Abstract
Objectives
People with mental disorders are more likely to smoke than the general population. The objective of this study is to develop a decision analytical model that estimates long-term cost-effectiveness of smoking cessation interventions in this population.
Methods
A series of Markov models were constructed to estimate average lifetime smoking-attributable inpatient cost and expected quality-adjusted life-years. The model parameters were estimated using a variety of data sources. The model incorporated uncertainty through probabilistic sensitivity analysis using Monte Carlo simulations. It also generated tables presenting incremental cost-effectiveness ratios of the proposed interventions with varying incremental costs and incremental quit rates. We used data from 2 published trials to demonstrate the model’s ability to make projections beyond the observational time frame.
Results
The average smoker’s smoking-attributable inpatient cost was 3 times higher and health utility was 5% lower than ex-smokers. The intervention in the trial with a statistically insignificant difference in quit rate (19% vs 25%; P=.2) showed a 45% to 49% chance of being cost-effective compared with the control at willingness-to-pay thresholds of £20 000 to £30 000/quality-adjusted life-years. The second trial had a significant outcome (quit rate 35.9% vs 15.6%; P<.001), and the corresponding probability of the intervention being cost-effective was 65%.
Conclusions
This model provides a consistent platform for clinical trials to estimate the potential lifetime cost-effectiveness of smoking cessation interventions for people with mental disorders and could help commissioners direct resources to the most cost-effective programs. However, direct comparisons of results between trials must be interpreted with caution owing to their different designs and settings.
Keywords: decision analytical model, long-term cost-effectiveness, mental disorders, smoking cessation
Highlights
-
•
People with mental disorders are more likely to smoke than the general population and are also likely to smoke more heavily, creating a significant health deficit and economic costs.
-
•
We developed a flexible decision analytical model that facilitates comparisons of the long-term cost-effectiveness of smoking cessation interventions for people with mental disorders. The model parameters were estimated using a variety of data sources, and it incorporated uncertainty through probabilistic sensitivity analysis using Monte Carlo simulations. We used data from 2 published trials to demonstrate the model’s ability to make projections beyond the observational time frame.
-
•
Compared with ex-smokers, the average smoker’s smoking-attributable cost was 3 times higher and their health utility was 5% lower. The decision analytical model provides a consistent platform for clinical trials to estimate the potential lifetime cost-effectiveness of smoking cessation interventions for people with mental disorders and could help commissioners direct resources to the most cost-effective programs. However, the direct comparisons of results between trials must be interpreted with caution owing to their different designs and settings.
Introduction
Tobacco smoking remains the leading preventable cause of morbidity and mortality worldwide.1 In the United Kingdom, although the prevalence of smoking in the general population fell from 45% in 1975 to 15% in 2018,2 smoking rates remain much higher among individuals with mental disorders (MDs): conditions that affect mood, thoughts, and behavior.3,4 In 2014, approximately 1 in 6 adults in England was classed as having a common MD (CMD), which includes various types of depression, and 34% of them were smokers.5,6 For those with severe MDs (SMDs), the smoking rate may exceed 50%.4,5 SMD here refers to serious psychological conditions that may cause insight and cognitive impairments, including bipolar disorder, schizophrenia and affective psychosis, and other non-organic psychotic disorders.5
People with MDs are more likely to smoke than the general population and are also likely to smoke more heavily.4,7 Despite their high levels of nicotine dependence, evidence suggests that the proportion of people with MDs who want to quit smoking is similar to that of the general population. Data from the Health Survey for England 2012 show that 69% of smokers taking psychoactive medications would like to quit smoking compared with 66% of those in the general population.4 Nevertheless, their smoking cessation rate remains much lower.8 High tobacco consumption and low quit rates can put smokers with MDs at a higher risk of developing smoking-related diseases (SRDs) and consequently constitute a significant economic burden (an estimated £719 million in 2009-2010) to the National Health Service (NHS).4,9
Many interventions are available to help people stop smoking, including pharmacotherapies (nicotine replacement therapy, varenicline, bupropion, and e-cigarette), intensive behavioral support, and combination therapies. Such smoking cessation interventions have proven to be highly cost-effective in both the short term and long term for the general population.10 Nevertheless, people with MDs are often actively excluded from clinical trials; consequently, evidence on the effectiveness and cost-effectiveness of smoking cessation interventions among this population is relatively scarce.11,12 Although some clinical trials have assessed the short-term cost-effectiveness of smoking cessation interventions in people with MDs, very few studies have considered the long-term cost-effectiveness of these interventions.13, 14, 15, 16 Within-trial cost-effectiveness analysis may fail to capture the full benefit of smoking cessation interventions, because the main benefits of quitting are improved health and healthcare cost saving that come from the reduced risks of developing SRDs in the long run.17,18
The aim of this article was to develop an adaptable and flexible decision model that facilitates comparisons of the long-term cost-effectiveness of smoking cessation interventions for people with MDs.
Methods
Model Structure
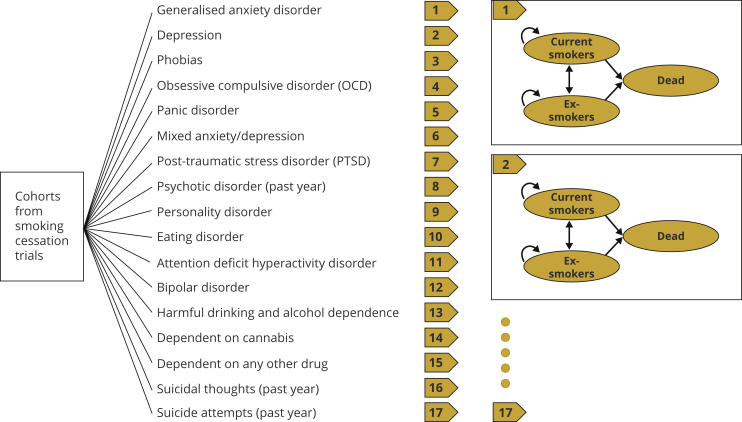
The model was designed to reflect the complexities and adaptability of the long-term impacts of smoking status on the costs of SRDs and quality-adjusted life-years (QALYs) in people with various MDs. A schematic representation of the model is shown in Figure 1.
Figure 1.
The schematic representation of the model.
The model first allows users to select a category of MD related to their clinical trial.
Here, we included 17 widely studied MDs based on data from the 2014 Adult Psychiatric Morbidity Survey (APMS).4,5 These 17 MDs covered the majority of the MDs identified in APMS where participants’ mental health and smoking status were both reported.5 For each MD, a Markov model was constructed to estimate lifetime smoking-attributable costs (SACs) and QALYs for patients with that particular MD; patients with multiple MDs were not included in this model.
This model includes people with MDs aged 35 years and more because evidence suggests that people who quit smoking before the age of 35 years are no more likely to experience negative health consequences than people who have never smoked.19,20 Meanwhile, the published relative risks of developing SRDs for smokers and ex-smokers used to project long-term outcomes were only available for people older than 35 years.21
The Markov models for the 17 MDs have the same 3-state structure: current smokers, ex-smokers, and death. For each intervention arm, the trial cohort enters the Markov model as current and ex-smokers according to the cessation rate, based on trial observations. Smokers and ex-smokers could transit to other states or remain in the same state at the end of each 1-year cycle, whereas the death state absorbs those who died within the cycle. Each state is associated with an annual smoking-attributable healthcare cost and a health outcome in terms of annual QALYs at the end of each cycle. The model runs until everyone dies or reaches the age of 90 years, which was considered to be a lifetime. SACs and QALYs are then summed up over all cycles separately to generate lifetime SACs and lifetime QALYs for each trial intervention, and the results of the interventions are compared.
Further modeling details are described in the Model Inputs section. For simplicity, it is assumed that once a cohort enters the model, participants will receive no further smoking cessation interventions. The model was programmed in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, www.microsoft.com).
Model Inputs
The model parameters were measured separately and stratified by age, gender, smoking status, and MDs whenever the data were available to accommodate all modeled cohorts. The same layout was used in the results tables reporting prevalence, costs, and QALYs. Further details can be found in the Appendix Tables (in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002) and are available from the authors upon request.
Transition probabilities
In the absence of evidence of the overall relapse rate in people with MDs, we employed a 10% annual relapse rate for 10 years after quitting and assumed no subsequent relapse after the 10th year, based on published systematic reviews and surveys in the general population.22, 23, 24 Because smokers might quit smoking without a cessation aid, an annual background or spontaneous abstinence rate of 4.1% was taken into account.25
Annual mortality rates were calculated by applying relevant weights to the mortality rates of the general population. First, the annual all-cause mortality rates were estimated by age and gender using data from deaths registered in England and Wales in 2017.26 Second, to adjust for the impact of smoking, the increased relative risks of mortality for smokers and ex-smokers were derived from the British doctors’ study, which had a 50-year follow-up, and applied to the all-cause mortality rates.18 Finally, to reflect that people with MDs have a higher risk of death than the general population, smoking-adjusted mortality rates were further multiplied by the increased relative risks of the 17 MDs, which were estimated based on data from various sources.27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 The parameters for transition probabilities applied in the model are summarized in Table 1.
Table 1.
Summary of the transition probabilities applied in the model.
| Parameters | Value (SE) | Source | |
|---|---|---|---|
| Quit rate | – | Based on the trials | |
| Probability of relapse (first 10 years) | 10.00% (3.06%) | 22, 23, 24 | |
| Background cessation rate | 4.1% (0.77%) | 25 | |
| Discount rate | 3.50% (0) | 45 | |
| Mortality | |||
| Age group | Mortality: males, smokers (SE) | Mortality: males, ex-smokers (SE) | 18,26 |
| 16-24 | 0.040% (0.000%) | 0.040% (0.000%) | |
| 25-34 | 0.070% (0.000%) | 0.070% (0.000%) | |
| 35-44 | 0.236% (0.001%) | 0.175% (0.001%) | |
| 45-54 | 0.811% (0.001%) | 0.515% (0.003%) | |
| 55-64 | 1.898% (0.004%) | 1.215% (0.006%) | |
| 65-74 | 5.070% (0.015%) | 3.030% (0.014%) | |
| 75+ | 25.516% (0.173%) | 15.214% (0.117%) | |
| Age group | Mortality: females, smokers (SE) | Mortality: females, ex-smokers (SE) | |
| 16-24 | 0.020% (0.000%) | 0.020% (0.000%) | |
| 25-34 | 0.035% (0.000%) | 0.035% (0.000%) | |
| 35-44 | 0.152% (0.000%) | 0.113% (0.000%) | |
| 45-54 | 0.528% (0.001%) | 0.335% (0.002%) | |
| 55-64 | 1.236% (0.003%) | 0.791% (0.004%) | |
| 65-74 | 3.339% (0.010%) | 1.996% (0.010%) | |
| 75+ | 21.154% (0.142%) | 12.613% (0.096%) | |
| Relative risks of mortality for the 17 mental disorders | |||
| Mental disorder | Relative risk of mortality compared with people without the condition (SE) | ||
| Generalized anxiety disorder | 1.61 (0.11) | 27 | |
| Depression | 1.52 (0.04) | 28 | |
| Phobias | 1.61 (0.11) | 27 | |
| OCD | 1.88 (0.36) | 29 | |
| Panic disorder | 1.61 (0.11) | 27 | |
| Mixed anxiety/depression | 1.61 (0.11) | 27 | |
| PTSD | 2.10 (0.23) | 30 | |
| Psychotic disorder (in the past year) | 2.60 (0.03) | 31 | |
| Personality disorder (males) | 5.00 (0.13) | 32 | |
| Eating disorder | 1.92 (0.27) | 33 | |
| ADHD | 2.07 (0.20) | 34 | |
| Bipolar disorder | 2.60 (0.03) | 31 | |
| Harmful drinking and alcohol dependence | 3.45 (0.27) | 35 | |
| Dependence on cannabis | 0.95 (0.07) | 36 | |
| Dependence on any other drug | 1.27 (0.13) | 36 | |
| Suicidal thoughts (past year) | 1.23 (0.03) | 37 | |
| Suicide attempts (past year) | 3.60 (0.08) | 38 | |
ADHD indicates attention-deficit/hyperactivity disorder; OCD, obsessive-compulsive disorder; PTSD, posttraumatic stress disorder; SE, standard error.
Prevalence of MDs and smoking
The prevalence of MDs and smoking were used to estimate the SACs incurred by people with different MDs. The prevalence of the 17 MDs was extracted from the 2014 APMS (Appendix 1 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002).5 For age groups where data regarding the prevalence of MD was unavailable, data from the adjacent lower age group were used instead. Owing to the lack of detailed information in the literature on smoking status in people with MDs, patient-level data from the latest wave of the APMS (2014) were requested through the UK Data Service (http://ukdataservice.ac.uk).19 The APMS data classified participants as current smokers, ex-smokers, and never-smokers. The proportion of smoking status was summarized by participants’ MDs and can be found in Appendix 2 (in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002).
SACs
The model allows the intervention and healthcare costs during the trial period to be input as a one-off cost. The annual healthcare costs over the projection period are prespecified by MD, smoking status, age group, and gender. In the Markov model, we used smoking-attributable hospital admissions costs as an approximation of additional healthcare costs for current smokers and ex-smokers compared with never-smokers. All model costs were expressed in British pounds and inflated to 2018 to 2019 year where necessary using the Hospital and Community Health Services Pay and Prices Inflation Index for costs incurred before 2016 and the NHS Cost Inflation Index for costs incurred in 2016 or later.39
Annual SAC was calculated following the cost-of-illness approach introduced by the World Health Organization Economics of Tobacco Toolkit.40 Equation 1 shows the formula used to calculate SAC. A total of 52 SRDs were included in the costing. These diseases were selected based on the 2018 Royal College of Physicians report wherein the authors summarized the relative risks of developing these SRDs for smokers or ex-smokers compared with never-smokers (Appendix 3 in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002).21
| (1) |
SACmsga denotes SACs for people with MD “m,” smoking status “s,” gender “g,” and age group “a”; pmsga denotes the prevalence of smoking status “s” in people with MD “m,” gender “g,” and age group “a”; rris denotes the relative risk of SRD “i” in people of smoking status “s” compared with the general population; THQmsgai denotes the number of annual hospital admissions for SRD “i” for people with MD “m,” gender “g,” and age group “a”; and UCi denotes the unit cost per hospital episode of SRD “i.”
For each SRD, the number of annual hospital admissions was extracted from the Hospital Episode Statistics41 for 2015 to 2016 by gender and age group. It was then multiplied by the smoking-attributable proportion of the same characteristics to calculate the number of smoking-attributable hospital admissions for each SRD in this group.
The set of unit costs per hospital episode for the SRDs was derived from the NHS reference cost and matched to the Hospital Episode Statistics data through the HRG4+ code to group workbook.21,42,43
The SACs of the SRDs were then calculated by multiplying unit costs by their respective numbers of smoking-attributable hospital admissions. For people with the same MD, smoking status, age group, and gender, the inpatient SACs of 52 SRDs were summed up to produce an estimated annual SAC.
QALYs
QALY is a generic measure of health benefit that combines both the quality and quantity of life lived.44 It is the preferred health outcome measure for economic evaluations recommended by many guidelines including the National Institute for Health and Care Excellence guide to methods of technology appraisal in the United Kingdom.45
QALYs are calculated based on health utility. Health utility is a summary score that assesses the value of various health statuses as measured by instruments such as EQ-5D, Health Utilities Index Mark 3, or SF-6D.44 In the absence of published health utilities for people with MD, we estimated utility scores for this population using data extracted from the APMS 2007 study.46 The APMS 2007 was the most recent wave of APMS surveys that collected data on patients’ smoking status alongside their health status using the 12-item Short Form Health Survey (SF-12) questionnaire. Following the methods proposed by Brazier et al,47, 48, 49 the reported SF-12 data were converted to the SF-6D utility score, where 1 represents perfect health, 0 represents death, and negative numbers represent states worse than death. Given the purpose of this study, the APMS SF-12 data used to calculate utility scores were restricted to people with MDs.
The utility scores at each time point were then used to generate QALYs using the area under the curve approach.50 For age groups where utility scores were unavailable for certain MDs in the APMS data, the average score for the same age group was used instead. Both future costs and health outcomes were discounted at an annual rate of 3.5%.45
Cost-Effectiveness Analysis and Probabilistic Sensitivity Analysis
The model conducts an incremental cost-effectiveness analysis comparing the lifetime SACs and QALYs of the smoking cessation interventions described in clinical trials. Model inputs require a range of trial data, including demographic information such as the MD of the target population, number of participating smokers, mean age, and gender composition of each intervention arm. The intervention-related variables include quit rates, mean intervention costs with standard error (SE), and mean QALYs with SE over the trial period.
If all required information is available, incremental cost-effectiveness ratios (ICERs) are calculated by dividing the difference in expected SACs by difference in expected QALYs between interventions. The ICERs can then be compared with the selected decision-making willingness-to-pay (WTP) threshold. The analysis takes the perspective of the NHS and personal social services over a lifetime horizon.
To assess uncertainty in parameter estimates, the model runs a probabilistic sensitivity analysis (PSA) using Markov chain Monte Carlo simulations. Appropriate distributions were selected from which random values for model parameters were drawn. A beta distribution was assigned for probabilities and a gamma distribution was assigned for QALYs and costs.51 A cost-effectiveness plane and cost-effectiveness acceptability curve, along with a summary message, are then generated based on the 10 000 Monte Carlo iterations.52
ICER Tables
Compared with smoking cessation intervention trials in the general population, the number of smoking cessation intervention trials focused on people with MDs is relatively small, and most such trials have not reported the costs and the QALYs of the interventions.11, 12, 13 In the absence of within-trial costs and QALYs for the existing trials or before the implementation of a new trial, the model also allows researchers and decision makers to assess the lifetime cost-effectiveness of the interventions, by generating a table to present the ICERs of the proposed interventions with varying hypothetical incremental costs and incremental quit rates for a specified subgroup of smokers by conducting a 2-way sensitivity analysis.
Application to Published Trial Data
For illustrative purposes, we used empirical data from published clinical trials as model inputs to demonstrate the model’s analyses. Two randomized controlled trials (RCTs) were chosen based on the most recent published systematic review of smoking cessation interventions for smokers with depression, one of the most prevalent MDs.53
The first trial, conducted by Hall et al,54 was the only trial to have published a cost-effectiveness article wherein intervention costs were reported.14 This trial included 322 participants and examined the efficacy of a staged care intervention compared with a brief contact control. The quit rates at the end of the 18-month follow-up were estimated at 19.1% for the control group and 24.6% for the intervention group (P = .2). The reported intervention costs, converted to 2018 to 2019 pounds sterling, were £3748 (SE £440) for the control and £4316 (SE £392) for the intervention.
The second trial, conducted by Anthenelli et al,55 had the largest sample size (525 smokers) of all RCTs in the review. It compared varenicline with a placebo. The carbon monoxide (CO)–confirmed continuous abstinence quit rate at 3 months was significantly higher in the varenicline group than in the placebo group (35.9% vs 15.6%; P < .001). This trial did not report any cost information; we estimated the average cost of the intervention (1 mg varenicline, twice daily for 12 weeks) at £163.80 per participant using data from the 2019 NHS prescription cost analysis.56
Neither trial reported any QALY-related outcomes; we assumed that the 2 groups in each trial were well balanced in terms of quality of life after randomization and thus that the incremental QALYs between groups within the trial periods were considered to be negligible owing to the limited follow-up period. Further details about the information extracted from the published trials and used to inform the model are presented in Table 2.
Table 2.
| Trial |
Group types |
|
|---|---|---|
| Hall’s RCT | Control group |
Intervention group |
| Brief contact control | Staged care intervention | |
| Model inputs | ||
| Number of smokers | 159 | 163 |
| Mean age | 42 | 42 |
| Gender (male, %) | 29% | 32% |
| Quit rate at 18 mo | 19% | 25% |
| Mean total cost (18 mo) | £3748 | £4316 |
| SE of the mean cost | £440 | £392 |
| QALYs | – | – |
| SE of mean QALYs | – | – |
| Model outputs | ||
| Intervention BC | Intervention SCC | |
| Mean lifetime SAC | £4951 | £5523 |
| Mean lifetime QALY gains | 12.547 | 12.564 |
| Lifetime ICER | £33 744/QALY | |
| Probability be cost-effective at WTP threshold | ||
| £20 000 | 55% | 45% |
| £30 000 | 51% | 49% |
| Anthenelli’s RCT | Control group |
Intervention group |
|---|---|---|
| Placebo | Varenicline | |
| Model inputs | ||
| Number of smokers | 269 | 256 |
| Mean age | 47 | 45 |
| Gender (male, %) | 37% | 38% |
| Quit rate at 3 mo | 16% | 36% |
| Mean intervention cost (3 mo) | £0 | £164 |
| SE of the mean cost | £0 | £0 |
| QALYs | – | – |
| SE of mean QALYs | – | – |
| Model outputs | ||
| Placebo | Varenicline | |
| Mean lifetime SAC | £1446 | £1563 |
| Mean lifetime QALY gains | 9.817 | 9.856 |
| Lifetime ICER | £3002/QALY | |
| Probability be cost-effective at WTP threshold | ||
| £20 000 | 35% | 65% |
| £30 000 | 35% | 65% |
BC indicates brief contact; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; RCT, randomized controlled trial; SAC, smoking-attributable cost; SCC, staged care intervention; SE, standard error; WTP, willingness to pay.
Results
Costs and QALYs
Based on the APMS 2014 data, the results show that smoking was about twice as prevalent among people with a CMD (31%) than among those without a diagnosis (17%) (Appendix 4 A in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002). The smoking rate was approximately 3 to 4 times higher among those with SMDs than those without (Appendix 4 B in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002). For example, smoking prevalence was 75% versus 17% respectively in people with and without drug dependence, 60% versus 19% in people with and without alcohol dependence, and 53% versus 19% in people with and without schizophrenia and bipolar disorders.
Appendix 5 (in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002) summarizes the mean annual SACs per person for people with 1 of the 17 selected MDs. SACs increased with age and varied by MD. SACs were substantially higher for smokers than ex-smokers and were highest among male smokers (£368). Generally, men had higher SACs than women within the same age group. SACs among male smokers and male ex-smokers ranged from £26 to £368 and £7 to £135, respectively, whereas in women, it was estimated at £21 to £305 for smokers and £5 to £100 for ex-smokers.
Appendix 6 (in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002) presents the mean utility scores for people with MDs by age, gender, and smoking status. Among people with MDs, the utility scores of ex-smokers were 0.03 to 0.05 higher than those who continued to smoke. This is equivalent to an average 5% increase in health utility gained from quitting smoking. The estimated utility scores were 0.02 to 0.04 higher for men than women in the same age and MD group. In general, health utility decreased with age; nevertheless, the scores for age groups 45 to 54 years and 55 to 64 years were very close.
Cost-Effectiveness Analysis Results of Selected RCTs
Both trial-based model inputs and cost-effectiveness analysis results from the model for the 2 selected trials are outlined in Table 2. For the trial by Hall et al54, the expected lifetime SAC and QALY gains per person were £4951 versus £5523 and 12.547 versus 12.564 for the brief contact and staged care interventions, respectively. The ICER of the intervention compared with the control was £33 744 per QALY. The results of the PSA for the trial by Hall et al54 are displayed in Figure 2. In the cost-effectiveness plane, the ICER iterations were scattered across 4 quadrants. The cost-effectiveness acceptability curve showed that the probability of the staged care being more cost-effective than brief contact was 45% to 49% at the WTP threshold range of £20 000 to £30 000 per QALY.
Figure 2.
CEP and CEAC to illustrate the results of the PSA (Hall’s trial).
Meanwhile, smokers from the study by Anthenelli et al55 who received varenicline gained an average of 0.039 more QALYs than those who received the placebo, at an increased cost of about £117, yielding a lifetime ICER of £3002 per QALY. The results of the PSA (Fig. 3) indicate that varenicline’s chance of being more cost-effective than placebo remained at 65% through the WTP threshold range of £20 000 to £30 000/QALY.
Figure 3.
CEP and CEAC to illustrate the results of the PSA (Anthenelli‘s trial).
Assuming both trial-related costs and quit rates data were unavailable, Table 3 presents an example of ICER tables for the cohort of patients from the trial by Hall et al.54 The potential lifetime ICERs were estimated using different combinations of incremental quit rate (ie, the quit rate in the intervention group minus the quit rate in the control group) and incremental cost (ie, the mean cost in the intervention group minus the mean cost in the control group). For example, if the expected incremental quit rate was 10%, using a WTP threshold of £20 000 per QALY, the intervention cost should be kept below £500 per person to ensure that the new intervention is more cost-effective than the control at a lifetime horizon. In contrast, if the trial has a budget of £500 per capita, an additional 10% or more incremental quit rate is required to ensure the new intervention’s long-term cost-effectiveness. Appendix 7 (in Supplemental Materials found at https://doi.org/10.1016/j.jval.2021.04.002) shows a more complete ICER table based on the cohorts from both Hall’s and Anthenelli’s studies.
Table 3.
ICER table for the cohort from Hall’s trial.
| Incremental quit rate (intervention – control) | Incremental cost (intervention – control) |
||||
|---|---|---|---|---|---|
| £100 | £300 | £500 | £700 | £900 | |
| 1% | £13 160 | £36 953 | £60 745 | £84 537 | £108 330 |
| 10% | £3809 | £11 687 | £19 565 | £27 443 | £35 322 |
| 25% | £1368 | £5093 | £8 819 | £12 544 | £16 269 |
| 50% | £344 | £2327 | £4310 | £6293 | £8277 |
| 75% | -£27 | £1324 | £2675 | £4026 | £5378 |
| 100% | −£219 | £806 | £1830 | £2855 | £3880 |
Note. This table presents the potential lifetime ICERs for given combinations of incremental quit rate in the first column (ie, the quit rate in the intervention group minus the quit rate in the control group) and incremental cost in the third row (ie, the mean cost in the intervention group minus the mean cost in the control group). For example, if we look at the fourth row and fourth column, a new intervention with an expected incremental quit rate of 10% and incremental cost of £500 has an ICER of £19 565 compared with the control intervention.
ICER indicates incremental cost-effectiveness ratio.
Discussion
The objective of this study was to construct a dynamic decision analytical model to assess the lifetime cost-effectiveness of smoking cessation interventions for people with various MDs. To the best of our knowledge, this is the first study of its kind to model the long-term cost-effectiveness of smoking cessation interventions in this population. The ability to make long-term prediction is important in evaluating smoking cessation programs because the benefits of quitting smoking do not always show immediately within the time frames of trials. Not considering the long-term benefits of quitting smoking, especially its impact on the risk of developing SRDs, may lead to erroneous conclusions about some potentially cost-effective interventions. Constructing models to predict the long-term economic and health impacts of each individual trial is impractical. Our model allows predefined trial cohorts to be projected and, therefore, provides a platform for timely and consistent comparison of smoking cessation programs. This model focuses on the cost-effectiveness of the studied trial interventions, so it is assumed that after the end of the trial period, participants received no further smoking cessation interventions.
Our results show that compared with people without MDs, smoking was twice as prevalent in people with CMDs and that smoking rate could reach as high as 75% for those with SMDs. This finding is consistent with those in the literature that people with MDs are more likely to smoke than the general population.4 Compared with ex-smokers, the healthcare SACs of people who continued to smoke were 3 times higher and their health utility was 5% lower. This indicates that smoking cessation may yield substantial cost savings and improved health-related quality of life for this vulnerable group. The result shows that the utility scores of ex-smokers were 0.03 to 0.05 higher than those of current smokers among people with MDs, a trend that is similar to the difference between ex-smokers and current smokers found in the general population in the literature.57,58 Although the model was demonstrated using data from England, it may be adapted for use in other countries where local data are available. For example, local SACs (calculated by multiplying the total health expenditure by smoking attribution proportion) and local QALYs may be used as model inputs to generate country-specific estimates for the interventions.
We used data from 2 published RCTs to demonstrate the model’s ability to make projections beyond the observational time frame and assess the impact of smoking cessation interventions well into the future. The model provided lifetime SAC and QALY gains for all trial interventions. ICERs were generated and compared with the selected WTP thresholds to establish cost-effectiveness. The model also quantified the uncertainty around the results using PSA where all parameters were simultaneously varied over chosen distributions. Other 1-way or 2-way sensitivity analyses to test the impact of individual parameters are also possible.
The PSA reflects the effects of the within-trial outcomes. For example, the difference in quit rate between the 2 arms of Hall’s trial was statistically insignificant (19% vs 25%; P = .2); the PSA results showed that the probability that the intervention was cost-effective was approximately 50%, indicating a high level of uncertainty, and hence, the results should be interpreted with caution. By contrast, in Anthenelli’s trial, a significant outcome (35.9% vs 15.6%; P < .001) led to more certain long-term results. Nevertheless, the direct comparisons of results between trials must be interpreted with caution owing to the difference in outcome measures. For example, the trial by Hall et al54 used a 7-day abstinence from cigarettes, verified by expired air CO at ≤10 ppm to define abstinence from smoking, whereas the study by Anthenelli et al55 employed a CO-validated continuous abstinence rate for the last 4 weeks.
In the absence of within-trial costs and QALYs, the model can also produce ICER tables for any target population with MDs. For heavily dependent smokers, such as people with MDs, more intensive levels of cessation interventions are required, and thus, trials could be expensive to run. The ICER table provides a useful tool to inform planning and budgeting for future trials.
Another strength of this model is the number of SRDs it considers. Few previous studies have considered SRDs in their models, and among those that have, only a small number of key SRDs such as lung cancer, chronic obstructive pulmonary disease, and coronary heart disease have been included.59, 60, 61 This model, in contrast, includes a wider range of 52 diseases associated with smoking, allowing both smoking- and MD-related morbidity and mortality to be adjusted simultaneously for long-term outcomes. Hence, the generated predictions are better able to capture the real costs and QALYs associated with MDs and smoking status than previous models.
Impact of Model Assumptions
The limitations of the model are mostly related to the assumptions made in its development. First, owing to the model structure design and the availability of evidence, this model only allows evaluations of interventions targeting smokers with a single MD. Such restriction is likely to exclude a good portion of study population, as approximately 30% of the participants identified with MD met the criteria for at least 2 different MDs based on the APMS 2007 survey.46 The assumption that participants only have 1 MD at a time may lead to an underestimation of participants’ SACs and overestimation of their QALYs. Nevertheless, this impact should be balanced across the 2 trial arms after randomization and should not affect the comparative cost-effectiveness of the 2 interventions.
Second, the SAC in this model covers costs incurred in inpatient settings only. Other types of primary care and community-based care were not included owing to data constraints. The assumption that primary care costs have the same distribution as hospital admissions costs may lead to an underestimation of SACs for people who continue to smoke. For instance, many SRDs are chronic conditions that are likely to be treated in outpatient settings. Nevertheless, it is unclear to what extent this assumption affects the predicted costs.
Another limitation is the comparability of QALY results. The QALYs used in this model were calculated from SF-6D instead of the more widely used EQ-5D owing to the limited availability of data for people with MDs. Therefore, any direct comparison between our model results and results based on EQ-5D in other studies should be made with caution. The limitations of the model, such as the lack of multiple MDs and primary care data in people with MDs, identified a gap in the literature and potential areas for future research. Updated models will be required to explore this gap once additional data become available.
Conclusions
People with MDs are more likely to smoke than the general population and are also likely to smoke more heavily. The presented model permits the comparison of the long-term cost-effectiveness of different programs to help people with MDs quit smoking. The model enables the consideration of lifetime SACs and health gains using the most recent evidence and provides useful information about what interventions should be commissioned to decision makers. However, the model’s assumptions and local population characteristics should be examined carefully before applying the model to specific studies, and direct comparisons of results between trials must be interpreted with caution owing to their different designs and settings.
Article and Author Information
Author Contributions:Concept and design: Wu, Gilbody, Parrott
Acquisition of data: Wu, Li, Wang
Analysis and interpretations of data: Wu, Li, Wang, Parrott
Drafting of the manuscript: Wu, Gilbody, Li, Wang, Parrott
Critical revision of the paper for important intellectual content: Wu, Gilbody, Li, Wang, Parrott
Statistical analysis: Wu, Li, Wang
Obtainingfunding: Gilbody, Parrott
Supervision: Gilbody, Parrott
Conflict of Interest Disclosures: The authors reported no conflict of interest.
Funding/Support: This work was funded jointly the National Institute for Health Research Yorkshire and Humber Applied Research Collaboration and the UK Centre for Tobacco and Alcohol Studies (UK Clinical Research Collaboration).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Acknowledgment: The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Footnotes
Supplemental data associated with this article can be found in the online version at https://doi.org/10.1016/j.jval.2021.04.002.
Supplemental Materials
References
- 1.World Health Organization . World Health Organization; Geneva: 2019. WHO Report on the Global Tobacco epidemic 2019. [Google Scholar]
- 2.Office for National Statistics Adult Smoking Habits in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2018
- 3.Royal College of Physicians . Royal College of Physicians; London: 2015. Fifty Years Since Smoking and Health. [Google Scholar]
- 4.Royal College of Physicians . Royal College of Physicians; London: 2013. Smoking and Mental Health. [Google Scholar]
- 5.McManus S., Bebbington P., Jenkins R., Brugha T. NHS Digital; Leeds: 2016. Mental Health and Wellbeing in England: Adult Psychiatric Morbidity Survey 2014. [Google Scholar]
- 6.Richardson S., McNeill A., Brose L.S. Smoking and quitting behaviours by mental health conditions in Great Britain (1993-2014) Addict Behav. 2019;90:14–19. doi: 10.1016/j.addbeh.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harker K., Cheeseman H. Action on Smoking and Health; London, United Kingdom: 2016. The Stolen Years: the Mental Health and Smoking Action Report. [Google Scholar]
- 8.Lasser K., Boyd J.W., Woolhandler S., Himmelstein D.U., McCormick D., Bor D.H. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q., Szatkowski L., Britton J., Parrott S. Economic cost of smoking in people with mental disorders in the UK. Tob Control. 2015;24(5):462–468. doi: 10.1136/tobaccocontrol-2013-051464. [DOI] [PubMed] [Google Scholar]
- 10.Parrott S., Godfrey C., Raw M., West R., McNeill A. Guidance for commissioners on the cost effectiveness of smoking cessation interventions. Thorax. 1998;53(suppl 5 Pt 2):S1–S38. [PMC free article] [PubMed] [Google Scholar]
- 11.Humphreys K., Blodgett J.C., Roberts L.W. The exclusion of people with psychiatric disorders from medical research. J Psychiatr Res. 2015;70:28–32. doi: 10.1016/j.jpsychires.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Gierisch J.M., Bastian L.A., Calhoun P.S., McDuffie J.R., Williams J.W., Jr. Smoking cessation interventions for patients with depression: a systematic review and meta-analysis. J Gen Intern Med. 2012;27(3):351–360. doi: 10.1007/s11606-011-1915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J., Fairhurst C., Peckham E. Cost-effectiveness of a specialist smoking cessation package compared with standard smoking cessation services for people with severe mental illness in England: a trial-based economic evaluation from the SCIMITAR+ study. Addiction. 2020;115(11):2113–2122. doi: 10.1111/add.15086. [DOI] [PubMed] [Google Scholar]
- 14.Barnett P.G., Wong W., Hall S. The cost-effectiveness of a smoking cessation program for out-patients in treatment for depression. Addiction. 2008;103(5):834–840. doi: 10.1111/j.1360-0443.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 15.González-Roz A., Weidberg S., García-Pérez Á., V Martínez-Loredo, R Secades-Villa One-year efficacy and incremental cost-effectiveness of contingency management for cigarette smokers with depression. Nicotine Tob Res. 2021;23(2):320–326. doi: 10.1093/ntr/ntaa146. [DOI] [PubMed] [Google Scholar]
- 16.Barnett P.G., Wong W., Jeffers A., SM Hall, JJ Prochaska Cost-effectiveness of smoking cessation treatment initiated during psychiatric hospitalization: analysis from a randomized, controlled trial. J Clin Psychiatry. 2015;76(10):e1285–e1291. doi: 10.4088/JCP.14m09016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drummond M., Sculpher M., Claxton K., Stoddart G.L., Torrance G.W. 4th ed. Oxford University Press; Oxford, United Kingdom: 2015. Methods for the Economic Evaluation of Health Care Programmes. [Google Scholar]
- 18.Doll R., Peto R., Boreham J., Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NatCen social research, University of Leicester . UK Data Service; Essex, United Kingdom: 2019. Mental Health and Wellbeing in England: Adult Psychiatric Morbidity Survey 2014. [Google Scholar]
- 20.Ostbye T., Taylor D.H., Jung S.H. A longitudinal study of the effects of tobacco smoking and other modifiable risk factors on ill health in middle-aged and old Americans: results from the Health and Retirement Study and Asset and Health Dynamics among the Oldest Old survey. Prev Med. 2002;34(3):334–345. doi: 10.1006/pmed.2001.0991. [DOI] [PubMed] [Google Scholar]
- 21.Royal College of Physicians . Royal College of Physicians; London, United Kingdom: 2018. Hiding in Plain Sight: Treating Tobacco Dependency in the NHS. [Google Scholar]
- 22.Hughes J.R., Peters E.N., Naud S. Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav. 2008;33(12):1516–1520. doi: 10.1016/j.addbeh.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins J., Hollingworth W., Campbell R. Long-term smoking relapse: a study using the British Household Panel Survey. Nicotine Tob Res. 2010;12(12):1228–1235. doi: 10.1093/ntr/ntq175. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services . US Department of Health and Human Services, Office on Smoking and Health; Atlanta, GA: 1990. The Health Benefits of Smoking Cessation. A Report of the US Surgeon General. [Google Scholar]
- 25.Hyland A., Li Q., Bauer J.E., Giovino G.A., Steger C., Cummings K.M. Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine Tob Res. 2004;6(suppl 3):S363–S369. doi: 10.1080/14622200412331320761. [DOI] [PubMed] [Google Scholar]
- 26.Office for National Statistics Death registrations summary tables - England and Wales 2017. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesreferencetables
- 27.Pratt L.A., Druss B.G., Manderscheid R.W., Walker E.R. Excess mortality due to depression and anxiety in the United States: results from a nationally representative survey. Gen Hosp Psychiatry. 2016;39:39–45. doi: 10.1016/j.genhosppsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuijpers P., Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72(3):227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 29.Meier S.M., Mattheisen M., Mors O., DE Schendel, Mortensen P.B., Plessen K.J. Mortality among persons with obsessive–compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268–274. doi: 10.1001/jamapsychiatry.2015.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boscarino J.A. Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service. Ann Epidemiol. 2006;16(4):248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.John A., McGregor J., Jones I. Premature mortality among people with severe mental illness - new evidence from linked primary care data. Schizophr Res. 2018;199:154–162. doi: 10.1016/j.schres.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Bjorkenstam E., Bjorkenstam C., Holm H., Gerdin B., Ekselius L. Excess cause-specific mortality in-patient-treated individuals with personality disorder: 25-year nationwide population-based study. Brit J Psychiatry. 2015;207(4):339–345. doi: 10.1192/bjp.bp.114.149583. [DOI] [PubMed] [Google Scholar]
- 33.Arcelus J., Mitchell A.J., Wales J., Nielsen S. Mortality rates in patients with anorexia nervosa and other eating disorders a meta-analysis of 36 studies. Arch Gen Psychiatry. 2011;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 34.Dalsgaard S., Ostergaard S.D., Leckman J.F., Mortensen P.B., Pedersen M.G. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190–2196. doi: 10.1016/S0140-6736(14)61684-6. [DOI] [PubMed] [Google Scholar]
- 35.Laramée P., Leonard S., Buchanan-Hughes A., S Warnakula, JB Daeppen, J Rehm Risk of all-cause mortality in alcohol-dependent individuals: a systematic literature review and meta-analysis. EBiomedicine. 2015;2(10):1394–1404. doi: 10.1016/j.ebiom.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker E.R., Pratt L.A., Schoenborn C.A., Druss B.G. Excess mortality among people who report lifetime use of illegal drugs in the United States: a 20-year follow-up of a nationally representative survey. Drug Alcohol Depend. 2017;171:31–38. doi: 10.1016/j.drugalcdep.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batterham P.J., Calear A.L., Mackinnon A.J., Christensen H. The association between suicidal ideation and increased mortality from natural causes. J Affect Disord. 2013;150(3):855–860. doi: 10.1016/j.jad.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Bergen H., Hawton K., Waters K. Premature death after self-harm: a multicentre cohort study. Lancet. 2012;380(9853):1568–1574. doi: 10.1016/S0140-6736(12)61141-6. [DOI] [PubMed] [Google Scholar]
- 39.Curtis L., Burns A. Personal Social Services Research Unit, University of Kent; Canterbury, United Kingdom: 2020. Unit Costs of Health and Social Care 2019. [Google Scholar]
- 40.World Health Organization . World Health Organization; Geneva, Switzerland: 2011. Economics of Tobacco Toolkit: Assessment of the Economic Costs of Smoking. [Google Scholar]
- 41.NHS Digital Hospital episodes statistics: hospital admitted patient care activity. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2015-16
- 42.NHS Digital Code to group: HRG4+ 2015/16 reference costs grouper. Health and social care information centre. http://content.digital.nhs.uk/casemix/costing
- 43.Department of Health and Social Services Reference costs 2015-16. https://www.govuk/government/publications/nhs-reference-costs-2015-to-2016
- 44.Brazier J., Ratcliffe J., Salomon J., Tsuchiya A. 2nd ed. Oxford University Press; Oxford, United Kingdom: 2017. Measuring and Valuing Health Benefits for Economic Evaluation. [Google Scholar]
- 45.National Institute for Health and Care Excellence . National Institute for Health and Clinical Excellence; London, United Kingdom: 2013. Guide to the Methods of Technology Appraisal 2013. [PubMed] [Google Scholar]
- 46.NHS Digital . Health and Social Care Information Centre; London, United Kingdom: 2009. Adult Psychiatric Morbidity in England, 2007 – Results of a Household Survey. [Google Scholar]
- 47.Brazier J.E., Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 48.Brazier J.E., Rowen D., Hanmer J. Revised SF-6D scoring programmes: a summary of improvements. PRO Newsl. 2008;40:14–15. [Google Scholar]
- 49.Kharroubi S.A., Brazier J.E., Roberts J., O’Hagan A. Modelling SF-6D health state preference data using a nonparametric Bayesian method. J Health Econ. 2007;26(3):597–612. doi: 10.1016/j.jhealeco.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Richardson G., Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ. 2004;13(12):1203–1210. doi: 10.1002/hec.901. [DOI] [PubMed] [Google Scholar]
- 51.Briggs A., Sculpher M., Claxton K. Oxford University Press; Oxford, United Kingdom: 2006. Decision Modelling for Health Economic Evaluation. [Google Scholar]
- 52.Fenwick E., Marshall D.A., Levy A.R., Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006;6(1):52. doi: 10.1186/1472-6963-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Secades-Villa R., Gonzalez-Roz A., Garcia-Perez A., Becona E. Psychological, pharmacological, and combined smoking cessation interventions for smokers with current depression: a systematic review and meta-analysis. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0188849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall S.M., Tsoh J.Y., Prochaska J.J. Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808–1814. doi: 10.2105/AJPH.2005.080382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anthenelli R.M., Morris C., Ramey T.S. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med. 2013;159(6):390–400. doi: 10.7326/0003-4819-159-6-201309170-00005. [DOI] [PubMed] [Google Scholar]
- 56.NHS Digital Prescription cost analysis - England 2019. Health and social care information centre. https://digital.nhs.uk/data-and-information/publications/statistical/prescription-cost-analysis/2018
- 57.Tillmann M., Silcock J. A comparison of smokers’ and ex-smokers’ health-related quality of life. J Public Health Med. 1997;19(3):268–273. doi: 10.1093/oxfordjournals.pubmed.a024629. [DOI] [PubMed] [Google Scholar]
- 58.Vogl M., Wenig C.M., Leidl R., S Pokhrel Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health. 2012;12(203):1–10. doi: 10.1186/1471-2458-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berg M.L., Cheung K.L., Hiligsmann M. Model-based economic evaluations in smoking cessation and their transferability to new contexts: a systematic review. Addiction. 2017;112(6):946–967. doi: 10.1111/add.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bolin K. Economic evaluation of smoking-cessation therapies: a critical and systematic review of simulation models. Pharmacoeconomics. 2012;30(7):551–564. doi: 10.2165/11590120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Bolin K., Wilson K., Benhaddi H. Cost-effectiveness of varenicline compared with nicotine patches for smoking cessation–results from four European countries. Eur J Public Health. 2009;19(6):650–654. doi: 10.1093/eurpub/ckp075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.