Abstract
The growing use of short-interfering RNA (siRNA)-based therapeutics for viral diseases reflects the most recent innovations in anti-viral vaccines and drugs. These drugs play crucial roles in the fight against many hitherto incurable diseases, the causes, pathophysiologies, and molecular processes of which remain unknown. Targeted liver drug delivery systems are in clinical trials. The receptor-mediated endocytosis approach involving the abundant asialoglycoprotein receptors (ASGPRs) on the surfaces of liver cells show great promise. We here review N-acetylgalactosamine (GalNAc)-siRNA conjugates that treat viral diseases such as hepatitis B infection, but we also mention that novel, native conjugate-based, targeted siRNA anti-viral drugs may also cure several life-threatening diseases such as hemorrhagic cystitis, multifocal leukoencephalopathy, and severe acute respiratory syndrome caused by coronaviruses and human herpes virus.
Keywords: Virus, Hepatitis B, SiRNA, GalNac conjugate, Hepatocytes
Graphical Abstract
1. Introduction
RNA molecular biology has become a prominent feature of medical research in recent years. Genome functional analysis, RNA processing and stability screening, transcriptional assays, and translational biology, virology, and cancer approaches exploit small (15–20 nucleotide) non-coding RNAs that regulate the expression of entire genomes [1]. These RNAs are subdivided by their biological roles or origins; short interfering RNAs (siRNAs) and microRNAs (miRNAs) play unique and important roles in RNA interference mechanism (RNAi) pathways [2], [3], [4], activated when cells encounter a double-stranded RNA (dsRNA) that is often a sign of (possibly fatal) infection [5]. We evaluate the anti-viral efficacies reported in clinical trials of N-acetylgalactosamine (GalNAc)-based siRNA treatments for several viral diseases including Hepatitis B. It is hoped that several targeting drug delivery systems will become available in the next few years.
2. RNAi mechanism
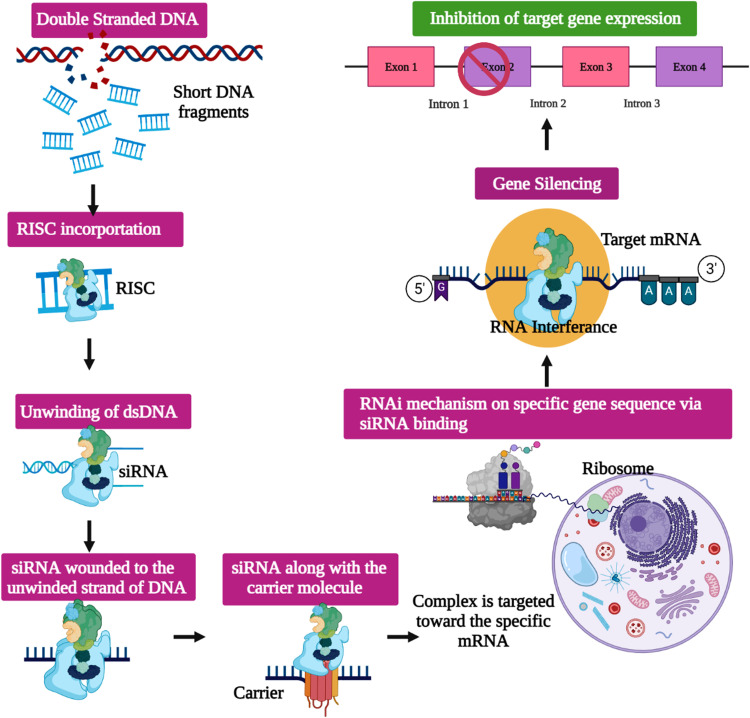
The RNAi mechanism was initially discovered as a form of miRNA-mediated silencing of the Caenorhabditis elegans genome [6]. A trigger RNA termed a long dsRNA or an miRNA primary transcript is cleaved and processed (by the RNase III enzymes Dicer and Drosha) into siRNAs with two-to-four-nucleotide overhangs on the 3′-ends of each strand [7], [8] ( Fig. 1). Then, the siRNAs are embedded in an effector complex termed the RNA-induced silencing complex (RISC) within which the siRNAs are matched via their stable 5′-ends, and then hybridize with the target mRNA sequence guided by the catalytic RISC protein, a member of the argonaut family (Ago2); ATP-dependent mRNA cleavage follows [9], [10], [11], [12].
Fig. 1.
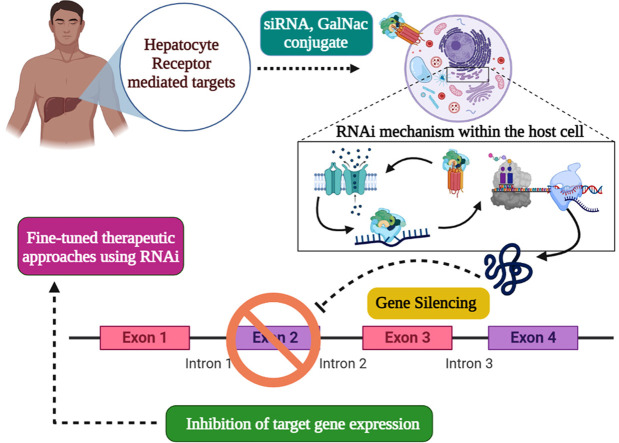
RNA interference mechanism via siRNA pathway – Diagrammatic representation of the RNAi mechanism within the host cell, via externally delivered siRNA complexes designed in order to knock-down the target gene, thereby leading to gene silencing.
Each siRNA is associated with an Ago2-family protein to form a sequence- specific gene-silencing ribonucleoprotein exhibiting specific base-pairing between the small (guide) RNA and its target mRNA sequence [13]. Recent studies on the molecular impacts of endogenous RNAi mechanisms have paved the way for use of siRNAs as nucleic acid medicines for several incurable diseases [14], [15], [16], [17]. Gene-targeting studies involving novel siRNAs have reduced immune activity after organ transfer and off-target serum stability and increased siRNA potency [18], [19]; it is possible to selectively control certain genes expressed in patients with serious genetic or viral diseases [20]. However, effective delivery of therapeutic siRNAs to target tissues remains challenging. Systemically injected nucleic acids must resist nuclease degradation in extracellular spaces, bypass renal clearance, evade sequestration by plasma proteins, avoid removal by the reticuloendothelial system, cross the capillary endothelium of the desired target cells via a paracellular or transcellular route, traverse the plasma membrane, escape the endolysosomal system prior to lysosomal degradation or re-export via exocytosis, and attain the required intracellular site of action. To date, most oligonucleotide therapeutics have focused on either local or liver delivery [21], [22], [23], [24].
3. Viruses
The virosphere is continuing to expand rapidly; new viruses are identified every year [25], [26]. The genetic-based classification of human virions is shown in Table 1. Although viral protein structures and associations are rather well-known, the effects of the environment and host replicative properties on viral infections remain poorly understood, as do the maintenance of protein structure and function over the course of evolution [28], [29], [30], [31], [32]. This convergent evolution of gene transfer has played key roles in distributing certain protein folding patterns throughout the orders of the phylogenetic tree, establishing pathologically distinct viruses. Viral pathogens have caused successive pandemics ( Table 2) [29], [33], [34], SARS CoV-2 is an excellent example [46]. The RNAi mechanism giving rise to antiviral siRNAs was first discovered using the single-stranded negative-sense RNA respiratory syncytial virus (RSV), a human pneumovirus causing respiratory tract infections [47]. Great efforts were then made to develop siRNA-based viral vaccines against DNA and RNA viruses [48], [49], [50], [51].
Table 1.
Classification of virus based on their genetic integrity for human specific virions [27].
| Type | Order/class/family | Family/subfamily | Genera | Species | Prominently disease-causing genus | Viral sp. | Disease | Host |
|---|---|---|---|---|---|---|---|---|
| ssDNA | ||||||||
| 1. | Parvoviridae | Densovirinae | 8 | 17 | – | – | – | Insects, shrimps and chordates |
| Hamaparvovirinae | 5 | 13 | – | – | – | – | ||
| Parvovirinae | 10 | > 50 | Erythroparvivirus | Erythrovirus B19 | Erythema infectiosum | Primates | ||
| Dependoparvovirus | Adeno-associated dependoparvovirus A 2 (AAV2) | Defective viruses | Affects humans and other primates with the help of a helper virus | |||||
| 2. | Circoviridae | – | 2 | > 50 | Circovirus | Porcine circovirus | Post weaning multi systemic wasting syndrome | Pigs |
| Cyclovirus | Human associated cyclovirus | Respiratory and neurological infections in humans | Mammals, birds and insects | |||||
| 3. | Anelloviridae | – | 14 | > 50 | Alpha, Beta, Gamma and the tatorquevirus | Torquevirus | May be associated with hepatitis, pulmonary diseases, hematologic disorders, myopathy and lupus | Humans and other primates |
| dsDNA | ||||||||
| 4. | Papovaviricetes | Papillomaviridae | 52 | > 50 | First papillomavirinae | > 100 sp. | HPV – 1 | Humans |
| Second papillomavirinae | ||||||||
| Polyomaviridae | 4 | > 50 | Alpha, beta, delta and gamma polyomaviruses | BK, JC and SV40 viruses | Hemorrhagic cystitis, multifocal leukoencephalopathy | Aves, humans and other primates | ||
| 5. | Rowavirales | Adenoviridae | 5 | > 50 | Mastadeno virus | Human adenovirus Serotypes | Mild respiratory, gastrointestinal and eye infections | Humans, mammals |
| 6. | Herpesvirales | AlloHerpesviridae | 4 | 13 | – | – | – | – |
| Herpesviridae | 13 | > 50 | Betaherpesvirinae |
Cytomegalovirus, Roseolo virus |
HHV5, HHV6 |
Human, monkeys | ||
| MalacoHerpesviridae | 2 | 2 | – | – | – | – | ||
| 7. | Poxviridae | Chordopoxvirinae | 18 | > 50 | Orthopoxvirus |
Vaccinia virus, Variola virus |
Smallpox, Respiratory diseases and Skin lesions | Human, mammals |
| Eentamopoxvirinae | 4 | > 50 | – | – | – | Insects | ||
| RT Viruses | ||||||||
| 8. | Hepadnaviridae | – | 5 | 18 | Orthohepadnavirus | Hepatitis B virus | Hepatitis, Hepatocellular carcinoma | Human, Mammals |
| 9. | Retroviridae | Orthoretroviridae | 6 | 49 | Lentivirus, aplpharetrovirus, deltaretrovirus |
Human immunodeficiency virus 1 and 2, Avian leukosis virus, Bovine leukemia virus |
AIDS, Malignancies, | Vertebrates |
| Spumaretrovirinae | 5 | 19 | Simiispumavirus | Simian foamy virus | Life-long persistent infections | Humans and mammals | ||
| dsRNA | ||||||||
| 10. | Reoviridae | Spinareovirinae | 9 | > 50 | Orthoreovirus | Mammalian orthoreovirus | Respiratory tract disease, gastroenteritis, biliary atresia | Mammals |
| ssRNA | ||||||||
| 11. | Coronaviridae | Letovirinae | 1 | 1 | – | – | ||
| Orthocoronavirinae | 4 | > 50 |
Alphacoronavirus, Betacoronavirus Gammacoronavirus, Deltacoronavirus |
CoV Strains | Mainly respiratory diseases (pneumonia) and gastroenteritis | Vertebrates | ||
| 12. | Picornavirales | Picornaviridae | 63 | > 50 | Enteroviruses | Poliovirus | Paralysis (non-polio, polio-type) | Human and Mammals |
| Aphthoviruses | Foot-and-mouth disease virus | Hand-foot-and-mouth disease | Mammals | |||||
| 13. | Articulavirales | Orthomyxoviridae | 7 | 9 | Influenza A virus | H1N1 | Acute febrile respiratory tract infection | Aquatic birds, Human, Pig, Horse, Seals |
| 14. | Bunyavirales | Hantaviridae | 7 | 45 | Hantaanorthohantavirus | Hantavirus | hemorrhagic fever, renal syndrome, pulmonary syndrome |
Humans and rodents |
| 15. | Mononegavirales | Paramyxoviridae | 14 | > 50 | Rubulavirus |
Measles morbillivirus, Mumps rubulavirus |
Measles and mumps | Humans, Apes, Pigs, Dogs |
| Rhabdoviridae | 30 | > 50 | Lyssavirus | Rabies lyssavirus | Fatal encephalitis | Humans and mammals | ||
| Pneumoviridae | 2 | 5 |
Metapneumovirus, Orthopneumovirus |
Human respiratory syncytial virus | Respiratory tract diseases | Human, cattle, rodents, birds | ||
| Filoviridae | 6 | 11 | Ebolavirus | Zaire ebolavirus | Hemorragic fever | Bats, Humans, primates | ||
| 16. | Unassigned | Deltavirus | 1 | 1 | Hepatitis delta virus | HDV | Hepatitis | Human, snakes, Birds |
Table 2.
The major virus-based flu pandemics and their impact on history.
| Name of the pandemic | Year | Deaths | First outbreak | Virus or Serotype | Refs. |
|---|---|---|---|---|---|
| Spanish Flu | 1918–1920 | 20–50 million | United States in 1918 | Influenza | [35] |
| Asian Flu | 1957–1958 | 2 million | China in 1956 | H2N2 subtype of the Influenza A virus | [36] |
| Hon-Kong Flu | 1968–1969 | 1 million | Hong Kong in 1968 | H3N2 strain of the Influenza A virus | [37] |
| Russian Flu | 1977–1978 | 1.5 million | Northern China in 1977 | Influenza A virus - H1N1 strain | [38] |
| Asiatic flu | 1989–1992 | 1 million | Bukhara of the Russian Empire in 1989 | H3N8 strain of the Influenza A virus subtype H2N2 | [39] |
| SARS CoV | 2002–2004 | > 1000 | Guangdong province of southern China in 2002 | SARS coronavirus of the CoV Strains | [40] |
| HIV/AIDS Pandemic | 2001–2012 | 36 million | Democratic Republic of the Congo in 1976 | HIV | [41] |
| Swine Flu | 2009–2010 | 12,469 | United States in 2009 | H1N1 influenza virus | [42] |
| Ebola outbreak | 2018–2020 | > 29,000 | North Kivu Province | Zaire ebolavirus | [43] |
| SARS CoV 2/ nCoV |
2019 | 847,986 | Wuhan, Hubei Province, China in 2020 | Respiratory Syncytial Virus- Coronavirus | [44] |
| Middle East respiratory syndrome coronavirus outbreak | 2020 | 2562 with 881 associated | Saudi Arabia in 2020 | MERS CoV of the CoV Strains | [45] |
4. Role of siRNAs in viral disease
siRNAs can be delivered to cells by viral and non-viral vectors. Synthetic siRNAs against the Influenza-A virus, incorporated in a lentiviral vector and driven by the polymerase U6 promotor, exhibited preventive and therapeutic effects when given intranasally to mice [52], [53], [54]. A synthetic siRNA against the coxsackievirus delivered to HeLA cells via oligofectamine-mediated transfection reduced viral replication [55], [56]. siRNAs developed against CoV-SARS (pSUPER and pSilent1-U6) transfected to cells with the aid of lipofectamine inhibited N gene expression; similarly, siRNA Pbs/U6 given intratracheally to mice reduced viral replication [57], [58]. A synthetic siRNA against the same RNA virus given intranasally to monkeys reduced infection and symptoms [59], [60]. Similarly, a Pcdna3/U6 siRNA against the food-and-mouth-disease virus (FMDV) transfected into BHK21 cells using lipofectamine inhibited viral protein 1 expression and reduced viral replication [61], [62]. In terms of DNA viruses, synthetic siRNAs against human papilloma virus (HPV) and hepatitis B virus (HBV) reduced viral numbers and inhibited S protein production in several in vitro studies using differentiating keratinocytes. Fugene compounds in HPV were targeting lipofectamine-transfected at short time intervals into Hep-G2 cells and ameliorated the effects of viral infection [63], [64], [65], [66], [67], [68], [69], [70], [71].
Chemically modified siRNAs targeting the Zaire ebolavirus (ZEBOV) prevented the synthesis of the viral polymerase, and proteins 24 and 35, in rhesus macaques [49]. TKM-100201 and TKM-130803 siRNAs against ZEBOV target the mRNAs encoding the polymerase-L membrane-associated viral protein 24 and the polymerase cofactor of viral protein 35 specific to the West African Makona strains when delivered via nanoparticles. However, the Phase II clinical trial results were poor, and the trial was terminated early [72], [73]. The FDA-approved (in 2028 and 2019) nucleic acid therapeutics based on antisense oligonucleotides include pegaptanib, mipomersen, eteplirsen, defibrotide, nusinersen, inotersen, patisiran, and givosiran [35], [72].
5. GalNAc-siRNA conjugates: a leading way for siRNA based antiviral therapeutics
siRNA-based treatments may be very valuable, but delivery remains problematic. Injected siRNAs may be degraded by nucleases in plasma, immune cells, and the kidney [74], [75], [76], [77], [78], greatly reducing the half-lives. Free siRNA does not readily cross cell membranes, given its negative charge and high molecular weight [79]. Occasionally, siRNAs may trigger non-specific side-effects. Nano-carriers and endosome-based delivery systems have been developed. Also, siRNAs have been modified via addition or removal of sugars, bases, or overhangs, and substitution of uridine residues [80], [81], [82]. GalNAc-siRNA conjugate is a trimer that binds firmly to a major hepatocellular protein, the asialoglycoprotein receptor (ASGPR) [83]. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry was used to assess the chemical integrities of GalNAc-siRNA conjugates. In preclinical studies, these conjugates successfully entered the livers of both rodents and nonhuman primates and are now the subjects of several clinical trials ( Table 3) [84]. Effects are evident when the trimer level exceeds 6 μg/mg ASGPR.
Table 3.
GalNAc–siRNA conjugate based clinical studies, their targets, action and other details.
| Drug | Condition | Target | Delivery/Mode | Phase | Status | Sponsors | Patents ID | Refs. |
|---|---|---|---|---|---|---|---|---|
| ARC-520 | Chronic HBV infection | Surface proteins | Intravenous injections | II | Terminated | Arrowhead Pharmaceuticals |
NCT02452528 NCT01872065 NCT02604212 |
[85] |
| HBV infection | Surface proteins | subcutaneously | II | Terminated | Arrowhead Pharmaceuticals |
NCT02577029 NCT02065336 NCT02604199 |
[85] | |
| ARC-521 | HBV infection | Viral DNA | subcutaneously | I | Terminated | Alnylam Pharmaceuticals | NCT02797522 | [85] |
| DCR-HBVS | Hepatitis B | HBV gene | GalNAc–siRNA conjugate | I/II | – | Dicerna Pharmaceuticals | NCT03772249 | [85] |
| ALN-HBV02 (VIR-2218) | Hepatitis B | HBV gene | GalNAc–siRNA conjugate |
I/II | – | Alnylam Pharmaceuticals |
NCT03672188 NCT02826018 |
[85] |
| AB-729 | Hepatitis B | HBV gene | GalNAc–siRNA conjugate | Preclinical | – | Arbutus Biopharma Corporation |
– | [86] |
| RBD1016 | Hepatitis B | HBV gene | GalNAc–siRNA conjugate | Preclinical | – | Suzhou Ribo Life ScienceCo., Ltd | – | [86] |
| JNJ-3989(ARO-HBV) | Hepatitis B | HNV viral proteins | GalNAc–siRNA conjugate | II | Completed/ Terminated | Arrowhead/JNJ | NCT03365947 NCT03982186 NCT04129554 | [85] |
| ARB-1467 | Hepatitis B | HBV gene | LNP | IIa | Completed | Arbutus Biopharma Corporation |
NCT02631096 | [85] |
| TKM-130803 | Ebola virus disease | Viral proteins | Intravenous infusion | II | Terminated | Arbutus Biopharma | PACTR201501000997429 | [73] |
| TKM-100201 (TKM-EBOV-001) |
Ebola virus disease | Viral proteins | Intravenous infusion | I | Terminated | Arbutus Biopharma Corporation | NCT01518881 | [85] |
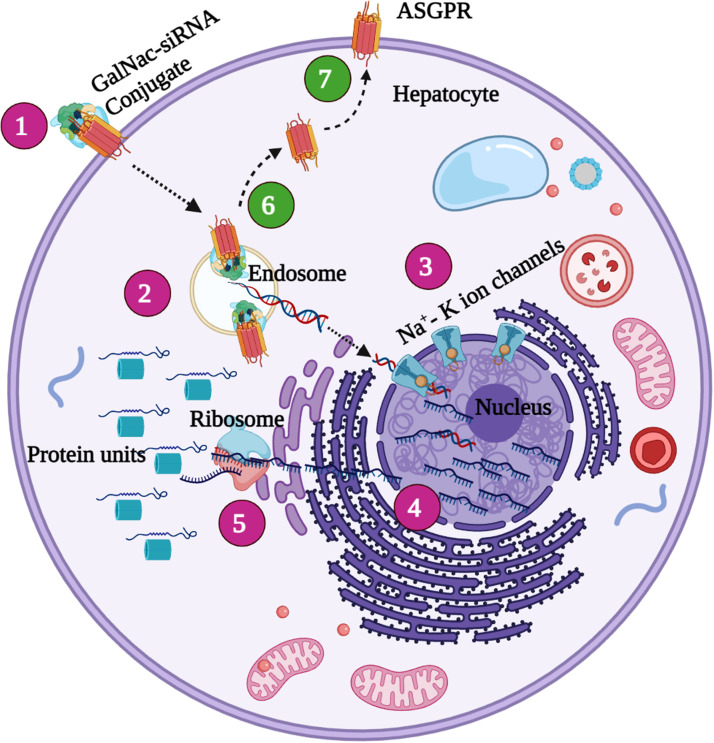
The liver constitutes one-third of the total reticuloendothelial mass of the human body, playing major roles in defense against a wide range of microorganisms [87]. Liver integrity is compromised by microbial pathogens that cause acute liver failure, hepatic fibrosis, and cirrhosis [88]. Hepatitis B is one such pathogen [89], [90]. It was earlier considered that the infection was incurable, but it can now be eradicated using nuclear-based anti‐viral drugs (NUCs) including lamivudine, telbivudine, adefovir, entecavir (ETV), tenofovirdisoproxil (TDF), and tenofoviralafenamide (TAF) [91], [92], [93], [94], [95], [96], [97]. RNAi-based drugs may treat several severe viral infections (including EBOLA infections) for which effective drugs and vaccines are lacking. GalNAc-siRNA conjugates bind to the approximately 106 ASGPR molecules on the sinusoidal cellular membrane of a hepatocyte. The conjugates are then endocytosed and accumulate in clathrin-coated pits [98]. When the pH falls, the conjugates are released into the cellular lumen and return to the cell surface [84]. Soon thereafter, the sialyl-GalNAc linkers are removed from siRNA, triggering transactivation of the RNA-binding protein and RNAi activity within cells ( Fig. 2).
Fig. 2.
GalNac-siRNA conjugate mediated gene silencing 1. The GalNac-siRNA binds to the ASGPR receptor molecule seen on the surface of the hepatocyte cellular membrane region firmly and enters the hepatocyte via the 2. endosome transfer by a process known as the endocytosis 3. The sialyl-GalNAc linkers are degraded from the siRNA molecule and are transferred into the nucleus thereby provoking desired alterations in the target gene site. 4. The altered gene expression is then processed through the process of translation and the 5. target protein is achieved successfully. 6. The free ASGPR receptor molecules are then recycled back to their original form and replaced in the 7. Cellular membrane surface for further functions to be carried out.
The first-generation drugs (the two synthetic siRNAs of ARC-520) [99] were delivered as GalNAc conjugates to patients with chronic HBV infection; the preclinical study of Arrowhead Pharmaceuticals is now entering the clinical phase. ARC-520 was well-tolerated in healthy volunteers (trials NCT02452528, NCT01872065, and NCT02604212) [100]. The drug targets the common regions at the 3′ UTR ends of HBV transcripts from episomal DNA; the drug is linked to a dynamic poly-conjugate (DPC) that facilitates cellular uptake by protecting it from degradation when given intravenously [101], [102].
The phase II trials (nos. NCT02577029, NCT02065336, and NCT02604199) revealed reduced HBsAg expression in patients taking nucleotide analogs [103], [104]. The second-generation ARC-521 reduced the expression of HBsAg and viral DNA in a phase I trial (NCT02797522) [105]. Unfortunately, the trial was discontinued after lethal toxicity developed in non-human primates. Further studies showed that a GalNac-conjugated siRNA targeting all HBV transcripts (JNJ-3989, formerly ARO-HBV) after subcutaneous administration exhibited fewer side-effects (trials NCT03982186, NCT04129554, and NCT03365947) [106], [107]. The next-generation hepatitis B treatment uses a mouse adeno-associated virus (AAV)- LNA ASO-GalNAc conjugate; this reduces the level of membrane surface HBsAg to the lowest value found to date [108]. GalNAc-conjugated siRNAs targeting Epstein-Barr virus, cytomegalovirus, herpes simplex virus, parvovirus, adenovirus, and SARS-associated coronaviruses have been reported, but have not yet been tested in the SARS CoV-2 context [109], [110], [111].
GalNAc-siRNA conjugates are simpler, smaller, and more defined than the LNP formulations. GalNAc-siRNA is synthesized using a solid-state method and chemically defined via MS [112]. Initially a neoglycopeptide (ah-GalNAc)3 was used to target a ligand composed of short, neutral, methyl phosphonate 8-mer oligonucleotides [113]. The linker length and sugar were then optimized, and a refined tris-galactoside structure used to deliver lipids and ASOs [114]. Recently, sequential conjugation of GalNAc residues via nucleosidic linkages has increased drug potencies [115], enhancing hepatocyte oligonucleotide delivery to ∼ 10-fold that of free systems in preclinical models [116].
Recent clinical trials using GalNAc-siRNA conjugates have been performed by Alnylam, Arrowhead, and Dicerna Pharmaceuticals. Alnylam is evaluating six GalNAc-siRNA conjugates in three phase III trials that widely target liver diseases. The first clinical trial evaluated revusiran (ALN-TTRsc) that targets the transthyretin (TTR) protein in an effort to treat TTR-mediated amyloidosis (trial nos. D2, D1–64, D1–65).
6. Conclusion
siRNAs that target key signaling genes may not only improve drug efficiencies but also enhance drug uptake and distribution by the native receptors based therapeutic approaches at cellular levels influencing chemical modifications in delivery mechanisms. We have reviewed the role played by GalNAc conjugates in oligonucleotide-based therapeutic approaches that exhibit great potential in targeted drug delivery system. Recently, ASO conjugated to 5′ nucleic acids has been shown to be maximally amenable to solid-phase synthesis, and to target hepatocytes more effectively, being tested against various disease conditions and more novel drugs based on native cell and tissue specific conjugates are yet to come in the future. These challenges serve to be the major achievements of pharmaceutical industries in the upcoming era.
Funding
This research received no external funding.
CRediT authorship contribution statement
Arun Meyyazhaganf: Conceptualization, Writing – review & editing, Coordinated the working group. Shanmughavel Piramanayagama: Conceptualization, Writing – review & editing. Balamuralikrishnan Balasubramanianb: Conceptualization, Writing – original draft, Selected bibliographic sources, Coordinated the working group, Writing – review & editing. Lokesh Thangamania: Writing – original draft. Karthika Pushparaje: Selected bibliographic sources, Figures. Murugesh Easwaranc: Selected bibliographic sources, Writing – review & editing. Jeyakumar Natarajand: Selected bibliographic sources, Writing – review & editing. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the DBT-Bioinformatics Centre at the Department of Bioinformatics, Bharathiar University for the computational facilities. Lokesh Thangamani acknowledge the financial support received through the award of ICMR-Senior Research Fellow (Award Letter No: BMI/11(01)/2020). All the authors were grateful and extended their appreciation to the authorities for their support.
References
- 1.Carthew R.W., Sontheimer E.J. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson R.C., Doudna J.A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaikkonen M.U., Lam M.T.Y., Glass C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011;90:430–440. doi: 10.1093/cvr/cvr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in caenorhabditiselegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 6.Lee R.C., Feinbaum R.L., Ambros V. The C. elegansheterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein E., Caudy A.A., Hammond S.M., Hannon G.J. Role for a bidentateribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 8.Kim D.H., Behlke M.A., Rose S.D., Chang M.S., Choi S., Rossi J.J. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat. Biotechnol. 2005;23:222–226. doi: 10.1038/nbt1051. [DOI] [PubMed] [Google Scholar]
- 9.Zamore P.D., Tuschl T., Sharp P.A., Bartel D.P. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/s0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 10.Choung S., Kim Y.J., Kim S., Park H.O., Choi Y.C. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem. Biophys. Res. Commun. 2006;342:919–927. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 11.Song J.-J. Crystal structure of argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. (80-.) [DOI] [PubMed] [Google Scholar]
- 12.Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Meister G., Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 14.Oh Y.K., Park T.G. siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Pal A., Ahmad A., Khan S., Sakabe I., Zhang C., Kasid U.N., Ahmad I. Systemic delivery of RafsiRNA using cationic cardiolipin liposomes silences Raf-1 expression and inhibits tumor growth in xenograft model of human prostate cancer. Int. J. Oncol. 2005;26:1087–1091. doi: 10.3892/ijo.26.4.1087. [DOI] [PubMed] [Google Scholar]
- 16.Landen C.N., Chavez-Reyes A., Bucana C., Schmandt R., Deavers M.T., Lopez-Berestein G., Sood A.K. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 17.Rubin M.A. Targeted therapy of cancer: new roles for pathologists—prostate cancer. Mod. Pathol. 2008;21(Suppl. 2):S44–S55. doi: 10.1038/modpathol.2008.11. [DOI] [PubMed] [Google Scholar]
- 18.Watts J.K., Deleavey G.F., Damha M.J. Chemically modified siRNA: tools and applications. Drug Discov. Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Guo P., Coban O., Snead N.M., Trebley J., Hoeprich S., Guo S., Shu Y. Engineering rna for targeted sirna delivery and medical application. Adv. Drug Deliv. Rev. 2010;62:650–666. doi: 10.1016/j.addr.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho W., Zhang X.Q., Xu X. Biomaterials in siRNA delivery: a comprehensive review. Adv. Healthc. Mater. 2016;5:2715–2731. doi: 10.1002/adhm.201600418. [DOI] [PubMed] [Google Scholar]
- 21.Shemesh C.S., Yu R.Z., Gaus H.J., Seth P.P., Swayze E.E., Bennett F.C., Geary R.S., Henry S.P., Wang Y. Pharmacokinetic and pharmacodynamic Investigations of ION-353382, a model antisense oligonucleotide: using alpha-2-macroglobulin and murinoglobulin double-knockout mice. Nucleic Acid Ther. 2016;26:223–235. doi: 10.1089/nat.2016.0607. [DOI] [PubMed] [Google Scholar]
- 22.Iversen F., Yang C., Dagnæs-Hansen F., Schaffert D.H., Kjems J., Gao S. Optimized siRNA-PEG conjugates for extended blood circulation and reduced urine excretion in mice. Theranostics. 2013;3:201–209. doi: 10.7150/thno.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geary R.S., Norris D., Yu R., Bennett C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015;87:46–51. doi: 10.1016/j.addr.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Finkel R.S., Chiriboga C.A., Vajsar J., Day J.W., Montes J., De Vivo D.C., Yamashita M., Rigo F., Hung G., Schneider E., Norris D.A., Xia S., Bennett C.F., Bishop K.M. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388:3017–3026. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 25.Li C.X., Shi M., Tian J.H., Lin X.D., Kang Y.J., Chen L.J., Qin X.C., Xu J., Holmes E.C., Zhang Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife. 2015;2015 doi: 10.7554/eLife.05378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi M., Lin X.D., Chen X., Tian J.H., Chen L.J., Li K., Wang W., Eden J.S., Shen J.J., Liu L., Holmes E.C., Zhang Y.Z. The evolutionary history of vertebrate RNA viruses. Nature. 2018;556:197–202. doi: 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 27.ICTV, (n.d.). 〈https://talk.ictvonline.org/taxonomy/〉. (Accessed 7 January 2021).
- 28.Luo M., Green T.J., Zhang X., Tsao J., Qiu S. Structural comparisons of the nucleoprotein from three negative strand RNA virus families. Virol. J. 2007;4:72. doi: 10.1186/1743-422X-4-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrescia N.G.A., Bamford D.H., Grimes J.M., Stuart D.I. Structure unifies the viral universe. Annu. Rev. Biochem. 2012;81:795–822. doi: 10.1146/annurev-biochem-060910-095130. [DOI] [PubMed] [Google Scholar]
- 30.Černý J., Bolfíková B.Č., Valdés J.J., Grubhoffer L., Růžek D. Evolution of tertiary structure of viral RNA dependent polymerases. PLoS One. 2014;9:96070. doi: 10.1371/journal.pone.0096070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laanto E., Mäntynen S., De Colibus L., Marjakangas J., Gillum A., Stuart D.I., Ravantti J.J., Huiskonen J.T., Sundberg L.R. Virus found in a boreal lake links ssDNA and dsDNA viruses. Proc. Natl. Acad. Sci. USA. 2017;114:8378–8383. doi: 10.1073/pnas.1703834114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahola T. New phylogenetic grouping of positive-sense RNA viruses is concordant with replication complex morphology. MBio. 2019;10 doi: 10.1128/mBio.01402-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bamford D.H., Burnett R.M., Stuart D.I. Evolution of viral structure. Theor. Popul. Biol. 2002;61:461–470. doi: 10.1006/tpbi.2002.1591. [DOI] [PubMed] [Google Scholar]
- 34.Oksanen H.M., Pietilä M.K., Sencilo A., Atanasova N.S., Roine E., Bamford D.H. Virus universe: can it be constructed from a limited number of viral architectures. Virus Essent. Agents Life. 2012:83–105. doi: 10.1007/978-94-007-4899-6_5. [DOI] [Google Scholar]
- 35.Martini M., Gazzaniga V., Bragazzi N.L., Barberis I. The Spanish Influenza Pandemic: a lesson from history 100 years after 1918. J. Prev. Med. Hyg. 2019;60 doi: 10.15167/2421-4248/jpmh2019.60.1.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson C. History lessons: the Asian Flu pandemic. Br. J. Gen. Pract. 2009;59:622–623. doi: 10.3399/bjgp09×453882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snacken R., Kendal A.P., Haaheim L.R., Wood J.M. The next influenza pandemic: lessons from Hong Kong, 1997. Emerg. Infect. Dis. 1999;5:195–203. doi: 10.3201/eid0502.990202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skog L., Hauska H., Linde A. The Russian influenza in Sweden in 1889-90: an example of Geographic Information System analysis. Euro Surveill. 2008;13 doi: 10.2807/ese.13.49.19056-en. [DOI] [PubMed] [Google Scholar]
- 39.Saunders-Hastings P.R., Krewski D. Reviewing the history of pandemic influenza: Understanding patterns of emergence and transmission. Pathogens. 2016;5 doi: 10.3390/pathogens5040066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng V.C.C., Lau S.K.P., Woo P.C.Y., Kwok Y.Y. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin. Microbiol. Rev. 2007;20:660–694. doi: 10.1128/CMR.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamptey P., Wigley M., Carr D., Collymore Y. Facing the HIV/AIDS pandemic. Popul. Bull. 2002;57 [Google Scholar]
- 42.Mukherjee S., Sen S., Nakate P., Moitra S. Management of swine flu (H1N1 Flu) outbreak and its treatment guidelines. Commun. Acquir. Infect. 2015;2 doi: 10.4103/2225-6482.166066. [DOI] [Google Scholar]
- 43.Rajak H., Jain D.K., Singh A., Sharma A.K., Dixit A. Ebola virus disease: past, present and future. Asian Pac. J. Trop. Biomed. 2015;5:337–343. doi: 10.1016/S2221-1691(15)30365-8. [DOI] [Google Scholar]
- 44.Shang W., Yang Y., Rao Y., Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. Npj Vaccin. 2020;5:18. doi: 10.1038/s41541-020-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aleanizy F.S., Mohmed N., Alqahtani F.Y., El Hadi Mohamed R.A. Outbreak of Middle East respiratory syndrome coronavirus in Saudi Arabia: a retrospective study. BMC Infect. Dis. 2017;17:23. doi: 10.1186/s12879-016-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coronavirus Disease (COVID-19) Situation Reports, (n.d.). 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports〉 (Accessed 7 January 2021).
- 47.Bitko V., Barik S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001;1:34. doi: 10.1186/1471-2180-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapadia S.B., Brideau-Andersen A., Chisari F.V. Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc. Natl. Acad. Sci. USA. 2003;100:2014–2018. doi: 10.1073/pnas.252783999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geisbert T.W., Lee A.C., Robbins M., Geisbert J.B., Honko A.N., Sood V., Johnson J.C., de Jong S., Tavakoli I., Judge A., Hensley L.E., MacLachlan I. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paavilainen H., Lehtinen J., Romanovskaya A., Nygårdas M., Bamford D.H., Poranen M.M., Hukkanen V. Topical treatment of herpes simplex virus infection with enzymatically created siRNA swarm. Antivir. Ther. 2017;22:631–637. doi: 10.3851/IMP3153. [DOI] [PubMed] [Google Scholar]
- 51.Villegas P.M., Ortega E., Villa-Tanaca L., Barrón B.L., Torres-Flores J. Inhibition of dengue virus infection by small interfering RNAs that target highly conserved sequences in the NS4B or NS5 coding regions. Arch. Virol. 2018;163:1331–1335. doi: 10.1007/s00705-018-3757-2. [DOI] [PubMed] [Google Scholar]
- 52.Ge Q., Filip L., Bai A., Nguyen T., Eisen H.N., Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc. Natl. Acad. Sci. USA. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tompkins S.M., Lo C.Y., Tumpey T.M., Epstein S.L. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc. Natl. Acad. Sci. USA. 2004;101:8682–8686. doi: 10.1073/pnas.0402630101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bennink J.R., Palmore T.N. The promise of siRNAs for the treatment of influenza. Trends Mol. Med. 2004;10:571–574. doi: 10.1016/j.molmed.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Yuan J., Cheung P.K.M., Zhang H.M., Chau D., Yang D. Inhibition of Coxsackievirus B3 replication by small interfering RNAs requires perfect sequence match in the central region of the viral positive strand. J. Virol. 2005;79:2151–2159. doi: 10.1128/jvi.79.4.2151-2159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn J., Jun E.S., Lee H.S., Yoon S.Y., Kim D., Joo C.-H., Kim Y.K., Lee H. A small interfering RNA targeting coxsackievirus B3 protects permissive HeLa cells from viral challenge. J. Virol. 2005;79:8620–8624. doi: 10.1128/jvi.79.13.8620-8624.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z., Ren L., Zhao X., Hung T., Meng A., Wang J., Chen Y.-G. Inhibition of severe acute respiratory syndrome virus replication by small interfering RNAs in mammalian cells. J. Virol. 2004;78:7523–7527. doi: 10.1128/jvi.78.14.7523-7527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu A., Zhang H., Zhang X., Wang H., Hu Q., Shen L., Schaffhausen B.S., Hou W., Li L. Attenuation of SARS coronavirus by a short hairpin RNA expression plasmid targeting RNA-dependent RNA polymerase. Virology. 2004;324:84–89. doi: 10.1016/j.virol.2004.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Li T., Fu L., Yu C., Li Y., Xu X., Wang Y., Ning H., Zhang S., Chen W., Babiuk L.A., Chang Z. Silencing SARS-CoV spike protein expression in cultured cells by RNA interference. FEBS Lett. 2004;560:141–146. doi: 10.1016/S0014-5793(04)00087-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Li B.J., Tang Q., Cheng D., Qin C., Xie F.Y., Wei Q., Xu J., Liu Y., Zheng B.J., Woodle M.C., Zhong N., Lu P.Y. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat. Med. 2005;11:944–951. doi: 10.1038/nm1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W., Yan W., Du Q., Fei L., Liu M., Ni Z., Sheng Z., Zheng Z. RNA interference targeting VP1 inhibits foot-and-mouth disease virus replication in BHK-21 cells and suckling mice. J. Virol. 2004;78:6900–6907. doi: 10.1128/jvi.78.13.6900-6907.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu M., Chen W., Ni Z., Yan W., Fei L., Jiao Y., Zhang J., Du Q., Wei X., Chen J., Liu Y., Zheng Z. Cross-inhibition to heterologous foot-and-mouth disease virus infection induced by RNA interference targeting the conserved regions of viral genome. Virology. 2005;336:51–59. doi: 10.1016/j.virol.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 63.Jiang M., Milner J. Selective silencing of viral gene E6 and E7 expression in HPV-positive human cervical carcinoma cells using small interfering RNAs. Methods Mol. Biol. 2005;292:401–420. doi: 10.1385/1-59259-848-x:401. [DOI] [PubMed] [Google Scholar]
- 64.Longworth M.S., Wilson R., Laimins L.A. HPV31 E7 facilitates replication by activating E2F2 transcription through its interaction with HDACs. EMBO J. 2005;24:1821–1830. doi: 10.1038/sj.emboj.7600651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giladi H., Ketzinel-Gilad M., Rivkin L., Felig Y., Nussbaum O., Galun E. Small interfering RNA inhibits hepatitis B virus replication in mice. Mol. Ther. 2003;8:769–776. doi: 10.1016/S1525-0016(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 66.Hamasaki K., Nakao K., Matsumoto K., Ichikawa T., Ishikawa H., Eguchi K. Short interfering RNA-directed inhibition of hepatitis B virus replication. FEBS Lett. 2003;543:51–54. doi: 10.1016/S0014-5793(03)00400-9. [DOI] [PubMed] [Google Scholar]
- 67.Konishi M., Wu C.H., Wu G.Y. Inhibition of HBV replication by siRNA in a stable HBV-producing cell line. Hepatology. 2003;38:842–850. doi: 10.1053/jhep.2003.50416. [DOI] [PubMed] [Google Scholar]
- 68.Shlomai A., Shaul Y. Inhibition of hepatitis B virus expression and replication by RNA interference. Hepatology. 2003;37:764–770. doi: 10.1053/jhep.2003.50146. [DOI] [PubMed] [Google Scholar]
- 69.Guo Y., Guo H., Zhang L., Xie H., Zhao X., Wang F., Li Z., Wang Y., Ma S., Tao J., Wang W., Zhou Y., Yang W., Cheng J. Genomic analysis of Anti-Hepatitis B Virus (HBV) activity by small interfering RNA and Lamivudine in stable HBV-producing cells. J. Virol. 2005;79:14392–14403. doi: 10.1128/jvi.79.22.14392-14403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carmona S., Ely A., Crowther C., Moolla N., Salazar F.H., Marion P.L., Ferry N., Weinberg M.S., Arbuthnot P. Effective inhibition of HBV replication in vivo by anti-HBx short hairpin RNAs. Mol. Ther. 2006;13:411–421. doi: 10.1016/j.ymthe.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 71.McCaffrey A.P., Nakai H., Pandey K., Huang Z., Salazar F.H., Xu H., Wieland S.F., Marion P.L., Kay M.A. Inhibition of hepatitis B virus in mice by RNA interference. Nat. Biotechnol. 2003;21:639–644. doi: 10.1038/nbt824. [DOI] [PubMed] [Google Scholar]
- 72.Cross R.W., Mire C.E., Feldmann H., Geisbert T.W. Post-exposure treatments for Ebola and Marburg virus infections. Nat. Rev. Drug Discov. 2018;17:413–434. doi: 10.1038/nrd.2017.251. [DOI] [PubMed] [Google Scholar]
- 73.Dunning J., Sahr F., Rojek A., Gannon F., Carson G., Idriss B., Massaquoi T., Gandi R., Joseph S., Osman H.K., Brooks T.J.G., Simpson A.J.H., Goodfellow I., Thorne L., Arias A., Merson L., Castle L., Howell-Jones R., Pardinaz-Solis R., Hope-Gill B., Ferri M., Grove J., Kowalski M., Stepniewska K., Lang T., Whitehead J., Olliaro P., Samai M., Horby P.W. Experimental treatment of Ebola Virus Disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med. 2016;13:1001997. doi: 10.1371/journal.pmed.1001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wittrup A., Lieberman J. Knocking down disease: a progress report on siRNA therapeutics. Nat. Rev. Genet. 2015;16:543–552. doi: 10.1038/nrg3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 76.Huang Y., Cheng Q., Ji J.L., Zheng S., Du L., Meng L., Wu Y., Zhao D., Wang X., Lai L., Cao H., Xiao K., Gao S., Liang Z. Pharmacokinetic behaviors of intravenously administered siRNA in glandular tissues. Theranostics. 2016;6:1528–1541. doi: 10.7150/thno.15246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang Y., Hong J., Zheng S., Ding Y., Guo S., Zhang H., Zhang X., Du Q., Liang Z. Elimination pathways of systemically delivered siRNA. Mol. Ther. 2011;19:381–385. doi: 10.1038/mt.2010.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rana T.M. Illuminating the silence: Understanding the structure and function of small RNAs. Nat. Rev. Mol. Cell Biol. 2007;8:23–36. doi: 10.1038/nrm2085. [DOI] [PubMed] [Google Scholar]
- 80.Prakash T.P., Graham M.J., Yu J., Carty R., Low A., Chappell A., Schmidt K., Zhao C., Aghajan M., Murray H.F., Riney S., Booten S.L., Murray S.F., Gaus H., Crosby J., Lima W.F., Guo S., Monia B.P., Swayze E.E., Seth P.P. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buchhaupt M., Peifer C., Entian K.D. Analysis of 2′-O-methylated nucleosides and pseudouridines in ribosomal RNAs using DNAzymes. Anal. Biochem. 2007;361:102–108. doi: 10.1016/j.ab.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Guzman-Aranguez A., Loma P., Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013;170:730–747. doi: 10.1111/bph.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kelin A.V., Milstein S., Taneja N., Oshea J., Shaikh S., Zhang L., Van Der Sluis R.J., Jung M.E., Akinc A., Hutabarat R., Kuchimanchi S., Fitzgerald K., Zimmermann T., Van Berkel T.J.C., Maier M.A., Rajeev K.G., Manoharan M. Multivalent N -acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 84.Springer A.D., Dowdy S.F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid. Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Home - ClinicalTrials.gov, (n.d.). 〈https://clinicaltrials.gov/〉 (Accessed 7 January 2021).
- 86.Zhou J., Wu Y., Wang C., Cheng Q., Han S., Wang X., Zhang J., Deng L., Zhao D., Du L., Cao H., Liang Z., Huang Y., Dong A. PH-Sensitive nanomicelles for high-efficiency siRNA delivery in vitro and in vivo: an insight into the design of polycations with robust cytosolic release. Nano Lett. 2016;16:6916–6923. doi: 10.1021/acs.nanolett.6b02915. [DOI] [PubMed] [Google Scholar]
- 87.Talwani R., Gilliam B.L., Howell C. Infectious diseases and the liver. Clin. Liver Dis. 2011;15:111–130. doi: 10.1016/j.cld.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi E.Y.K., Lamps L.W. Granulomas in the Liver, with a focus on infectious causes. Surg. Pathol. Clin. 2018;11:231–250. doi: 10.1016/j.path.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Moolla N., Kew M., Arbuthnot P. Regulatory elements of hepatitis B virus transcription. J. Viral Hepat. 2002;9:323–331. doi: 10.1046/j.1365-2893.2002.00381.x. [DOI] [PubMed] [Google Scholar]
- 90.Lamontagne R.J., Bagga S., Bouchard M.J. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163–186. doi: 10.20517/2394-5079.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Testoni B., Lebossé F., Scholtes C., Berby F., Miaglia C., Subic M., Loglio A., Facchetti F., Lampertico P., Levrero M., Zoulim F. Serum hepatitis B core-related antigen (HBcrAg) correlates with covalently closed circular DNA transcriptional activity in chronic hepatitis B patients. J. Hepatol. 2019;70:615–625. doi: 10.1016/j.jhep.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 92.Liu S., Zhou B., Valdes J.D., Sun J., Guo H. Serum Hepatitis B Virus RNA: a new potential biomarker for chronic Hepatitis B virus infection. Hepatology. 2019;69:1816–1827. doi: 10.1002/hep.30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tao Y., Wu D., Zhou L., Chen E., Liu C., Tang X., Jiang W., Han N., Li H., Tang H. Present and Future Therapies for Chronic Hepatitis B. Adv. Exp. Med. Biol. 2020:137–186. doi: 10.1007/978-981-13-9151-4_6. [DOI] [PubMed] [Google Scholar]
- 94.Nijampatnam B., Liotta D.C. Recent advances in the development of HBV capsid assembly modulators. Curr. Opin. Chem. Biol. 2019;50:73–79. doi: 10.1016/j.cbpa.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 95.Volz T., Allweiss L., Ben ḾBarek M., Warlich M., Lohse A.W., Pollok J.M., Alexandrov A., Urban S., Petersen J., Lütgehetmann M., Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J. Hepatol. 2013;58:861–867. doi: 10.1016/j.jhep.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019;20 doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 98.Huang Y. Preclinical and clinical advances of GalNAc-decorated nucleic acid therapeutics. Mol. Ther. Nucleic Acids. 2017;6:116–132. doi: 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rozema D.B., Lewis D.L., Wakefield D.H., Wong S.C., Klein J.J., Roesch P.L., Bertin S.L., Reppen T.W., Chu Q., Blokhin A.V., Hagstrom J.E., Wolff J.A. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schluep T., Lickliter J., Hamilton J., Lewis D.L., Lai C.L., Lau J.Y.N., Locarnini S.A., Gish R.G., Given B.D. Safety, tolerability, and pharmacokinetics of ARC-520 injection, an RNA interference-based therapeutic for the treatment of chronic Hepatitis B Virus Infection, in healthy volunteers. Clin. Pharmacol. Drug Dev. 2017;6:350–362. doi: 10.1002/cpdd.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Decorsière A., Mueller H., Van Breugel P.C., Abdul F., Gerossier L., Beran R.K., Livingston C.M., Niu C., Fletcher S.P., Hantz O., Strubin M. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531:386–389. doi: 10.1038/nature17170. [DOI] [PubMed] [Google Scholar]
- 102.Schroeder A., Levins C.G., Cortez C., Langer R., Anderson D.G. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuen M.F., Schiefke I., Yoon J.H., Ahn S.H., Heo J., Kim J.H., Lik Yuen Chan H., Yoon K.T., Klinker H., Manns M., Petersen J., Schluep T., Hamilton J., Given B.D., Ferrari C., Lai C.L., Locarnini S.A., Gish R.G. RNA interference therapy with ARC-520 results in prolonged Hepatitis B surface antigen response in patients with chronic Hepatitis B infection. Hepatology. 2020;72:19–31. doi: 10.1002/hep.31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foster D.J., Brown C.R., Shaikh S., Trapp C., Schlegel M.K., Qian K., Sehgal A., Rajeev K.G., Jadhav V., Manoharan M., Kuchimanchi S., Maier M.A., Milstein S. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol. Ther. 2018;26:708–717. doi: 10.1016/j.ymthe.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hassler M.R., Turanov A.A., Alterman J.F., Haraszti R.A., Coles A.H., Osborn M.F., Echeverria D., Nikan M., Salomon W.E., Roux L., Godinho B.M.D.C., Davis S.M., Morrissey D.V., Zamore P.D., AnanthKarumanchi S., Moore M.J., Aronin N., Khvorova A. Comparison of partially and fully chemically-modified siRNA in conjugate-mediated delivery in vivo. Nucleic Acids Res. 2018;46:2185–2196. doi: 10.1093/nar/gky037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schinazi R.F., Ehteshami M., Bassit L., Asselah T. Towards HBV curative therapies. Liver Int. 2018;38(Suppl. 1):S102–S114. doi: 10.1111/liv.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wooddell C.I., Yuen M.F., Chan H.L.Y., Gish R.G., Locarnini S.A., Chavez D., Ferrari C., Given B.D., Hamilton J., Kanner S.B., Lai C.L., Lau J.Y.N., Schluep T., Xu Z., Lanford R.E., Lewis D.L. Rnai-based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis b virus DNA is a source of hbsag. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Javanbakht H., Mueller H., Walther J., Zhou X., Lopez A., Pattupara T., Blaising J., Pedersen L., Albæk N., Jackerott M., Shi T., Ploix C., Driessen W., Persson R., Ravn J., Young J.A.T., Ottosen S. Liver-targeted anti-HBV single-stranded oligonucleotides with locked nucleic acid potently reduce hbv gene expression. Mol. Ther. Nucleic Acids. 2018;11:441–454. doi: 10.1016/j.omtn.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am. J. Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marjot T., Moolla A., Cobbold J.F., Hodson L., Tomlinson J.W. Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management. Endocr. Rev. 2020;41 doi: 10.1210/endrev/bnz009. [DOI] [PubMed] [Google Scholar]
- 111.Debacker A.J., Voutila J., Catley M., Blakey D., Habib N. Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug. Mol. Ther. 2020;28:1759–1771. doi: 10.1016/j.ymthe.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rajeev K.G., Nair J.K., Jayaraman M., Charisse K., Taneja N., O’Shea J., Willoughby J.L.S., Yucius K., Nguyen T., Shulga-Morskaya S., Milstein S., Liebow A., Querbes W., Borodovsky A., Fitzgerald K., Maier M.A., Manoharan M. Hepatocyte-specific delivery of siRNAs conjugated to novel non-nucleosidic trivalent N-acetylgalactosamine elicits robust gene silencing in vivo. ChemBioChem. 2015;16:903–908. doi: 10.1002/cbic.201500023. [DOI] [PubMed] [Google Scholar]
- 113.Hangeland J.J., Levis J.T., Lee Y.C., Ts’o P.O.P., Lee Y.C. Cell-type specific and ligand specific enhancement of cellular uptake of oligodeoxynucleosi de methylphosphonates covalently linked with a neoglycopeptide, YEE(ah-GalNAc)3. Bioconjug. Chem. 1995;6:695–701. doi: 10.1021/bc00036a006. [DOI] [PubMed] [Google Scholar]
- 114.Biessen E.A.L., Vietsch H., Rump E.T., Fluiter K., Kuiper J., Bijsterbosch M.K., Berkel T.J.C.Van. Targeted delivery of oligodeoxynucleotides to parenchymal liver cells in vivo. Biochem. J. 1999;340(Pt. 3):783–792. doi: 10.1042/0264-6021:3400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matsuda S., Keiser K., Nair J.K., Charisse K., Manoharan R.M., Kretschmer P., Peng C.G., Kel’in A.V., Kandasamy P., Willoughby J.L.S., Liebow A., Querbes W., Yucius K., Nguyen T., Milstein S., Maier M.A., Rajeev K.G., Manoharan M. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 116.Prakash T.P., Graham M.J., Yu J., Carty R., Low A., Chappell A., Schmidt K., Zhao C., Aghajan M., Murray H.F., Riney S., Booten S.L., Murray S.F., Gaus H., Crosby J., Lima W.F., Guo S., Monia B.P., Swayze E.E., Seth P.P. NAR breakthrough article targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]