Abstract
High-resolution non-invasive cetacean tagging systems can be used to investigate the influence of habitat characteristics and management factors on behavior by quantifying activity levels and distance traveled by bottlenose dolphins (Tursiops truncatus and Tursiops aduncus) in accredited zoos and aquariums. Movement Tags (MTags), a bio-logging device, were used to record a suite of kinematic and environmental information outside of formal training sessions as part of a larger study titled “Towards understanding the welfare of cetaceans in zoos and aquariums” (colloquially called the Cetacean Welfare Study). The purpose of the present study was to explore if and how habitat characteristics, environmental enrichment programs, and training programs were related to the distance traveled and energy expenditure of dolphins in accredited zoos and aquariums. Bottlenose dolphins in accredited zoos and aquariums wore MTags one day per week for two five-week data collection periods. Overall dynamic body acceleration (ODBA), a proxy for energy expenditure, and average distance traveled per hour (ADT) of 60 dolphins in 31 habitats were examined in relation to demographic, habitat, and management factors. Participating facilities were accredited by the Alliance for Marine Mammal Parks and/or Aquariums and the Association of Zoos & Aquariums. Two factors were found to be related to ADT while six factors were associated with ODBA. The results showed that enrichment programs were strongly related to both ODBA and ADT. Scheduling predictable training session times was also positively associated with ADT. The findings suggested that habitat characteristics had a relatively weak association with ODBA and were not related to ADT. In combination, the results suggested that management practices were more strongly related to activity levels than habitat characteristics.
Introduction
Bio-logging devices are tools which can be used to study animal kinematics and to monitor behavior. Bio-logging refers to the practice of attaching a miniaturized device that records and stores data related to an animal’s movement, behavior, physiology, and/or environment [1]. Bio-logging devices have been used to study the behavior and welfare of domesticated and non-domesticated animals in the wild as well as in farm, zoo, and aquarium environments. In domesticated species, they have been used to predict estrus in cows, quantify grazing patterns in cattle, identify heat stress indicators for swine, and examine welfare in both cattle and pigs [2–5]. In non-domesticated species, they have been used to study recumbency in giraffes under professional care [6] and they have also been used to study the migration patterns [7], nesting behaviors [8], and energy expenditure [9] of wild animals. Wearable tracking systems are an especially valuable tool for studying animals that are often out of view of direct observation such as wild cetaceans that spend their lives underwater and where sediment and algae can occlude them from view [10]. For cetaceans, bio-logging devices provide researchers a means of quantifying the impact of human activities [11–13], tracking behavior change [14–16], locating animals within the habitat [17, 18], capturing acoustics simultaneously with dynamic movement [19], and exploring diving behavior and fine-scale kinematics [20, 21].
High-resolution cetacean tagging systems tend to be non-invasive (e.g., attached via specially designed suction cups for cetaceans), and are designed to limit their effect on the animal’s hydrodynamics [22, 23]. These devices host a suite of onboard sensors that directly measure various kinematic and environmental data including accelerometers, magnetometers, gyroscopes, hydrophones, pressure sensors, thermometers, and global positioning systems. Parameters such as speed, acceleration, depth, diving behavior, and location can be derived from these sensors. The fine-scale data collected from these devices facilitate the investigation of fundamental questions in behavior, biology, and ecology [20, 24–26].
Bio-logging devices have been used broadly to study wild diving mammals [27] but their adoption for research on cetaceans within zoos and aquariums has been limited. Bottlenose dolphins (Tursiops truncatus) have been used to collect biomechanical data during prescribed tasks to characterize the effect of drag from the device on their metabolic rate, stroking effort, and swim speed [26, 28]. Shorter et al. [14] provides an account of an analysis of diving behavior and activity level of bottlenose dolphins under professional care. Using the bio-logging devices, they were able to detect day-scale changes in behavior. Consistent with studies using behavioral observation [29], the dolphins exhibited lower activity levels at night. In addition, they were able to establish time budgets for swimming types and characterize diving patterns.
Bio-logging tags are useful tools designed to quantify and monitor activity levels, which is an important indicator of welfare in combination with other physical and behavioral measures. Activity levels and locomotion are affected by various types of habitats and environmental enrichment and are used as an indicator of animal welfare [30–34]. Activity level increases and decreases may be a contributor to and an indicator of the physical health of an individual. Providing opportunities to exercise (i.e., active behaviors) can reduce the risk of developing illnesses and mitigate complications of current illnesses in several species [35–38]. In contrast, an extremely low activity level (i.e., lethargy) is considered an indicator of poor welfare [39–41]. In addition, locomotion, postural, and gait analyses have been used in welfare assessments to identify movement issues or lameness [42–44]. Bio-logging data has been used to identify specific behaviors, track activity levels over time, create activity budgets, and assess gait patterns [45–48]. Bio-logging tags are helpful tools in examining activity and locomotion because they generate fine-scale data on the movement of an individual. While gait has yet to be used in health assessments in dolphins, recent research has begun to establish bio-logging methods to quantify swimming patterns of bottlenose dolphins [14].
While these studies demonstrate the potential uses of bio-logging data by informing our understanding of dolphins under professional care, previous research has focused on a limited number of dolphins within the same habitat. However, dolphins under professional care live in habitats with a variety of features. They may be professionally managed zoo/aquarium habitats or professionally managed ocean habitats, consist of a single area or be comprised of multiple gated environments, and vary in depth, length, width, and shape. A comparative examination of how different environments may be related to activity is an open question. The purpose of the present study was to use data from a bio-logging device to explore if and how habitat characteristics, environmental enrichment programs, and training programs were related to the distance traveled and energy expenditure of dolphins in accredited zoos and aquariums.
Materials and methods
Ethics statement
This study was authorized by the management at each participating zoo and aquarium and, where applicable, was reviewed and approved by research committees. In addition, the study protocol was reviewed and approved by the U.S. Navy Marine Mammal Program Institutional Animal Care and Use Committee #123–2017.
Subjects and facilities
This study is part of a larger project entitled “Towards Understanding the Welfare of Cetaceans in Zoos and Aquariums” (colloquially called the Cetacean Welfare Study). Zoos and aquariums that were accredited in 2017 by the Alliance for Marine Mammal Parks and Aquariums and the Association of Zoos & Aquariums were eligible for participation in this portion of the larger Cetacean Welfare Study provided they cared for common bottlenose dolphins or Indo-Pacific bottlenose dolphins (Tursiops aduncus). Two animals from each participating facility were selected using a semi-random sampling design in order to create a balanced representation of the study population. In total, data were collected from 65 dolphins at 35 habitats. Participating habitats were located in Bermuda (n = 1), Hong Kong (n = 1), Jamaica (n = 2), Mexico (n = 15), Singapore (n = 1), Spain (n = 1), and the United States (n = 14). Information on the full dataset is given in S1 Appendix including sex, age, and total minutes recorded outside of formal training sessions.
Independent variables
Independent variables were selected to examine a range of habitat characteristics and management factors that could impact animal welfare. Independent variables and definitions are listed in Table 1. Definitions of terms and methods for calculating the synthesized independent variables as well as enrichment types are presented in Lauderdale et al [49].
Table 1. Independent variables included in the analysis.
| Variable | Definition | Values | Type of Variable |
|---|---|---|---|
| Demographic | |||
| Sex | Sex of the dolphin | Male/Female | Factor |
| Age | Age of the dolphin | Years | Covariate |
| Environmental Enrichment | |||
| Enrichment Diversity Index | Enrichment diversity index was created using the Shannon diversity index on the mean number of days each enrichment is provided at the facility | Index | Covariate |
| Enrichment Program Index | Enrichment program index is a standardized factor score created with scores on frequency of enrichment program components used at the facility using a polychoric PCA | Index | Covariate |
| Night Time Enrichment | Mean number of nights in a week that enrichment was provided to the dolphins at the facility | Number of Nights | Covariate |
| Enrichment Schedule | Categorical value indicating how enrichment was scheduled at the facility | Predictable, Semi-Random, Random | Factor |
| Frequency of New Enrichment | Categorical frequency that a facility provided the dolphins with new types/forms of enrichment | Weekly/Monthly, Twice a Year, Yearly/Year+ | Factor |
| Training | |||
| Dolphin Presentations | Mean number of dolphin presentations an individual dolphin participated in each week | Mean Number of Presentations | Covariate |
| Interaction Programs | Mean number of dolphin interaction programs an individual dolphin participated in each week | Mean Number of Interactions | Covariate |
| Training Duration | Mean amount of time each dolphin interacted with an animal care professional for presentations, interaction programs, training sessions, research, or other training activities each week | Hours | Covariate |
| Maximum Number of Interaction Guests | Maximum number of participants allowed for an interaction program for that facility | Number of Participants | Covariate |
| Training Schedule | Categorical variable indicating if the training schedule for the dolphins at that facility was predictable or semi-predictable | Predictable, Semi-Predictable | Factor |
| Habitat Characteristics | |||
| Day Time Spatial Experience | Proportionate volume of water the dolphin had access to based on the percentage of daytime hours spent in different habitats in each five-week data collection period | Megaliter | Covariate |
| Night Time Spatial Experience | Proportionate volume of water the dolphin had access to based on the percentage of night time hours spent in different habitats in each five-week data collection period | Megaliter | Covariate |
| 24 Hour Spatial Experience | Proportionate volume of water the dolphin had access to based on the percentage of hours throughout the entire day spent in different habitats in each five-week data collection period | Megaliter | Covariate |
| Length | The maximum straight length in any direction across any habitat the dolphin had access to in each five-week data collection period | m | Covariate |
| Depth | The maximum depth for any habitat the dolphin had access to in each five-week data collection period | m | Covariate |
| Habitat Type | Categorical variable indicating the dolphin was in a professionally managed zoo/aquarium habitat or a professionally managed ocean habitat | Zoo/Aquarium, Ocean | Factor |
| Number of Habitats | Maximum number of habitats (different enclosures) dolphin had access to in daytime hours during each five-week data collection period | Number of Habitats | Covariate |
| Social Management | Categorical variable indicating the type of social management practice for a dolphin during each five-week data collection period | Same Group, Split/Reunited, Rotated Subgroups | Factor |
| Neighboring Conspecifics | Categorical variable indicating if the dolphin had visual and auditory access to other dolphins without possibility of physical contact during each five-week data collection period | No, Yes | Factor |
Data collection
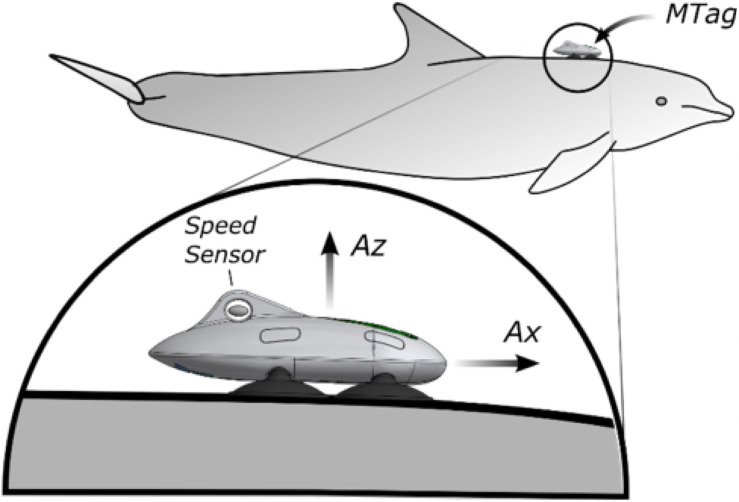
The Movement Tags (MTags) used in the study were 150 mm in length and 76 mm wide with four soft silicone cups (Figs 1 and 2). The primary housing of the MTag was 3D-printed using the FormLabs Form 2™ stereolithography process. The internal electronics were based on the Loggerhead Instruments OpenTag3 board. The OpenTag3 contained a nine degree-of-freedom (DOF) inertial measurement unit (IMU) with an accelerometer, gyroscope, and magnetometer. On-board sensors also recorded environmental temperature and pressure. Speed of the animal through water was measured using a magnetic micro-turbine mounted outside the tag housing and a 1-DOF Hall effect sensor (Fig 2). The IMU data from the accelerometer, gyroscope, and magnetometer were recorded at sampling rate of 50 Hz (i.e., samples per second) and the remaining sensors were recorded at a sampling rate of 5 Hz. The MTag was attached dorsally to the dolphin approximately 20 cm behind the blowhole with four silicone suction cups designed specifically for cetacean skin. Dolphins were trained to wear the MTags prior to the study in order to habituate the focal dolphin and their conspecifics to its presence. The MTags could be easily removed by animal care staff at any time. The MTags did not result in any damage to the skin and similar bio-logging devices have been used extensively with wild dolphins prior to application in this study.
Fig 1. A bottlenose dolphin wearing an MTag.
The MTag was secured to the dolphin approximately 20 cm behind the blowhole using four suction cups.
Fig 2. An illustration of the MTag.
Speed through the water was measured with an externally mounted speed sensor, and acceleration was measured with an internal 3-axis accelerometer.
Data were collected during five-week periods from July 2018 through November 2018 and from January through April 2019. MTags were deployed Tuesdays and Fridays using an alternating schedule for the two participants at each location. Each dolphin wore the MTag throughout its normal daily activities once a week.
Data analysis
Data from the accelerometer, gyroscope, and magnetometer on the MTags were used to estimate pitch, roll, and heading [50]. Relative pitch was calculated from the original pitch data by subtracting the low passed filtered static pitch component using a moving average filter with a window size of 1.5 seconds. Specific acceleration was calculated using information about the orientation of the MTag to subtract the gravitational acceleration measured by the accelerometers. To measure speed through water, the revolution rate of the micro-turbine sensor on the MTag was converted to speed through a linear calibration (slope-intercept). The calibration and verification for this speed sensor configuration is detailed in Gabaldon et al [51]. Measurement of speed through water was numerically integrated to estimate total distance traveled by the animal while wearing the tag. Overall dynamic body acceleration (ODBA) was also calculated from the accelerometer data, and was used to parameterize relative activity of the animal [52]. To quantify activity, a moving average with a two-second window was used on the ODBA values (Fig 3). A representative time series plot of these measurements and metrics is presented in Fig 4. Analyses were conducted in MATLAB 9.7.0 using custom scripts.
Fig 3. Representative summary measures of speed and ODBA.
Data was taken from two dolphins at different facilities. A two second moving average was used to calculate the parameters from ~1 hr of data from each dolphin.
Fig 4. A representative section of swimming data.
Surfacings are indicated with (*), and an example bought of swimming is highlighted. During this example, the animal uses a fluke and glide gait to move through the water.
Statistical analysis
MTag data were selected during times in which dolphins were outside of formal training sessions in order to explore dolphin behavior throughout the day. Demographic and management characteristics were evaluated for their association with ODBA and average distance traveled per hour (ADT). The distance was divided by the number of minutes recorded in the section and converted to a per hour rate. Statistical models were examined using generalized estimating equations (GEE) due to the non-normal distribution of the data. GEEs do not require data to be transformed which can preserve interpretability of the results [53, 54].
In addition to wearing the bio-logging devices, the dolphins were video recorded three times per week for 25 minutes over the course of the five-week period [55]. The minimum data criteria to be included in that study were that each dolphin would have at least 10 video observations and could be seen for the majority of those observations. This resulted in the 240-minute minimum criteria. The same criteria were used for the present study to remain consistent across manuscripts within the collection. Individuals with less than 240 minutes of MTag data recorded outside of formal training sessions in the 2018 and 2019 data collection periods were excluded from the analysis. If a dolphin had more than 240 minutes of data recorded in both the 2018 and 2019 data collection period, the 2018 data were used for analysis and the 2019 data were excluded. If only one of the two data collection periods had more than 240 minutes, the period with more than 240 minutes was retained for analysis and the other period was excluded. Data from a single five-week period were used due to the way GEEs handle missing data. Dolphins without data for both 2018 and 2019 would be entirely excluded from the analysis. These decisions were made to retain the robust sample size while ensuring the validity of the results. The goal was to prioritize an investigation of variability across accredited facilities rather than examining within subject variability. A chi-square test of significance and an independent t-test were used to determine if the age and sex demographics of the dolphins in the final data set were statistically different than the original group of dolphins sampled.
Predictive regression models were fitted using GEEs to allow for individual level analysis and to account for facility ID. Facility ID was considered a random effect with an independent correlation structure. Regression models were built first with univariate level predictors. Predictor variables with sample sizes less than three were not considered for further analysis. Predictor variables correlating (p < 0.15) with the dependent variable were retained for evaluation in building the multivariate models. The final multivariate model was selected based on the quasi-likelihood under the independence (QIC) value and the number of significant independent variables. Statistical analyses for the GEE regression models were conducted in SPSS 21. The final models that were considered with significant independent variables and the lowest QIC values are given in S1 File.
Results
Sixty dolphins in 31 habitats met the minimum criteria for inclusion based on minutes of data collected outside of formal training sessions. The sex (χ2(1, N = 125) = 3.623, p = 0.057) and age (t(123) = 0.542, p = 0.589) distribution of the group of participants included in the analyses were not significantly different than the group composition prior to excluding dolphins that did not meet the inclusion criteria. Of these, 57 individuals were common bottlenose dolphins and three were Indo-Pacific bottlenose dolphins. Dolphins that met the minimum criteria for inclusion ranged in age from 3 to 44 years at the start of data collection (mean 16.48 ± 9.84 SD). In total, 1053.35 hours of data (range: 255 to 2043 minutes per dolphin) were collected during periods in which the dolphin was outside of formal training sessions. On average, dolphins traveled 2.32 ± 1.13 km per hr. The mean ODBA value for all participants was 2.31 ± 0.63 m/s2. The mean maximum depth was 5.63 m for the professionally managed ocean habitats and 8.78 m for professionally managed zoo/aquarium habitats.
Demographic and management factors were evaluated in relation to ODBA and ADT. Univariate correlations where p < 0.15 were observed between ODBA and two demographic variables, three enrichment variables, two training variables, and seven habitat variables (Table 2). Univariate correlations where p < 0.15 were observed between ADT and one enrichment variable, one training variable, and seven habitat variables (Table 3). Descriptive statistics for independent variables included in the multivariate modeling process are presented in Table 4.
Table 2. Univariate correlations between ODBA and independent variables.
| Variables | Reference | n | Beta | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Sex | Ref a = Male | 35 | 0.000 | |
| Female | 25 | 0.372 | 0.020* | |
| Age | 60 | -0.027 | 0.003* | |
| Environmental Enrichment | ||||
| Enrichment Diversity Index | 60 | 0.031 | 0.800 | |
| Enrichment Program Index | 60 | 0.022 | 0.741 | |
| Night Time Enrichment | 60 | -0.059 | 0.073^ | |
| Enrichment Schedule | Ref = Predictable | 8 | 0.000 | |
| Semi-Random | 44 | 0.272 | 0.018* | |
| Random | 8 | -4.320 | 0.015* | |
| Frequency of New Enrichment | Ref = Year+ | 2 | 0.000 | |
| Twice a Year | 16 | -0.145 | 0.213 | |
| Monthly/ Weekly | 42 | 0.246 | 0.029* | |
| Training | ||||
| Dolphin Presentations | 60 | 0.012 | 0.185 | |
| Interaction Programs | 60 | 0.008 | 0.527 | |
| Training Duration | 60 | 0.033 | 0.000* | |
| Maximum Number Interaction Guests | 60 | 0.009 | 0.356 | |
| Training Schedule | Ref = Predictable | 29 | 0.000 | |
| Semi-Predictable | 31 | -0.391 | 0.013* | |
| Habitat Characteristics | ||||
| Day Time Spatial Experience | 60 | 0.049 | 0.000* | |
| Night Time Spatial Experience | 60 | 0.047 | 0.000* | |
| 24 Hour Spatial Experience | 60 | 0.049 | 0.000* | |
| Length | 60 | 0.007 | 0.049* | |
| Depth | 60 | -0.044 | 0.005* | |
| Habitat Type | Ref = Zoo/Aquarium Habitat | 35 | 0.000 | |
| Ocean Habitat | 25 | 0.244 | 0.112^ | |
| Number of Habitats | 60 | -0.087 | 0.018* | |
| Social Management | Ref = Same Group | 29 | 0.000 | |
| Split/Reunited at Night | 20 | 0.100 | 0.575 | |
| Rotated Subgroups | 11 | -0.226 | 0.295 | |
| Neighboring Conspecifics | Ref = No Visual Access | 35 | 0.000 | |
| Visual/Auditory Access | 25 | -0.212 | 0.186 | |
^p-value < 0.15 used as threshold significant level for model building
*p-value < 0.05
a The reference value (Ref =) was the baseline value used when calculating univariate correlations with these binary variables.
Table 3. Univariate correlations between distance traveled and independent variables.
| Variables | Reference | n | Beta | p-value |
|---|---|---|---|---|
| Demographic | ||||
| Sex | Ref a = Male | 35 | 0.000 | |
| Female | 25 | 0.281 | 0.311 | |
| Age | 60 | 0.008 | 0.596 | |
| Environmental Enrichment | ||||
| Enrichment Diversity Index | 60 | -0.177 | 0.485 | |
| Enrichment Program Index | 60 | 0.018 | 0.901 | |
| Night Time Enrichment | 60 | -0.026 | 0.621 | |
| Enrichment Schedule | Ref = Predictable | 8 | 0.000 | |
| Semi-Random | 44 | 0.494 | 0.203 | |
| Random | 8 | -0.307 | 0.462 | |
| Frequency of New Enrichment | Ref = Year+ / Yearly | 2 | 0.000 | |
| Twice a Year | 16 | 0.347 | 0.158 | |
| Monthly / Weekly | 42 | 0.946 | <0.001* | |
| Training | ||||
| Dolphin Presentations | 60 | 0.001 | 0.909 | |
| Interaction Programs | 60 | 0.012 | 0.553 | |
| Training Duration | 60 | -0.007 | 0.632 | |
| Maximum Number Interaction Guests | 60 | 0.014 | 0.336 | |
| Training Schedule | Ref = Predictable | 29 | 0.000 | |
| Semi-Predictable | 31 | -0.697 | 0.012* | |
| Habitat Characteristics | ||||
| Day Time Spatial Experience | 60 | -0.003 | 0.852 | |
| Night Time Spatial Experience | 60 | -0.020 | 0.244 | |
| 24 Hour Spatial Experience | 60 | -0.011 | 0.467 | |
| Length | 60 | -0.002 | 0.608 | |
| Depth | 60 | -0.004 | 0.878 | |
| Habitat Type | Ref = Zoo/Aquarium Habitat | 35 | 0.000 | |
| Ocean Habitat | 25 | 0.077 | 0.796 | |
| Number of Habitats | 60 | 0.024 | 0.684 | |
| Social Management | Ref = Same Group | 29 | 0.000 | |
| Split/Reunited at Night | 20 | 0.528 | 0.095^ | |
| Rotated Subgroups | 11 | 0.342 | 0.402 | |
| Neighboring Conspecifics | Ref = No Visual Access | 35 | 0.000 | |
| Visual/Auditory Access | 25 | -0.504 | 0.074^ | |
^p-value < 0.15 used as threshold significant level for model building
*p-value < 0.05
a The reference value (Ref =) was the baseline value used when calculating univariate correlations with these binary variables.
Table 4. Descriptive statistics for the independent variables included in the multivariate modeling process.
| Independent Variable | Reference | n | Mean | SD | Min | Max | Median |
|---|---|---|---|---|---|---|---|
| Sex | Refa = Male | 35 | - | - | - | - | - |
| Female | 25 | - | - | - | - | - | |
| Age | 60 | 16.48 | 9.84 | 3.00 | 44.00 | 14.00 | |
| Enrichment Schedule | Ref = Predictable | 8 | - | - | - | - | - |
| Semi-Random | 44 | - | - | - | - | - | |
| Random | 8 | - | - | - | - | - | |
| Frequency of New Enrichment | Ref = Year+ / Yearly | 2 | - | - | - | - | - |
| Twice a Year | 16 | - | - | - | - | - | |
| Monthly / Weekly | 42 | - | - | - | - | - | |
| Training Schedule | Ref = Predictable | 29 | - | - | - | - | - |
| Semi-Predictable | 31 | - | - | - | - | - | |
| Day Time Spatial Experience | 60 | 2931.47 | 4361.90 | 38.12 | 25200.00 | 1700.00 | |
| Depth | 60 | 7.47 | 4.39 | 3.00 | 18.11 | 5.00 |
a The reference value (Ref =) was the baseline value used when calculating univariate correlations with these binary variables.
The final multivariate model for ODBA included sex, age, enrichment schedule, day time spatial experience, frequency of new enrichment, and depth (Table 5). ODBA was higher for females when compared to males (β = 0.32, p = 0.02). ODBA values decreased as the age of the dolphin increased (β = -0.02, p = 0.01; i.e., older dolphins had lower ODBA values) and dolphins provided with enrichment on a random schedule had lower ODBA values than those provided enrichment on a predictable schedule (β = -0.35, p = 0.03). ODBA values were higher for dolphins receiving new enrichment on a monthly/weekly schedule than dolphins receiving new enrichment on a yearly/year+ schedule (β = 0.30, p = 0.01). ODBA values increased as day time spatial experience increased (β = 0.03, p < 0.01). ODBA values decreased as the maximum depth of the habitat increased (β = -0.03, p = 0.05).
Table 5. Results for the full multivariate model examining ODBA.
| Variable | Beta | Std error | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|---|
| (Intercept) | 2.26 | 0.18 | 1.92 | 2.61 | <0.01 |
| Sex: Male | - | - | - | - | - |
| Sex: Female | 0.32 | 0.13 | 0.06 | 0.58 | 0.02* |
| Age | -0.02 | 0.01 | -0.04 | -0.01 | 0.01* |
| Enrichment Schedule: Predictable | - | - | - | - | - |
| Enrichment Schedule: Semi-Random | 0.19 | 0.13 | -0.06 | 0.43 | 0.13 |
| Enrichment Schedule: Random | -0.35 | 0.16 | -0.67 | -0.04 | 0.03* |
| Frequency of New Enrichment: Yearly / Year+ | - | - | - | - | - |
| Frequency of New Enrichment: Twice per year | 0.22 | 0.13 | -0.03 | 0.48 | 0.09 |
| Frequency of New Enrichment: Monthly / Weekly | 0.30 | 0.12 | 0.07 | 0.53 | 0.01* |
| Day Time Spatial Experience | 0.03 | 0.01 | 0.01 | 0.04 | <0.01* |
| Depth | -0.03 | 0.01 | -0.05 | 0.00 | 0.05* |
*p-value < 0.05
The final multivariate model for ADT included the frequency of new enrichment and training schedule (Table 6). ADT values were higher for dolphins who received new enrichment on a twice per year schedule than dolphins who received new enrichment on a yearly/year+ schedule (β = 0.62, p < 0.01). ADT values were higher for dolphins participating in training sessions on semi-predictable schedules than those participating on predicable schedules (β = -0.56, p = 0.04). Descriptive statistics for independent variables included in the multivariate modeling process are presented in Table 4.
Table 6. Results for the full multivariate model examining average distance traveled per hour.
| Variable | Beta | Std error | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|---|
| (Intercept) | 2.73 | 0.23 | 2.29 | 3.18 | <0.01 |
| Frequency of New Enrichment: Yearly / Year+ | - | - | - | - | - |
| Frequency of New Enrichment: Twice per year | 0.62 | 0.20 | 0.22 | 1.02 | <0.01* |
| Frequency of New Enrichment: Monthly / Weekly | 0.21 | 0.27 | -0.32 | 0.73 | 0.44 |
| Training Schedule: Predictable | - | - | - | - | - |
| Training Schedule: Semi-Predicable | -0.56 | 0.27 | -1.09 | -0.02 | 0.04* |
*p-value < 0.05
Discussion
This research marks the first large scale study to investigate bottlenose dolphin activity and energy expenditure using high resolution wearable data tagging systems in accredited zoos and aquariums. The participants provided data from a multitude of habitats and individuals thereby enabling robust conclusions.
ODBA (i.e., overall dynamic body acceleration) and ADT (i.e., average distance traveled per hour) represent two metrics that can be used to quantify activity and characterize two ways in which bottlenose dolphins use their habitat. While ODBA is not a validated indicator of welfare, the results suggested that several demographic, enrichment, and habitat characteristic variables were related to ODBA. Two enrichment-related factors (i.e., enrichment schedule and frequency of new enrichment) played a significant role in our final model. Supplying novel enrichment on a monthly/weekly schedule were associated with higher ODBA values than on a yearly/year+ schedule. Dolphins receiving enrichment on a random schedule had lower ODBA values than those receiving enrichment on a predictable schedule. Predictability of favorable events is thought to be an important part of animal welfare [56]. Events can be predictable based on temporal schedules or by establishing signals that precede the event. Watters and colleagues [57] found that moderate predictability in food delivery times stimulated species-specific behaviors and reduced anticipatory behaviors when compared to completely predictable and completely unpredictable schedules. One possible reason for the finding that ODBA had a negative relationship with random enrichment schedules may be that dolphins remained near areas where enrichment is provided and were more vigilant in a nearby area than active throughout the habitat during those times. If this is behavior commonly seen by animal care staff with a specific group or individual, they could mitigate this by establishing a signal that precedes the addition of enrichment objects. Based on findings from other analyses from the Cetacean Welfare Study, group active behaviors and interacting with conspecifics were higher for dolphins receiving enrichment on a predictable schedule than those receiving enrichment on a random schedule [10]. Group active behaviors and interacting with conspecifics were also higher for dolphins receiving new enrichment on a monthly/weekly schedule and/or a biannual schedule when compared those receiving new enrichment on a yearly/year+ schedule [10, 55]. These findings suggested ODBA, ADT, and social behaviors may be intertwined.
Two space use factors were included in the final ODBA model. Access to larger volumes of water during the day were positively related to ODBA values, and deeper habitats were negatively related to ODBA values. Similarly, the data from the Cetacean Welfare Study also indicated that behavioral diversity decreased as the maximum depth of the habitat increased [58]. Importantly, ODBA values were derived from acceleration measurements, which are related to changes in the speed of the animal and other forces, like water impacting the MTag as it moves through the air/water interface while surfacing. More surfacing events during a given amount of time would increase the noise in the acceleration measurements masking the acceleration of the animal. However, deeper depths may have been associated with lower ODBA values because swimming at the bottom and ascent portion of a dive sequence may require less active swimming than what is required for multiple short surfacing events [14]. ODBA values are lowest during gliding portions of swimming gates and during ascent as dolphins use their positive buoyancy to reduce the need to actively fluke. The ability to dive deeper and utilize gliding patterns may result in a small reduction in ODBA when compared to periods of continuous fluking. Given the small beta coefficients for the relationship between day time spatial experience and ODBA, even substantial increases in space would yield only minimal increases in ODBA values. This suggested that larger volumes of water were not the primary driver of activity when compared to animal management factors.
Small decreases in ODBA were associated with increased age (i.e., older dolphins). This is consistent with previous reports that high energy activities such as play are more common in younger dolphins [59]. As age increased, use of the top third of the habitat decreased [60] suggesting that older dolphins may use the bottom of the habitat for solitary or group swimming. Females were found to have significantly higher ODBA values than males across facilities. This finding has not been previously reported and both females and males are generally considered to be highly social [61, 62]. As such, this is an important avenue of future research. While day time spatial experience, depth, and age were included in the ODBA final model, the associations were weak based on the beta coefficients when compared to sex and the enrichment variables. Thus, the frequency with which new environmental enrichment was given and the schedule it was supplied on had a stronger relationship to activity levels than the size of the habitat.
The dolphins in the present study traveled an average of 2.32 km per hr. Previous research has shown dolphins in Sarasota Bay, Florida swim at a mean speed of approximately 1.2 km per hour during the day [63]. Wild bottlenose dolphins on the Pacific Coast of the United States have been recorded swimming between 11 and 47 km over a 24 hour period [64]. This would equate to between 0.46 km per hr and 1.96 km per hr which are well below the rate found in the current study. While data for this work were collected only during daytime hours, it has been reported that wild bottlenose dolphins are active throughout the night whereas dolphins under human care reduce their activity comparatively [65, 66]. Prior reports suggest that dolphins under human care have reduced levels of activity during nighttime hours [14, 29]. It is possible this pattern is related to the fact that training sessions are generally concentrated during daytime hours. Future research should examine the influence of the number and spacing of training sessions (i.e., when the dolphins receive the majority of their diet) on ODBA and ADT.
Previous research has found that dolphins spent more time floating when in a smaller, zoo/aquarium habitat than in a larger ocean habitat during the day [67, 68]. However, habitat characteristics were not found to be related to ADT in the present study. The final multivariate model for ADT included the frequency of new enrichment and the training schedule. Supplying new environmental enrichment items twice per year was associated with higher ADT. Novelty and resistance to habituation are important factors to consider when implementing an enrichment program [69]. Semi-predictable training schedules were associated with lower distance traveled values when compared to predictable schedules. One hypothesis is that predictable training schedules allowed dolphins to express specific behaviors at appropriate times outside of formal training sessions in relation to the schedule. In addition, other findings from the Cetacean Welfare Study suggested that behavioral diversity was higher for dolphins participating in training sessions on a semi-predictable training schedule than those participating in training sessions on a random schedule [55]. Semi-predictable training schedules may result in dolphins maintaining vigilance in anticipation of the next session thereby reducing the distance they swim outside of formal training sessions.
Limitations of the study include the potential effects of wearing the MTags. While dolphins were trained and habituated to wearing the MTags prior to the onset of data collection, it is possible that the dolphin participants or their conspecifics were modifying their behavior due to its presence. Additionally, the MTag adds drag to dolphin’s fusiform body and may have resulted in changes in behavior. DTAGs, a larger but similarly shaped tag, do not affect bottlenose dolphins oxygen consumption, physical activity ratios, cost of transport, or locomotor costs during structured swimming tasks [26]. However, the DTAGs may have affected swimming speed as dolphins swam significantly slower (11%) when wearing DTAGs during the structured swimming task in that study [26]. It is possible that dolphins in the present study also slowed their swimming in order to compensate for the MTag despite its smaller size. Additionally, the ADT values may be an underestimate to the actual distance traveled. This parameter was recorded using a micro-turbine mounted on top of the MTag that spun as the dolphins swam through the water. Thus, when the dolphin is swimming at the surface of the water, the turbine is not spinning and recording revolutions. Dolphins who spent larger portions of time with the MTag above water would result in artificially lower ADT values.
Other factors that may influence ODBA and ADT include the animals diet, training schedule, social composition, and dominance structure of the group. For example, dominant individuals might influence the speed and directionality of the group. The results may also be limited by the inclusion of both Tursiops aduncus and Tursiops truncatus. Future research should investigate potential differences in activity levels between the subspecies while under professional care. Another limitation was data loss due to water damage to some of the MTags. Several dolphins were removed from the study because the MTags were water damaged before they met the minimum inclusion criteria. MTags are a relatively new technology and MTag deployments on this scale are unprecedented. Thus, it was unsurprising that, on occasion, a device would be damaged due to the closure system that protected the internal components. Future designs of the MTag will operate wirelessly to remove the possibility of the internal components receiving water damage.
The goal of the present study was to use bio-logging tags to investigate if and how habitat characteristics and management factors were related to the activity levels and distanced traveled by bottlenose dolphins in accredited zoos and aquariums. The results showed that enrichment programs played a significant role in both ODBA and ADT. Scheduling predictable training session times was also associated with higher ADT. The findings suggested that habitat characteristics had a relatively weak association with ODBA and were not related to ADT. In combination, the results suggested that management practices were more influential on activity levels than habitat characteristics. These data revealed important relationships between dolphins’ swimming behavior and a facility’s management practices as well as habitat characteristics. These findings demonstrated that the data obtained by bio-logging devices has the potential to inform managers and veterinarians of changes in health and welfare in real time, allowing for rapid veterinary and behavioral interventions aimed at promoting optimal health and welfare. Future research should investigate the types of activities and swimming styles in relation to normal behavior.
Supporting information
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
We would like to start by thanking Joy Mench and Melinda Conners for their significant efforts at the beginning of the project assisting with project design and review of methods. We would also like to thank Jocelyn Woods for all of her efforts in behavioral coding, data entry, and data processing. In addition, special thanks to the Alliance for Marine Mammal Parks and Aquariums and the Association of Zoos & Aquariums for support throughout the duration of the project. We would also like to thank Sarah Breen-Bartecki and Bill Zeigler for their continued support and Rita Stacey and the Seven Seas staff for all of their original efforts in testing the MTags. We would like to acknowledge Cheryl Meehan, Joy Mench, Kathy Carlstead, Jennifer Hogan, and the additional co-authors of the Epidemiological Investigations of North American Zoo Elephant Welfare Collection for providing a framework for aspects of the present study. Finally, a sincere thank you to the people at each of the following facilities for participating in this study: Cabo Dolphins, Chicago Zoological Society–Brookfield Zoo, Delphinus Playa Mujeres, Delphinus Puerto Morelos, Delphinus Punta Cancún, Delphinus Riviera Maya, Delphinus Xcaret, Delphinus Xel-Há, Dolphin Adventure, Dolphin Discovery Akumal, Dolphin Discovery Cozumel, Dolphin Discovery Dreams, Dolphin Discovery Isla Mujeres, Dolphin Discovery Mahahual, Dolphin Discovery Maroma, Dolphin Discovery Moon Palace, Dolphin Discovery Ocho Rios, Dolphin Discovery Riviera Maya, Dolphin Island–Resorts World Sentosa, Dolphin Quest Hawaii, Dolphin Quest Oahu, Dolphin Quest Bermuda, Georgia Aquarium/Marineland Dolphin Adventure, Gulf World Marine Park, Gulfarium Marine Adventure Park, Indianapolis Zoo, Interactive Aquarium Cancun, John G. Shedd Aquarium, Lisbon Zoo Portugal, Loro Parque and Loro Parque Fundación, Mystic Aquarium, National Aquarium, Ocean Park Corporation, Sea Life Park Hawaii, Sea World Parks, Texas State Aquarium, The Mirage Dolphin Habitat and The Mirage Casino-Hotel LLC, The Seas® Epcot® Walt Disney World® Resort, and the U.S. Navy Marine Mammal Program.
Data Availability
In order to protect the identification of the facilities and animals included in this study, findings require data access restrictions at the individual-level. All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The work was funded by a National Leadership Grant (MG-30-17-0006-17) from the Institute of Museum and Library Services (www.imls.gov) to the Chicago Zoological Society. Additional financial support was provided to the Chicago Zoological Society by the Indianapolis Zoo, The Seas® Epcot® Walt Disney World® Resort, Dolphin Island – Resorts World Sentosa, Texas State Aquarium, Loro Parque and Loro Parque Fundación, SeaWorld Busch Gardens Conservation Fund and the Chicago Zoological Society Women’s Board. Chicago Zoological Society provided financial support for LM salary, University of California Irvine provided financial support for DG salary and University of Florida provided financial support for MW salary through Clearwater Marine Aquarium and Florida Fish and Wildlife Conservation Commission state funding. Author’s roles are specified in the author contributes’ section of the manuscript.
References
- 1.Rutz C, Hays GC. New frontiers in biologging science. Bio Lett. 2009;289–92. doi: 10.1098/rsbl.2009.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talukder S, Thomson PC, Kerrisk KL, Clark CE, Celi P. Evaluation of infrared thermography body temperature and collar-mounted accelerometer and acoustic technology for predicting time of ovulation of cows in a pasture-based system. Theriogen. 2015Mar1;83(4):739–48. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DW, Trotter MG, Knight CW, Thomas MG. Use of GPS tracking collars and accelerometers for rangeland livestock production research. Trans Anim Sci. 2018Feb;2(1):81–8. doi: 10.1093/tas/txx006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koltes JE, Koltes DA, Mote BE, Tucker J, Hubbell DS. Automated collection of heat stress data in livestock: new technologies and opportunities. Trans Anim Sci. 2018Sep;2(3):319–23. doi: 10.1093/tas/txy061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapa JM, Maschat K, Iwersen M, Baumgartner J, Drillich M. Accelerometer systems as tools for health and welfare assessment in cattle and pigs–a review. Behav Proc. 2020Oct10:104262. doi: 10.1016/j.beproc.2020.104262 [DOI] [PubMed] [Google Scholar]
- 6.Razal CB, Bryant J, Miller LJ. Monitoring the behavioral and adrenal activity of giraffe (Giraffa camelopardalis) to assess welfare during seasonal housing changes. Anim Behav Cogn. 2017;4(2):154–64. [Google Scholar]
- 7.Teo SL, Boustany A, Dewar H, Stokesbury MJ, Weng KC, Beemer S, et al. Annual migrations, diving behavior, and thermal biology of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar Biol. 2007Mar1;151(1):1–8. [Google Scholar]
- 8.Morrone SC. Biologging the behavior of the Chocoan River turtle, Rhinoclemmys nasuta (Testudines: Geoemydidae). Doctoral dissertation, University of Louisiana at Monroe. 2017.
- 9.Ware C, Trites AW, Rosen DA, Potvin J. Averaged propulsive body acceleration (APBA) can be calculated from biologging tags that incorporate gyroscopes and accelerometers to estimate swimming speed, hydrodynamic drag and energy expenditure for Steller sea lions. PloS one. 2016;11(6). doi: 10.1371/journal.pone.0157326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller LJ, Lauderdale LK, Mellen JD, Walsh MT, Granger DA. Assessment of animal management and habitat characteristics impacting social behavior in bottlenose dolphins across zoological facilities. PLOS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowacek DP, Thorne LH, Johnston DW, Tyack PL. Responses of cetaceans to anthropogenic noise. Mamm Rev. 2007Apr;37(2):81–115. [Google Scholar]
- 12.Southall BL, Bowles AE, Ellison WT, Finneran JJ, Gentry RL, Greene CR Jr, et al. Marine mammal noise-exposure criteria: initial scientific recommendations. Bioacous. 2008Jan1;17(1–3):273–5. [Google Scholar]
- 13.Tyack PL. Human-generated sound and marine mammals. Phys Tod. 2009Nov1;62(11):39–44. [Google Scholar]
- 14.Shorter K, Shao Y, Ojeda L, Barton K, Rocho‐Levine J, van der Hoop J, et al. A day in the life of a dolphin: Using bio‐logging tags for improved animal health and well‐being. Mar Mamm Sci. 2017Jul;33(3):785–802. [Google Scholar]
- 15.Zhang D, Shorter KA, Rocho-Levine J, van der Hoop J, Moore M, Barton K. Behavior inference from bio-logging sensors: A systematic approach for feature generation, selection and state classification. In Proc ASME 2018 Dyn Sys Con Conf. 2018 Sept 30; Vol. 2.
- 16.Sibal R, Zhang D, Rocho-Levine J, Shorter KA, Barton K. Bidirectional LSTM recurrent neural network plus hidden Markov model for wearable sensor based dynamic state estimation. In Proc ASME 2019 Dyn Sys Con Conf. 2019 Oct 8; Vol. 2.
- 17.Gabaldon J, Zhang D, Barton K, Johnson-Roberson M, Shorter KA, A framework for enhanced localization of marine mammals using auto-detected video and wearable sensor data fusion. In 2017 IEEE/RSJ Int Conf Intell Rob Syst (IROS) 2017 Sept 24:2505–10.
- 18.Zhang D, Gabaldon J, Lauderdale LK, Johnson-Roberson M, Miller LJ, Barton K, et al. Localization and tracking of uncontrollable underwater agents: Particle filter based fusion of on-body IMUs and stationary cameras. In 2019 IEEE Int Conf Rob Auto (ICRA) 2019May20:6575–81. [Google Scholar]
- 19.Johnson MP, Tyack PL. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Ocean Engin. 2003Apr2;28(1):3–12. [Google Scholar]
- 20.Arranz P, Benoit-Bird K, Friedlaender AS, Hazen EL, Goldbogen JA, Stimpert AK, et al. Diving behavior and fine-scale kinematics of free-ranging Risso’s dolphins foraging in shallow and deep-water habitats. Front Ecol Evol. 2019;7:53. [Google Scholar]
- 21.Watwood SL, Miller PJ, Johnson M, Madsen PT, Tyack PL. Deep‐diving foraging behaviour of sperm whales (Physeter macrocephalus). Appl Anim Etho. 2006May1;75(3):814–25. [DOI] [PubMed] [Google Scholar]
- 22.Giovani F, Anderson E, Garborg CS, Murray M, Johnson M, Moore MJ, et al. From the track to the ocean: using flow control to improve marine bio-logging tags for cetaceans. PloS one. 2017;12, no. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, van der Hoop JM, Petrov V, Rocho-Levine J, Moore MJ, Shorter KA. Simulated and experimental estimates of hydrodynamic drag from bio-logging tags. Mar Mamm Sci. 2020Jan; 36:136–57 [Google Scholar]
- 24.Nowacek DP, Johnson MP, Tyack PL. North Atlantic right whales (Eubalaena glacialis) ignore ships but respond to alerting stimuli. Proc Roy Soc Lon. Ser B: Bio Sci. 2004Feb7;271(1536):227–31. doi: 10.1098/rspb.2003.2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyack P, Gordon J, Thompson D. Controlled exposure experiments to determine the effects of noise on marine mammals. Mar Technol Soc J. 2003Dec1;37(4):41–53. [Google Scholar]
- 26.van der Hoop JM, Fahlman A, Hurst T, Rocho-Levine J, Shorter KA, Petrov V, et al. Bottlenose dolphins modify behavior to reduce metabolic effect of tag attachment. J Exp Biol. 2014Dec1;217(23):4229–36. doi: 10.1242/jeb.108225 [DOI] [PubMed] [Google Scholar]
- 27.Ropert-Coudert Y, Beaulieu M, Hanuise N, Kato A. Diving into the world of biologging. Endan Spec Res. 2009Apr27;10:21–7. [Google Scholar]
- 28.Akiyama Y, Matsuda Y, Sakurai N, Sato K. Evaluation of wave drag on bottlenose dolphin Tursiops truncatus from swimming effort. Coast Mar Sci. 2015;38:42–6. [Google Scholar]
- 29.Gnone G, Benoldi C, Bonsignori B, Fognani P. Observations of rest behaviours in captive bottlenose dolphins (Tursiops truncatus). Aquat Mamm. 2001;27(1):29–33. [Google Scholar]
- 30.Hoff MP, Powell DM, Lukas KE, Maple TL. Individual and social behavior of lowland gorillas in outdoor exhibits compared with indoor holding areas. App Anim Beh Sci. 1997Nov1;54(4):359–70. [Google Scholar]
- 31.Hopper LM, Shender MA, Ross SR. Behavioral research as physical enrichment for captive chimpanzees. Zoo Bio. 2016Jul;35(4):293–7. doi: 10.1002/zoo.21297 [DOI] [PubMed] [Google Scholar]
- 32.Macedonia J M. Effects of housing differences upon activity budgets in captive sifakas (Propithecus verreauxi). Zoo Bio. 1987;6(1), 55–67. [Google Scholar]
- 33.Posta B, Huber R, Moore DE III. The effects of housing on zoo elephant behavior: A quantitative case study of diurnal and seasonal variation. Int J Comp Psy. 2013;26(1). [Google Scholar]
- 34.Skibiel AL, Trevino HS, Naugher K. Comparison of several types of enrichment for captive felids. Zoo Bio. 2007Sep;26(5):371–81. doi: 10.1002/zoo.20147 [DOI] [PubMed] [Google Scholar]
- 35.Fowler ME, Csuti B, Sargent EL, Bechert US. An overview of foot conditions in Asian and African elephants. The elephant’s foot: Prevention and care of foot conditions in captive Asian and African elephants. 2001;3–7. [Google Scholar]
- 36.Honda A, Sogo N, Nagasawa S, Shimizu T, Umemura Y. High-impact exercise strengthens bone in osteopenic ovariectomized rats with the same outcome as sham rats. J App Physio. 2003Sep;95(3):1032–7. doi: 10.1152/japplphysiol.00781.2002 [DOI] [PubMed] [Google Scholar]
- 37.Kuhar CW, Fuller GA, Dennis PM. A survey of diabetes prevalence in zoo‐housed primates. Zoo Bio. 2013Jan;32(1):63–9. doi: 10.1002/zoo.21038 [DOI] [PubMed] [Google Scholar]
- 38.Metsios GS, Stavropoulos-Kalinoglou A, Veldhuijzen van Zanten JJ, Treharne GJ, Panoulas VF, Douglas KM, et al. Rheumatoid arthritis, cardiovascular disease and physical exercise: a systematic review. Rheum. 2008Mar1;47(3):239–48. doi: 10.1093/rheumatology/kem260 [DOI] [PubMed] [Google Scholar]
- 39.Meagher R. Is boredom an animal welfare concern?. Anim Welf. 2018Feb1;28(1):21–32. [Google Scholar]
- 40.Inglis IR. Towards a cognitive theory of exploratory behaviour. In: Archer J and Birke LIA (eds) Exploration in Animals and Man.Van Nostrand Reinhold: London, UK. 1983; 72–117. [Google Scholar]
- 41.Mason GJ, Latham N. Can’t stop, won’t stop: is stereotypy a reliable animal welfare indicator?. Anim Welf. 2004;13. [Google Scholar]
- 42.De Passille AM, Jensen MB, Chapinal N, Rushen J. Use of accelerometers to describe gait patterns in dairy calves. J Dairy Sci. 2010Jul1;93(7):3287–93. doi: 10.3168/jds.2009-2758 [DOI] [PubMed] [Google Scholar]
- 43.Ringgenberg N, Bergeron R, Devillers N. Validation of accelerometers to automatically record sow postures and stepping behaviour. App Anim Beh Sci. 2010Dec1;128(1–4):37–44. [Google Scholar]
- 44.Sheldon JD, Adkesson MJ, Allender MC, Balko JA, Bailey RS, Langan JN, et al. Objective gait analysis in humboldt penguins (spheniscus humboldti) using a pressure-sensitive walkway. J Zoo Wild Med. 2020Jan;50(4):910–6. doi: 10.1638/2019-0054 [DOI] [PubMed] [Google Scholar]
- 45.Nishizawa H, Noda T, Yasuda T, Okuyama J, Arai N, Kobayashi M. Decision tree classification of behaviors in the nesting process of green turtles (Chelonia mydas) from tri-axial acceleration data. J Etho. 2013Sep1;31(3):315–22. [Google Scholar]
- 46.Soltis J, Wilson RP, Douglas-Hamilton I, Vollrath F, King LE, Savage A. Accelerometers in collars identify behavioral states in captive African elephants Loxodonta africana. End Spec Res. 2012Sep28;18(3):255–63. [Google Scholar]
- 47.Watanabe S, Izawa M, Kato A, Ropert-Coudert Y, Naito Y. A new technique for monitoring the detailed behaviour of terrestrial animals: a case study with the domestic cat. App Anim Beh Sci. 2005Oct1;94(1–2):117–31. [Google Scholar]
- 48.Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, et al. A new technique for monitoring the behaviour of free-ranging Adelie penguins. J Exp Bio. 2001Feb15;204(4):685–90. [DOI] [PubMed] [Google Scholar]
- 49.Lauderdale LK, Mellen JD, Walsh MT, Grainger DA, & Miller LJ. Environmental enrichment, training, and habitat characteristics of common bottlenose dolphins (Tursiops truncatus) and Indo-Pacific bottlenose dolphins (T. aduncus). PLOS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madgwick S. An efficient orientation filter for inertial and inertial/magnetic sensor arrays. Uni Brist UK. 2010;25:113–18. [Google Scholar]
- 51.Gabaldon J, Turner EL, Johnson-Roberson M, Barton K, Johnson M, Anderson EJ, et al. Integration, calibration, and experimental verification of a speed sensor for swimming animals. IEEE Sens J. 2019May15;19(10):3616–25. [Google Scholar]
- 52.Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, et al. Moving towards acceleration for estimates of activity‐specific metabolic rate in free‐living animals: the case of the cormorant. J Anim Ecol. 2006Sep;75(5):1081–90. doi: 10.1111/j.1365-2656.2006.01127.x [DOI] [PubMed] [Google Scholar]
- 53.Kowalski J, Tu XM. Modern applied U-statistics. John Wiley & Sons; 2008Jan28. [Google Scholar]
- 54.Tang W, He H, Tu XM. Applied categorical and count data analysis. CRC Press; 2012Jun4. [Google Scholar]
- 55.Miller LJ, Lauderdale LK, Mellen JD, Granger DA, Walsh MT. Impact of animal management and habitat characteristics on two potential indicators of welfare for bottlenose dolphins under professional care. PLOS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bassett L, Buchanan-Smith HM. Effects of predictability on the welfare of captive animals. Appl Anim Behav Sci. 2007Feb1;102(3–4):223–45. [Google Scholar]
- 57.Watters JV, Miller JT, Sullivan TJ. Note on optimizing environmental enrichment: A study of fennec fox and zoo guests. Zoo Biol. 2011Nov;30(6):647–54. doi: 10.1002/zoo.20365 [DOI] [PubMed] [Google Scholar]
- 58.Paulos RD, Trone M, Kuczaj SA II,. Play in wild and captive cetaceans. Int J Comp Psy. 2010;23(4). [Google Scholar]
- 59.Lauderdale LK, Shorter KA, Zhang D, Gabaldon J, Mellen JD, Grainger DA, et al. Habitat characteristics and animal management factors associated with habitat use by bottlenose dolphins in zoological environments. PLOS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connor RC, Smolker RA, Richards AF. Dolphin alliances and coalitions. Coalitions and alliances in humans and other animals. 1992;414–43. [Google Scholar]
- 61.Wells RS. The role of long-term study in understanding the social structure of a bottlenose dolphin community. In Dolphin societies: Discoveries and puzzles. 1991:199–225. [Google Scholar]
- 62.Mate BR, Rossbach KA, Nieukirk SL, Wells RS, Blair Irvine A, Scott MD, et al. Satellite‐monitored movements and dive behavior of a bottlenose dolphin (Tursiops truncatus) in Tampa Bay, Florida. Mar Mamm Sci. 1995Oct;11(4):452–63. [Google Scholar]
- 63.Defran RH, Weller DW, Kelly DL, Espinosa MA. Range characteristics of Pacific coast bottlenose dolphins (Tursiops truncatus) in the Southern California Bight. Mar Mamm Sci. 1999Apr;15(2):381–93. [Google Scholar]
- 64.Irvine AB, Scott MD, Wells RS, Kaufmann JH. Movements and activities of the Atlantic bottlenose dolphin, Tursiops truncatus, near Sarasota, Florida. Fish Bull. 1981Jan1;79(4):671–88. [Google Scholar]
- 65.Shane SH, Wells RS, Würsig B. Ecology, behavior and social organization of the bottlenose dolphin: a review. Mar Mamm Sci. 1986Jan;2(1):34–63. [Google Scholar]
- 66.Ruiz CU, Sánchez A, Maldonado FG. Social and individual behavior of a group of bottlenose dolphins (Tursiops truncatus) in open and closed facilities. Vet Méx. 2009;40(4). [Google Scholar]
- 67.Blasio AL, Pérez RV, Pardo MR, Maldonado FG. Maintenance behaviour and cortisol levels in bottlenose dolphins (Tursiops truncatus) in closed and open facilities. Vet Méx. 2012;43(2):103–12. [Google Scholar]
- 68.Ugaz C, Valdez RA, Romano MC, Galindo F. Behavior and salivary cortisol of captive dolphins (Tursiops truncatus) kept in open and closed facilities. J Vet Beh. 2013Jul1;8(4):285–90. [Google Scholar]
- 69.Kuczaj S, Lacinak T, Fad O, Trone M, Solangi M, Ramos J. Keeping environmental enrichment enriching. Inter J Comp Psy. 2002;15(2). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
In order to protect the identification of the facilities and animals included in this study, findings require data access restrictions at the individual-level. All relevant data are within the manuscript and its Supporting information files.