Abstract
On January 30, 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic a worldwide emergency. Worldwide there have been 170 million cases of the resulting disease coronavirus 2019 (COVID-19), of those, 3.53 million have resulted in death. The Food and Drug Administration (FDA) with Mayo Clinic as the lead institution authorized COVID-19 convalescent plasma (CCP) for treatment of SARS-CoV-2 infection. Effective therapeutic window for CCP administration had yet to be defined. We addressed this gap by characterizing longitudinal biologic response and clinical outcomes of COVID-19 patients treated with CCP. Primary outcome was discharged to home/home health.
Keywords: covid-19, convalescent plasma, sars-cov-2, discharge to home, infusion
Introduction
On January 30, 2020, the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic a worldwide emergency. Worldwide there have been 114 million cases of the resulting disease coronavirus 2019 (COVID-19) of those, 2.54 million have resulted in death. At pandemic debut, available treatment was limited to supportive care as no approved therapy or vaccination was available. This treatment vacuum motivated the utilization of convalescent plasma infusion to complement the antibody response.
Passive immunization has been successfully used to treat infectious diseases since the 1890s [1,2]. Convalescent plasma (CP) was used to treat Spanish Influenza A (H1N1), severe acute respiratory syndrome in 2003 caused by SARS-associated coronavirus (SARS-CoV), and Influenza A pandemic in 2009 [3,4]. Underpinning CP treatment is that subjects recovering from viral infection marshalled an effective antibody response. And donated CP administered to an infected individual anticipating transfused antibodies would affect sufficient passive immunity to reduce symptoms and mortality risk.
Results from small case series conducted during the prior Middle East Respiratory Syndrome (MERS) and SARS-CoV documented CP to be safe, well-tolerated and promoted faster viral clearance, particularly when given early in the disease course [5]. The primary hypothesized mechanism of action of CP in COVID-19 pathogenesis involves antibody neutralization downregulating the hyperinflammatory response evoked by SARS-CoV-2 ribonucleic acid (RNA) [6,7]. Also, the thought was that transfused antibodies passively increased tissue repair decreasing and/or obviating complications and progression to death [8-11]. Additional preliminary clinical evidence suggested that CP might benefit individuals with SARS-CoV-2 infection and symptom onset suggestive of COVID-19.
The Food and Drug Association (FDA) with Mayo Clinic as the lead institution authorized COVID-19 convalescent plasma (CCP) for treatment of CoV-2 infection. Effective therapeutic window for CCP administration had yet to be defined. We addressed this gap by characterizing longitudinal biologic response and clinical outcomes of COVID-19 patients treated with CCP.
Materials and methods
Study design and population
This retrospective longitudinal study analyzed electronic medical record data, including but not limited to characteristics and laboratory test findings from 197 patients consecutively admitted between March 28 and August 5, 2020. Among those, 92 and 105, respectively, received CCP infusion within 48h of versus 48h after hospitalization. Primary outcome was discharged to home/home health and secondary outcome was longitudinal CRP levels post CCP infusion.
Sarasota Memorial Hospital Institutional Review Board authorized consenting COVID-19 inpatients to participate in the national CCP protocol. Written informed consent was obtained from every CCP recipient or their legal guardian. Internal medicine resident physicians identified, contacted, and facilitated logistical pathways for CCP donation in collaboration with the community blood bank.
Enrolled patients were at least 18 years old with laboratory confirmed SARS-CoV-2 infection admitted for treatment of severe or life-threatening COVID-19. Severe disease was defined as the presence of at least one of the following characteristics: dyspnea, respiratory rate of 30 breaths per minute or more, oxygen saturation (SpO2) equal to or less than 93%, partial pressure of arterial oxygen to fraction of inspired oxygen less than 300 or development of lung infiltrates with more than 50% involvement within 24-48 hours (h). Life-threatening disease was defined as the development of at least one of the following: respiratory failure, septic shock or multiple organ dysfunction or failure.
Data analysis
Analyses contrasted patients who underwent CCP infusion within or more than 48h after admission. Primary outcome was discharged to home/home health. Continuous data summarized as median (interquartile range [IQR]) were compared using Kruskal-Wallis test or two-way analysis of variance (ANOVA). Discrete data were compared with Pearson’s chi-square test. Two-tailed p < 0.05 was significant.
Results
The study included 197 patients consecutively admitted between March 28, 2020 and August 5, 2020. Of those, 92 received CCP infusion within 48h and 105 received CCP infusion after 48h. Median age in the group that received CCP within 48h was 67 years and 63 years in the group who received CCP after 48h. Of the 197 patients, 57% were male. Intergroup comorbidities were distributed similarly among both groups for the exception of body mass index (BMI) (32.7 (27-40)) vs. (29.4 (26-37) kg/m2, p < 0.0001) (Table 1). Distribution of COVID-19 directed pharmacologic treatment was also similar amongst both groups.
Table 1. Baseline characteristics of consecutive patients with COVID-19.
IQR: interquartile range; N: number; BMI: body mass index; CRP: C-reactive protein; s: seconds; SpO2: oxygen saturation; aPTT: activated partial thromboplastin time.
| Characteristics | Convalescent plasma > 48 hours | Convalescent plasma < 48 hours |
| (N = 105) | (N = 92) | |
| Age, median (IQR), | 67 (59-78) | 63 (50-71) |
| Sex, No. (%) | ||
| Male | 59 (56) | 54 (59) |
| Female | 46 (44) | 38 (41) |
| Race, No. (%) | ||
| White | 66 (63) | 57 (62) |
| Black | 15 (14) | 9 (10) |
| Other | 24 (23) | 26 (28) |
| Anthropometrics, median (IQR) | ||
| Height (cm) | 166 (160-177) | 168 (161-178) |
| Weight (kg) | 87.4 (70.6-104.0) | 97.3 (78.0-112.2) |
| BMI (kg/m2) | 29.4 (26-37) | 32.7 (27-40) |
| Vital signs, median (IQR) | ||
| Body temperature (°F) | 98.3 (98.0-98.9) | 98.5 (98.1-99.0) |
| Inspired O2 (%) | 70 (51-90) | 80 (58-100) |
| SpO2 (%) | 95 (93-97) | 95 (93-97) |
| Respiratory rate, /min | 19 (18-21) | 20 (19-21) |
| Heart rate, /min | 77 (66-86) | 80 (70-93) |
| Systolic blood pressure, mmHg | 129 (117-143) | 129 (117-140) |
| Coexisting diseases, No. (%) | ||
| Hypertension | 60 (59) | 38 (43) |
| Diabetes | 43 (41) | 28 (30) |
| Obesity | 43 (41) | 45 (51) |
| Kidney disease | 19 (19) | 18 (20) |
| Depression | 16 (16) | 12 (14) |
| Neurological disease | 14 (14) | 13 (15) |
| Congestive heart failure | 11(11) | 9 (10) |
| COVID-19 pharmacotherapy | ||
| Dexamethasone | 86 (82) | 73 (79) |
| Remdesivir | 65 (62) | 64 (70) |
| Azithromycin | 17 (16) | 21 (23) |
| Tocilizumab | 13 (12) | 12 (13) |
| Hydroxychloroquine | 7 (7) | 4 (4) |
| Laboratory tests, median (IQR) | ||
| Inflammatory biomarkers | ||
| CRP, mg/dL | 9.3 (4.3-15.6) | 11.90 (7.03-15.90) |
| Ferritin, ng/mL | 592 (302-1165) | 657 (259-1290) |
| Lactate dehydrogenase, U/L | 326 (274-428) | 366 (301-469) |
| Procalcitonin, ng/mL | 0.12 (0.05-0.98) | 0.14 (0.05-0.59) |
| Triglycerides, mg/dL | 200(118-297) | 161 (99-236) |
| Liver and kidney function | ||
| Aminotransferase, U/L | ||
| Alanine | 38 (22-60) | 39 (23-68) |
| Aspartate | 42 (29-61) | 41 (31-68) |
| Creatinine, mg/dL | 1.1 (0.8-1.5) | 1.1 (0.9-1.4) |
| Complete blood cells count | ||
| White blood cells, /mL | 6.80 (5.20-10.10) | 7.05 (5.35-9.08) |
| Neutrophil, /mL | 5.18 (3.70-7.95) | 5.49 (3.90-7.20) |
| Lymphocyte, /mL | 19 (12-28) | 18 (12-25) |
| Hemoglobin, gm/dL | 13.2 (11.3-14.7) | 12.1 (14.2-15.4) |
| Coagulation profiles | ||
| D-dimer, mg/mL | 1.00 (0.72-2.04) | 1.02 (0.66-2.01) |
| Prothrombin time, s | 11.3 (10.8-12.0) | 11.4 (10.7-12.3) |
| aPTT, s | 30.2 (27.3-32.9) | 30.8 (26.4-32.9) |
| Cardiac biomarkers | ||
| Creatine Kinase Total, U/L | 131 (63-271) | 132 (70-381) |
| Troponin I, ng/mL | 0.02 (0.02-0.13) | 0.02 (0.02-0.06) |
| Pro-BNP, pg/mL | 1071 (219-3141) | 225 (58-3004) |
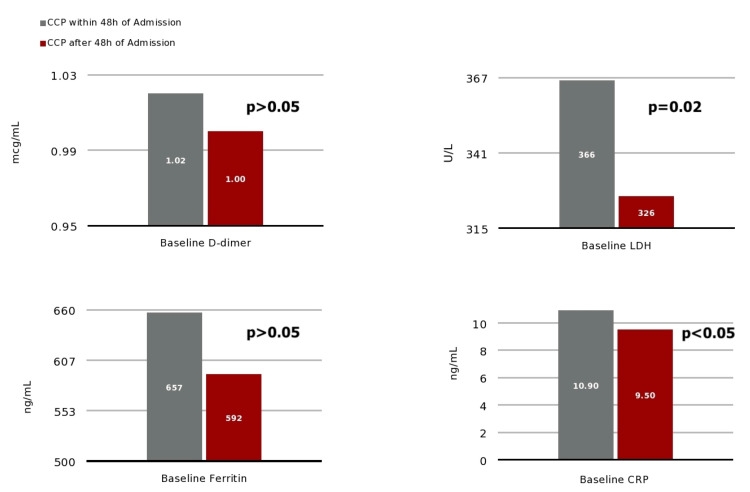
Initial admission vital signs and laboratory test results were not different between groups (p>.05) including temperature (98.4 (98.0-99.0) °F); SpO2 (95, (93-97) %); C-reactive protein (CRP) (2.6 (0.3-2.7) mg/dL); D-dimer (1.01 (0.69-2.03) mg/L); and ferritin (624 (281-1228) ng/mL) (Figure 1).
Figure 1. Baseline values for D-dimer, LDH, ferritin and CRP, respectively, for patients who received CCP within 48h of admission vs. 48h after admission.
LDH: lactate dehydrogenase; CRP: C-reactive protein; CCP: COVID-19 convalescent plasma.
Time from admission to CCP infusion was 32.4 (22.0-41.2) vs. 81.7 (61.3-130.0) hours who respectively received CCP infusion within vs after 48h, p < 0.0001. Admission lactate dehydrogenase (LDH) was (366 (301-469) U/L) vs. (326 (274-428) U/L, p = 0.02) (Figure 2).
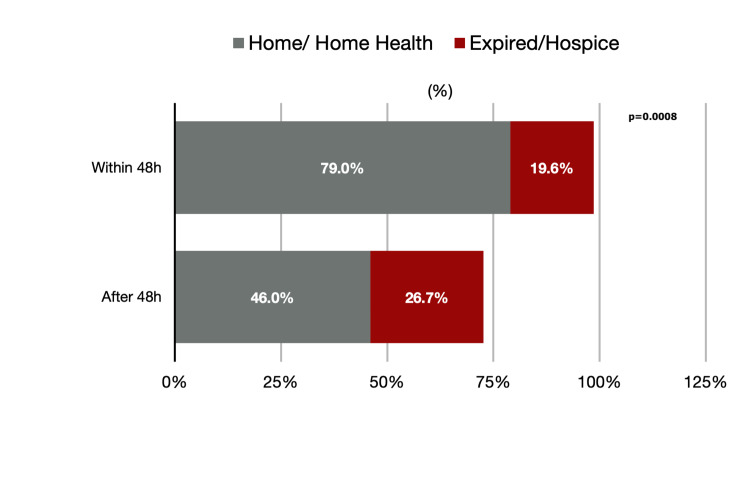
Figure 2. Percentage of patients discharged home/home health vs. expired/hospice.
Patients intubated included 25/92 (27%) vs. 29/105 (28%), p = 0.34. Days of mechanical ventilation were 8.0 (5.0-11.8) vs. 11.3 (4.5-17.9), p = 0.16. Hospital length of stay was 8.5 (4.9-15.2) vs. 13.0 (6.5-18.9) days, p = 0.03 (Table 2). Longitudinal CRP levels within 48h pre-, 24h post- and 25-48 post-CCP infusion, respectively, were (10.9 (7.1-16.7) vs. 9.5 (3.9-15.9)) (p = 0.12), 7.5 (4.9-15.1) vs. 6.9 (3.3-12.6) (p = 0.20), and 4.4 (2.7-9.0) vs. 6.1 (4.0-12.0), (p = 0.02) (Figure 3).
Table 2. Outcomes.
ICU: intensive care unit; LOS: length of stay; N = number; IQR: interquartile range; DC: discharge.
| Clinical outcomes | Convalescent plasma > 48h | Convalescent plasma < 48h |
| (N = 105) | (N = 92) | |
| ICU admission, No. (%) | 40 (38) | 34 (37) |
| ICU days, median (IQR) | 9.1 (3.0-16.6) | 7.8 (2.9-12.1) |
| Intubation, No. (%) | 30 (29) | 23 (25) |
| Ventilation days, median (IQR) | 11.3 (4.5-17.9) | 8.0 (5.0-11.8) |
| Survivor not Hospice LOS | 11.0 (5.9-18.7) | 7.0 (4.7-12.9) |
| Overall LOS, median (IQR) | 13.0 (6.5-18.9) | 8.5 (4.9-15.2) |
| Discharge to home, No. (%) | 51 (49) | 67 (73) |
| Expired or Hospice DC, No. (%) | 28 (26.7) | 18 (19.6) |
Figure 3. Longitudinal CRP levels within 48h, 24h post- and 25-48 post-CCP infusion, respectively. Two-way ANOVA demonstrated greater overall reduction in CRP when CCP was infused within 48h, p = 0.16.
CRP: C-reactive protein; CCP: COVID-19 convalescent plasma; ANOVA: analysis of variance.
Primary outcome was discharged to home/home health which was 73/92 (79%) vs. 49/105 (46%), p = 0.005.
Discussion
Between March 28 and August 5, 2020, Sarasota Memorial Hospital participated in the Mayo Clinic-led national FDA expanded access program providing access to convalescent plasma protocol. Throughout this period, data from 197 participants were analyzed and contrasted among those who received CCP infusion within or more than 48h after admission with a confirmed COVID-19 infection. We observed a post-CCP reduction in C-reactive protein, lower hospital length of stay and increase in discharge directly from hospital to home/home health in patients who received CCP within 48h of admission rather than later in hospitalization. Moreover, earlier CCP treatment resulted in 7% fewer patients who died or were discharged to hospice.
In December 2019, a new member of Coronaviridae family, SARS-CoV-2 was detected in Wuhan, China primarily manifesting as a respiratory illness [3]. Since then, multiple studies have been conducted exploring varied treatments for this lethal disease which has led to nearly 114 million of positive COVID-19 cases and 500,000 deaths in the United States. Presented with state of emergency accompanied by no available treatment and scarce medical resources; multiple centers around the world instituted CCP in a relatively timely manner previously shown effective during the SARS-CoV and Spanish Flu pandemic [3].
Initial evidence demonstrated CCP to be most beneficial when administered soon after SARS-CoV-2 infection. A randomized, double-blinded, placebo-controlled trial evaluated disease progression in 80 patients who received convalescent plasma within 72h after onset of mild COVID-19 symptoms vs. placebo [9]. Disease progression was advancement to severe respiratory disease defined as respiratory rate more than 30 breaths per min and/or arterialized blood oxygen saturation less than 93% breathing ambient air. Of the 80 patients, 31% of the patients who received placebo vs. 16% who received CCP progressed to severe respiratory disease [9]. Life-threatening respiratory disease was observed in only 5% of CCP patients vs. 12% in the placebo group. This study concluded that the administration of CCP to infected patients within 72h after the onset of symptoms reduce the risk of progression to severe respiratory disease by 48% [9]. CCP has demonstrated benefit in the clearance of SARS-CoV-2 in individuals who are symptomatic with COVID-19, including immunocompromised patients [8,10,11,12]. A cohort study of 966 patients with hematologic cancer and COVID-19 showed that after CP treatment, there was a significantly improved 30-day mortality [13].
CCP transfusion is associated with a reduction in inflammatory markers, such as CRP [8,10,14]. In a case series, 20 patients were treated with CCP compare to 20 controls matched with severe or life-threatening COVID-19 infection [15]. Results showed a marked reduction in CRP levels seven days after CCP infusion compared to control group. Discharges were similar amongst both groups, but mortality was higher in the control group. No patient died if they received CCP prior to seven days of hospitalization [15]. Another study reported lack of benefit when CCP was administered later (median of 21.5 days after diagnosis) for SARS-CoV-2, which supports that earlier treatment may be of critical importance [16]. Our longitudinal CRP observations corroborate and extend evidence favoring least “door to treatment” time.
Our data should be interpreted with some caveats. We conducted a monocenter pragmatic investigation. CCP was administered before FDA required titer labeling. Therefore, we couldn’t establish if antibody titers in CCP transfused across patient groups were equivalently distributed. In addition, SARS-CoV-2 serologic testing was unreliable restricting the assessment of whether a patient exhibited an impaired humoral response.
Conclusions
Our study was conducted while no anti-viral treatment was approved by the FDA for patients hospitalized with COVID-19. We evince CCP treatment within 48h of admission was associated a reduction in hyperinflammation and hospital length of stay in patients more obese with higher LDH levels with greater benefit for discharge to home/home health benefit and reduction in composite outcome of hospital mortality or discharge to hospice. Convalescent plasma has shown to be an effective treatment when given soon after SARS-CoV-2 infection. Unfortunately, in hospitalized patients with COVID-19, infusion of CCP late in the course of illness provides no observed benefit, as reported with other anti-viral agents.
Acknowledgments
The authors would like to thank Sarasota Memorial Hospital and the Internal Medicine Residency for all their contributions in making this manuscript possible.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Mark J. Magenheim, MD MPH, Medical Director IRB and Ashley Butler, CIP, Coordinator of the Institutional Review Board issued approval 21-COVID-30. Thank you for your submission of New Project materials for this research study. This research study qualifies for Expedited Review, as it involves no greater than minimal risk to subjects, and involves a retrospective review of medical records. The primary purpose of this study was to assess the relative impact of time from hospitalization to infusion of convalescent COVID-19 antibody-positive plasma (CCP) versus putative blood biomarkers of inflammation and organ injury evoked by patients hospitalized with a CoV-2 infection. Sarasota Memorial Hospital Institutional Review Board has APPROVED your submission. This approval is based on an appropriate risk/benefit ratio and a study design wherein the risks have been minimized. All research must be conducted in accordance with this approved submission.
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Convalescent plasma transfusion for the treatment of COVID-19: systematic review. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. J Med Virol. 2020;92:1475–1483. doi: 10.1002/jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 3.Convalescent plasma in Covid-19: possible mechanisms of action. Rojas M, Rodríguez Y, Monsalve DM, et al. Autoimmun Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Hung IF, To KK, Lee CK, et al. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expanded Access to Convalescent Plasma for the Treatment of Patients with COVID-19. [May;2021 ];https://www.uscovidplasma.org/-/media/kcms/gbs/patient-consumer/documents/2020/05/27/21/23/covid-19-plasma-eap.pdf 2020
- 6.Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Imai M, Iwatsuki-Horimoto K, Hatta M, et al. Proc Natl Acad Sci U S A. 2020;117:16587–16595. doi: 10.1073/pnas.2009799117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Generation of a broadly useful model for COVID-19 pathogenesis, vaccination and treatment. Sun J, Zhuang Z, Zheng J, et al. Cell. 2020;182:734–743. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Klassen SA, Senefeld JW, Johnson PW, et al. Mayo Clin Proc. 2021;96:1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Early high-titer plasma therapy to prevent severe Covid-19 in older adults. Libster R, Pérez Marc G, Wappner D, et al. https://pubmed.ncbi.nlm.nih.gov/33406353/ N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Convalescent plasma for the treatment of severe COVID-19 infection in cancer patients. Tremblay D, Seah C, Schneider T, et al. Cancer Med. 2020;9:8571–8578. doi: 10.1002/cam4.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Determinants of COVID-19 disease severity in patients with cancer. Robilotti EV, Babady NE, Mead PA, et al. Nat Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency. [Published online November 10] Senefeld JW, Klassen SA, Ford SK, et al. BMJ. 2020 [Google Scholar]
- 13.Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. Thompson MA, Henderson JP, Shah PK, et al. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nature and dimensions of the systemic hyper-inflammation and its attenuation by convalescent plasma in severe COVID-19. Bandopadhyay P, Rozario R, Lahiri A, et al. J Infect Dis. 2021 doi: 10.1093/infdis/jiab137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Use of convalescent plasma in hospitalized patients with COVID-19: case series. Hegerova L, Gooley TA, Sweerus KA, et al. Blood. 2020;136:759–762. doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Wooding DJ, Bach H. Clin Microbiol Infect. 2020;26:1436–1446. doi: 10.1016/j.cmi.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]