Abstract
One concern as lung cancer screening (LCS) is implemented is that patients will be screened who are too ill to benefit. Poor exercise capacity (EC) predicts adverse outcomes following lung resection.
Objective:
Describe the distribution of EC among smokers eligible for LCS and examine associations with comorbidities.
Methods:
Cross-sectional analysis of baseline data from a randomized controlled trial of tobacco treatment in the context of LCS. Participants responded regarding limitations in moderate activities, ability to climb stairs, and frequency of dyspnea on a scale from never/almost never to all or most of the time. Responses were assigned a numeric score and summed to categorize exercise limitation. Associations between poor EC and key comorbidities were examined using adjusted logistic regression.
Results:
660 participants completed a survey with the following characteristics: 64.4% male, 89.5% white, mean age 64.5. Overall EC categories were: good 39.0%, intermediate 41.6%, and poor 19.4%. Prevalence of poor EC was higher among patients with COPD (OR 4.62 95%CI 3.05–7.02), heart failure (OR 3.07 95%CI 1.62–5.82) and cardiovascular disease (OR 2.24, 95%CI 1.45–3.47), and was highest among patients with multimorbidity. Among patients with COPD and heart failure, 57% had poor and 0% had good EC. In adjusted logistic regression, only COPD and Charlson comorbidity index remained significantly associated with poor EC.
Conclusions:
Many patients eligible for LCS reported poor EC, with increased odds of poor EC among patients with comorbidities. More research is needed to determine how to best integrate EC and comorbidity into eligibility and shared decision-making conversations.
Keywords: lung cancer screening, exercise capacity, functional capacity, comorbidity, multimorbidity, cancer screening, lung cancer
Microabstract
Poor exercise capacity (EC) predicts adverse cardiopulmonary complications following lung resection, may preclude curative surgery and dilute the benefit of the screening intervention. Patients ineligible for surgery may instead be eligible for stereotactic body radiation therapy (SBRT). Six hundred and sixty individuals who smoke and are eligible for lung cancer screening self-reported their exercise capacity, of whom 19.4% reported poor exercise capacity. Poor exercise capacity was strongly associated with chronic obstructive pulmonary disease. Exercise capacity may need to be integrated into eligibility determinations and during the shared decision-making process for lung cancer screening.
Background:
Lung cancer screening (LCS) with low-dose computed tomography is gradually being implemented as a strategy to decrease mortality from lung cancer. It is effective through the detection and treatment of cancers at an earlier stage.1 If appropriately treated, early stage cancers have a high cure rate and five-year survival.2 As LCS is implemented in non-trial settings, one concern is that patients will be screened who are too ill to maximally benefit due to limited life expectancy or the inability to undergo recommended procedures and treatments.3 Screening these patients for lung cancer may expose them to unnecessary individual risks and dilute the population-level benefits of LCS, while also increasing costs and strain on the health system.
At present, the gold standard treatment for early stage lung cancers is resection with lobectomy—a surgery that requires significant pulmonary and cardiovascular reserve to undergo safely.4 Though mortality outcomes using modern surgical approaches are lower,5 lobectomy in general carries significant potential morbidity and mortality. Among older LCS-eligible patients with lung cancer, 30-day mortality following lobectomy exceeded 2%.6 Patients with excessive operative risk instead receive other treatments such as stereotactic body radiation therapy (SBRT) or sub-lobar resection, which may not provide equivalent survival outcomes.7 Therefore, patients with screen-identified early stage cancers who cannot safely undergo surgeries or diagnostic procedures may not realize the full benefits of early detection and treatment.
Poor pre-operative exercise capacity is one of several factors that predicts worse post-lobectomy outcomes and higher frequency of adverse cardiopulmonary complications.8 In one study, patients who climbed less than 12 meters in a stair climbing test (about three flights of stairs) had 13 times higher mortality than patients who climbed 22 meters (about 5 flights of stairs).9 The American College of Cardiology/American Heart Association joint guideline on preoperative testing recommends pre-operative assessment of exercise capacity (EC) by history for all patients, with additional testing needed for those able to perform fewer than 4 METs (metabolic equivalents) of activity who are planned for thoracic surgery.10 Activities such as moderate housework or walking at a brisk pace are approximately 4 METs, while heavy housework or hill-climbing indicate performance of more than 4 METs.11 Therefore, patients who report dyspnea with moderate activities, cannot climb several flights of stairs, or report shortness of breath throughout their daily activities are likely to have an EC of less than 4 METs, placing them at higher risk of adverse outcomes from thoracic surgery. Nearly all thoracic surgeons consider EC in their evaluations of potential surgical patients.12 Thus EC plays a critical role in deciding whether a patient is fit to undergo curative lung resection.13
To qualify for reimbursement by the Centers for Medicare and Medicaid,14 the decision to refer a patient for LCS is made only after a process of shared decision-making (SDM). This process is meant to weigh the risks and benefits of LCS in the context of the patient’s personal lung cancer risk, values, preferences, medical history and comorbidities. SDM helps deliver LCS to patients who are not only motivated and informed, but who are also appropriate candidates medically. Many older smokers have comorbidities which might complicate or preclude diagnostic procedures or surgical lung cancer treatment, and which are likely associated with EC. Compared to medical comorbidities, EC is less frequently reported in routine clinical care. Though several publications have examined the prevalence of comorbidities in the LCS population,15, 16 the distribution of exercise limitations in the LCS population is largely unknown.
We sought to assess self-reported exercise limitations among smokers eligible for LCS and to examine the associations with key comorbidities and tobacco use history. We hypothesized that poor EC and high burden of dyspnea would be strongly associated with medical comorbidities, and that multiple co-morbidities would increase the odds of reporting a poor EC. A portion of the results were presented at the American Thoracic Society Meeting 2020.17
Methods:
We conducted a cross-sectional analysis of data drawn from the baseline survey from the Program for Lung Cancer Screening and Tobacco Cessation (PLUTO) Trial. The PLUTO trial is a sequential multiple assignment randomized trial examining outcomes of longitudinal tobacco cessation treatment provided in the context of LCS.18 The study was approved by the IRB at each institution (University of Minnesota Fairview/MHealth, CPRC2015NTLS048, Allina Health, 1187771,and the Minneapolis VA Health Care System, 4619-B).
Population:
Participants were current smokers from one of three medical systems in the Minneapolis/St. Paul, Minnesota metropolitan area, deemed to be eligible for LCS. Eligibility criteria for LCS was determined according to the United States Preventive Services Task Force recommendations19 and included: age 55 to 80, at least 30 pack-years of smoking, and no personal history of lung cancer. Enrollment was limited to current smokers. Potential participants were identified electronically by either having an order placed by their provider for LCS or evidence in the electronic health record of LCS eligibility per age and smoking history. LCS eligibility was then confirmed on study enrollment. All participants provided written informed consent. Baseline data collection occurred between November 2016 and October 2019.
Measures and Survey Procedures:
Participants completed data collection by phone including tobacco use history, demographics, and medical history including a self-reported Charlson comorbidity index (CCI). The CCI was developed to estimate 10-year mortality on the basis of chronic comorbid disease and includes 19 conditions.20 We utilized a version adapted for survey administration.21 Participants were asked several questions pertaining to respiratory symptoms and health status derived from the Short Form 12 Survey (SF-12)22 and the Chronic Obstructive Pulmonary Disease (COPD) Assessment Test.23 We selected three items from these scales most directly pertaining to EC and dyspnea (i.e. those items that most closely resembled the queries used by clinicians to assess a patient’s EC in clinic). An item specifying limitations due to dyspnea was included to differentiate limitations in activities that were unrelated to cardiopulmonary causes, such as due to musculoskeletal pain or mobility issues. Participants were asked: “Does your health now limit your ability to perform moderate activities such as vacuuming or moving a table? Does your health now limit your ability to climb several flights of stairs? How often are you short of breath?” Each response was assigned a numeric value from 0 to 2, with zero representing the best EC/least symptoms.
Outcome:
Participants were grouped into three categories of EC based on their summed responses to the three items: good (0–1), intermediate (2–4), and poor (5–6).
Predictors:
Predictors included heaviness of smoking and comorbidities expected to impact EC or with a prevalence of at least 10% in our population, which included: COPD, cardiovascular disease (CVD), congestive heart failure (CHF), diabetes, CCI (categorized into quartiles), history of localized cancer, pack-years, cigarettes per day, and key combinations of comorbidities. Pack-years were computed by calculating the years smoked based on age at initiation subtracting both longest quit time and peak smoking time, then multiplying years smoked at peak by peak cigarettes per day and adding years smoked times usual cigarettes per day. For statistical analysis, pack-years was divided into two levels by the median value. Current cigarettes per day were measured by patient report.
Statistical analysis:
Baseline characteristics were summarized by frequencies and percentages for categorical items and means and standard deviations (SD) for quantitative variables. Key demographics, heaviness of smoking, and specific comorbidities were reported by EC category. Logistic regressions were fit to assess the association between the predictors and being in the poorest EC category. We fit separate logistic models for each of these comorbidities and key combinations. Each regression was adjusted for race (white or minority race), gender, age in years and education (high school or less, some college or college graduate). Pack-years, cigarettes smoked/day and history of cancer were excluded from the final models due to lack of association, and diabetes was excluded due to colinearity with other variables. We created a final adjusted model including each comorbidity and CCI. Results were reported as estimated odds ratios with 95% confidence intervals. All statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). All statistical tests were two-sided; p-values less than 0.05 were considered statistically significant. Given that some participants were eligible but did not have an active LCS referral, we completed a sensitivity analysis restricted to those with a current referral for LCS.
Results:
660 patients completed baseline data collection (Figure 1). Participants were primarily male (64.4%), white (89.5%), and had a mean age of 64.5 years. Most participants had income less than $65,000 and less than a bachelor’s degree of education. Participants were heavy smokers with a mean of 50.9 pack-years of smoking, currently smoking a mean of 17.6 cigarettes per day. Co-morbidities were common, with 33% of participants reporting COPD, 8% CHF and 24% CVD. Most participants reported a Charlson comorbidity index >2. 73.8% had an active referral for LCS. (Table 1).
Figure 1:
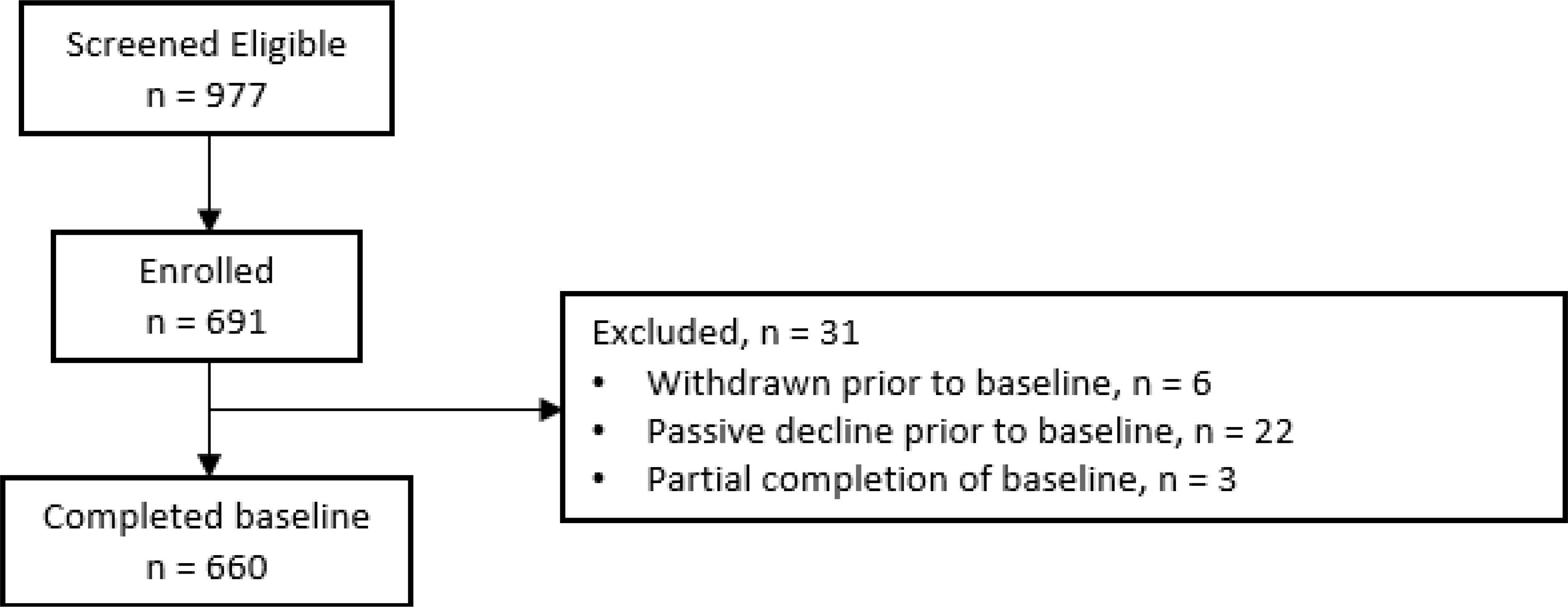
Results of patient selection
Table 1:
Baseline Characteristics of participants, current smokers eligible for lung cancer screening (N=660)
| Characteristic | n (%) |
|---|---|
|
| |
| Age (years), mean (SD) | 64.4 (5.8) |
| Gender, n (%) | |
| Female | 231 (35.0) |
| Male | 429 (65.0) |
| Hispanic ethnicity, n (%) | 6 (0.9) |
| Race, n (%) | |
| Other | 12 (1.8) |
| Black | 33 (5.0) |
| White | 583 (89.0) |
| More than one race | 27 (4.1) |
| Education, n (%) | |
| High school or less | 229 (34.7) |
| Vocational training | 71 (10.8) |
| Associates degree/Some college | 214 (32.4) |
| Undergraduate/graduate degree | 145 (22.0) |
| Married/Domestic partner, n (%) | 294 (44.8) |
| Household income*, n (%) | |
| <$15,000 | 85 (14.1) |
| $15,000 – $34,999 | 142 (23.6) |
| $35,000 – $64,999 | 178 (29.5) |
| >$65,000 | 198 (32.8) |
| Cigarettes per day, mean (SD) | 17.6 (8.6) |
| Pack-years of smoking, mean (SD) | 50.7 (23.1) |
| Comorbidities, n (%) | |
| COPD | 216 (32.9) |
| Cardiovascular Disease | 158 (24.0) |
| Congestive Heart Failure | 54 (8.2) |
| History of Localized Cancer | 73 (11.2) |
| Diabetes | 117 (17.8) |
| Charlson Comorbidity Index | |
| 1–2 | 237 (36.8) |
| 3–4 | 226 (35.1) |
| 5 or more | 181 (28.1) |
| Referred for Lung Cancer Screening | 487 (73.8) |
Abbreviations: COPD: Chronic obstructive pulmonary disease
57 participants were missing household income. 16 were missing Charlson Index. Other variables had <2% missingness.
Assessing the individual items, almost a third of subjects (31.1%) reported “a lot” of limitation in climbing several flights of stairs, and 28.5% reported “a lot” of limitation in performing moderate daily activities such as vacuuming, while 20.7% reported feeling out of breath all or most of the time (Figure 2). Only 20.1% of subjects reported no limitations in any category, while 8.3% reported the maximum amount of limitation for all three items (Figure 3).
Figure 2:
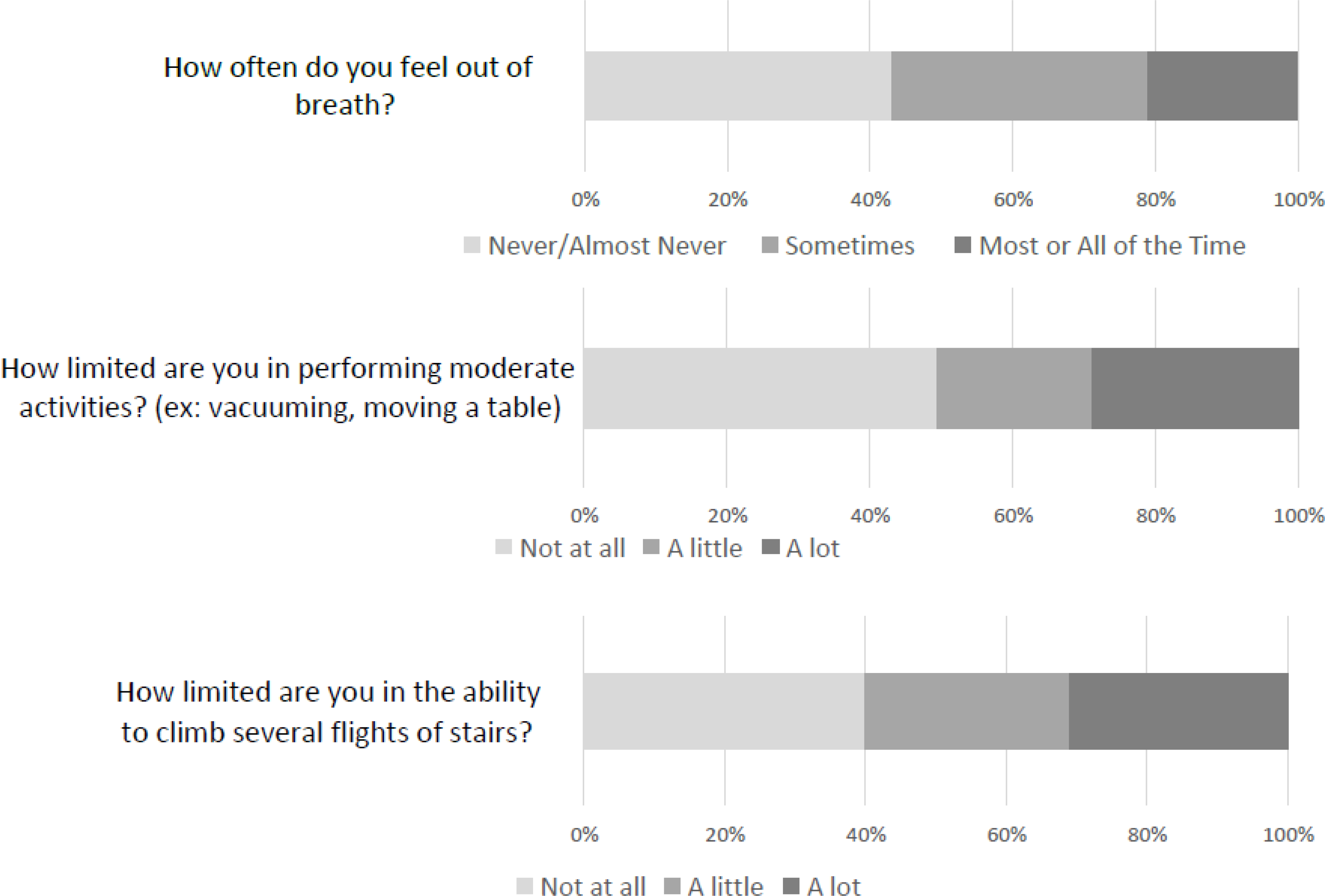
Distribution of responses to items assessing exercise capacity among smokers eligible for lung cancer screening
Figure 3:
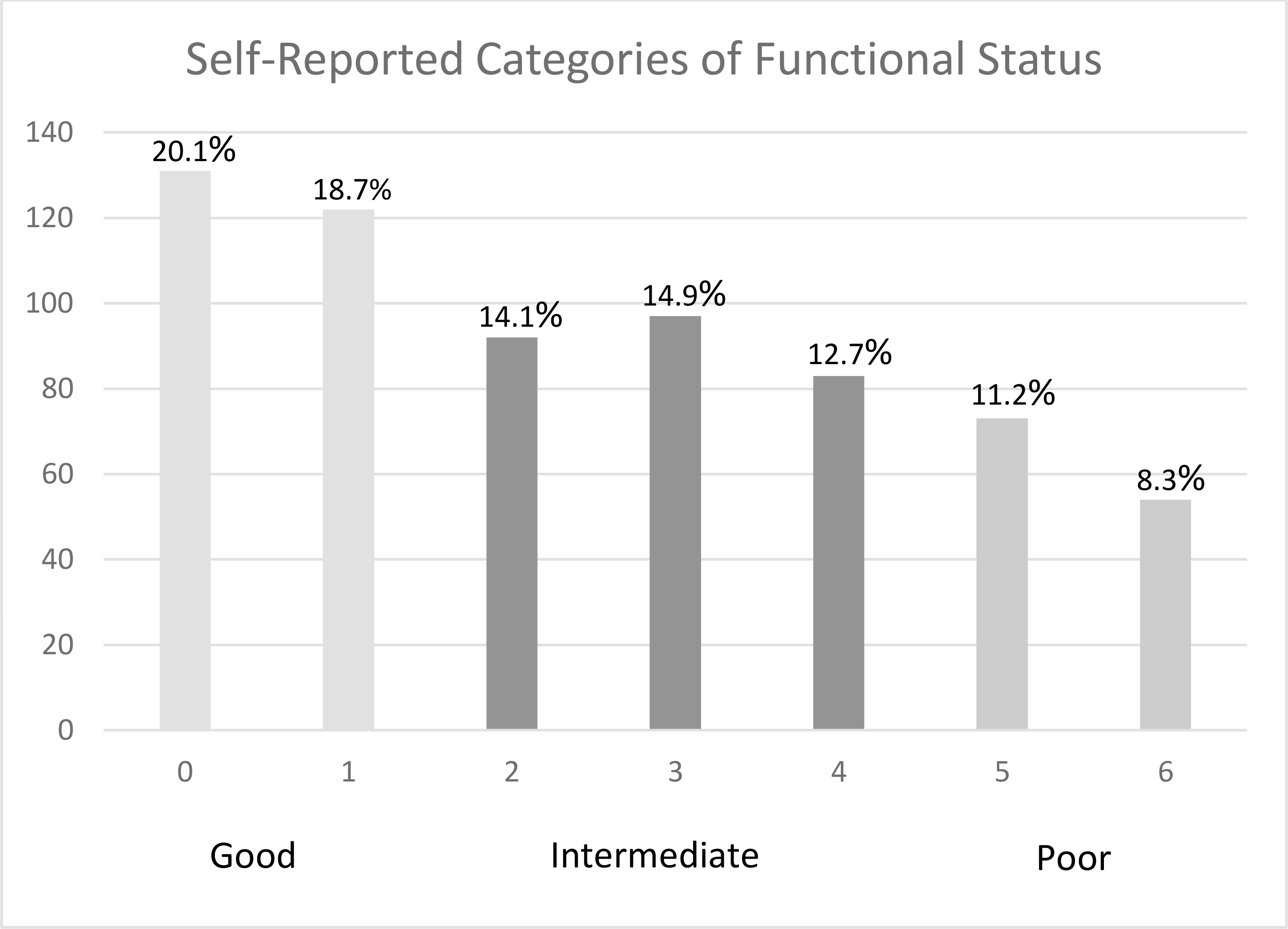
Frequency of self-reported exercise capacity categories among smokers eligible for lung cancer screening
EC score was available for 659 patients. The overall distribution of EC categories was: good 39.0% (257/659), intermediate 41.6% (274/659) and poor 19.4% (128/659). Qualitatively, the distribution of EC varied by the presence of comorbidities, where patients with comorbidities more frequently reported poor or intermediate EC. Among patients with none of COPD, CHF or CVD, a majority of patients (52.1%) reported good EC, while just 9.3% reported poor EC. Among subjects with COPD, 36.2% reported poor EC while only 18.8% reported good EC, with a similar distribution among subjects with CHF. Subjects with multiple comorbidities in combination with COPD more frequently reported poor EC. Among subjects with both COPD and CHF, no subjects reported good EC and over half (57%) reported poor EC. Among subjects with COPD and CVD, only 9.5% reported good EC and 44.4% reported poor EC. Patients with higher CCI were modestly more likely to report poor EC (29.3% of 5+ vs 11% of 1–2) as were patients with diabetes (29.1% poor EC vs 31% good EC). History of localized cancer showed little association with EC. The distribution of good and intermediate EC varied somewhat with pack-years of smoking and current cigarettes per day, but neither of these variables was associated with poor EC (Table 2).
Table 2:
Distribution of categories of functional capacity among smokers eligible for lung cancer screening, stratified by comorbidities and pack-years of smoking (n=659)
| Variable | Functional score (mean, SD) | Good* N (%) | Intermediate N (%) | Poor N (%) |
|---|---|---|---|---|
|
| ||||
| Overall | 2.5 (1.9) | 253 (38.8) | 272 (41.6) | 127 (19.4) |
| No COPD, CHF or CVD (n=334) | 1.8 (1.7) | 174 (52.1) | 129 (38.6) | 31 (9.3) |
| COPD (n=213) | 3.5 (1.9) | 40 (18.8) | 96 (45.1) | 77 (36.2) |
| CHF (n=54) | 3.3 (1.9) | 11 (20.4) | 25 (46.3) | 18 (33.3) |
| CVD (n=158) | 3.0 (1.9) | 45 (28.5) | 69 (43.7) | 44 (27.9) |
| History of localized Cancer (n=73) | 3.0 (1.9) | 20 (27.4) | 37 (50.7) | 16 (21.9) |
| Diabetes (n=117) | 3.1 (1.9) | 31 (26.5) | 52 (44.4) | 34 (29.1) |
| COPD+CHF (n=23) | 4.5 (1.2) | 0 (0.0) | 10 (43.5) | 13 (56.5) |
| COPD+CVD (n=63) | 3.9 (1.7) | 6 (9.5) | 29 (46.0) | 28 (44.4) |
| CVD+CHF (n=34) | 3.2 (1.9) | 8 (23.5) | 15 (44.1) | 11 (32.4) |
| COPD+CHF+CVD (n=14) | 4.4 (1.2) | 0 (0.0) | 6 (42.9) | 8 (57.1) |
| Charlson Comorbidity Index | ||||
| 1–2 (n=237) | 1.8 (1.8) | 124 (52.3) | 87 (36.7) | 26 (11.0) |
| 3–4 (n=226) | 2.5 (1.9) | 89 (39.4) | 92 (40.7) | 45 (19.9) |
| 5+ (n=181) | 3.2 (1.9) | 42 (23.3) | 86 (47.5) | 53 (29.3) |
| Pack-years | ||||
| ≤47 (n=320) | 2.3 (1.9) | 144 (45.0) | 117 (36.6) | 59 (18.4) |
| >47 (n=323) | 2.7 (1.9) | 106 (32.8) | 152 (47.1) | 65 (20.1) |
Abbreviations: COPD: chronic obstructive pulmonary disease, CVD: Cardiovascular disease, CHF: Congestive heart failure
Good was defined as score of 0 to 1, Intermediate as 2 to 4 and Poor as 5 to 6.
In adjusted logistic regression models for each individual comorbidity, when compared to those without the comorbidity, subjects with COPD (OR 4.62 95%CI 3.05–7.02), were the most likely to report poor EC, followed by CHF (OR 3.07, 95%CI 1.62–5.82), CVD (OR 2.24, 95% CI 1.4503.47), and diabetes (OR 2.17 95% CI 1.35–3.48). Higher CCI was also associated with poor EC (OR 2.48 95% CI 1.83–3.35), while history of localized cancer showed no association. When compared to those without COPD and CHF, patients with the combination of COPD and CHF were the most likely to report poor EC (OR 7.42, 95% CI 3.08–17.89), with no significant additive effect of the addition of CVD. (Table 3a). In a model including COPD, CHF, CVD, and CCI, only COPD and CCI remained significantly associated with poor EC.
Table 3:
Adjusted associations of key medical comorbidities and multi-morbidity with poor exercise capacity among smokers eligible for lung cancer screening (n=647)
| Comorbidity | Odds Ratio | (95% CI) | P-value |
|---|---|---|---|
|
| |||
| 3a. Separate logistic regression models for each individual comorbidity or combination* | |||
|
| |||
| COPD | 4.62 | 3.05, 7.02 | <0.001 |
| CHF | 3.07 | 1.62, 5.82 | 0.001 |
| CVD | 2.24 | 1.45, 3.47 | <0.001 |
| Localized Cancer | 1.21 | 0.65, 2.26 | 0.61 |
| Diabetes | 2.17 | 1.35, 3.48 | 0.001 |
| COPD+CHF | 7.42 | 3.08, 17.89 | <0.001 |
| COPD+CVD | 4.46 | 2.55, 7.80 | <0.001 |
| CVD+CHF | 2.87 | 1.32, 6.26 | 0.008 |
| COPD+CHF+CVD | 7.23 | 2.39, 21.86 | 0.001 |
| CCI (categorical) | 2.48 | 1.83, 3.35 | <0.001 |
|
| |||
| 3b. Final logistic regression model including comorbidities and Charlson Comorbidity Index* | |||
|
| |||
| COPD | 3.54 | 2.23, 5.62 | <0.001 |
|
| |||
| CHF | 1.94 | 0.94, 3.98 | 0.072 |
|
| |||
| CVD | 1.31 | 0.76, 2.25 | 0.325 |
|
| |||
| CCI (categorical) | 1.58 | 1.08, 2.31 | 0.019 |
Adjusted for age, sex, race (white vs minority race), educational attainment. Diabetes excluded from final model due to colinearity with CCI. Localized cancer excluded due to lack of association with exercise capacity.
Abbreviations: COPD: Chronic obstructive pulmonary disease, CHF: Congestive heart failure, CVD: cardiovascular disease, CCI: Charlson Comorbidity Index
In sensitivity analysis restricted to patients referred for LCS, inference and magnitude of associations were very similar for the models of each individual comorbidity. In the fully adjusted model, CHF was significantly associated with poor EC (OR 2.38, 95% CI 1.07–5.32) though CCI was not (OR 1.3, 95% CI 0.81–2.10).
Discussion:
Within this cohort of smokers eligible for LCS, the prevalence of dyspnea and limitations in self-reported EC was high, and poor EC was strongly associated with medical comorbidities, primarily COPD. Many patients reported limitations in stair climbing, performance of moderate activities, and shortness of breath throughout the day, with 1 in 12 reporting the highest limitations for all three measures. In particular, the combination of COPD and CHF was very strongly associated with poor EC, confirming that multimorbidity plays a role in limited EC in older LCS-eligible smokers.
Several LCS guidelines explicitly state that participants be “in good health”24 without comorbidities that limit life expectancy, and able to tolerate diagnostic procedures for screen-detected findings and treatment for early stage lung cancer.25 However, there is no clear consensus as to what constitutes an adequate health status to participate in LCS, as most of these statements are qualitative. In general, routine cancer screening is recommended for patients with at least a 10 year life expectancy.26 Considering that only about a quarter of patients with CHF survive to 10 years, comorbidities are clearly important to consider when conducting SDM visits for LCS.27
Due to shared risk factors of age and smoking, COPD was highly prevalent in this study population. COPD presents a conundrum with respect to LCS eligibility, as it both independently increases the likelihood of developing lung cancer,28 but also decreases the likelihood of long term survival after treatment,29 and also impacts surgical candidacy. The combination of CHF and COPD conferred the highest likelihood of poor EC. Each condition contributes to limited aerobic exercise capacity through different physiologic mechanisms that are additive. Indeed no patients with both conditions reported a good EC and most reported a poor one. COPD and other tobacco and age-related conditions present a competing risk of death that may limit the benefit of the screening intervention.
Beyond the association with comorbidities, EC is an important indicator of health status and is an independent predictor of life expectancy. In some studies of patients with COPD, the presence of dyspnea with activities was more strongly predictive of mortality than FEV1.30 This is important as COPD is significantly under-diagnosed in the lung cancer screening population. Various measures of functional and exercise capacity have been found to be independently predictive of death among patients with heart failure,31 lung cancer,32 COPD,33 and overall.34 Unlike medical diagnoses, EC, whether measured by self-report or formal testing, is not routinely recorded in medical settings. Therefore, it likely does not play a large role in the shared decision-making process, but may still be a relevant factor for patients to consider when deciding to be screened. Shared decision-making as described by the Centers for Medicare and Medicaid decision memo requires a documented visit which includes discussion of eligibility, benefits and harms, and “the impact of comorbidities and ability or willingness to undergo diagnosis and treatment” prior to furnishing an order.35 EC may need to be considered in this context. Professional organizations have called for better integration of health status into SDM, including the need for better research in this area.36 Specific guidance on the impact of EC or comorbidities that should prompt more specific assessment of EC would be helpful.
While lobectomy remains the gold-standard treatment for early stage lung cancer, SBRT is increasingly used for non-operative candidates.37 SBRT has yet to be proven equally effective when compared to lobectomy,7 though trials are ongoing. The use of SBRT is highly relevant when considering LCS in populations with high rates of comorbidity and poor EC. Many patients even with severe COPD, CHF or major exercise limitations can undergo SBRT with relative safety. However, including these patients in a lung cancer screening program may not be appropriate on a wider population level. Patients with higher comorbidity burden who are ineligible for surgery have significantly higher short and long-term mortality rates following lung cancer treatment and were very underrepresented in the NLST, whose participants were relatively healthy.38 These competing risks are nuanced and are challenging to consider during a brief shared decision-making encounter. Ideally, when completing the shared decision-making process, a clinician would weigh each of these factors and make an informed recommendation to the patient, which would then allow a patient to make a decision that is congruent with their values and the medical facts. The USPSTF has recommended expanding LCS to younger patients with lighter smoking history, which may shift the LCS population towards younger, healthier patients.39 Future work should focus on assessing the impact of comorbidity, multimorbidity and EC on LCS outcomes to help inform the process of shared decision-making and future LCS guidelines.
Our study has limitations. Reports of dyspnea and exercise limitation were by self-report and did not provide information as to why patients were limited, for example due to musculoskeletal issues. We attempted to improve specificity by including a separate item on dyspnea. The strong association of our measures with medical comorbidities suggests these items are valid. We would typically expect patients to over-report EC, therefore self-report is likely to underestimate the true prevalence of poor EC.40 Our study population was limited to current smokers who were willing to quit smoking within the next three months, which may impact generalizability. We found no significant associations with pack-years of smoking or heaviness of smoking and poor EC. Older former smokers with heavy tobacco use history, such as in the LCS population, are likely to have a similar distribution of medical comorbidities and symptoms, and at least half of screen-eligible patients are current smokers. Comorbidities were measured by self-report, and some patients may not be fully aware of their medical history. Finally, our population were majority male and white, which may limit generalizability outside of these populations.
Conclusion:
In summary, many individuals who smoke and are eligible for LCS report significant dyspnea and limitations in EC. This has important implications for LCS programs and medical systems, underscoring the need to offer LCS to patients who are likely to benefit from it. More research is needed to clarify whether the benefits of LCS are maintained for sicker patients, and how assessments of EC can be incorporated into eligibility assessments and shared decision-making for lung cancer screening.
Poor exercise capacity is common among individuals who smoke and are eligible for lung cancer screening, with only 39% reporting good exercise capacity.
Poor exercise capacity is strongly associated with chronic obstructive pulmonary disease and the presence of multiple comorbid conditions including congestive heart failure.
More research is needed as to how exercise capacity should be considered when completing shared decision-making for lung cancer screening.
Acknowledgements:
AMJ had full access to all of the data takes responsibility for the content and accuracy of the manuscript including data and analysis. ACM completed primary drafting of the manuscript and contributed to study design, analysis and interpretation of the results. AB, KS, SSF, AJR, DMV, and BJL contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript. BJL completed primary analysis. All authors approved the final manuscript.
The Department of Veterans Affairs and the National Cancer Institute did not have a role in the conduct of the study, in the collection, management, analysis, interpretation of data, or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
Work was funded by NCI R01CA141527 (Adaptive Interventions for Smoking Cessation in Lung Cancer Screening Programs). Dr. Melzer is supported by a VA HSR&D CDA HX003067-01A1.
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Medical Center, Minneapolis/USA.
Funding:
Work was funded by NCI R01CA141527 (Adaptive Interventions for Smoking Cessation in Lung Cancer Screening Programs). Dr. Melzer is supported by a VA HSR&D CDA HX003067-01A1. Work was also supported by resources from the Minneapolis VA Health Care System.
Footnotes
The authors report no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
A portion of the findings were presented virtually at the American Thoracic Society Virtual Meeting, 9/2020.
Clinicaltrials.gov registration NCT02597491.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ou S-HI, Zell JA, Ziogas A and Anton-Culver H. Prognostic factors for survival of stage I nonsmall cell lung cancer patients. Cancer. 2007; 110: 1532–41. [DOI] [PubMed] [Google Scholar]
- 3.Rivera MP, Tanner NT, Silvestri GA, et al. Incorporating Coexisting Chronic Illness into Decisions about Patient Selection for Lung Cancer Screening. An Official American Thoracic Society Research Statement. Am J Resp Crit Care Med. 2018; 198: e3–e13. [DOI] [PubMed] [Google Scholar]
- 4.Guidelines on the selection of patients with lung cancer for surgery. Thorax. 2001; 56: 89–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai H, Natt B, Kim S and Bime C. Decreased In-Hospital Mortality after Lobectomy Using Video-assisted Thoracoscopic Surgery Compared with Open Thoracotomy. Ann Am Thorac Soc. 2017; 14: 262–6. [DOI] [PubMed] [Google Scholar]
- 6.Iñiguez CEB, Armstrong KW, Cooper Z, et al. Thirty-day mortality after lobectomy in elderly patients eligible for lung cancer screening. Ann Thorac Surg. 2016; 101: 541–6. [DOI] [PubMed] [Google Scholar]
- 7.Bryant AK, Mundt RC, Sandhu AP, et al. Stereotactic body radiation therapy versus surgery for early lung cancer among US veterans. Ann Thorac Surg. 2018; 105: 425–31. [DOI] [PubMed] [Google Scholar]
- 8.Bolliger CT, Jordan P, Solèr M, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Resp Crit Care Med. . 1995; 151: 1472–80. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli A, Refai M, Xiume F, et al. Performance at symptom-limited stair-climbing test is associated with increased cardiopulmonary complications, mortality, and costs after major lung resection. Ann Thorac Surg. 2008; 86: 240–7; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JL, Antman EM, Harold JG, et al. Clinical practice guidelines on perioperative cardiovascular evaluation: collaborative efforts among the ACC, AHA, and ESC. Circulation. 2014; 130: 2213–4. [DOI] [PubMed] [Google Scholar]
- 11.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 12.Clark JM, Marrufo AS, Kozower BD, et al. Cardiopulmonary testing before lung resection: what are thoracic surgeons doing? Ann Thorac Surg. 2019; 108: 1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non–small cell lung cancer J Thorac Cardiovasc Surg. 2002; 123: 280–7. [DOI] [PubMed] [Google Scholar]
- 14.Healthcare.gov. Preventive Care Benefits for Adults. https://www.healthcare.gov/preventive-care-adults/ (Accessed May 5,2021)
- 15.Howard DH, Richards TB, Bach PB, Kegler MC and Berg CJ. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer. 2015; 121: 4341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young RP, Duan F, Chiles C, et al. Airflow limitation and histology shift in the National Lung Screening Trial. The NLST-ACRIN cohort substudy. Am J Resp Crit Care Med. 2015; 192: 1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melzer AC, Begnaud A, Schertz KL, et al. Functional Status Among Current Smokers Referred for Lung Cancer Screening: Distribution and Association with Key Comorbidities. C99 LUNG CANCER SCREENING - INSIGHTS ON RISK ASSESSMENT, COMORBIDITIES, AND ACCESS. p. A6001–A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu SS, Rothman AJ, Vock DM, et al. Program for lung cancer screening and tobacco cessation: Study protocol of a sequential, multiple assignment, randomized trial. Contemp Clin Trials. 2017; 60: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014; 160: 330–8. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry S, Jin L and Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005; 43: 607–15. [DOI] [PubMed] [Google Scholar]
- 21.Katz JN, Chang LC, Sangha O, Fossel AH and Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996; 34: 73–84. [DOI] [PubMed] [Google Scholar]
- 22.Huo T, Guo Y, Shenkman E and Muller K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: a report from the wellness incentive and navigation (WIN) study. Health Qual Life Outcomes. 2018; 16: 34-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones P, Harding G, Wiklund I, Berry P and Leidy N. Improving the process and outcome of care in COPD: development of a standardised assessment tool. Prim Care Respir J. 2009; 18: 208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wender R, Fontham ET, Barrera E, et al. American Cancer Society lung cancer screening guidelines. Cancer. 2013; 63: 106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzone PJ, Silvestri GA, Patel S, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2018; 153: 954–85. [DOI] [PubMed] [Google Scholar]
- 26.Salzman B, Beldowski K and de la Paz A. Cancer Screening in Older Patients. Am Fam Physician. 2016; 93: 659–67. [PubMed] [Google Scholar]
- 27.Taylor CJ, Ordóñez-Mena JM, Roalfe AK, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ. 2019; 364: l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durham AL and Adcock IM. The relationship between COPD and lung cancer. Lung cancer. 2015; 90: 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W, Dou S, Dong W, et al. Impact of COPD on prognosis of lung cancer: from a perspective on disease heterogeneity. Int J Chron Obstruct Pulmon Dis. 2018; 13: 3767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura K, Izumi T, Tsukino M and Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002; 121: 1434–40. [DOI] [PubMed] [Google Scholar]
- 31.Grodin JL, Hammadah M, Fan Y, Hazen SL and Tang WHW. Prognostic Value of Estimating Functional Capacity With the Use of the Duke Activity Status Index in Stable Patients With Chronic Heart Failure. J Card Fail. 2015; 21: 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung cancer. 2012; 76: 248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowen JB, Votto JJ, Thrall RS, et al. Functional Status and Survival Following Pulmonary Rehabilitation. Chest. 2000; 118: 697–703. [DOI] [PubMed] [Google Scholar]
- 34.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011; 305: 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439 N). Baltimore, MD: Centers for Medicare and Medicaid, U.S. Department of Health and Human Services. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.Accessed 20 Oct 2017. [Google Scholar]
- 36.Rivera MP, Tanner NT, Silvestri GA, et al. Incorporating Coexisting Chronic Illness into Decisions about Patient Selection for Lung Cancer Screening. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018; 198: e3–e13. [DOI] [PubMed] [Google Scholar]
- 37.Hobbs CJ, Ko SJ, Paryani NN, et al. Stereotactic Body Radiotherapy for Medically Inoperable Stage I-II Non-Small Cell Lung Cancer: The Mayo Clinic Experience. Mayo Clin Proc. 2018; 2: 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanner NT, Dai L, Bade BC, Gebregziabher M and Silvestri GA. Assessing the Generalizability of the National Lung Screening Trial: Comparison of Patients with Stage 1 Disease. Am J Resp Crit Care Med. 2017; 196: 602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021; 325: 962–70. [DOI] [PubMed] [Google Scholar]
- 40.Li MHG, Bolshinsky V, Ismail H, Ho K-M, Heriot A, Riedel B. Comparison of Duke Activity Status Index with cardiopulmonary exercise testing in cancer patients. J Anesth. 2018; 32: 576–84. [DOI] [PubMed] [Google Scholar]


