To the Editor:
The COVID-19 pandemic is still raging across the world and vaccination is expected to lead us out of this pandemic. Although the efficacy of the vaccines is beyond doubt, as many vaccines were granted expedited approval, safety still remains a concern.1 In light of this, we read with great interest a recent article by Bril et. al.2 They described a case of autoimmune hepatitis (AIH) possibly triggered by COVID-19 vaccination. However, as the patient was 3-months post-partum a true causal relationship is difficult to determine.3 We hereby describe a case of severe AIH in a pre-morbidly well patient after the first dose of Moderna-COVID-19 vaccine (mRNA-1273).
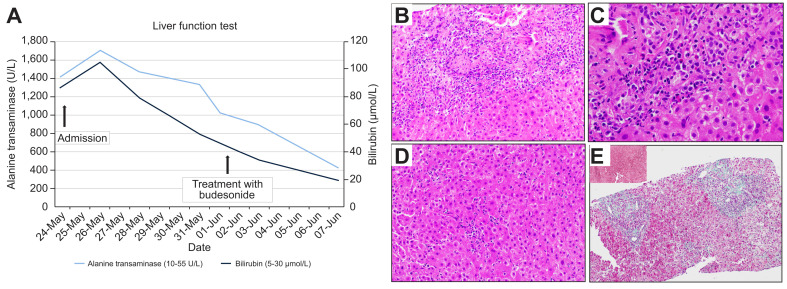
A 56-year-old woman received her first dose of Moderna-COVID-19 vaccine (mRNA-1273) on 14-April 2021. She developed intense malaise and flu-like symptoms the next day, owing to which she later decided against receiving the recommended second dose. Six weeks after vaccination, she presented with a 1-week history of jaundice, feeling unwell and persistent anorexia. Apart from her regular medication (rosuvastatin 10 mg daily) started 9 years ago, she was not on any other prescription or traditional medication/supplements. Prior to admission, her last liver function test was performed in 2019 and was normal. On the day of presentation, her physical examination was normal except for scleral icterus. The initial laboratory studies showed that she had hepatocellular injury (albumin 46 g/L, bilirubin 102 μmol/L, alkaline phosphatase 298 U/L, alanine aminotransferase [ALT] 1,701 U/L, aspartate aminotransferase 1,124 U/L, international normalized ratio 1.0) fulfilling Hy’s law.4 Acute viral hepatitis (hepatitis A, hepatitis B, hepatitis C, hepatitis E, cytomegalovirus, Epstein-Barr virus, herpes simplex virus) was excluded and cross-sectional abdominal imaging was unremarkable. Antinuclear antibody and anti-smooth muscle antibody were positive and serum IgG level was raised (32.6 G/L). The trend in bilirubin and ALT levels is illustrated in Fig. 1 A. Liver biopsy demonstrated portal inflammation with interface hepatitis, accompanied by conspicuous lobular inflammation with the presence of plasma cell aggregates, rosette formation and apoptotic hepatocytes (Fig. 1B–D). The histological features are in-keeping with an AIH-type pattern. Eosinophils were also identified, raising the possibility of drug association. Early young fibrosis is noted in the portal regions and would be compatible with recent subacute injury (Fig. 1E).
Fig. 1.
Liver function test and histology of liver biopsy.
(A) Graph showing the trend of bilirubin (μmol/L) and alanine aminotransferase (U/L) before and after starting treatment with budesonide on 1-June 2021. (B) H&E-stained section of liver biopsy at 200x magnification showing a portal tract with portal inflammation and interface damage. (C) Higher magnification (400x) of the portal and interface inflammatory cells showing aggregates of plasma cells, lymphocytes and few eosinophils. (D) Within the liver parenchyma (200x magnification), there is lobulitis with clusters of plasma cells, rosette formation and apoptotic hepatocytes. (E) The Masson’s trichrome stain (100x magnification) showed pale green staining of the portal regions with a lack of elastic fibres seen on orcein stain (inset, 100x magnification), consistent with recent young fibrosis. Elastic fibres appear black on orcein stains and are usually absent in normal portal tracts. Old fibrosis type collagen contains elastic fibres and is a useful indicator of chronicity on histology. (This figure appears in color on the web.)
The details of lab work are presented in Table S1 and S2. The pre-treatment revised original AIH score5 was 16. The patient was started on budesonide which led to rapid clinical and biochemical improvement (Fig. 1A).
COVID-19 infection is not known to impact outcomes of patients with AIH, but little is known about the relationship between COVID-19 vaccines and AIH.6 Drug-induced AIH (DI-AIH) is often a diagnostic challenge.7 , 8 Features favouring DI-AIH include history of recent drug exposure, absence of advanced fibrosis on liver histology and lack of relapse after stopping immunosuppressants.5 , 6 While our patient is still undergoing steroid therapy, other features are suggestive of DI-AIH. However, a few considerations require particular attention. Although statins are well known to cause DI-AIH, the median latency in statin-induced AIH is around 150 days.9 Given the temporal sequence, results of biochemistry, immunological assays (Table S1) and typical histological changes on liver biopsy, diagnosis of DI-AIH due to the COVID19 vaccine is most plausible. While DI-AIH is rare,10 the diagnosis of vaccine-related DI-AIH carries 2 important clinical implications. First, the re-exposure to a second dose of COVID-19 vaccine might trigger a fulminant AIH flare and second, prolonged immunosuppression may not be required. Thus, an accurate diagnosis and being aware of this rare complication of COVID mRNA vaccines is vital. DI-AIH after COVID-19 vaccination has rarely been reported to date (2), which might be due to either minimal awareness of this condition or the fact that patients without icteric disease do not usually present to hospitals.
Our report differs from that of Bril et. al. 2 in a few important domains. Firstly, our patient is not post-partum, which could be seen as a confounder3 emphasizing that DI-AIH post COVID-19 vaccination might have been a causal relationship rather than just an association. Secondly, our patient was inoculated with the Moderna-COVID-19 vaccine (mRNA-1273) and not the Pfizer-BioNTech vaccine. However, both are mRNA vaccines. The mechanism by which the mRNA vaccines could cause DI-AIH is explained by Bril et al. in their report2 and we hold the same view that molecular mimicry might be the potential mechanism underlying these mRNA vaccine-induced autoimmune conditions.
In conclusion, we would like to emphasize that clinicians need to remain vigilant and should consider DI-AIH secondary to mRNA vaccines in patients with similar presentation. However, this rare complication of the Moderna-COVID-19 vaccine (mRNA-1273) should not deter people from getting vaccinated.
Financial support
All the authors have no financial support statement to make.
Authors’ contributions
Concept: CKT, RK; Data Collection: CKT,LMW; Manuscript Writing: CKT, YJW; LWM; RK; Critical Reviewing: All. Guarantor of article and data interpretation: LWM, RK.
Conflict of interest
All authors do not have any potential conflict of interest to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.06.009.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capecchi P.L., Lazzerini P.E., Brillanti S. Comment to the letter of Bril F et al. “Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) Vaccine: causality or casualty?”. J Hepatol. 2021;75:994–995. doi: 10.1016/j.jhep.2021.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robles-Diaz M., Lucena M.I., Kaplowitz N., Stephens C., Medina-Cáliz I., González-Jimenez A., et al. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147(1):109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez F., Berg P.A., Bianchi F.B., Bianchi L., Burroughs A.K., Cancado E.L., et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 6.Marjot T., Buescher G., Sebode M., Barnes E., Barritt A.S., Armstrong M.J., et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74(6):1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mack C.L., Adams D., Assis D.N., Kerkar N., Manns M.P., Mayo M.J., et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020 Aug;72(2):671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu; clinical practice guideline panel: chair; panel members; EASL governing board representative: EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 2019 Jun;70(6):1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Russo M.W., Hoofnagle J.H., Gu J., Fontana R.J., Barnhart H., Kleiner D.E., et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60(2):679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manns M.P., Czaja A.J., Gorham J.D., Krawitt E.L., Mieli-Vergani G., Vergani D., et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.