Abstract
Ozone (O3) is a criteria air pollutant known to increase the morbidity and mortality of cardiopulmonary diseases. This occurs through a pulmonary inflammatory response characterized by increased recruitment of immune cells into the airspace, pro-inflammatory cytokines, and pro-inflammatory lipid mediators. Recent evidence has demonstrated sex-dependent differences in the O3-induced pulmonary inflammatory response. However, it is unknown if this dimorphic response is evident in pulmonary lipid mediator metabolism. We hypothesized that there are sex-dependent differences in lipid mediator production following acute O3 exposure. Male and female C57BL/6J mice were exposed to 1 part per million O3 for 3 h and were necropsied at 6 or 24 h following exposure. Lung lavage was collected for cell differential and total protein analysis, and lung tissue was collected for mRNA analysis, metabololipidomics, and immunohistochemistry. Compared with males, O3-exposed female mice had increases in airspace neutrophilia, neutrophil chemokine mRNA, pro-inflammatory eicosanoids such as prostaglandin E2, and specialized pro-resolving mediators (SPMs), such as resolvin D5 in lung tissue. Likewise, precursor fatty acids (arachidonic and docosahexaenoic acid; DHA) were increased in female lung tissue following O3 exposure compared with males. Experiments with ovariectomized females revealed that loss of ovarian hormones exacerbates pulmonary inflammation and injury. However, eicosanoid and SPM production were not altered by ovariectomy despite depleted pulmonary DHA concentrations. Taken together, these data indicate that O3 drives an increased pulmonary inflammatory and bioactive lipid mediator response in females. Furthermore, ovariectomy increases susceptibility to O3-induced pulmonary inflammation and injury, as well as decreases pulmonary DHA concentrations.
Keywords: air pollution, lipids, lung, inflammation, sex differences
Ozone (O3) is a criteria pollutant that contributes to more than 1 million deaths worldwide each year (Malley et al., 2017). Currently, the United States Environmental Protection Agency’s (EPA) standard for maximum acceptable level of ambient O3 is 70 parts per billion (ppb); however, ambient levels are reported to exceed this standard, particularly in urban settings (Devlin et al., 1991; Malley et al., 2017). Increases in ambient O3 levels are reported to decrease pulmonary function, induce airway inflammation, and exacerbate chronic lung diseases. Recent findings have shown that women, compared with men, are more susceptible to several respiratory diseases known to be exacerbated by O3 including asthma, chronic obstructive pulmonary disease, and pulmonary hypertension (Humbert et al., 2010; Pinkerton et al., 2015; Schatz and Camargo, 2003). Furthermore, female asthmatics (ages 10–17) were more likely to be hospitalized from increases in ambient O3 levels when compared with male asthmatics in the same age range (Sheffield et al., 2015). Although health effects of O3 have been reported by multiple studies, the biological mechanisms contributing to O3-induced pulmonary inflammation, and why these responses vary between males and females, are still understudied.
Pulmonary inflammation and injury induced by O3 are characterized by increased innate immune cell infiltration (including macrophages and neutrophils) into the airspace, pro-inflammatory cytokine and chemokine production, and microvascular injury (Birukova et al., 2019; Hollingsworth et al., 2007; Kilburg-Basnyat et al., 2018). Sex-dependent differences in the pulmonary inflammatory response to O3 have recently been elucidated in rodent models. In female mice, O3 exposure (2 parts per million; ppm) increases lung damage, airspace neutrophilia, and pulmonary expression of the pro-inflammatory cyto/chemokines interleukin (IL)-6, IL-1β, macrophage inflammatory protein-1α (MIP-1α), MIP-2α, MIP-3α, and monocyte chemoattractant protein-1 (MCP-1) compared with males (Birukova et al., 2019; Fuentes et al., 2019b; Kan et al., 2008). However, the mechanisms by which sex influences the O3-induced inflammatory response remain unknown.
O3 is also known to influence pulmonary lipid metabolism. Omega-3 and omega-6 fatty acids arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are mostly acquired through maternal transfer (across the placenta or via breast milk) and/or synthesized through diet (Koletzko et al., 2008). They can then be metabolized to bioactive lipid mediators by enzymes 5-lipoxygenase (5-LOX), 12/15-LOX, cyclooxygenase-2 (COX-2), and cytochrome P450 (Chiurchiu et al., 2018). O3 has been shown to increase the production of AA-derived lipid mediators, termed eicosanoids, including prostaglandin E2 (PGE2), 6-keto-PGF1-α, thromboxane B2 (TXB2), PGD2, leukotriene B4 (LTB4), and LTD4 (Madden et al., 1991). Recently, our group published that O3 exposure in male mice decreases pulmonary and systemic production of DHA-derived pro-resolving metabolites, termed specialized pro-resolving mediators (SPMs), which are a novel class of oxylipins known to promote tissue homeostasis and the resolution of inflammation (Kilburg-Basnyat et al., 2018). The majority of these data collected on O3 and pulmonary lipid metabolism, including our own, did not consider sex as a biologic variable. Therefore, it is still unknown if sex can influence pulmonary lipid metabolism which is known to drive inflammation and/or the resolution of inflammation after O3 exposure.
In addition to AA, EPA, and DHA-derived lipid mediators, sex hormones have also been shown to influence pulmonary inflammation. This is best supported by studies demonstrating effects with gonadectomy and/or sex hormone supplementation (Fuentes et al., 2019a; Fussbroich et al., 2020; Osgood et al., 2019). This effect appears to be related to sex hormone-mediated effects on lipid metabolites and their enzymes. In models of inflammatory diseases (myocardial infarction, obesity, dry eye, etc.), 5-LOX and COX-2 were downregulated in female mice. Additionally, it has been suggested that sex hormones (ie, estrogens) influence the production of lipid metabolites and lipid metabolizing enzymes COX-1, COX-2, and 5-LOX (Chatterjee et al., 2018; Chillingworth et al., 2006; Chistyakov et al., 2018; Torosyan et al., 2006), and alter eicosanoid production in pulmonary inflammatory models (Cai et al., 2012; Ho et al., 2008; Li et al., 2014). Furthermore, the production of DHA-derived SPMs and their precursors 14-hydroxydocosahexaenoic acid (14-HDHA), resolvin D4 (RvD4), and/or resolvin D5 (RvD5) appear to be increased in female mice (Crouch et al., 2019; Gao et al., 2018; Pullen et al., 2020). Presently, the role of pro-inflammatory lipid production and metabolism in sex-mediated differences in O3-induced pulmonary inflammation and injury is unknown.
In the present study, we investigated how sex influences pulmonary inflammation including pro-inflammatory and pro-resolving lipid mediator production following O3 exposure. These data demonstrate sex-dependent variations in pulmonary inflammatory markers including increased pulmonary neutrophilia and expression of neutrophil recruiting chemokines (C-X-C motif) ligand 1 (CXCL1) and CXCL2. Additionally, distinct sex-dependent differences were observed in several pro-inflammatory eicosanoids including increased PGF2α and PGE2 and in DHA-derived SPMs such as RvD5. Furthermore, ovariectomy led to increased susceptibility to O3-induced inflammation while causing decreased lung DHA concentrations. The results of this study indicate a significant sex-dependent variation in pulmonary lipid metabolism that may influence O3-induced lung inflammation.
MATERIALS AND METHODS
Animals
Male and female C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, Maine) at 8 weeks of age. In an additional set of experiments, ovariectomized (OVX) female or sham surgery female C57BL/6J mice (surgery performed at 7 weeks of age) were purchased from Jackson Laboratories at 8 weeks of age. OVX was confirmed by uterus weights at time of necropsy (data not shown). All experiments were performed in accordance with the National Institutes of Health guidelines and approved by the East Carolina University Institutional Animal Care and Use Committee.
Murine in vivo exposures
Mice were placed in stainless steel wire exposure chambers inside a stainless chamber and exposed to filtered air (FA) or O3 at a concentration of 1 ppm for 3 h. O3 was generated by directing 100% oxygen through an ultraviolet light generator and mixed with a humidified FA supply prior to entering the exposure chamber. Temperature, humidity, and O3 concentration in chamber were monitored continuously (Teledyne API T400, San Diego, California).
Bronchoalveolar lavage fluid collection and analysis
The right lung of each mouse was lavaged in situ 3 times with weight-specific volumes (26.25 ml/kg body weight) using ice-cold phosphate-buffered saline (PBS) (Gibco Life Technologies, Grand Island, New York) as described previously (Kilburg-Basnyat et al., 2018). Bronchoalveolar lavage fluid (BALF) samples were also processed as described previously (Birukova et al., 2019; Kilburg-Basnyat et al., 2018). BAL supernatant was collected and analyzed for total protein using the Pierce BCA Protein-Assay Kit (Thermo Scientific, Hercules, California). Red blood cells were lysed in the cell pellets, re-suspended in 1 ml PBS + 10% fetal bovine serum, and total cell counts were performed using a hemocytometer (Hausser Scientific, Horsham, Pennsylvania). Each sample was then centrifuged using a Cytospin 4 Cytocentrifuge (Thermo Fisher Scientific, Waltham, Pennsylvania) onto slides and stained with a Shandon Kwik-Diff Stains (Thermo Fisher Scientific). Cell differential counts were determined by morphology by evaluating 200 cells per mouse. BALF supernatant was also collected to quantify PGE2 by enzyme-linked immunosorbent assay (ELISA; Cayman Chemical, Ann Arbor, Michigan).
RNA isolation and quantitative polymerase chain reaction
The left lung was harvested, flash frozen, and total RNA was isolated using a Qiagen RNeasy Mini Kit (Qiagen, Valencia, California). RNA concentrations were determined using the NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, Delaware). RNA was reverse transcribed using a high-capacity RNA to cDNA kit (Thermo Fisher) and quantitative polymerase chain reaction (qPCR) was performed with a Taqman assay kit (Invitrogen, Waltham, Massachusetts) or PowerUP SYBR Green kit (Applied Biosystems, Foster City, California). Genes were amplified with Applied Biosystems StepOnePlus real-time polymerase chain reaction (RT-PCR) kit (Life Technologies). Fold changes in mRNA expression were calculated using the 2-ΔΔCt method and Ct values. Samples were normalized to 18S or GAPDH as described previously (Sullivan et al., 2017). See Supplementary Table 1 for Invitrogen primer (Waltham, Massachusetts) and for Taqman primer (Applied Biosystems) information. See Supplementary Table 2 for housekeeping gene stability.
Immunohistochemistry
From animals not used for qPCR, the left lung was perfused with 10% neutral buffered formalin fixative and allowed to fix for 24 h prior to processing. Lungs were processed, embedded in paraffin, and 5 μm sections were mounted on slides. Immunohistochemistry for COX-2 was performed as described previously (Messenger et al., 2018). Positive COX-2 immunostaining was enumerated by light microscopy (15 fields/lobe) by a blinded veterinary pathologist as described previously (McPeek et al., 2019) using the grading scheme described in Supplementary Table 3.
Lipid mediator sample preparation
All standards and internal standards used for reverse-phase high-performance liquid chromatography (HPLC) tandem mass spectrometry (LC-MS/MS) analysis of lipid mediators were purchased from Cayman Chemical (Ann Arbor, Michigan). All HPLC solvents and extraction solvents were HPLC grade.
Left lung tissue samples were homogenized, and lipid mediators were isolated with modifications from a prior protocol (Armstrong et al., 2020). Tissue samples were preweighed and transferred into a dry ice chilled, preweighed TissueLyser tube (Qiagen, Hilden, Germany) with a 5 mm stainless steel ball. About 1.0 ml of −20°C chilled methanol and 10 µl of internal or reference standard solution (Supplementary Table 4) were added and the samples were homogenized at 50 Hz for 5 min. The sample was then centrifuged at 14 000 RPM for 10 min at 4°C. The supernatant was removed and transferred to a new 1.5-ml microcentrifuge tube and dried in a speed vac until completely dry. The dried sample was then reconstituted with 1.0 ml of 10% methanol. The samples were then loaded on a Strata-X 33 mm 30 mg/1 ml solid-phase extraction (SPE) column (Phenomenex, Torrance, California) preconditioned with 1.0 ml of methanol followed by 1.0 ml of water. The SPE column was then washed with 10% methanol and then eluted directly into a reduced surface activity/maximum recovery glass autosampler vial with 1.0 ml of methyl formate. The methyl formate was evaporated completely from the vial with a stream of nitrogen and then the SPE cartridge was eluted with 1.0 ml of methanol directly into the same autosampler vial. The methanol was evaporated to dryness with a stream of nitrogen and then the sample was reconstituted with 20 ml of ethanol. The samples were analyzed immediately or frozen at −70°C until analysis.
Liquid chromatography-mass spectrometry
Quantitation of lipid mediators was performed using LC-MS/MS as described previously (Armstrong et al., 2020; Kilburg-Basnyat et al., 2018). Briefly, an extracted sample was injected onto an Agilent trapping column to trap the lipid mediators. The flow was reversed to elute the trapped lipid mediators onto an Agilent Eclipse Plus analytical column. Mass spectrometric analysis was performed on an Agilent 6490 triple quadrupole mass spectrometer (Agilent, Santa Clara, California) in negative ionization mode. Data for lipid mediators were acquired in dynamic MRM mode using experimentally optimized collision energies obtained by flow injection analysis of authentic standards (Supplementary Table 4). Calibration curves for each lipid mediator were constructed using Agilent Masshunter Quantitative Analysis software. Samples were quantitated using the calibration curves to obtain the on-column concentration, followed by multiplication of the results by the appropriate dilution factor to obtain the concentration in pg/mg of wet tissue.
Fatty acid analysis in lung tissue
Lipids were organically extracted using the Folch method as described previously (Folch et al., 1957; Kilburg-Basnyat et al., 2018). Briefly, lipids were extracted from the organic phase of homogenized lung tissue, methylated, saturated with sodium chloride solution, and suspended in toluene for gas chromatography (GC) analysis (Basile et al., 2011). Craft Technologies (Wilson, North Carolina) performed the extraction and fatty acid analysis of lung samples.
Statistical analysis
Group sizes ranged from 3 to 15 and data points were pooled from up to 3 experiments when possible. Data are expressed as means ± standard error of the mean (SEM). Data were analyzed using parametric 2-way ANOVA (Kruskal-Wallis test) using sex (male/female or sham/OVX) as one factor and exposure (FA/O3) as the other factor. This was followed by comparison using a post-hoc Tukey’s multiple comparison test to correct for multiple comparisons either within factors or between factors depending on if there was statistically significant interaction. Additionally, comparison of lipid metabolites or fatty acids was performed by 2-way ANOVA with time as one factor (FA considered “0 h” time point) and sex as the other factor. Then, t test was used when only comparing between sex within a single time point (Figure 3 and Supplementary Tables 5–8). Statistical analysis was conducted using statistical hypothesis testing in GraphPad Prism 8.0 (San Diego, California). A value of p < .05 was considered significant. To calculate fold change of eicosanoids, all values were increased by 1 unit to prevent the impossibility of dividing by zero (Supplementary Figure 3). Outliers were determined by the regression and outlier removal (ROUT) method.
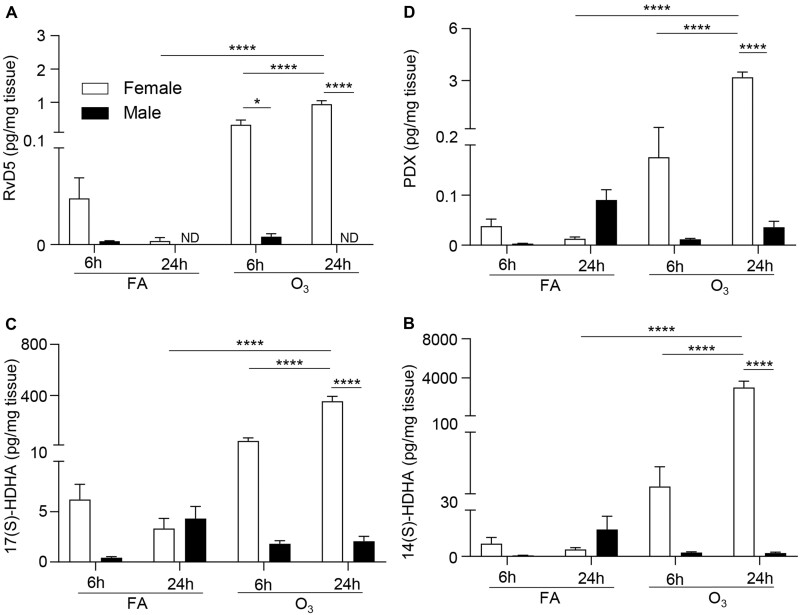
Figure 3.
O3 exposure increases pulmonary levels of DHA-derived SPMs in females but not males. Female and male mice were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 6 or 24 h following exposure. Lung tissue was analyzed for (A) RvD5, (B) PDX, (C) 17(S)-HDHA, and (D) 14(S)-HDHA, at 6 and 24 h post exposure. *p < .05, **p < .01, ****p < .0001, ND = not detectable; n = 6 per group.
RESULTS
O3-Induced Pulmonary Neutrophilia Varies by Sex
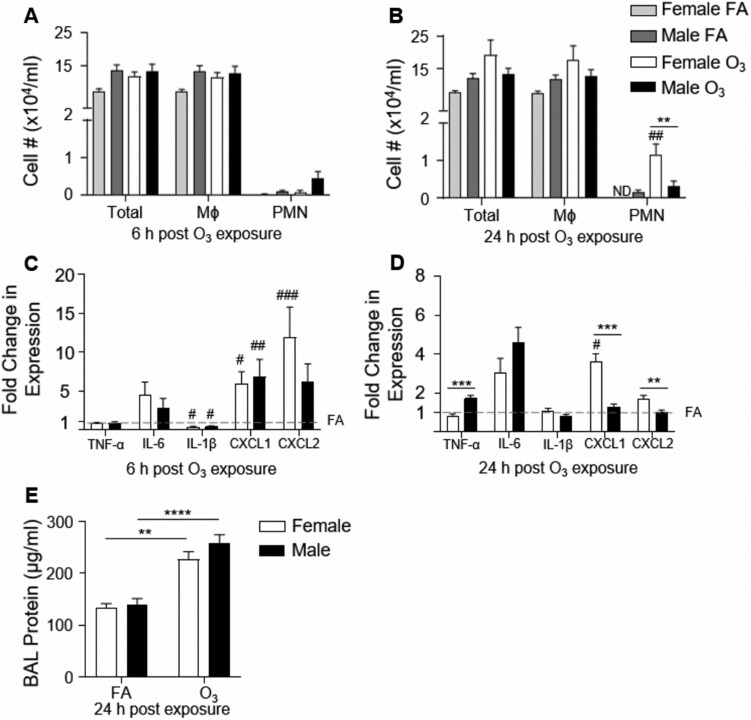
To establish sex differences following acute O3 exposure, we exposed male and female mice to FA or 1 ppm O3 for 3 h and assessed lung inflammation and injury 6 and 24 h post exposure. Following FA exposure, there were no differences in BAL cell differentials or protein between males and females. At 6 h following an acute O3 exposure, there were no increases in immune cells in the airspace of males or females when compared with FA controls (Figure 1A). O3-exposed males and females did not have any alterations in cellular influx 6 h following acute O3 exposure (Figure 1A; PMN, p = .16). However, at 24 h post-O3 exposure, airspace neutrophilia was significantly increased in females compared with FA controls whereas there were no differences between O3 and FA-exposed males (Figure 1B). Between males and females, there was a 3.7-fold increase in neutrophils in the airspace 24 h post exposure in females (Figure 1B). Additionally, total protein in BAL was quantified to reflect pulmonary microvascular injury. Although BAL protein was increased following O3 inhalation, it was not significantly different between males and females (Figure 1E).
Figure 1.
Females have an increased pulmonary inflammatory response to O3 but no differences in lung injury. Female and male mice were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied 6 or 24 h following exposure. Bronchoalveolar lavage (BAL) was analyzed for (A) cell differentials 6 h post exposure, (B) cell differentials 24 h post exposure (n = 9–15 per group), and lung tissue was harvested and analyzed for mRNA expression of TNF-α, IL-6, IL-1β, CXCL1, and CXCL2 at (C) 6 h post exposure, and (D) 24 h post exposure. The dashed line at y = 1 represents the FA value that fold change expression is normalized to. BAL was also analyzed for (E) total protein. #p < .05, ##p < .01, ###p < .001 compared with same sex FA group. *p < .05, **p < .01, ***p < .001, ****p < .0001 compared between males and females in the same exposure group (either FA or O3); n = 6–15 per group for BAL analysis. n = 5–15 per group for mRNA expression analysis. ND = not detectable.
To further define the pulmonary inflammatory response, whole lung pro-inflammatory cyto/chemokines expression was assessed to define sex-dependent effects on O3-induced cyto/chemokine production. There were no sex-dependent differences in cyto/chemokine expression in the FA exposure groups (Supplementary Figure 1). At 6 h post-O3 exposure, TNF-α and IL-6 were unchanged from FA controls (all expression was normalized to sex respective FA housekeeping gene) in both male and female mice; however, IL-1β was decreased in males and females (Figure 1C and Supplementary Figure 1A). Neutrophil chemokines CXCL1 and CXCL2 were increased in females compared with FA, whereas only CXCL1 was increased in males (Figure 1C and Supplementary Figure 1A). There were no statistically significant differences between males and females 6 h post-O3 exposure. At 24 h post-O3 exposure, CXCL1 was increased in O3-exposed females compared with FA, and IL-1β returned to equivalent with FA in both males and females (Figure 1D and Supplementary Figure 1B). Between sexes, 24 h post-O3 inhalation TNF-α expression was increased in males compared with females whereas CXCL1 and CXCL2 were increased in female lung tissue compared with males (Figure 1D). These data demonstrate that in our model of acute O3 exposure, there are sex differences in pulmonary inflammation (airspace neutrophilia, expression of TNF-α, CXCL1, CXCL2) whereas markers of pulmonary injury (BAL protein) had no significant differences between males and females.
Sex-Dependent Differences in the Pulmonary Lipidome
Eicosanoids including PGE2, LTB4, 6-keto-PGF1α, and 12S-hydroxyeicosateraenoic acid (HETE) have been associated with inflammation, often in a sex-dependent manner (Chatterjee et al., 2018; Chillingworth et al., 2006; Chistyakov et al., 2018; Chung et al., 2019; Torosyan et al., 2006). To evaluate eicosanoids in the dimorphic O3-induced response, we evaluated PGE2 levels in BALF by ELISA and PGE2 levels were significantly increased in females 24 h after O3 exposure compared with males (Supplementary Figure 2). Interestingly, fold changes from FA to O3 exposure were near equivalent between sexes (females 2.9, males 3.4). PGE2 was not statistically different between males and females post-FA exposure, this implies that increased baseline levels contributed to increased levels post-O3 exposure in females. We further investigated this eicosanoid production with LC-MS/MS analysis of lung tissue from male and female mice exposed to FA or O3 (Figure 2A and Supplementary Table 5). Several eicosanoids were increased in females compared with males exposed to FA including 15S-HETE (Figure 2B), PGF2α (Figure 2C), 11(12)-epoxyeicosatrienoic acid (11(12)-EET), 13-13-Oxo-9E, 11E-octadecadienoic acid (13-OxoODE), and 9-OxoODE. This trend was also observed 6 h following O3 exposure with increases in 10 of the 18 lipids in female lung tissue when compared with exposed male mice. At 24 h following O3 exposure, 15 of 18 lipids were significantly increased including 12S-HETE (Figure 2D), 15S-HETE, PGF2α, PGE2 (Figure 2E), 13-HODE (Figure 2F), and 9-HODE (Figure 2G) which were 100 to 1000-fold increased compared with males (Supplementary Figure 3). In contrast, pulmonary production of 11(12)-EET was decreased, and LTB4 and 14(15)-EET were not altered in females compared with males 24 h post-O3 exposure.
Figure 2.
Pulmonary levels of pro-inflammatory lipid mediators are augmented in females compared with males following O3 exposure. Male and female mice were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 6 or 24 h following exposure. (A) Lung tissue was analyzed for the listed lipid mediators 6 and 24 h post exposure by LC/MS-MS. Selected lipids were graphed over time: (B) 15S-HETE, (C) PGF2α, (D) 12S-HETE, (E) PGE2, (F) 13-HODE, and (G) 9-HODE. *p < .05,****p < .0001; n = 6 per group.
Although eicosanoids are known to drive the pulmonary inflammatory response, SPMs have been associated with an anti-inflammatory response and promoting the resolution of inflammation (Serhan, 2014). DHA-derived SPMs are synthesized primarily through 15-LOX, followed by several intermediate molecules and combinations of 5-LOX activity, peroxidation, and hydrolysis (Serhan and Petasis, 2011). A notable SPM pathway is DHA oxidation through 15-LOX to create 17(S)-HDHA which 5-LOX then oxidizes to RvD5. The intermediate 14(S)-HDHA which leads to maresin-1, and protectin DX (PDX) can also be synthesized from 15-LOX mediated DHA oxidation (Serhan and Levy, 2018). Due to the potent pro-resolving capabilities of these SPMs, we also evaluated if there were sex-dependent differences in the pulmonary production of SPMs or SPM precursors following O3 exposure. Following FA exposure, there were no significant differences in SPMs or SPM precursors between males and females. However, 6 h post-O3 exposure pulmonary RvD5 levels were significantly increased in females compared with males (Figure 3A). At 24 h post-O3 exposure, pulmonary levels of RvD5, PDX (Figure 3B), 17(S)-HDHA (Figure 3C), and 14(S)-HDHA (Figure 3D) were significantly increased in females compared with males (Figure 3). These findings demonstrate that O3 induces a dimorphic response of both pro-inflammatory and pro-resolving lipid mediators in the lung that correlates with pulmonary inflammation.
Pulmonary Lipid Mediator Enzymes Do Not Differ Between Sexes
To explore potential mechanisms driving the sex differences in pulmonary lipid metabolism following O3 exposure, we evaluated the expression/production of enzymes known to regulate the production of lipid metabolites in lung tissue. The predominant enzymes responsible for the production of pro-inflammatory lipid mediators and SPMs are COX-2, 5-LOX, and 12/15-LOX (Serhan and Petasis, 2011). We scored pulmonary COX-2 immunostaining at 6 h (Figure 4A and Supplementary Figure 4) and 24 h (Figure 4B and Supplementary Figure 4) post-O3 exposure. In addition, we measured pulmonary gene expression of 5-LOX and 12/15-LOX (Figs. 4C and 4D). There were no sex differences in the prevalence or expression of any of the lipid mediator enzymes analyzed in whole lung tissue (Figure 4). These data indicate that sex differences in pulmonary lipid mediator production are not driven by differences in the expression of common lipid metabolizing enzymes following O3 exposure.
Figure 4.
Sex does not alter the pulmonary expression of lipid metabolizing enzymes after O3 exposure. Female and male mice were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 6 or 24 h following exposure. Lung tissue was processed for immunohistochemistry and stained for COX-2 at (A) 6 h post exposure, and (B) 24 h post exposure. Slides were graded by a veterinary pathologist according to the grading scheme described in Supplementary Table 3. RNA from lung tissue was harvested and analyzed for mRNA expression of 5-LOX and 12/15-LOX at (C) 6 h post exposure, and (D) 24 h post exposure. The dashed line at y = 1 represents the FA value that fold change expression is normalized to. n = 3–6 per group.
Parent Fatty Acid Profile in the Lung is Different Between Sexes
Given that eicosanoids are metabolized from parent polyunsaturated fatty acids, we then focused on potential sex-dependent differences in pulmonary fatty acids. Females generally have higher levels of circulating DHA, although it is currently unknown if this correlates to lung tissue levels at baseline or during inflammation (Kitson et al., 2010). Therefore, we quantified a panel of fatty acids in lung tissue in FA-exposed mice and mice following O3 exposure. In FA-exposed animals, stearic acid (Supplementary Table 6), DHA (Figure 5A), and AA (Figure 5B) were increased in female lungs compared with males. At 24 h following exposure, both DHA and AA were increased in O3-exposed females compared with FA females whereas only DHA was increased in O3-exposed males compared with FA males. Furthermore, both DHA and AA were increased in female lungs after O3 exposure compared with O3-exposed males. Baseline differences between FA males and females may explain this difference post-O3 exposure. However, it should be noted that DHA increased more in female lungs (+17%) than in male lungs (+11%) after O3 exposure, and AA did not increase in males after O3 exposure. This suggests that increased pulmonary eicosanoids and resolvins in females may be the result of increased production of their parent fatty acids in females.
Figure 5.
DHA and AA accumulate in female lungs more than male lungs following O3 exposure. Mice were exposed to 1 ppm O3 for 3 h or filtered air (FA) and necropsied at 24 h following exposure. Whole lung tissue homogenate was analyzed for (A) docosahexaenoic acid (DHA) and (B) arachidonic acid (AA). Lung tissue from different animals under the same experimental conditions was harvested and analyzed for mRNA expression of (C) ELOVL2. The dashed line at y = 1 represents the FA value that fold change expression is normalized to. #p < .05, compared with same sex FA group. *p < .05, ***p < .001, ****p < .0001; n = 6 per group.
We next measured the pulmonary gene expression of fatty acid desaturase 1 (FADS1), FADS2, elongation of very long-chain fatty acids-like 2 (ELOVL2), and ELOVL5 in the lungs to evaluate the contribution of long-chain polyunsaturated fatty acid synthesizing enzymes toward alterations in DHA and AA seen in female lung tissue. There were no differences between FA-exposed males and females. Then, ELOVL2 gene expression was increased in female lung tissue 6 h post-O3 exposure compared with FA females (Figure 5C). Furthermore, ELOVL2 was increased in females compared with males 6 h post-O3 exposure. ELOVL2 then returned to levels equivalent to FA-exposed animals 24 h after O3 exposure in both females and males. At 6 h post-O3 exposure, no differences were seen in FADS1, FADS2 was decreased in both females and males, and ELOVL5 was increased in both females and males in the lungs (Supplementary Figure 5A). At 24 h post-O3 exposure, there were no differences in any of the measured enzymes (Supplementary Figure 5B). This increase in ELOVL2 6 h post-O3 exposure suggests females are actively synthesizing polyunsaturated fatty acids, perhaps contributing to the increased pulmonary DHA measured following O3 exposure.
Ovariectomy Increases O3-Induced Pulmonary Inflammation
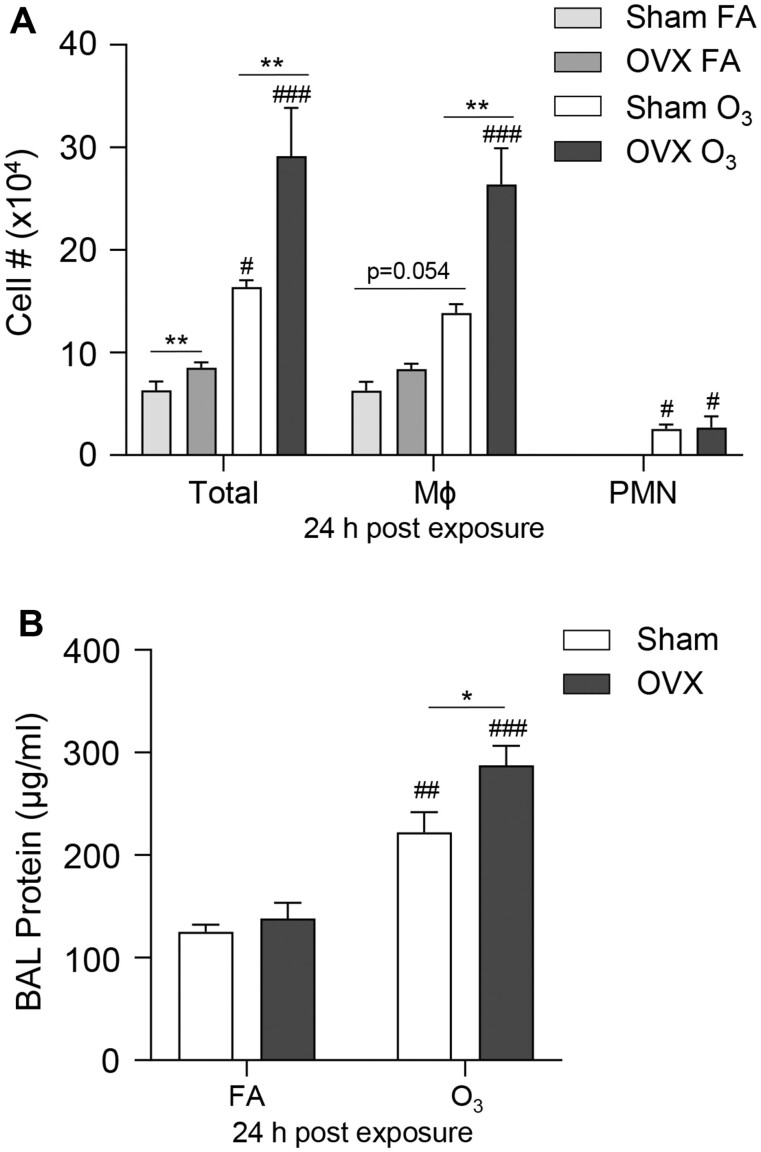
Because we observed sex differences in O3-induced pulmonary inflammation, we hypothesized that female sex hormones could contribute to these differences. To test this hypothesis, OVX female mice and sham female mice were exposed to FA or O3. We observed that FA-exposed OVX mice had increases in total cells in their BAL compared with FA-exposed sham mice (Figure 6A). At 24 h post-O3 exposure, sham and OVX mice had increased BAL total cell counts, macrophages, neutrophils, and total protein compared with FA controls (Figure 6). When comparing OVX and sham mice exposed to O3, there were increases in total cells, macrophages, and total BAL protein in the OVX mice. These findings indicate that the loss of ovaries and their hormone production leads to higher susceptibility to O3-induced lung inflammation and injury.
Figure 6.
Ovariectomy increases pulmonary macrophage influx and injury following O3 exposure. Female mice that underwent either sham surgery (Sham) or ovariectomy (OVX) were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 24 h following exposure. BAL was collected and analyzed for (A) cell differential counts, and (B) total protein concentration at 24 h post exposure. #p < .05, ##p < .01, ###p < .001 compared with same sex FA group. *p < .05, **p < .01 compared between males and females; n = 6 per group.
Ovariectomy Does Not Alter Pulmonary Lipid Mediator Production but Does Reduce Fatty Acids Levels
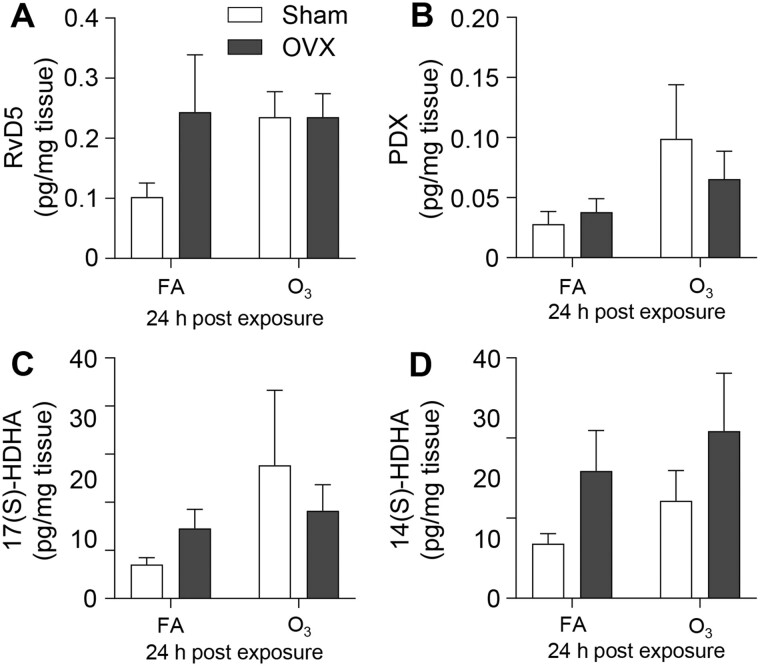
To evaluate if OVX alters pulmonary lipid mediator or fatty acid levels following O3 exposure, lung tissue was assessed from sham and OVX mice following FA or O3 exposure. In FA-exposed mice, there were largely no differences in pulmonary eicosanoids except for 9-OxoODE, which was increased in lung tissue from OVX females (Figure 7, Supplementary Table 7). FA-exposed mice had no statistically significant differences in SPMs, and following O3 exposure, there were no differences between sham and OVX mice in the pulmonary eicosanoid, SPM, or SPM precursor concentrations (Figure 7, Supplementary Table 7 and Figure 8). Conversely, OVX decreased pulmonary DHA levels after FA and 24 h following O3 exposure (Figure 9). These findings show that O3-induced pulmonary eicosanoid and SPM production in females is independent of ovarian hormone production. However, ovariectomy altered pulmonary concentrations of DHA, which is a precursor to many SPMs.
Figure 7.
The lung lipid mediator profile following O3 exposure is not altered by ovariectomy. Female mice that underwent sham surgery (Sham) or ovariectomy (OVX) were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 24 h following exposure. Lung tissue was analyzed for the listed lipid mediators by LC-MS/MS 24 h post exposure. n = 5 per group.
Figure 8.
Ovariectomy does not alter pulmonary SPM production following O3 exposure. Female mice that underwent sham surgery (Sham) or ovariectomy (OVX) were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 24 h following exposure. Lungs were collected and analyzed for (A) RvD5 (B) PDX, (C) 17(S)-HDHA, and (D) 14(S)-HDHA, at 24 h post exposure. n = 3–5 per group.
Figure 9.
Ovariectomy decreased pulmonary DHA levels following O3 exposure. Female mice that underwent sham surgery (Sham) or ovariectomy (OVX) were exposed to 1 ppm O3 or filtered air (FA) for 3 h and necropsied at 24 h following exposure. Lung tissue was collected and analyzed for DHA levels 24 h post exposure. *p < .05, **p < .01 compared between males and females; n = 3–5 per group.
DISCUSSION
Elevation of ambient O3 is a major public health concern with ambient O3 concentrations above accepted standards accounting for 3,880 excess annual deaths (Cromar et al., 2019). Recent findings have indicated that the risks associated with O3 exposure are elevated in females (Fuentes et al., 2018); however, the mechanism of this disparity between sexes is currently unknown. In this study, our data demonstrate that females have increased pulmonary inflammation and considerable elevations of several pro-inflammatory and pro-resolving lipid mediators following acute O3 exposure. Additionally, we determined that lipid metabolizing enzymes COX-2, 5-LOX, and 12/15-LOX in whole lung tissue were not different between sexes. However, parent fatty acid levels (AA and DHA) were elevated in female lung tissue, potentially contributing to the dimorphic response following O3. Additionally, O3-induced pulmonary lipid mediator production was independent of ovarian hormone production whereas pulmonary inflammation, pulmonary injury, and pulmonary levels of DHA were influenced by ovarian hormones. Taken together, these data suggest that sex drives differences in pulmonary fatty acids which could contribute to O3-induced pulmonary inflammation.
Sex differences have been noted previously in the O3-induced pulmonary inflammatory response with increased airspace neutrophilia and neutrophil recruiting chemokines in females (Cabello et al., 2015; Fuentes et al., 2019b; Vancza et al., 2009). We supported and expanded upon this research by examining an earlier time point, demonstrating a clear sex-specific difference in lipid mediator responses, and elucidating the influence of ovariectomy on this model. We chose to evaluate acute time points post exposure (6 and 24 h) to better capture the pulmonary lipid mediator response which is known to happen early on in the pulmonary inflammatory response (Bates, 1995). Choosing these earlier time points allowed us to better characterize when sex differences occur in the pulmonary inflammatory response to O3. It is important to note that some of our findings are different than what has been reported. For instance, at the time points and O3 dose we utilized, we did not observe sex differences in IL-6 or IL-1β expression. However, it has been previously shown that females have increased production of these cytokines 24 h post O3 (Cabello et al., 2015). These discrepancies in findings could be attributed to differences in O3 doses (1 ppm vs 2 ppm) as well as females being in different stages of the estrus cycle when the exposure was performed (Fuentes et al., 2019a). Additionally, we found that OVX leads to increased pulmonary cellular influx and injury which contradicts a previous study where OVX protected females from O3-induced pulmonary inflammation and injury (Fuentes et al., 2019b). Curiously, in our study, the noted cellular influx was primarily macrophages in OVX mice and we also show that females had increased neutrophil influx in comparison to males. Some evidence indicates that estrogens are necessary for adequate neutrophil recruitment (Inigo et al., 2020; Le et al., 2018). Therefore, OVX may lead to a shift toward monocyte/macrophage recruitment over neutrophil recruitment. Given all these data, further investigation is needed to elucidate the role of estrogens/ovarian hormones in the O3-induced pulmonary inflammatory responses. Overall, our data along with published work confirm that females have a more exacerbated pulmonary inflammatory response to O3 inhalation at multiple time points and doses (Birukova et al., 2019).
A factor that may contribute to the O3-induced dimorphic pulmonary inflammatory response is lipid mediator production. Production and metabolism of lipid mediators have been shown to vary between males and females in several inflammatory models such as rheumatoid arthritis, systemic lupus erythematosus (Beeson, 1994), obesity (Crouch et al., 2019), and asthma (Pace and Werz, 2020). However, there is limited research on how the production of these lipid mediators following lung injury/inflammation are influenced by sex. In this study, several eicosanoids (AA-derived oxylipins) were increased in female lung tissue compared with males before and after O3 exposure. Although multiple studies have demonstrated eicosanoid production increases in the lung during the pulmonary inflammatory response, most studies only examined males. To the best of our knowledge, this is the first study to report that sex influences the eicosanoid profiles in the lung when unchallenged and following inflammation (Alfaro et al., 2007; Hazbun et al., 1993; Miller et al., 1987). This dimorphic eicosanoid response could be a result of multiple biological processes, including those that are unique to females. Eicosanoids derived from AA are generally considered to induce inflammation (Chiurchiu et al., 2018), induce neutrophil chemotaxis (Fredman et al., 2016; Lammermann et al., 2013), and augment pro-inflammatory cytokine production (Aoki and Narumiya, 2012; Narumiya and Furuyashiki, 2011). Therefore, increased pulmonary eicosanoid levels noted in females could be a consequence of the increased inflammatory response noted after O3 exposure. However, given the FA and acute (6 h post-O3 exposure) increase in eicosanoid levels in the female lung, we hypothesize that the increased eicosanoids may drive the increased pulmonary inflammatory responses. Interestingly, OVX did not alter pulmonary eicosanoid production indicating that this phenomenon is independent of female hormone production and is perhaps intrinsic to females or could be driven by other sex hormones including testosterone. Overall, these findings suggest that increases in eicosanoids in females may play a significant role in the pulmonary inflammatory response to O3.
Along with the increased pro-inflammatory eicosanoids, we found that DHA-derived SPM precursors 14(S)-HDHA, 17(S)-HDHA, SPMs PDX, and RvD5 were increased in female lung tissue compared with males following O3 inhalation. Although measured concentrations of SPMs were relatively low compared with their precursors, near equivalent doses have demonstrated beneficial effects in the lung (Codagnone et al., 2018; Cox et al., 2015; Ganesan et al., 2020; Hu et al., 2019; Tan et al., 2018). The pulmonary levels of these SPM precursors and SPMs were again unchanged with OVX regardless of exposure indicating that this response is independent of female hormone production. SPM precursors and SPMs are well documented to drive an anti-inflammatory response and promote resolution (Barden et al., 2016; Chiurchiu et al., 2016; Li et al., 2020; Ramon et al., 2012). However, sex differences in the pulmonary SPM production have yet to be evaluated. Our laboratory has previously reported that male mice have a significant decrease in 14(S)-HDHA and PDX following O3 exposure; however, females were not evaluated (Kilburg-Basnyat et al., 2018). Recently, sex differences in SPM production have been reported in other models of inflammation and injury (Crouch et al., 2019; Lim et al., 2020; Parashar et al., 2020). In obesity models, decreased SPMs and decreased expression of SPM generating enzymes 5-LOX and 12/15-LOX were noted in obese males but not females (Crouch et al., 2019). In our O3 model, we noted that 5-LOX and 12/15-LOX expression in the lung did not correlate with increased SPMs/SPM precursors in females. Possible explanations for this could be that the time points analyzed (6 and 24 h) may not have captured changes in mRNA expression of these enzymes as well as activity might have changed without altering gene expression. It is also possible that there are sex differences in the cell-specific or subcellular localization of these enzymes that were not detected in the current whole lung tissue analysis. It has previously been reported that macrophages from females have increased nuclear 5-LOX when compared with macrophages isolated from males (Crouch et al., 2019; Pace et al., 2017; Rossi et al., 2014). Regardless of enzymatic activity, increased SPM production in females suggests that females should be more protected from O3. However, we show that females have increased pulmonary inflammation compared with males. We suggest that the augmented pulmonary SPMs in females is a compensatory mechanism caused by increased pulmonary neutrophilia and pro-inflammatory eicosanoid production. This is supported by the relatively abundant increases in eicosanoids in female lungs at the 6 h time point whereas only RvD5 was increased at that time. Furthermore, females had increased AA as well as DHA whereas males only had increased DHA after O3 exposure. The increase of AA and its metabolites may have contributed to the increased pulmonary inflammation in females that was not seen in males. Further investigations will focus on how sex influences pulmonary eicosanoid and SPM biosynthesis.
The mechanisms contributing to increased pulmonary eicosanoid and SPM precursor/SPMs in females is currently unknown, although differences in parent fatty acids may contribute to this mechanism. Our data indicate that parent fatty acids AA and DHA were increased in female lungs both in FA-exposed mice and after O3 exposure. Females are known to have increased plasma levels of DHA compared with males (Bakewell et al., 2006; Crowe et al., 2008; Metherel et al., 2009), which has been attributed to an increased capacity to endogenously synthesize DHA from α-linolenic acid via ELOVL2 activity (Burdge et al., 2002; Burdge and Wootton, 2002; Metherel et al., 2021). This increased DHA was supported by increased pulmonary ELOVL2 gene expression 6 h post-O3 exposure. It has been hypothesized that this is an evolutionary adaptation of females to provide DHA in utero to offspring for fetal development during pregnancy (Burdge and Postle, 1994; Innis, 2000; Otto et al., 2001; Postle et al., 1995). Additionally, estrogens have been shown to activate an Elovl2 promoter (Gonzalez-Bengtsson et al., 2016). These data correlate with our findings that DHA was decreased in the lungs of OVX females both after FA and O3 exposure. It should be noted that the sex differences in pulmonary inflammation were minor and biological relevance could be challenged. We argue that although these differences were minor, they correlated significantly with differences in pulmonary eicosanoids and SPM/SPM precursor production that may be the result of increased parental fatty acid concentrations in the lung. In OVX mice, there was decreased DHA in the lungs which correlated with increased lung injury. Furthermore, Birukova et al. demonstrated clear physiological differences between sexes which highlights the biological relevance of sex differences and O3 exposure (Birukova et al., 2019). Although our lipid mediator findings are somewhat correlative, we clearly demonstrate significant differences in bioactive oxylipins during the dimorphic pulmonary inflammatory response to O3.
There are several avenues for future research as our work had some limitations. For instance, one limitation is that this study was performed without syncing the female mouse estrus cycles. It has been reported that the stage of estrus can influence the pulmonary inflammatory response (Fuentes et al., 2019a). Thus, future studies will need to further examine the contribution of ovarian hormones using our model. In addition, It has been previously reported that castrated males have decreased airway hyperresponsiveness and pulmonary inflammation in response to O3 exposure (Osgood et al., 2019); however, it is unknown if castration influences the lipid mediator response after O3 exposure. Future studies will evaluate the O3-induced pulmonary lipid metabolism response in castrated males as well as the impact of male/female hormone replacement. Future investigation may also focus on DHA supplementation to OVX mice with the hypothesis that DHA supplementation will prevent the increased pulmonary inflammation and injury seen in OVX mice. Another limitation to this work is variability between exposures. For example, DHA increased in the lungs of females after O3 exposure, whereas this increase did not reach statistical significance in the sham surgery mice. In addition, our previous work demonstrated decreased SPMs in males after O3 exposure while this was not observed in the current study. A potential explanation for this variability is differences in cell membrane incorporation of DHA where DHA may be more or less bioactive in immune or structural cells. This variability may be masked by evaluating total lung concentration of DHA where little to no differences in whole lung tissue hides drastic changes in specific myeloid populations that more directly influence the immune response. Investigation into the cause of these variable responses will be a focus of future investigations to better understand the mechanisms of O3-induced inflammation.
In conclusion, our data reveal sex differences in pulmonary lipid mediators and fatty acid levels following O3 exposure. These findings correlated with an increased pulmonary inflammatory response in females, suggesting eicosanoid and SPM production influences and/or contributes to the dimorphic O3-induced pulmonary inflammatory response. Furthermore, we show that apparent DHA-driven resolution of pulmonary inflammation is dependent on ovarian hormones. Therefore, the data presented here add insights into the understanding of how sex contributes to the pulmonary inflammatory response.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institutes of Health (R01ES031378 to K.M.G. and S.R.S., R01ES020897 and P30ES025128 to J.C.B., T32ES007046 to D.Y., R01ES027574 to R.M.T.); National Center for Research and Resources (1S10OD010366); and Colorado Clinical and Translational Sciences Institute (UL1 RR025780 to N.R., J.M., and M.A.).
DECLARATION of CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
REFERENCES
- Alfaro M. F., Walby W. F., Adams W. C., Schelegle E. S. (2007). Breath condensate levels of 8-isoprostane and leukotriene B4 after ozone inhalation are greater in sensitive versus nonsensitive subjects. Exp. Lung Res. 33, 115–133. [DOI] [PubMed] [Google Scholar]
- Aoki T., Narumiya S. (2012). Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 33, 304–311. [DOI] [PubMed] [Google Scholar]
- Armstrong M., Manke J., Nkrumah-Elie Y., Shaikh S. R., Reisdorph N. (2020). Improved quantification of lipid mediators in plasma and tissues by liquid chromatography tandem mass spectrometry demonstrates mouse strain specific differences. Prostaglandins Other Lipid Mediat. 151, 106483. [DOI] [PubMed] [Google Scholar]
- Bakewell L., Burdge G. C., Calder P. C. (2006). Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br. J. Nutr. 96, 93–99. [DOI] [PubMed] [Google Scholar]
- Barden A. E., Moghaddami M., Mas E., Phillips M., Cleland L. G., Mori T. A. (2016). Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent. Fatty Acids 107, 24–29. [DOI] [PubMed] [Google Scholar]
- Basile F., Sibray T., Belisle J. T., Bowen R. A. (2011). Analysis of lipids from crude lung tissue extracts by desorption electrospray ionization mass spectrometry and pattern recognition. Anal. Biochem. 408, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J. (1995). Eicosanoids, fatty acids and neutrophils: Their relevance to the pathophysiology of disease. Prostaglandins Leukot. Essent. Fatty Acids 53, 75–86. [DOI] [PubMed] [Google Scholar]
- Beeson P. B. (1994). Age and sex associations of 40 autoimmune diseases. Am. J. Med. 96, 457–462. [DOI] [PubMed] [Google Scholar]
- Birukova A., Cyphert-Daly J., Cumming R. I., Yu Y. R., Gowdy K. M., Que L. G., Tighe R. M. (2019). Sex modifies acute ozone-mediated airway physiologic responses. Toxicol. Sci. 169, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Burdge G. C., Jones A. E., Wootton S. A. (2002). Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 88, 355–363. [DOI] [PubMed] [Google Scholar]
- Burdge G. C., Postle A. D. (1994). Hepatic phospholipid molecular species in the guinea pig. Adaptations to pregnancy. Lipids 29, 259–264. [DOI] [PubMed] [Google Scholar]
- Burdge G. C., Wootton S. A. (2002). Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 88, 411–420. [DOI] [PubMed] [Google Scholar]
- Cabello N., Mishra V., Sinha U., DiAngelo S. L., Chroneos Z. C., Ekpa N. A., Cooper T. K., Caruso C. R., Silveyra P. (2015). Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L1150–L1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Zhou J., Webb D. C. (2012). Estrogen stimulates th2 cytokine production and regulates the compartmentalisation of eosinophils during allergen challenge in a mouse model of asthma. Int. Arch. Allergy Immunol. 158, 252–260. [DOI] [PubMed] [Google Scholar]
- Chatterjee A., Roy D., Guevara P., Pal R., Naryan M., Roychowdhury S., Das S. (2018). Arachidonic acid induces the migration of mda-mb-231 cells by activating raft-associated leukotriene B4 receptors. Clin. Cancer Drugs 5, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chillingworth N. L., Morham S. G., Donaldson L. F. (2006). Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R327–R334. [DOI] [PubMed] [Google Scholar]
- Chistyakov D. V., Azbukina N. V., Astakhova A. A., Goriainov S. V., Chistyakov V. V., Sergeeva M. G. (2018). Sex-mediated differences in LPS induced alterations of TNFalpha, IL-10 expression, and prostaglandin synthesis in primary astrocytes. Int. J. Mol. Sci. 19, 2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V., Leuti A., Dalli J., Jacobsson A., Battistini L., Maccarrone M., Serhan C. N. (2016). Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 8, 353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiu V., Leuti A., Maccarrone M. (2018). Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 9, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E. J., Reedy J. L., Kwon S., Patil S., Valle L., White A. O., Citrin D. E. (2019). 12-lipoxygenase is a critical mediator of type II pneumocyte senescence, macrophage polarization and pulmonary fibrosis after irradiation. Radiat. Res. 192, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codagnone M., Cianci E., Lamolinara A., Mari V. C., Nespoli A., Isopi E., Mattoscio D., Arita M., Bragonzi A., Iezzi M., et al. (2018). Resolvin D1 enhances the resolution of lung inflammation caused by long-term pseudomonas aeruginosa infection. Mucosal. Immunol. 11, 35–49. [DOI] [PubMed] [Google Scholar]
- Cox R. Jr, Phillips O., Fukumoto J., Fukumoto I., Parthasarathy P. T., Arias S., Cho Y., Lockey R. F., Kolliputi N. (2015). Enhanced resolution of hyperoxic acute lung injury as a result of aspirin triggered resolvin D1 treatment. Am. J. Respir. Cell. Mol. Biol. 53, 422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromar K. R., Gladson L. A., Ewart G. (2019). Trends in excess morbidity and mortality associated with air pollution above American Thoracic Society-Recommended Standards, 2008-2017. Ann. Am. Thorac. Soc. 16, 836–845. [DOI] [PubMed] [Google Scholar]
- Crouch M. J., Kosaraju R., Guesdon W., Armstrong M., Reisdorph N., Jain R., Fenton J., Shaikh S. R. (2019). Frontline science: A reduction in DHA-derived mediators in male obesity contributes toward defects in select B cell subsets and circulating antibody. J. Leukoc. Biol. 106, 241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe F. L., Skeaff C. M., Green T. J., Gray A. R. (2008). Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br. J. Nutr. 99, 168–174. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., McDonnell W. F., Mann R., Becker S., House D. E., Schreinemachers D., Koren H. S. (1991). Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am. J. Respir. Cell. Mol. Biol. 4, 72–81. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G. H. (1957). A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- Fredman G., Hellmann J., Proto J. D., Kuriakose G., Colas R. A., Dorweiler B., Connolly E. S., Solomon R., Jones D. M., Heyer E. J., et al. (2016). An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat. Commun. 7, 12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes N., Cabello N., Nicoleau M., Chroneos Z. C., Silveyra P. (2019a). Modulation of the lung inflammatory response to ozone by the estrous cycle. Physiol. Rep. 7, e14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes N., Nicoleau M., Cabello N., Montes D., Zomorodi N., Chroneos Z. C., Silveyra P. (2019b). 17beta-estradiol affects lung function and inflammation following ozone exposure in a sex-specific manner. Am. J. Physiol. Lung Cell. Mol. Physiol. 317, L702–L716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes N., Roy A., Mishra V., Cabello N., Silveyra P. (2018). Sex-specific microRNA expression networks in an acute mouse model of ozone-induced lung inflammation. Biol .Sex Differ. 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussbroich D., Colas R. A., Eickmeier O., Trischler J., Jerkic S. P., Zimmermann K., Gopel A., Schwenger T., Schaible A., Henrich D., et al. (2020). A combination of LCPUFA ameliorates airway inflammation in asthmatic mice by promoting pro-resolving effects and reducing adverse effects of EPA. Mucosal Immunol. 13, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan R., Henkels K. M., Shah K., De La Rosa X., Libreros S., Cheemarla N. R., Serhan C. N., Gomez-Cambronero J. (2020). D-series resolvins activate phospholipase D in phagocytes during inflammation and resolution. Faseb J. 34, 15888–15906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Su J., Zhang Y., Chan A., Sin J. H., Wu D., Min K., Gronert K. (2018). Dietary DHA amplifies lxa4 circuits in tissues and lymph node PMN and is protective in immune-driven dry eye disease. Mucosal Immunol. 11, 1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Bengtsson A., Asadi A., Gao H., Dahlman-Wright K., Jacobsson A. (2016). Estrogen enhances the expression of the polyunsaturated fatty acid elongase elovl2 via eralpha in breast cancer cells. PLoS One 11, e0164241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazbun M. E., Hamilton R., Holian A., Eschenbacher W. L. (1993). Ozone-induced increases in substance P and 8-epi-prostaglandin F2 alpha in the airways of human subjects. Am. J. Respir. Cell. Mol. Biol. 9, 568–572. [DOI] [PubMed] [Google Scholar]
- Ho C. C., Ling Y. C., Chang L. W., Tsai H. T., Tsai M. H., Lin P. (2008). 17-beta estradiol and hydroxyestradiols interact via the Nf-kappa b pathway to elevate cyclooxygenase 2 expression and prostaglandin E2 secretion in human bronchial epithelial cells. Toxicol. Sci. 104, 294–302. [DOI] [PubMed] [Google Scholar]
- Hollingsworth J. W., Maruoka S., Li Z., Potts E. N., Brass D. M., Garantziotis S., Fong A., Foster W. M., Schwartz D. A. (2007). Ambient ozone primes pulmonary innate immunity in mice. J. Immunol. 179, 4367–4375. [DOI] [PubMed] [Google Scholar]
- Hu X., Shen H., Wang Y., Zhang L., Zhao M. (2019). Aspirin-triggered resolvin D1 alleviates paraquat-induced acute lung injury in mice. Life Sci. 218, 38–46. [DOI] [PubMed] [Google Scholar]
- Humbert M., Sitbon O., Chaouat A., Bertocchi M., Habib G., Gressin V., Yaici A., Weitzenblum E., Cordier J. F., Chabot F., et al. (2010). Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122, 156–163. [DOI] [PubMed] [Google Scholar]
- Inigo M. R., Amorese A. J., Tarpey M. D., Balestrieri N. P., Jones K. G., Patteson D. J., Jackson K. C., Torres M. J., Lin C. T., Smith C. D., et al. (2020). Estrogen receptor-alpha in female skeletal muscle is not required for regulation of muscle insulin sensitivity and mitochondrial regulation. Mol. Metab. 34, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis S. M. (2000). The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev. Neurosci. 22, 474–480. [DOI] [PubMed] [Google Scholar]
- Kan H., London S. J., Chen G., Zhang Y., Song G., Zhao N., Jiang L., Chen B. (2008). Season, sex, age, and education as modifiers of the effects of outdoor air pollution on daily mortality in Shanghai, China: The Public Health and Air Pollution in Asia (PAPA) study. Environ. Health Perspect. 116, 1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburg-Basnyat B., Reece S. W., Crouch M. J., Luo B., Boone A. D., Yaeger M., Hodge M., Psaltis C., Hannan J. L., Manke J., et al. (2018). Specialized pro-resolving lipid mediators regulate ozone-induced pulmonary and systemic inflammation. Toxicol. Sci. 163, 466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson A. P., Stroud C. K., Stark K. D. (2010). Elevated production of docosahexaenoic acid in females: Potential molecular mechanisms. Lipids 45, 209–224. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Lien E., Agostoni C., Bohles H., Campoy C., Cetin I., Decsi T., Dudenhausen J. W., Dupont C., Forsyth S., et al. (2008). The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Perinat. Med. 36, 5–14. [DOI] [PubMed] [Google Scholar]
- Lammermann T., Afonso P. V., Angermann B. R., Wang J. M., Kastenmuller W., Parent C. A., Germain R. N. (2013). Neutrophil swarms require ltb4 and integrins at sites of cell death in vivo. Nature 498, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le G., Novotny S. A., Mader T. L., Greising S. M., Chan S. S. K., Kyba M., Lowe D. A., Warren G. L. (2018). A moderate oestradiol level enhances neutrophil number and activity in muscle after traumatic injury but strength recovery is accelerated. J. Physiol. 596, 4665–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lee P. S., Sun Y., Gu X., Zhang E., Guo Y., Wu C. L., Auricchio N., Priolo C., Li J., et al. (2014). Estradiol and mtorc2 cooperate to enhance prostaglandin biosynthesis and tumorigenesis in TSC2-deficient lam cells. J. Exp. Med. 211, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. F., Hao H., Tu W. S., Guo N., Zhou X. Y. (2020). Maresins: Anti-inflammatory pro-resolving mediators with therapeutic potential. Eur. Rev. Med. Pharmacol. Sci. 24, 7442–7453. [DOI] [PubMed] [Google Scholar]
- Lim C. S., Porter D. W., Orandle M. S., Green B. J., Barnes M. A., Croston T. L., Wolfarth M. G., Battelli L. A., Andrew M. E., Beezhold D. H., et al. (2020). Resolution of pulmonary inflammation induced by carbon nanotubes and fullerenes in mice: Role of macrophage polarization. Front. Immunol. 11, 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden M. C., Eling T. E., Dailey L. A., Friedman M. (1991). The effect of ozone exposure on rat alveolar macrophage arachidonic acid metabolism. Exp. Lung Res. 17, 47–63. [DOI] [PubMed] [Google Scholar]
- Malley C. S., Henze D. K., Kuylenstierna J. C. I., Vallack H. W., Davila Y., Anenberg S. C., Turner M. C., Ashmore M. R. (2017). Updated global estimates of respiratory mortality in adults >/=30years of age attributable to long-term ozone exposure. Environ. Health Perspect. 125, 087021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek M., Malur A., Tokarz D. A., Lertpiriyapong K., Gowdy K. M., Murray G., Wingard C. J., Fessler M. B., Barna B. P., Thomassen M. J. (2019). Alveolar macrophage abcg1 deficiency promotes pulmonary granulomatous inflammation. Am. J. Respir. Cell. Mol. Biol. 61, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messenger Z. J., Hall J. R., Jima D. D., House J. S., Tam H. W., Tokarz D. A., Smart R. C. (2018). C/EBPbeta deletion in oncogenic Ras skin tumors is a synthetic lethal event. Cell Death Dis. 9, 1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherel A. H., Armstrong J. M., Patterson A. C., Stark K. D. (2009). Assessment of blood measures of n-3 polyunsaturated fatty acids with acute fish oil supplementation and washout in men and women. Prostaglandins Leukot. Essent. Fatty Acids 81, 23–29. [DOI] [PubMed] [Google Scholar]
- Metherel A. H., Irfan M., Klingel S. L., Mutch D. M., Bazinet R. P. (2021). Higher increase in plasma DHA in females compared to males following EPA supplementation may be influenced by a polymorphism in elovl2: An exploratory study. Lipids 56, 211–228. [DOI] [PubMed] [Google Scholar]
- Miller P. D., Ainsworth D., Lam H. F., Amdur M. O. (1987). Effect of ozone exposure on lung functions and plasma prostaglandin and thromboxane concentrations in guinea pigs. Toxicol. Appl. Pharmacol. 88, 132–140. [DOI] [PubMed] [Google Scholar]
- Narumiya S., Furuyashiki T. (2011). Fever, inflammation, pain and beyond: Prostanoid receptor research during these 25 years. Faseb J. 25, 813–818. [DOI] [PubMed] [Google Scholar]
- Osgood R. S., Kasahara D. I., Tashiro H., Cho Y., Shore S. A. (2019). Androgens augment pulmonary responses to ozone in mice. Physiol. Rep. 7, e14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S. J., van Houwelingen A. C., Badart-Smook A., Hornstra G. (2001). Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. Am. J. Clin. Nutr. 73, 302–307. [DOI] [PubMed] [Google Scholar]
- Pace S., Pergola C., Dehm F., Rossi A., Gerstmeier J., Troisi F., Pein H., Schaible A. M., Weinigel C., Rummler S., et al. (2017). Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J. Clin. Invest. 127, 3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace S., Werz O. (2020). Impact of androgens on inflammation-related lipid mediator biosynthesis in innate immune cells. Front. Immunol. 11, 1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar K., Schulte F., Hardt M., Baker O. J. (2020). Sex-mediated elevation of the specialized pro-resolving lipid mediator levels in a Sjogren’s syndrome mouse model. Faseb J. 34, 7733–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton K. E., Harbaugh M., Han M. K., Jourdan Le Saux C., Van Winkle L. S., Martin W. J. 2nd, Kosgei R. J., Carter E. J., Sitkin N., Smiley-Jewell S. M., et al. (2015). Women and lung disease. Sex differences and global health disparities. Am. J. Respir. Crit. Care Med. 192, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle A. D., Al M. D., Burdge G. C., Hornstra G. (1995). The composition of individual molecular species of plasma phosphatidylcholine in human pregnancy. Early Hum. Dev. 43, 47–58. [DOI] [PubMed] [Google Scholar]
- Pullen A. B., Kain V., Serhan C. N., Halade G. V. (2020). Molecular and cellular differences in cardiac repair of male and female mice. J. Am. Heart Assoc. 9, e015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon S., Gao F., Serhan C. N., Phipps R. P. (2012). Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189, 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A., Pergola C., Pace S., Radmark O., Werz O., Sautebin L. (2014). In vivo sex differences in leukotriene biosynthesis in zymosan-induced peritonitis. Pharmacol. Res. 87, 1–7. [DOI] [PubMed] [Google Scholar]
- Schatz M., Camargo C. A. Jr (2003). The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann. Allergy Asthma Immunol. 91, 553–558. [DOI] [PubMed] [Google Scholar]
- Serhan C. N. (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Levy B. D. (2018). Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 128, 2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Petasis N. A. (2011). Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield P. E., Zhou J., Shmool J. L., Clougherty J. E. (2015). Ambient ozone exposure and children’s acute asthma in New York City: A case-crossover analysis. Environ. Health 14, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E. M., Fix A., Crouch M. J., Sparagna G. C., Zeczycki T. N., Brown D. A., Shaikh S. R. (2017). Murine diet-induced obesity remodels cardiac and liver mitochondrial phospholipid acyl chains with differential effects on respiratory enzyme activity. J. Nutr. Biochem. 45, 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Chen L., Wang Y. X., Hu L. S., Xiong W., Shang Y., Yao S. L. (2018). Protectin DX exhibits protective effects in mouse model of lipopolysaccharide-induced acute lung injury. Chin. Med. J. 131, 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosyan Y., Dobi A., Naga S., Mezhevaya K., Glasman M., Norris C., Jiang G., Mueller G., Pollard H., Srivastava M. (2006). Distinct effects of annexin a7 and p53 on arachidonate lipoxygenation in prostate cancer cells involve 5-lipoxygenase transcription. Cancer Res. 66, 9609–9616. [DOI] [PubMed] [Google Scholar]
- Vancza E. M., Galdanes K., Gunnison A., Hatch G., Gordon T. (2009). Age, strain, and gender as factors for increased sensitivity of the mouse lung to inhaled ozone. Toxicol. Sci. 107, 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.