Abstract
Little is known about temporal trends of pregnant women’s exposures to environmental phenols and parabens. We quantified four phenols (bisphenol A [BPA], bisphenol F, bisphenol S, triclosan), four parabens (butyl paraben, ethyl paraben [ETPB], methyl paraben [MEPB], propyl paraben [PRPB]) and triclocarban in 760 urine samples collected during 2007–2014 from 218 California pregnant women participating in a high-familial risk autism spectrum disorder cohort. We applied multiple regression to compute least square geometric means of urinary concentrations and computed average annual percent changes. We compared our urinary concentrations with those of other study populations to examine geographic variations in pregnant women’s exposure to these target compounds. Urinary concentrations of BPA, MEPB, ETPB, and PRPB in this study population decreased over the study period [percent change per year (95% confidence interval): −5.7% (−8.2%, −3.2%); −13.0% (−18.1%, −7.7%); −5.5% (−11.0%, 0.3%); −13.3% (−18.3%, −8.1%), respectively] and were consistently lower than those in pregnant women in other U.S. regions during the same study period. In recent years, certain phenols and parabens with known adverse health effects are being regulated or replaced with alternatives, which explain decreased body burdens observed in this study population. Either the national regulations or the advocacy campaigns in California may have influenced exposures or consumer product choices.
Keywords: bisphenols, geographic variations, personal care products, regulations, social forces, temporal changes
Graphical Abstract
1. Introduction
Some environmental phenols, parabens and triclocarban (TCC) are widely used in various consumer and personal care products.1, 2 Bisphenols are used as plasticizers, and other phenols and TCC are commonly used as antimicrobial preservatives in toothpaste, soaps, and detergents.2–4 Parabens are primarily used as preservatives in shampoos, lotions, sunscreens, deodorants, cosmetics and pharmaceutical products.5 Because products containing phenols, parabens and TCC are widely used, they are detected in U.S. household dust 6–8 and in urine of a large, representative sample of the U.S. population as well as pregnant women, infants, and young children.9–15
Prenatal exposure to phenols, parabens and TCC is of public health concern because epidemiologic studies showed prenatal exposure to some phenols, parabens and TCC to be associated with adverse health effects in pregnant women or their offspring, such as poor birth outcomes,16, 17 decreased female fecundity,18 and increased childhood obesity.19 Prenatal exposure to phenols, parabens and TCC is suspected to have potential adverse neurodevelopmental effects, leading to increased risk of non-typical brain development,10 externalizing behaviors,20 and decreased cognitive test scores.21 Laboratory animal studies also showed that bisphenol A (BPA), bisphenol S (BPS), and bisphenol F (BPF) can disrupt female reproductive systems.22–26 These findings, combined with the fact that phenols, parabens and TCC are detected in cord blood,27–29 support that higher prenatal exposure to these compounds could result in elevated risk of having adverse outcomes in pregnant women and their offspring.
Health concern and toxicity associated with prenatal exposure to some phenols, parabens and TCC led to regulatory efforts that restricted their production and inclusion in products used by women of reproductive age, infants, or young children. The U.S. Food and Drug Administration (FDA) banned the use of BPA in baby bottles and spill-proof cups, including their lids in 201230 and restricted the use of BPA-based epoxy resins in packaging materials used for infant formula in 2013.31 Thus, manufacturers are replacing BPA with BPS or BPF to comply with the U.S. regulations on BPA.32, 33 The U.S. FDA also banned the use of triclosan (TCS) and TCC in hand and body soaps in 2016.34 Parabens are not regulated in the U. S., but after the California Safe Cosmetics Program mandates that cosmetic companies disclose chemical ingredients in beauty and personal care products sold in California since 2007,35 exposure to parabens may have been decreased in the general U.S. population. In addition, the European Union (EU), Japan, and the Southeast Asian nations set maximum paraben concentrations in cosmetics and do not permit some parabens in any cosmetic product.36–38 As a result of these regulations and following market changes, urinary concentrations of some phenols and parabens decreased over time in the general population of the U.S.39 and Germany40 and in Danish young men.41 However, few studies examined temporal trends of pregnant women’s exposure to phenols, parabens and TCC.
There are many factors that play a role in understanding pregnant women’s exposure to phenols, parabens and TCC. For example, pregnant women tended to have lower urinary biomarker concentrations of these compounds than non-pregnant women,42–44 possibly because of changes in their use in cosmetics and other personal care products after becoming pregnant. Pregnant women’s exposure to these compounds differed by sociodemographic characteristics and use frequency of products potentially containing these compounds before urine collection.45–54 In our previous study,55 we learned that broad social forces may have influenced temporal trends and geographic variations in pregnant women’s phthalate exposure. Thus, the same study approach may help identify similar broad social forces related to pregnant women’s phenol, paraben and TCC exposure.
In this study, we quantified four phenols (BPA, BPF, BPS, TCS), four parabens (butyl paraben (BUPB), ethyl paraben (ETPB), methyl paraben (MEPB), and propyl paraben (PRPB)), and TCC to assess temporal trends of their urinary concentrations in California pregnant women during 2007–2014. To understand whether broad social forces affected exposure to the target study compounds in the U. S., we compared our measured urinary concentrations with those of other pregnancy cohorts in Massachusetts and Puerto Rico as well as those of pregnant and non-pregnant women in the U.S. National Health and Nutrition Examination Survey (NHANES).
2. Methods
2.1. Study population
This study included women participating in MARBLES (Markers of Autism Risk in Babies – Learning Early Signs), a pregnancy cohort study in California beginning in 2006.56 MARBLES enrolls pregnant women who have a child with autism spectrum disorder (ASD) and thus are at high risk (~20%) for delivering another infant who develops ASD.57 MARBLES families are recruited from lists of children receiving services for ASD through the California Department of Developmental Services, from other studies, by self- or other referrals and various clinics. Details of study design, eligibility criteria for inclusion, recruitment, exposure data, sample size, and developmental diagnosis are available elsewhere.56
For the current study, we selected 218 women who provided first morning voids (FMVs) and/or 24-hour urine samples during pregnancy between 2007 and 2014. Among 218 women, 15 women participated in this study for two different pregnancies. All urine samples included in this study were collected from a total of 233 unique pregnancies from 218 women. This study was approved by the institutional review boards for the State of California and the University of California Davis (UC Davis). Participants provided informed consent prior to collection of data.
2.2. Urine sample collection
Women in the MARBLE Study were instructed to collect three FMVs (taken one week apart) and one 24-hour urine sample during each trimester of pregnancy. Participants were asked to place their urine samples in home freezers or refrigerators; these biosamples were collected during home visits and then transported to UC Davis, where they were thawed, aliquoted, and stored at −80 °C until analysis. To reduce sample analysis cost, for women who provided three or more samples within a trimester, we selected the first FMV as an individual sample and pooled remaining samples (including 24-hour samples if any) for that trimester.43, 58 After pooling, 760 samples (383 FMVs, 116 24-hour samples, 261 pools) from 233 pregnancies remained for chemical analysis. Although all women were asked to collect four samples (i.e., three FMVs and one 24-hour samples) per trimester, many did not. Thus, only 116 24-hour samples were available from 233 pregnancies. The details of collecting urine samples and methods for pooling multiple samples are described elsewhere.43, 58 The type and number of urine samples collected and analyzed in this study are summarized in the Supporting Information (SI, Figure S1).
2.3. Biomarker quantification
We shipped the urine samples in a 1-mL aliquot to the Laboratory of Exposure Assessment and Development for Environmental Research (LEADER), Rollins School of Public Health, at Emory University for biomarker analysis. At Emory University, we quantified urinary concentrations for our nine target compounds using two liquid chromatographic-tandem mass spectrometric (LC-MS/MS) methods. Details of analytical methods are described elsewhere10 and are also presented in the SI.
In the current study, the average relative percent difference (RPD) of repeated measures of quality controls (QCs) was below 11%, depending on the analyte and QC concentration. The laboratory also analyzed 16 blind duplicates for quality assurance. Replicate analyses for individual pairs of duplicate samples exhibited good agreement. The average RPD of 8 pairs of blind duplicates was 15%, ranging from 9% to 22%, depending on the analyte. The limits of detection (LOD) for BPA, BPF, BPS, TCC, TCS, BUPB, ETPB, MEPB, and PRPB were 0.8, 2.5, 0.5, 1.0, 15.0, 1.5, 0.5, 0.5, and 1.0 nanograms per milliliter (ng/mL), respectively (Table 1).
Table 1.
Distribution of specific gravity-corrected concentrations of phenols, parabens and TCC [ng/mL] in 760 urine samples (383 FMVs, 116 24-hour samples, 261 pools) collected from 233 pregnancies.
| Compounds | LOD [ng/mL] | % detect | All samples | FMV | Pool | 24-hr | FMV versus pools a | FMV versus 24-hr a | 24-hr versus pools a | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Percentiles | |||||||||||
| 5th | 50th | 95th | 50th | 50th | 50th | ||||||
| BPA | 0.8 | 59 | <LOD | 1.0 | 4.5 | 1.0 | 1.1 | 1.0 | 0.42 | 0.35 | 0.17 |
| BPF | 2.5 | 17 | <LOD | <LOD | 9.9 | <LOD | <LOD | <LOD | - | - | - |
| BPS | 0.5 | 14 | <LOD | <LOD | 2.8 | <LOD | <LOD | <LOD | - | - | - |
| TCC | 1.0 | 5 | <LOD | <LOD | 2.8 | <LOD | <LOD | <LOD | - | - | - |
| TCS | 15.0 | 32 | <LOD | <LOD | 285.5 | <LOD | <LOD | <LOD | - | - | - |
| MEPB | 0.5 | 96 | 0.6 | 38.9 | 388.0 | 36.2 | 41.4 | 48.9 | 0.78 | 0.60 | 0.58 |
| ETPB | 0.5 | 50 | <LOD | 0.7 | 44.3 | 0.7 | 0.8 | 0.7 | 0.90 | 0.54 | 0.54 |
| PRPB | 1.0 | 78 | <LOD | 8.3 | 98.6 | 8.5 | 7.8 | 8.2 | 0.34 | 0.94 | 0.43 |
| BUPB | 1.5 | 8 | <LOD | <LOD | 7.5 | <LOD | <LOD | <LOD | - | - | - |
Abbreviation: limit of detection (LOD), bisphenol A (BPA), bisphenol F (BPF), bisphenol S (BPS), triclocarban (TCC), triclosan (TCS), methyl paraben (MEPB), ethyl paraben (ETPB), propyl paraben (PRPB), butyl paraben (BUPB)
P-value from the Wilcoxon rank-sum test of the null hypothesis that two populations have the same distribution with the same median.
2.4. Correction for urinary dilution
We measured specific gravity (SG) of each analyzed urine sample with a digital handheld refractometer (Atago Co., Ltd., Tokyo, Japan) at UC Davis. We then corrected urinary concentrations of our target compounds for urinary dilution using the following formula:59 CSG = C[(1.012 – 1)/(SG-1)], where CSG is the SG-corrected urinary concentration (in ng/mL), C is the measured urinary concentration (in ng/mL), 1.012 is the median SG of all analyzed urine samples, and SG is the specific gravity of each sample.
2.5. Statistical analysis
We performed all statistical analyses using R version 3.6.160 and several packages.61–63 For all analyzed compounds, we provided summary statistics of SG-corrected urinary concentrations. For all other statistical analyses requiring sufficient detection of the samples, we only included compounds detected in 50% or greater of all samples. For concentrations below the LOD, we assigned a value of the LOD divided by the square root of 2.64, 65
To test for significant changes in urinary concentrations of the target compounds over our study period, we selected a priori six population characteristics that may influence exposure to our target compounds:45–54 race/ethnicity (non-Hispanic White, Hispanic, others), age at delivery (35 years, >35 years), education (less than college degree, Bachelor’s degree, graduate or professional degree), pre-pregnancy body mass index (BMI) (underweight/normal, overweight, obese), parity (1, >1), and homeownership (yes, no). Then, we performed multiple linear regression analyses. We used natural log-transformed urinary concentrations in the regression to account for their skewed distributions. To estimate the least square mean (LSM) which is the mean of natural log-transformed urinary concentrations for each sampling year, we used the estimated regression coefficients and the computed yearly-specific fractions or average of the selected covariates. Then, we computed the least square geometric mean (LSGM) of urinary concentrations for each sampling year as exp(LSM), with 95% confidence intervals (CIs) as exp(LSM ± 1.97·SELSM), where SELSM is the standard error of the LSM.66 We also computed average annual percent changes of LSGMs, using the equation [exp(β) – 1] × 100% with 95% CIs as [exp(β ± 1.97·SEβ) – 1], where β is the time-related regression coefficient and SEβ is the standard error of the time-related regression coefficients.66
We examined differences in urinary concentrations among three types of urine samples (i.e., FMV, 24-hour, pool) and among subgroups of population characteristics (e.g., race/ethnicity, age at delivery). To test for monotonic (or unadjusted) temporal trends in urinary concentrations over our study period, we performed the Mann-Kendall test and computed the Kendall’s tau correlation coefficient (τ) between sampling dates and urinary concentrations.
One of our study goals is to understand broad social forces that may influence exposure to our target compounds in the United States. Thus, to compare urinary concentrations in our study population with those in other study populations, we computed geometric means (GMs) of urinary concentrations for MARBLES pregnant women and NHANES’s pregnant and non-pregnant women (20 to 50 years of age).67 For pregnant women in Massachusetts, we used GMs during 2007–2009 from the LIFECODES cohort.68 For pregnant women in Puerto Rico, we used GMs during 2011–2016 from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort.45 Sampling time, sample size, participants’ age range, LODs, and GMs of urinary concentrations for each cohort are available in Table S1. Although the PROTECT, LIFECODES, and MARBLES studies measured SG-corrected concentrations while the NHANES studies did not conduct any correction, other studies have demonstrated that medians and temporal variability among SG-corrected concentrations and uncorrected concentrations are similar.69, 70
3. Results
3.1. Population characteristics
The average age of the participating women at delivery was 34.9 years old, ranging from 20.5 to 49.2 years old (Table S2). The women included in the study were 55% White, 21% Hispanic, and 24% other (3% Black, 18% Asian, and 3% multiracial). Approximately 43% of the women were normal or underweight and 44% of the women did not have a bachelor’s degree or a higher degree. Summary statistics of other population characteristics are available in Table S2.
3.2. Urinary concentrations of target compounds
Among the nine studied analyte compounds, four compounds were detected in 50% or more of all urine samples: BPA (59%), MEPB (96%), ETPB (50%), and PRPB (78%) (Table 1). The other five compounds (i.e., BPF, BPS, TCC, TCS, BUPB) were detected in less than 35% of the samples. For BPA, MEPB, ETPB and PRPB, concentrations were not significantly different (p-value > 0.05) in pair-wise comparisons of the three sample types (i.e., FMV versus pool, FMV versus 24-hour, pool versus 24-hour), indicating that FMVs can be interpreted together with 24-hour samples and pools in the current study. The detection frequency of BPA decreased from 67% to 42%, while that of BPS increased from 0% to 32% during the study period (Table 2). The detection frequency of MEPB, ETPB, and PRPB varied during the study period: 91–98% for MEPB, 40–61% for ETPB, and 71–88% for PRPB (Table 2). The highest median of SG-corrected concentrations was observed for MEPB (38.9 ng/mL), followed by PRPB, BPA, and ETPB (8.3, 1.0, and 0.7 ng/mL, respectively).
Table 2.
Detection frequencies of phenols, parabens and TCC and the number of samples for each sampling year.
| Year | No. of all samples | % of FMVs | % of Pools | % of 24-hour samples | Detection frequency (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | BPF | BPS | TCC | TCS | MEPB | ETPB | PRPB | BUPB | |||||
| 2007 | 51 | 51 | 38 | 11 | 67 | 22 | 0 | 12 | 25 | 96 | 61 | 88 | 8 |
| 2008 | 121 | 58 | 34 | 8 | 63 | 22 | 5 | 3 | 30 | 98 | 56 | 88 | 11 |
| 2009 | 181 | 54 | 30 | 16 | 58 | 18 | 9 | 4 | 38 | 96 | 46 | 71 | 9 |
| 2010 | 112 | 48 | 35 | 17 | 59 | 13 | 14 | 4 | 38 | 96 | 51 | 82 | 5 |
| 2011 | 67 | 48 | 38 | 14 | 55 | 10 | 6 | 3 | 33 | 96 | 40 | 76 | 7 |
| 2012 | 65 | 46 | 31 | 24 | 62 | 17 | 26 | 5 | 43 | 95 | 54 | 78 | 11 |
| 2013 | 106 | 49 | 36 | 15 | 62 | 17 | 26 | 2 | 22 | 94 | 46 | 72 | 5 |
| 2014 | 57 | 36 | 47 | 17 | 42 | 9 | 32 | 14 | 23 | 91 | 51 | 77 | 4 |
Abbreviation: bisphenol A (BPA), bisphenol F (BPF), bisphenol S (BPS), triclocarban (TCC), triclosan (TCS), methyl paraben (MEPB), ethyl paraben (ETPB), propyl paraben (PRPB), butyl paraben (BUPB)
3.3. Temporal trends of urinary phenol and paraben concentrations in California pregnant women in a high-familial risk ASD cohort
After adjusting for the selected covariates, the LSGMs of BPA, MEPB, ETPB, and PRPB in California pregnant women in a high-familial risk ASD cohort decreased over the study period [average percent change of the LSGM per year (95% CI): −5.7% (−8.2%, −3.2%); −13.0% (−18.1%, −7.7%); −5.5% (−11.0%, 0.3%); −13.3% (−18.3%, −8.1%), respectively] (Figure 1). For MEPB and PRPB, larger than 60% and 50% of the LSGM decrease occurred from 2007 to 2008, respectively. Temporal trends of unadjusted concentrations from the Mann-Kendall trend test were similar to those of adjusted concentrations from the regression (Figure S2). Urinary concentrations of BPA (τ = −0.11, p-value <0.01), MEPB (τ = −0.14, p-value <0.01), ETPB (τ = −0.06, p-value = 0.01), and PRPB (τ = −0.13, p-value <0.01) decreased over the study period.
Figure 1.
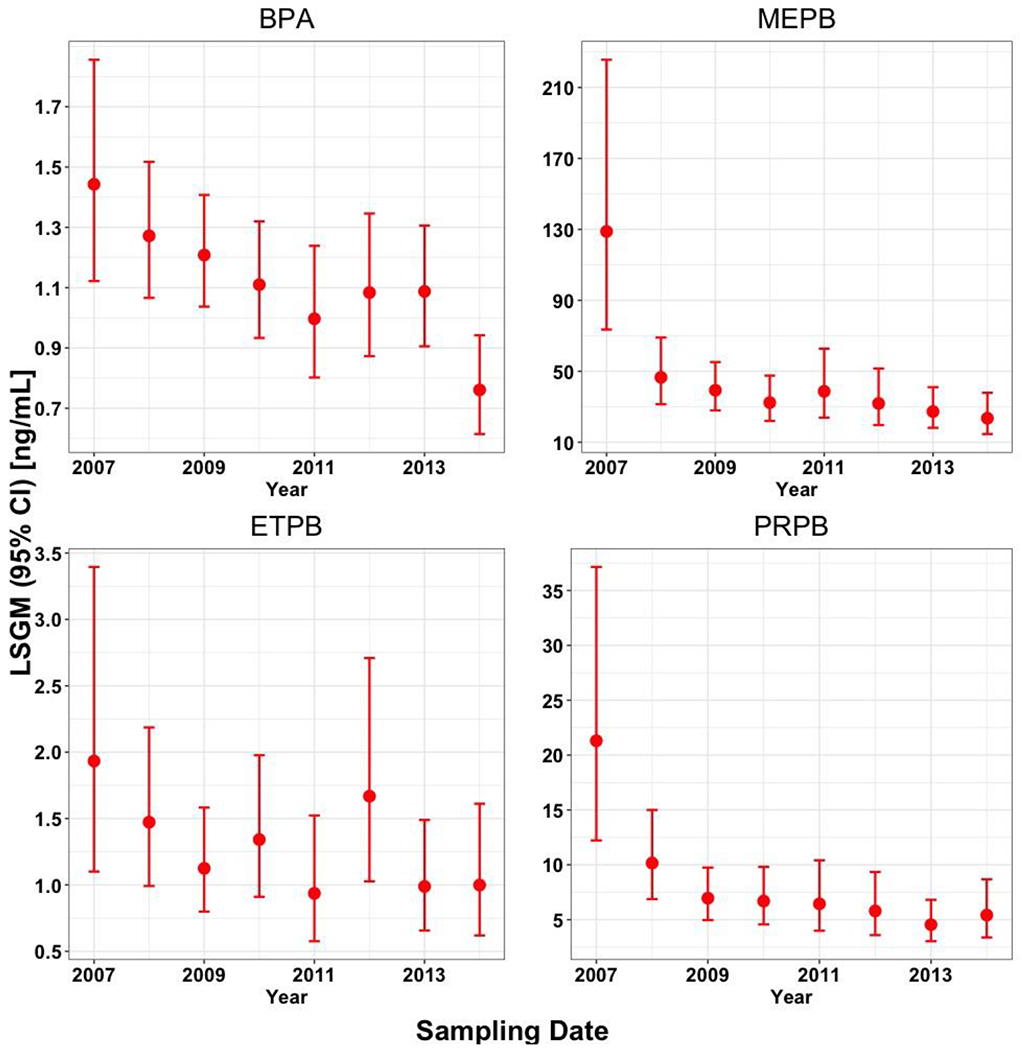
Temporal trends in measured urinary concentrations (ng/mL) of bisphenol A (BPA), methyl paraben (MEPB), ethyl paraben (ETPB), and propyl paraben (PRPB) in 760 urine samples collected from California pregnant women in a high-familial risk ASD cohort during 2007–2014. The urinary concentrations were adjusted for SG, sampling year, race/ethnicity, pre-pregnancy body mass index (BMI), education, age at delivery, homeownership, and parity. Data points represent LSGMs (least square geometric means or adjusted geometric means) and error bars represent 95% confidence intervals (CIs).
3.4. Temporal trends of urinary concentrations of phenols, parabens and TCC in all study populations
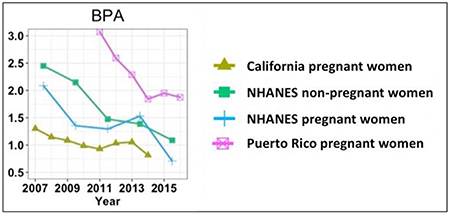
Overall, GM concentrations of BPA, MEPB, ETPB, and PRPB in California pregnant women in a high-familial risk ASD cohort were consistently lower than those in NHANES pregnant and non-pregnant women as well as pregnant women in Massachusetts and Puerto Rico during the same study period (Figure 2). Compared to NHANES non-pregnant women, GM concentrations in NHANES pregnant women were lower for BPA during most of the NHANES cycles. Puerto Rico pregnant women had higher GM concentrations of BPA, TCC, TCS, and BUPB than the current study population or NHANES pregnant and non-pregnant women during the same study period, while they had lower GM concentrations of BPF and BPS than NHANES non-pregnant women. Compared to other study populations, Puerto Rico pregnant women had relatively fast decreasing trends in GM concentrations of BPA, MEPB, ETPB, and PRPB, while they had relatively fast increasing trends in GM concentrations of BPS. MEPB and ETPB had a sharp decrease between 2007 and 2009 in our study and in other studies from the same time period. PRPB sharply declined in the earlier part of the study period in our study but did not decline much in other studies. Note that because of the relatively small sample size of NHANES pregnant women for each sampling year (Table S1), the results on their temporal trends and GM concentrations should be interpreted with caution.
Figure 2.
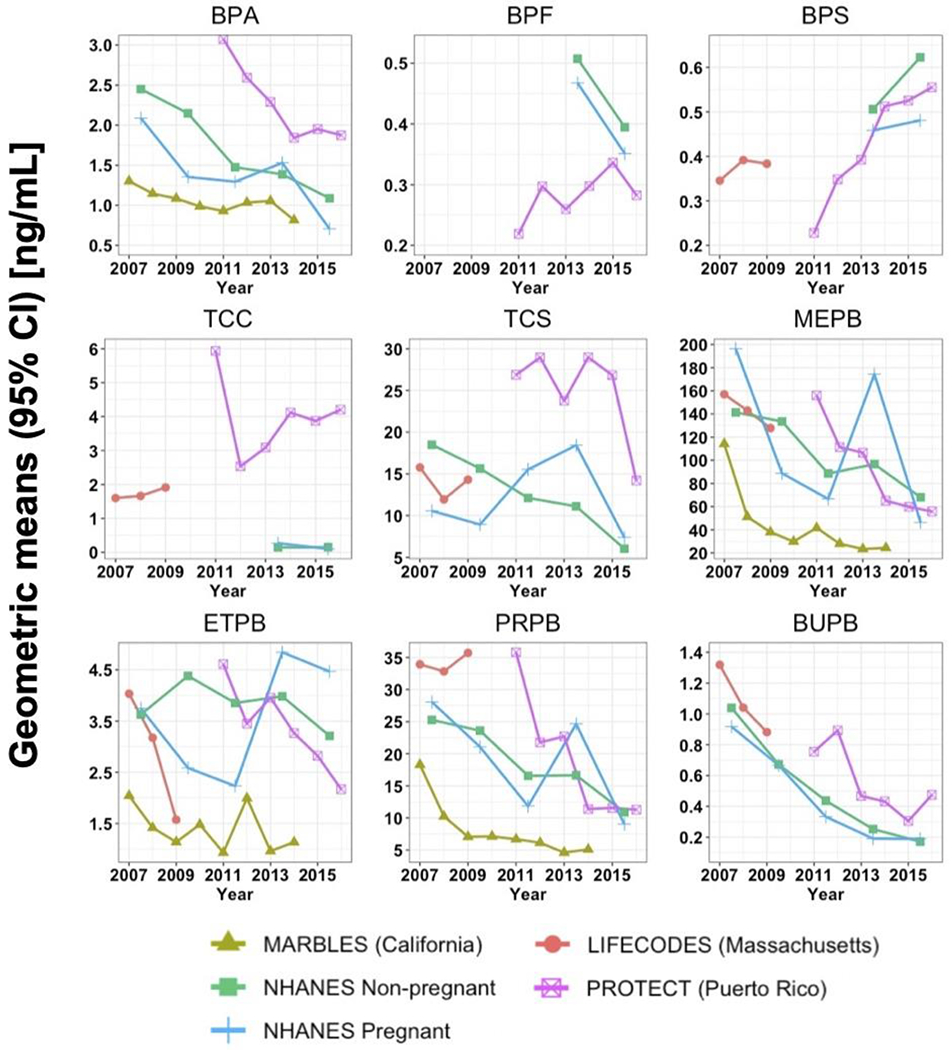
Geometric means (GMs) of urinary concentrations of bisphenol A (BPA), bisphenol F (BPF), bisphenol S (BPS), triclocarban (TCC), triclosan (TCS), methyl paraben (MEPB), ethyl paraben (ETPB), propyl paraben (PRPB), and butyl paraben (BUPB) [ng/mL] among different study cohorts between 2007 and 2016. GMs for MARBLES, PROTECT and LIFECODES were only adjusted for specific gravity and those for NHANES were not adjusted. Only non-pregnant women aged 20 to 50 years were included in U.S. NHANES non-pregnant women. Urinary concentrations for NHANES pregnant women were only available in a small sample size (range: 18–26 pregnant women), depending on the NHANES cycle, and thus should be interpreted with caution. BPS, BPF, TCC, TCS and BUPB were detected in less than 50% of the samples in MARBLES. BPA and BPF were not measured in LIFECODES. BPF, BPS, TCC were not measured in the NHANES during 2007–2012.
3.5. Urinary concentrations with different subgroups of population characteristics
For the four compounds detected in 50% or greater of the samples in the current study (i.e., BPA, MEPB, ETPB, PRPB), medians of urinary concentrations differed by several population characteristics (Table S3). Hispanic women had the highest median for three parabens (p-value < 0.05). Women with pre-pregnant BMI in a normal/underweight range had the highest median of BPA, MEPB, ETPB and PRPB. Women without a college degree had the highest median of BPA and PRPB. Women who owned a home (considered as a proxy of higher socioeconomic status) had lower medians for three parabens and a higher median for BPA than those who did not.
When examining trends in urinary concentrations of the four compounds (i.e., BPA, MEPB, ETPB, PRPB) within specific subgroups of population characteristics during the study period, three parabens (MEPB, ETPB, PRPB) did not change in Hispanic women or in women with pre-pregnant BMI in an overweight range (Table 3). BPA did not change in women with pre-pregnant BMI in an obese range or in women with a graduate or professional degree. ETPB also did not change in women in other race/ethnicity group, in women with pre-pregnant BMI in a normal/underweight range, in women without a college degree, in women who delivered at older ages (> 35 years old) or in women who did not own a home. Both ETPB and MEPB did not change in women with one parity. However, because the sample size for each demographic variable is not equally distributed over the years, these findings need to be interpreted with caution.
Table 3.
Trends in SG-corrected urinary concentrations [ng/mL] in 760 urine samples collected during 2007–2014.
| BPA | MEPB | ETPB | PRPB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | tau a | p-value b | tau | p-value | tau | p-value | tau | p-value |
| Race/ethnicity | |||||||||
| White (non-Hispanic) | 425 | −0.07 | 0.03 | −0.19 | <0.01 | −0.14 | <0.01 | −0.15 | <0.01 |
| Hispanic | 173 | −0.18 | <0.01 | 0.02 | 0.74 | 0.06 | 0.24 | −0.06 | 0.23 |
| Other c | 162 | −0.14 | 0.01 | −0.15 | 0.01 | 0.00 | 0.95 | −0.15 | 0.01 |
| Pre-pregnancy BMI | |||||||||
| Normal/ underweight | 335 | −0.08 | 0.04 | −0.15 | <0.01 | −0.07 | 0.05 | −0.19 | <0.01 |
| Overweight | 241 | −0.20 | <0.01 | −0.07 | 0.09 | 0.03 | 0.50 | −0.02 | 0.62 |
| Obese | 172 | −0.02 | 0.68 | −0.20 | <0.01 | −0.15 | <0.01 | −0.15 | <0.01 |
| Education | |||||||||
| Less than college degree | 361 | −0.14 | <0.01 | −0.08 | 0.02 | 0.01 | 0.77 | −0.08 | 0.02 |
| Bachelor’s degree | 278 | −0.11 | 0.01 | −0.19 | <0.01 | −0.12 | <0.01 | −0.16 | <0.01 |
| Graduate or professional degree | 121 | −0.10 | 0.12 | −0.20 | <0.01 | −0.16 | 0.01 | −0.18 | <0.01 |
| Age at delivery | |||||||||
| ≤ 35 years old | 404 | −0.13 | <0.01 | −0.15 | <0.01 | −0.08 | 0.02 | −0.14 | <0.01 |
| > 35 years old | 356 | −0.09 | 0.02 | −0.12 | <0.01 | −0.02 | 0.56 | −0.11 | <0.01 |
| Homeownership | |||||||||
| Yes | 280 | −0.12 | <0.01 | −0.12 | <0.01 | −0.10 | 0.02 | −0.09 | 0.03 |
| No | 466 | −0.12 | <0.01 | −0.13 | <0.01 | −0.02 | 0.56 | −0.15 | <0.01 |
| Parity | |||||||||
| 1 | 49 | −0.26 | 0.01 | −0.12 | 0.24 | −0.02 | 0.84 | −0.22 | 0.02 |
| >1 | 697 | −0.10 | <0.01 | −0.14 | <0.01 | −0.06 | 0.01 | −0.13 | <0.01 |
The number of missing values for pre-pregnancy BMI, homeownership, and parity are 12, 14, and 14, respectively.
Kendall’s tau from the Mann-Kendall trend test: negative and positive tau represent decrease and increase in urinary concentrations, respectively, over time.
P-values from the Mann-Kendall trend test less than 0.05 are highlighted in bold.
Includes Black (3%), Asian (18%), and multiracial (3%).
4. Discussion
In the current study, we examined temporal trends of urinary concentrations of phenols, parabens and TCC in California pregnant women participating in a high-familial risk ASD cohort. We observed that GM decreased from 1.44 to 0.78 (46%) for BPA, from 111.42 to 21.18 (81%) for MEPB, from 2.02 to 1.15 (43%) for ETPB, and from 18.00 to 5.39 (70%) for PRPB between 2007 and 2014. The detection frequency of BPS, a BPA replacement, increased from 0% to 32% during the same study period. When comparing our urinary concentrations with those in other study populations, urinary concentrations of BPA, MEPB, ETPB, and PRPB in California pregnant women were lower than those in other study populations during the same study period.
Overall, the decreasing trends of BPA, MEPB, and PRPB urinary concentrations in California pregnant women were also observed in NHANES’s non-pregnant women (Figure 2). Although we did not test differences in urinary concentrations among studies for each calendar year, we observed that the GMs of these three compounds and ETPB in California pregnant women were lower than those in NHANES women, both pregnant and non-pregnant, during the same period. Beause our participating women already have a child with ASD, they may have more concerns about products they were using such as personal care products or cosmetics. Thus, this comparision needs to be interpreted with caution. In addition, because California has been leading in protecting human health from chemical exposure via advocacy campaigns or legislation,35, 71 it is possible that California women may have been choosing products that do not contain chemicals such as our target compounds more frequently than women in other U.S. states. The decreasing trends of BPA in California pregnant women and NHANES’s women might be attributed to regulations against the use of BPA in cell phone cases, certain food contact polycarbonate products and thermal papers.72 Regulations enforced in other countries (e.g., EU, Japan) since the late 2000s may also play a role, at least in part, in the decreasing trends of urinary paraben concentrations among U.S. pregnant women, because the U.S. is the second largest importer for cosmetics in the world and approximately half of the imported cosmetics are originated from EU or Japan where some parabens are currently under regulations.73 Decreasing trends of MEPB, ETPB, and BUPB were observed in Massachusetts pregnant women,68 but this result should be interpreted cautiously because of a relatively short period of sample collection (i.e., 2007–2009). Note that BUPB was detected in fewer than 10% of the samples in the current study. Ashrap et al. also observed decreasing trends of two phenols (i.e., BPA, TCS) and four parabens (i.e., MEPB, ETPB, PRPB, BUPB) during 2011–2016 in pregnant women of Puerto Rico, a part of the U.S. territory.45 Thus, the decreasing trends in urinary concentrations of some phenols and parabens among U.S. study populations correspond temporally with domestic and/or international legislative actions or advocacy campaigns for reducing exposure to these compounds.
When comparing our annual average urinary concentrations of the target compounds with those observed in other study populations, we found that the levels and temporal trends of the concentrations were different across regions within the U.S. during the same period (Figure 2). For example, pregnant women in Puerto Rico had higher BPA, MEPB, ETPB, and PRPB concentrations than California pregnant women. In addition, compared to NHANES’s women, pregnant women in Puerto Rico had higher TCC, TCS, and BUPB concentrations but had lower concentrations of BPF, a substitute for BPA. Puerto Rico is a U.S. territory, but its median household income in 2019 was low (~$20,000) compared to that of California (~$80,000) and the entire U.S. (~$65,000).74 In addition, racial composition was different between California (39.4% Hispanic) and Puerto Rico (98.7% Hispanic).75 Thus, the considerable difference in household income and racial composition between Puerto Rico and other U.S. states may have contributed to the differences in exposure patterns (e.g., cosmetic use frequencies) and the magnitude of exposure sources (e.g., type of products containing BPA, MEPB, ETPB, and PRPB) for the study compounds. Two states, California and Massachusetts, had comparable median household incomes in 2019 ($78,105, $87,707, respectively), however, the Hispanic population was approximately three times higher in California (39.4%) than Massachusetts (12.4%). Nevertheless, we observed that Massachusetts pregnant women had higher MEPB, ETPB, and PRPB concentrations than California pregnant women during the same study period.
Trends in detection frequencies or urinary concentrations for BPA versus BPS were in opposite directions among all study populations, including the current study, NHANES women, and Puerto Rico pregnant women. These trends may have been influenced by nationwide regulatory efforts for reducing exposure to BPA 30, 31 and corresponding emergence of BPS as a substitute of BPA.32, 33 As experimental research showed that prenatal exposure to BPS has a neurotoxicity potential and abnormal behavioral function,76 monitoring the trend of pregnant women’s BPS exposure such as this study may help open up avenues for potential intervention of BPS exposure in the vulnerable populations (e.g., women of reproductive age, infants). For BPF, a clear trend was not observed in the detection frequency in California pregnant women during 2007–2014 (Table 2) and in the urinary concentrations in Puerto Rico pregnant women during 2011–2016 (Figure 2), but we observed a decreasing trend in NHANES’s women during 2013–2016 (Figure 2).
We observed potential determinants affecting urinary concentrations and their temporal trends of BPA and three parabens (Table S3, Table 3). For example, Hispanic pregnant women tended to have higher concentrations of three parabens (i.e., MEPB, ETPB, PRPB) than pregnant women in the two other subgroups of race/ethnicity, which was observed in a previous study of the U.S. general population using NHANES data.77 Compared to non-Hipanic White pregnant women, Hispanic pregnant women in our study had a lower rate of owning a home (54% versus 64%) or having at least a bachelor’s degree (28% versus 64%). In addition, because we observed higher BPA concentrations among women who owned a home than those who did not, exposures to our target compounds might be associated with socioeconomic status. Women with pre-pregnancy BMI in a normal/underweight range had the highest concentrations of three parabens (i.e., MEPB, ETPB, PRPB), which was observed in other studies,78, 79 which may have reflected differences in paraben metabolism between women with differing BMI and/or exposure (e.g., diet or product use).79 We observed higher ETPB concentrations in older women (> 35 years old) than in younger women (≤ 35 years old). These differences may be due to increased use of personal care products and cosmetics with age80 or from decreased metabolic or execretion rates with age.81, 82
Strengths of this study include concentrations of phenols, parabens and TCC in urine collected from pregnant women over a relatively long sample collection time (during 2007–2014). This enabled us to observe temporal trends in pregnant women’s exposure to these compounds after regulations or advocacy campaigns since the late 2000s to mid 2010s. In addition, from comparison of these compounds among mutiple U.S. study populations including NHANES women, we could find the potential effect of socioeconomic status, racial composition and broad social forces such as state advocacy campagins on pregnant women’s exposure to phenols, parabens and TCC. When comparing urinary concentrations between NHANES pregnant women and non-pregnant women, we found that pregnant women had consistently lower concentrations of BPA, BPF, and BPS than non-pregnant women during most of the NHANES study cycles. We note that the NHANES pregnant women data need to be interpreted cautiously because of their small sample size. However, this might be further evidence that pregnant women may have changed their use in cosmetics and other personal care products after becoming pregnant.42–44
Some limitations should be noted for this study. First, the exposure levels observed in our pregnancy cohort may not be representative of other U.S. pregnancy cohorts and California pregnant women, because our study participants may have changed their use of personal care products or cosmetics to reduce the risk of having another child with ASD. Second, because over two thirds of our urine samples were FMVs, which were collected a long time after product use or the last meal (i.e., approximately 9 hours of sleeping),83 this may have resulted in lower concentrations of BPA, MEPB, ETPB, and PRPB in our populations than NHANES women. In addition, the LODs were approximately 5 to 12 times higher in the current study than the other three studies (Table S1). Thus, it is likely that BPF, BPS, TCC, TCS, and BUPB were less frequently detected in our samples than the other three studies. Lastly, the relationship between several population characteristics and urinary concentrations (Table S3) should be interpreted with caution, because our target compounds are quickly excreted in urine with elimination half-lives of less than one day.84, 85
In summary, this study showed decreasing trends in urinary concentrations of BPA, MEPB, ETPB, and PRPB and increasing detection frequencies of BPS, a substitute for BPA, in California pregnant women during 2007–2014. This may reflect the U.S. nationwide regulation efforts and/or California advocacy campaigns since the late 2000s to mid 2010s for reducing exposure to phenols, parabens and TCC in women of reproductive age and infants. Comparison of our urinary concentrations with those in other U.S. study populations allowed us to observe geographic variations in pregnant women’s exposure to some phenols and parabens, which may be associated with differences in regulatory status, socioeconomic status, and racial composition as well as potential changes in product use after being pregnant. As other BPA alternatives that were not targeted for analysis in the current study (i.e., bisphenol A bis (2,3-dihydroxypropyl) ether, bisphenol A (3-chloro-2-hydroxypropyl) (2,3-dihydroxypropyl) ether, and bisphenol AF) were detected in U.S. residential indoor dust collected during 2015–2016,6, 8 future studies may benefit by measuring those BPA alternatives to gain insight into trends in total bisphenol exposure. As transparent product composition information is not readily available from all manufacturers, characterizing comprehensive temporal biomarker trends of phenols, parabens, and TCC in pregnant women will help us to understand the effects of regulations or advocacy compaigns on exposure trends and to identify chemicals with similar use or functions in products that are increasing over time.
Supplementary Material
Synopsis:
This study showed that body burden of some phenols and parabens in the U.S. pregnant women decreased over time, which appeared to reflect regulations or campaigns.
Acknowledgments
We would like to thank the MARBLES study participants for making this research possible. We would also like to acknowledge Grace Lee and Priya D’Souza for their contribution to laboratory analyses at Emory University’s LEADER. We also acknowledge Dr. Kelly K. Ferguson at the National Institute of Environmental Health Sciences (NIEHS) and Dr. John D. Meeker at the University of Michigan School of Public Health who kindly provided annual GM biomarker concentrations measured in LIFECODES and PROTECT studies, respectively. Lab and epidemiological data for the MARBLES cohort are hosted at the CHEAR Data Center Repository (https://cheardatacenter.mssm.edu/) under the following DOIs: 10.36043/CHEAR-2016-1449-UEP_Trim1, 10.36043/CHEAR-2016-1449-UEP_Trim2_3, 10.36043/CHEAR-2016-1449-Covars, 10.36043/CHEAR-2016-1449-Demo, 10.36043/CHEAR-2016-1449-Outcome,10.36043/CHEAR-2016-1449-Spec.
Funding
This research was supported by grants from the National Institutes of Health (R21-ES025551, R21-ES028131, R01-ES020392, R24-ES028533, P30-ES023513, P01-ES011269, U2C-ES026555, U2C-ES026560, P30-ES019776, U54-HD079125, UH3-OD023342), the U.S. Environmental Protection Agency (83543201), and the UC Davis MIND Institute.
Footnotes
Supporting Information
Details of biomarker quantification methods, geometric means of phenol, paraben and TCC urinary concentrations (ng/mL) from various study populations (Table S1), characteristics of study population (Table S2), medians of SG-corrected phenol and paraben urinary concentrations (ng/mL) by population characteristics (Table S3), type and number of urine samples analyzed in this study (Figure S1), and SG-corrected phenol and paraben urinary concentrations (ng/mL) in log 10 scale during our study period and p-values from the Mann-Kendall trend test (Figure S2).
Conflict of interest
The authors declare that they have no actual or potential competing financial interest.
Ethics approval and consent to participate
The MARBLES study protocol and this study were approved by the institutional review boards for the State of California, the University of California Davis (UC Davis), and the University of Texas Arlington (UT Arlington). Participants provided written informed consent before collection of any data.
References
- 1.Sanchis Y; Coscolla C; Corpas-Burgos F; Vento M; Gormaz M; Yusa V; Bettermilk project, Biomonitoring of bisphenols A, F, S and parabens in urine of breastfeeding mothers: Exposure and risk assessment. Environ Res 2020, 185, 109481. [DOI] [PubMed] [Google Scholar]
- 2.Calafat AM; Valentin-Blasini L; Ye X, Trends in Exposure to Chemicals in Personal Care and Consumer Products. Curr Environ Health Rep 2015, 2, 348–355. [DOI] [PubMed] [Google Scholar]
- 3.Liao C; Liu F; Kannan K, Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 2012, 46, 6515–6522. [DOI] [PubMed] [Google Scholar]
- 4.Bedoux G; Roig B; Thomas O; Dupont V; Le Bot B, Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ Sci Pollut Res Int 2012, 19, 1044–1065. [DOI] [PubMed] [Google Scholar]
- 5.Soni MG; Carabin IG; Burdock GA, Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 2005, 43, 985–1015. [DOI] [PubMed] [Google Scholar]
- 6.Kim K; Shin HM; Wong L; Young TM; Bennett DH, Temporal variability of indoor dust concentrations of semivolatile organic compounds. Indoor Air 2020, 31: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitro SD; Dodson RE; Singla V; Adamkiewicz G; Elmi AF; Tilly MK; Zota AR, Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-Analysis of U.S. Studies. Environ Sci Technol 2016, 50, 13661–13672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin HM; Moschet C; Young TM; Bennett DH, Measured concentrations of consumer product chemicals in California house dust: Implications for sources, exposure, and toxicity potential. Indoor Air 2020, 30, 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aker AM; Johns L; McElrath TF; Cantonwine DE; Mukherjee B; Meeker JD, Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ Int 2018, 113, 341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkoski JM; Busgang SA; Bixby M; Bennett D; Schmidt RJ; Barr DB; Panuwet P; Gennings C; Hertz-Picciotto I, Prenatal phenol and paraben exposures in relation to child neurodevelopment including autism spectrum disorders in the MARBLES study. Environ Res 2019, 179, 108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger K; Coker E; Rauch S; Eskenazi B; Balmes J; Kogut K; Holland N; Calafat AM; Harley K, Prenatal phthalate, paraben, and phenol exposure and childhood allergic and respiratory outcomes: Evaluating exposure to chemical mixtures. Sci Total Environ 2020, 725, 138418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson KK; Lan Z; Yu Y; Mukherjee B; McElrath TF; Meeker JD, Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates. Environ Int 2019, 131, 104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaKind JS; Naiman DQ, Temporal trends in bisphenol A exposure in the United States from 2003–2012 and factors associated with BPA exposure: Spot samples and urine dilution complicate data interpretation. Environ Res 2015, 142, 84–95. [DOI] [PubMed] [Google Scholar]
- 14.Mervish N; McGovern KJ; Teitelbaum SL; Pinney SM; Windham GC; Biro FM; Kushi LH; Silva MJ; Ye X; Calafat AM; Wolff MS; Bcerp, Dietary predictors of urinary environmental biomarkers in young girls, BCERP, 2004–7. Environ Res 2014, 133, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calafat AM; Weuve J; Ye X; Jia LT; Hu H; Ringer S; Huttner K; Hauser R, Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 2009, 117, 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philippat C; Mortamais M; Chevrier C; Petit C; Calafat AM; Ye X; Silva MJ; Brambilla C; Pin I; Charles MA; Cordier S; Slama R, Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 2012, 120, 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang R; Chen MJ; Ding GD; Chen XJ; Han XM; Zhou K; Chen LM; Xia YK; Tian Y; Wang XR, Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut 2013, 178, 115–120. [DOI] [PubMed] [Google Scholar]
- 18.Velez MP; Arbuckle TE; Fraser WD, Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril 2015, 103, 1011–1020. [DOI] [PubMed] [Google Scholar]
- 19.Buckley JP; Herring AH; Wolff MS; Calafat AM; Engel SM, Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children's Environmental Health Study. Environ Int 2016, 91, 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun JM; Yolton K; Dietrich KN; Hornung R; Ye X; Calafat AM; Lanphear BP, Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009, 117, 1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson-Browne MS; Papandonatos GD; Chen A; Calafat AM; Yolton K; Lanphear BP; Braun JM, Identifying Vulnerable Periods of Neurotoxicity to Triclosan Exposure in Children. Environ Health Perspect 2018, 126, 057001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guignard D; Gayrard V; Lacroix MZ; Puel S; Picard-Hagen N; Viguie C, Evidence for bisphenol A-induced disruption of maternal thyroid homeostasis in the pregnant ewe at low level representative of human exposure. Chemosphere 2017, 182, 458–467. [DOI] [PubMed] [Google Scholar]
- 23.Stroheker T; Chagnon MC; Pinnert MF; Berges R; Canivenc-Lavier MC, Estrogenic effects of food wrap packaging xenoestrogens and flavonoids in female Wistar rats: a comparative study. Reprod Toxicol 2003, 17, 421–432. [DOI] [PubMed] [Google Scholar]
- 24.Ji K; Hong S; Kho Y; Choi K, Effects of bisphenol s exposure on endocrine functions and reproduction of zebrafish. Environ Sci Technol 2013, 47, 8793–8800. [DOI] [PubMed] [Google Scholar]
- 25.Peretz J; Vrooman L; Ricke WA; Hunt PA; Ehrlich S; Hauser R; Padmanabhan V; Taylor HS; Swan SH; VandeVoort CA; Flaws JA, Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 2014, 122, 775–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siracusa JS; Yin L; Measel E; Liang S; Yu X, Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod Toxicol 2018, 79, 96–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geer LA; Pycke BFG; Waxenbaum J; Sherer DM; Abulafia O; Halden RU, Association of birth outcomes with fetal exposure to parabens, triclosan and triclocarban in an immigrant population in Brooklyn, New York. J Hazard Mater 2017, 323, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pycke BF; Geer LA; Dalloul M; Abulafia O; Halden RU, Maternal and fetal exposure to parabens in a multiethnic urban U.S. population. Environ Int 2015, 84, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towers CV; Terry PD; Lewis D; Howard B; Chambers W; Armistead C; Weitz B; Porter S; Borman CJ; Kennedy RC; Chen J, Transplacental passage of antimicrobial paraben preservatives. J Expo Sci Environ Epidemiol 2015, 25, 604–607. [DOI] [PubMed] [Google Scholar]
- 30.Food and Drug Administration (FDA), Indirect Food Additives: Polymers. Federal Register, 2012; Vol. 77, pp 41899–41902.
- 31.Food and Drug Administration (FDA), Indirect Food Additives: Adhesives and Components of Coatings. Federal Register, 2013; Vol. 78, pp 41840–41843.
- 32.Rochester JR; Bolden AL, Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect 2015, 123, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehmler HJ; Liu B; Gadogbe M; Bao W, Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration (FDA), Safety and Effectiveness of Consumer Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Federal Register, 2016; Vol. 81, pp 61106–61130. [PubMed]
- 35.California Safe Cosmetic Act (CSCA). Public Law 2005, https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/CSCP/Pages/CSCP.aspx. [accessed March 05, 2021].
- 36.EC Regulation (EC) NO 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (2009). Official Journal of the European Union: 2009. [Google Scholar]
- 37.EC Regulation (EU) No 1004/2014 amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and the Council on cosmetic products. (2014).
- 38.Association of Southeast Asian Nations (ASEAN), The ASEAN harmonized cosmetic regulatory scheme, Annexes of the ASEAN cosmetic directive. 2003. https://www.asean.org/wp-content/uploads/2012/10/20707.pdf [accessed May 10, 2021].
- 39.Han C; Lim YH; Hong YC, Ten-year trends in urinary concentrations of triclosan and benzophenone-3 in the general U.S. population from 2003 to 2012. Environ Pollut 2016, 208, 803–810. [DOI] [PubMed] [Google Scholar]
- 40.Moos RK; Koch HM; Angerer J; Apel P; Schroter-Kermani C; Bruning T; Kolossa-Gehring M, Parabens in 24 h urine samples of the German Environmental Specimen Bank from 1995 to 2012. Int J Hyg Envir Heal 2015, 218, 666–674. [DOI] [PubMed] [Google Scholar]
- 41.Frederiksen H; Nielsen O; Koch HM; Skakkebaek NE; Juul A; Jorgensen N; Andersson AM, Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int J Hyg Envir Heal 2020, 223, 93–105. [DOI] [PubMed] [Google Scholar]
- 42.Marie C; Cabut S; Vendittelli F; Sauvant-Rochat MP, Changes in Cosmetics Use during Pregnancy and Risk Perception by Women. Int J Env Res Pub He 2016, 13, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin HM; Bennett DH; Barkoski J; Ye X; Calafat AM; Tancredi D; Hertz-Picciotto I, Variability of urinary concentrations of phthalate metabolites during pregnancy in first morning voids and pooled samples. Environ Int 2019, 122, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodruff TJ; Zota AR; Schwartz JM, Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 2011, 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashrap P; Watkins DJ; Calafat AM; Ye X; Rosario Z; Brown P; Velez-Vega CM; Alshawabkeh A; Cordero JF; Meeker JD, Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: Predictors and trends. Environ Int 2018, 121, 990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortensen ME; Calafat AM; Ye X; Wong LY; Wright DJ; Pirkle JL; Merrill LS; Moye J, Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children's Study. Environ Res 2014, 129, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun JM; Just AC; Williams PL; Smith KW; Calafat AM; Hauser R, Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol 2014, 24, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun JM; Kalkbrenner AE; Calafat AM; Bernert JT; Ye X; Silva MJ; Barr DB; Sathyanarayana S; Lanphear BP, Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect 2011, 119, 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadaei S; Pourzamani H; Ebrahimpour K; Feizi A; Daniali SS; Kelishadi R, Investigating determinants of parabens concentration in maternal urine. Hum Ecol Risk Asses 2020, 27, 1–19. [Google Scholar]
- 50.Fisher M; MacPherson S; Braun JM; Hauser R; Walker M; Feeley M; Mallick R; Berube R; Arbuckle TE, Paraben Concentrations in Maternal Urine and Breast Milk and Its Association with Personal Care Product Use. Environ Sci Technol 2017, 51, (7), 4009–4017. [DOI] [PubMed] [Google Scholar]
- 51.Mahalingaiah S; Meeker JD; Pearson KR; Calafat AM; Ye X; Petrozza J; Hauser R, Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect 2008, 116, (2), 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meeker JD; Cantonwine DE; Rivera-Gonzalez LO; Ferguson KK; Mukherjee B; Calafat AM; Ye X; Anzalota Del Toro LV; Crespo-Hernandez N; Jimenez-Velez B; Alshawabkeh AN; Cordero JF, Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environ Sci Technol 2013, 47, (7), 3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polinski KJ; Dabelea D; Hamman RF; Adgate JL; Calafat AM; Ye X; Starling AP, Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ Res 2018, 162, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakhi AK; Sabaredzovic A; Papadopoulou E; Cequier E; Thomsen C, Levels, variability and determinants of environmental phenols in pairs of Norwegian mothers and children. Environ Int 2018, 114, 242–251. [DOI] [PubMed] [Google Scholar]
- 55.Shin HM; Dhar U; Calafat AM; Nguyen V; Schmidt RJ; Hertz-Picciotto I, Temporal Trends of Exposure to Phthalates and Phthalate Alternatives in California Pregnant Women during 2007–2013: Comparison with Other Populations. Environ Sci Technol 2020, 54, 13157–13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hertz-Picciotto I; Schmidt RJ; Walker CK; Bennett DH; Oliver M; Shedd-Wise KM; LaSalle JM; Giulivi C; Puschner B; Thomas J; Roa DL; Pessah IN; Van de Water J; Tancredi DJ; Ozonoff S, A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study. Environ Health Perspect 2018, 126, 117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ozonoff S; Young GS; Carter A; Messinger D; Yirmiya N; Zwaigenbaum L; Bryson S; Carver LJ; Constantino JN; Dobkins K; Hutman T; Iverson JM; Landa R; Rogers SJ; Sigman M; Stone WL, Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics 2011, 128, e488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin HM; Schmidt RJ; Tancredi D; Barkoski J; Ozonoff S; Bennett DH; Hertz-Picciotto I, Prenatal exposure to phthalates and autism spectrum disorder in the MARBLES study. Environ Health 2018, 17, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauser R; Meeker JD; Park S; Silva MJ; Calafat AM, Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 2004, 112, 1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2014. http://www.R-project.org/. [Google Scholar]
- 61.Endres CJ nhanesA: NHANES Data Retrieval, R package version 0.6.5.3; 2021. [Google Scholar]
- 62.Lenth RV, Least-Squares Means: The R Package lsmeans. J Stat Softw 2016, 69, 1–33. [Google Scholar]
- 63.Wickham H, ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York: 2016. [Google Scholar]
- 64.Antweiler RC, Evaluation of Statistical Treatments of Left-Censored Environmental Data Using Coincident Uncensored Data Sets. II. Group Comparisons. Environ Sci Technol 2015, 49, 13439–46. [DOI] [PubMed] [Google Scholar]
- 65.Hornung RW e. a., Estimation of average concentrations in the presence of nondetectable values. J Occup Environ Hyg 1990, 5, 46–51. [Google Scholar]
- 66.Zota AR; Calafat AM; Woodruff TJ, Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect 2014, 122, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention (CDC), Fourth National Report on Human Exposure to Environmental Chemicals. Vol 1, pp 1–866.
- 68.Ferguson KK; Meeker JD; Cantonwine DE; Mukherjee B; Pace GG; Weller D; McElrath TF, Environmental phenol associations with ultrasound and delivery measures of fetal growth. Environ Int 2018, 112, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quiros-Alcala L; Eskenazi B; Bradman A; Ye X; Calafat AM; Harley K, Determinants of urinary bisphenol A concentrations in Mexican/Mexican--American pregnant women. Environ Int 2013, 59, 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Philippat C; Wolff MS; Calafat AM; Ye XY; Bausell R; Meadows M; Stone J; Slama R; Engel SM, Prenatal Exposure to Environmental Phenols: Concentrations in Amniotic Fluid and Variability in Urinary Concentrations during Pregnancy. Environ Health Persp 2013, 121, 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zota AR; Singla V; Adamkiewicz G; Mitro SD; Dodson RE, Reducing chemical exposures at home: opportunities for action. J Epidemiol Commun H 2017, 71, 937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.California Environmental Protection Agency. 2021. Proposition 65: Safe Drinking Water and Toxic Enforcement Act of 1986. Public Law. https://oehha.ca.gov/proposition-65 [accessed May 11, 2021].
- 73.International Trade Centre, Trade Map. 2021https://www.trademap.org/Index.aspx [accessed May 10, 2021]
- 74.Guzman GG, Household Income: 2019. U.S. Census Bureau, 2020. https://www.census.gov/library/publications/2020/demo/p60-270.html [accessed Jan 05, 2021].
- 75.U.S. Census Bureau. 2019. U.S. Census Bureau QuickFacts. https://www.census.gov/quickfacts [accessed May 7, 2021].
- 76.Naderi M; Kwong RWM, A comprehensive review of the neurobehavioral effects of bisphenol S and the mechanisms of action: New insights from in vitro and in vivo models. Environ Int 2020, 145, 106078. [DOI] [PubMed] [Google Scholar]
- 77.Calafat AM; Ye X; Wong LY; Bishop AM; Needham LL, Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ Health Perspect 2010, 118, 679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koeppe ES; Ferguson KK; Colacino JA; Meeker JD, Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007–2008. Sci Total Environ 2013, 445, 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meeker JD; Yang T; Ye XY; Calafat AM; Hauser R, Urinary Concentrations of Parabens and Serum Hormone Levels, Semen Quality Parameters, and Sperm DNA Damage. Environ Health Persp 2011, 119, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang HS; Kyung MS; Ko A; Park JH; Hwang MS; Kwon JE; Suh JH; Lee HS; Moon GI; Hong JH; Hwang IG, Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ Res 2016, 146, 245–251. [DOI] [PubMed] [Google Scholar]
- 81.Shimokata H; Kuzuya F, Aging, basal metabolic rate, and nutrition. Nihon Ronen Igakkai Zasshi 1993, 30, 572–576. [DOI] [PubMed] [Google Scholar]
- 82.Mangoni AA; Jackson SH, Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004, 57, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu XM; Bennett DH; Lee K; Cassady DL; Ritz B; Hertz-Picciotto I, Longitudinal variability of time-location/activity patterns of population at different ages: a longitudinal study in California. Environ Health 2011, 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fransway AF; Fransway PJ; Belsito DV; Yiannias JA, Paraben Toxicology. Dermatitis 2019, 30, 32–45. [DOI] [PubMed] [Google Scholar]
- 85.Stahlhut RW; Welshons WV; Swan SH, Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ Health Perspect 2009, 117, 784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


