Abstract
Inhibiting matrix metalloproteinase (MMP) activity has been considered as a potential therapeutic treatment that may modify the outcome for osteoarthritis (OA), a disease governed by abnormalities in the balance between MMPs and their inhibitors. Due to unexpected tissue fibrosis in early-phase clinical trials with some MMP inhibitors, possible divergent effects of inhibiting MMP activity on different cells are hypothesized. Therefore, we evaluated the effects of MMP inhibition on cells relevant to cartilage tissue engineering by culturing them in vitro in poly(ethylene glycol) diacrylate hydrogels to create 3D representations of cartilage tissue while allowing for local and direct administration of inhibitors. Mesenchymal stem cells demonstrated an inhibitor concentration-dependent decrease in extracellular matrix (ECM) deposition, while normal chondrocytes were mainly affected at the highest concentration of inhibitors. In contrast, the concomitant treatment of chondrocytes from patients with OA resulted in an increase in glycosaminoglycan content only in the presence of both inhibitors and anabolic growth factors. The observed upregulation of bone markers, however, indicates a delicate balance that must be addressed to therapeutically treat OA chondrocytes to stimulate more ECM production without errant bone formation. In conclusion, this study suggests that MMPs have complex interactions in both pathobiology and homeostasis.
Keywords: osteoarthritis, matrix metalloproteinases, mesenchymal stem cells, chondrocytes, extracellular matrix, inhibitors
1. Introduction
The process of new tissue development and mature tissue remodelling is a balance of both extracellular matrix (ECM) production and degradation. Matrix metalloproteinases (MMPs) are a large family of zinc-dependent enzymes that play a role during these processes, as they degrade components of the ECM, thus producing changes in matrix composition, mechanical properties and bioactivity (Bramono et al., 2004; Clark and Parker, 2003). MMPs are also critical in stem cell differentiation and ultimately new tissue production (Chun et al., 2006). Alterations in the balance of matrix production and MMP-related degradation are a prevalent characteristic of numerous diseases, including osteoarthritis (OA). However, the specific roles of MMPs during cartilage tissue development and the pathogenesis of OA remain unclear (Murphy and Nagase, 2008).
Tissue-engineering research generally focuses on building matrix as quickly as possible to restore tissue form and function. There has been limited investigation into the role of matrix degradation in tissue engineering, despite degradation and remodelling being critical components of normal embryological development (Balbin et al., 2001; Kinoh et al., 1996). Clinical applications of tissue engineering may be especially appropriate for the treatment of underlying diseases characterized by abnormal matrix destruction. OA is one such disease, characterized by cartilage tissue damage driven by inflammatory cytokines and mediators and excessive matrix destruction caused by MMPs (Goldring and Goldring, 2004, 2007; Goldring, 2000). Understanding the functional roles of MMPs in both normal tissue growth and aberrant tissue repair is critical to better comprehending the OA disease process and to further the development of tissue-engineering approaches as a possible therapeutic intervention for OA.
In this study, the effects of inhibiting MMP activity were evaluated in three different cell sources relevant for cartilage tissue engineering and joint homeostasis: bone marrow-derived mesenchymal stem cells (MSCs), normal chondrocytes and chondrocytes derived from patients diagnosed with OA. Since the effects of MMP activity on different cell types cultured under 3D conditions are relatively unknown, all the cells used in this study were encapsulated in poly(ethylene glycol) diacrylate (PEGDA) hydrogels, a three-dimensional (3D) scaffold with few cellular or extracellular matrix interactions that supports cartilage production in vitro and in vivo (Sharma et al., 2007; Williams et al., 2003). We hypothesize that inhibiting the activity of the MMPs will affect the phenotype and ECM production of the different cell types in the hydrogels to varying degrees. These results will be crucial for elucidating the functional role of MMP inhibition on different cells while developing therapeutic modalities for reducing disease and simultaneously promoting tissue regeneration.
2. Materials and methods
2.1. Cell isolation
Bone marrow-derived mesenchymal stem cells (MSCs) were isolated from male goats and cultured until the end of passage 5, as previously described (Williams et al., 2003). Normal articular chondrocytes were removed from the patellofemoral groove and femoral condyles of 5–8 week-old calves, as previously described (Kim et al., 2003). Human OA articular chondrocytes were taken from the condyles and tibial plateaux of patients undergoing total knee arthroplasty. All human samples were received from the National Disease Resource Institution (Philadel-phia, PA, USA) according to an IRB-approved protocol. The ages of the human donors were 54 and 59 years.
All cartilage explants were digested on an orbital shaker for 16–20 h at 37 °C with 5% CO2 in a collagenase solution composed of 0.17% w/v type II collagenase (Worthington Biochemical, Lakewood, NJ, USA) in high-glucose DMEM with 6% FBS. Upon completion of digestion, the filtrate was passed through a 70 μm strainer and cells were rinsed thoroughly with phosphate buffer saline and 100 unit/ml penicillin and 100 μg/ml steptomycin (PBS-PS) before preparation for experiments.
Primary normal chondrocytes were used for hydrogel encapsulation, while OA chondrocytes were always cultured in monolayer with chondrocyte growth medium for a single passage to obtain sufficient cell numbers before encapsulation.
2.2. Cell encapsulation in poly(ethylene-glycol) diacrylate
Three-dimensional constructs were formed by encapsulating cells in poly(ethylene glycol) diacrylate (PEGDA), using ultraviolet (UV) photopolymerization. Cells were mixed in a 10% PEGDA solution in PBS-PS at a density of 20 million cells/ml. Using a cylindrical mould with a diameter of about 5 mm, the cell/polymer solution was mixed with the photoinitiator (Irgacure 2959), which was initially dissolved in 70% ethanol to create a final concentration of 0.05% w/v within the solution and then photopolymerized with UV light at 3 mW/cm2 with a wavelength of 365 nm for 5 min. Upon removal from the cylindrical moulds, the constructs were transferred to 24-well plates and cultured with MMP inhibitors (Figure 1A) in either chondrocyte growth medium or chondrogenic medium with transforming growth factor-β1 (TGFβ1), depending on the experiment. All constructs were cultured on an orbital shaker at 37 °C with 5% CO2 for 3 weeks.
Figure 1.
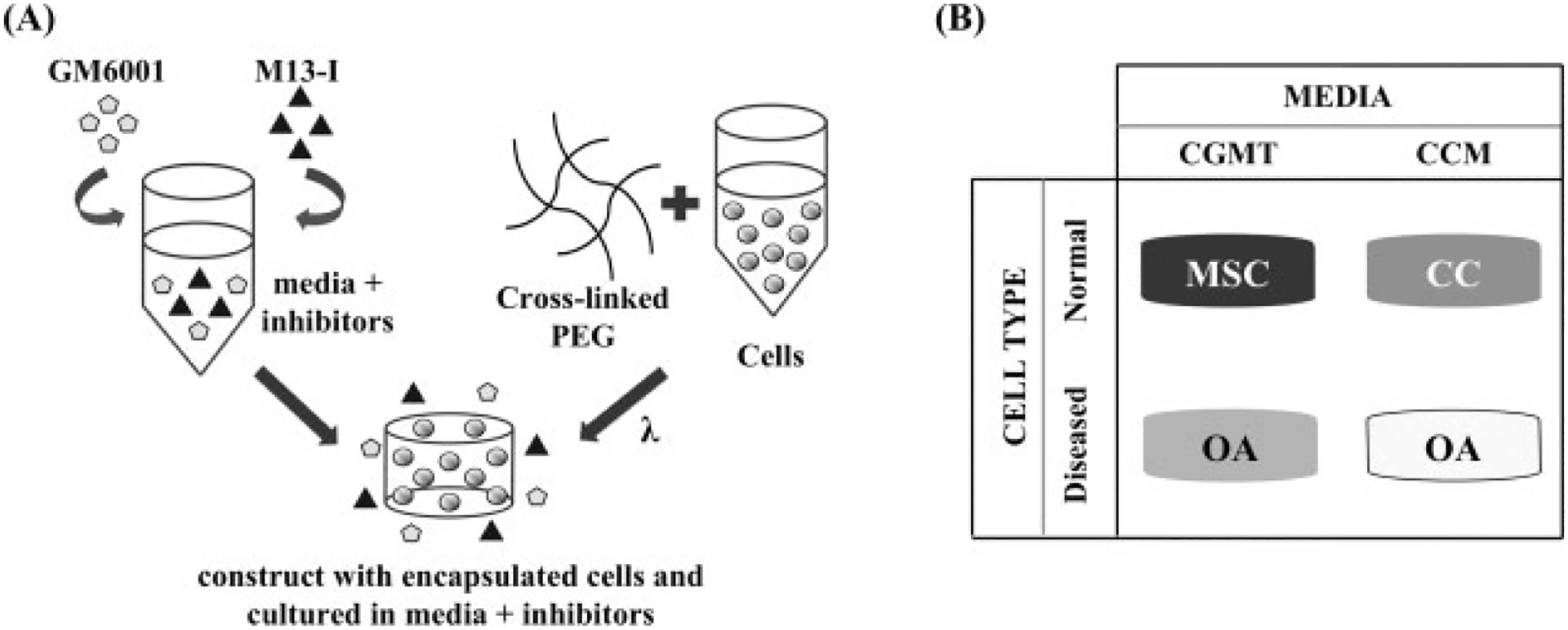
Experimental set-up for inhibiting MMP activity in different cell types. (A) GM6001 and a synthetic MMP-13 inhibitor were added exogenously into the media and then administered to cells that were encapsulated in crosslinked PEGDA through UV photopolymerization. (B) The four different conditions of this study included administering MMP inhibitors to MSCs and OA chondrocytes cultured in chondrogenic medium with TGFβ1 (CGMT) and normal chondrocytes (CC) and OA chondrocytes cultured in chondrocyte growth medium (CCM)
2.3. Media conditions
Chondrocyte growth medium (CCM) consisted of high-glucose Dulbecco’s modified Eagle’s medium (DMEM), 10% FBS (Hyclone Laboratories), 0.1 mM MEM non-essential amino acids solution (Invitrogen), 10 mM HEPES (Invitrogen), 50 μg/ml ascorbic acid (Sigma), 0.4 mM proline (Sigma), 1 mM sodium pyruvate (Invitrogen) and 100 unit/ml penicillin and 100 μg/ml streptomycin (Invitrogen). Chondrogenic medium with TGFβ1 (CGMT) consisted of high-glucose DMEM, 100 nM dexamethasone (Sigma), 40 μg/ml proline (Sigma), 50 μg/ml ascorbic acid-2-phosphate (Sigma), 0.9 mM sodium pyruvate (Invitrogen), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen), 1% ITS+ Universal Culture Supplement Premix (BD Bioscience) and 10 ng/ml TGFβ1 (RDI Fitzgerald). The TGFβ1 was added to the chondrogenic medium at every medium change. All medium changes occurred every 2–3 days.
2.4. Inhibition of MMP activity and experimental conditions
Two MMP inhibitors (MMPis) were used in this experiment: GM6001 (EMD Bioscience), which is a broad-spectrum inhibitor that effectively targets MMP-1, -2, -3, -8 and -9, and a synthetic inhibitor that selectively inhibits MMP-13 (EMD Bioscience; denoted M13-I). The carrier vehicle for the inhibitors was dimethyl sulphoxide (DMSO; Sigma). For every medium change, both inhibitors were administered exogenously into the surrounding medium to achieve final concentrations of 0 (or control), 1, 10 or 25 μM of both inhibitors. The final concentration of the DMSO that was exposed to the cells was 0.1%. The control consisted of only 0.1% DMSO with no inhibitors.
The experimental conditions are summarized in Figure 1B. MSCs were cultured with CGMT and the four concentrations of MMP inhibitors. Normal chondrocytes were cultured in CCM with the four concentrations of MMP inhibitors. One set of OA chondrocytes were cultured with CCM and the four concentrations of MMP inhibitors. The second set of OA chondrocytes was cultured in CGMT with three concentrations of MMP inhibitors (0, 10 and 25 μM).
2.5. Zymography
Pre-cast gelatin zymogram gels (Bio-Rad) were used to detect gelatinase activity from the supernatant of cultured constructs. Bicinchoninic acid (BCA) assay was conducted to determine protein content, and equal quantities of protein were loaded into the pre-cast gels. After electrophoresis, the gels were renatured and developed through Bio-Rad buffers. Coomassie blue dye was used to stain the gels, and the destaining solution revealed the gelatinase activity. Images of the gels were inverted so that dark zones indicate MMP activity. MMP-2 (gelatinase A) activity was confirmed by the molecular weight of a MMP-2 standard (Anaspec), as well as inhibition of the activity with GM6001.
2.6. Biochemical analysis
Constructs were harvested at day 21, lyophilized for 48 h and then homogenized with papain digestion buffer (Worthington Biomedical, Lakewood, NJ, USA) and digested for 16 h at 60 °C, as previously described (Williams et al., 2003). DNA content was measured using fluorescence from the low-assay Hoechst 33 258 dye (Molecular Probes, Eugene, OR, USA). Varying concentrations of calf thymus DNA (Invitrogen) were used to generate the standard curve. All standards and sample values were measured on a fluorometer at 365 nm excitation and 458 nm emission, and DNA values were normalized to the dry weights of the respective constructs. Glycosaminoglycan (GAG) quantity was determined with the dimethylmethylene blue (DMMB) dye assay. Chondroitin sulphate C was used to generate the standard curve, and all standards and papain samples were mixed with the dye, while absorbance was measured at 525 nm on an UV-vis spectrophotometer. Total collagen content was calculated with the hydroxyproline assay. Briefly, papain-digested samples were hydrolysed with HCl for 18 h at 115 °C, mixed with methyl red and titrated with varying concentrations of NaOH and HCl. trans-4-Hydroxy-L-proline (Sigma Aldrich, St. Louis, MO, USA) was used to generate the hydroxproline standard curve, and both standards and samples were mixed with chloramine-T hydrate and p-dimethylamino ben-zaldehyde. Absorbance values were measured at 550 nm and the ratio of 1:10 hydroxyproline:collagen was used to calculate total collagen content. For each of the assays, ECM content was normalized to the respective DNA content.
2.7. Histology
For histological staining, constructs were fixed in 4% paraformaldehyde and then dehydrated in increasing concentrations of ethanol before being placed in xylene. Constructs were then embedded in paraffin and sliced in 5 μm thick sections on the microtome. The sections were stained with Safranin-O/fast green (SchoLAR Chemistry) to observe GAG production, and immunostained with type II collagen primary antibody, using a 1:100 dilution factor (RDI Fitzgerald).
2.8. RNA extraction and polymerase chain reaction
The constructs were harvested and homogenized in TRIzol Reagent (Invitrogen) before extraction following the manufacturer’s instructions. Complementary DNA was then formed using the reverse-transcriptase Superscript First-Strand Synthesis kit (Invitrogen). Quantitative PCR was conducted on the following genes:
aggrecan (human/goat primers: forward (F), CACGATGCCTTTCACCACGAC; reverse (R), TGCGGGTCAACAGTGCCTATC; bovine primers: F, CATCGGGCTTGCCAGAGTT; R, ACTGGTGTCCACGAACGTAATG)
type II collagen (F, GAAACCATCAATGGTGGCTTCC; R, CGATAACAGTCTTGCCCCACTT)
cbfa-1 (F, CCACCCGGCCGAACTGGTCC; R, CCTCG TCCGCTCCGGCCCACA)
osteopontin (F, GGACTCCATTGACTCGAACG; R, TACTGGATGTCAGGTCTGCG)
bone sialoprotein (F, AGATGACAGTTCAGAAGAGG; R, TGTGTGCTGTTGGTACTGGT)
β-actin (F, TGGCACCACACCTTCTACAATGAGC; R, GCACAGCTTCTCCTTAATGTCACGC)
All genes were analysed in triplicate and normalized to β-actin, using the 2−ΔΔct method (Livak and Schmittgen, 2001). The reactions were conducted on the ABI Prism 7700 Sequence Detection System (Perkin Elmer/Applied Biosystems, Rotkreuz, Switzerland), using SYBR Green PCR Master Mix (ABI).
2.9. Statistical analysis
All analyses were done in triplicate or n = 3, and analysed with Student’s t-test for pairwise comparison. Statistical significance was noted for all p values <0.05: *p < 0.05; **p < 0.01; ***p < 0.001. All chart data represented in the figures depict the mean and error bars represent one standard deviation (SD).
3. Results
3.1. Inhibition of MMP activity in MSCs undergoing chondrogenesis
Cells encapsulated in PEGDA hydrogel were cultured in the presence of two MMP inhibitors (MMPis), GM6001 and a synthetic MMP-13 inhibitor to target MMP-1, -2, -3, -8, -9 and -13 simultaneously. Zymography demonstrated decreasing MMP activity as MMPi concentrations increased. Specifically, gelatin zymography conducted on the supernatant collected at day 21 of culture demonstrated a dose-dependent decrease in MMP-2 activity of both latent (~72 kDa) and active (~68 kDa) forms of the protease as MMPi concentrations increased from 0 to 25 μM (Figure 2A).
Figure 2.
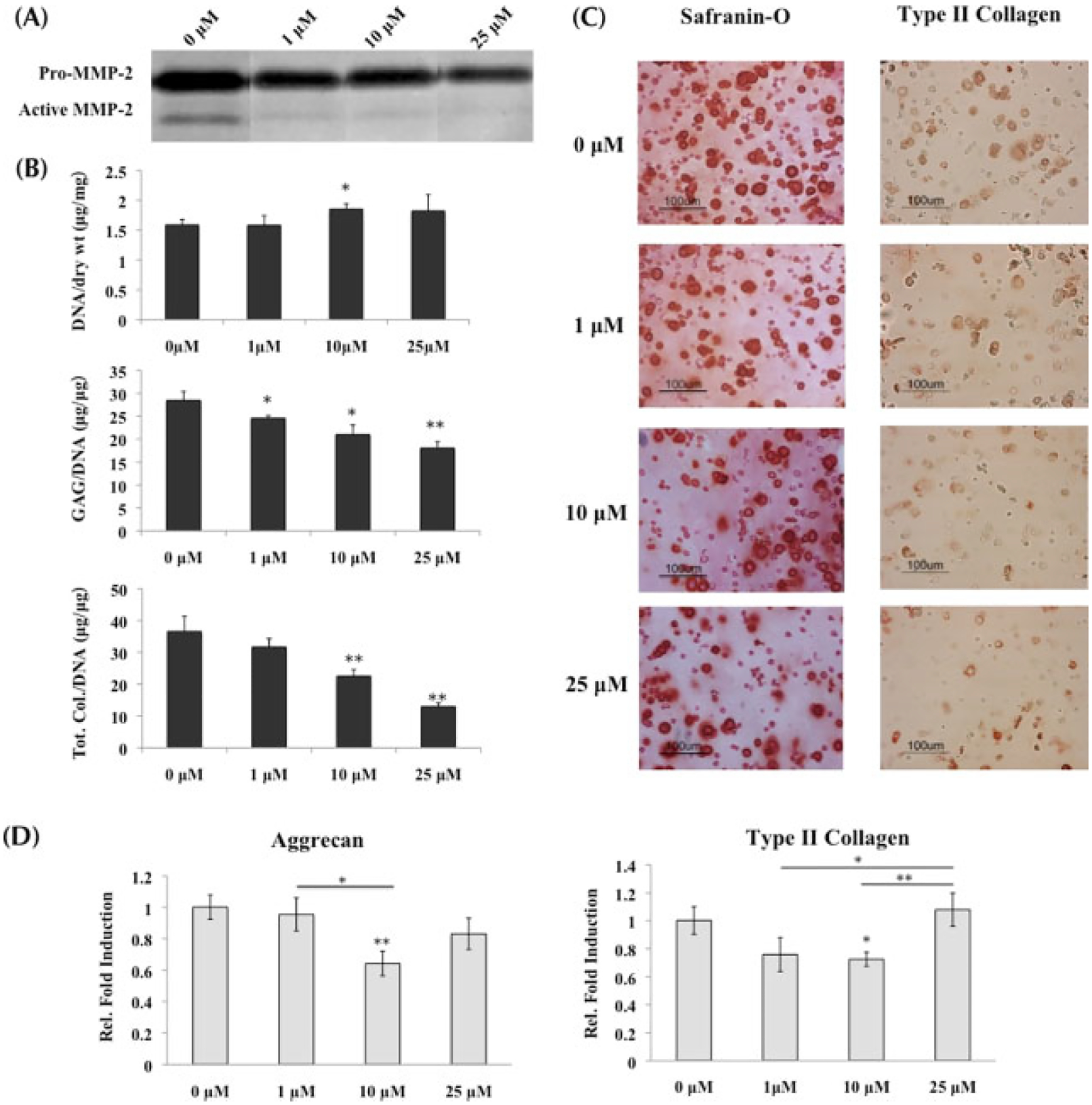
MSCs undergoing chondrogenesis with inhibition of MMP activity; *statistical significance from control or 0 μM, unless otherwise specified. (A) Gelatin zymography conducted on supernatant collected at day 21 verified the decrease of MMP-2 activity, both latent (denoted by ‘pro’; ~72 kDa) and active (~68 kDa), as inhibitor concentrations increased. (B) GAG and total collagen content (n = 3) decreased significantly as MMPis increased, with the most significant changes at 10 and 25 μM. Both sets of data were normalized to DNA content. (C) Histological staining with Safranin-O and type II collagen immunostaining confirmed the decrease in ECM content from (B). (D) Real-time PCR (n = 3) of aggrecan and type II collagen indicated that at 10 μM, transcription of both genes was significantly downregulated when compared to control. However, at 25 μM, transcription increased to being comparable to the control, suggesting that the MSCs were compensating for the low ECM production by increasing gene transcription
MMPis had a significant impact on tissue production by stem cells. Administering both MMPis to MSCs undergoing chondrogenic differentiation resulted in decreased GAG and total collagen content, which was normalized to DNA (Figure 2B). Specifically, there was a concentration-dependent decrease of the ECM with increasing MMPi concentration, which culminated in a 37% drop of GAG content and 65% drop of total collagen content in the presence of 25 μM MMPis when compared to control. Histological staining for Safranin-O and type II collagen immunolabelling supported the biochemical results, showing a decrease in staining for both GAG and type II collagen with MMP inhibition (Figure 2C).
Quantitative PCR of cartilage-specific ECM components also supported the decrease in tissue production with MMP inhibition. Aggrecan gene expression was downregulated at 10 μM compared to encapsulated cells with either 0 or 1 μM inhibitor exposure, and then returned to comparable expression level after MSCs were cultured with 25 μM MMPis (Figure 2D). Type II collagen was also downregulated significantly at 10 μM while returning to control expression at 25 μM.
3.2. Inhibiting MMP activity in normal chondrocytes
MMP activity in normal chondrocytes was also successfully downregulated in the presence of MMPis. Gelatin zymography on the supernatant collected on day 21 of culture demonstrated a dose-dependent decrease in MMP-2 activity as the MMPi concentrations increased from 0 to 25 μM (Figure 3A).
Figure 3.
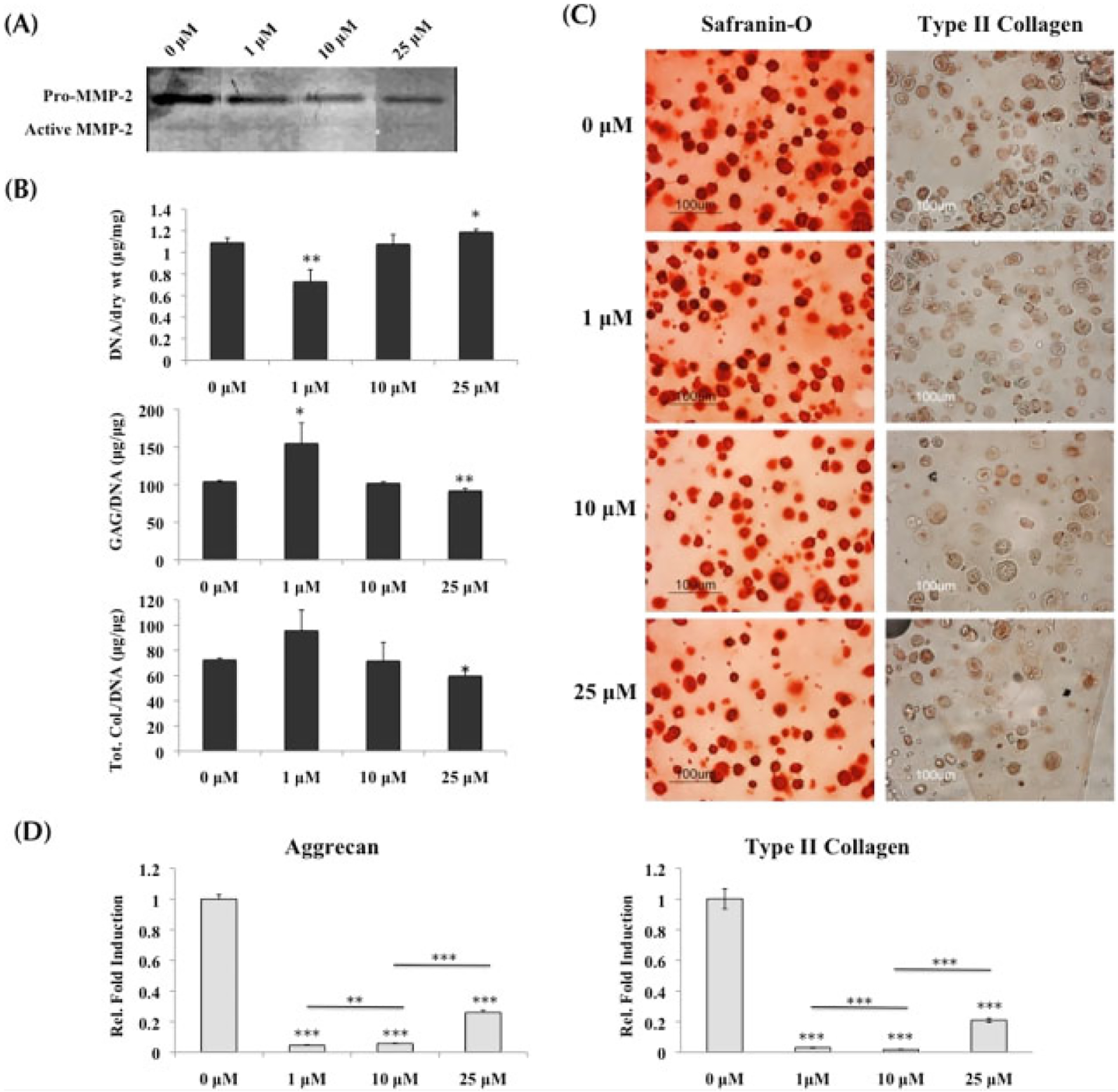
Normal chondrocytes cultured in growth medium with MMP inhibitors; *statistical significance from control or 0 μM, unless otherwise specified. (A) Gelatin zymography on supernatant collected at day 21 demonstrated the decrease in MMP-2 activity as inhibitor concentrations increased. (B) Quantification of biochemical analysis (n = 3) indicated that at 1 μM GAG content was increased, while at 25 μM both GAG and total collagen content were significantly decreased when compared to control. Both sets of data were normalized to DNA content. (C) Safranin-O staining and type II collagen immunostaining demonstrated a decreasing trend of matrix production as the MMPi concentrations increased. (D) Real-time PCR (n = 3) indicated that at 1 and 10 μM, both aggrecan and type II collagen were significantly downregulated from control, while at 25 μM a small upregulation in transcription occurred. However, it was still significantly lower than control
Normal chondrocytes demonstrated similar trends to the MSCs when producing tissue matrix in the presence of MMPis. While there was an increase in ECM production at the lowest concentration of inhibitors, both GAG and total collagen content per DNA were significantly decreased at 25 μM inhibitors, resulting in a 12% drop of GAG content and a 18% drop of total collagen content when compared to untreated chondrocytes (Figure 3B). Histological staining for Safranin-O and type II collagen immunolabelling supported the decreasing trend in ECM production as MMPi concentrations increased (Figure 3C).
Transcription for cartilage-specific ECM genes was significantly downregulated when MMP proteolytic activity was inhibited (Figure 3D). Both aggrecan and type II collagen dropped significantly in the presence of 1 and 10 μM MMPis. Although a slight increase in transcription was observed with 25 μM of MMPis, it was still notably lower than control.
3.3. Inhibiting MMP activity in OA chondrocytes cultured in growth medium
Similar to the MSCs and normal chondrocytes, the presence of MMPis resulted in decreasing MMP activity in OA chondrocytes. Gelatin zymography indicated that increasing MMPi concentrations resulted in less MMP-2 activity in the supernatant collected at day 21 of culture (Figure 4A). However, no significant change from control was observed in ECM production when OA chondrocytes were cultured in growth medium and treated with MMPis (Figure 4B). This observation was confirmed in histological staining of Safranin-O and type II collagen immunolabelling (Figure 4C).
Figure 4.
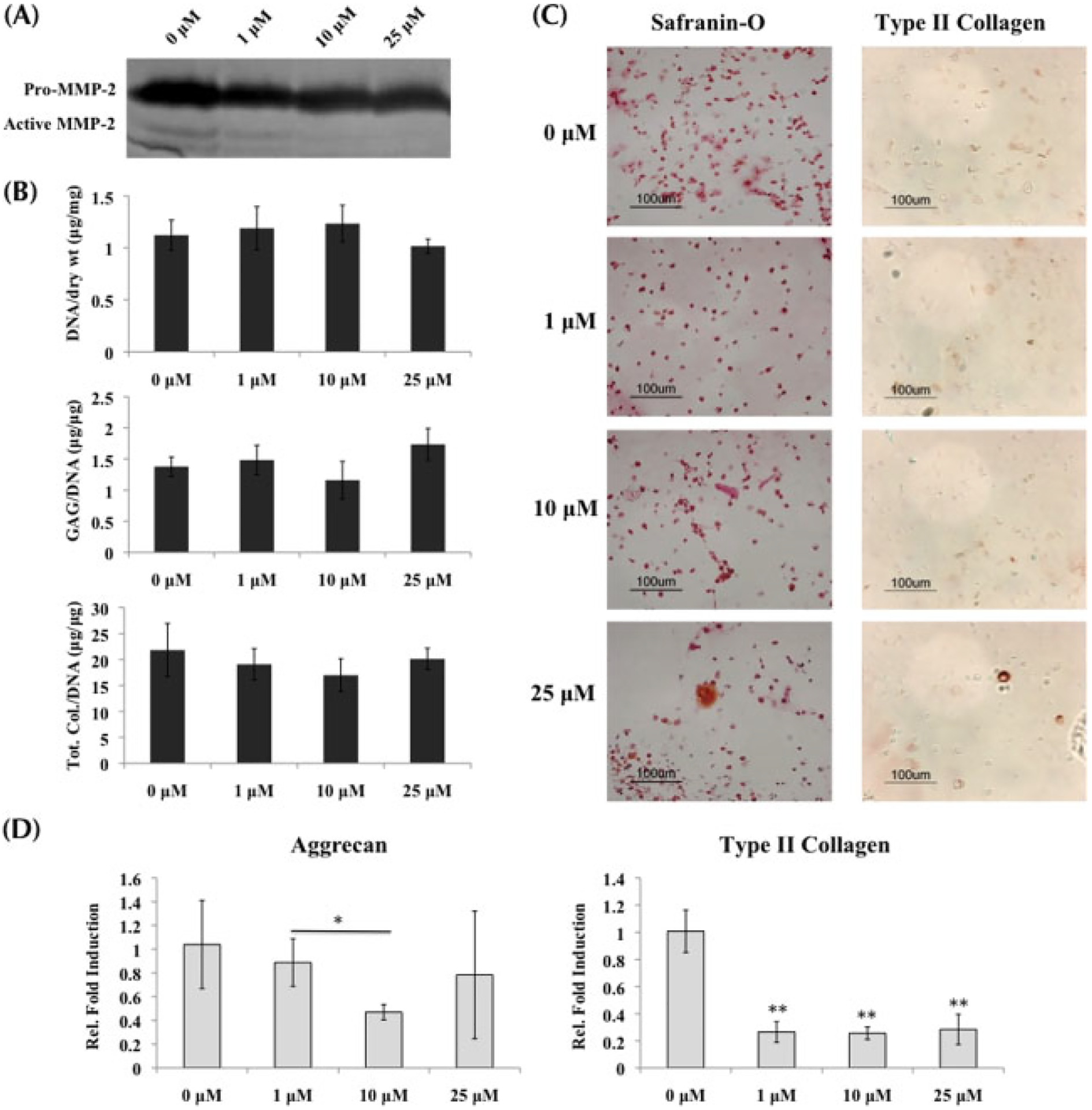
OA chondrocytes cultured in growth medium with MMP inhibitors; *statistical significance from control or 0 μM, unless otherwise specified. (A) Gelatin zymography verified the decreasing activity of MMP-2 in supernatant collected at day 21 as MMPi concentrations increased. (B) Biochemical analysis (n = 3) indicated no change from control in both GAG and total collagen content per DNA when MMPis were administered. (C) Histological staining for GAG and type II collagen supported the ECM results in (B). (D) Real-time PCR (n = 3) indicated that aggrecan transcription was downregulated in the presence of 10 μM of MMPis, while type II collagen transcription was decreased in the presence of all MMPi concentrations
Quantified expressions of cartilage-specific genes indicated a slight downregulation in aggrecan expression after 10 μM MMPis, while type II collagen was decreased at all three concentrations of MMPis (Figure 4D). However, when analysing bone markers, both cbfa-1, an indicative marker of hypertrophy, and osteopontin were significantly decreased in the presence of MMPis (Figure 6A, B). Bone sialoprotein was downregulated only at the highest concentration of MMPis (Figure 6C).
Figure 6.
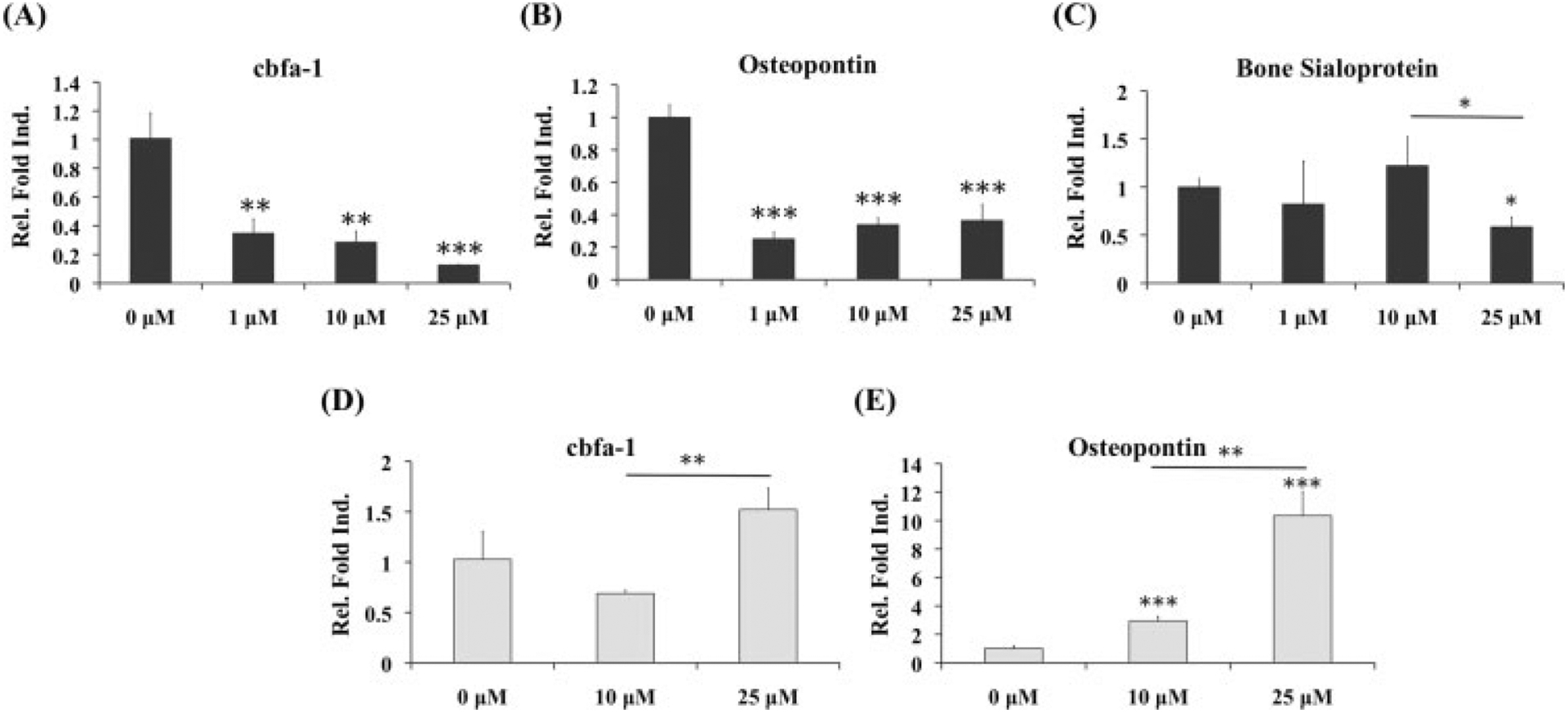
Real-time PCR of osteogenic markers for OA chondrocytes cultured with MMP inhibitors; *statistical significance from control or 0 μM, unless otherwise specified; n = 3. (A–C) When OA chondrocytes were cultured in growth medium, cbfa-1 and osteopontin were significantly downregulated in the presence of all concentrations of MMPis. Bone sialoprotein was only decreased at the highest concentration. (D, E) After culture in chondrogenic medium with TGFβ1, both cbfa-1 and osteopontin demonstrated increasing trends in the presence of MMPis, with the most significant changes in osteopontin
3.4. Inhibiting MMP activity in OA chondrocytes cultured in chondrogenic medium with TGFβ1
While MMP activity was downregulated in the presence of MMPis (Figure 5A), culturing diseased chondrocytes in chondrogenic medium with TGFβ1 resulted in a significant increase in GAG content, with no change in total collagen content when normalized to DNA (Figure 5B). Moreover, an increase in Safranin-O staining supports the observation that GAG production increased in OA chondrocytes in the presence of chondrogenic medium (Figure 5C).
Figure 5.
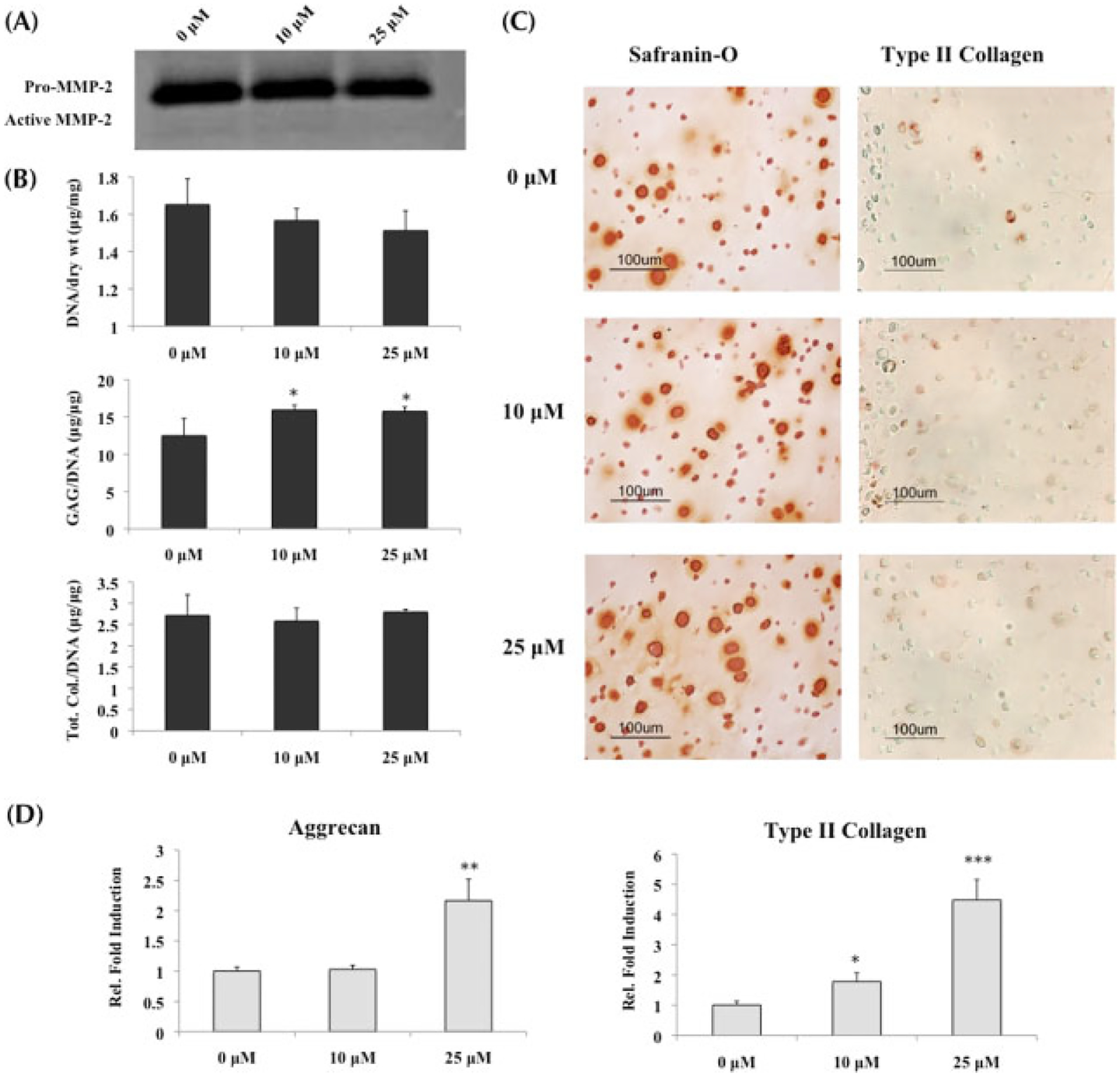
OA chondrocytes cultured in chondrogenic medium with TGFβ1 and MMP inhibitors; *statistical significance from control or 0 μM, unless otherwise specified. (A) Gelatin zymography conducted on supernatant collected at day 21 demonstrated a decrease in MMP-2 activity in the presence of MMPis. (B) Biochemical analysis (n = 3) of ECM content normalized to DNA indicated that only GAG production was increased in the presence of MMPis, while no change was observed in total collagen content. (C) Histological staining for GAG and type II collagen supported the biochemical results of (B). (D) Real-time PCR (n = 3) indicated significant upregulations in both aggrecan and type II collagen gene expression, especially in the presence of 25 μM MMPis
Consistent with the increased GAG production, aggrecan gene expression was upregulated in the presence of 25 μM MMPis (Figure 5D). Although no change was observed in total collagen content, type II collagen gene expression was upregulated in a concentration-dependent manner. Contrary to what was observed with OA chondrocytes cultured in growth medium alone, there was a significant increase in osteopontin gene expression as well as an increasing trend in cbfa-1 when the cells were exposed to MMPis (Figures 6D, E).
4. Discussion
Inhibiting MMP activity has been proposed as a potential therapeutic treatment in diseases with increased MMP activity, including certain locally invasive cancers and arthritis (Hoekstra et al., 2001). However, many of the therapeutic attempts with MMPis have resulted in severe, unintended side-effects, such as musculoskeletal syndrome, most likely induced through the oral and systemic administration of synthesized inhibitors during clinical trials and in animal models (Coussens et al., 2002; Johnson et al., 1998; Krzeski et al., 2007; Leff et al., 2003; Renkiewicz et al., 2003; Sparano et al., 2004). Therefore, the purpose of this study was to focus on the effects of inhibiting MMP activity through local and direct administration, and to analyse how different cell types are affected phenotypically as well as during tissue formation. Numerous studies have demonstrated PEGDA to be an appropriate scaffold for in vitro culture of various cell types, and it provided a suitable environment to study the effects of MMP inhibition through efficient diffusion while creating a realistic representation of cartilage tissue (Sharma et al., 2007; Williams et al., 2003).
MMPs are categorized into different families, mainly based on the matrix substrate that is cleaved (Visse and Nagase, 2003). A significant portion of osteoarthritic research has focused on MMPs that target proteoglycans (MMP-3), type II collagen fibrils (MMP-13) and gelatin fragments (MMP-2 and -9) (Hsieh et al., 2004; Lin et al., 2004; Mitchell et al., 1996; Neuhold et al., 2001). Our study was composed of experiments that preliminarily focused on analysing the effects of MMP inhibition in tissue engineering. Two different inhibitors were used to simultaneously target major proteases in OA: GM6001, a broad-spectrum MMPi with selectivity for MMP-1, -2, -3, -8 and -9 and a synthetic MMP-13 specific inhibitor.
Our studies suggest that MMP activity is important during normal cartilage tissue development and in OA pathogenesis. The severe hindrance of tissue generation in MSCs differentiating towards the chondrogenic lineage with the inhibition of MMPs indicates the importance of proteolytic activity in matrix production as well as matrix breakdown. The recovery of aggrecan and type II collagen gene transcriptions with 25 μM MMPis suggests that at high concentrations of inhibitors, MSCs may compensate for the significant decrease in matrix content by upregulating anabolic transcription. These data thus support the idea that an imbalance that favours less proteolytic activity can be detrimental to tissue development, which has been observed by other researchers (Chun et al., 2006; Bertram et al., 2009; Connelly et al., 2008).
Normal chondrocytes responded similarly to the inhibition of MMP activity as MSCs, although with less severity. Only the highest concentration of MMPis resulted in a significant decrease in matrix content. However, anabolic gene transcription was also severely downregulated with a slight upregulation at 25 μM MMPis, indicating that the cells could also be compensating for decreased ECM production. Chondrocytes from normal cartilage tissue have a naturally low metabolic rate, which is accompanied by low MMP activity (Ulrich-Vinther et al., 2003; Freed et al., 1997; Murphy et al., 2002). Therefore, the presence of only 1 μM MMPis could have been enough to decrease the low MMP activity without affecting the ability of the cells to produce matrix. This could potentially explain the increase in ECM production at the lowest concentration of inhibitors, even though transcription was significantly downregulated. However, proteolysis is still crucial during mature tissue remodelling, as indicated by the decrease in ECM production in the presence of 25 μM MMPis.
While the lack of proteolytic activity can be detrimental for normal cartilage tissue development, inhibition of MMP activity could potentially have a positive effect on diseased chondrocytes that have abnormal MMP levels (Baragi et al., 2009). When OA chondrocytes were cultured in growth medium, no change was observed in actual ECM production, although anabolic gene expression demonstrated similar trends as MSCs and normal chondrocytes. Removal of the diseased cells from their natural degradative and inflammatory environment into neutral PEGDA culture could potentially explain the gene expression trend. However, it has been previously demonstrated that chondrocyte growth medium does not support matrix production by OA chondrocytes (Li et al., unpublished data), and the lack of change in ECM with only MMP inhibitors suggests that there could be a need for increasing anabolic processes in addition to the inhibition of MMP catabolic activity to increase matrix content and ultimately tissue production. Therefore, we cultured OA chondrocytes in chondrogenic medium containing the anabolic growth factor TGFβ1 during MMP inhibition, and we observed an increase in only GAG matrix content with no change in total collagen content. This indicates that inhibiting MMP activity is not enough to treat OA chondrocytes, and that the synergy of downregulating catabolism and upregulating anabolism can potentially increase some ECM production from diseased cells. Literature has cited that aggrecan breakdown, which occurs at the earlier stages of OA, indicates a reversible stage of cartilage degradation (Clark and Parker, 2003; Karsdal et al., 2008; Shingleton et al., 1996). Once collagen breakdown begins, osteoarthritis becomes irreversible, leading only to a cascade of increased ECM depletion. This could account for the lack of change in total collagen content from OA chondrocytes in the presence of the MMPis. In addition, the irreversible stage of OA suggests that there could be an intrinsic alteration within the phenotype of the diseased chondrocytes that prevents the production of collagen fibrils. This could potentially explain why there was an increase in type II collagen gene expression but no translated increase in collagen production. Taken together, the data allude to the fact that culturing OA chondrocytes in biomaterials with chondrogenic differentiation medium and MMP inhibitors can potentially promote proteoglycan matrix production. However, changes in bone-related markers were also observed simultaneously.
An interesting observation is how differently ECM production was affected in MSCs and OA chondrocytes when exposed to MMP inhibitors and TGFβ1. Matrix production was significantly reduced in MSCs, while OA chondrocytes had limited ECM production. These results suggest that the effects of MMP inhibition in combination with TGFβ1 greatly vary, depending on the cell type. Previous studies in the laboratory have demonstrated that OA chondrocytes still retained their upregulated degradative and inflammatory transcriptional state when cultured in vitro in PEGDA (Li et al., unpublished data). Therefore, we hypothesize that MMP inhibitors and TGFβ1 together were able to stimulate the GAG matrix production in OA chondrocytes, as they already had a naturally higher catabolic activity due to their diseased state. While TGFβ1 is important during chondrogenesis of mesenchymal stem cells (Johnstone et al., 1998), we hypothesize that the decrease in proteolytic activity provided a more detrimental environment for the non-diseased cells, as it is important during normal matrix remodelling and thus hindered tissue generation from the MSCs.
In addition to the increase in GAG matrix production, upregulated changes in bone-related markers from OA chondrocytes were also observed simultaneously when treated with both MMPis and TGFβ1. This indicates that treating OA requires more than just attempting to increase ECM production, but also balancing it with processes that characterize the disease’s pathogenesis. OA has also been labelled as a disease that affects the bone (Felson and Neogi, 2004; Rogers et al., 2004). Hypertropic bony osteophytes are commonly observed in patients suffering from OA, along with aberrant evidence of subchondral sclerosis and increased bone turnover in the subchondral bony architecture (Kijowski et al., 2006; Hayami et al., 2006; Salaffi et al., 2003). The physical and biological crosstalk between cartilage and bone is a critical component of joint homeostasis and diseases with bone overgrowth, causing significant morbidity. Therefore, analysis of osteogenic markers is important to evaluate when studying cells from OA patients (Kim et al., 1999; Petersson et al., 1998; Pullig et al., 2000). Gene expressions of bone markers were decreased in the presence of MMPis only when OA chondrocytes were not stimulated with TGFβ1, thus supporting previous observations that MMPs and hypertrophy are related processes during osteoarthritic development (D’Angelo et al., 2000). In contrast, OA cells stimulated to make more matrix through TGFβ1 expressed an upregulation of osteopontin, as well as an increasing trend in cbfa-1 between 10 and 25 μM of inhibitors. Previous literature has indicated that the presence of TGFβ1 may play an important role in increasing osteophyte formation (Scharstuhl et al., 2002). Therefore, while the growth factor stimulated more cartilage ECM production from diseased chondrocytes, the chances of osteophyte formation are also increased. Regulating the different mechanisms that dictate the progression of OA is a delicate balance that involves more than just inhibiting proteolytic activity, and will require further investigation on the various physiological processes that modulate osteoarthritis. Most importantly, strategies that simply focus on increasing cartilage formation in diseased cells may also be stimulating abnormal bone formation.
In conclusion, we observed that affecting tissue remodelling via MMPs plays an important role in tissue production in both normal and diseased cells, and that MMPs are critical components of tissue development and formation in stem cells and normal chondrocytes. One of the challenges we faced was the accessibility of cells from human donors, especially in getting different cell types. Cells from different sources were used, and one monolayer passage of OA chondrocytes was conducted in order to obtain sufficient cell numbers for the in vitro studies. Previous research has demonstrated that OA chondrocytes have the ability to redifferentiate in 3D after expanding them through monolayer passage (Tallheden et al., 2005). Therefore, we expanded the OA chondrocytes for only one passage before encapsulation to limit phenotypic changes due to dedifferentiation (Li et al., unpublished data). While using all human cells would have been ideal, using easily accessible cell lines in this preclinical and preliminary study provided compelling data that demonstrate the differences in tissue development and gene transcription from different cell types when exposed to MMP inhibitors. This also indicates that directly inhibiting MMP activity may have a role in the potential treatment of OA and in the development of matrix tissue and differentiation of chondrocytes for tissue engineering. However, it also appears that growth factors, cytokines and other local factors may play important roles in determining the net effects of MMP inhibition. This work forms a foundation for future experiments with human cells that will delve further into the use of MMP inhibitors and anabolic growth factors when treating OA chondrocytes. It also highlights the importance for additional studies in this area, such as elucidating the specific inhibition of certain MMPs either individually or simultaneously during different stages of cartilage tissue development to better enhance tissue regeneration from different cell types.
Acknowledgements
This body of work was supported by the NIH/National Institute of Biomedical Imaging and Bioengineering (Grant No. R01EB005517), the NIH/National Institute of Dental Craniofacial Research (Grant No. R01DE016887), and the NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant No. R01AR054005).
References
- Balbin M, Fueyo A, Knauper V, et al. 2001; Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem 276: 10253–10262. [DOI] [PubMed] [Google Scholar]
- Baragi VM, Becher G, Bendele AM, et al. 2009; A new class of potent matrix metalloproteinase 13 inhibitors for potential treatment of osteoarthritis: evidence of histologic and clinical efficacy without musculoskeletal toxicity in rat models. Arthritis Rheum 60: 2008–2018. [DOI] [PubMed] [Google Scholar]
- Bertram H, Boeuf S, Wachters J, et al. 2009; Matrix metalloprotease inhibitors sup-press initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev 18: 881–892. [DOI] [PubMed] [Google Scholar]
- Bramono DS, Richmond JC, Weitzel PP, et al. 2004; Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res 428: 272–285. [DOI] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, et al. 2006; A pericellular collagenase directs the three-dimensional development of white adi-pose tissue. Cell 125: 577–591. [DOI] [PubMed] [Google Scholar]
- Clark IM, Parker AE. 2003; Metalloproteinases: their role in arthritis and potential as therapeutic targets. Expert Opin Ther Targets 7: 19–34. [DOI] [PubMed] [Google Scholar]
- Connelly JT, Wilson CG, Levenston ME. 2008; Characterization of proteoglycan production and processing by chondrocytes and BMSCs in tissue engineered constructs. Osteoarthr Cartilage 16: 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. 2002; Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295: 2387–2392. [DOI] [PubMed] [Google Scholar]
- D’Angelo M, Yan Z, Nooreyazdan M, et al. 2000; MMP-13 is induced during chondrocyte hypertrophy. J Cell Biochem 77: 678–693. [PubMed] [Google Scholar]
- Felson DT, Neogi T. 2004; Osteoarthritis: is it a disease of cartilage or of bone? Arthritis Rheum 50: 341–344. [DOI] [PubMed] [Google Scholar]
- Freed LE, Langer R, Martin I, et al. 1997; Tissue engineering of cartilage in space. Proc Natl Acad Sci USA 94: 13885–13890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring MB. 2000; The role of the chondrocyte in osteoarthritis. Arthritis Rheum 43: 1916–1926. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Goldring SR. 2007; Osteoarthritis. J Cell Physiol 213: 626–634. [DOI] [PubMed] [Google Scholar]
- Goldring SR, Goldring MB. 2004; The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 427: (suppl): S27–36. [DOI] [PubMed] [Google Scholar]
- Hayami T, Pickarski M, Zhuo Y, et al. 2006; Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone 38: 234–243. [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Eskens FA, Verweij J. 2001; Matrix metalloproteinase inhibitors: current developments and future perspectives. Oncologist 6: 415–427. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Chu SC, et al. 2004; Expression changes of gelatinases in human osteoarthritic knees and arthroscopic debridement. Arthroscopy 20: 482–488. [DOI] [PubMed] [Google Scholar]
- Johnson LL, Dyer R, Hupe DJ. 1998; Matrix metalloproteinases. Curr Opin Chem Biol 2: 466–471. [DOI] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, et al. 1998; In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238: 265–272. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Madsen SH, Christiansen C, et al. 2008; Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther 10: R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijowski R, Blankenbaker D, Stanton P, et al. 2006; Correlation between radiographic findings of osteoarthritis and arthroscopic findings of articular cartilage degeneration within the patellofemoral joint. Skeltal Radiol 35: 895–902. [DOI] [PubMed] [Google Scholar]
- Kim IS, Otto F, Zabel B, et al. 1999; Regulation of chondrocyte differentiation by Cbfa1. Mech Dev 80: 159–170. [DOI] [PubMed] [Google Scholar]
- Kim TK, Sharma B, Williams CG, et al. 2003; Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthr Cartilage 11: 653–664. [DOI] [PubMed] [Google Scholar]
- Kinoh H, Sato H, Tsunezuka Y, et al. 1996; MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J Cell Sci 109: 953–959. [DOI] [PubMed] [Google Scholar]
- Krzeski P, Buckland-Wright C, Balint G, et al. 2007; Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res Ther 9: R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff RL, Elias I, Ionescu M, et al. 2003; Molecular changes in human osteoarthritic cartilage after 3 weeks of oral administration of BAY 12–9566, a matrix metalloproteinase inhibitor. J Rheumatol 30: 544–549. [PubMed] [Google Scholar]
- Lin PM, Chen CT, Torzilli PA. 2004; Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3 and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthr Cartilage 12: 485–496. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001; Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, et al. 1996; Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest 97: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Knauper V, Atkinson S, et al. 2002; Matrix metalloproteinases in arthritic disease. Arthritis Res 4(suppl 3): S39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Nagase H. 2008; Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol 4: 128–135. [DOI] [PubMed] [Google Scholar]
- Neuhold LA, Killar L, Zhao W, et al. 2001; Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest 107: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson IF, Boegard T, Svensson B, et al. 1998; Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol 37: 46–50. [DOI] [PubMed] [Google Scholar]
- Pullig O, Weseloh G, Gauer S, et al. 2000; Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol 19: 245–255. [DOI] [PubMed] [Google Scholar]
- Renkiewicz R, Qiu L, Lesch C, et al. 2003; Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis Rheum 48: 1742–1749. [DOI] [PubMed] [Google Scholar]
- Rogers J, Shepstone L, Dieppe P. 2004; Is osteoarthritis a systemic disorder of bone? Arthritis Rheum 50: 452–457. [DOI] [PubMed] [Google Scholar]
- Salaffi F, Carotti M, Stancati A, et al. 2003; Radiographic assessment of osteoarthritis: analysis of disease progression. Aging Clin Exp Res 15: 391–404. [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, Glansbeek HL, van Beunin-gen HM, et al. 2002; Inhibition of endogenous TGF-β during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol 169: 507–514. [DOI] [PubMed] [Google Scholar]
- Sharma B, Williams CG, Khan M, et al. 2007; In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg 119: 112–120. [DOI] [PubMed] [Google Scholar]
- Shingleton WD, Hodges DJ, Brick P, et al. 1996; Collagenase: a key enzyme in collagen turnover. Biochem Cell Biol 74: 759–775. [DOI] [PubMed] [Google Scholar]
- Sparano JA, Bernardo P, Stephenson P, et al. 2004; Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group trial E2196. J Clin Oncol 22: 4683–4690. [DOI] [PubMed] [Google Scholar]
- Tallheden T, Bengtsson C, Brantsing C, et al. 2005; Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther 7: R560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Vinther M, Maloney MD, Schwarz EM, et al. 2003; Articular cartilage biology. J Am Acad Orthop Surg 11: 421–430. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. 2003; Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92: 827–839. [DOI] [PubMed] [Google Scholar]
- Williams CG, Kim TK, Taboas A, et al. 2003; In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9: 679–688. [DOI] [PubMed] [Google Scholar]


