Abstract
Background: Medication adherence is widely recognized as an essential component of chronic disease management, yet only 50% of patients take their medication as prescribed. Newer technologies have the potential to improve medication adherence. Objective: To conduct a pilot study estimating the impact of a pharmacy-dispensed electronic reminder cap (SMARxT cap), which also records cap openings, on medication adherence and blood pressure (BP). Methods: After a 30-day run-in period, 28 individuals were randomized to receive a SMARxT or placebo cap on each BP medication. The primary outcome was adherence measured via (1) the medication possession ratio, (2) number of cap openings, and (3) self-report. The secondary outcome was the average of 2 BP readings at 6 months. Mean changes from baseline to 6 months were compared between the 2 groups. Results: The medication possession ratio increased 2.7% in the SMARxT cap group and decreased 1.1% in the control group (P = .13), and cap openings increased 11.9% in the SMARxT cap group and 9.9% in the control group (P = .83). Self-reported adherence increased 1.1 points in the SMARxT cap group and 0.8 points in the control group (P = .64). Systolic BP decreased 8.2 mm Hg in the SMARxT cap group and 2.8 mm Hg in the placebo cap group (P = .35), and diastolic BP decreased to 6.2 mm Hg in the SMARxT cap group and was unchanged in the placebo cap group (P = .06). Conclusions: Use of SMARxT cap showed nonsignificant improvement in medication adherence and BP lowering. This technology has potential to characterize and improve medication-taking behavior.
Keywords: medication adherence, hypertension, blood pressure, electronic reminder devices, electronic medication packaging
Background
Medication adherence is widely recognized as an essential component of effective disease state management, yet research demonstrates that less than 50% take their medication as prescribed, and typically fewer than half of the prescribed doses are taken at all.1 Adherence to medications for chronic diseases are particularly problematic because these medications must be taken over a long duration of time, and the direct effect of taking or missing doses often are not readily perceived by the patient. This is particularly problematic for the treatment of hypertension—more than one third of patients with prescriptions for medications have uncontrolled blood pressure (BP), and nonadherence is hypothesized to be a major contributing factor.2,3 In fact, a recent study found that more than half of resistant hypertension could be attributed to patients’ poor adherence to medications.4 Annually, nonadherence costs an estimated $290 billion to the US health care system.5 At the individual level, annual costs attributed to all-cause nonadherence are estimated to range from $5271 to $52 341 per person.6 Reasons for nonadherence are multifactorial and include lack of access to medications, adverse effects associated with the medication, poor understanding of the dosing instructions or the need for adherence, and concerns about cost.7 Research also confirms that forgetfulness is also a key factor, accounting for more than 50% of nonadherence.8-11
Multiple adherence-enhancing strategies have been evaluated, yet studies routinely find inconsistent evidence of their effects on adherence.12,13 In addition to medication therapy management programs and other clinician-based interventions, technology now plays a larger role in promoting patient adherence. One review of electronic reminder systems identified short-term effectiveness of electronic reminder devices (ERDs), yet another found insufficient evidence for broad recommended use of a variety of ERDs.14,15 A recent large-scale study of low-cost reminder devices without monitoring, including a standard pillbox, pill bottle strip with toggles, and a digital timer cap, found no difference in adherence among nonadherent patients taking medication(s) for chronic conditions.16 However, electronic monitoring systems, which often include reminder functionality, have been shown to affect adherence and biomarkers in several diseases.17-21 In fact, digital displays that combine an audible reminder with the last time of container opening are associated with improved adherence.22 Differences among components of ERDs, the quality of existing studies, and variance in adherence measurement and associated patient outcomes are some of the many challenges associated with estimating the impact of these devices.
This pilot study aimed to estimate the effect of an inexpensive pharmacy-dispensed electronic reminder cap, the SMARxT cap—an ERD that includes an audible alarm and display of the last container opening, on medication adherence and BP. We hypothesized that patients with hypertension receiving the SMARxT cap would exhibit higher medication adherence and lower BP than patients receiving placebo caps.
Methods
Study Setting
This study was conducted with patients from 3 northwest Indiana locations that are part of a regional chain of 22 community pharmacies (Fagen Pharmacies) located in Indiana and Illinois. These pharmacies were selected based on their commitment to participating in the study, ability of the chain to add more pharmacies to the study if required, and proximity to study personnel. Each of the pharmacies was open for business approximately 65 hours per week and employed 1 to 2 full-time and 2 to 3 part-time pharmacists and 5 to 7 technicians. The pharmacies filled an average of 1800 prescriptions weekly and served a relatively diverse population of patients relative to the overall population of the region. All study procedures were approved by the Purdue University Human Research Protection Program.
Study Design
In this single-blind, randomized, controlled pilot study, consented participants receiving 1 or more antihypertensive medications from 3 participating community pharmacies were randomized to receive either a SMARxT cap or matching placebo cap affixed to all of their antihypertensive medication vials. Patients returned their SMARxT or placebo cap to the pharmacy and received a new cap monthly.
SMARxT Cap System
This electronic reminder cap system, which fits standard prescription bottles, is programmed during the dispensing process within the pharmacy. The caps are programmed using a device attached to the pharmacy dispensing system, which automatically matches the prescribing instructions to the cap. The SMARxT cap is programmed to alert the patient with beeps and flashes when each dose of the medication is due to be taken. The device also has a visual timer (Figure 1) that counts the minutes and hours between doses. Each time the bottle is opened, the timer resets to zero. The cap records the time at opening and is an indicator of dosing, which is used to estimate adherence to the prescribed regimen. The matching placebo cap physically resembles the SMARxT cap and also records bottle openings but does not exhibit the audio or visual alerts. The cost of each SMARxT cap is around $10 each.
Figure 1.
Image of SMARxT cap (left) and placebo cap (right) on a prescription medication bottle.
Patient Recruitment
Subjects were recruited using a combination of active and passive strategies in the pharmacies. Passive strategies included posters and bag stuffers about the study. Active strategies included discussion with patients receiving antihypertensive medications from the pharmacists and technicians. To confirm interest and ensure eligibility, patients who were receiving antihypertensive medication(s) from 1 of the 3 pharmacy sites and were interested in the study participated in an initial face-to-face screening with a research assistant. A BP of >140/90 mm Hg for patients without diabetes or >130/80 mm Hg for patients with diabetes or chronic kidney disease was necessary for trial consideration. Additional inclusion criteria included aged 18 to 85 years, receiving at least 1 antihypertensive medication with no change in the regimen or dose in the past 4 weeks, and receiving antihypertensive medication(s) regularly from the pharmacy during the past 6 months with no plans to switch pharmacies in the next year.
Patients were excluded if they had stage 3 hypertension (BP ≥180/110 mm Hg), a myocardial infarction or stroke within the past 6 months prior to enrollment, New York Heart Association Class III or IV heart failure, unstable angina, serious renal disease requiring dialysis, serious hepatic disease, pregnancy, poor prognosis with a life expectancy estimated at less than 1 year, dementia or cognitive impairment preventing them from taking their medications without assistance, a score of <6 on the Rapid-PC screen for cognitive impairment, or were unable to open child-resistant medication caps or requested non-child-resistant medication caps.
Data Collection
Prior to randomization, all eligible and consented patients completed a 30-day run-in period during which a placebo cap was placed on each BP medication. The purpose of the run-in period was to gather a baseline assessment of cap-related adherence. At the end of the run-in period, additional baseline adherence measure data were collected including prescription refill data for the prior 6 months and self-reported medication adherence. At this time, we excluded patients with a baseline adherence of >80% to all of their BP medications based on the results of the run-in electronic cap adherence. A baseline BP was also collected at the end of the run-in period and prior to randomization.
Participants were randomized to either the placebo cap or SMARxT cap group and were monitored for 6 months. All patients in the SMARxT cap group received additional verbal and written information from the pharmacist regarding the SMARxT cap’s features and alerting functions. At the 6-month follow-up assessment, self-reported medication adherence, prescription refill data for the 6-month study period, electronic cap-opening data, and BP were collected for all patients using both an in-person interview and review of pharmacy records.
Measures
Primary outcome measures included 3 estimations of medication adherence: (1) the medication possession ratio (MPR), (2) electronic cap adherence, and (3) self-reported medication adherence. First, the MPR was estimated using prescription refill records spanning the 6 months prior to study enrollment for the baseline measure and during the 6-month study period. Specifically, the MPR was calculated as the sum of the days’ supply of medication divided by the number of days in the refill interval. Medication-specific MPRs were calculated and averaged for all subjects’ BP medication.23 An MPR of ≥0.8 represents good adherence.24
Second, cap adherence was estimated using the proportion of time that a patient takes his or her medication(s) at their specified dosing time, measured from electronic cap-opening data. The proportion of doses taken “on time” was calculated using 10% as the maximum deviation window permitted from the time the dose was scheduled to be taken in order for a dose to be counted as “on time.” This percentage was averaged across all 6 months for each medication.25
Third, self-reported adherence was measured using the Morisky Medication Adherence Scale (MMAS-8), which is an 8-item questionnaire that measures self-reported adherence based on medication-taking behavior. MMAS-8 scores range from 0 to 8, with scores of <6 reflecting poor adherence, 6 to <8 reflecting medium adherence, and 8 reflecting high adherence.26-28
Secondary outcomes included mean changes in systolic BP (SBP) and diastolic BP (DBP) between groups from baseline to 6 months. At each time point, BP was measured 3 consecutive times in the pharmacy by a trained research assistant using an automated BP device (Omron) following guidelines from the American Heart Association.29 The average of the second and third measurements was used as the BP reading.
Statistical Analyses
Descriptive statistics were used to characterize the baseline demographics of each group, and independent samples t-tests were used to compare the mean change in each primary and secondary outcome from baseline to 6 months between the placebo and SMARxT cap groups. Given that this was a pilot study, sample size calculations are noncontributory. P values of <.05 were considered to be statistically significant. Data were analyzed using SPSS Statistics software.
Results
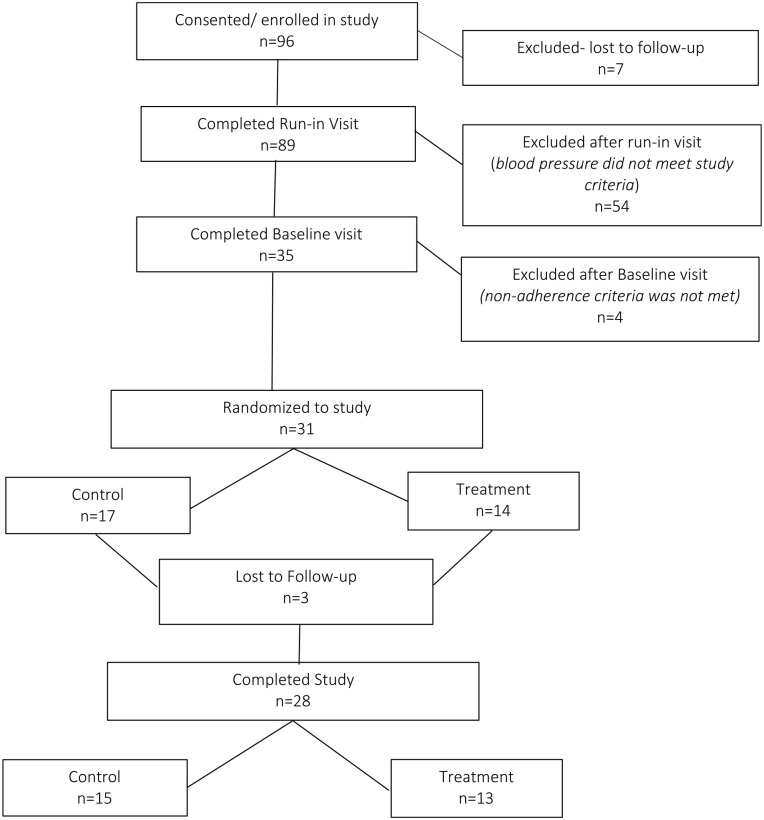
Of 28 patients enrolled in the study, 13 were randomized to the SMARxT cap group and 15 to the placebo cap group (Figure 2). No statistically significant differences were observed between groups at baseline (Table 1). At 6 months, the medication adherence estimates (MPR, cap adherence, and MMAS-8 score) were higher in the SMARxT cap group compared with the placebo cap group, but the improvements were not statistically significant (Table 2). MPR increased 2.7 percentage points in the SMARxT cap group and decreased 1.1 percentage points in the placebo cap group (P = .13) from baseline to 6 months. Cap adherence increased 11.9% in the SMARxT cap group and 9.9% in the placebo cap group from baseline to 6 months (P = .83). From baseline to 6 months, self-reported adherence (MMAS-8 score) increased 1.1 points in the SMARxT cap group and 0.8 points in the placebo cap group (P = .64).
Figure 2.
Flow diagram of patient enrollment.
Table 1.
Baseline Characteristics (n = 28).
| Characteristics | Control (n = 15) | Intervention (n = 13) | P a |
|---|---|---|---|
| Age (years), mean ± SD | 62.8 ± 13.0 | 57.8 ± 9.9 | .27 |
| Male gender, n (%) | 6 (40%) | 6 (46.2%) | .75 |
| Race, n (%) | .78 | ||
| White | 9 (60%) | 9 (69.2%) | |
| Black | 5 (33.3%) | 3 (23.1%) | |
| Other | 1 (6.7%) | 1 (7.7%) | |
| Married, n (%) | 4 (26.7%) | 5 (38.5%) | .52 |
| Education status, n (%) | .75 | ||
| Less than high school | 1 (6.7%) | 0 | |
| High school | 8 (53.3%) | 7 (53.8%) | |
| Some college/completed college | 6 (40%) | 6 (46.2%) | |
| Employment status, n (%) | .68 | ||
| Employed | 2 (13.3%) | 2 (15.4%) | |
| Out of work | 2 (13.3%) | 3 (23.1%) | |
| Retired | 6 (40%) | 3 (23.1%) | |
| Disabled | 4 (26.7%) | 5 (38.5%) | |
| Other | 1 (6.7%) | 0 | |
| Comorbid conditions | |||
| Body mass index, mean ± SD | 31.2 ± 7.1 | 32.6 ± 7.5 | .61 |
| Current tobacco use, n (%) | 6 (40%) | 4 (30.8%) | .79 |
| Asthma, n (%) | 5 (33.3%) | 3 (23.1%) | .57 |
| Arthritis, n (%) | 10 (66.7%) | 10 (76.9%) | .57 |
| Diabetes, n (%) | 2 (13.3%) | 6 (46.2%) | .07 |
| “Heart trouble,” n (%) | 4 (26.7%) | 2 (15.4%) | .49 |
| Stroke, n (%) | 1 (6.7%) | 1 (7.7%) | .88 |
| Systolic blood pressure (mm Hg), mean ± SD | 142.9 ± 19.7 | 132.2 ± 25.2 | .22 |
| Diastolic blood pressure (mm Hg), mean ± SD | 84.0 ± 11.8 | 84.7 ± 10.8 | .87 |
| Blood pressure medications, mean ± SD | 2.3 ± 1.2 | 2.2 ± 1.1 | .82 |
| Adherence measures | |||
| Medication possession ratiob for all blood pressure medications, mean ± SD | 92.7 ± 6.8 | 89.1 ± 9.8 | .27 |
| Cap adherencec, mean ± SD | 55.1 ± 27.3 | 60.7 ± 33.0 | .63 |
| MMAS-8 scored, mean ± SD | 5.7 ± 1.3 | 6.2 ± 1.1 | .29 |
Calculated using χ2 test, Fisher’s exact test, or student’s t test, as appropriate.
Medication possession ratio (MPR) refers to the proportion of days that a patient had blood pressure medication available to take during the 6 months prior to study enrollment (based on prescription refill records).
Cap adherence refers to the proportion of time that a patient takes his or her medication at their specified dosing time; measured from cap-opening data.
Table 2.
Medication Adherence Results (n = 28).
| Control group (n = 15) |
Intervention group (n = 13) |
Δa | P b | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Change | Baseline | 6 months | Change | |||
| MPR %, mean ± SD | 92.7 ± 6.8 | 91.7 ± 8.6 | −1.1 ± 6.0 | 89.1 ± 9.8 | 91.8 ± 8.9 | 2.7 ± 6.7 | 3.8 | .13 |
| Cap adherence %, mean ± SD | 55.1 ± 27.3 | 65.0 ± 28.0 | 9.9 ± 22.5 | 60.7 ± 33.0 | 72.6 ± 28.3 | 11.9 ± 26.8 | 2.0 | .83 |
| MMAS-8 score, mean ± SD | 5.7 ± 1.3 | 6.5 ± 1.5 | 0.8 ± 1.3 | 6.2 ± 1.1 | 7.2 ± 1.1 | 1.1 ± 1.4 | 0.3 | .64 |
Abbreviation: MPR, medication possession ratio; MMAS, morisky medication adherence scale.
Refers to the difference between the change from baseline to 6 months in the control and intervention groups.
Refers to the comparison of change from baseline to 6 months between the control and intervention groups using an independent samples t test.
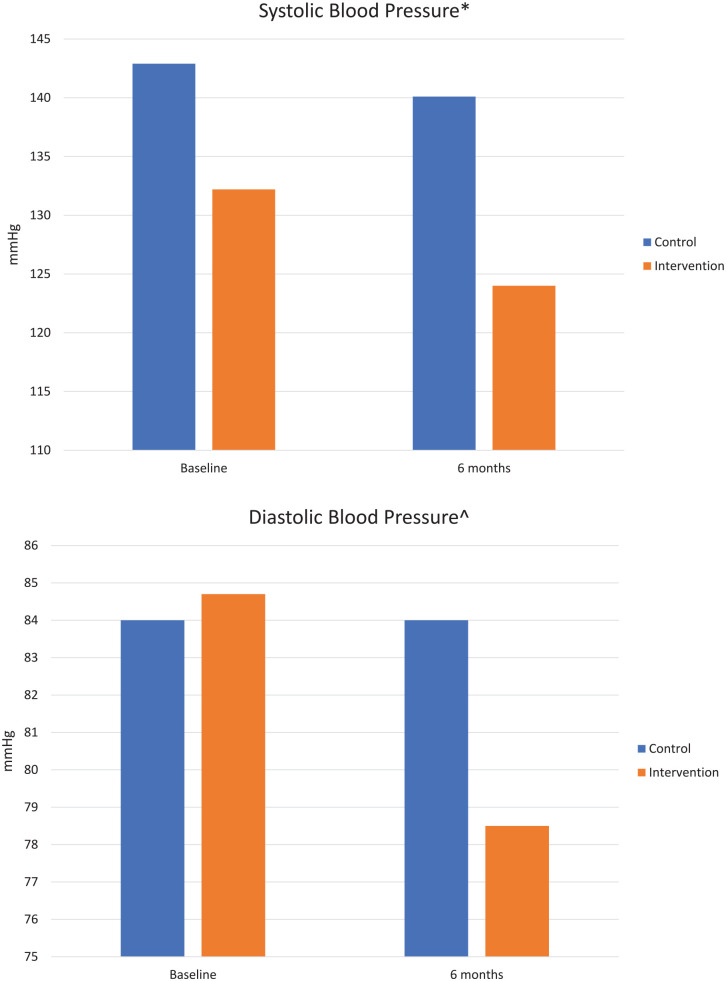
From baseline to 6 months, BP measures indicated SBP decreased by 8.2 mm Hg in the SMARxT cap group and 2.8 mm Hg in the placebo cap group from baseline to 6 months (P = .35; Figure 3). For DBP, a decrease of 6.2 mm Hg was observed in the SMARxT cap group versus no change in the placebo cap group (P = .06).
Figure 3.
Blood pressure results (n = 28).
* P = .35 for the comparison of systolic blood pressure change from baseline to 6 months, calculated using an independent samples t test.
^ P = .06 for the comparison of diastolic blood pressure change from baseline to 6 months, calculated using an independent samples t test.
Discussion
Findings from this pilot study indicate that electronic reminder packaging may play a role in improving patients’ adherence to their antihypertensive medication(s). Our data support evidence from other studies suggesting that ERDs can modestly improve patient adherence and short-term BP. Published data on results of ERD use are variable. In one review of studies on ERDs with audio visual reminders, 3 of 7 studies showed significant improvement on adherence in the short-term, and defined as a follow-up period of less than 6 months.14 Within this review, one study focused on patients with hypertension (n = 71) who used a reminder alarm card (credit card–sized card with audible reminder alarm). Adherence measured by pill count was similar between those who used the alarm card and those who did not during months 1 and 2. The difference was statistically significant at 3 months (87.3% control group vs 97.3% intervention group; P = .011).30 Two other studies examined ERDs in patients with hypertension and did not find significant improvement in adherence.31,32 Furthermore, a recent study found that neither electronic pill bottles nor bidirectional text messaging improved adherence enough to affect BP or that adherence was not the primary driver of hypertension control.33 When combined with our findings, additional studies to determine the components of ERDs most likely to improve antihypertension adherence and BP are needed.
Another review of 37 studies of ERDs, of which 9 studies focused on hypertension, concluded that many varieties of ERDs exist and there are limited data supporting their use. There were more data supporting ERD models that were more complex and those that were integrated into health care delivery. Useful features of devices appeared to be the ability to monitor adherence using both digital displays and audio alarms and store data.22 While the SMARxT caps used in this pilot study were integrated into the pharmacy-dispensing process and included the ability to monitor and store adherence information with digital and audio alarms, the improvement in adherence over 6 months was small, and it varied according to the measurement method.
Adherence sensitivity might be improved with electronic monitoring. This method is useful when the timing of doses is likely to affect drug efficacy or adverse effects, such as for certain medications such as contraceptives and antiretrovirals.34,35 There also might be additional medications for which the timing of nonadherence impacts therapeutic outcomes, such as was shown in one study with patients taking lipid-lowering drugs.36 The effect of taking medication(s) exactly at the specified time each day is limited for antihypertensive drugs but bedtime dosing might be important for achieving outcomes.37 Studies have shown that more optimal-dosing execution leads to improved persistence with chronic medications over time.35
Adherence is a complex issue that often requires a combination of reminder-type devices plus other support dependent on electronic monitoring (eg, text messages for missed doses) to overcome unintentional nonadherence, and other interventions, like counseling by a pharmacist to help overcome intentional nonadherence. Electronic reminder caps themselves require minimal clinician intervention and are less resource intensive than other strategies, but they can be included in more complex adherence strategies.22 It also is possible that reminder features of the cap need improvement and individualization. Additionally, some patients might benefit more from an alternative electronic reminder system to be actively alerted to take their medication, for example, through electronic mobile phone messages rather than through a system physically incorporated into the medication packaging. Historically, ERDs have not been covered by health insurance, which has been a barrier to more widespread use.34 The cost of the ERD used in this project is minimal (around $10 each), which would allow insurers to benefit from its use by lowering the overall cost of care.38,39 However, cost-effectiveness studies of these devices are needed.
Study Limitations
One major limitation of this pilot study was the small number of participants, which diminished our ability to draw conclusions about the impact of the caps on adherence and/or blood pressure. Our sample size was not adequate to detect statistically significant changes in BP. However, the difference in decrease in both SBP and DBP in the SMARxT cap group was clinically meaningful. This suggests the need for future studies in larger populations. The results of this study could be useful to estimate effect size and calculate a sample size in future trials. In previous surveys, patient interest in using the device was very high, but only a small percentage of the patients recruited for this project responded to the invitation to participate. As mentioned, other studies of ERDs also have been limited by small numbers of subjects, a problem that highlights the need to better understand the reasons why patients are reluctant to adopt an ERD and tailor recruitment strategies to overcome this barrier.
Additionally, the baseline measures of adherence were high, suggesting that a ceiling effect likely limited our ability to observe more dramatic improvements in adherence. Similarly, our simple randomized design, while balancing most of our measures of baseline characteristics, may not have accounted for other unobserved characteristics that affect adherence. The caps used in this study had some technical development issues, which delayed recruitment of some subjects and prevented inclusion of others. Finally, while electronic monitoring has been shown to provide a more accurate and detailed representation of actual dosing than other methods (eg, self-report and pharmacy claims data), this system is still an indirect adherence measure, because the opening of the medication bottle is assumed to be an indicator of a dose being taken by the patient.35,40
Conclusions
Use of SMARxT cap showed nonsignificant improvement in medication adherence and BP lowering. In addition to serving as a reminder device, this health technology can potentially provide clinicians with an additional source of data to characterize patient medication-taking behavior. Further research with a larger sample size is needed to more accurately estimate the impact of the SMARxT and other ERDs on patient adherence and health outcomes.
Footnotes
Authors’ Note: Presented as a poster at the American Pharmacist Association Annual Meeting in 2016.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Downey and Mr Klink have ownership interest in the electronic cap that was part of this study, and they are employed by Concordance Health Solutions. The other authors have no conflicts of interest to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute on Aging, NIH. Small Business Innovation Research, Grant Number 2R44AG039178-02A1.
ORCID iD: Alan J. Zillich  https://orcid.org/0000-0002-7495-3678
https://orcid.org/0000-0002-7495-3678
References
- 1.World Health Organization. Commitment to long-term therapies: evidence to support operations. Published 2003. Accessed May 4, 2021. https://www.who.int/chp/knowledge/publications/adherence_report_fin.pdf
- 2.Centers for Disease Control and Prevention. High blood pressure. Accessed May 4, 2021. https://www.cdc.gov/bloodpressure/index.htm
- 3.Chobanian AV, Bakris GL, Black HR, et al. ; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Published December 1, 2003. Accessed May 4, 2021. https://www.ahajournals.org/doi/10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed]
- 4.Florczak E, Tokarczyk B, Warchol-Celinska E, et al. Assessment of adherence to treatment in patients with resistant hypertension using toxicological serum analysis. A subgroup evaluation of the RESIST-POL study. Pol Arch Med Wewn. 2015;125:65-72. doi: 10.20452/pamw.2648 [DOI] [PubMed] [Google Scholar]
- 5.New England Healthcare Institute. Thinking outside the pillbox: a system-wide approach to improving patient medication adherence for chronic disease. Published August 2009. Accessed May 4, 2021. https://www.nehi.net/writable/publication_files/file/pa_issue_brief_final.pdf
- 6.Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8:e016982. doi: 10.1136/bmjopen-2017-016982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200-209. doi: 10.1097/01.mlr.0000114908.90348.f9 [DOI] [PubMed] [Google Scholar]
- 8.National Community Pharmacists Association. Take as directed: a prescription not followed. Research conducted by The Polling Company; 2007. [Google Scholar]
- 9.Samet JH, Libman H, Steger KA, et al. Compliance with zidovudine therapy in patients infected with human immunodeficiency virus, type 1: a cross-sectional study in a municipal hospital clinic. Am J Med. 1992;92:495-502. doi: 10.1016/0002-9343(92)90746-X. [DOI] [PubMed] [Google Scholar]
- 10.Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med. 2002;11:185-198. [PubMed] [Google Scholar]
- 11.Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256:568-576. doi: 10.1007/s00415-009-0096-y [DOI] [PubMed] [Google Scholar]
- 12.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157:785-795. doi: 10.7326/0003-4819-157-11-201212040-00538 [DOI] [PubMed] [Google Scholar]
- 13.Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127-135. doi: 10.2147/PPA.S26314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vervloet M, Linn AJ, Van Weert JCM, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19:696-704. doi: 10.1136/amiajnl-2011-000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA. 2014;312:1237-1247. doi: 10.1001/jama.2014.10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhry NK, Krumme AA, Erocle PM, et al. Effect of reminder devices on medication adherence: the remind randomized clinical trial. JAMA Intern Med. 2017;177:624-631. doi: 10.1001/jamainternmed.2016.9627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao D, Xie L, Wang T, Wang T. A meta-analysis of the use of electronic reminders for patient adherence to medication in chronic disease care. J Telemed Telecare. 2015;21:3-13. doi: 10.1177/1357633X14541041 [DOI] [PubMed] [Google Scholar]
- 18.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417-1423. doi: 10.1086/323201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Lewis JJ, Zhang H, et al. Effectiveness of electronic reminders to improve medication adherence in tuberculosis patients: a cluster-randomised trial. PLoS Med. 2015;12: e1001876. doi: 10.1371/journal.pmed.1001876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968-977. doi: 10.7326/0003-4819-134-10-200105150-00011 [DOI] [PubMed] [Google Scholar]
- 21.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33:74-109. doi: 10.1016/j.clinthera.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 22.Checchi K. Electronic medication packaging devices and medication adherence: a systematic review. JAMA. 2014;312:1237-1247. doi: 10.1001/jama.2014.10059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105-116. doi: 10.1016/S0895-4356(96)00268-5 [DOI] [PubMed] [Google Scholar]
- 24.Chernew ME, Shah MR, Wegh A, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood). 2008;27:103-112. doi: 10.1377/hlthaff.27.1.103 [DOI] [PubMed] [Google Scholar]
- 25.Tu W, Morris AB, Li J, et al. Association between adherence measurements of metoprolol and health care utilization in older patients with heart failure. Clin Pharmacol Ther. 2005;77:189-201. doi: 10.1016/j.clpt.2004.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348-354. doi: 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in hypertensive seniors. Am J Manag Care. 2009;15:59-66. [PMC free article] [PubMed] [Google Scholar]
- 28.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol. 2011;64:255-257. doi: 10.1016/j.jclinepi.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e [DOI] [PubMed] [Google Scholar]
- 30.da Costa FA, Guerreiro JP, de Melo MN, et al. Effect of reminder cards on compliance with antihypertensive medication. Int J Pharm Pract. 2005;13:205-211. doi: 10.1211/ijpp.13.3.0006 [DOI] [Google Scholar]
- 31.Christensen A, Osterberg LG, Hansen EH. Electronic monitoring of patient adherence to oral antihypertensive medical treatment: a systematic review. J Hypertens. 2009;27:1540-1551. doi: 10.1097/HJH.0b013e32832d50ef [DOI] [PubMed] [Google Scholar]
- 32.Santschi V, Wuerzner G, Schneider MP, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007;63:1179-1184. doi: 10.1007/s00228-007-0364-7 [DOI] [PubMed] [Google Scholar]
- 33.Mehta SJ, Volpp KG, Troxel AB, et al. Electronic pill bottles or bidirectional text messaging to improve hypertension medication adherence (Way 2 Text): a randomized clinical trial. J Gen Intern Med. 2019;34:2397-2404. doi: 10.1007/s11606-019-05241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487-497. doi: 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 35.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275-301. doi: 10.1146/annurev-pharmtox-011711-113247 [DOI] [PubMed] [Google Scholar]
- 36.Walter P, Arnet I, Romanens M, Tsakiris DA, Hersberger KE. Pattern of timing adherence could guide recommendations for personalized intake schedules. J Pers Med. 2012;2:267-276. doi: 10.3390/jpm2040267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermida RC, Crespo JJ, Dominguez-Sardina M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565-4576. doi: 10.1093/eurheartj/ehz754 [DOI] [PubMed] [Google Scholar]
- 38.Roebuck MC, Dougherty JS, Kaestner R, Miller LM. Increased use of prescription drugs reduces medical costs in Medicaid populations. Health Aff (Millwood). 2015;34:1586-1593. doi: 10.1377/hlthaff.2015.0335 [DOI] [PubMed] [Google Scholar]
- 39.Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff (Millwood). 2011;30:91-99. doi: 10.1377/hlthaff.2009.1087 [DOI] [PubMed] [Google Scholar]
- 40.Denhaerynck K, Schafer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol. 2008;8:5. doi: 10.1186/1471-2288-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]