Abstract
Intracranial penetration during attempted nasotracheal intubation is a potentially devastating complication, which should be carefully evaluated and the risk should be addressed in neonatal resuscitation trainings.
Keywords: brain injury, child development, cribriform plate perforation, intubation, neonate
Intracranial penetration during attempted nasotracheal intubation is a potentially devastating complication, which should be carefully evaluated and the risk should be addressed in neonatal resuscitation trainings.
1. BACKGROUND
Misplacement of the endotracheal (ET) tube into the brain during attempted nasotracheal intubation is a rare, but serious complication. Three neonates with iatrogenic perforation of the lamina cribrosa following intended nasotracheal intubation as well as a review of the literature are presented. Previously, only two further cases of ET tube misplacement have been reported. Three patients were transferred to our neurosurgical department after failed nasotracheal intubation. In every patients, intubation was complicated by large amounts of blood in the oral cavity postnatally. Brain lesions were detected by cerebral ultrasound and confirmed by MRI. All infants required neurosurgical intervention. The resulting encephalocele may be associated with seizures and impaired long‐term neurodevelopmental outcome. Our case series and previous reports underline the importance of an appropriate technical skills training. Therefore, we suggest to address this possible adverse event in neonatal resuscitation courses and simulation‐based team trainings. In addition, we advise to carefully examine brain lesions on ultrasound, especially in preterm infants with a history of difficult intubation.
Endotracheal intubation is a common procedure in neonatal intensive care units.1 Although a series of local injuries such as acute and chronic trauma to the nose, mouth, pharynx, larynx, and even trachea are described due to endotracheal intubation,2 it is still unclear whether the orotracheal or the nasotracheal route should be preferred.1
Intracranial penetration during attempted nasotracheal intubation represents a rare but potentially devastating complication and a significant effort should be made to avoid it.3 In the current literature, just two cases of intracranial penetration of the endotracheal tube during an attempt of nasotracheal intubation are reported.3, 4
Here, we report three cases of iatrogenic perforation of the lamina cribrosa after nasotracheal intubation in 3 different tertiary centers, subsequently treated in our center. In all patients, the procedure was performed by an experienced consultant in a perinatal center where nasotracheal intubation is performed routinely. We describe diagnostic procedures and treatment options with a focus on neurodevelopmental long‐term outcome. Finally, our cases are compared with those previously reported in the literature.
2. CASE PRESENTATION
2.1. Case 1
This preterm infant was born at 256/7 weeks GA with a birth weight of 600g. He was the first twin from a monochorionic monoamniotic pregnancy, delivered by cesarean section (CS), due to twin to twin transfusion syndrome stage three. In the absence of spontaneous breathing efforts, non‐invasive positive pressure ventilation (NIPPV) was initiated. A first intubation attempt was difficult due to large amounts of residual blood in the oral cavity after CS. Thus, visualization of the vocal cords was initially impaired. NIPPV was provided via a facial mask and chest compressions were performed between the 4th and 8th minute of life. Despite effective NIPPV, the clinical situation did not improve and the placement of an endotracheal tube became necessary. At 14 minutes after birth, an endotracheal tube size 2.0 mm was correctly inserted via the nasopharyngeal route. Apgar score was 1/2/4.
Head ultrasound performed on the 1st day of life (DOL) showed an IVH°I and a linear hyperechogenic area within the left fronto‐parietallobe, which gradually decreased in intensity during the first months of life and could no longer be seen at 3 months of age.
The MRI at three months showed an IVH°II and a periventricular alteration above the cella media, which showed a linear extension in the direction of the vertex. This alteration corresponded to the lesion previously described on ultrasound. EEG was normal for age. An extensive neurodevelopmental examination revealed merely a moderate axial hypotonia, without other neurological signs. The boy was discharged home at four months of age. At 16 months, he presented with a first cluster of focal epileptic seizures, originated from the left frontal lobe. The infant was admitted to the hospital after the third seizure spell, all characterized by arousal from sleep, alteration of contact, and slight paresis of the right hand during the night. On EEG, characteristic high amplitude focal activity was demonstrated above the left frontal lobe, with a duration of 2–9 minutes and spontaneous resolution. On cranial MRI, a defect of the left lamina cribrosa with a suspected herniation of brain tissue into the ethmoid sinuses of about 2 × 1.5 cm was demonstrated. Initially, the infant was treated with levetiracetam leading to seizure control. After surgical repair of the skull base defect at 18 months of age, no further seizures were recorded. Levetiracetam treatment was stopped at 27 months of age without seizure recurrence. Neurodevelopmental evaluation by Bayley Scales of Infant and Toddler Development, 3rd Edition (BSITD‐III) at 31 months showed a normal cognitive (PDI = 110), motor (MDI = 89), and language development (LDI = 88).
2.2. Case 2
The extremely low gestational age infant was delivered by CS due to maternal eclampsia at 243/7 weeks GA with a birth weight of 490g. Endotracheal intubation and surfactant administration were performed in the delivery room immediately after birth. Apgar score was 1/1/3. Head ultrasound on the first DOL showed no abnormalities, but on the 7th DOL, an IVH°I was detected in the right ventricle. At 11th DOL in presence of signs of ET tube obstruction, the tube was replaced. After adequate analgosedation, a new ET tube was inserted through one nostril. However, on laryngoscopy, the tube could not be visualized at the pharyngeal level. The ET tube was immediately withdrawn and another tube was inserted without difficulties.
Head ultrasound demonstrated a parenchymal lesion above the left lateral ventricle and a suspected blood clot in the left frontal horn. At 12 weeks of age, a cerebrospinal fluid (CSF) fistula was suspected due to an increasing serous secretion from the left nostril. The fluid showed a glucose level >65mg/dl and positive ß2‐transferrin, both findings of indicating a CSF fistula with rhinorrhea. Cranial MRI at 16 weeks showed a left‐sided lamina cribrosa defect associated with a fronto‐ethmoidal encephalocele, a herniation of the inferior gyrus frontalis and fluid effusion into the nasal cavity. These findings supported the final diagnosis of CSF fistula. After suspected seizures characterized by apnea and convulsions of all extremities, treatment with phenobarbitone was started.
At three months of age, the infant underwent a surgical correction of the defect, which was performed by an endoscopic resection of the encephalocele and a seal with Tachosil® (fibrin sealant patch, Takeda, Germany). The MRI after surgical repair showed soft tissue covering the bone defect and the complete closure of the passage to the upper left nasal cavity.
At 17 months, the boy's head circumference was 46 cm (<3. P). Motor assessment revealed unilateral cerebral palsy, classified as level I on the Gross Motor Function Classification System (GMFCS). There were no signs of visual or hearing impairment. Despite adequate seizures control, BSITD‐III showed neurodevelopmental impairment with an MDI of 50 and a PDI of 52.
2.3. Case 3
This preterm infant was delivered by CS at 35 2/7 weeks GA (birth weight: 2500g) due to acute hemorrhage from a placenta praevia. Since initial CPAP management was insufficient respiratory stabilization, intubation was attempted. Large amounts of placental blood obstructed the oral cavity resulting in a frustrating attempt of intubation. Thus, a second attempt of stabilization on CPAP was successfully performed. Apgar score was 6/6/6. An ET tube misplacement during the intubation attempt was suspected because of continuous minor bleeding from the right nostril. On brain ultrasound, a 4.5 cm lesion in the right frontal lobe and a thalamic hemorrhage were detected, which were both confirmed on MRI (Figure 1). After referral to our center, on the third DOL endoscopic repair with TachoSil® (Takeda, Germany) was successfully performed and liquorrhoea stopped. On follow‐up, MRIs recurrence of an encephalocele was observed. A microsurgical resection of the encephalocele via a transcranial subfrontal approach was performed at the age of 6 months. Neurosurgical intervention required a reconstruction of the dural defect with a periosteal flap and fibrin glue. After surgical repair, the child showed a normal neurodevelopment.
FIGURE 1.
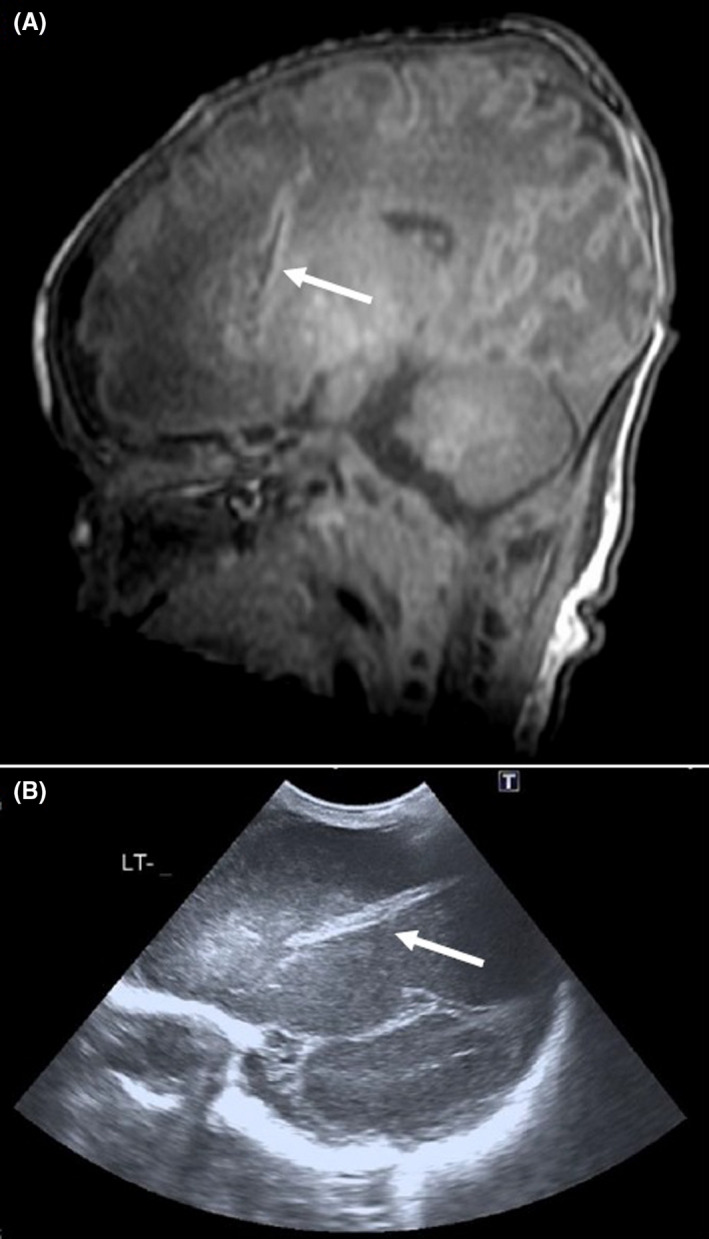
Linear hyperechogenic area on the left frontoparietal area at (A) MRI and (B) ultrasonography (patient 3)
At 24 months, the boy's head circumference was 49.7 cm (<25.–50. P). MDI score was 97 and PDI was 81 (BSITD‐III).
2.3.1. Previously reported cases
To our knowledge, only two cases of accidental perforation of the lamina cribrosa due to intubation attempts are reported in the literature. The first case was a preterm infant, born at 29 weeks gestation, described by Cameron and Lupton in 19934; the second case was an infant born at 28 weeks gestation presented by de Vries in 2014.3 In both cases, the nasal ET tube was not visible in the oral cavity when the laryngoscope was inserted. In both infants, the lesions were detected on cranial ultrasound examination. The infant described by Cameron and Lupton showed a developmental delay with generalized hypotonia at eight months of age.4 The infant reported by De Vries et al. developed a porencephalic cyst with posthemorrhagic hydrocephalus. A ventriculostomy was performed followed by a ventriculoperitoneal shunt. At the age of 2 years and 9 months, the infant showed an MDI of 90 and a PDI of 102.3
3. DISCUSSION
We present the largest case series of traumatic perforation of the lamina cribrosa after nasotracheal intubation with subsequent brain lesions in neonates. None of these newborns showed coagulation alterations or presented major bleedings, nor acute neurological signs at the time of the injury. In neonatology, endotracheal intubation is sometimes necessary and may be lifesaving in some patients. However, the trend toward lower rates of endotracheal intubation during the initial stabilization of preterm infants over the last decades may also lead to less training of the technical skills necessary for successful intubation.5 In addition, intubation of very preterm infants may be particularly difficult, both because of the small size of the patient and the fact that the procedure is often performed in emergencies.5
To date, there is no clear evidence whether the orotracheal route is more suitable than the nasotracheal route, since both offer advantages and disadvantages. Whereas orotracheal intubation may be easier to perform, fixation of the ET tube might be more challenging. In summary, in many NICUs, the preferred route depends on individual and institutional experience and local practices.
Based on the three cases presented here and the two previously published patients, neonatologists and doctors in training need to know about the risk of lamina cribrosa perforation following attempted nasotracheal intubation. Besides medical history, cranial ultrasound plays a crucial role for diagnosis. In fact, in all five patients a typical linear hyperintense area starting from the region of the lamina cribrosa and protruding into the frontal lobe was demonstrated (Figure 1). We also suggest to rule out lamina cribrosa perforation in any infant in whom similar alterations are found on brain ultrasound, especially those with a history of a difficult nasotracheal intubation.
Clinical manifestations varied between patients (Table 1). Whereas in one neonate the parenchymal lesion became clinically evident only at 17 months of age with the insurgence of seizures, in the second case, brain injury was detected much earlier, at 12 weeks of age. Also the timing for surgical interventions differed widely among patients. Luckily, in our case series, in all three infants, neurosurgical interventions resulted in the complete repair of the lesion. The infant reported by de Vries et al. developed a posthemorrhagic hydrocephalus with a mild neurodevelopmental delay at the age of 2 years and 9 months.3 The case described by Cameron and Lupton developed hydrocephalus with a porencephalic cyst, developmental delay, and hypotonia.4 In both cases, placement of a ventriculoperitoneal shunt was necessary.3, 4
TABLE 1.
Reported cases of perforation of cribriform lamina
| Author | GA (w) | BW (g) | Age at perforation (days) | Ultrasound positive | Seizures | Hydrocephalus | Neurosurgical intervention | MDI | PDI |
|---|---|---|---|---|---|---|---|---|---|
| Cameron | 29 | 1370 | 1 | YES | NO | YES | YES | NA* | NA* |
| De Vries | 28+4 | 1260 | 1 | YES | 2yrs 6mo+ | YES | YES | 90 | 102 |
| Case 1 | 25+6 | 600 | 1 | YES | YES | NO | YES | 89 | 110 |
| Case 2 | 24+3 | 490 | 11 | YES | YES | NO | YES | 50 | 52 |
| Case 3 | 35+2 | 2500 | 1 | YES | NO | NO | YES | 97 | 81 |
*Developmental delay with persistent head lag and generalized hypotonia. +Status epilepticus due to sepsis meningitis caused by Streptococcus pneumoniae (not directly related to the initial lesion).
It is challenging to evaluate the effect of the lesion itself on long‐term neurodevelopmental outcome, since two of our infants were born extremely preterm. Prematurity is an independent risk factor for an impaired neurodevelopmental outcome. The infant described by Cameron and Lupton showed a “developmental delay with generalized hypotonia.” However, neurodevelopmental testing (like BSITD‐III) was not reported.4 Four out of five described neonates were very low birth weight preterm infants, a condition which is associated with a higher risk for an impaired neurodevelopmental outcome. Nevertheless, BSITD‐III was markedly reduced only in one infant, which was the smallest preterm and therefore at highest risk for developmental delay.
In summary, perforation of the lamina cribrosa is a rare, but potentially severe complication of nasotracheal intubation, since it leads to acute symptoms and may be associated with an impaired long‐term neurodevelopmental outcome. In addition, all reported infants required neurosurgical intervention.
After the publication of the COIN trial,6 non‐invasive respiratory support is often preferred even in very preterm infants.7 As a possible consequence, operator's intubation skills might get lost over time since endotracheal intubation is generally less often performed. Therefore, the misplacement of the ET tube might occur more often. Hence, we underline the importance of performing the procedure correctly. As already indicated in the paper by de Vries et al.,3 care should be taken, never to apply force on the ET tube while passing it through the nasal cavity, especially, if any resistance is encountered. We also suggest, as recommended by Cameron & Lupton, to insert a softer guide, for example, a suctioning tube into the ET tube.4 This permits to insert a thinner and softer guide first and to insert the ET tube only after the tip of the nasogastric tube is visible in the oropharynx.
The increased use of non‐invasive ventilation to stabilize even very preterm infants leads pediatric residents to encounter difficulties in learning neonatal intubation. Therefore, misplacement of the ET tube might occur more often. This increases the importance of technical skills training on manikins. Planning such trainings with emphasis on nasotracheal intubation could be helpful in order that all the personnel involved in intubation acquires the necessary skills.7 In addition, we suggest addressing this possible complication in neonatal resuscitation courses and in simulation‐based team trainings.
4. CONCLUSIONS
Although sometimes necessary and lifesaving, nasotracheal intubation in rare cases may cause a perforation of the lamina cribrosa and subsequent brain lesions. These injuries might lead to short‐term symptoms and may be associated with an impaired long‐term neurodevelopmental outcome. Cranial ultrasound plays a key role in diagnosis, since it is highly sensitive and easily repeatable. Moreover, it clearly shows the abovementioned lesions.
We recommend addressing this possible adverse event in neonatal resuscitation and simulation‐based team trainings. Also, we advise to evaluate similar lesions in cranial ultrasound carefully, especially in infants with a history of difficult intubation.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
FL, AS, LP, and AF have been involved in drafting the manuscript and have made substantial contributions to acquisition of data. All authors have made substantial contributions to conception and design and acquisition of data and revising the manuscript critically for important intellectual content. All authors have been involved in drafting the manuscript and revising it critically for important intellectual content.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
The parents or guardians of the patients have given written consent to publish the cases (including publication of images).
ACKNOWLEDGMENTS
Published with written consent of the patient.
Lupi F, Staffler A, Parmeggiani L, et al. Lamina cribrosa perforation during nasotracheal intubation in neonates: case series and review of the literature. Clin Case Rep. 2021;9:e04650. 10.1002/ccr3.4650
Funding information
No funding sources
DATA AVAILABILITY STATEMENT
All original data presented are available through the first and the corresponding author.
REFERENCES
- 1.Spence K, Barr P. Nasal versus oral intubation for mechanical ventilation of newborn infants. Cochrane Database Syst Rev. 2000;(2):CD000948. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberton NRC. Pulmonary diseases of the newborn. In: Roberton NRC, ed. Textbook of Neonatology. Melbourne: Churchill Livingston, 2005:1257‐1258. [Google Scholar]
- 3.de Vries MJ, Sival DA, van Doormaal‐Stremmelaar EF, Ter Horst HJ. Traumatic perforation of the lamina cribrosa during nasal intubation of a preterm infant. Pediatrics. 2014;133(3):e762‐e765. [DOI] [PubMed] [Google Scholar]
- 4.Cameron D, Lupton BA. Inadvertent brain penetration during neonatal nasotracheal intubation. Arch Dis Childhood. 1993;69:79‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell CPF. Intubation difficulty in neonatology: are you experienced? Arch Dis Child Fetal Neonatal Ed. 2019;104(5):F458‐F460. http://dx.doi.org.emedien.ub.uni‐muenchen.de/10.1136/archdischild‐2018‐316711 [DOI] [PubMed] [Google Scholar]
- 6.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet J‐M, Carlin JB, for the COIN Trial Investigators . Nasal CPAP or Intubation at Birth for Very Preterm Infants. N Engl J Med. 2008;358:700‐708. [DOI] [PubMed] [Google Scholar]
- 7.Foglia EE, Ades A, Sawyer T, et al. Neonatal intubation practice and outcomes: an international registry study. Pediatrics. 2019;143(1):e20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All original data presented are available through the first and the corresponding author.


