Abstract
Post-COVID-19 infection symptoms such as mental fog, tachycardia, and extreme fatigue are just a few of the symptoms wreaking havoc on patients’ lives. Patients with long-term symptoms following COVID-19 are being called long haulers. To date, long haulers are receiving little to no guidance from physicians on their lingering COVID-19 symptoms with limited treatment options available. Zofin is an acellular biologic that contains the extracellular vesicle (EV) fraction of human amniotic fluid and is under investigation for use as a COVID-19 therapeutic. We obtained FDA and IRB approval to investigate the therapeutic use of Zofin in a single long hauler patient case experiencing prolonged shortness of breath and respiratory impairment. Administration of the EV product was shown to be safe. Furthermore, demonstrated respiratory improvements through chest X ray images and oxygen saturation measurement. The single patient IND studies were completed without any reported adverse events or safety concerns. Furthermore, these completed studies demonstrate the feasibility and a therapeutic potential of amniotic fluid-derived EVs for COVID-19 long hauler intervention.
Keywords: COVID-19, Extracellular vesicles, Perinatal, Long-hauler, Long COVID
1. Introduction
Patients suffering from active infections of COVID-19 have more to worry about than the acute condition. Even after testing negative, many patients are finding themselves with lingering symptoms such as mental fog, tachycardia, and extreme fatigue [1,2]. Some are finding themselves unable to successfully complete activities of daily living, citing lingering COVID-19 symptoms as the culprit. The long-term sequelae following COVID-19 is being called long COVID, or ‘long haul COVID’ [3]. These long hauler COVID patients have limited treatment options available.
Extracellular vesicle-based therapies are actively being investigated for COVID-19 infections due to the observed immunomodulatory and anti-inflammatory effects [4]. Zofin is a novel biologic that is derived from the soluble nanoparticle and extracellular vesicle fraction of human amniotic fluid. The manufactured Zofin therapeutic is an acellular product characterized to contain growth factors, cytokines, and chemokines as well as extracellular vesicles and exosomes secreted from perinatal tissues. Therefore, our proposed therapeutic intervention utilizes cell-derived and tissue-secreted paracrine factors, rather than the cells themselves, as the active drug components. Zofin is currently under investigation as a novel COVID-19 therapeutic [5]. However, the use of amniotic fluid-derived paracrine factors, including proteins and extracellular vesicles, have not been tested for the treatment of COVID-19 long hauler symptoms.
In this case report study, a 55-year-old Caucasian male was diagnosed with shortness of breath, fevers of unknown origin, total malaise and arthropathies on June 8, 2020. The patient experienced vomiting, diarrhea, and headaches, as well as loss of smell. Repetitive COVID-19 PCR nasal swabs came back negative, although antibody tests came back positive. With this, the patient was given a formal COVID-19 diagnosis and then spent 7 weeks hospitalized. During this time, the patient was moved into the ICU twice, both instances for a period of over a week. While the patient was never intubated, he was on BIPAP to address the shortness of breath. While hospitalized, the patient was treated with Zosym, Erythromycin, Remdesavir, IV steroids, and a 10-day course of oral Dexamethasone. At discharge, the patient was prescribed daily evening nebulizer treatments of 2.5mg Albuterol Sulfate every 6 hours or when needed.
Two months after initial hospital admission, the patient was still experiencing symptoms—most prominently, sustained and prolonged shortness of breath. This resulted in severe fatigue that restricted the patient's ability to perform daily living activities. As a result, the treating physician diagnosed this patient as COVID-19 long hauler and requested a compassionate use trial to administer Zofin.
Prior to treatment initiation, the patient experienced extreme shortness of breath while going up stairs, however, was not able to go downstairs at all. This shortness of breath also resulted in the patient feeling extremely air hungry while eating or talking. At the time, the patient was also experiencing viral pneumonitis, acute respiratory distress, meningitis/encephalitis, and hyperglycemia while on steroids. Here within, we present a completed case report study of the investigational use of Zofin in this COVID-19 long hauler patient. The aim of this study was to demonstrate investigational product safety and feasibility for the COVID-19 long hauler population.
2. Materials and methods
Ethics: This study involves a human participant that was reviewed and approved by the FDA and the Independent Review Board Western Institutional Review Board. The patient's consent to participate in the study and for data publication was obtained at the treatment site by the patient using an IRB approved informed consent form. This single patient IND was submitted under the approved parent IND #19881 and FDA approval was issued with the following IND # 25888. IRB approval was issued by letter of acknowledgement from Western Institutional Review Board.
Therapeutic Intervention: The therapeutic intervention studied in this case report was an acellular biologic called Zofin. Zofin was manufactured by Organicell Regenerative Medicine, Inc. in Miami, FL as previously described [5]. The Zofin product was derived from human amniotic fluid donated from consenting adults during routine, planned cesarean sections under IRB approved donor screening (IRB approval agency: IRCM). The final Zofin product was released by Organicell Regenerative Medicine, Inc. after meeting the release criteria requirements. The specific release criteria parameters for the product administered in these treatments were: sterility (14-day cultures: no growth for aerobic, anaerobic, and fungal contamination), endotoxin (<0.05 EU/mL), nanoparticle composition (concentration = 3.26 × 1011/mL, mode particle size = 90.2nm), protein concentration (2.83mg/mL), and hyaluronic acid concentration (261ng/mL). Zofin was stored frozen and shipped on dry ice to the treatment location following validated storage and shipping methods.
Patient Standard Care and Administration of Therapeutic Intervention: The patient was treated at Advanced Regenerative Therapies in Savanah, GA. Three 1ml doses of Zofin were administered intravenously on Day 0, 4, and 8 in an outpatient setting. The treating physician followed up with the patient daily during treatment and continued to monitor progress on follow up visits; Day 14, 21, 28, and 60. The patient was monitored for 30 minutes immediately following the IV infusion. ChartPad Software (Technomad), a cloud-based electronic data capture platform, was used to collect patient data.
Chest X-Ray: Chest X-Ray (CXR) was used to acquire imaging to evaluate, identify, and monitor lung abnormalities [6]. After images were acquired, analysis was performed by the treating physician at Advanced Regenerative Therapies, Savanah GA. CXR reports were generated to outline the clinical findings.
Pulse Oximetry Testing: A pulse oximeter was placed on the index finger and the blood oxygen saturation was recorded at each visit.
Laboratory and Biomarker Testing: Blood collection and testing occurred at 0, 4, 8, 14, 21, and 28 days after initiation of Zofin therapy to assess standard blood panels and for concentration of D-Dimer, CRP, IL2, IL6, and TNFα. Blood panels and biomarkers were completed at the laboratory facility at St. Joseph/Candler Hospital, Savannah, GA.
Patient Perspective: The patient's perspective and self-reported effects were obtained directly from the physician's record. Patient observations and visit summaries were recorded and stored by the physician using ChartPad Software.
3. Results
3.1. Progression of chest X ray findings
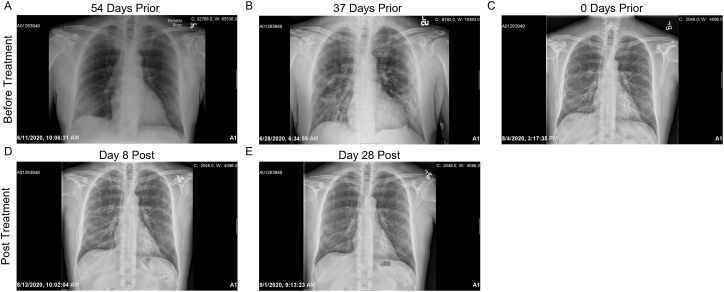
54 days prior to therapeutic intervention, chest X ray (CXR) demonstrate patchy infiltrate in the right lower lobe and a clear left lung. There were no pleural effusions. (Fig. 1A, Table 1). These findings worsened 37 days prior to treatment with moderate bilateral patchy infiltrates overall slightly less consolidated. No pleural abnormality or pneumothorax were found. (Fig. 1B, Table 1). At baseline, the day of first Zofin treatment, CXR demonstrated bilateral pneumonia (Fig. 1C, Table 1). On day 4, a decrease in small consolidations in both lungs was reported. As well, there were no new pulmonary abnormalities, pneumothorax, or pleural effusion (Table 1). On Day 8, CXR reports indicated there was no change and minimal thickening of the interstitium. (Fig. 1D, Table 1). On day 14, the CXR showed subtle interstitial markings resolved and now appeared normal (Table 1). On day 21, the CXR report detailed that the patient's lungs were well-inflated and clear (Table 1). On day 28, CXR reports lungs were well-inflated and clear with no acute cardiopulmonary disease or abnormality (Fig. 1E, Table 1). On day 60, CXR was not ordered, and the patient was not limited by activity and returned to work. Day 4, Day 14, and Day 21 CXR images not shown.
Fig. 1.
Representative Chest X Ray Images: A) CXR 54 days prior to treatment B) CXR 37 days prior to treatment C) CXR on the day of treatment D) CXR 8 days post treatment E) CXR 28 days post treatment.
Table 1.
CXR reports.
| Time Point | Report Findings |
|---|---|
| 54 Days Prior | Patchy infiltrate in the right lower lobe, the left lung is clear. There are no pleural effusions. |
| 37 Days Prior | Moderate bilateral patchy infiltrates overall slightly less consolidated. No pleural abnormality or pneumothorax unchanged. |
| Baseline | Bilateral pneumonia |
| Day 4 | Small consolidations present in both lungs had decreased. As well, there were no new pulmonary abnormalities, pneumothorax, or pleural effusion |
| Day 8 | No change and minimal thickening of the interstitium |
| Day 21 | The patient's lungs were clear. At the time there was no infiltrate, atelectasis, or pleural effusion |
| Day 28 | Well-inflated and clear. No pneumothorax or pleural effusion was present and heart size was normal |
3.2. Oxygen saturation results
Oxygen saturation measured at baseline was low at 95% pre-treatment. On day 4, oxygen saturation increased to 97–98%. Oxygen saturation remained normal on day 8 in the 97–98% range and continued to be measured at normal levels through day 60.
3.3. Laboratory and Biomarker Testing
Blood labs and biomarker testing was completed at each study time point to monitor any abnormalities or changes in systemic inflammation. The majority of lab results were normal at baseline and remained within the normal reference range throughout the time course (Table 2). Platelets were elevated at baseline and returned into the normal range by day 8. Monocyte count was elevated at baseline and remined elevated throughout the time course. Inflammatory biomarkers (TNF-α, IL-6, D-Dimer, and CRP) were not elevated in this patient.
Table 2.
Laboratory results.
| Lab | Ref. Range | Day 0 | Day 4 |
Day 8 |
Day 14 |
Day 21 |
Day 28 |
Day 60 |
|---|---|---|---|---|---|---|---|---|
| WBC | 3.4–10.6 K/mm3 | 7.46 | 7.9 | 7.46 | 7.53 | 8.64 | 10.12 | 6.63 |
| RBC | 3.8–5.4 M/mm3 | 4.6 | 4.8 | 4.7 | 4.8 | 4.7 | 4.6 | 4.8 |
| Hemoglobin | 12.9–16.1 g/dL | 14.6 | 15.1 | 14.8 | 15.2 | 14.8 | 15 | 15.3 |
| Hematocrit | 38.0–47.0% | 43.9 | 44.8 | 43.5 | 43.6 | 43 | 42.7 | 44 |
| MCV | 80-100 Fl | 94.6 | 94.1 | 92 | 90.6 | 91.7 | 92 | 91.3 |
| MCH | 27.0–33.0 pg | 31.5 | 31.7 | 31.3 | 31.6 | 31.6 | 32.3 | 31.7 |
| MCHC | 32–37.5 g/dL | 33.3 | 33.7 | 34 | 34.9 | 34.4 | 35.1 | 34.8 |
| RDW | 11.5–15.8% | 14.1 | 13.80 | 13.6 | 13.4 | 13.7 | 13.3 | 12.2 |
| Platelets | 150–450 K/mm3 | 498 | 503 | 401 | 332 | 301 | 349 | 349 |
| Neutrophils | 36–75.2% | 46.5 | 56.60 | 52.3 | 57.7 | 54.9 | 59.6 | 52.8 |
| Lymphocytes | 20.5–51.1% | 38.7 | 30.90 | 33 | 31.2 | 29.2 | 23.8 | 34.7 |
| Monocytes | 1–9.3% | 10.9 | 9.90 | 11.5 | 8.6 | 11.8 | 9.7 | 10 |
| Eosinophils | 0.9–6.0% | 2 | 1.40 | 1.9 | 1.3 | 2.9 | 5.6 | 1.4 |
| Basophils | 0.3–1.5% | 1.1 | 1.00 | 0.9 | 0.9 | 1 | 1 | 0.9 |
| Sodium | 136–145 mmol/L | 142 | 141 | 142 | 139 | 138 | 140 | 139 |
| Potassium | 3.3–5.1 mmol/L | 4 | 4.3 | 4.1 | 4.1 | 4.3 | 4.2 | 4.1 |
| Chloride | 98–107 mmol/L | 105 | 107 | 108 | 106 | 106 | 107 | 106 |
| CO2 | 21–32 mmol/L | 29 | 25 | 25 | 25 | 24 | 27 | 23 |
| Glucose | 74–106 mg/dL | 82 | 96 | 104 | 97 | 101 | 79 | 102 |
| Urea Nitrogen | 9–23 mg/dL | 10 | 12 | 12 | 12 | 16 | 16 | 13 |
| Creatinine | 0.70–1.30 mg/dL | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Calcium | 8.7–10.4 mg/dL | 10.3 | 10.1 | 9.8 | 10 | 10 | 9.9 | 9.9 |
| Total Protein | 5.7–8.2 g/dL | 6.7 | 6.5 | 6.6 | 6.8 | 6.7 | 6.5 | 6.7 |
| Albumin | 3.2–4.8 g/dL | 4.4 | 4.3 | 4.2 | 4.3 | 4.3 | 4.2 | 4.3 |
| Total Bilirubin | 0.3–1.2 mg/dL | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 |
| Alkaline Phosphatase | 46–116 U/L | 89 | 88 | 82 | 84 | 83 | 78 | 81 |
| Aspartate Aminot. (AST) | <34 U/L | 21 | 21 | 9 | 18 | 19 | 22 | 23 |
| Alanine Aminotrans (ALT) | 10–49 U/L | 35 | 28 | 22 | 22 | 19 | 17 | 31 |
| eGFR AF AMER | ≥60 mL/min/1.73m2 | >60 | >60 | >60 | >60 | >60 | >60 | >60 |
| eGFR NON-AF AMER | ≥60 mL/min/1.73m2 | >60 | >60 | >60 | >60 | >60 | >60 | >60 |
| Anion Gap | 5.0–15.0 mEq/L | 8 | 9 | 9 | 8 | 8 | 6 | 10 |
| TNF-α | 0.56–1.40 pg/mL | 0.83 | 0.86 | 0.81 | 0.74 | 0.69 | 0.66 | 0.56 |
| Interleukin-6 | ≤2 pg/mL | 1.4 | <2 | <2 | <2 | <2 | <2 | <2 |
| d-Dimer | ≤0.5 μg/mL FEU | <0.27 | 0.36 | N/A | 0.37 | 0.3 | <0.27 | 0.28 |
| C-Reactive Protein | <1.0 mg/dL | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 | <0.4 |
*Bold text indicates out of normal range value.
3.4. Patient's perspective
Following infusion of the first dose and up until the second dose (day 4), the patient reported feeling much better. During his physical therapy sessions, his exercise tolerance improved, and he was able to go up two flights of stairs without stopping. Improvements were also noted in his ability to lie flat to sleep and his overall sense of well-being. On day 4, the second infusion date, the patient's greatest limitation was still shortness of breath with some dyspnea with exertion. When following up with the patient, he reported no general malaise but fatigued following activities of daily living. At this point, the patient was wide awake and much more vibrant compared to being depressed and withdrawn prior to study initiation. Following his second dose, the patient began taking the necessary steps to return to work by taking basic life support training. Meanwhile, the patient was experiencing decreased air hungriness and was able to sleep flat without difficulty. Most of all, the patient was able to exercise to fatigue, at which time, he did not desaturate. On day 8, the patient reported feeling 75% back to normal prior to receiving the third Zofin infusion. By the day 14 visit, the patient reported big improvements since the third infusion with improvements in the ability to sleep and a general return to normal life activities. On day 18, noticeable changes in the ability to exercise without fatigue and a lack of desaturation was notable. By day 21, the patient reported feeling as if they made a full recovery that continued to improve by day 60 in which the patient was strong, upbeat and happy. At this point, the patient was unlimited in activity and at work.
4. Discussion
This completed case study is the first demonstration of human amniotic fluid-derived extracellular vesicles and soluble factors as a safe therapeutic for the recovery from long hauler complications induced by COVID-19 infection. The intravenous and multi-dose administration of Zofin was found to be safe and well tolerated without the report of any serious adverse events.
The long hauler patient describe in this case report had become infected with COVID-19 2 months prior to treatment and suffered from continued respiratory distress. At baseline, bilateral pneumonia with shortness of breath were the primary inhibitory factors that lead to the long hauler diagnosis. There was no elevation in blood laboratory results or inflammatory biomarkers that could be linked to the persistent symptoms. The patient began to experience improvements in shortness of breath complications early in the treatment protocol and continued to improve throughout. By the conclusion of the study period, the patient had returned to normal with no observation of impairments or respiratory distress.
Novel biologic medicines such as stem cell and extracellular vesicles have been studied prior to the COVID-19 pandemic as therapeutic agents of tissue repair. Extracellular vesicles, in particular, have proven to become a front runner for commercial development. As acellular, cell-derived secretions, extracellular vesicles carry both protein and nucleotides, such as microRNA, that have demonstrated promising therapeutic candidates due to the anti-inflammatory and tissue regenerative effects shown across various pre-clinical models [[7], [8], [9]]. Based on pre-clinical data of exosome treatments in the lung, it is hypothesized that the respiratory effects of extracellular vesicles is particularly useful in mitigating symptoms associated with respiratory distress, and to promote the induction of endogenous tissue repair through repair of alveolar tissue and inflammatory immune cell modulation [10,11].
The molecular composition of Zofin, particularly the nanoparticle population that includes perinatal-secreted extracellular vesicles and exosomes, has strong potential as a COVID-19 therapeutic and may have been a contributing factor to this patient's recovery [12]. However, this single patient case report study is limited due to the lack of an experimental control group that would be required to determine biologic efficacy. Furthermore, the treatment of patients in an outpatient setting makes it difficult to collect a wide range of clinical data points. Despite the experimental limitations, analysis of the CXR reports, oxygen saturation, and self-reported effects demonstrated a potential efficacy of the administered treatment. To date, the patient's health is restored to normal without any reported respiratory distress, shortness of breath, and post-COVID complications.
5. Conclusions
In conclusion, our positive experience with this long hauler patient further warrants larger scale testing to further determine the safety and therapeutic effects of Zofin in this patient population and demonstrates a potential therapeutic treatment option for the growing number of COVID-19 long hauler patients.
Author Contributions
M.M., G.S. A.K. X.X Conceptualization, project administration. A.M, G.H. data curation, validation. M.B., A.M, M.M, A.A, A.K, X.X. writing—review and editing methodology, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
Clinical Trial Sponsor: Organicell Regenerative Medinie. No additional external funding to report.
Institutional Review Board Statement: This study involves a human participant that was reviewed and approved by the FDA and the Independent Review Board Western Institutional Review Board. This single patient IND was submitted under the approved parent IND #19881 and FDA approval was issued with the following IND # 25888. IRB approval was issued by letter of acknowledgement from Western Institutional Review Board.
Informed Consent Statement: The patient's consent to participate in the study and for data publication was obtained at the treatment site by the patient using an IRB approved informed consent form.
Data Availability Statement: The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
Declaration of competing interest
MM and GS report personal fees and equity from Organicell Regenerative Medicine, Inc., outside the submitted work. In addition, MM has a patent COMPOSITIONS COMPRISING NANOPARTICLES, METHOD OF MAKING AND USES THEREOF pending. MB, GH, and AA report personal fees from Organicell Regenerative Medicine, Inc., outside the submitted work. AM is a medical advisor to Organicell Regenerative Medicine. AK and XX discloses a relationship with AssureImmune Cord Blood Bank that includes equity.
Acknowledgment
This case report study was funded by the study sponsor, Organicell Regenerative Medicine. There was no external funding provided.
References
- 1.Baig A.M. Deleterious outcomes in long-hauler COVID-19: the effects of SARS-CoV-2 on the CNS in chronic COVID syndrome. ACS Chem. Neurosci. 2020;11(24):4017–4020. doi: 10.1021/acschemneuro.0c00725. [DOI] [PubMed] [Google Scholar]
- 2.Rubin R. JAMA; 2020. As Their Numbers Grow, COVID-19 "Long Haulers" Stump Experts. [DOI] [PubMed] [Google Scholar]
- 3.Callard F., Perego E. How and why patients made Long Covid. Soc. Sci. Med. 2021;268:113426. doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoury M. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur. Respir. J. 2020;55(6) doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitrani M.I. Case report: administration of amniotic fluid-derived nanoparticles in three severely Ill COVID-19 patients. Front. Med. 2021;8(242) doi: 10.3389/fmed.2021.583842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobi A. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin. Imag. 2020;64:35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barile L. Roles of exosomes in cardioprotection. Eur. Heart J. 2017;38(18):1372–1379. doi: 10.1093/eurheartj/ehw304. [DOI] [PubMed] [Google Scholar]
- 8.Williams A.M. Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain bar-rier integrity in swine model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2020;88(2):207–218. doi: 10.1097/TA.0000000000002563. [DOI] [PubMed] [Google Scholar]
- 9.Ha D.H. Mesenchymal stem/stromal cell-derived exosomes for immunomodulatory therapeutics and skin regen-eration. Cells. 2020;9(5) doi: 10.3390/cells9051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu A. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expet Opin. Biol. Ther. 2020;20(2):125–140. doi: 10.1080/14712598.2020.1689954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah T.G., Predescu D., Predescu S. Mesenchymal stem cells-derived extracellular vesicles in acute respiratory distress syndrome: a review of current literature and potential future treatment options. Clin. Transl. Med. 2019;8(1):25. doi: 10.1186/s40169-019-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuchiya A. Therapeutic potential of mesenchymal stem cells and their exosomes in severe novel coronavirus disease 2019 (COVID-19) cases. Inflamm. Regen. 2020;40:14. doi: 10.1186/s41232-020-00121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]