Abstract
Patients with continuous-flow left ventricular assist devices have a high risk of gastrointestinal bleeding (GIB) and recurrent bleeding. Studies have shown octreotide can reduce the risk of GIB. This retrospective, case-crossover study, evaluated the efficacy of octreotide for the prevention of recurrent GIB in patients with left ventricular assist devices between August 2008 and October 2018. A total of 32 patients received octreotide and were included in the study. Hospital admission for GIB was evaluated before and after the initiation of octreotide. Each case served as his/her own control. Most patients were on a reduced aspirin dose (56.2%) and had a reduced international normalized ratio goal (59.4%) before starting monthly octreotide. The most common dose of long-acting octreotide was 30 mg every 28 days. Overall, octreotide decreased the frequency of GIB (4.3 vs. 0.9 events/year, p < 0.001). Nineteen (59.4%) patients did not have a subsequent gastrointestinal bleed. Of the 13 patients who rebled after initiation of octreotide, the frequency of events decreased by 2.6 bleeds per patient per year (4.8 vs. 2.2; p = 0.043). In high-risk patients who have failed conventional therapy, octreotide can be useful for the prevention of recurrent GIB.
Keywords: left ventricular assist device, continuous-flow left ventricular assist device, gastrointestinal bleed, octreotide, bleed
Gastrointestinal bleeding (GIB) is one of the most common complications in patients on continuous-flow left ventricular assist device (CF-LVAD) support and is associated with increased morbidity and mortality.1 The incidence of GIB after implantation of a CF-LVAD ranges between 15.4% and 61.0%.2,3
The pathophysiology of GIB in patients with CF-LVADs is multifactorial. The risk factors for increased likelihood of GIB in patients with CF-LVADs include chronic anticoagulation, antiplatelet agents, device physiology causing high shear stress resulting in acquired Von Willebrand disease, impaired platelet aggregation, formation of arteriovenous malformations, angiodysplastic lesions, and intestinal hypoperfusion.2–6 Various interventions to reduce the incidence of GIB include reduced antiplatelet dose, lower target international normalized ratio (INR) goals, decreased pump speed to allow for aortic valve opening, use of proton pump inhibitors or histamine-2 receptor antagonists, treatment with thalidomide, danazole, or octreotide.1,7–10
Octreotide is a somatostatin analog, FDA approved for acromegaly, diarrhea associated with carcinoid tumors and vasoactive intestinal peptide (VIP) secreting neurogenic tumors. Octreotide is used off-label for treatment and secondary prevention of GIB. A few observational studies have shown a benefit with the use of long-acting release octreotide (LAR) for the prevention of recurrent GIB (Table 1).11–14 The largest of the studies included 51 patients with a 180 day follow-up in comparison to a historical cohort.12
Table 1.
Key Studies Utilizing LAR in CF-LVAD Patients
| Devices | Number of Patients | Octreotide Regimen | Outcomes | |
|---|---|---|---|---|
| Juricek et al.11 | HMII | 30 | IM LAR depot 20 mg every 4 weeks | Decreased frequency of GIB 3.4 ± 3.1 to 0.7 ± 1.3 events/year; p < 0.001 |
| HVAD Heartassist | ||||
| Shah et al.12 | HMII | 51 | IM LAR depot (37) or SQ daily (14) for 6 months | Increased freedom from rebleeding GIB for the octreotide group, 75 ± 6% vs 52 ± 8%, p < 0.01 |
| Malhotra et al.13 | HMII | 8 | IM LAR depot 20 mg every 4 weeks for 16 weeks | No episodes of GIB observed or reported |
| Smallfield et al.14 | Devices not specified | 34 | IM LAR depot monthly or SQ daily (doses not specified) | IM LAR depot monthly had lower rate of bleeding compared to SQ daily (29% vs. 42%) |
CF-LVAD, continuous-flow left ventricular assist device; GIB, gastrointestinal bleed; HMII, HeartMate II; HVAD, HeartWare HVAD System; LAR, long-acting release.
We conducted a retrospective cohort study evaluating the use of LAR at a single center over a 10 year period examining the incidence of GIB prior and following LAR therapy.
Materials and Methods
Patient Population
A retrospective, crossover study was conducted at Sentara Norfolk General Hospital. The study inclusion criteria included patients aged 18 years and older, CF-LVAD placement from August 2008 to October 2018, a GIB requiring hospital admission, standard-of-care treatment for secondary prophylaxis, and treatment with monthly LAR. Patients were excluded if they missed greater than two consecutive doses of LAR (Table 2). The study was approved by the Eastern Virginia Medical School Institutional Review Board.
Endpoints
Each patient served as their own control to compare GIB rates. The control period was time from CF-LVAD implant to initiation of LAR (pre-LAR), whereas the comparison period was time from the initiation of LAR (post-LAR) until the end of follow-up. The primary endpoint was bleeding events per patient per year pre-LAR in comparison with post-LAR. A bleeding event was defined as a GIB resulting in hospitalization. Although subject to clinical assessment, patients were generally admitted to the hospital for a decrease of 2 g/dl of hemoglobin or signs of a GIB, such as a positive fecal occult blood sample. After evidence of GIB, colonoscopies, and endoscopies were routinely performed. If no bleeding source was identified, additional invasive procedures such as spiral enteroscopy would be performed.
Institution-Specific Protocols
Patients on CF-LVAD support were initially placed on aspirin 325 mg daily, warfarin with a goal INR of 2–3, and a proton pump inhibitor or histamine-2 receptor antagonist. Regimens after first bleed were then individualized per patient. Modifications to the INR goal, antiplatelet regimen, pump speed, or initiation of octreotide were considered before discharge at the discretion of the attending cardiologist. Per protocol, patients initiate octreotide when modifiable causes for bleeding (e.g., supratherapeutic INR or a lesion requiring cauterization) have been fixed. Patients started on octreotide continue monthly or more frequently until transplant or the patient expires.
Statistical Analysis
Paired categorical data were evaluated using the McNemar’s test. For continuous data, the paired t-test or Wilcoxon signed-rank test was utilized. We then performed a mixed effects analysis of variance (ANOVA) model and included a random effect for patient ID to account for correlation between pre and post measurements within the same patient. In this three-way full factorial ANOVA, we evaluated the impact of octreotide, a reduced INR goal, and reduced aspirin dose on the outcome of GIB frequency. A Kaplan–Meier analysis with a log rank test was utilized to determine the impact of LAR on the freedom from rebleeding. Statistical analysis was completed with IBM SPSS statistics software version 24.
Results
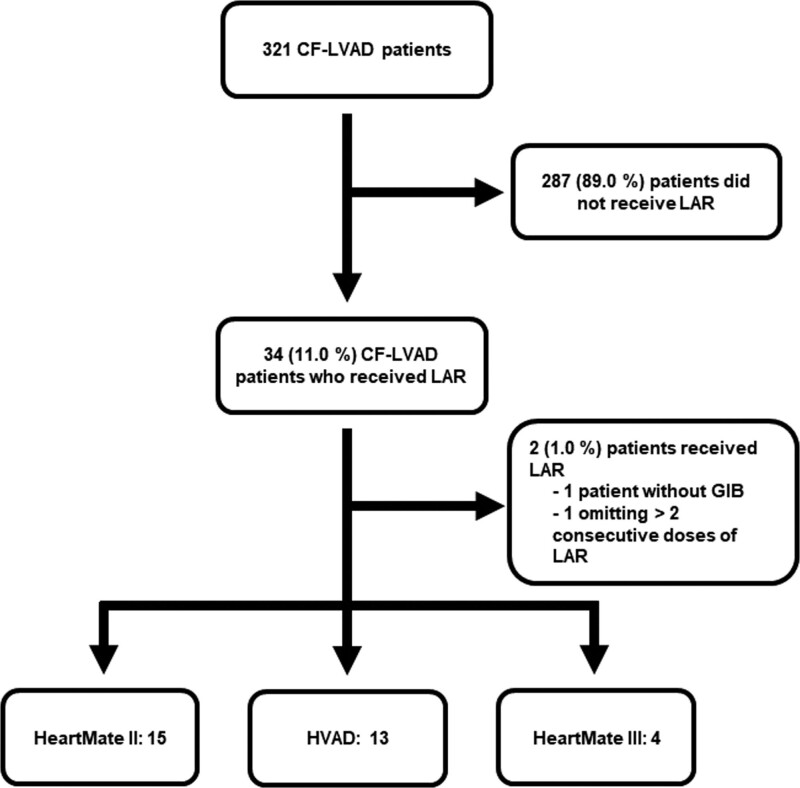
Thirty-four patients out of 321 CF-LVAD implants during the study period developed a GIB and were initiated on LAR. Two of these patients were excluded, one due to receiving LAR for epistaxis and another due to missing greater than two consecutive doses of LAR. Pharmacokinetically when three consecutive doses are missed (3 months), the intramuscular drug is completely eliminated from the body. A total of 32 patients were included in the analysis, 15 (46.9%) had HeartMate II (Abbott, IL), 13 (40.7%) HVAD (Medtronic, MN), and 4 (12.4%) HeartMate III (Abbott, IL) (Figure 1).
Figure 1.
Patient selection.
Baseline characteristics of the patients at the time of admission before the initiation of LAR are shown in Table 2. As this was a refractory patient population, most pre-LAR patients were on a reduced dose aspirin (56.2%), lower INR goal (59.4%), and were on a proton pump inhibitor (93.8%).
Table 2.
Patient Characteristics
| Patient Demographics | |
|---|---|
| Gender: male, n (%) | 25 (78.1) |
| Age at LVAD implantation, mean ± SE | 61.2 ± 1.2 |
| Body mass index, mean ± SE | 30.4 ± 0.91 |
| Race, n (%) | |
| Caucasian | 16 (50.0) |
| African American | 16 (50.0) |
| Past medical history, n (%) | |
| Hypertension | 26 (81.3) |
| Ischemic etiology | 23 (71.9) |
| Atrial fibrillation | 21 (65.6) |
| History of ventricular tachycardia | 21 (65.6) |
| Diabetes mellitus | 18 (56.3) |
| History of prior bleeding events | 13 (40.6) |
| History of prior thrombotic events | 9 (28.1) |
| Current infection | 3 (9.4) |
| LVAD, n (%) | |
| Heartmate II | 15 (46.9) |
| HVAD | 13 (40.7) |
| Heartmate III | 4 (12.4) |
| CF-LVAD rotations per minute, mean ± SE | |
| Heartmate II | 9,211.3 ± 105.9 |
| HVAD | 2,821.5 ± 61.0 |
| Heartmate III | 5,550.0 ± 236.3 |
| Destination therapy, n (%) | 17 (53.1) |
| INR at the time of the bleed before LAR, mean ± SE | 2.2 ± 0.094 |
| Aspirin, n (%) | |
| None | 8 (25.0) |
| 81 mg | 9 (28.1) |
| 162 mg | 1 (3.1) |
| 325 mg | 14 (43.8) |
| Angiotensin-converting enzyme inhibitor/angiotensin II receptor antagonist use, n (%) | 19 (59.4) |
| Digoxin use, n (%) | 4 (12.5) |
| Acid suppression therapy, n (%) | |
| Proton pump inhibitor | 30 (93.8) |
| Histamine-2 receptor antagonist | 2 (6.2) |
CF-LVAD, continuous-flow left ventricular assist device; HMII; HVAD; LAR, long-acting release.
All patients had a therapeutic INR at the time of GIB before LAR initiation. Characteristics related to GIB events and their management can be found in Table 3. Post-LAR there were more patients on a reduced aspirin dose (p = 0.04) and lower INR goal (p = 0.02). There was no difference between patient follow-up time pre-LAR and post-LAR (393.5 vs. 470.3 days, p = 0.4).
Table 3.
Patient Management Parameters Pre-LAR and Post-LAR
| Interventions | Pre-LAR | Post-LAR | p |
|---|---|---|---|
| Recurrent bleeding management | |||
| Reduced aspirin dose (<325 mg), n (%) | 18 (56.2) | 25 (78.1) | 0.04 |
| Aspirin, n (%) | |||
| None | 8 (25.0) | 18 (56.2) | 0.04 |
| 81 mg | 9 (28.1) | 7 (21.9) | |
| 162 mg | 1 (3.1) | 0 (0) | |
| 325 mg | 14 (43.8) | 7 (21.9) | |
| Reduced INR goal (INR range 2–3), n (%) | 19 (59.4) | 26 (81.3) | 0.02 |
| Dose of LAR (mg), median, [interquartiles] | 30.0 [20.0–40.0] | ||
| Dose frequency of LAR (days), median, [interquartiles] | 28 [21–28] | ||
| Angiotensin-converting enzyme inhibitor/angiotensin II receptor antagonist use, n (%) | 19 (59.4) | 15 (46.9) | 0.22 |
| Digoxin use, n (%) | 4 (12.5) | 5 (15.6) | 1.00 |
| CF-LVAD support parameters and durations | |||
| Reduced CF-LVAD rotations per minute, n (%) | 3 (9.4) | ||
| Patient follow-up (days), mean ± SE | 393.5 ± 73.6 | 470.3 ± 57.8 | 0.4 |
| Outcome measures | |||
| Patients with recurrent GIB, n (%) | 32 (100) | 13 (41) | <0.01 |
| Number of GIB median, [interquartiles] | 2.0 [1–3] | 0 [0–1] | <0.01 |
| Frequency of GIB per year, median, [interquartiles] | 2.95 [1.2–5.7] | 0 [0–1.4] | <0.001 |
CF-LVAD, continuous-flow left ventricular assist device; GIB, gastrointestinal bleed; INR, international normalized ratio; LAR, long-acting release.
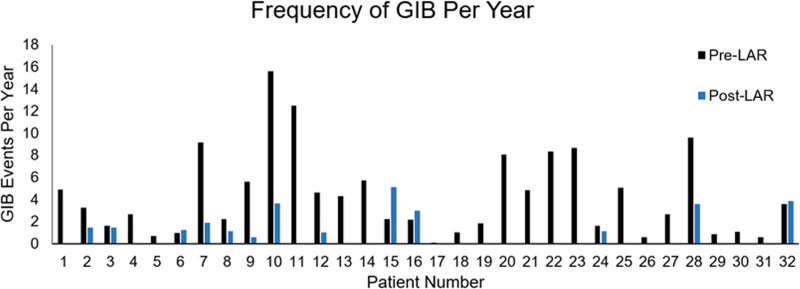
Before the initiation of LAR, patients had an average of 4.3 ± 3.8 GIB events per year (median 2.0 GIB events per year), whereas post-LAR patients had an average of 0.9 ± 1.4 GIB events per year (median 0 GIB events per year). Our study showed that LAR significantly reduced the frequency of GIB events per year, p <0.001 (Figure 2). Most patients (87.5%, 28/32) had a positive response to LAR with a decrease in admissions for GIB. After initiating LAR, 59.4% (19/32) did not have a subsequent GIB. Of the 13 patients that bled after the initiation of LAR, the frequency of events decreased by 2.6 bleeds per patient per year compared with pre-LAR (2.2 vs. 4.8; p = 0.043).
Figure 2.
Frequency of GIB pre-LAR and post-LAR. GIB, gastrointestinal bleed; INR, international normalized ratio.
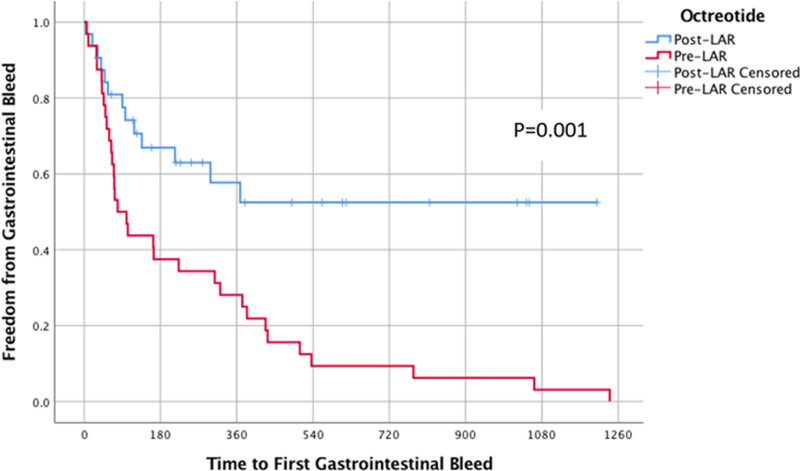
Among the three devices studied, there was no difference in GIB frequency (p = 0.54) or effect of LAR (p = 0.28). A multivariate, mixed modeling analysis demonstrated that LAR significantly reduced GIB frequency (p < 0.01), whereas a reduced INR goal and reduced aspirin dose had no effect (p= 0.85, p = 0.54) (Tables 4 and 5). The Kaplan–Meier curves showed the time to first GIB for both pre-LAR and post-LAR. The 90 day freedom from GIB in pre-LAR was 50%, whereas in the same time frame, the post-LAR had 78% freedom from GIB (Figure 3).
Table 4.
Overall F-Tests From the Mixed Modeling Full Factorial ANOVA
| Variable | F | Sig |
|---|---|---|
| Intercept | 42.0 | 1 |
| Octreotide | 24.47 | <0.01 |
| INR reduction (yes/no) | 0.04 | 0.85 |
| Aspirin reduction (yes/no) | 0.39 | 0.54 |
| INR × aspirin | 0.07 | 0.79 |
| Octreotide × INR | 1.77 | 0.20 |
| Octreotide × aspirin | 0.02 | 0.89 |
| Octreotide × INR × aspirin | 0.01 | 0.93 |
INR, international normalized ratio.
Table 5.
Estimated Magnitude of Main Effects
| Variable | Estimate | 95% CI | p |
|---|---|---|---|
| Octreotide | −0.31 | −0.43 to −0.18 | <0.01* |
| INR reduction (yes/no) | −0.014 | −0.16 to 0.14 | 0.85 |
| Aspirin reduction (yes/no) | −0.046 | −0.20 to 0.10 | 0.54 |
INR, international normalized ratio.
Figure 3.
Kaplan–Meier graph displaying freedom from GIB. GIB, gastrointestinal bleed.
Of the 32 patients included in this study, two patients developed a pump thrombosis. Patient 1 had a HeartMate II that had been on LAR for 4 months, had an INR of 1.7 (INR goal 1.5–2.0) and was on aspirin 325 mg daily at the time of admission. Patient 2 had a HeartMate II that had been on LAR over 2 years, had an INR of 3.20 (INR goal 2.0–3.0), and on aspirin 81 mg daily at the time of admission. Both patients had a reduction in their anticoagulant or antiplatelet, making it difficult to ascertain the cause of the pump thrombosis.
Discussion
Despite improvements with CF-LVADs, GIB continues to be a vexing problem. This retrospective, case-crossover study aimed to evaluate the efficacy of LAR for prevention of recurrent GIB. Mehra et al.17 found that the incidence of GIB to be 0.31 and 0.49 GIB events per patient per year in HeartMate II and HeartMate III devices, respectfully. Our patients had a higher incidence of 4.3 ± 3.8 GIB events per patient per year. When analyzing the study’s incidence of GIB, we could consider that our patient population is higher risk for GIB compared with the average patient population. In our study, LAR prevented recurrent GIB in 59.4% of patients and showed an overall reduction of GIB in 87.5% of patients. Upon a multivariate analysis, only LAR significantly reduced the frequency of GIB, while reduced INR goal and reduced aspirin dose had no effect.
Previous studies have demonstrated the efficacy and safety of octreotide for recurrent GIB. Shah et al.12 used a historical cohort from the original HeartMate II trials and found that the use of octreotide in HeartMate II patients with or without prior GIB more than a 6 month period resulted in significantly fewer recurrent GIB (24% vs. 43%, p = 0.04) and increased freedom from rebleed (75% vs 52 ± 8%, p < 0.01). Our study mirrored these results at 6 months with fewer recurrent GIB (31% vs. 63%) and increased freedom from rebleed (67% vs. 38%). While on LAR, 13 patients had recurrent GIB and the majority of bleeds (10/13) occurred in the first 6 months. Despite this rebleeding, LAR decreased the frequency of GIB. Four patients did not respond to LAR and continued to have GIB.
Another study by Juricek et al.11 determined monthly LAR used in patients with HeartMate II, HVAD, and Heartassist CF-LVADs significantly reduced the frequency of GIB (3.0 ± 2.4 vs. 0.7 ± 1.3 GIB events/year, p < 0.05). Our study confirms these results with a similar decrease in GIB events/year (4.3 ± 3.8 vs. 0.9 ± 1.4, p < 0.001). Additionally, our study further elaborates on previous results evaluating outcomes with HeartMate III, continuing LAR longer than 12 months, and the impacts of adding LAR, adjusting aspirin dose, and INR goal on GIB. The use of octreotide resulted in reduction of GIB frequency and prevention of refractory GIB in patients with CF-LVADs.
Octreotide is known to be associated with adverse effects such as hyperglycemia, bradycardia, hypertension, diarrhea, and injection site reactions. None of the patients in this study had side effects that were attributed to octreotide. Cost is another factor in the decision-making process as an average dose of octreotide of 20 mg is $5,117.69 USD per month, limiting use to patients with insurance coverage. At this time, most insurance plans that serve the patients around our center in the United States appear to cover LAR, although prior authorizations may take up to 14 days delaying initiation of therapy. The use of octreotide decreased frequency of hospitalization for GIB in 87.5% of patients. The costs of LAR may be offset with reduced hospitalizations and could be appropriate in patients with multiple admissions for GIB.
Modifications to patient regimens such as reduction in aspirin dose, reduction in goal INR, and changes in CF-LVAD settings have been utilized in an attempt to reduce the frequency of GIB; however, these treatments may increase the risk for thrombotic events.15,16 Use of octreotide in this population may decrease readmissions rates and increase days spent out of the hospital.11 There are several inherent limitations in this study. This was a retrospective single-center and relatively small study, including 32 patients that received LAR at our center. On average, these patients had at least 2 GIB before initiation of octreotide when adjustments in aspirin dose and target INR failed to prevent recurrent GIB. This leads to a selection bias, identifying only patients with the most frequent GIB who failed conventional therapies. Another limitation would include simultaneous medication interventions such as reduced aspirin doses or INR goals, which confounds the impact of LAR on our results. There were additional gastrointestinal interventions concurrently performed on these patients when a source of bleeding was found that could also impact the results. Additionally, the changes in clinical practice over the long study time frame cannot be taken into account. Our results should be considered in the context of the refractory population studied. Efforts were taken to identify and eliminate confounders to prevent impact on results.
Conclusion
Overall, our study demonstrates that LAR is associated with a significant decrease in the frequency of GIB in high-risk patients with CF-LVADs. This study adds to prior literature and adds insight with additional follow-up time and the inclusion of the Heartmate III. Future prospective studies, including a randomized controlled trial, are needed to validate these findings.
Acknowledgment
Dr. Trent Gaugler, PhD, Associate Professor of Mathematics at Lafayette University.
Footnotes
Disclosure: Dr. Baran has consulting income from Abiomed, Getinge, Livanova, MC3, and speaking honoraria from Novartis and Pfizer. Dr. Herre has consulting income from LifeNet Health and receives grant support from Analytics 4 Life, Boston Scientific and DCRI. Dr. Yehya has speaking honoraria from Akcea therapeutics, CareDx, and Zoll. The other authors have no conflicts of interest to report.
Contributor Information
Tyler J. Wilson, Email: tjwilso1@sentara.com.
David A. Baran, Email: DABARAN@sentara.com.
John M. Herre, Email: JMHERRE@sentara.com.
Chad M. Cameron, Email: CMCAMERO@sentara.com.
Amin Yehya, Email: AXYEHYA@sentara.com.
Amanda I. Ingemi, Email: AIINGEMI@sentara.com.
References
- 1.Guha A, Eshelbrenner CL, Richards DM, Monsour HP, Jr: Gastrointestinal bleeding after continuous-flow left ventricular device implantation: review of pathophysiology and management. Methodist Debakey Cardiovasc J 2015.11: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey L, Holley CT, John R: Gastrointestinal bleed after left ventricular assist device implantation: Incidence, management, and prevention. Ann Cardiothorac Surg 2014.3: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsano J, Desai J, Chang S, Chau M, Pochapin M, Gurvits GE: Characteristics of gastrointestinal bleeding after placement of continuous-flow left ventricular assist device: A case series. Dig Dis Sci 2015.60: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal A, Pant R, Kumar S, et al. : Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg 2012.93: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JA, Paone G, Nemeh HW, et al. : Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant 2012.31: 715–718. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein DJ, Aaronson KD, Tatooles AJ, et al. : Gastrointestinal bleeding in recipients of the heartware ventricular assist system. JACC Heart Fail 2015.303–313. [DOI] [PubMed] [Google Scholar]
- 7.Crow S, John R, Boyle A, et al. : Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg 2009.137: 208–215. [DOI] [PubMed] [Google Scholar]
- 8.Loyaga-Rendon RY, Hashim T, Tallaj JA, et al. : Octreotide in the management of recurrent gastrointestinal bleed in patients supported by continuous flow left ventricular assist devices. ASAIO J 2015.61: 107–109. [DOI] [PubMed] [Google Scholar]
- 9.Draper K, Kale P, Martin B, Kelly Cordero R, Ha R, Banerjee D: Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: case series and treatment protocol. J Heart Lung Transplant 2015.34: 132–134. [DOI] [PubMed] [Google Scholar]
- 10.Schettle S, Bawardy BA, Asleh R, et al. : Danazol treatment of gastrointestinal bleeding in left ventricular assist device-supported patients. J Heart Lung Transplant 2018.37: 1035–1037. [DOI] [PubMed] [Google Scholar]
- 11.Juricek C, Imamura T, Nguyen A, et al. : Long-acting octreotide reduces the recurrence of gastrointestinal bleeding in patients with a continuous-flow left ventricular assist device. J Card Fail 2018.24: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah KB, Gunda S, Emani S, et al. : Multicenter evaluation of octreotide as secondary prophylaxis in patients with left ventricular assist devices and gastrointestinal bleeding. Circ Heart Fail 2017.10:e004500. [DOI] [PubMed] [Google Scholar]
- 13.Malhotra R, Shah KB, Chawla R, et al. : Tolerability and biological effects of long-acting octreotide in patients with continuous flow left ventricular assist devices. ASAIO J 2017.63: 367–370. [DOI] [PubMed] [Google Scholar]
- 14.Smallfield GB, Gunda S, Emani S, et al. : A multicenter evaluation of octreotide for ventricular assist device related gastrointestinal bleeding. J Heart Lung Transplant 2016.35: S245. [Google Scholar]
- 15.Teuteberg JJ, Slaughter MS, Rogers JG, et al. ; ADVANCE Trial Investigators: The HVAD left ventricular assist device: risk factors for neurological events and risk mitigation strategies. JACC Heart Fail 2015.3: 818–828. [DOI] [PubMed] [Google Scholar]
- 16.Lea JC, Floroff CK, Ingemi AI, Zeevi GR: Impact of time in therapeutic range after left ventricular assist device placement: a comparison between thrombus and thrombus-free periods. J Thromb Thrombolysis 2019.47: 361–368. [DOI] [PubMed] [Google Scholar]
- 17.Mehra MR, Uriel N, Naka Y, et al. ; MOMENTUM 3 Investigators: A fully magnetically levitated left ventricular assist device - final report. N Engl J Med 2019.380: 1618–1627. [DOI] [PubMed] [Google Scholar]