Abstract
Background
Asthma is an important reason for hospitalization in children aged under five years. Information about the current status of asthma in Iranian children can help the Iranian health sector plan carefully and prevent asthma incidence by educating the families. The present systematic review and meta-analysis is aimed at estimating asthma prevalence in Iranian children and adolescents.
Method
Data were found using keywords such as prevalence, epidemiology, asthma, adolescent, children, pediatrics, Iran in Web of Science, Scopus, PubMed, Cochrane, and Embase databases. Three national databases, including Magiran, Barakat Pharmed Co (Iran medex), and Scientific Information Databank (SID) were searched until 1 October 2020. Cross-sectional and original studies were included in the study, and then, quality assessment was done using the National Institutes of Health's Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. A pooled estimated prevalence of asthma was calculated using Der Simonian-Laird random model. Egger's test was used to evaluate publication bias. The data were analyzed using the STATA software version 16.
Results
30 studies were selected and investigated. The prevalence of asthma in children and adolescents was 6% and 8%, and the prevalence in boys and girls was 9% and 8%, respectively. Among the asthma symptoms, wheezing had the most prevalence (17% in children and 19% in adolescents) and sleep disturbance had the lowest prevalence (6% in children and 6% in adolescents).
Conclusion
The prevalence of asthma in Iranian children and adolescents is lower than in the world. Existing strategies should be pursued followed. Also, guidelines for asthma control and prevention should be considered in the future.
1. Background
Asthma is an airway inflammatory disease characterized by variable symptoms like chest tightness, breathlessness, wheeze, and cough. Asthma is one of the leading chronic respiratory diseases in children in the world. This disease is one of the main reasons for hospitalizations of children under five years old and has been increasing in recent years [1].
The estimations suggest that more than 300 million people are affected by asthma worldwide, and more than 400 million persons will experience asthma in the future. The prevalence of asthma in children varies according to geographical variation. However, the global prevalence is from 9.1% to 9.5% in children and from 9.1% to 10.4% in adolescents [2].
It seems that factors such as age, sex, economic status, genetics, and exposure to pollutants can affect asthma prevalence [3]. Asthma causes growth disturbance, increased health care costs, decreased quality of life, absence at school, etc. Implementation of proper strategies for recognizing the epidemiology of asthma and, consequently, appropriate treatment of the disease has effectively decreased disease burden [4].
The prevalence of asthma symptoms in children and adolescents increased worldwide, in recent years, particularly in low-middle income countries [5]. Many studies have been performed to estimate the prevalence of asthma in children and adolescents in various cities in Iran. Heidarnia et al. evaluated 19 studies between 1998 and 2003; all of them used ISSAC protocol. The lowest and the highest prevalence were reported in Kerman (2.7%) and Tehran (35.4%), respectively [6]. The overall prevalence of asthma was estimated to be 13.14%. In another meta-analysis, Ghaffari et al. evaluated 27 studies between 1992 and 2012. They reported asthma prevalence to be 2.7% and 3.5% in children aged 6-7 and 13-14 years, respectively [7]. These two meta-analyses showed a noticeable difference in the total asthma prevalence; also, there is no updated information about asthma status in Iranian children in recent years.
Information about the current status of asthma in Iranian children can help the Iranian health sector plan carefully and prevent asthma incidence by educating the families. It can also help neighboring countries to control the incidence of this disease because management of the health of this generation is important for any country. Due to Iran's geographical variety, the prevalence of this disease varies from region to region. As there is no updated comprehensive study to evaluate asthma prevalence in Iranian children and adolescents, the present systematic review and meta-analysis was performed to estimate asthma prevalence in children and adolescents in Iran.
2. Method and Material
The present study was performed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) instruction [8] to examine asthma prevalence among children and adolescents in Iran.
2.1. Search Strategy
Data were found using keywords such as asthma, prevalence, epidemiology, children, pediatrics, adolescent, Iran in Scopus, PubMed, Web of Science, Cochrane and Embase databases.
For PubMed databases, this syntax was used: (Prevalence[Mesh] OR Prevalence[TIAB] OR Epidemiology[Mesh] OR Epidemiology[TIAB]) AND (Asthma[Mesh] OR Asthma∗[TIAB]) AND (Child[Mesh] OR Child∗[TIAB] OR “Adult Children”[Mesh] OR “Adult Children”[TIAB] OR Pediatrics[Mesh] OR Pediatrics∗[TIAB]) AND (Iran[Mesh] OR Iran∗[Text word]). Also, all the big cities searched separately to find the possible related studies. Three national databases, including Magiran, Barakat Pharmed Co (Iran medex), and Scientific Information Databank (SID) were searched until 1 October 2020. All articles published in internationally accredited journals, national conferences, and all available dissertations that attempted to assess the prevalence of asthma in different parts of the country were collected. All references were reviewed manually.
2.2. Inclusion Criteria
Studies that evaluated asthma prevalence in Iranian children were reviewed. The instrument used for data collection was ISSAC or SF-questionnaires, which study participants or their parents completed. Children were categorized as 0 to 10 years old, and those aged 11 to 19 were classified as adolescents. Cross-sectional and original studies were included in the study, and review articles or letter to editors was removed.
2.3. Exclusion Criteria
Unrelated topics, non-Iranian subjects, nonstandard questionnaires, incomplete data, and studies not defined the age group were excluded from the present study. Also, review articles and case-control studies were excluded.
2.4. Quality Assessment
In this step, the papers were independently evaluated by two authors. For quality assessment, we used the National Institutes of Health's Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, which resolve past versions' flaws. In this questionnaire, scoring is shown by a scale of “No,” “Yes,” “cannot be determined”, “Not Applicable,” and “Not reported” [9].
2.5. Data Extraction
The data extracted from all articles were used in the present study and qualified as a checklist. This checklist includes the author's name, type of study, year of publication, assessment tool, study location, sample size, age, gender, asthma prevalence, and associated risk factors.
2.6. Subgrouping the Outcome
In this study, the prevalence of asthma in children, adolescents, girls, and boys was considered the primary outcome. Other related information such as geographical area and asthma symptoms were considered as the secondary outcome.
2.7. Statistical Analysis
A pooled estimated prevalence of asthma was calculated using Der Simonian-Laird random model [10], and the results were reported by 95% confidence interval (CI). To examine the heterogeneity, I2 threshold was used [11]. Also, subgroup was done based on asthma symptoms and geographical areas. For finding the possible reasons of heterogeneity, metaregression was used based on the sample size and publication years. Sensitivity analysis was performed to ensure the stability of the results. Egger's test was used to evaluate publication bias [12]. The data were analyzed using the STATA software version 16.
3. Results
3.1. Search Results
In the first stage, 1270 articles were found; by reviewing the articles, 1090 duplicate and irrelevant articles with inadequate data and other exclusion factors were removed and finally, 30 articles were selected for this systematic review study (Figure 1).
Figure 1.
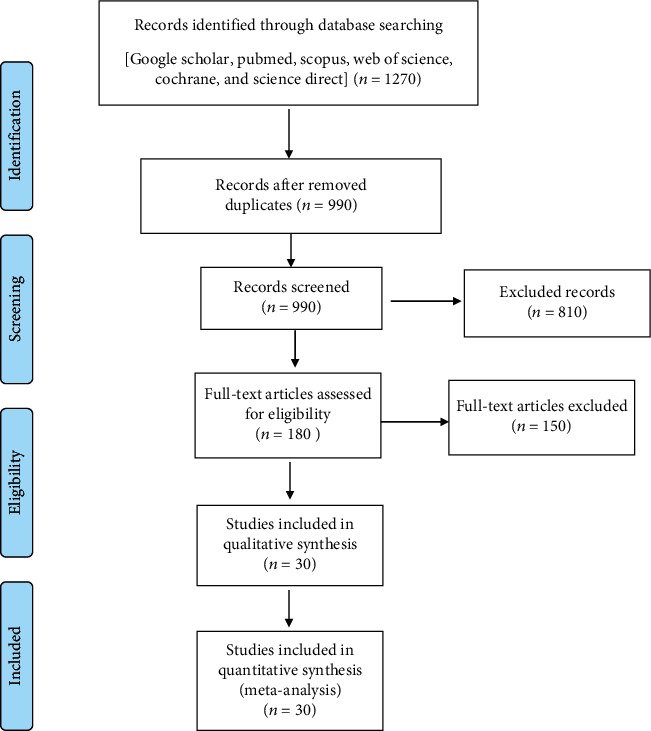
Flowchart describing the study design process.
3.2. Quality Assessment Results
This study used the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. The studies' assessment showed that most of the studies had satisfactory quality and low-risk bias; however, most of them did not mention blinding of the outcome assessors. Most of the studies did not mention the research's timing in the title (Table 1).
Table 1.
The score of the studied based on National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
| Author/studies | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Boskabady and Karimian | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | ✓ | ✓ | NR | ✓ | ✓ |
| Tootoonchi | × | ✓ | ✓ | ✓ | ✓ | NA | CD | × | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Hatami et al. | ✓ | ✓ | ✓ | ✓ | NR | NR | ✓ | ✓ | ✓ | NA | ✓ | CD | ✓ | ✓ |
| Habibi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Zohal and Hasheminasab | ✓ | ✓ | ✓ | ✓ | ✓ | NA | NR | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Ghazi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Masjedi et al. | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Abbasi | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Bazzazi et al. | × | ✓ | ✓ | ✓ | ✓ | NA | NR | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Karimi et al. | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Bidad et al. | ✓ | NR | × | ✓ | ✓ | NA | ✓ | × | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Shakurnia et al. | × | ✓ | ✓ | ✓ | ✓ | NA | NR | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Rahimi Rad et al. | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Fadaeizadeh et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Mohammadzadeh et al. | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Sahebi and Shabestray | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Rajaeifard et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | NR | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Zobeiri | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Tavacol et al. | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Hassanzadeh et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | CD | ✓ | NA | × | NR | ✓ | ✓ |
| Hajavi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Gooya et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Farrokhi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Nasiri Kalmarzi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Ghozikali et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Zamanfar et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Mehravar et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Assadi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Khazaei et al. | × | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
| Fazlollahi et al. | ✓ | ✓ | ✓ | ✓ | ✓ | NA | ✓ | ✓ | ✓ | NA | ✓ | NR | ✓ | ✓ |
3.3. Descriptive Results
Descriptive information of all studies was shown in Table 2. There are 30 studies in this table, of which the lowest prevalence of asthma in children was 2% in Masjedi et al.'s study in Tehran. The lowest prevalence of asthma in adolescents was 1% in the study of Hatami et al. in Bushehr. The highest prevalence of asthma in children was 32% in Tehran and 37% in adolescents. The majority of the study population was between 1,000 and 10,000. Only two studies had a population of less than 1,000. All studies use ISSAC questionnaire to gather the data. Most studies were performed in Tehran province (8 studies).
Table 2.
Characteristics of all eligible asthma prevalence studies in children and adolescents in Iran.
| Author name | Year | City (province) | Method | Prevalence in children | Prevalence in adolescent | Prevalence in girl | Prevalence in boy | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | n | % | n | % | n | % | n | |||||
| Boskabady and Karimian | 2000 | Mashhad (Khorasan Razavi) | ISSAC | _ | _ | 22 | 1201 | 25 | 618 | 19 | 583 | [[27]] |
| Tootoonchi | 2001 | Tehran (Tehran) | ISSAC | 4 | 24 | _ | _ | 3 | 9 | 5 | 15 | [[28]] |
| Hatami et al. | 2002 | Bushehr (Bushehr) | ISSAC | _ | _ | 1 | 19 | _ | _ | _ | _ | [[29]] |
| Habibi et al. | 2002 | Kerman (Kerman) | ISSAC | 3 | 28 | 3 | 32 | 2 | 27 | 3 | 33 | [[30]] |
| Zohal and Hasheminasab | 2003 | Ghazvin (Ghazvin) | ISSAC | 2 | 47 | 2 | 57 | 2 | 45 | 2 | 59 | [[31]] |
| Ghazi et al. | 2003 | Tehran (Tehran) | ISSAC | 32 | 215 | 37 | 507 | 34 | 343 | 37 | 379 | [[32]] |
| Masjedi et al. | 2004 | Tehran (Tehran) | ISSAC | 2 | 64 | 3 | 80 | 3 | 80 | 2 | 64 | [[33]] |
| Abbasi | 2005 | Rasht (Gilan) | ISSAC | 7 | 213 | 5 | 136 | 5 | 144 | 7 | 205 | [[34]] |
| Bazzazi et al. | 2006 | Gorgan (Golestan) | ISSAC | _ | _ | 7 | 196 | 6 | 86 | 8 | 110 | [[35]] |
| Karimi et al. | 2006 | Yazd (Yazd) | ISSAC | _ | _ | 4 | 118 | _ | _ | _ | _ | [[36]] |
| Bidad et al. | 2006 | Tehran (Tehran) | ISSAC | _ | _ | 7 | 212 | 6 | 99 | 9 | 106 | [[37]] |
| Shakurnia et al. | 2007 | Ahvaz (Khuzestan) | ISSAC | 7 | 96 | 10 | 144 | _ | _ | _ | _ | [[38]] |
| Rahimi Rad et al. | 2007 | Urmia (West Azarbaijan) | ISSAC | _ | _ | 2 | 62 | 1 | _ | 3 | _ | [[39]] |
| Fadaeizadeh et al. | 2008 | Tehran, Rasht (Tehran, Gilan) | ISSAC | 5 | 283 | 4 | 222 | _ | _ | _ | _ | [[40]] |
| Mohammadzadeh et al. | 2008 | Babol (Mazandaran) | ISSAC | 3 | 91 | 4 | 128 | 4 | 119 | 5 | 141 | [[41]] |
| Sahebi and Shabestray | 2009 | Tabriz (East Azarbaijan) | ISSAC | _ | _ | 2 | 30 | _ | _ | _ | _ | [[42]] |
| Rajaeifard et al. | 2010 | Yasuj (Kohgiluyeh and Boyer-Ahmad) | ISSAC | 10 | 62 | _ | _ | 7 | 23 | 13 | 39 | [[43]] |
| Zobeiri | 2011 | Kermanshah (Kermanshah) | ISSAC | 2 | 63 | 3 | 99 | 4 | 110 | 2 | 52 | [[44]] |
| Tavacol et al. | 2011 | Ahvaz (Khuzestan) | ISSAC | 4 | 35 | 6 | 54 | _ | _ | _ | _ | [[45]] |
| Hassanzadeh et al. | 2011 | Shiraz (Fars) | ISSAC | _ | _ | 4 | 115 | 3 | 51 | 4 | 64 | [[46]] |
| Hajavi et al. | 2011 | Gonabad (Khorasan Razavi) | ISSAC | _ | _ | 3 | 54 | _ | _ | _ | _ | [[47]] |
| Gooya et al. | 2014 | Bushehr (Bushehr) | ISSAC | 6 | 11 | 15 | 34 | 13 | 38 | 6 | 7 | [[48]] |
| Farrokhi et al. | 2014 | Bushehr (Bushehr) | ISSAC | 7 | 86 | 8 | 85 | _ | _ | _ | _ | [[49]] |
| Nasiri Kalmarzi et al. | 2016 | Kurdistan | ISSAC | 5 | 80 | 4 | 75 | _ | _ | _ | _ | [[50]] |
| Ghozikali et al. | 2016 | Tabriz (East Azarbaijan) | ISSAC | _ | _ | 12 | 142 | _ | _ | 12 | 142 | [[51]] |
| Zamanfar et al. | 2016 | Mazandaran | ISSAC | _ | _ | 12 | 362 | 9 | 140 | 16 | 222 | [[52]] |
| Mehravar et al. | 2016 | Golestan | ISSAC | 11 | 84 | 22 | 200 | 18 | 156 | 15 | 128 | [[53]] |
| Assadi et al. | 2017 | Bushehr (Bushehr) | ISSAC | 4 | 20 | 7 | 38 | 5 | 25 | 5 | 33 | [[54]] |
| Khazaei et al. | 2018 | Tehran (Tehran) | ISSAC | 4 | 40 | 8 | 82 | 6 | 63 | 6 | 59 | [[55]] |
| Fazlollahi et al. | 2018 | Tehran (Tehran) | ISSAC | 9 | 1543 | 12 | 2090 | 10 | _ | 12 | _ | [[56]] |
3.4. The Overall Prevalence of Asthma
Table 3 shows the prevalence of asthma in the four groups of children, adolescents, boys, and girls in the selected articles. As shown in Table 3, the prevalence of asthma in children was 7% (95% CI: 5-9) with heterogeneity of 98.37% (Figure 2). The prevalence of asthma in adolescents was 9% (95% CI: 7-11) with heterogeneity of 99.32% (Figure 3). The prevalence of asthma in girl and boy were 8% (95% CI: 6-10) and 9% (95% CI: 7-11), respectively. The odds ratio for male/female and children/adolescents was 0.13 and 0.82, respectively. Sensitivity analysis showed that the results before and after this analysis did not change, and the findings were stable.
Table 3.
Overall results of the prevalence of asthma in Iranian children and adolescent.
| Item | Prevalence | P value | I 2 | OR | Number of study |
|---|---|---|---|---|---|
| Children | 6% (5–8) | <.001 | 98.37% | 0.82 (0.64-1.06) | 19 |
| Adolescent | 8% (6–11) | <.001 | 99.32% | 28 | |
| Girl | 8% (6–10) | <.001 | 99.02% | 0.13 (-0.04-0.30) | 20 |
| Boy | 9% (7–11) | <.001 | 99.18% | 21 |
Figure 2.
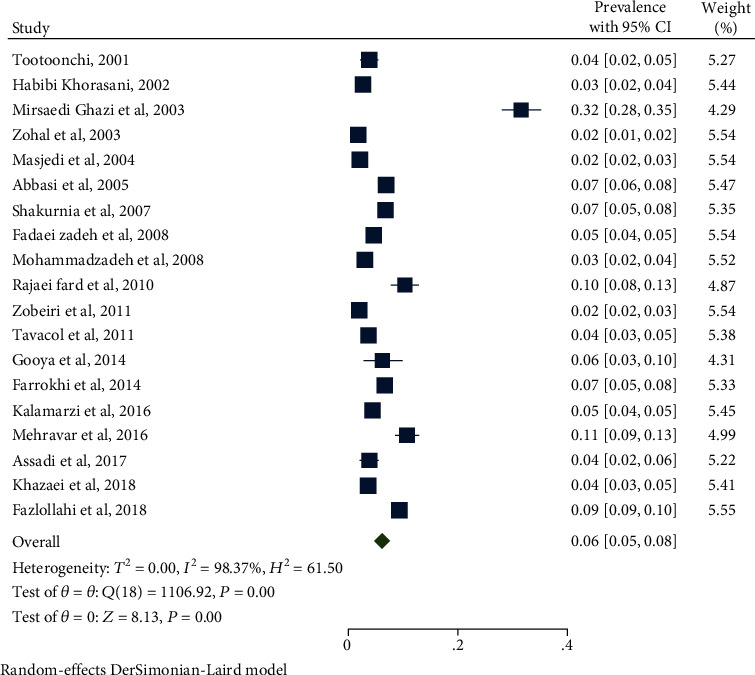
Prevalence of asthma in Iranian children (0-10).
Figure 3.
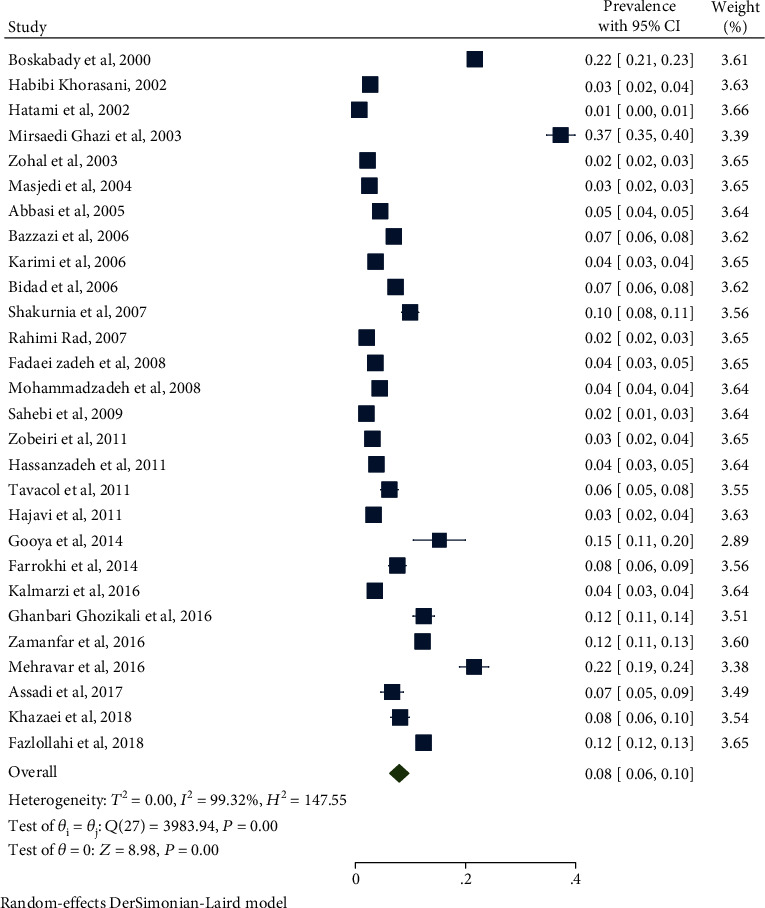
Prevalence of asthma in Iranian adolescent (11–19).
3.5. The Prevalence of Asthma Based on Geographical Areas
Iran's geographic areas were divided into five groups: North, South, Central, West, and East. The outbreak of asthma in the studies in each region was estimated as presented in Table 4. The highest prevalence of asthma in children and adolescents were in the Center and the East, respectively. The lowest prevalence of asthma was in children and adolescents in the West.
Table 4.
The prevalence of asthma by geographical area of Iran.
| Region | Studies | Sample | Heterogeneity | |||
|---|---|---|---|---|---|---|
| All | % | I2 | P value | |||
| Children | Central | 7 | 30425 | 8 | 99.30% | <0.001 |
| North | 3 | 6881 | 7 | 97.64% | <0.001 | |
| South | 7 | 5955 | 6 | 79.62% | <0.001 | |
| East | _ | _ | _ | _ | _ | |
| West | 2 | 4823 | 3 | 94.82% | <0.001 | |
|
| ||||||
| Adolescent | Central | 8 | 37130 | 9 | 99.58% | <0.001 |
| North | 5 | 12605 | 10 | 98.53% | <0.001 | |
| South | 8 | 11175 | 6 | 97.83% | <0.001 | |
| East | 2 | 7160 | 13 | 99.85% | <0.001 | |
| West | 5 | 10954 | 4 | 99.51% | <0.001 | |
3.6. The Prevalence of Asthma Symptoms
As shown in Table 5, the outbreak of asthma symptoms and their effects were evaluated in this study. Among asthma symptoms in children and adolescents, wheezing had the most prevalence (17% in children and 19% in adolescents) and sleep disturbance had the lowest prevalence (6% in children and 6% in adolescents).
Table 5.
Asthma symptoms in Iranian children and adolescent.
| Item | Prevalence | P value | I 2 | OR | Number of study | |
|---|---|---|---|---|---|---|
| Wheezing | Children | 17% (13–20) | <.001 | 98.65% | 0.78 (0.64-0.95) | 16 |
| Adolescent | 19% (15–23) | <.001 | 99.58% | 23 | ||
| Boy | 19% (15–23) | <.001 | 98.83% | 0.04 (-0.18-0.26) | 16 | |
| Girl | 18% (15–21) | <.001 | 98.61% | 18 | ||
|
| ||||||
| Dry cough | Children | 11% (9–14) | <.001 | 98.47% | 0.61 (0.46-0.80) | 15 |
| Adolescent | 17% (15–20) | <.001 | 98.85% | 21 | ||
| Boy | 18% (15–21) | <.001 | 98.03% | 0.17 (-0.01-0.35) | 14 | |
| Girl | 15% (12–17) | <.001 | 98.41% | 14 | ||
|
| ||||||
| Exercise wheezing | Children | 7% (5–9) | <.001 | 99.03% | 0.33 (0.26-0.41) | 13 |
| Adolescent | 16% (13–19) | <.001 | 98.94% | 18 | ||
| Boy | 17% (12–22) | <.001 | 98.73% | 4.31 (3.79-4.82) | 13 | |
| Girl | 12% (9–14) | <.001 | 99.55% | 13 | ||
|
| ||||||
| Wheezing attack | Children | 13% (9–17) | <.001 | 98.43% | 0.82 (0.78-0.86) | 5 |
| Adolescent | 14% (9–18) | <.001 | 99.25% | 7 | ||
| Boy | 15% (7–22) | <.001 | 98.87% | 0.15 (0.06-0.24) | 4 | |
| Girl | 14% (7–21) | <.001 | 98.85% | 4 | ||
|
| ||||||
| Sleep disturbance | Children | 6% (4–7) | <.001 | 96.28% | 1.03 (0.93-1.14) | 11 |
| Adolescent | 6% (4–7) | <.001 | 98.02% | 14 | ||
| Boy | 8% (6–11) | <.001 | 98.02% | 0.06 (-0.05-0.18) | 7 | |
| Girl | 7% (5–9) | <.001 | 98.48% | 7 | ||
Publication bias of the asthma prevalence was significant (P < 0.001) (Figure 4). The prevalence of asthma by year (Figure 5) and sample size (Figure 6) was not significant (P > 0.05).
Figure 4.
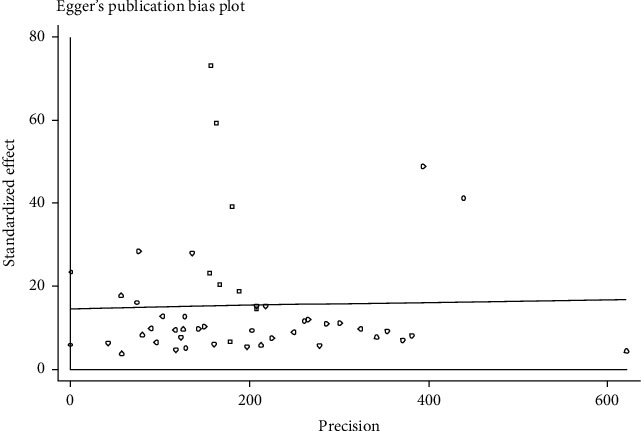
Publication bias in the studied researches (children and adolescent).
Figure 5.
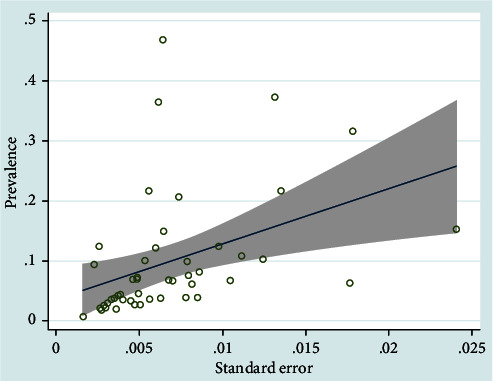
Metaregression for prevalence of asthma and years of studies (children and adolescent).
Figure 6.
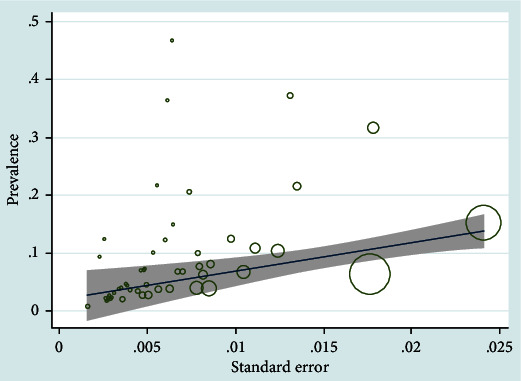
Metaregression for prevalence of asthma and sample size of studies (children and adolescent).
4. Discussion
The purpose of this systematic review and meta-analysis was to evaluate the prevalence of asthma among children and adolescents in Iran. This systematic review and meta-analysis investigated 30 studies from 2000 to 2020. The global outbreak of asthma has been increasing over the past decades. As a chronic disease that usually starts in early childhood, it imposes a heavy burden on these people's lives, caregivers, and the community. Despite significant progress in health care in the last decades, there is still ongoing disagreement between countries.
The prevalence of asthma in Iranian children and adolescents was 6% (95% CI: 5-8) and 8% (95% CI: 6-11). The global prevalence of asthma in children and adolescents has increased. Prevalence of ever asthma worldwide was 9.1% to 9.5% in children and 9.1% to 10.4% in adolescents [2]. The prevalence of asthma in children was reported to be 10.6%, 10.1%, 5.35%, 7.4%, 23.8%, and 10.7% in Oman (2016), Brazil (2019), India (2016), Thailand (2018), Costa Rica (2019), and Northern Portugal (2016), respectively. The prevalence of asthma in adolescents was 19.8%, 6.05%, 6.1%, and 25.9% in Oman, India, Thailand, and Costa Rica, respectively [13–18]. The prevalence of asthma in Iran in the present study is lower than the reported value in other countries (such as Brazil, Oman, Costa Rica, and Portugal), but it was higher than Indian children and adolescents in Thailand and India. Many types of researches in Iran have examined the prevalence of asthma, leading to control of asthma incidence. Differences in variables such as climate, lifestyle, nutrition, ethnicity, and cultures in Iran compared to other countries can also affect asthma prevalence.
Many studies have been performed to estimate the prevalence of asthma in children and adolescents in various cities in Iran. Heidarnia et al. evaluated 19 studies between 1998 and 2003, and the overall prevalence of asthma was estimated to be 13.14%, which was higher than our study [6]. In another meta-analysis, Ghaffari et al. evaluated 27 studies between 1992 and 2012. They reported asthma prevalence to be 2.7% and 3.5% in children aged 6-7 and 13-14 years, respectively [7]. In Hassanzadeh et al.'s study, just guidance school children were evaluated between 1997 and 2009. The overall outbreak was 3.9% [19]. Varmaghani et al. evaluated 10 studies between 1990 and 2015; they reported the asthma prevalence to be 8.80% [20]. Compared with the present results, these reports showed that asthma prevalence increased in recent years in Iran.
According to the present systematic review and meta-analysis, the prevalence of asthma in adolescents was higher than in children (OR = 0.82, CI: 0.64-1.06, and P < 0.001). This was consistent with several studies, such as Soto-Martínez et al., Al-Herz, de Oliveira et al., and Singh et al. [13, 17]. This may be because adolescents spend more time outside the home and are more exposed to contaminants and other allergens and pollen. This finding may also be due to changes during puberty, since hormonal changes at this age can affect the incidence and severity of asthma [3]. Smoking as well is more common in this age group and can increase the prevalence of asthma. Other causes of increased asthma prevalence in adolescents could include increased consumption of foods and drinks containing preservatives.
In this systematic review and meta-analysis, the prevalence of asthma was found to be higher in boys (9%, 95% CI: 7-11) than in girls (8%, 95% CI: 6-10) (OR = 0.13, CI: -0.04-0.30, and P < 0.001). Several studies were similar to the present study, such as the study of Chinratanapisit et al. in Bangkok, and other studies in Turkey and Korea [16, 21, 22]. Also in Japan, the prevalence of asthma was higher in boys (6%) than girls (4%) [23]. These gender differences might be ascribed to a narrower airways caliber in males than females in early life due to different hormonal factors. Other reasons include physical and social differences. Since smoking is more prevalent in boys than in girls, it can influence this disease [24].
The other causes of the difference in asthma prevalence include climate dispersal in a different region of Iran. In the present systematic review and meta-analysis, among region subgroups, the highest and lowest prevalence of asthma in children was 8% and 3% in the Center and the West of Iran, respectively. Also, the highest and lowest prevalence of asthma in adolescent were 13% in the East and 4% in the West of Iran. As a result, the central region's prevalence was higher, which may be due to air pollution and contaminants in central populated cities such as Tehran. According to Lashanizand and Gholamrezaie's study, atmospheric conditions directly and indirectly affect the prevalence of asthma, and ambient moisture reduces asthma attacks, according to the present study. It can be concluded that the northern and southern regions of the sea margin have suitable ambient humidity, which results in a lower prevalence of asthma in these regions [25]. In addition to climate change, air pollution and vehicle overcrowding in crowded cities, diet in different areas, and genetic changes can also affect asthma prevalence. Also, scattering vegetation in different cities and exposure to pollen in different seasons can affect asthma prevalence. As shown in the study of Zhang et al., SO2, relative humidity and sunshine are associated with asthma prevalence [26].
Descriptive information including wheezing, dry cough, sleep disturbance, wheezing attacks, and exercise wheeze was estimated in the present study. The prevalence of asthma symptoms in children and adolescents worldwide increased from 11.1% to 11.6% and from 13.2% to 13.7%, respectively, between ISAAC phase I and phase III. According to the present systematic review and meta-analysis, wheezing prevalence was higher in Iranian adolescents than in children (17% in children and 19% in adolescents). In a study in Thailand (Southeast Asia), wheezing was reported 14.6% in children and 12.5% in adolescents [16]. A study by Mallol et al. reported the prevalence of wheezing from 11.11% in 1994 to 13.4% in 2015 among adolescents [3]. It was 35.1% in children and 35.4% in adolescent in Costa Rica [17]. The prevalence of wheezing in Thailand was lower than in the present study but higher in Brazil and Costa Rica than in the present study. It was higher in adolescents than children in the present study; this is in line with Costa Rica study. The prevalence of dry cough was higher in adolescents than in children (11% in children and 17% in adolescents). The prevalence of dry cough in a study in Thailand (24.2% in children and 29.9 in adolescents) was similar to the present study; however, the result of Costa Rica study (28.5% in children and 24.4% in adolescent) was different with our result [16, 17]. In Mallol et al.'s study (2015), it was reported to be 33.1% in adolescents. The prevalence of dry cough in the present study was lower than studies done in Thailand, Costa Rica, and South-Santiago. The prevalence of exercise-induced wheezing was higher in adolescents than in children (7% in children and 16% in adolescents). In Chinratanapisit et al.'s study in Thailand, it was 3% in children and 14.8% in adolescents [16], which was higher than the amount reported in the present study.
The prevalence of wheezing attacks was significantly higher in adolescents than in children, and this finding is in line with a study in Thailand [16]. Having stress during this period of life is more than childhood and can affect asthma wheezing attacks. The prevalence of wheezing attacks in the present study was lower than in children and higher than in adolescents in Thailand. As shown, the prevalence of asthma symptom in adolescent was higher than children. The reasons can also be attributed to the increased prevalence of asthma in adolescents than children.
Publication bias of the asthma prevalence was significant. The quality of the papers affects the bias. Most of the studies did not mention blinding of the outcome assessors. Also, most of them did not mention the research's timing in the title. The results may affect by some limitation in the researches. Also, heterogeneity was encountered perhaps due to various center settings, populations enrolled, etc. In some parts of the country, we do not find any research; few studies have been done in other parts. This limitation may affect the results and cause publication bias. This study was not registered in PROSPERO.
5. Conclusion
Overall, the present study showed that asthma prevalence in Iranian children and adolescents is lower than in regions in the world. Given that the disease affects the quality of life of the studied age group and the quality of their education and economic situation, the existing strategies should be pursued as well, on the provision of medicines and the cost of treatment. Also, guidelines for asthma control should be considered in the future, such as providing asthma medicines throughout the country (especially villages), measures to reduce air pollution, and training parents to quit smoking, alternatively, smoking in a place far away from their children.
Acknowledgments
We thank to those who helped us in writing of this manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
All the authors contributed equally.
References
- 1.Bush A., Fleming L. Diagnosis and management of asthma in children. BMJ. 2015;350, article h996 doi: 10.1136/bmj.h996. [DOI] [PubMed] [Google Scholar]
- 2.Ferrante G., La Grutta S. The burden of pediatric asthma. Frontiers in Pediatrics. 2018;6 doi: 10.3389/fped.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallol J., Aguirre V., Mallol-Simmonds M., Matamala- Bezmalinovic A., Calderón-Rodriguez L., Osses-Vergara F. Changes in the prevalence of asthma and related risk factors in adolescents: three surveys between 1994 and 2015. Allergologia et Immunopathologia. 2019;47(4):313–321. doi: 10.1016/j.aller.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Taylor Y. J., Tapp H., Shade L. E., Liu T.-L., Mowrer J. L., Dulin M. F. Impact of shared decision making on asthma quality of life and asthma control among children. Journal of Asthma. 2018;55(6):675–683. doi: 10.1080/02770903.2017.1362423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zar H. J., Levin M. E. Challenges in treating pediatric asthma in developing countries. Pediatric Drugs. 2012;14(6):353–359. doi: 10.2165/11597420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Heidarnia M. A., Entezari A., Moein M., Mehrabi Y., Pourpak Z. Prevalence of asthma symptom in Iran: a meta-analysis. Research-in-Medicine. 2007;31(3):217–225. [Google Scholar]
- 7.Ghaffari J., Aarabi M. The prevalence of pediatric asthma in the Islamic Republic of Iran: a review and meta-analysis. Journal of Pediatrics Review. 2013;1(1):2–11. [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D. G., for the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339, article b2535 doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute. Study quality assessment tools. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 10.DerSimonian R., Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Higgins J. P., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Herz W. A systematic review of the prevalence of atopic diseases in children on the Arabian Peninsula. Medical Principles and Practice. 2018;27(5):436–442. doi: 10.1159/000493267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira T. B., Moscon J. G., do Nascimento Ferreira E. N., da Veiga A. B. G. Prevalence of symptoms of asthma and allergic rhinitis in children in Southern Brazil: a ten-year monitoring study. Journal of Asthma. 2020;57(4):373–380. doi: 10.1080/02770903.2019.1573253. [DOI] [PubMed] [Google Scholar]
- 15.Singh S., Sharma B. B., Sharma S., Sabir M., Singh V., ISAAC collaborating investigators Prevalence and severity of asthma among Indian school children aged between 6 and 14 years: associations with parental smoking and traffic pollution. Journal of Asthma. 2016;53(3):238–244. doi: 10.3109/02770903.2015.1087558. [DOI] [PubMed] [Google Scholar]
- 16.Chinratanapisit S., Suratannon N., Pacharn P., Sritipsukho P., Vichyanond P. Prevalence and severity of asthma, rhinoconjunctivitis and eczema in children from the Bangkok area: the Global Asthma Network (GAN) Phase I. Asian Pacific Journal of Allergy and Immunology. 2019;37 doi: 10.12932/ap-120618-0336. [DOI] [PubMed] [Google Scholar]
- 17.Soto-Martínez M., Yock-Corrales A., Camacho-Badilla K., et al. The current prevalence of asthma, allergic rhinitis, and eczema related symptoms in school-aged children in Costa Rica. Journal of Asthma. 2019;56(4):360–368. doi: 10.1080/02770903.2018.1455860. [DOI] [PubMed] [Google Scholar]
- 18.Branco P., Nunes R., Alvim-Ferraz M., et al. Asthma prevalence and risk factors in early childhood at Northern Portugal. Revista Portuguesa de Pneumologia (English Edition) 2016;22(3):146–150. doi: 10.1016/j.rppnen.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Hassanzadeh J., Mohammadbeigi A., Moussavizadeh A., Akbari M. Asthma prevalence in Iranian guidance school children, a descriptive meta-analysis. Journal of Research in Medical Sciences. 2012;17(3) [PMC free article] [PubMed] [Google Scholar]
- 20.Varmaghani M., Farzadfar F., Sharifi F., et al. Prevalence of asthma, COPD, and chronic bronchitis in Iran: a systematic review and meta-analysis. Iranian Journal of Allergy, Asthma, and Immunology. 2016;15(2):93–104. [PubMed] [Google Scholar]
- 21.Ones U., Akcay A., Tamay Z., Guler N., Zencir M. Rising trend of asthma prevalence among Turkish schoolchildren (ISAAC phases I and III) Allergy. 2006;61(12):1448–1453. doi: 10.1111/j.1398-9995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 22.Hong S., Son D. K., Lim W. R., et al. The prevalence of atopic dermatitis, asthma, and allergic rhinitis and the comorbidity of allergic diseases in children. Environmental Health and Toxicology. 2012;27 doi: 10.5620/eht.2012.27.e2012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusunoki T., Morimoto T., Nishikomori R., et al. Changing prevalence and severity of childhood allergic diseases in Kyoto, Japan, from 1996 to 2006. Allergology International. 2009;58(4):543–548. doi: 10.2332/allergolint.09-OA-0085. [DOI] [PubMed] [Google Scholar]
- 24.Almqvist C., Worm M., Leynaert B. Gender’ wgoGLW. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2007;63(1):47–57. doi: 10.1111/j.1398-9995.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 25.Lashanizand M., Gholamrezaie S. Study of relationship between climatic variables and incidence of asthma attacks leading to hospitalization in the children's hospital of Khorramabad city. Scientific Magazine Yafte. 2013;15(1):51–58. [Google Scholar]
- 26.Zhang J., Dai J., Yan L., et al. Air pollutants, climate, and the prevalence of pediatric asthma in urban areas of China. BioMed Research International. 2016;2016 doi: 10.1155/2016/2935163.2935163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boskabady M. H., Karimian M. R. Prevalence of asthma symptoms among secondary school students (aged 11–16 years) in the city of Mashhad (north east of Iran) Archives of Iranian Medicine. 2000;3(4):165–169. [Google Scholar]
- 28.Tootoonchi P. Prevalence of asthma, related symptoms and risk factors in children younger than 5 years. Acta Medica Iranica. 2004:450–454. [Google Scholar]
- 29.Hatami G., Amir A. E., Najafi A., et al. Prevalence of asthma and asthma-related symptoms among 13-14yr. School children in Bushehr-ISSAC; 2003. [Google Scholar]
- 30.Habibi K. A., Janghorbani M., Gozashti H. Prevalence of asthma in elementary school children in Kerman in 1999. Journal of Kerman University of Medical Sciences. 2002;9(4):184–193. [Google Scholar]
- 31.Zohal M., Hasheminasab R. Prevalence of asthma among school-age children in Qazvin (2003) Journal of Inflammatory Disease. 2006;9(4):64–68. [Google Scholar]
- 32.Ghazi B. M. The prevalence of asthma among the students (7-18 years old) in Tehran during 2002-2003. Iranian Journal of Allergy, Asthma and Immunology. 2004;3:89–92. [PubMed] [Google Scholar]
- 33.Masjedi M. R., Fadaizadeh L., Najafizadeh K., Dokouhaki P. Prevalence and severity of asthma symptoms in children of the Tehran-ISAAC study. Pediatric Asthma, Allergy & Immunology. 2004;17(4):244–250. doi: 10.1089/pai.2004.17.244. [DOI] [PubMed] [Google Scholar]
- 34.Abbasi R. Z. Prevalence of asthma symptoms in children. Journal of Guilan University of Medical Sciences. 2006 [Google Scholar]
- 35.Bazzazi H., Gharagozlou M., Kassaiee M., Parsikia A., Zahmatkesh H. The prevalence of asthma and allergic disorders among school children in Gorgan. Journal of Research in Medical Sciences. 2007;12(1):28–33. [Google Scholar]
- 36.Karimi M., Mirzaei M., Ahmadieh M. H. Acetaminophen use and the symptoms of asthma, allergic rhinitis and eczema in children. Iranian Journal of Allergy, Asthma and Immunology. 2006;5:63–67. [PubMed] [Google Scholar]
- 37.Bidad K., Anari S., Aghamohammadi A., Pourpak Z., Moayeri H. Prevalence of asthma related to BMI in adolescents in Tehran, Iran, 2004–2005. European Journal of Pediatrics. 2007;166(5):453–454. doi: 10.1007/s00431-006-0259-0. [DOI] [PubMed] [Google Scholar]
- 38.Shakurnia A. H., Assar S., Afra M., Latifi M. Prevalence of asthma among schoolchildren in Ahvaz, Islamic Republic of Iran. Eastern Mediterranean Health Journal. 2010;16(6):651–656. doi: 10.26719/2010.16.6.651. [DOI] [PubMed] [Google Scholar]
- 39.Rahimi Rad M., Hejazi M., Behrouzian R. Asthma and other allergic diseases in 13-14-year-old schoolchildren in Urmia: an ISAAC study. Eastern Mediterranean Health Journal. 2007;13(5):1005–1016. doi: 10.26719/2007.13.5.1005. [DOI] [PubMed] [Google Scholar]
- 40.Fadaeizadeh L., Saeidfar K., Najafizadeh K., Masjedi M. R. Evaluation of agreement between video and written questionnaires for asthma symptoms among children of Tehran: ISAAC study. Journal of Shahid Sadoughi University of Medical Sciences and Health Services. 2008;16(2) [Google Scholar]
- 41.Mohammadzadeh I., Ghafari J., Savadkoohi R. B., Tamaddoni A., Dooki M. R. E., Navaei R. A. The prevalence of asthma, allergic rhinitis and eczema in north of Iran. Iranian Journal of Pediatrics. 2008;18(2):117–122. [Google Scholar]
- 42.Sahebi L., Shabestary M. S. The prevalence of asthma, allergic rhinitis, and eczema among middle school students in Tabriz (northwestern Iran) Turkish Journal of Medical Sciences. 2011;41(5):927–938. [Google Scholar]
- 43.Rajaeifard A., Moosavi Zadeh A., Pourmahmoudi A., Naeimi E., Hadinia A., Karimi A. Evaluation of prevalence and related factors of pediatric asthma in children under six years old with logistic regression and probit. Armaghane danesh. 2011;16(3):272–281. [Google Scholar]
- 44.Zobeiri M. Prevalence, risk factors and severity of asthma symptoms in children of Kermanshah, IRAN: ISAAC phase I, II. Acta Medica Iranica. 2011;49:184–188. [PubMed] [Google Scholar]
- 45.Tavacol H., Rahimi Z., Cheraghi M., Ghatfan F., Baji Z., Rahmani H. Original paper A cross-sectional study of prevalence and risk factors for childhood asthma in Ahvaz city, Iran. Advances in Dermatology and Allergology. 2015;32(4):268–273. doi: 10.5114/pdia.2015.53322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassanzadeh J., Basiri F., Mohammad-Beigi A. Prevalence of asthma symptoms and allergic diseases with ISSAC method in children, Shiraz 2009. Zahedan Journal of Research in Medical Sciences. 2012;13(8):35–39. [Google Scholar]
- 47.Hajavi J., Tolide-ie H. R., Rastgoie Chavoshlu S., Salehi Rezve M., Modoodi Yaghooti M., Rahimi J. Do rural and urban children have different prevalence of allergic disorders in Gonabad? Quarterly of Horizon of Medical Sciences. 2012;18(2):21–26. [Google Scholar]
- 48.Gooya M., Shirkani A., Tahmasebi R., et al. Prevalence of asthma and allergic diseases and its risk factors in school children aged (6-7 and 13-14 years) in Assalouyeh City, Bushehr Province based on III ISAAC protocol phase I, in 2014. Iranian South Medical Journal. 2017;20(1):57–69. [Google Scholar]
- 49.Farrokhi S., Gheybi M. K., Movahhed A., et al. Prevalence and risk factors of asthma and allergic diseases in primary schoolchildren living in Bushehr, Iran: phase I, III ISAAC protocol. Iranian Journal of Allergy, Asthma and Immunology. 2014;13:348–355. [PubMed] [Google Scholar]
- 50.Nasiri Kalmarzi R., Shekari A., Tajik M., et al. The prevalence of asthma symptoms in elementary and middle school students in Kurdistan Province, the West of Iran. International Journal of Pediatrics. 2016;4(2):p. 1330. [Google Scholar]
- 51.Ghozikali M. G., Ansarin K., Naddafi K., et al. Prevalence of asthma and associated factors among male late adolescents in Tabriz, Iran. Environmental Science and Pollution Research. 2018;25(3):2184–2193. doi: 10.1007/s11356-017-0553-6. [DOI] [PubMed] [Google Scholar]
- 52.Zamanfar D., Ghaffari J., Behzadnia S., Yazdani-charati J., Tavakoli S. The prevalence of allergic rhinitis, eczema and asthma in students of guidance schools in Mazandaran Province, Iran. Open Access Macedonian Journal of Medical Sciences. 2016;4(4):619–623. doi: 10.3889/oamjms.2016.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehravar F., Rafiee S., Bazrafshan B., Khodadost M. Prevalence of asthma symptoms in Golestan schoolchildren aged 6–7 and 13–14 years in Northeast Iran. Frontiers of Medicine. 2016;10(3):345–350. doi: 10.1007/s11684-016-0462-y. [DOI] [PubMed] [Google Scholar]
- 54.Assadi T., Gheybi M., Shirkani A., Movahed A., Khoddami S., Ashourinejad A., et al. Study of prevalence and risk factors of asthma and allergic diseases among school children (6-7 and 13-14 years) based on ISAAC protocol in Jam City, Bushehr Province in 2014. Iranian South Medical Journal. 2017;19(6):972–981. [Google Scholar]
- 55.Khazaei Z., Goodarzi E., Farbakhsh F., Darvishi I., Dehghani S. L., Faraji M. Prevalence of asthma and the related-symptoms in children and adolescences; a cross-sectional study. Immunopathologia Persa. 2018;4(2, article e28) doi: 10.15171/ipp.2018.28. [DOI] [Google Scholar]
- 56.Fazlollahi M. R., Najmi M., Fallahnezhad M., et al. Paediatric asthma prevalence: the first national population-based survey in Iran. The Clinical Respiratory Journal. 2019;13(1):14–22. doi: 10.1111/crj.12975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


