Abstract
Bisphenol A (BPA) is a high-production volume chemical used to manufacture consumer and medical-grade plastic products. Due to its ubiquity, the general population can incur daily environmental exposure to BPA, whereas heightened exposure has been reported in intensive care patients and industrial workers. Due to health concerns, structural analogs are being explored as replacements for BPA. This study aimed to examine the direct effects of BPA on cardiac electrophysiology compared with recently developed alternatives, including BPS (bisphenol S) and BPF (bisphenol F). Whole-cell voltage-clamp recordings were performed on cell lines transfected to express the voltage-gated sodium channel (Nav1.5), L-type voltage-gated calcium channel (Cav1.2), or the rapidly activating delayed rectifier potassium channel (hERG). Cardiac electrophysiology parameters were measured using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) and intact, whole rat heart preparations. BPA was the most potent inhibitor of fast/peak (INa-P) and late (INa-L) sodium channel (IC50 = 55.3, 23.6 µM, respectively), L-type calcium channel (IC50 = 30.8 µM), and hERG channel current (IC50 = 127 µM). Inhibitory effects on L-type calcium channels were supported by microelectrode array recordings, which revealed a shortening of the extracellular field potential (akin to QT interval). BPA and BPF exposures slowed atrioventricular (AV) conduction and increased AV node refractoriness in isolated rat heart preparations, in a dose-dependent manner (BPA: +9.2% 0.001 µM, +95.7% 100 µM; BPF: +20.7% 100 µM). BPS did not alter any of the cardiac electrophysiology parameters tested. Results of this study demonstrate that BPA and BPF exert an immediate inhibitory effect on cardiac ion channels, whereas BPS is markedly less potent. Additional studies are necessary to fully elucidate the safety profile of bisphenol analogs on the heart.
Keywords: bisphenol, plastics, cardiac electrophysiology, comprehensive in vitro proarrhythmia assay (CiPA)
Bisphenol A (BPA) is a high-production volume chemical, with roughly 8 million metric tons used each year to manufacture polycarbonate plastics (eg, food and beverage containers, medical devices), epoxy resins (eg, aluminum can liners), and in thermal printing applications (PR newswire, 2016; Shelby, 2008). Human exposure to BPA can occur daily, and as a result, biomonitoring studies have detected BPA in 91–99% of the general population (Calafat et al., 2005; Chen et al., 2016a; Lehmler et al., 2018; Vandenberg et al., 2010, 2007). Although environmental exposure to BPA occurs at a relatively low dose (Koch and Calafat, 2009; Vandenberg et al., 2007, 2010), occupational (Bousoumah et al., 2021; Hines et al., 2018; Ribeiro et al., 2017), and clinical environments can result in exceedingly high BPA exposure (Calafat et al., 2009; Duty et al., 2013; Gaynor et al., 2019; Huygh et al., 2015; Testai et al., 2016). Indeed, BPA was detected in 60% of neonatal intensive care unit (NICU) supplies, including items used for feeding, bandages, breathing support, intravenous, and parenteral infusion (Iribarne-Durán et al., 2019). Clinical exposure can also result in heightened and/or prolonged BPA exposure in young patients, due to an underdeveloped metabolic system (Calafat et al., 2009). In the NICU setting, premature infants had urinary BPA levels that ranged from 1.6 to 946 µg/l (Calafat et al., 2009) and the degree of exposure was linked to high-intensity treatment that required multiple (plastic) medical devices (Duty et al., 2013). Furthermore, higher urinary concentrations of BPA were detected in very low birth weight infants, as compared with term infants, likely due to increased medical device usage (Strømmen et al., 2021). Adult ICU patients were found to have urinary BPA levels that ranged from 6.1 to 680 µg/l and serum levels ranged from 2.6 to 255 µg/l when undergoing extracorporeal membrane oxygenation in conjunction with continuous veno-venous hemofiltration (Huygh et al., 2015). Similarly, urinary BPA levels increased approximately 4-fold in adult cardiac surgery patients postoperatively, as compared with preoperative levels (Shang et al., 2019).
BPA exposure is concerning, particularly in sensitive patient populations, as accumulating evidence suggests that BPA exerts a negative impact on cardiovascular health (Bae et al., 2012; Bae and Hong, 2015; Han and Hong, 2016; Melzer et al., 2010, 2012). A 10-year longitudinal study found that BPA exposure was associated with a 46–49% higher hazard ratio for cardiovascular and all-cause mortality (Bao et al., 2020). Furthermore, epidemiological studies have reported associations between BPA exposure and an increased risk of myocardial infarction, hypertension, coronary and peripheral artery disease, and a decrease in heart rate variability (reviewed previously; Posnack, 2014; Ramadan et al., 2020). Experimental studies have noted that BPA exposure can antagonize ion channels, impair electrical conduction, and precipitate triggered arrhythmias (Belcher et al., 2012; Deutschmann et al., 2013; Feiteiro et al., 2018; Michaela et al., 2014; Posnack et al., 2015; Wang et al., 2011; Yan et al., 2011). In vitro studies performed in kidney, neuronal, and smooth muscle cells have shown that BPA inhibits T-type and L-type calcium channel current (Deutschmann et al., 2013; Feiteiro et al., 2018; Michaela et al., 2014). In cardiac tissue, such an alteration in calcium channel current would alter nodal cell depolarization, atrioventricular (AV) conduction, and the plateau phase of the cardiac action potential. Furthermore, BPA exposure can disrupt intracellular calcium handling, resulting in calcium leak from the sarcoplasmic reticulum and an increased propensity for triggered arrhythmias (Gao et al., 2013; Liang et al., 2014; Ramadan et al., 2018). Of interest, BPA exposure was observed to increase calcium-mediated triggered activity and ventricular arrhythmias in females (but not males) subjected to catecholamine stress (Patel et al., 2015). Notably, such alterations in calcium handling were attenuated in an estrogen-receptor knockout model (Yan et al., 2011), suggesting that BPA-induced effects may be sex-specific. To the best of our knowledge, experimental studies examining the impacts of bisphenol chemicals using human cardiomyocyte models are lacking.
With increasing health concerns, structurally similar chemicals are being explored as replacements for BPA (Chen et al., 2016a). Two such substitutes, bisphenol S (BPS) and bisphenol F (BPF), are used to manufacture consumer products that don “BPA-free” labeling. For example, BPS is used to produce polyethersulfone plastic food containers, medical-grade products, epoxy resins, and is found in thermal printing applications (Lehmler et al., 2018; Chen et al., 2016a). Unfortunately, many of these alternative chemicals are considered “regrettable substitutions,” as BPS and BPF may exert biological effects that are similar to BPA (Kojima et al., 2019; Moon, 2019; Trasande, 2017). To date, it is unclear whether BPA alternatives are less cardiotoxic, as recent work suggests that BPS and BPF may also impair cardiac function (Ferguson et al., 2019; Gao et al., 2015; Mu et al., 2019). Recent biomonitoring studies have observed an uptick in BPS and BPF exposure in the general population as BPA is phased out and replaced (Lehmler et al., 2018), highlighting the urgent need to investigate the impact of BPA analogs on cardiac physiology.
We compared the cardiac electrophysiology effects of BPA, BPS, and BPF using whole-cell voltage-clamp experiments to identify the half-maximal inhibitory concentration (IC50) of 4 key cardiac ion channels, highlighted by the comprehensive in vitro proarrhythmia assay (CiPA) initiative (Colatsky et al., 2016; Sager et al., 2014). Follow-up studies on human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) were performed using microelectrode array (MEA) recordings. Importantly, hiPSC-CM have been widely adopted as a tool for preclinical safety testing to measure alterations in cardiac automaticity, conduction velocity, depolarization, and repolarization time (Chen et al., 2016b). To aid in the translation of our in vitro cell studies, we employed an intact, whole rat heart preparation for direct assessment of cardiac electrophysiology. We previously reported that BPA treatment prolongs AV conduction in isolated rat heart preparations during sinus rhythm (Posnack et al., 2014), accordingly, we hypothesized that inhibitory effects of bisphenol chemicals on calcium current would impair AV conduction and increase refractoriness in cardiac preparations. Furthermore, we hypothesized that BPS and/or BPF exposure would have less effect on cardiac electrophysiology due to differences in chemical structure that may increase the potency of BPA for voltage-gated channels. The latter is supported by a study by Deutschmann et al. (2013), which reported a direct interaction between BPA and the extracellular part of voltage-gated calcium channels with structure-effect analyses suggesting that its angulated orientation may promote this interaction.
MATERIALS AND METHODS
Reagents
Chemical reagents were purchased from Sigma Aldrich (St Louis, MO). BPA (CAS #80-05-7), BPS (CAS #80-09-1), and BPF (CAS #620-92-8) (≥98% purity, analytical standard) stock solutions were prepared in 99+% dimethyl sulfoxide (DMSO). Working concentrations were prepared directly in iCell culture media (Fujifilm cellular dynamics; Madison, Wisconsin) for voltage-clamp recordings and MEA studies or Krebs-Henseleit (KH) crystalloid buffer (Reilly et al., 2020) for intact heart preparations to obtain a final concentration between 0.001 and 100 µM BPA, BPS, or BPF. The range of concentrations was selected to mimic human environmental exposure (1–100 nM), maximum clinical or occupational exposure (≤ 10 µM), and supraphysiological exposure levels (> 10 µM) (Bousoumah et al., 2021; Ramadan et al., 2020). A wider range of doses were employed for whole-cell voltage-clamp recordings, when necessary, to pinpoint the half half-maximal inhibitory concentration (0.01–3 mM). To reduce the risk of environmental contamination, nonpolycarbonate bisphenol-free plastic was used for experiments (including Tygon tubing [Saint-Gobain in North America; Malvern, Pennsylvania], polystyrene cell culture plates [Axion biosystems; Atlanta, Georgia], and glass tissue bath [Radnoti; Covina, California]).
Whole-cell voltage-clamp recordings
Environmental chemicals can alter the cardiac action potential, electrical conduction, or elicit arrhythmias through inhibition of sodium, calcium, and potassium ion currents. To test the effects of bisphenol chemicals on cardiac currents, Nav1.5, Cav1.2, and hERG channel recordings were performed using stably transfected cell lines, as previously described (Jaimes et al., 2019). For each measurement, cells were exposed to bisphenol-containing solutions for approximately 5–10 min, until currents reached stability. For Nav1.5 recordings, the extracellular solution included 137 mM sodium chloride, 10 mM dextrose, 10 mM HEPES, 4 mM potassium chloride, 1 mM magnesium chloride, and 1 mM calcium chloride. The intracellular solution consisted of 120 mM cesium hydroxide, 120 mM aspartic acid, 10 mM egtazic acid, 10 mM cesium chloride, 10 mM HEPES, 5 adenosine 5ʹ-triphosphate magnesium salt, and 0.4 mM guanosine 5ʹ-triphosphate salt. The voltage protocol was approximately 1 sec in duration, repeated at 0.1 Hz. Sodium channel recordings were performed using human embryonic kidney cells (HEK293) transfected with Nav1.5 cDNA. Cells were repolarized from −95 to −120 mV for 200 ms, depolarized from −120 to −15 mV for 40 ms, and then further depolarized to +40 mV for 200 ms. This was followed immediately by a voltage ramp-down phase from +40 to −95 mV for 100 ms. Neurotoxin-2 (ATXII, 20 nmol/l) was included in the extracellular solution to induce Nav1.5 late current, as previously described (Mantegazza et al., 1998). Tetrodotoxin (30 µM) was applied at the end of each recording to determine the current baseline. Cav1.2 recordings were performed using Chinese hamster ovary cells (CHO) stably transfected with Cav1.2 cDNA. Cells were depolarized from −80 to 0 mV for 40 ms, further depolarized to +30 mV for 200 ms, followed by a voltage ramp-down phase from +30 to −80 mV for 100 ms. Recording stability was assessed by applying the voltage protocol in the control solution for 12 consecutively recorded traces with < 10% difference. hERG recordings were performed using HEK293 cells stably transfected with hERG cDNA. The extracellular solution included 130 mM sodium chloride, 12.5 mM dextrose, 10 mM HEPES, 5 mM potassium chloride, 1 mM magnesium chloride hexahydrate, and 1 mM calcium chloride. The intracellular solution consisted of 120 potassium-gluconate, 20 mM potassium chloride, 10 mM HEPES, 5 egtazic acid, and 1.5 adenosine 5ʹ-triphosphate magnesium salt. The voltage protocol was 5 sec in duration, repeated at 0.1 Hz. Cells were depolarized −80 to +40 mV for 500 ms, followed by a voltage ramp-down phase from +40 to −80 mV for 100 ms. An hERG potassium channel blocker (10 µM E-4031) was applied at the end of each recording to determine the baseline. Recordings were collected before and after bisphenol chemical exposure; chemical potency was calculated by dividing the steady-state current amplitude by the average amplitude from the last 5 traces measured in the control solution to calculate the fractional block. This was plotted against the bisphenol chemical concentration tested, fitted with the Hill Equation to generate a half-maximal inhibitory concentration (IC50) and the Hill coefficient.
Human cardiomyocyte MEA recordings
hiPSC-CM (iCell cardiomyocytes2, female donor #01434, Fujifilm cellular dynamics; Madison, Wisconsin) were plated onto fibronectin-coated MEAs at a density of 50–75 000 cells/well (24-well plate, Axion Biosystems; Atlanta, Georgia). Cells were defrosted in iCell cardiomyocyte plating media in a cell culture incubator (37°C, 5% CO2) for 2 h, thereafter cells were cultured in iCell maintenance media for 4–7 days, per the manufacturer’s instructions. Treatment groups included 0.01% DMSO (vehicle), 0.01–100 µM BPA, BPS, or BPF. Cells were treated for 15 min, and then extracellular field potential signals were recorded in response to external stimulation (1–2 Hz). Extracellular field potential duration (FPD) was measured and rate corrected with the Fridericia formula (FPDc). Disturbances in the recorded waveform can be used to predict the identity of ion channels impacted by chemical exposure, with FPD analogous to an in vitro QT interval that correlates with action potential duration at 50% repolarization (Asakura et al., 2015; Clements, 2016). Although hiPSC-CM can display an immature phenotype as compared with adult cardiomyocytes, recent comparative studies have demonstrated good correlation between FPDc and clinical trial QTc prolongation in response to 26 different drugs when using commercially available hiPSC-CM cell lines (iCell and Cor4.U) (Blinova et al., 2017).
Animals
Animal protocols were approved by the Institutional Animal Care and Use Committee at Children’s National Research Institute and followed the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. Bisphenol chemicals are xenoestrogens that may cause exaggerated cardiac effects in females (Ben-Jonathan and Steinmetz, 1998; Yan et al., 2011); accordingly, experiments were performed using female Sprague Dawley rats, aged 3–4 months (strain NTac: SD, from NIH Genetic Resource stock, Taconic Biosciences; Germantown, New York). Animals were housed in conventional acrylic rat cages in the Research Animal Facility, under standard environmental conditions (12:12 h light:dark cycle, 18°C–25°C, 30%–70% humidity). Each animal served as its own control, with electrophysiology measurements collected at baseline and again after treatment.
Intact heart preparations
Animals were anesthetized with 3% isoflurane; the chest was opened, the heart was rapidly excised, and the aorta was cannulated. The isolated, intact heart was then transferred to a temperature-controlled (37°C) constant-pressure (70 mmHg) Langendorff-perfusion system. Excised hearts were perfused with a modified KH buffer bubbled with carbogen, as previously described (Jaimes et al., 2019; Swift et al., 2020). Pseudo-electrocardiograms (ECG) were recorded in lead II configuration, and biosignals were acquired in iox2 and analyzed in ecgAUTO (EMKA technologies; Sterling, Virginia). Isolated hearts remained stable with minimal fluctuations in heart rate or electrophysiology parameters following 0.01% DMSO media perfusion (vehicle; Figure 1). To account for animal variability, ECG recordings were collected during control media perfusion (15 min) and in response to bisphenol exposure (15 min). Similarly, electrophysiology measurements (see below) were measured at baseline, after 15 min chemical exposure, and again after 15 min washout with KH media. Results of 2-dimensional hiPSC-CM studies may/may not translate to a whole heart with specialized anatomy, cell populations (atrial, nodal, ventricular), spatial tissue heterogeneity, a coordinated conduction system, and increased metabolic demand. Accordingly, an intact heart model can aid in the translational impact of our findings—and also allows for a direct comparison to the existing bisphenol chemical literature (Belcher et al., 2015; Ferguson et al., 2019; Gear et al., 2017; Yan et al., 2011).
Figure 1.
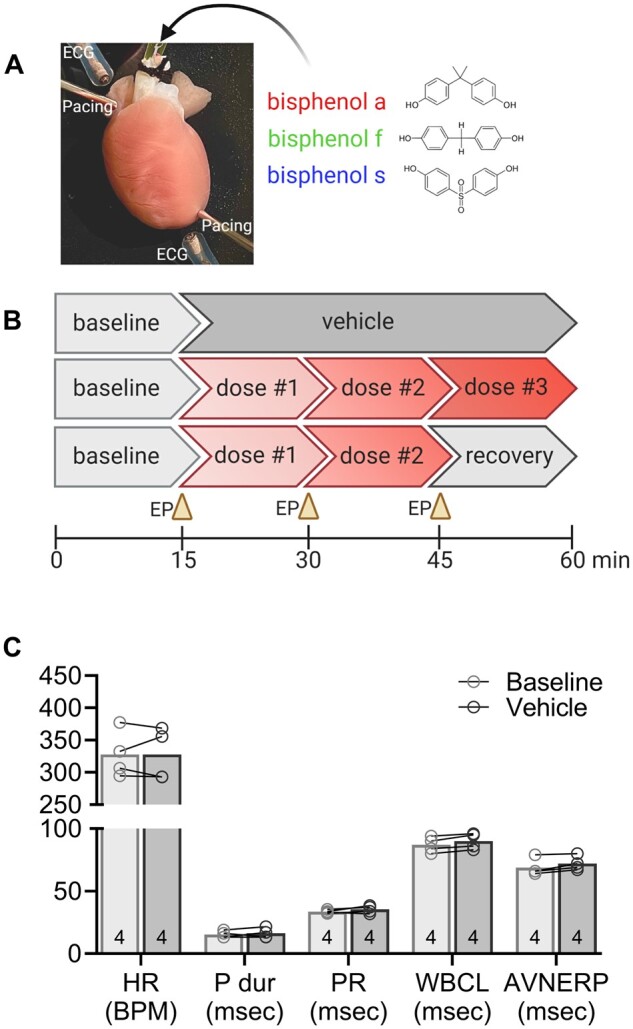
Experimental design and vehicle control parameters. A, Langendorff-perfused rat heart shown with pacing electrodes on the right atria and apex, and monopolar electrodes placed to record electrocardiograms. B, The schematic timeline depicts perfusion protocols used in the study, including (top) vehicle control exposure, (middle) bisphenol chemical dose response, and (bottom) bisphenol chemical dose response and subsequent recovery. C, Cardiac electrophysiology parameters are consistent over time, and similar between baseline and vehicle (0.01% DMSO) exposure. Values reported as mean ± SD. Statistical significance determined by RM-ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph (n = 4). Abbreviations: ECG, location of electrocardiogram electrode; EP, electrophysiology protocol; HR, heart rate; BPM, beats per minute; P dur, P wave duration; PR, PR interval; WBCL, Wenckebach cycle length; AVNERP, atrioventricular node effective refractory period; msec, milliseconds; DMSO, dimethyl sulfoxide.
Electrophysiology measurements
A pacing electrode was positioned on the right atrium for assessment of AV conduction time, AV node refractory period (AVNERP), and Wenckebach cycle length (WBCL) (Swift et al., 2019). WBCL was defined as the shortest S1-S1 pacing interval that resulted in 1:1 AV conduction. AVNERP was defined as the shortest S1-S2 pacing interval that resulted in 1:1 AV conduction. Electrophysiology studies were performed using a Bloom Classic electrophysiology stimulator (Fischer Medical; Wheat Ridge, Colorado) set to a pacing current 1.5× the minimum pacing threshold, with a 1 ms monophasic pulse width. For each parameter, the pacing cycle length (PCL) was decremented to pinpoint the PCL before loss of capture was observed.
Statistical analysis
Results are reported as mean ± standard deviation. Data normality was assessed by Shapiro-Wilk testing (GraphPad Prism, GraphPad Software; San Diego, California). Statistical analysis was performed using one-way analysis of variance (ANOVA) for MEA recordings or 2-way analysis of variance with repeated measures (RM-ANOVA) to compare baseline versus treatment in whole heart experiments. Significance was defined by an adjusted p-value (q < 0.1) after correction for multiple comparisons using a 2-stage linear step-up procedure to control the false discovery rate (0.1) as described by Benjamini et al. 2006). Significance is denoted in the figures with an asterisk (*).
RESULTS
BPA Exerts a Greater Inhibitory Effect on Ion Channels, Compared with BPS or BPF
Whole-cell voltage-clamp recordings were performed on cells transfected with 1 of 4 cardiac ion channels, as highlighted by CiPA (Colatsky et al., 2016; Sager et al., 2014). Currents were evoked and recorded before and after exposure to BPA, BPS, or BPF, and a half-maximal inhibitory concentration (IC50) was computed by testing the respective potency to each ion channel. Collectively, BPA had the highest affinity for each ion channel tested, compared with both BPF and BPS, and current suppression was concentration-dependent (Figures 2 and 3). Peak sodium current (INa-P) was suppressed with an IC50 of 55.3 μM BPA, 232 μM BPF, and 1090 μM for BPS; late sodium current (INa-L) was suppressed at lower doses, with a computed IC50 of 23.6 μM BPA, 100 μM BPF, and 369 μM BPS (Figs. 2A and 2B). In ventricular tissue, INa-P is responsible for action potential upstroke (phase 0), and INa-L is involved in the plateau phase (phase 2). Accordingly, inhibition of INa-P is likely to slow depolarization and electrical conduction, whereas inhibition of INa-L can shorten the action potential duration. L-type calcium channel current (ICaL) was also the most sensitive to BPA exposure, with an IC50 of 30.8 μM, compared with 76 μM BPF and 333 μM BPS (Figure 3A). Calcium current (ICaL) plays a prominent role in the plateau phase of the ventricular action potential and also contributes to the action potential upstroke in nodal cells. Inhibition of ICaL can slow sinus rate, delay AV conduction, increase AV refractoriness, and shorten the ventricular myocyte action potential. Finally, the rapid delayed rectifier potassium current (IKr) was suppressed at higher bisphenol concentrations, with a measured IC50 of 127 μM BPA, 209 μM BPF, and 633 μM BPS (Figure 3B). Bisphenol exposure could suppress IKr and prolong cardiac repolarization (phase 3) at high concentrations.
Figure 2.
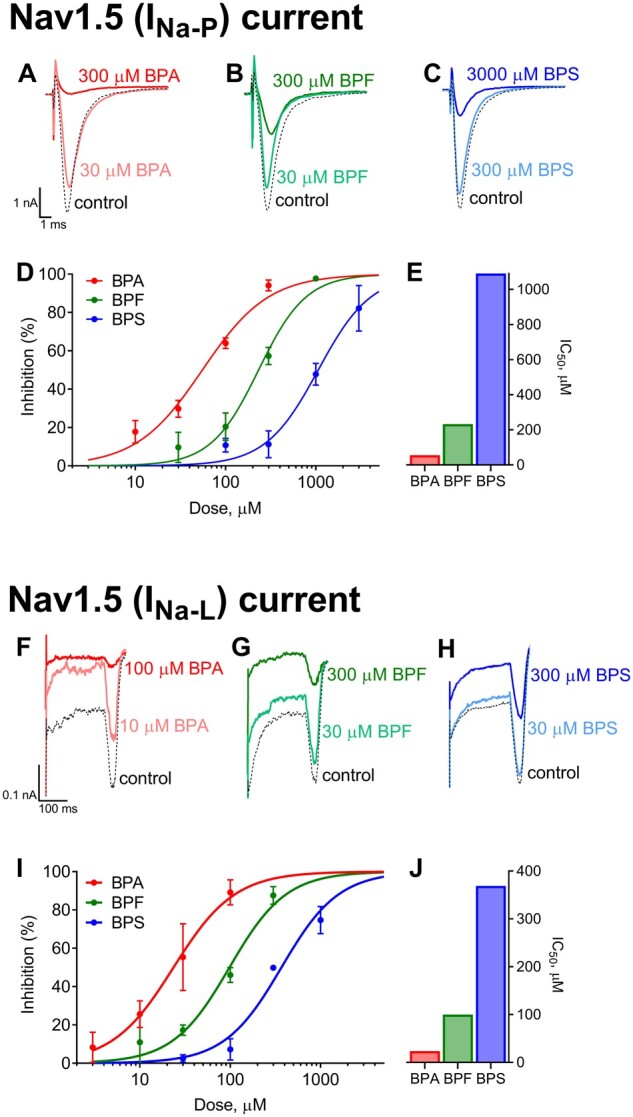
Bisphenol inhibition of sodium currents. Whole-cell voltage-clamp recordings of fast/peak sodium current (INa-P) following acute (5–10 min) exposure to (A) BPA, (B) BPF, or (C) BPS. D, Dose-dependent inhibition of INa-P (mean ± SD). E, Calculated IC50 values are shown. Whole-cell voltage-clamp recordings of late sodium current (INa-L) following exposure to (F) BPA, (G) BPF, or (H) BPS. I, Dose-dependent inhibition of INa-L (mean ± SD). J, Calculated IC50 values are shown. n = 4 independent cell recordings per study. Abbreviations: BPA, bisphenol A; BPS, bisphenol S; BPF, bisphenol F.
Figure 3.
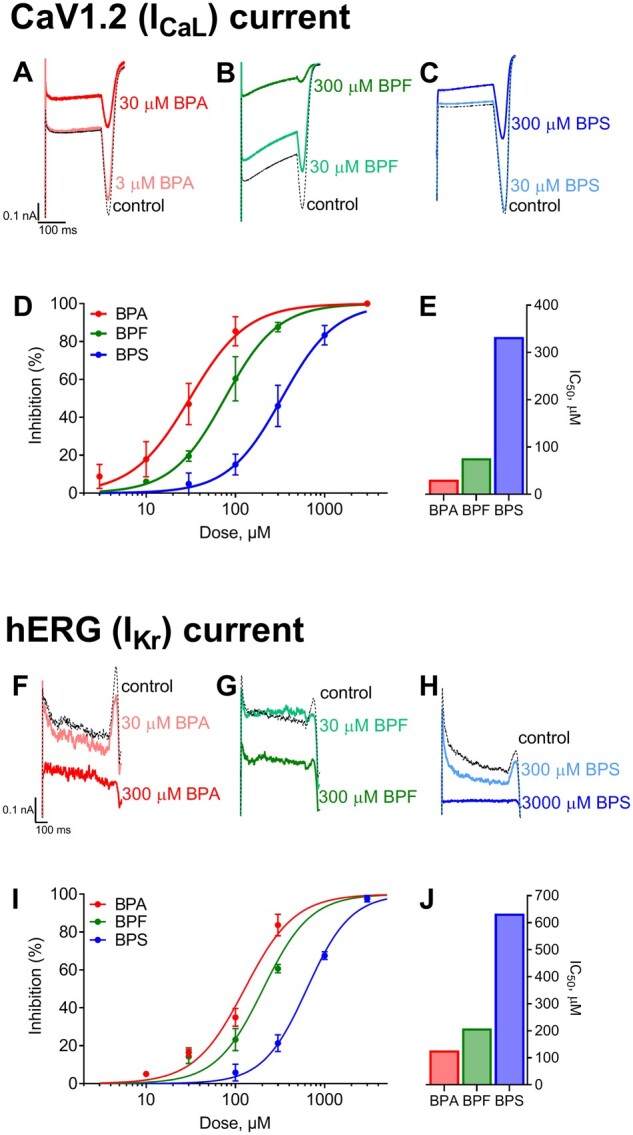
Bisphenol inhibition of calcium and potassium currents. Whole-cell voltage-clamp recordings of L-type calcium current (ICaL) following acute (5–10 min) exposure to (A) BPA, (B) BPF, or (C) BPS. D, Dose-dependent inhibition of ICaL (mean ± SD). E, Calculated IC50 values are shown. Whole-cell voltage-clamp recordings of hERG current (IKr) following exposure to (F) BPA, (G) BPF, or (H) BPS. I, Dose-dependent inhibition of IKrL (mean ± SD). J, Calculated IC50 values are shown. n = 4 independent cell recordings per study.
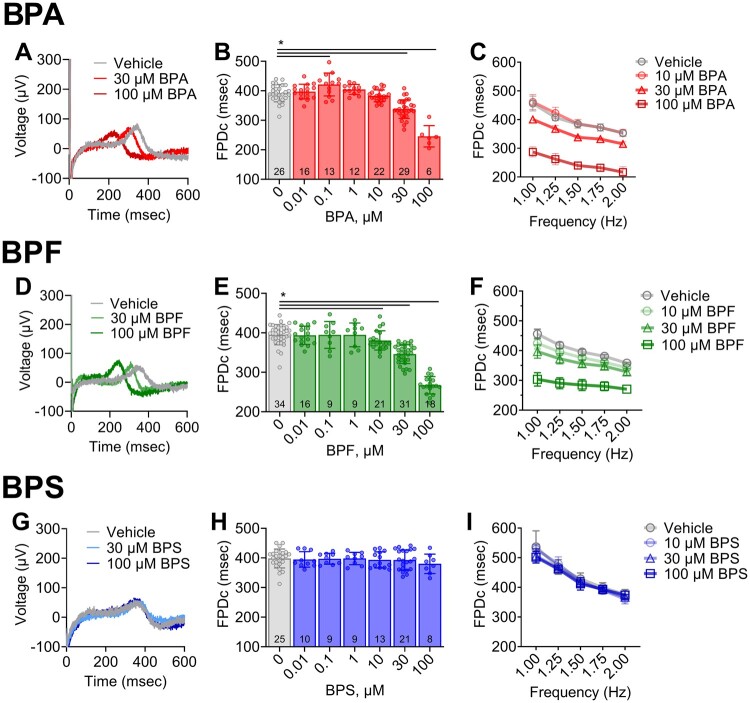
BPA and BPF Exposures Alter the Extracellular Field Potential of Human Cardiomyocytes
To further investigate the effects of bisphenol chemicals on cardiac electrophysiology, we employed hiPSC-CM that express key cardiac ion channels (Edwards and Louch, 2017). hiPSC-CM were cultured atop microelectrodes and extracellular field potentials were recorded (Figs. 4A, 4D, and 4G), with FPD measurements analogous to an in vitro QT interval that correlates with the action potential duration (Asakura et al., 2015; Clements, 2016). Acute BPA exposure resulted in a slight nonmonotonic dose response (Figure 4B), wherein no effect on FPDc was observed at the lowest BPA dose tested (0.01 µM), and a 7.5% increase in FPDc was observed at 0.1 µM BPA (q < 0.005). Notably, low-dose effects have previously been reported for BPA and other endocrine-disrupting chemicals that can present with a nonmonotonic dose response (Birnbaum, 2012; Gao et al., 2015; Liang et al., 2014; Vandenberg, 2014). At higher BPA doses, FPDc shortened significantly compared with the vehicle (13.8% at 30 µM, 37.3% at 100 µM, q < 0.0001). Low-dose effects were not observed for either BPF or BPS (Figs. 4E and 4H). However, BPF exposure resulted in FPDc shortening at higher concentrations (3.7% at 10 µM [q < 0.05], 12.5% at 30 µM [q < 0.0001], 32.4% at 100 µM [q < 0.0001]). Treatment with BPS did not alter FPDc at any of the tested concentrations. FPDc restitution curves were generated by increasing the pacing frequency (1–2 Hz). A frequency-dependent effect was not observed for BPA in hiPSC-CM, although BPF exhibited a slight reverse-use dependency with FPDc shortening more prominent at lower frequencies (Figs. 4C and 4F).
Figure 4.
Bisphenol chemical effects on cardiomyocyte field potential duration. A, Representative traces of extracellular field potentials recorded from hiPSC-CM following acute (15 min) exposure to vehicle, 30, or 100 µM BPA. B, Field potential duration (corrected using Fridericia formula: “FPDc”) shortens with increasing BPA exposure; single pacing frequency (1.5 Hz). C, FPDc restitution curve at multiple pacing frequencies (1–2 Hz). D, Local field potential traces following exposure to vehicle, 30, or 100 µM BPF. E, FPDc shortens with increasing BPF exposure (1.5 Hz). F, FPDc restitution curve (1–2 Hz). G, Local field potential traces following exposure 30–100 µM BPS. H, FPDc remains constant with increasing BPS exposure (1.5 Hz). I, FPDc restitution curve (1–2 Hz). Values reported as mean ± SD. *q < 0.05 as determined by ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph.
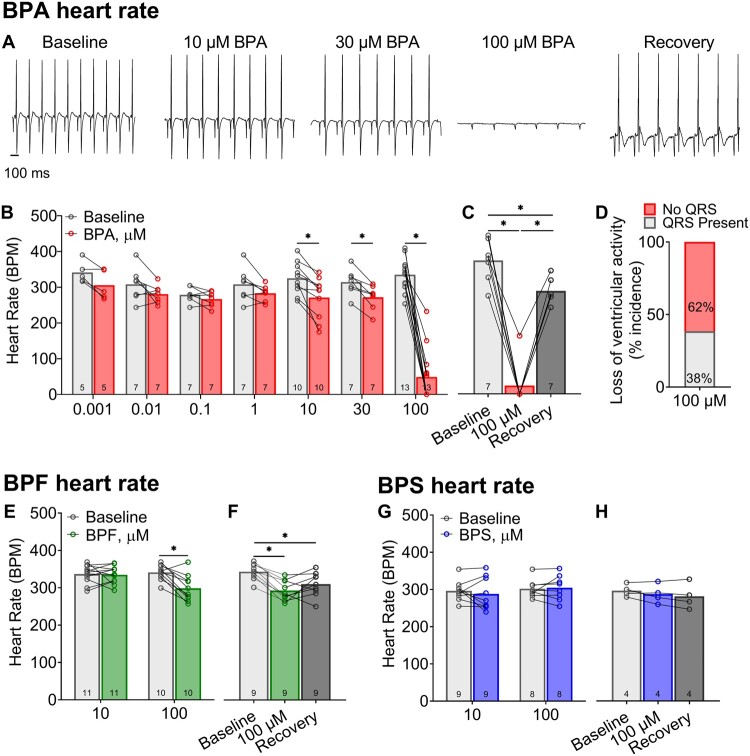
BPA and BPF Exposures Slow Heart Rate
To aid in the translation of our in vitro findings, we quantified the acute effects of BPA, BPF, and BPS on cardiac electrophysiology using an ex vivo intact heart preparation. Heart preparations exhibited normal sinus rhythm when perfused with control KH media (327.7 ± 36.8 BPM) and KH media supplemented with vehicle (327.6 ± 40.3 BPM; Figure 1C). BPA exposure resulted in a measurable decline in heart rate, which may be partly attributed to calcium channel current inhibition. Sinus rate slowed by 16.6% (q < 0.01) and 85.4% (q < 0.0001) after exposure to 10 and 100 µM BPA, respectively (relative to baseline recording; Figs. 5A and 5B). This depressive effect culminated in the cessation of ventricular electrical activity in 62% of heart preparations treated with 100 µM BPA (Figure 5D). Heart rate slowing was immediate, yet reversible, as sinus rhythm recovered quickly after removal of BPA and replacement with control media perfusion. A nonmonotonic BPA dose-response relationship was not observed. Heart rate also slowed by 12.5% (q < 0.001) after exposure to 100 µM BPF, the only dose to significantly affect automaticity (Figure 5E). Conversely, no significant change in sinus rhythm or rate was observed after exposure to BPS at the concentrations tested (Figure 5G).
Figure 5.
Bisphenol chemical effects on heart rate. A, Representative ECG recordings from Langendorff-perfused hearts at baseline, acute (15 min) exposure to BPA, or recovery (100 µM BPA exposure, followed by 15 min washout). B, BPA exposure results in sinus rate slowing, beginning at 10 µM BPA. C, Heart rate slowing after 100 µM BPA exposure largely recovers after washout (15 min). D, Heart rate measurements at high BPA doses were confounded by intermittent 3rd degree heart block, with loss of ventricular electrical activity. E, Heart rate slowing following BPF exposure occurred at the highest concentration tested (100 µM BPF). F, Heart rate slowing after 100 µM BPF recovered slightly after washout. G and H, BPS exposure had no discernable effect on heart rate. Values reported as mean ± SD. *q < 0.05 as determined by RM-ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph.
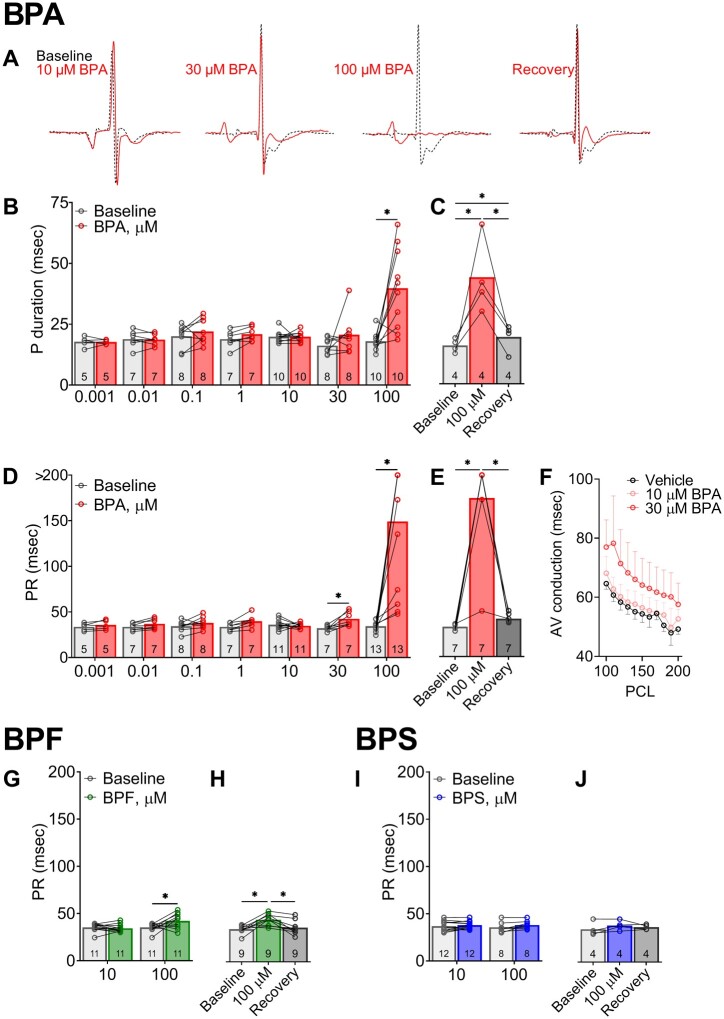
BPA and BPF Exposures Slow AV Conduction
Heart preparations exhibited stable atrial and AV conduction with control media perfusion (15.3 ± 2.8 ms P duration, 33.6 ± 1.4 ms PR interval), and during perfusion with media supplemented with vehicle (16.3 ± 4.0 msec P duration, 35.3 ± 3.1 ms PR interval). Concurrent with heart rate slowing due to BPA exposure, we also observed significant lengthening of both the P duration and PR interval. A prolonged P duration was only observed at the highest BPA dose (39.8 ± 16.6 msec at 100 µM BPA, Figure 6B), whereas the PR interval progressively lengthened with higher BPA concentrations (Figs. 6A and 6D). BPA exposure resulted in variable degrees of AV block, ranging from 1st degree to intermittent 3rd degree AV block (Figure 6D). Notably, the acute effect of BPA on AV conduction was reversible with a rapid recovery of the PR interval time after 15-min washout (Figure 6E). Atrial pacing was implemented, and AV conduction slowing persisted at multiple PCL (Figure 6F). BPF exposure also lengthened the PR interval, albeit the effect was much less pronounced (+19.6% 100 µM BPF). There was no observable change in AV conduction following BPS exposure at the tested concentrations (Figure 6I).
Figure 6.
Bisphenol chemical effects on atrial and atrioventricular conduction during sinus rhythm. A, Representative ECG waveform from Langendorff-perfused hearts at baseline, acute BPA exposure (15 min), or recovery (100 µM BPA exposure, followed by 15 min washout). Each waveform pair recorded from the same animal, before and after exposure. B, P duration indicates longer atrial depolarization time at the highest BPA dose (100 µM). C, Slowed atrial conduction after 100 µM BPA exposure recovers after washout. D, The progressive lengthening of PR duration indicates slowed AV conduction following 30–100 µM BPA exposure, often resulting in intermittent 3rd degree heart block (denoted by data point > 200 ms). E, AV conduction slows after 100 µM BPA exposure and recovers after washout. F, AV conduction slowing persists with external pacing to correct for heart rate. G, Atrioventricular conduction slowing occurs at 100 µM BPF concentration and (H) recovers after washout. I and J, BPS exposure had no discernable effect on atrioventricular conduction time. Values reported as mean ± SD. *q < 0.05 as determined by RM-ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph. Abbreviation: PCL, pacing cycle length.
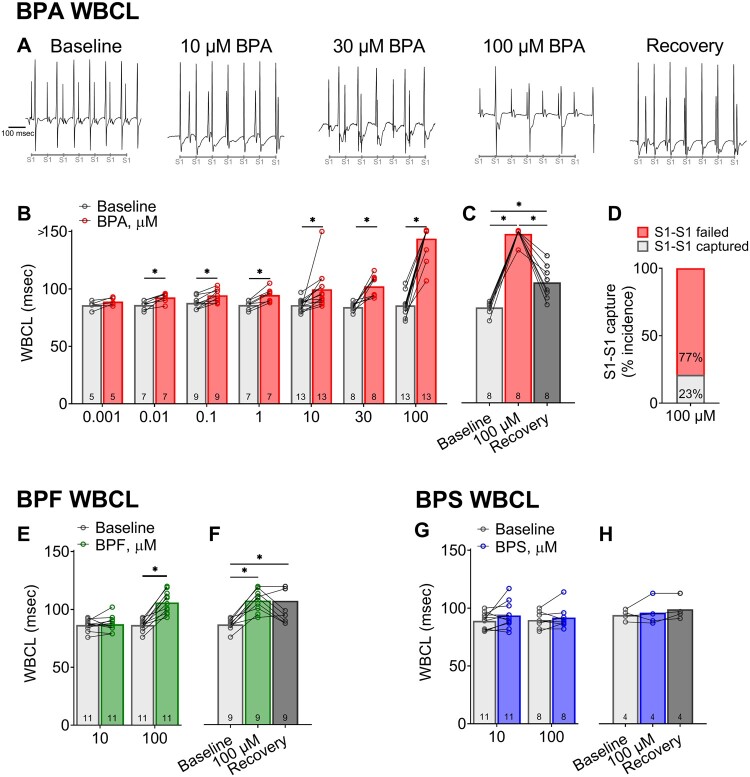
BPA and BPF Exposures Increase AV Nodal Refractoriness
To further investigate slowed AV conduction in the presence of bisphenols, incremental atrial pacing was implemented to pinpoint the Wenckebach phenomenon. WBCL was comparable between control media perfusion (87 ± 6.2 msec) and during perfusion with vehicle (90 ± 6.5 msec). However, it proved to be a highly sensitive parameter for bisphenol-induced slowing of AV conduction (Figs. 7A and 7B). BPA exposure altered WBCL in a dose-dependent manner beginning at a low nanomolar concentration (+7.6% 0.01 µM BPA [q < 0.1]; +68% 100 µM BPA [q < 0.0001]), suggesting a lengthening of the relative refractory period. Pinpointing an accurate WBCL after 100 µM BPA exposure was confounded by the loss of capture at the slowest cycle length tested (150 ms; Figure 7C). BPF exposure had a moderate effect on WBCL, but only at the highest concentration tested (22.5% 100 µM BPF [q < 0.0001]). BPS exposure did not alter WBCL at the tested concentrations, which was in agreement with PR interval measurements during sinus rhythm (Figure 7G). Extrastimulus pacing was also performed to measure the effective refractory period of the AV node. Similar to WBCL measurements, BPA exposure increased AV node refractoriness in a dose-dependent manner (Figs. 8A and 8B), beginning with modest changes at a low nanomolar concentration (+9.2% 0.001 µM BPA [q < 0.05]) and increasing thereafter (+95.7% 100 µM BPA [q < 0.0001]). BPF exposure had a moderate effect on AVNERP, but only at the highest concentration tested (20.7% 100 µM BPF [q < 0.0001]). BPS exposure did not alter AVNERP at the tested concentrations (Figure 8G).
Figure 7.
Bisphenol chemical effects on atrioventricular conduction in response to atrial pacing. A, Representative ECG recordings during atrial pacing show failure to capture in BPA-treated hearts, indicating slowed atrioventricular conduction. The timing of S1-S1 pulses (90 msec) is indicated below. B, Longer Wenckebach cycle length (WBCL) following exposure to BPA concentrations (0.01–100 µM), as compared with baseline. Note: Complete Heart block denoted by measurement > 150 msec (longest S1 pacing interval tested). C, Longer WBCL after 100 µM BPA exposure largely recovers after 15-min washout. D, WBCL measurements at high BPA doses were confounded by complete heart block. E, 100 µM BPF exposure results in longer WBCL. F, Moderate recovery in atrioventricular conduction after BPF washout. G and H, No change in WBCL was observed after exposure to 10–100 µM BPS. Values reported as mean ± SD. *q < 0.05 as determined by RM-ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph. Abbreviation: ECG, electrocardiogram.
Figure 8.
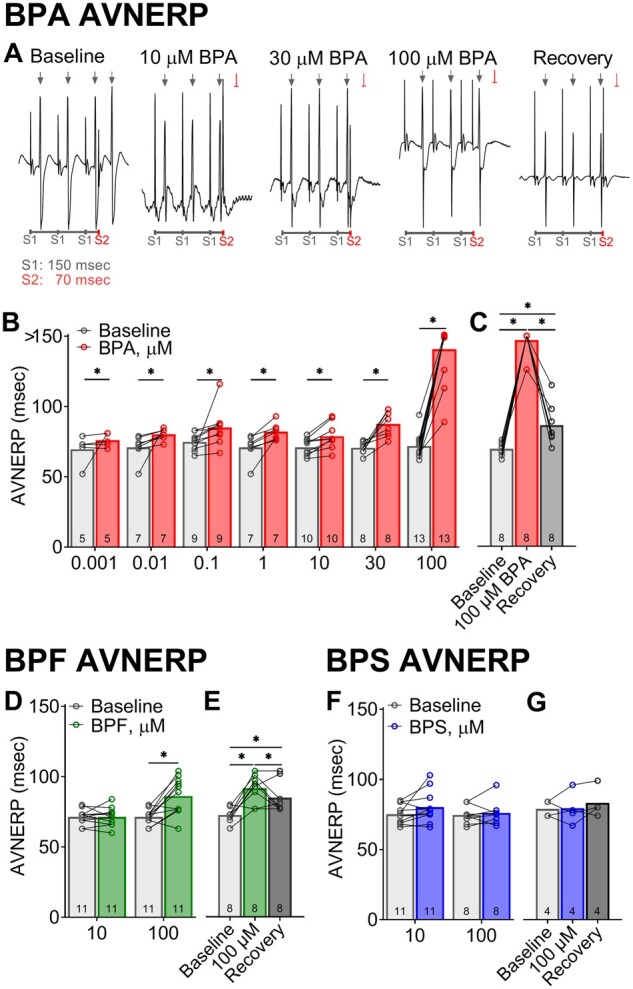
Bisphenol chemical effects on atrioventricular nodal refractoriness in response to atrial pacing. A, Representative ECG recordings during atrial pacing show capture (↓) and failure to capture ( ) in response to S1-S2 pacing (150, 70 ms). B, Longer atrioventricular nodal effective refractory period (AVNERP) following exposure to BPA concentrations (1–100 µM), as compared with baseline. Note: Complete heart block denoted by measurement > 150 msec (S1 pacing interval). C, Longer AVNERP after 100 µM BPA exposure recovers after 15-min washout. D, BPF exposure results in longer AVNERP at the highest dose tested. E, Moderate recovery of AVNERP after BPF washout. F and G, No change in AVNERP was observed after exposure to 10–100 µM BPS. Values reported as mean ± SD. *q < 0.05 as determined by RM-ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph.
) in response to S1-S2 pacing (150, 70 ms). B, Longer atrioventricular nodal effective refractory period (AVNERP) following exposure to BPA concentrations (1–100 µM), as compared with baseline. Note: Complete heart block denoted by measurement > 150 msec (S1 pacing interval). C, Longer AVNERP after 100 µM BPA exposure recovers after 15-min washout. D, BPF exposure results in longer AVNERP at the highest dose tested. E, Moderate recovery of AVNERP after BPF washout. F and G, No change in AVNERP was observed after exposure to 10–100 µM BPS. Values reported as mean ± SD. *q < 0.05 as determined by RM-ANOVA with multiple comparisons testing (0.1 FDR). Number of replicates indicated in each bar graph.
DISCUSSION
This is the first study to compare the acute effects of BPA, BPS, and BPF on cardiac electrophysiology using both in vitro and ex vivo cardiac preparations. In the described study, we demonstrate that BPA is the most potent inhibitor of sodium, calcium, and potassium channel currents—as compared with the chemical alternatives, BPS and BPF. In the context of industrial or clinical environments, individuals can present with urinary BPA concentrations that are exceedingly high—reaching 4–8 µM (Calafat et al., 2009; He et al., 2009; Wang et al., 2012). Accordingly, we tested a range of concentrations that encompass environmental, clinical, occupational, and supraphysiological exposures (Bousoumah et al., 2021; Ramadan et al., 2020). We found that BPA exerted the greatest effect on automaticity and AV conduction—which may be mediated by inhibitory effects on calcium current. Electrical disturbances were largely focused on nodal and AV conduction, with negligible effects on cardiac repolarization or arrhythmia susceptibility. Results of this study suggest that acute BPS exposure is less disruptive to cardiac electrophysiology, as compared with BPA or BPF. Epidemiological or clinical studies are needed to investigate an association between bisphenol chemical exposure and bradycardia or slowed AV conduction; moreover, bisphenol chemical exposure could exert an additive effect in patients with subclinical AV node abnormalities, ischemic conditions, or in patients being treated for cardiac arrhythmias (eg, beta-blocker usage). Additional mechanistic studies are required to fully elucidate the safety profile of bisphenol chemicals on cardiac electrical and mechanical function, and report on the chronic effects of bisphenol exposure.
Bisphenol Chemicals and Calcium Ion Homeostasis
Of the bisphenol chemicals tested in this study, BPA was the most potent inhibitor of L-type calcium channels with an IC50 = 30.8 µM. This finding is in agreement with the literature, which reported an immediate inhibitory effect of BPA on T-type calcium channels in human embryonic kidney cells (IC50 = 6–33 µM, depending on channel subtype [Michaela et al., 2014]). Similarly, Deutschmann et al. (2013) reported that BPA rapidly and reversibly inhibited calcium current through L-, N-, P/Q-, R-, and T-type calcium channels in rat endocrine cells, dorsal root ganglion, cardiomyocytes, and transfected human embryonic kidney cells (IC50 = 26–35 µM). Studies suggest that the inhibitory effects of bisphenol chemicals on calcium channel current are influenced by the chemical structure and bridge between the 2 phenol rings, with reduced inhibitory effects anticipated for BPS and BPF (Deutschmann et al., 2013). In cardiac tissue, calcium channels play an important role in nodal cell depolarization, AV conduction, the plateau phase of the cardiac action potential, and contractility. Indeed, recent studies have shown that BPA can alter cardiac electrophysiology, likely through a calcium-dependent mechanism. Sinus bradycardia and delayed electrical conduction have been reported after BPA exposure, using in vivo and ex vivo models (Belcher et al., 2015; Patel et al., 2015; Posnack et al., 2014; Valokola et al., 2019). Patel et al. (2015) observed conduction slowing in BPA-exposed animals subjected to catecholamine stress, although this effect was limited to females. BPA-induced heart rate slowing was also reported in in vivo studies conducted by Belcher et al. (2015), although the authors noted that this effect might be attributed to autonomic dysregulation. In addition to electrophysiology disturbances, BPA exposure has been shown to alter intracellular calcium handling, which can increase calcium leak from the sarcoplasmic reticulum (Yan et al., 2011), increase the incidence of calcium-mediated arrhythmias, and precipitate calcium amplitude alternans (Ramadan et al., 2018). Studies suggest that intracellular calcium handling may be influenced by post-translational modifications of key calcium proteins via an estrogen-mediated mechanism (Liang et al., 2014).
Bisphenol Chemicals and Sodium Channel Current
Similar to calcium channel inhibition, we found that BPA was the most potent inhibitor of fast (INa-P) and late (INa-L) sodium channel (IC50 = 55.3 and 23.6 µM, respectively). This finding is in agreement with previously published studies, which reported that BPA blocks fast voltage-gated sodium channels in transfected HEK cells (IC50 = 25 µM; O’Reilly et al., 2012) and isolated dorsal root ganglion neurons (IC50 = 40 mM; Wang et al., 2011). These effects were rapid, reversible, and dose-dependent (Wang et al., 2011). Moreover, in isolated ganglion neurons, the described effects were attenuated with protein kinase A (PKA) or protein kinase C (PKC) inhibitors, suggesting an underlying protein kinase-dependent pathway. In cardiac tissue, the fast voltage-gated sodium channel (INa-P; tetrodotoxin-sensitive) is responsible for the action potential upstroke, and blockade is likely to reduce the rate of depolarization and slow conduction velocity. Late-sodium channel current (tetrodotoxin-insensitive) is active during the action potential plateau phase, and blockade is expected to shorten repolarization time (Horváth et al., 2020). This highlights the importance of performing electrophysiology studies using cardiac models (eg, human cardiomyocytes, isolated whole heart, in vivo studies) in conjunction with whole-cell recordings, given the dynamic nature of cardiac electrophysiology.
Bisphenol Chemicals and Estrogen-Receptor Signaling
Since BPA is classified as a xenoestrogen, alterations in cardiac function may also be attributed to its interaction with estrogen receptors. In the presented study, we utilized female cardiac preparations because previous reports have indicated that BPA-induced effects on calcium handling and electrical instabilities can be heightened in female animals, exacerbated in the presence of estradiol, and attenuated in estrogen-receptor-beta knockout animals (Belcher et al., 2012; Yan et al., 2011). Studies have shown that 17β-estradiol alone can rapidly and reversibly inhibit sodium and calcium channels in a concentration-dependent manner (Lee et al., 2002; Wang et al., 2013). Accordingly, the effects of BPA on cardiac electrophysiology may be mediated by both direct interaction with ion channels at the cell membrane and/or intracellular signaling pathways precipitated by estrogen-receptor binding. Although BPS and BPF have also been shown to display estrogenic activity comparable to BPA (Kojima et al., 2019; Moreman et al., 2017), in the presented study, we identified clear differences in the potency of BPA, BPS, and BPF as it relates to ion channel inhibition and cardiac electrophysiology parameters.
Cardiotoxic Profile of BPA Analogs
Biomonitoring studies have recently reported an uptick in BPS and BPF exposure in the general population, as manufacturers begin to phase out and replace BPA in some consumer and medical products. For example, data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES) detected BPA, BPS, and BPF in 96%, 90%, and 67% of urinary samples from the general population (Lehmler et al., 2018). Nevertheless, very little is known about the effects of these substitute chemicals on cardiovascular health, and whether they offer a superior safety profile. Using a zebrafish model, Qiu et al. (2020) reported that BPS exposure results in transcriptional changes that can increase inflammation, alter cardiac morphology, and decrease heart rate. In a rodent cardiac model, BPS-treatment alone was shown to increase heart rate, whereas the addition of catecholamine stress increased the propensity for premature ventricular contractions and calcium-mediated triggered activity (Gao et al., 2015). The authors noted that the observed effect on cardiac electrophysiology was sex-specific and mediated via estrogen-receptor-beta signaling, which alters the phosphorylation status of key calcium handling proteins. Notably, the same group has reported nearly identical effects with BPA treatment, which suggests that the 2 chemicals may act via a common mechanism (Yan et al., 2011). Variable results between species could be attributed to intrinsic differences in ion channel expression and/or ectothermic regulation of zebrafish. In a separate study by Ferguson et al. (2019), BPS or BPA-treatment rapidly reduced mechanical function in heart preparations, but slightly different post-translational modifications were observed in myofilament proteins. Investigations into the cardiac effects of BPF are even more limited, with a single report noting a decrease in the heart rate of zebrafish following BPF-exposure (Mu et al., 2019). The current study was focused on cardiac electrophysiology outcomes; therefore, future work is still needed to assess the impact of BPA, BPF, and BPS on myocardial contractility.
To the best of our knowledge, our study is the first to compare the acute effects of BPA, BPS, and BPF exposure on cardiac ion channels and electrophysiology. We aimed to identify the IC50 concentrations for BPA, BPS, and BPF on key cardiac ion channels highlighted by the CiPA initiative—and validate the effect of those concentrations on human cardiomyocyte and intact heart preparations. Collectively, we observed that BPA exposure has a more potent effect on cardiac electrophysiology, as compared with the chemical substitutes BPF and BPS. Our results suggest that BPS may be a reasonable alternative, particularly for medical devices used to treat vulnerable patient populations at increased risk for bisphenol chemical exposure. Nevertheless, limitations to our study should be considered. First, we included a number of models in our study, but additional mechanistic work is necessary to fully elucidate the safety profile of bisphenol chemicals—including the impact on intracellular targets, genomic and proteomic expression profiles (subacute or chronic studies), and further investigation of in vivo conditions with crosstalk between systems (autonomic regulation, metabolism, hormonal fluctuations). Second, there are notable differences in cardiac electrophysiology between species (eg, ion channel expression, sinus rate, action potential morphology), which should be noted when considering the translation of experimental studies to humans. Third, our study was focused on the acute effects of bisphenol chemicals on cardiac electrophysiology and the chronic impact of such exposures remains unclear. Finally, future work is necessary to investigate a full range of bisphenol chemical exposures beyond those reported here—as endocrine-disrupting chemicals can present with a nonmonotonic dose response (Birnbaum, 2012; Gao et al., 2015; Liang et al., 2014; Vandenberg, 2014).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by the National Institutes of Health (R01HL139472 to N.G.P.), Children’s National Heart Institute, Sheikh Zayed Institute for Pediatric Surgical Innovation, and the Children’s National Research Institute. This publication was also supported by the Gloria and Steven Seelig family.
REFERENCES
- Asakura K., Hayashi S., Ojima A., Taniguchi T., Miyamoto N., Nakamori C., Nagasawa C., Kitamura T., Osada T., Honda Y., et al. (2015). Improvement of acquisition and analysis methods in multi-electrode array experiments with iPS cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 75, 17–26. [DOI] [PubMed] [Google Scholar]
- Bae S., Hong Y.-C. (2015). Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hypertension 65, 313–319. [DOI] [PubMed] [Google Scholar]
- Bae S., Kim J. H., Lim Y.-H., Park H. Y., Hong Y.-C. (2012). Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension 60, 786–793. [DOI] [PubMed] [Google Scholar]
- Bao W., Liu B., Rong S., Dai S. Y., Trasande L., Lehmler H. J. (2020). Association between bisphenol A exposure and risk of all-cause and cause-specific mortality in US adults. JAMA Netw. Open 3, e2011620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher S. M., Chen Y., Yan S., Wang H.-S. S. (2012). Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology 153, 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher S. M., Gear R. B., Kendig E. L. (2015). Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology 156, 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Krieger A. M., Yekutieli D. (2006). Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93, 491–507. [Google Scholar]
- Ben-Jonathan N., Steinmetz R. (1998). Xenoestrogens: The emerging story of bisphenol A. Trends Endocrinol. Metab. 9, 124–128. [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S. (2012). Environmental chemicals: Evaluating low-dose effects. Environ. Health Perspect. 120, a143–a144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova K., Stohlman J., Vicente J., Chan D., Johannesen L., Hortigon-Vinagre M. P., Zamora V., Smith G., Crumb W. J., Pang L., et al. (2017). Comprehensive translational assessment of human-induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol. Sci. 155, 234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousoumah R., Leso V., Iavicoli I., Huuskonen P., Viegas S., Porras S. P., Santonen T., Frery N., Robert A., Ndaw S. (2021). Biomonitoring of occupational exposure to bisphenol A, bisphenol S and bisphenol F: A systematic review. Sci. Total Environ. 783, 146905. [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Kuklenyik Z., Reidy J. A., Caudill S. P., Ekong J., Needham L. L. (2005). Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 113, 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M., Weuve J., Ye X., Jia L. T., Hu H., Ringer S., Huttner K., Hauser R. (2009). Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 117, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Kannan K., Tan H., Zheng Z., Feng Y.-L., Wu Y., Widelka M. (2016a). Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 50, 5438–5453. [DOI] [PubMed] [Google Scholar]
- Chen I. Y., Matsa E., Wu J. C. (2016b). Induced pluripotent stem cells: At the heart of cardiovascular precision medicine. Nat. Publ. Gr. 13, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. (2016). Multielectrode array (MEA) assay for profiling electrophysiological drug effects in human stem cell-derived cardiomyocytes. Curr. Protoc. Toxicol. 68, 22.4.1–22.4.32. [DOI] [PubMed] [Google Scholar]
- Colatsky T., Fermini B., Gintant G., Pierson J. B., Sager P., Sekino Y., Strauss D. G., Stockbridge N. (2016). The comprehensive in vitro proarrhythmia assay (CiPA) initiative—Update on progress. J. Pharmacol. Toxicol. Methods 81, 15–20. [DOI] [PubMed] [Google Scholar]
- Deutschmann A., Hans M., Meyer R., Häberlein H., Swandulla D. (2013). Bisphenol A inhibits voltage-activated Ca(2+) channels in vitro: Mechanisms and structural requirements. Mol. Pharmacol. 83, 501–511. [DOI] [PubMed] [Google Scholar]
- Duty S. M., Mendonca K., Hauser R., Calafat A. M., Ye X., Meeker J. D., Ackerman R., Cullinane J., Faller J., Ringer S. (2013). Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics 131, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. G., Louch W. E. (2017). Species-dependent mechanisms of cardiac arrhythmia: A cellular focus. Clin. Med. Insights. Cardiol. 11, 1179546816686061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiteiro J., Mariana M., Glória S., Cairrao E. (2018). Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci. 43, 579–586. [DOI] [PubMed] [Google Scholar]
- Ferguson M., Lorenzen-Schmidt I., Pyle W. G. (2019). Bisphenol S rapidly depresses heart function through estrogen receptor-β and decreases phospholamban phosphorylation in a sex-dependent manner. Sci. Rep. 9, 15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Liang Q., Chen Y., Wang H.-S. S. (2013). Molecular mechanisms underlying the rapid arrhythmogenic action of bisphenol A in female rat hearts. Endocrinology 154, 4607–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Ma J., Chen Y., Wang H.-S. (2015). Rapid responses and mechanism of action for low-dose bisphenol S on ex vivo rat hearts and isolated myocytes: Evidence of female-specific proarrhythmic effects. Environ. Health Perspect. 123, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor J. W., Ittenbach R. F., Calafat A. M., Burnham N. B., Bradman A., Bellinger D. C., Henretig F. M., Wehrung E. E., Ward J. L., Russell W. W., et al. (2019). Perioperative exposure to suspect neurotoxicants from medical devices in newborns with congenital heart defects. Ann. Thorac. Surg. 107, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear R., Kendziorski J. A., Belcher S. M. (2017). Effects of bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: A CLARITY-BPA study. Toxicol. Lett. 275, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Hong Y.-C. (2016). Bisphenol A, hypertension, and cardiovascular diseases: Epidemiological, laboratory, and clinical trial evidence. Curr. Hypertens. Rep. 18, 11. [DOI] [PubMed] [Google Scholar]
- He Y., Miao M., Wu C., Yuan W., Gao E., Zhou Z., Li D.-K. (2009). Occupational exposure levels of bisphenol A among Chinese workers. J. Occup. Health 51, 432–436. [DOI] [PubMed] [Google Scholar]
- Hines C. J., Christianson A. L., Jackson M. V., Ye X., Pretty J. R., Arnold J. E., Calafat A. M. (2018). An evaluation of the relationship among urine, air, and hand measures of exposure to bisphenol A (BPA) in US manufacturing workers. Ann. Work Expo. Heal. 62, 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth B., Hézső T., Kiss D., Kistamás K., Magyar J., Nánási P. P., Bányász T. (2020). Late sodium current inhibitors as potential antiarrhythmic agents. Front. Pharmacol. 11, 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygh J., Clotman K., Malarvannan G., Covaci A., Schepens T., Verbrugghe W., Dirinck E., Van Gaal L., Jorens P. G. (2015). Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ. Int. 81, 64–72. [DOI] [PubMed] [Google Scholar]
- Iribarne-Durán L. M., Artacho-Cordón F., Peña-Caballero M., Molina-Molina J. M., Jiménez-Díaz I., Vela-Soria F., Serrano L., Hurtado J. A., Fernández M. F., Freire C., et al. (2019). Presence of bisphenol A and parabens in a neonatal intensive care unit: An exploratory study of potential sources of exposure. Environ. Health Perspect. 127, 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes R., McCullough D., Siegel B., Swift L., McInerney D., Hiebert J., Perez-Alday E. A., Trenor B., Sheng J., Saiz J., et al. (2019). Plasticizer interaction with the heart: Chemicals Used in plastic medical devices can interfere with cardiac electrophysiology. Circ. Arrhythmia Electrophysiol. 12, e007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. M., Calafat A. M. (2009). Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. B Biol. Sci. 364, 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Takeuchi S., Sanoh S., Okuda K., Kitamura S., Uramaru N., Sugihara K., Yoshinari K. (2019). Profiling of bisphenol A and eight of its analogues on transcriptional activity via human nuclear receptors. Toxicology 413, 48–55. [DOI] [PubMed] [Google Scholar]
- Lee D., Chai Y., Lee E., Kim K., Nah S., Oh T., Rhim H. (2002). 17Beta-estradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 70, 2047–2059. [DOI] [PubMed] [Google Scholar]
- Lehmler H. J., Liu B., Gadogbe M., Bao W. (2018). Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. adults and children: The National Health and Nutrition Examination Survey 2013-2014. ACS Omega 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q., Gao X., Chen Y., Hong K., Wang H.-S. S. (2014). Cellular mechanism of the nonmonotonic dose response of bisphenol A in rat cardiac myocytes. Environ. Health Perspect. 122, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantegazza M., Franceschetti S., Avanzini G. (1998). Anemone toxin (ATX II)-induced increase in persistent sodium current: Effects on the firing properties of rat neocortical pyramidal neurones. J. Physiol. 507, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D., Osborne N. J., Henley W. E., Cipelli R., Young A., Money C., McCormack P., Luben R., Khaw K. T., Wareham N. J., et al. (2012). Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 125, 1482–1490. [DOI] [PubMed] [Google Scholar]
- Melzer D., Rice N. E., Lewis C., Henley W. E., Galloway T. S. (2010). Association of urinary bisphenol a concentration with heart disease: Evidence from NHANES 2003/06. PLoS One 5, e8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaela P., Mária K., Silvia H., Ľubica L., L’ubica L. (2014). Bisphenol A differently inhibits CaV3.1, Ca V3.2 and Ca V3.3 calcium channels. Naunyn Schmiedebergs Arch. Pharmacol. 387, 153–163. [DOI] [PubMed] [Google Scholar]
- Moon M. K. (2019). Concern about the safety of bisphenol A substitutes. Diabetes Metab. J. 43, 46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreman J., Lee O., Trznadel M., David A., Kudoh T., Tyler C. R. (2017). Acute toxicity, teratogenic, and estrogenic effects of bisphenol A and its alternative replacements bisphenol S, bisphenol F, and bisphenol AF in zebrafish embryo-larvae. Environ. Sci. Technol. 51, 12796–12805. [DOI] [PubMed] [Google Scholar]
- Mu X., Liu J., Yuan L., Yang K., Huang Y., Wang C., Yang W., Shen G., Li Y. (2019). The mechanisms underlying the developmental effects of bisphenol F on zebrafish. Sci. Total Environ. 687, 877–884. [DOI] [PubMed] [Google Scholar]
- O’Reilly A. O., Eberhardt E., Weidner C., Alzheimer C., Wallace B. A., Lampert A. (2012). Bisphenol a binds to the local anesthetic receptor site to block the human cardiac sodium channel. PLoS One 7, e41667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B. B., Raad M., Sebag I. A., Chalifour L. E. (2015). Sex-specific cardiovascular responses to control or high fat diet feeding in C57bl/6 mice chronically exposed to bisphenol A. Toxicol. Rep. 2, 1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack N. G. (2014). The adverse cardiac effects of di(2-ethylhexyl)phthalate and bisphenol A. Cardiovasc. Toxicol. 14, 339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack N. G., Brooks D., Chandra A., Jaimes R., Sarvazyan N., Kay M. W. (2015). Physiological response of cardiac tissue to Bisphenol A: Alterations in ventricular pressure and contractility. Am. J. Physiol. Heart Circ. Physiol. 309, H267–H275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnack N. G., Jaimes R., Asfour H., Swift L. M., Wengrowski A. M., Sarvazyan N., Kay M. W., Jaimes R., Asfour H., Swift L. M. (2014). Bisphenol A exposure and cardiac electrical conduction in excised rat hearts. Environ. Health Perspect. 122, 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PR newswire. (2016) Global Bisphenol-A Market Overview 2016-2022. Available at: https://www.prnewswire.com/news-releases/bisphenol-a--a-global-market-overview-300282092.html.
- Qiu W., Chen B., Greer J. B., Magnuson J. T., Xiong Y., Zhong H., Andrzejczyk N. E., Zheng C., Schlenk D. (2020). Transcriptomic responses of bisphenol S predict involvement of immune function in the cardiotoxicity of early life-stage zebrafish (Danio rerio). Environ. Sci. Technol. 54, 2869–2877. [DOI] [PubMed] [Google Scholar]
- Ramadan M., Cooper B., Posnack N. G. (2020). Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res. 112, 1362–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan M., Sherman M., Jaimes R., Chaluvadi A., Swift L., Posnack N. G. N. G., Posnack N. G. N. G. (2018). Disruption of neonatal cardiomyocyte physiology following exposure to bisphenol-A. Sci. Rep. 8, 7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly M., Bruno C. D., Prudencio T. M.Ciccarelli N., Guerrelli D., Nair R., Ramadan M., Luban N. L. C., Posnack N. G. (2020). Potential consequences of the red blood cell storage lesion on cardiac electrophysiology. J. Am. Heart Assoc. 9, e017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro E., Ladeira C., Viegas S. (2017). Occupational exposure to bisphenol A (BPA): A reality that still needs to be unveiled. Toxics 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager P. T., Gintant G., Turner J. R., Pettit S., Stockbridge N. (2014). Rechanneling the cardiac proarrhythmia safety paradigm: A meeting report from the Cardiac Safety Research Consortium. Am. Heart J. 167, 292–300. [DOI] [PubMed] [Google Scholar]
- Shang J., Corriveau J., Champoux-Jenane A., Gagnon J., Moss E., Dumas P., Gaudreau E., Chevrier J., Chalifour L. E. (2019). Recovery from a myocardial infarction is impaired in male C57bl/6 N mice acutely exposed to the bisphenols and phthalates that escape from medical devices used in cardiac surgery. Toxicol. Sci. 168, 78–94. [DOI] [PubMed] [Google Scholar]
- Shelby M. D. (2008). NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR MON vvii–ix. 1–64 passim. [PubMed] [Google Scholar]
- Strømmen K., Lyche J. L., Moltu S. J., Müller M. H. B., Blakstad E. W., Almaas A. N., Sakhi A. K., Thomsen C., Nakstad B., Rønnestad A. E., et al. (2021). High urinary concentrations of parabens and bisphenol A in very low birth weight infants. Chemosphere 271, 129570. [DOI] [PubMed] [Google Scholar]
- Swift L., Jaimes R., McCullough D., Burke M., Reilly M., Maeda T., Zhang H., Ishibashi N., Rogers J., Posnack N. G. (2019). Optocardiography and electrophysiology studies of ex vivo Langendorff-perfused hearts. J. Vis. Exp. 153, doi: 10.3791/60472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift L. M., Burke M., Guerrelli D., Reilly M., Ramadan M., McCullough D., Prudencio T., Mulvany C., Chaluvadi A., Jaimes R., et al. (2020). Age-dependent changes in electrophysiology and calcium handling: Implications for pediatric cardiac research. Am. J. Physiol. Circ. Physiol. 318, H354–H365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testai E., Ms Scientific Committee SCENIHR. Electronic address: SANTE-C2-SCENIHR@ec.europa.eu, Hartemann P., Rodríguez-Farre E., Rastogi S. C., Bustos J., Gundert-Remy U., Hensten A., Kopperud H. M., Olea N., Piersma A., et al. (2016) The safety of the use of bisphenol A in medical devices. Regul. Toxicol. Pharmacol. 79, 106–107. [DOI] [PubMed]
- Trasande L. (2017). Exploring regrettable substitution: Replacements for bisphenol A. Lancet Planet. Health 1, e88–e89. [DOI] [PubMed] [Google Scholar]
- Valokola M. G., Karimi G., Razavi B. M., Kianfar M., Jafarian A. H., Jaafari M. R., Imenshahidi M. (2019). The protective activity of nanomicelle curcumin in bisphenol A-induced cardiotoxicity following subacute exposure in rats. Environ. Toxicol. 34, 319–329. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N. (2014). Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose Response 12, 259–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Chahoud I., Heindel J. J., Padmanabhan V., Paumgartten F. J. R., Schoenfelder G. (2010). Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 118, 1055–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Hauser R., Marcus M., Olea N., Welshons W. V. (2007). Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Wang F., Hua J., Chen M., Xia Y., Zhang Q., Zhao R., Zhou W., Zhang Z., Wang B. (2012). High urinary bisphenol A concentrations in workers and possible laboratory abnormalities. Occup. Environ. Med. 69, 679–684. [DOI] [PubMed] [Google Scholar]
- Wang Q., Cao J., Hu F., Lu R., Wang J., Ding H., Gao R., Xiao H. (2013). Effects of estradiol on voltage-gated sodium channels in mouse dorsal root ganglion neurons. Brain Res. 1512, 1–8. [DOI] [PubMed] [Google Scholar]
- Wang Q., Cao J., Zhu Q., Luan C., Chen X., Yi X., Ding H., Chen J., Cheng J., Xiao H. (2011). Inhibition of voltage-gated sodium channels by bisphenol A in mouse dorsal root ganglion neurons. Brain Res. 1378, 1–8. [DOI] [PubMed] [Google Scholar]
- Yan S., Chen Y., Dong M., Song W., Belcher S. M., Wang H. S. (2011). Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 6, e25455. [DOI] [PMC free article] [PubMed] [Google Scholar]