779780943305. [DOI] [PubMed] [Google Scholar]
- 297.Furuya K, Omura M, Kudo S, Sugiura W, Azuma H. 2008. Recognition profiles of microsporidian Encephalitozoon cuniculi polar tube protein 1 with human immunoglobulin M antibodies. Parasite Immunol 30:13–21. 10.1111/j.1365-3024.2007.00988.x. [DOI] [PubMed] [Google Scholar]
- 298.Achbarou A, Ombrouck C, Gneragbe T, Charlotte F, Renia L, Desportes-Livage I, Mazier D. 1996. Experimental model for human intestinal microsporidiosis in interferon gamma receptor knockout mice infected by Encephalitozoon intestinalis. Parasite Immunol 18:387–392. 10.1046/j.1365-3024.1996.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 299.Khan IA, Moretto M. 1999. Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect Immun 67:1887–1893. 10.1128/.67.4.1887-1893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Moretto MM, Lawlor EM, Khan IA. 2010. Lack of interleukin-12 in p40-deficient mice leads to poor CD8+ T-cell immunity against Encephalitozoon cuniculi infection. Infect Immun 78:2505–2511. 10.1128/IAI.00753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Khan IA, Schwartzman JD, Kasper LH, Moretto M. 1999. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J Immunol 162:6086–6091. [PubMed] [Google Scholar]
- 302.Moretto MM, Khan IA, Weiss LM. 2012. Gastrointestinal cell mediated immunity and the microsporidia. PLoS Pathog 8:e1002775. 10.1371/journal.ppat.1002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Moretto MM, Harrow DI, Khan IA. 2015. Effector CD8 T cell immunity in microsporidial infection: a lone defense mechanism. Semin Immunopathol 37:281–287. 10.1007/s00281-015-0482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Moretto MM, Weiss LM, Combe CL, Khan IA. 2007. IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection. J Immunol 179:2485–2492. 10.4049/jimmunol.179.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Moretto MM, Harrow DI, Hawley TS, Khan IA. 2015. Interleukin-12-producing CD103+ CD11b− CD8+ dendritic cells are responsible for eliciting gut intraepithelial lymphocyte response against Encephalitozoon cuniculi. Infect Immun 83:4719–4730. 10.1128/IAI.00820-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lawlor EM, Moretto MM, Khan IA. 2010. Optimal CD8 T-cell response against Encephalitozoon cuniculi is mediated by Toll-like receptor 4 upregulation by dendritic cells. Infect Immun 78:3097–3102. 10.1128/IAI.00181-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Fischer J, Suire C, Hale-Donze H. 2008. Toll-like receptor 2 recognition of the microsporidia Encephalitozoon spp. induces nuclear translocation of NF-kappaB and subsequent inflammatory responses. Infect Immun 76:4737–4744. 10.1128/IAI.00733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gigley JP, Khan IA. 2011. Plasmacytoid DC from aged mice down-regulate CD8 T cell responses by inhibiting cDC maturation after Encephalitozoon cuniculi infection. PLoS One 6:e20838. 10.1371/journal.pone.0020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ramanan P, Pritt BS. 2014. Extraintestinal microsporidiosis. J Clin Microbiol 52:3839–3844. 10.1128/JCM.00971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Didier ES, Weiss LM. 2011. Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis 24:490–495. 10.1097/QCO.0b013e32834aa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Desportes-Livage I, Datry A. 2005. Infections à microsporidies, Isospora et Sarcocystis. EMC Malad Infect 2:178–196. 10.1016/j.emcmi.2005.08.001. [DOI] [Google Scholar]
- 312.Conteas CN, Berlin OGW, Ash LR, Pruthi JS. 2000. Therapy for human gastrointestinal microsporidiosis. Am J Trop Med Hyg 63:121–127. 10.4269/ajtmh.2000.63.121. [DOI] [PubMed] [Google Scholar]
- 313.Weber R, Muller A, Spycher MA, Opravil M, Ammann R, Briner J. 1992. Intestinal Enterocytozoon bieneusi microsporidiosis in an HIV-infected patient—diagnosis by ileo-colonoscopic biopsies and long-term follow up. Clin Investig 70:1019–1023. 10.1007/BF00180312. [DOI] [PubMed] [Google Scholar]
- 314.Molina J-M, Sarfati C, Beauvais B, Lemann M, Lesourd A, Ferchal F, Casin I, Lagrange P, Modigliani R, Derouin F, Modal J. 1993. Intestinal microsporidiosis in human immunodeficiency virus-infected patients with chronic unexplained diarrhea—prevalence and clinical and biologic features. J Infect Dis 167:217–221. 10.1093/infdis/167.1.217. [DOI] [PubMed] [Google Scholar]
- 315.Pol S, Romana CA, Richard S, Amouyal P, Desportes-Livage I, Carnot F, Pays JF, Berthelot P. 1993. Microsporidia infection in patients with the human immunodeficiency virus and unexplained cholangitis. N Engl J Med 328:95–99. 10.1056/NEJM199301143280204. [DOI] [PubMed] [Google Scholar]
- 316.Espern A, Morio F, Miegeville M, Illa H, Abdoulaye M, Meyssonnier V, Adehossi E, Lejeune A, Phung DC, Besse B, Gay-Andrieu F. 2007. Molecular study of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon intestinalis among human immunodeficiency virus-infected patients from two geographical areas: Niamey, Niger, and Hanoi, Vietnam. J Clin Microbiol 45:2999–3002. 10.1128/JCM.00684-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.del Aguila C, Lopez-Velez R, Fenoy S, Turrientes C, Cobo J, Navajas R, Visvesvara GS, Croppo GP, Da Silva AJ, Pieniazek NJ. 1997. Identification of Enterocytozoon bieneusi spores in respiratory samples from an AIDS patient with a 2-year history of intestinal microsporidiosis. J Clin Microbiol 35:1862–1866. 10.1128/JCM.35.7.1862-1866.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Hartskeerl RA, Schuitema AR, van Gool T, Terpstra WJ. 1993. Genetic evidence for the occurrence of extra-intestinal Enterocytozoon bieneusi infections. Nucleic Acids Res 21:4150–4150. 10.1093/nar/21.17.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Orenstein JM, Gaetz HP, Yachnis AT, Frankel SS, Mertens RB, Didier ES. 1997. Disseminated microsporidiosis in AIDS: are any organs spared? AIDS 11:385–386. [PubMed] [Google Scholar]
- 320.Sheth SG, Bates C, Federman M, Chopra S. 1997. Fulminant hepatic failure caused by microsporidial infection in a patient with AIDS. AIDS 11:553–554. [PubMed] [Google Scholar]
- 321.Schwartz DA, Sobottka I, Leitch GJ, Cali A, Visvesvara GS. 1996. Pathology of microsporidiosis: emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med 120:173–188. [PubMed] [Google Scholar]
- 322.Soule JB, Halverson AL, Becker RB, Pistole MC, Orenstein JM. 1997. A patient with acquired immunodeficiency syndrome and untreated Encephalitozoon (Septata) intestinalis microsporidiosis leading to small bowel perforation. Response to albendazole. Arch Pathol Lab Med 121:880–887. [PubMed] [Google Scholar]
- 323.Zender HO, Arrigoni E, Eckert J, Kapanci Y. 1989. A case of Encephalitozoon cuniculi peritonitis in a patient with AIDS. Am J Clin Pathol 92:352–356. 10.1093/ajcp/92.3.352. [DOI] [PubMed] [Google Scholar]
- 324.Mertens RB, Didier ES, Fishbein MC, Bertucci DC, Rogers LB, Orenstein JM. 1997. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod Pathol 10:68–77. [PubMed] [Google Scholar]
- 325.Kester KE, Turiansky GW, McEvoy PL. 1998. Nodular cutaneous microsporidiosis in a patient with AIDS and successful treatment with long-term oral clindamycin therapy. Ann Intern Med 128:911–914. 10.7326/0003-4819-128-11-199806010-00009. [DOI] [PubMed] [Google Scholar]
- 326.Kester KE, Visvesara GS, McEvoy P. 2000. Organism responsible for nodular cutaneous microsporidiosis in a patient with AIDS. Ann Intern Med 133:925. 10.7326/0003-4819-133-11-200012050-00028. [DOI] [PubMed] [Google Scholar]
- 327.Schwartz DA, Visvesvara GS, Leitch GJ, Tashjian L, Pollack M, Holden J, Bryan RT. 1993. Pathology of symptomatic microsporidial (Encephalitozoon hellem) bronchiolitis in the acquired immunodeficiency syndrome: a new respiratory pathogen diagnosed from lung biopsy, bronchoalveolar lavage, sputum, and tissue culture. Hum Pathol 24:937–943. 10.1016/0046-8177(93)90106-q. [DOI] [PubMed] [Google Scholar]
- 328.Sobottka I, Albrecht H, Schafer H, Schottelius J, Visvesvara GS, Laufs R, Schwartz DA. 1995. Disseminated Encephalitozoon (Septata) intestinalis infection in a patient with AIDS: novel diagnostic approaches and autopsy-confirmed parasitological cure following treatment with albendazole. J Clin Microbiol 33:2948–2952. 10.1128/JCM.33.11.2948-2952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Tosoni A, Nebuloni M, Ferri A, Bonetto S, Antinori S, Scaglia M, Xiao L, Moura H, Visvesvara GS, Vago L, Costanzi G. 2002. Disseminated microsporidiosis caused by Encephalitozoon cuniculi III (dog type) in an Italian AIDS patient: a retrospective study. Mod Pathol 15:577–583. 10.1038/modpathol.3880566. [DOI] [PubMed] [Google Scholar]
- 330.Fournier S, Liguory O, Sarfati C, David-Ouaknine F, Derouin F, Decazes J, Molina J. 2000. Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med 1:155–161. 10.1046/j.1468-1293.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 331.Galvan AL, Sanchez AM, Valentin MA, Henriques-Gil N, Izquierdo F, Fenoy S, del Aguila C. 2011. First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J Clin Microbiol 49:1301–1306. 10.1128/JCM.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Tanveer N, Barman S. 2015. Microsporidium infection and perforation peritonitis: a rare association. Indian J Sex Transm Dis AIDS 36:188–191. 10.4103/0253-7184.167173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Willson R, Harrington R, Stewart B, Fritsche T. 1995. Human immunodeficiency virus 1-associated necrotizing cholangitis caused by infection with Septata intestinalis. Gastroenterology 108:247–251. 10.1016/0016-5085(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 334.Belcher JW, Jr, Guttenberg SA, Schmookler BM. 1997. Microsporidiosis of the mandible in a patient with acquired immunodeficiency syndrome. J Oral Maxillofac Surg 55:424–426. 10.1016/S0278-2391(97)90142-7. [DOI] [PubMed] [Google Scholar]
- 335.Dore GJ, Marriott DJ, Hing MC, Harkness JL, Field AS. 1995. Disseminated microsporidiosis due to Septata intestinalis in nine patients infected with the human immunodeficiency virus: response to therapy with albendazole. Clin Infect Dis 21:70–76. 10.1093/clinids/21.1.70. [DOI] [PubMed] [Google Scholar]
- 336.Terada S, Reddy KR, Jeffers LJ, Cali A, Schiff ER. 1987. Microsporidan hepatitis in the acquired immunodeficiency syndrome. Ann Intern Med 107:61–62. 10.7326/0003-4819-107-1-61. [DOI] [PubMed] [Google Scholar]
- 337.Filho MM, Ribeiro HB, Paula LJ, Nishioka SA, Tamaki WT, Costa R, Siqueira SF, Kawakami JT, Higuchi ML. 2009. Images in cardiovascular medicine. Endocarditis secondary to microsporidia: giant vegetation in a pacemaker user. Circulation 119:e386–e388. 10.1161/CIRCULATIONAHA.108.817312. [DOI] [PubMed] [Google Scholar]
- 338.Kicia M, Wesolowska M, Kopacz Z, Kvac M, Sak B, Sokulska M, Cebulski K, Hendrich AB, Pozowski A. 2018. Disseminated infection of Encephalitozoon cuniculi associated with osteolysis of hip periprosthetic tissue. Clin Infect Dis 67:1228–1234. 10.1093/cid/ciy256. [DOI] [PubMed] [Google Scholar]
- 339.Bergquist NR, Stintzing G, Smedman L, Waller T, Andersson T. 1984. Diagnosis of encephalitozoonosis in man by serological tests. Br Med J (Clin Res Ed) 288:902–902. 10.1136/bmj.288.6421.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Ditrich O, Chrdle A, Sak B, Chmelik V, Kubale J, Dykova I, Kvac M. 2011. Encephalitozoon cuniculi genotype I as a causative agent of brain abscess in an immunocompetent patient. J Clin Microbiol 49:2769–2771. 10.1128/JCM.00620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Weber R, Kuster H, Visvesvara GS, Bryan RT, Schwartz DA, Luthy R. 1993. Disseminated microsporidiosis due to Encephalitozoon hellem—pulmonary colonization, microhematuria, and mild conjunctivitis in a patient with AIDS. Clin Infect Dis 17:415–419. 10.1093/clinids/17.3.415. [DOI] [PubMed] [Google Scholar]
- 342.Franzen C, Mueller A, Salzberger B, Fatkenhauer G, Diehl V, Schrappe M. 1996. Chronic rhinosinusitis in patients with AIDS: potential role of microsporidia. AIDS (London) 10:687–688. 10.1097/00002030-199606000-00022. [DOI] [PubMed] [Google Scholar]
- 343.Lewis NL, Francis IC, Hawkins GS, Coroneo MT. 2003. Bilateral microsporidial keratoconjunctivitis in an immunocompetent non-contact lens wearer. Cornea 22:374–376. 10.1097/00003226-200305000-00018. [DOI] [PubMed] [Google Scholar]
- 344.Friedberg DN, Stenson SM, Orenstein JM, Tierno PM, Charles NC. 1990. Microsporidial keratoconjunctivitis in acquired immunodeficiency syndrome. Arch Ophthalmol 108:504–508. 10.1001/archopht.1990.01070060052047. [DOI] [PubMed] [Google Scholar]
- 345.Metcalfe TW, Doran RM, Rowlands PL, Curry A, Lacey CJ. 1992. Microsporidial keratoconjunctivitis in a patient with AIDS. Br J Ophthalmol 76:177–178. 10.1136/bjo.76.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lowder CY, Meisler DM, McMahon JT, Longworth DL, Rutherford I. 1990. Microsporidia infection of the cornea in a man seropositive for human immunodeficiency virus. Am J Ophthalmol 109:242–244. 10.1016/S0002-9394(14)76005-2. [DOI] [PubMed] [Google Scholar]
- 347.Rastrelli PD, Didier E, Yee RW. 1994. Microsporidial keratitis. Opthalmol Clin North Am 7:617–631. [Google Scholar]
- 348.Anderson NW, Muehlenbachs A, Arif S, Bruminhent J, Deziel PJ, Razonable RR, Wilhelm MP, Metcalfe MG, Qvarnstrom Y, Pritt BS. 2019. A fatal case of disseminated microsporidiosis due to Anncaliia algerae in a renal and pancreas allograft recipient. Open Forum Infect Dis 6:ofz285. 10.1093/ofid/ofz285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Watts MR, Chan RC, Cheong EY, Brammah S, Clezy KR, Tong C, Marriott D, Webb CE, Chacko B, Tobias V, Outhred AC, Field AS, Prowse MV, Bertouch JV, Stark D, Reddel SW. 2014. Anncaliia algerae microsporidial myositis. Emerg Infect Dis 20:185–191. 10.3201/eid2002.131126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Coyle CM, Weiss LM, Rhodes LV 3rd, Cali A, Takvorian PM, Brown DF, Visvesvara GS, Xiao L, Naktin J, Young E, Gareca M, Colasante G, Wittner M. 2004. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N Engl J Med 351:42–47. 10.1056/NEJMoa032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Weiss LM, Keohane EM. 1999. Microsporidia at the turn of the millenium: Raleigh 1999. J Eukaryot Microbiol 46:3S–5S. 10.1111/j.1550-7408.1999.tb06055.x. [DOI] [PubMed] [Google Scholar]
- 352.Cali A, Neafie R, Weiss LM, Ghosh K, Vergara RB, Gupta R, Takvorian PM. 2010. Human vocal cord infection with the microsporidium Anncaliia algerae. J Eukaryot Microbiol 57:562–567. 10.1111/j.1550-7408.2010.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Visvesvara GS, Moura H, Leitch GJ, Schwartz DA, Xiao LX. 2005. Public health importance of Brachiola algerae (Microsporidia)—an emerging pathogen of humans. Folia Parasitol (Praha) 52:83–94. 10.14411/fp.2005.011. [DOI] [PubMed] [Google Scholar]
- 354.Sutrave G, Maundrell A, Keighley C, Jennings Z, Brammah S, Wang M-X, Pamphlett R, Webb CE, Stark D, Englert H. 2018. Anncaliia algerae microsporidial myositis, New South Wales, Australia. Emerg Infect Dis 24:1528. 10.3201/eid2408.172002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Visvesvara GS, Belloso M, Moura H, Da SA, Moura IN, Leitch GJ, Schwartz DA, Chevez-Barrios P, Wallace S, Pieniazek NJ, Goosey JD. 1999. Isolation of Nosema algerae from the cornea of an immunocompetent patient. J Eukaryot Microbiol 46:10S. [PubMed] [Google Scholar]
- 356.Sood AB, Debiec MR, Yeh S, Grossniklaus HE, Randleman JB. 2016. Microsporidial stromal keratitis and endophthalmitis in an immunocompetent patient. J Ophthalmic Inflamm Infect 6:30. 10.1186/s12348-016-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Weiss LM. 2014. Clinical syndromes associated with microsporidiosis, p 371–401. In Weiss LM, Becnel JJ (ed), Microsporidia: pathogens of opportunity. Wiley Blackwell, Oxford, United Kingdom. [Google Scholar]
- 358.Margileth AM, Strano AJ, Chandra R, Neafie R, Blum M, McCully RM. 1973. Disseminated nosematosis in an immunologically compromised infant. Arch Pathol 95:145–150. [PubMed] [Google Scholar]
- 359.Davis RM, Font RL, Keisler MS, Shadduck JA. 1990. Corneal microsporidiosis: a case report including ultrastructural observations. Ophthalmology 97:953–957. 10.1016/S0161-6420(90)38016-4. [DOI] [PubMed] [Google Scholar]
- 360.Font RL, Samaha AN, Keener MJ, Chevez-Barrios P, Goosey JD. 2000. Corneal microsporidiosis: report of case, including electron microscopic observations. Ophthalmology 107:1769–1775. 10.1016/s0161-6420(00)00285-2. [DOI] [PubMed] [Google Scholar]
- 361.Font RL, Su GW, Matoba AY. 2003. Microsporidial stromal keratitis. Arch Ophthalmol 121:1045–1047. 10.1001/archopht.121.7.1045. [DOI] [PubMed] [Google Scholar]
- 362.Rauz S. 2004. Ultrastructural examination of two cases of stromal microsporidial keratitis. J Med Microbiol 53:775–781. 10.1099/jmm.0.45524-0. [DOI] [PubMed] [Google Scholar]
- 363.Huang H-Y, Wu C-L, Lin S-H, Lin W-C, Huang F-C, Hung J-H, Tseng S-H. 2020. Microsporidial stromal keratitis: characterisation of clinical features, ultrastructural study by electron microscopy and efficacy of different surgical modalities. Br J Ophthalmol 104:1613–1620. 10.1136/bjophthalmol-2019-315094. [DOI] [PubMed] [Google Scholar]
- 364.Sulaiman IM, Matos O, Lobo ML, Xiao L. 2003. Identification of a new microsporidian parasite related to Vittaforma corneae in HIV-positive and HIV-negative patients from Portugal. J Eukaryot Microbiol 50:586–590. 10.1111/j.1550-7408.2003.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 365.Reddy AK, Balne PK, Gaje K, Garg P. 2011. PCR for the diagnosis and species identification of microsporidia in patients with keratitis. Clin Microbiol Infect 17:476–478. 10.1111/j.1469-0691.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- 366.Weber R, Bryan RT. 1994. Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis 19:517–521. 10.1093/clinids/19.3.517. [DOI] [PubMed] [Google Scholar]
- 367.Hollister WS, Canning EU, Weidner E, Field AS, Kench J, Marriott DJ. 1996. Development and ultrastructure of Trachipleistophora hominis n.g., n.sp. after in vitro isolation from an AIDS patient and inoculation into athymic mice. Parasitology 112:143–154. 10.1017/S0031182000065185. [DOI] [PubMed] [Google Scholar]
- 368.Chupp GL, Alroy J, Adelman LS, Breen JC, Skolnik PR. 1993. Myositis due to Pleistophora (Microsporidia) in a patient with AIDS. Clin Infect Dis 16:15–21. 10.1093/clinids/16.1.15. [DOI] [PubMed] [Google Scholar]
- 369.Ledford DK, Overman MD, Gonzalvo A, Cali A, Mester SW, Lockey RF. 1985. Microsporidiosis myositis in a patient with the acquired immunodeficiency syndrome. Ann Int Med 102:628–630. 10.7326/0003-4819-102-5-628. [DOI] [PubMed] [Google Scholar]
- 370.Curry A, Beeching NJ, Gilbert JD, Scott G, Rowland PL, Currie BJ. 2005. Trachipleistophora hominis infection in the myocardium and skeletal muscle of a patient with AIDS. J Infect 51:e139–e144. 10.1016/j.jinf.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 371.Vavra J, Yachnis AT, Shadduck JA, Orenstein JM. 1998. Microsporidia of the genus Trachipleistophora—causative agents of human microsporidiosis: description of Trachipleistophora anthropophthera n. sp. (Protozoa: Microsporidia). J Eukaryot Microbiol 45:273–283. 10.1111/j.1550-7408.1998.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 372.Pariyakanok L, Jongwutiwes S. 2005. Keratitis caused by Trachipleistophora anthropopthera. J Infect 51:325–328. 10.1016/j.jinf.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 373.Grau A, Valls ME, Williams JE, Ellis DS, Muntané MJ, Nadal C. 1996. Myositis caused by Pleistophora in a patient with AIDS. Med Clin (Barc) 107:779–781. (In Spanish.) [PubMed] [Google Scholar]
- 374.Macher AR, Neafie R, Angritt P, Tuur S. 1988. Microsporidia myositis and the acquired immunodefiency syndrome (AIDS): a four year followup. Ann Intern Med 109:343–344. 10.7326/0003-4819-109-4-343_1. [DOI] [PubMed] [Google Scholar]
- 375.Cali A, Takvorian PM. 2003. Ultrastructure and development of Pleistophora ronneafiei n. sp., a microsporidium (Protista) in the skeletal muscle of an immune-compromised individual. J Eukaryot Microbiol 50:77–85. 10.1111/j.1550-7408.2003.tb00237.x. [DOI] [PubMed] [Google Scholar]
- 376.Connors WJ, Carson JA, Chan WW, Parkins MD. 2017. Albendazole-responsive disseminated Tubulinosema acridophagus in a patient with chronic lymphocytic leukaemia. Clin Microbiol Infect 23:684–685. 10.1016/j.cmi.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 377.Cali A, Meisler D, Lowder C, Lembach R, Ayers L, Takvorian P, Rutherford I, Longworth D, McMahon J, Bryan R. 1991. Corneal microsporidioses: characterization and identification. J Protozool 38:215S–217S. [PubMed] [Google Scholar]
- 378.Silveira H, Canning EU. 1995. In vitro cultivation of the human microsporidium Vittaforma corneae: development and effect of albendazole. Folia Parasitol (Praha) 42:241–250. [PubMed] [Google Scholar]
- 379.Garcia LS. 2002. Laboratory identification of the microsporidia. J Clin Microbiol 40:1892–1901. 10.1128/jcm.40.6.1892-1901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Beckers PJ, Derks GJ, Gool T, Rietveld FJ, Sauerwein RW. 1996. Encephalocytozoon intestinalis-specific monoclonal antibodies for laboratory diagnosis of microsporidiosis. J Clin Microbiol 34:282–285. 10.1128/JCM.34.2.282-285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Conteas C, Donovan J, Berlin O, Sowerby T, La RM. 1997. Comparison of fluorescence and standard light microscopy for diagnosis of microsporidia in stools of patients with AIDS and chronic diarrhoea. AIDS 11:386. [PubMed] [Google Scholar]
- 382.Weber R, Bryan RT, Owen RL, Wilcox CM, Gorelkin L, Visvesvara GS, The Enteric Opportunistic Infections Working Group . 1992. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. N Engl J Med 326:161–166. 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 383.Ryan NJ, Sutherland G, Coughlan K, Globan M, Doultree J, Marshall J, Baird RW, Pedersen J, Dwyer B. 1993. A new trichrome-blue stain for detection of microsporidial species in urine, stool, and nasopharyngeal specimens. J Clin Microbiol 31:3264–3269. 10.1128/JCM.31.12.3264-3269.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kokoskin E, Gyorkos TW, Camus A, Cedilotte L, Purtill T, Ward B. 1994. Modified technique for efficient detection of microsporidia. J Clin Microbiol 32:1074–1075. 10.1128/JCM.32.4.1074-1075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Moura H, Schwartz DA, Bornay-Llinares F, Sodre FC, Wallace S, Vivesvara GS. 1997. A new and improved “quick-hot gram-chromotrope” technique that differentially stains microsporidian spores in clinical samples, including paraffin-embedded tissue sections. Arch Pathol Lab Med 121:888–893. [PubMed] [Google Scholar]
- 386.Weber R, Schwartz DA, Deplazes P. 1999. Laboratory diagnosis of microsporidiosis, p 315–362. In Wittner M, Weiss LM (ed), The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 387.van Gool T, Snijders F, Reiss P, Eeftinck Schattenkerk JK, van den Bergh Weerman MA, Bartelsman JF, Bruins JJ, Canning EU, Dankert J. 1993. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J Clin Pathol 46:694–699. 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Didier ES, Orenstein JM, Aldras A, Bertucci D, Rogers LB, Janney FA. 1995. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol 33:3138–3145. 10.1128/JCM.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.DeGirolami PC, Ezratty CR, Desai G, McCullough A, Asmuth D, Wanke C, Federman M. 1995. Diagnosis of intestinal microsporidiosis by examination of stool and duodenal aspirate with Weber's modified trichrome and Uvitex 2B strains. J Clin Microbiol 33:805–810. 10.1128/JCM.33.4.805-810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Peterson TS, Spitsbergen JM, Feist SW, Kent ML. 2011. Luna stain, an improved selective stain for detection of microsporidian spores in histologic sections. Dis Aquat Org 95:175–180. 10.3354/dao02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Sheoran AS, Feng X, Singh I, Chapman-Bonofiglio S, Kitaka S, Hanawalt J, Nunnari J, Mansfield K, Tumwine JK, Tzipori S. 2005. Monoclonal antibodies against Enterocytozoon bieneusi of human origin. Clin Diagn Lab Immunol 12:1109–1113. 10.1128/CDLI.12.9.1109-1113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Singh I, Sheoran AS, Zhang Q, Carville A, Tzipori S. 2005. Sensitivity and specificity of a monoclonal antibody-based fluorescence assay for detecting Enterocytozoon bieneusi spores in feces of simian immunodeficiency virus-infected macaques. Clin Diagn Lab Immunol 12:1141–1144. 10.1128/CDLI.12.10.1141-1144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Thellier M, Breton J. 2008. Enterocytozoon bieneusi in human and animals, focus on laboratory identification and molecular epidemiology. Parasite 15:349–358. 10.1051/parasite/2008153349. [DOI] [PubMed] [Google Scholar]
- 394.Procop GW. 2007. Molecular diagnostics for the detection and characterization of microbial pathogens. Clin Infect Dis 45:S99–S111. 10.1086/519259. [DOI] [PubMed] [Google Scholar]
- 395.Ghosh K, Weiss LM. 2009. Molecular diagnostic tests for microsporidia. Interdiscip Perspect Infect Dis 2009:926521. 10.1155/2009/926521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Velasquez JN, Carnevale S, Labbe JH, Chertcoff A, Cabrera MG, Oelemann W. 1999. In situ hybridization: a molecular approach for the diagnosis of the microsporidian parasite Enterocytozoon bieneusi. Human Pathol 30:54–58. 10.1016/S0046-8177(99)90300-3. [DOI] [PubMed] [Google Scholar]
- 397.Fedorko DP, Hijazi YM. 1996. Application of molecular techniques to the diagnosis of microsporidial infection. Emerg Infect Dis 2:183. 10.3201/eid0203.960304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Katzwinkel-Wladarsch S, Deplazes P, Weber R, Loescher T, Rinder H. 1997. Comparison of polymerase chain reaction with light microscopy for detection of microsporidia in clinical specimens. Eur J Clin Microbiol Infect Dis 16:7–10. 10.1007/BF01575111. [DOI] [PubMed] [Google Scholar]
- 399.Fedorko DP, Nelson NA, Cartwright CP. 1995. Identification of microsporidia in stool specimens by using PCR and restriction endonucleases. J Clin Microbiol 33:1739–1741. 10.1128/JCM.33.7.1739-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Ombrouck C, Ciceron L, Biligui S, Brown S, Marechal P, van Gool T, Datry A, Danis M, Desportes-Livage I. 1997. Specific PCR assay for direct detection of intestinal microsporidia Enterocytozoon bieneusi and Encephalitozoon intestinalis in fecal specimens from human immunodeficiency virus-infected patients. J Clin Microbiol 35:652–655. 10.1128/JCM.35.3.652-655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Müller A, Stellermann K, Hartmann P, Schrappe M, Fätkenheuer G, Salzberger B, Diehl V, Franzen C. 1999. A powerful DNA extraction method and PCR for detection of microsporidia in clinical stool specimens. Clin Diagn Lab Immunol 6:243–246. 10.1128/CDLI.6.2.243-246.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Polley SD, Boadi S, Watson J, Curry A, Chiodini PL. 2011. Detection and species identification of microsporidial infections using SYBR Green real-time PCR. J Med Microbiol 60:459–466. 10.1099/jmm.0.026781-0. [DOI] [PubMed] [Google Scholar]
- 403.Wolk D, Schneider S, Wengenack N, Sloan L, Rosenblatt J. 2002. Real-time PCR method for detection of Encephalitozoon intestinalis from stool specimens. J Clin Microbiol 40:3922–3928. 10.1128/JCM.40.11.3922-3928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Carville A, Mansfield K, Widmer G, Lackner A, Kotler D, Wiest P, Gumbo T, Sarbah S, Tzipori S. 1997. Development and application of genetic probes for detection of Enterocytozoon bieneusi in formalin-fixed stools and in intestinal biopsy specimens from infected patients. Clin Diagn Lab Immunol 4:405–408. 10.1128/CDLI.4.4.405-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Hester JD, Alan Lindquist HD, Bobst AM, Schaefer FW. 2000. Fluorescent in situ detection of Encephalitozoon hellem spores with a 6‐carboxyfluorescein‐labeled ribosomal RNA‐targeted oligonucleotide probe. J Eukaryot Microbiol 47:299–308. 10.1111/j.1550-7408.2000.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 406.Goguel J, Katlama C, Sarfati C, Maslo C, Leport C, Molina JM. 1997. Remission of AIDS-associated intestinal microsporidiosis with highly active antiretroviral therapy. AIDS 11:1658–1659. [PubMed] [Google Scholar]
- 407.Foudraine NA, Weverling GJ, van Gool T, Roos MT, de Wolf F, Koopmans PP, van den Broek PJ, Meenhorst PL, van Leeuwen R, Lange JM, Reiss P. 1998. Improvement of chronic diarrhoea in patients with advanced HIV-1 infection during potent antiretroviral therapy. AIDS 12:35–41. 10.1097/00002030-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 408.Martins SA, Muccioli C, Belfort R, Jr, Castelo A. 2001. Resolution of microsporidial keratoconjunctivitis in an AIDS patient treated with highly active antiretroviral therapy. Am J Ophthalmol 131:378–379. 10.1016/S0002-9394(00)00810-2. [DOI] [PubMed] [Google Scholar]
- 409.Maggi P, Larocca AM, Quarto M, Serio G, Brandonisio O, Angarano G, Pastore G. 2000. Effect of antiretroviral therapy on cryptosporidiosis and microsporidiosis in patients infected with human immunodeficiency virus type 1. Eur J Clin Microbiol Infect Dis 19:213–217. 10.1007/s100960050461. [DOI] [PubMed] [Google Scholar]
- 410.Miao YM, Awad-El-Kariem FM, Franzen C, Ellis DS, Muller A, Counihan HM, Hayes PJ, Gazzard BG. 2000. Eradication of cryptosporidia and microsporidia following successful antiretroviral therapy. J Acquir Immune Defic Syndr 25:124–129. 10.1097/00042560-200010010-00006. [DOI] [PubMed] [Google Scholar]
- 411.Sriaroon C, Mayer CA, Chen L, Accurso C, Greene JN, Vincent AL. 2008. Diffuse intra-abdominal granulomatous seeding as a manifestation of immune reconstitution inflammatory syndrome associated with microsporidiosis in a patient with HIV. AIDS Patient Care STDS 22:611–612. 10.1089/apc.2008.0004. [DOI] [PubMed] [Google Scholar]
- 412.Costa SF, Weiss LM. 2000. Drug treatment of microsporidiosis. Drug Resist Updat 3:384–399. 10.1054/drup.2000.0174. [DOI] [PubMed] [Google Scholar]
- 413.Molina JM, Tourneur M, Sarfati C, Chevret S, de Gouvello A, Gobert JG, Balkan S, Derouin F. 2002. Fumagillin treatment of intestinal microsporidiosis. N Engl J Med 346:1963–1969. 10.1056/NEJMoa012924. [DOI] [PubMed] [Google Scholar]
- 414.Molina J-M, Goguel J, Sarfati C, Chastang C, Desportes-Livage I, Michiels J-F, Maslo C, Katlama C, Cotte L, Leport C, Raffi F. 1997. Potential efficacy of fumagillin in intestinal microsporidiosis due to Enterocytozoon bieneusi in patients with HIV infection: results of a drug screening study. AIDS 11:1603–1610. 10.1097/00002030-199713000-00009. [DOI] [PubMed] [Google Scholar]
- 415.Dunand VA, Hammer SM, Rossi R, Poulin M, Albrecht MA, Doweiko JP, DeGirolami PC, Coakley E, Piessens E, Wanke CA. 1997. Parasitic sinusitis and otitis in patients infected with human immunodeficiency virus: report of five cases and review. Clin Infect Dis 25:267–272. 10.1086/514536. [DOI] [PubMed] [Google Scholar]
- 416.Diesenhouse MC, Wilson LA, Corrent GF, Visvesvara GS, Grossniklaus HE, Bryan RT. 1993. Treatment of microsporidial keratoconjunctivitis with topical fumagillin. Am J Ophthalmol 115:293–298. 10.1016/s0002-9394(14)73578-0. [DOI] [PubMed] [Google Scholar]
- 417.Weber R, Sauer B, Spycher MA, Deplazes P, Keller R, Ammann R, Briner J, Luthy R. 1994. Detection of Septata intestinalis in stool specimens and coprodiagnostic monitoring of successful treatment with albendazole. Clin Infect Dis 19:342–345. 10.1093/clinids/19.2.342. [DOI] [PubMed] [Google Scholar]
- 418.Molina JM, Chastang C, Goguel J, Michiels JF, Sarfati C, Desportes-Livage I, Horton J, Derouin F, Modai J. 1998. Albendazole for treatment and prophylaxis of microsporidiosis due to Encephalitozoon intestinalis in patients with AIDS: a randomized double-blind controlled trial. J Infect Dis 177:1373–1377. 10.1086/515268. [DOI] [PubMed] [Google Scholar]
- 419.Han B, Weiss LM. 2018. Therapeutic targets for the treatment of microsporidiosis in humans. Expert Opin Ther Targets 22:903–915. 10.1080/14728222.2018.1538360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Li J, Katiyar SK, Hamelin A, Visvesvara GS, Edlind TD. 1996. Tubulin genes from AIDS-associated microsporidia and implications for phylogeny and benzimidazole sensitivity. Mol Biochem Parasitol 78:289–295. 10.1016/s0166-6851(96)02628-x. [DOI] [PubMed] [Google Scholar]
- 421.Katiyar S, Edlind T. 1997. In vitro susceptibilities of the AIDS-associated microsporidian Encephalitozoon intestinalis to albendazole, its sulfoxide metabolite, and 12 additional benzimidazole derivatives. Antimicrob Agents Chemo 41:2729–2732. 10.1128/AAC.41.12.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Akiyoshi DE, Weiss LM, Feng X, Williams BA, Keeling PJ, Zhang Q, Tzipori S. 2007. Analysis of the beta-tubulin genes from Enterocytozoon bieneusi isolates from a human and rhesus macaque. J Eukaryot Microbiol 54:38–41. 10.1111/j.1550-7408.2006.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Dieterich DT, Lew EA, Kotler DP, Poles MA, Orenstein JM. 1994. Treatment with albendazole for intestinal disease due to Enterocytozoon bieneusi in patients with AIDS. J Infect Dis 169:178–183. 10.1093/infdis/169.1.178. [DOI] [PubMed] [Google Scholar]
- 424.Higgins M, Kent M, Moran J, Weiss L, Dawe S. 1998. Efficacy of the fumagillin analog TNP-470 for Nucleospora salmonis and Loma salmonae infections in chinook salmon Oncorhynchus tshawytscha. Dis Aquat Org 34:45–49. 10.3354/dao034045. [DOI] [PubMed] [Google Scholar]
- 425.Coyle C, Kent M, Tanowitz HB, Wittner M, Weiss LM. 1998. TNP-470 is an effective antimicrosporidial agent. J Infect Dis 177:515–518. 10.1086/517390. [DOI] [PubMed] [Google Scholar]
- 426.Bukreyeva I, Angoulvant A, Bendib I, Gagnard JC, Bourhis JH, Dargere S, Bonhomme J, Thellier M, Gachot B, Wyplosz B. 2017. Enterocytozoon bieneusi microsporidiosis in stem cell transplant recipients treated with fumagillin. Emerg Infect Dis 23:1039–1041. 10.3201/eid2306.161825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Desoubeaux G, Maakaroun-Vermesse Z, Lier C, Bailly E, Morio F, Labarthe F, Bernard L, Chandenier J. 2013. Successful treatment with fumagillin of the first pediatric case of digestive microsporidiosis in a liver-kidney transplant. Transpl Infect Dis 15:E250–E259. 10.1111/tid.12158. [DOI] [PubMed] [Google Scholar]
- 428.Anwar-Bruni DM, Hogan SE, Schwartz DA, Wilcox CM, Bryan RT, Lennox JL. 1996. Atovaquone is effective treatment for the symptoms of gastrointestinal microsporidiosis in HIV-1-infected patients. AIDS 10:619–623. 10.1097/00002030-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 429.Dionisio D, Sterrantino G, Meli M, Trotta M, Milo D, Leoncini F. 1995. Use of furazolidone for the treatment of microsporidiosis due to Enterocytozoon bieneusi in patients with AIDS. Recenti Prog Med 86:394–397. [PubMed] [Google Scholar]
- 430.Coca M, Kim J, Shenoy S, Chévez-Barrios P, Kapur M. 2016. Microsporidial stromal keratitis: successful treatment with topical voriconazole and oral itraconazole. Cureus 8:e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Rossi P, Urbani C, Donelli G, Pozio E. 1999. Resolution of microsporidial sinusitis and keratoconjunctivitis by itraconazole treatment. Am J Ophthalmol 127:210–212. 10.1016/s0002-9394(98)00352-3. [DOI] [PubMed] [Google Scholar]
- 432.Sridhar MS, Sharma S. 2003. Microsporidial keratoconjunctivitis in a HIV-seronegative patient treated with debridement and oral itraconazole. Am J Ophthalmol 136:745–746. 10.1016/S0002-9394(03)00391-X. [DOI] [PubMed] [Google Scholar]
- 433.Didier ES, Maddry JA, Brindley PJ, Stovall ME, Didier PJ. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev Anti Infect Ther 3:419–434. 10.1586/14787210.3.3.419. [DOI] [PubMed] [Google Scholar]
- 434.Bicart-Sée A, Massip P, Linas M-D, Datry A. 2000. Successful treatment with nitazoxanide of Enterocytozoon bieneusi microsporidiosis in a patient with AIDS. Antimicrob Agents Chemother 44:167–168. 10.1128/aac.44.1.167-168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cabello RR, Guerrero LR, García MRM, Cruz AG. 1997. Nitazoxanide for the treatment of intestinal protozoan and helminthic infections in Mexico. Trans R Soc Trop Med Hyg 91:701–703. 10.1016/S0035-9203(97)90531-9. [DOI] [PubMed] [Google Scholar]
- 436.Rossignol J, Maisonneuve H. 1984. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nana infections. Am J Trop Med Hyg 33:511–512. 10.4269/ajtmh.1984.33.511. [DOI] [PubMed] [Google Scholar]
- 437.Sharpstone D, Rowbottom A, Francis N, Tovey G, Ellis D, Barrett M, Gazzard B. 1997. Thalidomide: a novel therapy for microsporidiosis. Gastroenterology 112:1823–1829. 10.1053/gast.1997.v112.pm9178672. [DOI] [PubMed] [Google Scholar]
- 438.Bacchi CJ, Weiss LM, Lane S, Frydman B, Valasinas A, Reddy V, Sun JS, Marton LJ, Khan IA, Moretto M. 2002. Novel synthetic polyamines are effective in the treatment of experimental microsporidiosis, an opportunistic AIDS-associated infection. Antimicrob Agents Chemother 46:55–61. 10.1128/aac.46.1.55-61.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Pleshinger J, Weidner E. 1985. The microsporidian spore invasion tube. IV. Discharge activation begins with pH-triggered Ca2+ influx. J Cell Biol 100:1834–1838. 10.1083/jcb.100.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Sobottka I, Bartscht K, Schäfer P, Weitzel T, Schottelius J, Kock N, Laufs R. 2002. In vitro activity of polyoxin D and nikkomycin Z against Encephalitozoon cuniculi. Parasitol Res 88:451–453. 10.1007/s00436-002-0592-9. [DOI] [PubMed] [Google Scholar]
- 441.Bigliardi E, Bernuzzi AM, Corona S, Gatti S, Scaglia M, Sacchi L. 2000. In vitro efficacy of nikkomycin Z against the human isolate of the microsporidian species Encephalitozoon hellem. Antimicrob Agents Chemother 44:3012–3016. 10.1128/AAC.44.11.3012-3016.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Didier ES, Maddry JA, Kwong CD, Green LC, Snowden KF, Shadduk J. 1998. Screening of compounds for antimicrosporidial activity in vitro. Folia Parasitol 45:129–139. [PubMed] [Google Scholar]
- 443.Didier ES, Bowers L, Stovall ME, Kuebler D, Mittleider D, Brindley PJ, Didier PJ. 2005. Antimicrosporidial activity of (fluoro) quinolones in vitro and in vivo. Folia Parasitol 52:173–181. 10.14411/fp.2005.022. [DOI] [PubMed] [Google Scholar]
- 444.García-Torres I, Hernández-Alcántara G, Molina-Ortiz D, Caballero-Salazar S, Olivos-García A, Nava G, López-Velázquez G, Enríquez-Flores S. 2018. First characterization of a microsporidial triosephosphate isomerase and the biochemical mechanisms of its inactivation to propose a new druggable target. Sci Rep 8:8591. 10.1038/s41598-018-26845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Schwartz DA, Bryan RT. 1999. The microsporidial infections: progress in epidemiology and prevention, p 73–98. In Scheld WM, Craig WA, Hughes JM (ed), Emerging Infections 3. ASM Press, Washington, DC. [Google Scholar]
- 446.Shadduck JA, Polley MB. 1978. Some factors influencing the in vitro infectivity and replication of Encephalitozoon cuniculi. J Protozool 25:491–496. 10.1111/j.1550-7408.1978.tb04174.x. [DOI] [PubMed] [Google Scholar]
- 447.Albrecht H, Sobottka I. 1997. Enterocytozoon bieneusi infection in patients who are not infected with human immunodeficiency virus. Clin Infect Dis 25:344–344. 10.1086/516920. [DOI] [PubMed] [Google Scholar]
- 448.Weiser J. 1993. Early experiences with microsporidia of man and mammals. Folia Parasitol 40:257–260. [PubMed] [Google Scholar]
- 449.Modigliani R, Bories C, Le Charpentier Y, Salmeron M, Messing B, Galian A, Rambaud J, Lavergne A, Cochand-Priollet B, Desportes I. 1985. Diarrhoea and malabsorption in acquired immune deficiency syndrome: a study of four cases with special emphasis on opportunistic protozoan infestations. Gut 26:179–187. 10.1136/gut.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Orenstein JM. 1991. Microsporidiosis in the acquired immunodeficiency syndrome. J Parasitol 77:843–864. 10.2307/3282733. [DOI] [PubMed] [Google Scholar]
- 451.Sandfort J, Hannemann A, Gelderblom H, Stark K, Owen RL, Ruf B. 1994. Enterocytozoon bieneusi infection in an immunocompetent patient who had acute diarrhea and who was not infected with the human immunodeficiency virus. Clin Infect Dis 19:514–516. 10.1093/clinids/19.3.514. [DOI] [PubMed] [Google Scholar]
- 452.Mohanty A, Mitra S, Das S, Priyadarshini S, Sahu SK. 2020. Clinical profile of bilateral microsporidial keratoconjunctivitis in healthy individuals—a case series with long-term follow-up. Cornea 39:902–908. 10.1097/ICO.0000000000002297. [DOI] [PubMed] [Google Scholar]
- 453.Cali A, Takvorian PM. 1999. Developmental morphology and life cycles of the microsporidia, p 85–128. In Wittner M, Weiss LM (ed), The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 454.Kotler DP, Orenstein JM. 1999. Clinical syndromes associated with microsporidia, p 258–292. In Wittner M, Weiss LM (ed), The microsporidia and microsporidiosis. ASM Press, Washington, DC. [Google Scholar]
- 455.Ghosh K, Schwartz D, Weiss LM. 2014. Laboratory diagnosis of microsporidiosis, p 421–456. In Weiss LM, Becnel JJ (ed), Microsporidia: pathogens of opportunity. Wiley Blackwell, Oxford, United Kingdom. [Google Scholar]
- 456.Monaghan SR, Rumney RL, Vo NTK, Bols NC, Lee LEJ. 2011. In vitro growth of microsporidia Anncaliia algerae in cell lines from warm water fish. In Vitro Cell Dev Biol Anim 47:104–113. 10.1007/s11626-010-9366-3. [DOI] [PubMed] [Google Scholar]
- 457.Sprague V. 1974. Nosema connori n. sp., a microsporidian parasite of man. Trans Am Microsc Soc 93:400–403. 10.2307/3225442. [DOI] [PubMed] [Google Scholar]
- 458.Vávra J, Bedrník P, Cinátl J. 1972. Isolation and in vitro cultivation of the mammalian microsporidian Encephalitozoon cuniculi. Folia Parasitol (Praha) 19:349–354. [PubMed] [Google Scholar]
- 459.Colbourn NI, Hollister WS, Curry A, Canning EU. 1994. Activity of albendazole against Encephalitozoon cuniculi in vitro. Eur J Protistol 30:211–220. 10.1016/S0932-4739(11)80031-3. [DOI] [Google Scholar]
- 460.Ghosh K, Nieves E, Keeling P, Cali A, Weiss LM. 2012. A new vesicular compartment in Encephalitozoon cuniculi. Microbes Infect 14:324–328. 10.1016/j.micinf.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Fasshauer V, Gross U, Bohne W. 2005. The parasitophorous vacuole membrane of Encephalitozoon cuniculi lacks host cell membrane proteins immediately after invasion. Eukaryot Cell 4:221–224. 10.1128/EC.4.1.221-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Joseph J, Sharma S. 2009. In vitro culture of various species of microsporidia causing keratitis: evaluation of three immortalized cell lines. Indian J Med Microbiol 27:35–39. 10.1016/S0255-0857(21)01750-3. [DOI] [PubMed] [Google Scholar]
- 463.Shadduck JA. 1969. Nosema cuniculi: in vitro isolation. Science 166:516–517. 10.1126/science.166.3904.516. [DOI] [PubMed] [Google Scholar]
- 464.Haro M, Del Aguila C, Fenoy S, Henriques-Gil N. 2003. Intraspecies genotype variability of the microsporidian parasite Encephalitozoon hellem. J Clin Microbiol 41:4166–4171. 10.1128/jcm.41.9.4166-4171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Haro M, Del Aguila C, Fenoy S, Henriques-Gil N. 2006. Variability in infection efficiency In vitro of different strains of the microsporidian Encephalitozoon hellem. J Eukaryot Microbiol 53:46–48. 10.1111/j.1550-7408.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 466.Khanduja S, Ghoshal U, Agarwal V, Pant P, Ghoshal UC. 2017. Identification and genotyping of Enterocytozoon bieneusi among human immunodeficiency virus infected patients. J Infect Public Health 10:31–40. 10.1016/j.jiph.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 467.Breton J, Bart-Delabesse E, Biligui S, Carbone A, Seiller X, Okome-Nkoumou M, Nzamba C, Kombila M, Accoceberry I, Thellier M. 2007. New highly divergent rRNA sequence among biodiverse genotypes of Enterocytozoon bieneusi strains isolated from humans in Gabon and Cameroon. J Clin Microbiol 45:2580–2589. 10.1128/JCM.02554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Didier ES. 1997. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob Agents Chemother 41:1541–1546. 10.1128/AAC.41.7.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Watson AK, Williams TA, Williams BAP, Moore KA, Hirt RP, Embley TM. 2015. Transcriptomic profiling of host-parasite interactions in the microsporidian Trachipleistophora hominis. BMC Genomics 16:983. 10.1186/s12864-015-1989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Franzen C, Salzberger B. 2008. Analysis of the beta-tubulin gene from Vittaforma corneae suggests benzimidazole resistance. Antimicrob Agents Chemo 52:790–793. 10.1128/AAC.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Sokolova YY, Bowers LC, Alvarez X, Didier ES. 2019. Encephalitozoon cuniculi and Vittaforma corneae (Phylum Microsporidia) inhibit staurosporine-induced apoptosis in human THP-1 macrophages in vitro. Parasitology 146:569–579. 10.1017/S0031182018001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Leitch G, Shaw A, Colden-Stanfield M, Scanlon M, Visvesvara G. 2005. Multinucleate host cells induced by Vittaforma corneae (Microsporidia). Folia Parasitol 52:103–110. 10.14411/fp.2005.013. [DOI] [PubMed] [Google Scholar]
- 473.Bryan RT, Cali A, Owen RL, Spencer HC. 1991. Microsporidia: opportunistic pathogens in patients with AIDS. Prog Clin Parasitol 2:1–26. [PubMed] [Google Scholar]
- 474.Pinnolis M, Egbert PR, Font RL, Winter FC. 1981. Nosematosis of the cornea. Case report, including electron microscopic studies. Arch Ophthalmol 99:1044–1047. 10.1001/archopht.1981.03930011044012. [DOI] [PubMed] [Google Scholar]
- 475.Ashton N, Wirasinha PA. 1973. Encephalitozoonosis (nosematosis) of the cornea. Br J Ophthalmol 57:669–674. 10.1136/bjo.57.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]