To the Editor:
Kidney transplantation remains limited by organ shortage, thus increasing focus on improving deceased donor kidney use.1,2 With modern direct-acting antivirals (DAAs), transplantation of kidneys from viremic donors with positive hepatitis C virus nucleic acid testing (HCV-NAT+) represents a major breakthrough with the potential to significantly increase the number of transplants, as kidneys from HCV+ donors were historically at high risk for discard.3–5 Accumulating recent publications have demonstrated the safety and efficacy of HCV-NAT+ deceased donor kidney transplantation in recipients with or without HCV (Table S1).
We sought to determine whether the increasing evidence for HCV-NAT+ kidney use is reflected in the intentions and practices at US transplant centers by examining bypass filter settings, which centers use to restrict the organ offers they receive to only those that match their practice preferences. The UNOS Organ Center assists with placing organs from donors with unusual circumstances that may have difficulty being placed locally, and to expedite placement of these organs, transplant centers indicate in advance via electronic filters the donor/organ characteristics that they will not consider for transplantation for any of their waitlisted patients. These filters result in centers being bypassed for all regional and national offers from the UNOS Organ Center, but not local or other offers directly from an organ procurement organization. Centers set bypass filters for 48+ donor characteristics; the HCV-NAT+ filter has been available since August 10, 2015. We examined UNOS data on temporal changes in HCV-NAT+ filter use among 245 kidney transplant centers through July 31, 2019, and transplantation of HCV-NAT+ kidneys (detailed methods in Item S1).
Within 1 month of the first use of the HCV-NAT+ filter, 14% of centers had recorded a response, of which two-thirds (representing 9% of all centers) opted out of receiving these offers. Within a year, 22% of all centers had opted out; a proportion that increased to 32% approximately 4 years after filter introduction (Fig 1). The proportion of centers explicitly indicating willingness to consider these offers increased from 5% at 1 month to 38% by 4 years. By OPTN policy, the remaining centers with no response to this filter continued to receive HCV-NAT+ offers by default. Of the 73 centers with no response to the HCV-NAT+ filter as of July 31, 2019, a total of 26 (36%) had performed at least 1 kidney transplantation from an HCV-NAT+ deceased donor into an HCV-seronegative recipient. A similar proportion of centers actively opting in to these offers (38%) performed at least 1 such transplantation, whereas only 11% of centers opting out had transplanted a (presumably local) HCV-NAT+ kidney into a negative recipient (Table S2).
Figure 1.
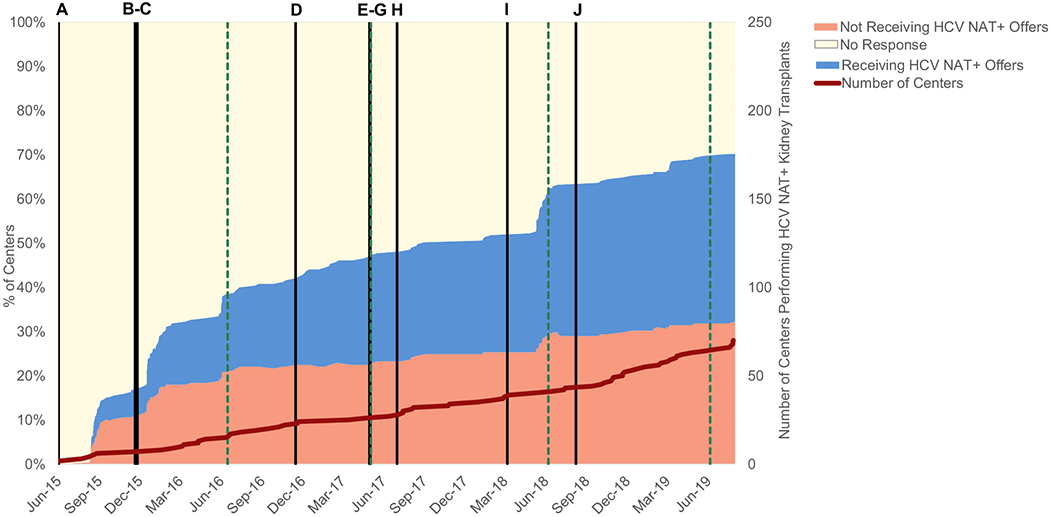
Kidney transplant center responses to the HCV-NAT+ national offer filter over time. The colored areas represent proportions of centers opting to automatically bypass these offers, receive them, or with no response. Black vertical bars indicate the publication date of major studies (key in Table S1), and green vertical dashed bars indicate the date of the American Transplant Congress each year. The red line indicates the cumulative count of centers performing at least 1 kidney transplant from an HCV-NAT+ donor to an HCV-seronegative recipient.
The count of centers transplanting at least 1 HCV-NAT+ kidney into an HCV-seronegative recipient increased steadily since March 31, 2015 (when donor HCV-NAT+ status was first systematically collected), reaching 70 of 245 (29%) by July 31, 2019. Recovery and use of HCV-NAT+ kidneys increased since 2018, with the discard rate falling from 44% in the first quarter of 2018 to 21% in the third quarter of 2019 to match the overall kidney discard rate (Fig S1), although HCV-NAT+ donors may differ from other donors in additional characteristics that we did not directly compare. Centers receiving HCV-NAT+ kidney offers had larger waitlist and transplant volumes (Table S2) and were located throughout the United States (Fig 2A), although more HCV-NAT+ transplantations to seronegative recipients occurred in the eastern half of the country (Fig 2B).
Figure 2.
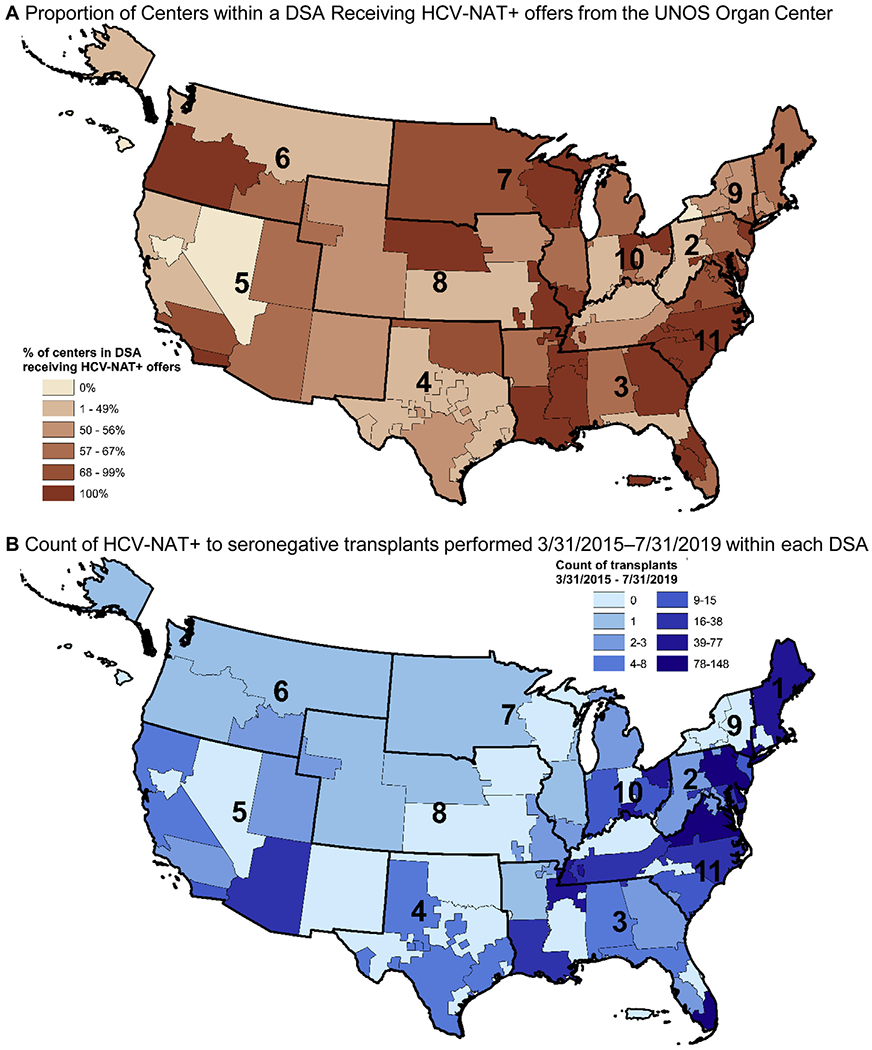
Geographic distribution of centers receiving offers and transplanting kidneys from HCV-NAT+ deceased donors as of July 31, 2019, by donation service area (DSA).
These findings demonstrate continued reluctance by a large minority of centers to transplant kidneys from HCV-NAT+ donors despite mounting evidence of safety and efficacy of their use, as well as the increasing use of these organs by other centers.6 Centers may be unwilling to consider offers from HCV-NAT+ donors due to concerns over insurance coverage for expensive DAAs, uncertainty in long-term safety/outcomes, or their ability to manage consent or potential complications of HCV infection posttransplantation. Although bypass filters may improve allocation efficiency, programs’ bypass filter selections are not currently publicly reported, and bypassed offers are excluded from the calculations used to compare programs’ organ use in the SRTR (Scientific Registry of Transplant Recipients)–reported offer acceptance ratios. As a result, patients seeking to avoid the suboptimal outcomes of remaining on the waiting list are often unaware of which donors/offers a given center has opted out of and the potential adverse impact on their probability of undergoing transplantation sooner.7,8 Varying approaches to filtering organ offers likely contribute to previously observed center-level heterogeneity in the use of kidneys at risk for discard.9
Patients prefer to be waitlisted at centers with the shortest wait times, including centers that use innovative approaches to increase access to transplantation.10 Our findings suggest that diffusion and adoption of innovative clinical practice is slower and less universal than expected in the context of a severe organ shortage. Examining bypass filter settings allows an earlier look at changes in centers’ attitudes toward HCV-NAT+ transplantation than transplant rates alone, which also depend on the availability of HCV-NAT+ donors and having a matched willing recipient. Despite increasing evidence of the safety of transplanting HCV-NAT+ organs, our results demonstrate that 32% of transplant centers currently choose to opt out of receiving regional/national offers from HCV-NAT+ donors.
Supplementary Material
Acknowledgements:
The authors thank Sarah Taranto for assistance in understanding the data elements used in this analysis.
Support:
SAH is supported by the National Center for Advancing Translational Sciences (KL2 TR001874). SM is supported by NIH grants R01-DK114893, U01-DK116066, and R01-MD014161. The funders had no role in study design, data collection, analysis, or reporting; or the decision to submit for publication.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: Disclaimer: The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN or the US Government.
References
- 1.Cooper M, Formica R, Friedewald J, et al. Report of National Kidney Foundation Consensus Conference to Decrease Kidney Discards. Clin Transplant. 2019;33(1):e13419. [DOI] [PubMed] [Google Scholar]
- 2.Reese PP, Harhay MN, Abt PL, Levine MH, Halpern SD. New solutions to reduce discard of kidneys donated for transplantation. J Am Soc Nephrol. 2016;27(4):973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitsky J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017;17(11):2790–2802. [DOI] [PubMed] [Google Scholar]
- 4.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting hepatitis C-positive kidneys. N Engl J Med. 2015;373(4):303–305. [DOI] [PubMed] [Google Scholar]
- 5.Mohan S, Chiles MC, Patzer RE, et al. Factors leading to the discard of deceased donor kidneys in the United States. Kidney Int. 2018;94(1):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potluri VS, Goldberg DS, Mohan S, et al. National trends in utilization and 1-year outcomes with transplantation of HCV-viremic kidneys. J Am Soc Nephrol. 2019;30(10):1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain SA, King KL, Pastan S, et al. Association between declined offers of deceased donor kidney allograft and outcomes in kidney transplant candidates. JAMA Netw Open. 2019;2(8):e1910312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation. 2002;74(10): 1377–1381. [DOI] [PubMed] [Google Scholar]
- 9.Brennan C, Husain SA, King KL, et al. A donor utilization index to assess the utilization and discard of deceased donor kidneys perceived as high risk. Clin J Am Soc Nephrol. 2019;14(11): 1634–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain SA, Brennan C, Michelson A, et al. Patients prioritize waitlist over posttransplant outcomes when evaluating kidney transplant centers. Am J Transplant. 2018;18(11):2781–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


