Abstract
Difficult diagnosis is due to rarity of the case. TT or TE echocardiography is sufficient to make a correct diagnosis. The risk of embolism or coronary ostia occlusion should guide the decision for surgery.
Keywords: aorta and great vessels, aortic surgery, cardiac tumors
Difficult diagnosis is due to rarity of the case. TT or TE echocardiography is sufficient to make a correct diagnosis. The risk of embolism or coronary ostia occlusion should guide the decision for surgery.
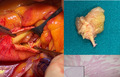
1. INTRODUCTION
We report an exceedingly rare case of aortic wall papillary fibroelastoma simulating unstable angina, diagnosed with echocardiography and CT scan and surgically successfully removed.
Primary aortic wall tumors are very rare and consist mainly of malignant tumors.1 Papillary fibroelastomas (PFEs) are benign neoformations usually originating from the valvular endocardium, consisting of non‐vascularized papillary tissue surrounded by an endothelium layer.2 The localization of PFE on the aortic wall is extremely rare.3, 4 The clinical presentation of aortic PFE is usually related to embolization with myocardial infarction and/or stroke.4, 5 We report a case of aortic wall PFE simulating unstable angina, diagnosed with transthoracic and transesophageal echocardiography (TTE/TEE) and surgically removed.
2. CASE REPORT
A 73‐year‐old woman, hypertensive and diabetic, came to ER with typical angina symptoms. The patient had undergone PTCA with DES on the anterior descending and first diagonal coronary arteries, following acute myocardial infarction, 3 years before. Subsequently, one and a half years later a further coronary artery examination, performed after a chest pain episode with positive ECG, showed a good success of the previous PTCAs. No other coronary lesions were found, and nothing was detected in the ascending aorta. At the current admission, the ECG showed ST depression in II‐III‐aVF that spontaneously resolved and there was no significant HS troponin enzymatic release. At a routine TTE, the presence of a mass in the upper part of the right coronary sinus just above the right coronary ostium was noted. A TEE was quickly performed confirming the presence of a mobile mass of about 1 × 1.5 cm in diameter with irregular profile (Figure 1A). A CT scan was then performed confirming the location, aspect, and size of the mass (Figure 1B). Although the patient remained asymptomatic, the mobility of the mass associated with the previous episode of chest pain made us put an indication to urgent surgery.
FIGURE 1.
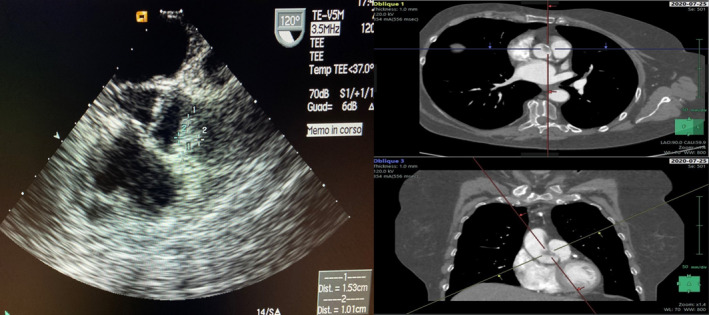
A, TEE showing a mobile, irregular mass of 1 × 1.5 cm diameters in right coronary sinus. B, CTA scan confirming the presence of the mass in ascending aorta
After a median sternotomy, the extracorporeal circulation was started by cannulation of the ascending aorta and left atrium. After aortic clamping, longitudinal aortic root exposure was obtained. A transparent gelatinous mass was found, implanted by a small peduncle in the upper part of the right coronary sinus slightly laterally to the coronary ostium, which appeared partially covered by the mass itself (Figure 2A). The tumor was excised at the base, without the need to remove part of the aortic root (Figure 2B). The total CPB time was 32 min and the cross‐clamp 16 min. The postoperative course was uncomplicated, and the patient discharged home on the seventh POD. Before discharge, a new coronary artery angiography showed an unchanged finding. The histopathological examination confirmed that the mass was a papillary fibroelastoma without thrombotic component (Figure 2C).
FIGURE 2.
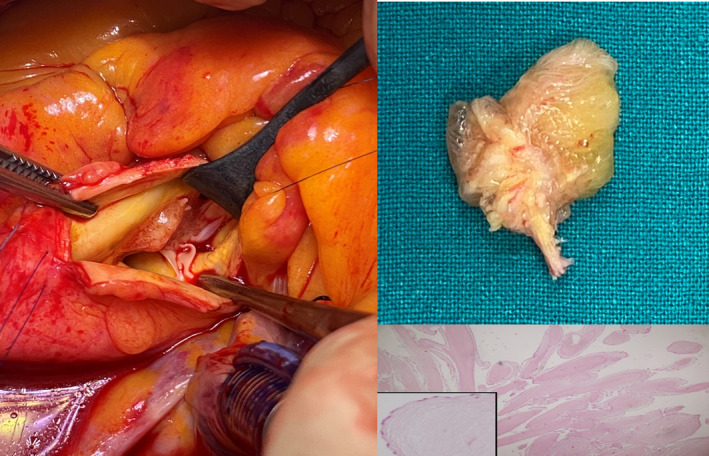
A, Surgical view of the mass. B, Macroscopic view of the resected mass. C, The histology shows individual fronds consisting of a core of hyalinized hypocellular stroma that is rich in elastic fibers and a lining of hyperplastic endocardial cells (magnification ratio:200:1–400:1. Hematoxylin‐eosin staining)
3. DISCUSSION
PFEs are second in frequency after myxomas, or ever more common, among primary cardiac tumors.6 Some authors reported higher rates of PFEs, and they have been considered more common than myxomas in recent publications.6, 7 They present pathologically with the appearance of a sea anemone with leafy arms, starting from a stalked central core,5 consisting of avascular papillary tissue. The 88% of PFE originate from the valvular surfaces and the remaining 12% from other cardiac localizations6; aortic localization is extremely rare and can occur in all aortic segments. To the best of our knowledge, only few cases of aortic wall PFE have been described in literature.3, 8, 9
In aortic wall PFE, although the majority of patients remain asymptomatic, clinical onset usually occurs with cerebral ischemic or coronary symptoms due to embolization or partial or total coronary occlusion.3, 4, 8, 9 The diagnosis is very difficult mainly due to their low frequency and consequently the lack of knowledge of the case; this is the unique case of aortic PFE reported in our center after about 20,000 cardiac interventions. In addition, presentation with angina symptoms in a patient with previous PTCA caused diagnostic confusion. Usually, transthoracic echocardiography (TTE) is sufficient to allow a diagnosis with high sensitivity and specificity (88.9% and 87.8%, respectively) where TEE has high sensitivity (76.6%) even for PFEs smaller than 0.2 cm.10 The execution of a CT scan can be useful to define the diagnosis itself which, due to its extreme rarity, is hardly immediately evaluated. In fact, in our case the typical angina symptoms would probably have led us to perform a new coronary angiogram, with the associated embolic risk, given the difficulty to think of a PFE of the aorta. The presence of the mass, already evident in TTE, led us to the execution of TEE which allowed a diagnosis with high probability; the CT confirmed the size and position of the mass allowing us to place the clinical suspicion and the consequent surgical indication to the removal. Coronary angiography was not performed to avoid mobilization of the mass.
There are no current guidelines for the treatment of PFE. In patients with embolic events, surgery is strongly recommended.11 In asymptomatic patients, the opinions are different and are based on mass mobility, position, concomitant surgery, and embolic risk. Preoperative CT coronary angiography can be performed to evaluate coronary artery lesions needing revascularization instead of coronary angiography that carries embolization risk. We believe that, since the tumor is positioned in the aortic flow, surgery should always be indicated for the potential embolic risk, except in asymptomatic high‐risk patients. Given the benign nature of the tumor, a simple but accurate excision is sufficient. After surgical resection, no recurrence of the tumor has ever been reported8.
4. CONCLUSIONS
PFEs of the aortic wall are exceptionally rare and can be found accidentally or show symptoms of cerebral or cardiac ischemia. The diagnosis is particularly difficult mainly due to the lack of experience of the operators. TTE/TEE is the method of choice for their diagnosis while CT scan or MRI can be helpful in the diagnostic doubt. Surgical resection, where possible, is mandatory due to the risk of embolism and is generally low‐risk surgery. Our case highlights how clinical suspicion should be placed even when the mass is in such atypical positions (Video S1).
CONFLICT OF INTEREST
No conflict of interest to declare.
AUTHOR CONTRIBUTIONS
PM, GM, and GE involved in concept/design. PM, PC, II, ADR, GC, and DT drafted the article. SI, GM, and AC critically revised the article.
PATIENT CONSENT
The written consent for publication was released by the patient.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was obtained from the director of A.O.U. San Giovanni di Dio e Ruggi D’Aragona Hospital. Formal approval is not necessary to report a case as "Case Report" in our hospital.
Supporting information
Video S1
ACKNOWLEDGEMENTS
Published with written consent of the patient.
Masiello P, Catalano A, Mastrogiovanni G, et al. Surgical removal of an exceedingly rare papillary fibroelastoma of the aortic wall causing unstable angina. Clin Case Rep. 2021;9:e04688. 10.1002/ccr3.4688
Funding information
The authors declare that no funding sources were utilized
Data Availability Statement
The dataset generated during the current study are not publicly available because of privacy regulation but are available from the corresponding author on reasonable request.
REFERENCES
- 1.Das AK, Reddy KS, Suwanjindar P, et al. Primary tumors of the aorta. Ann Thorac Surg. 1996;62:1526‐1528. [DOI] [PubMed] [Google Scholar]
- 2.Burke A. Tumors of the heart and great vessels. Atlas Tumor Pathol. 1996;16:171‐179. [Google Scholar]
- 3.Yerebakan C, Liebold A, Steinhoff G, Skrabal CA. Papillary fibroelastoma of the aortic wall with partial occlusion of the right coronary ostium. Ann Thorac Surg. 2009;87:1953‐1954. [DOI] [PubMed] [Google Scholar]
- 4.Rolf T, Iglesias JF, Tozzia P, von Segessera LK. Acute myocardial infarction caused by coronary embolization of a papillary fibroelastoma of the thoracic ascending aorta. Interact Cardiovasc Thorac Surg. 2010;11:676‐678. [DOI] [PubMed] [Google Scholar]
- 5.Klarich KW, Enriquez‐Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997;30:784‐790. [DOI] [PubMed] [Google Scholar]
- 6.Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW. Neoplasia and the heart: pathological review of effects with clinical and radiological correlation. J Am Coll Cardiol. 2018;72(2):202‐227. [DOI] [PubMed] [Google Scholar]
- 7.Tamin SS, Maleszewski JJ, Scott CG, et al. Prognostic and bioepidemiologic implications of papillary fibroelastomas. J Am Coll Cardiol. 2015;65(22):2420‐2429. [DOI] [PubMed] [Google Scholar]
- 8.Anand S, Sydow N, Janardhanan R. Papillary fibroelastoma diagnosed through multimodality cardiac imaging: a rare tumour in an uncommon location with review of literature. BMJ Case Rep. 2017;2017(8):bcr‐2017‐219327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez‐Santos JM, Arnaiz‐Garcıa ME, Vargas‐Fajardo MC, Arribas‐Jimenez A. Aortic wall papillary fibroelastoma. J Thorac Cardiovasc Surg. 2013;146:e1‐3. [DOI] [PubMed] [Google Scholar]
- 10.Sun JP, Asher CR, Yang XS, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001;103:2687‐2693. [DOI] [PubMed] [Google Scholar]
- 11.Gowda RM, Khan IA, Nair CK, Metha NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003;146:404‐410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Data Availability Statement
The dataset generated during the current study are not publicly available because of privacy regulation but are available from the corresponding author on reasonable request.


