Abstract
Background
Cognitive impairment has been shown to predict falls risk in older adults. The ability to step accurately is necessary to safely traverse challenging terrain conditions such as uneven or slippery surfaces. However, it is unclear how well those with cognitive impairment can step accurately to avoid such hazards, and what specific aspects of cognition predict stepping ability in different patient populations.
Methods
Healthy older adults (NC), patients with Mild Cognitive Impairment with only memory impairment (MCI- EF) or memory and executive function impairments (MCI+ EF) and early Alzheimer's patients (AD) were timed as they performed a stepping accuracy test with increasing cognitive demand (Walking Trail Making Test; W-TMT) which required stepping on instrumented targets with either increasing sequential numbers (W-TMT A) or alternating sequential numbers and letters (W-TMT B).
Results
After accounting for age and baseline walking speed, AD and MCI+ EF were significantly slower than the NC and MCI-EF groups on the task with the highest cognitive demand, W-TMT B (interaction effect F=6.781, p<0.0001). No group differences were noted on the W-TMT A task which was less cognitively demanding. Neuropsychological measures of executive functioning were associated with slower W-TMT B performance, while memory, visual attention and visual spatial skills were not (Adjusted R2=0.42).
Conclusions
Executive function is important for stepping performance particularly under more complex environmental conditions.
Introduction
Patients with Alzheimer's disease (AD) are at higher risk for falling and suffer more serious injuries as compared to their healthy age counterparts (1, 2). Although extrapyramidal symptoms and general health status contribute to fall risk, dementia severity has also consistently been shown to be an independent factor (3, 4).
In healthy elderly adults, executive functions (e.g. attention control, working memory/problem solving) are important for successful completion of many balance and walking tasks including postural maintenance, obstacle avoidance, and ambulation (5, 6, 7), and are related to fall history (8), whereas other cognitive skills such as language and basic memory ability are less involved (5). It remains unclear whether a similar relationship between these cognitive domains and balance and walking exists in patients with significant cognitive impairment. Recent dual task studies have shown impairments in motor functions under divided attention conditions in patients with AD. Specifically, AD patients exhibit reduced walking speed (9, 10, 11) and gait variability (10) compared to healthy age matched controls. Hauer and colleagues (12) also found similar reduced divided attention performance during a postural stability task in AD patients as compared to older fallers with no cognitive impairment. These studies are suggestive of similar cognitive relationships to mobility in both healthy controls and patients with AD, at least early on in the disease process.
Attempting to isolate specific cognitive factors that impact mobility performance in AD can be difficult due to the global nature of deficits in this population. Declines in memory and language are hallmarks of AD, although recent studies have demonstrated that many AD patients also show executive functioning deficits early on in the disease (13). With the prevalence of impairments in multiple domains, it can be difficult to determine which cognitive factor is more important to gait in AD. One approach would be to compare groups of persons with different, circumscribed cognitive deficits to better understand how specific cognitive systems impact gait performance (5). Patients with Mild Cognitive Impairment (MCI), for example, do not meet criteria for dementia, yet have documented and often circumscribed cognitive deficits on testing, including MCI with purely amnestic disorder (MCI- EF) and those with additional deficits most often including executive functioning impairment (MCI+ EF).
Although an understanding of the role of specific aspects of cognition to mobility and increased fall risk is clearly important, it is only one piece of the puzzle. Situational demands are also key factors. An understanding of the interaction between cognitive and environmental demands will allow better identification of those individuals at highest risk of falling, as well as the conditions under which this will likely occur (5). Studies have shown that older adults with and without cognitive impairment have greater difficulty in comparison to young when engaging in more complex motor tasks such as avoiding obstacles or balancing on a raised beam (14, 15, 16). Declines in divided attention performance (i.e., walking while performing a simultaneous verbal task) may in part be mediated by an executive function (17, 18, 19). In order to explore the interaction of environment and cognition more fully, our group designed a stepping accuracy task with increasing cognitive demands: the Walking Trail Making Test (W-TMT) (20). These walkways were locomotor analogs of a standard neuropsychological measure, the Trail Making test (TMT) which has been shown to be a good predictor of fall-related injuries in community dwelling individuals (21) The TMT test was used as a model because it allows for a direct comparison between visual attention and search skills and executive/set shifting processes. Age differences on the walking trails have been demonstrated as the complexity of the walking task increases, with the greatest difference seen on the most complex walkway requiring executive set shifting skills (20).
The aim of this study was to examine performance on a stepping accuracy task with increasing cognitive demand in patient groups with different cognitive impairments (i.e., MCI, AD). We hypothesized that the MCI+ EF group would perform similarly to early AD subjects on the walkway tasks, while MCI – EF patients would perform similarly to healthy older individuals. Further, performance on the walkway tasks would be differentially related to cognitive measures of executive functioning, and not other cognitive domains.
Methods
Subjects
Three groups of community dwelling older adults were tested: 12 healthy older adults (NC), 26 patients with MCI and 15 patients with mild AD. All patients were diagnosed at the research consensus conference for the Michigan Alzheimer's Disease Research Center (MADRC), with MCI being diagnosed based on Peterson criteria (22). The MCI patients were further subdivided into those with only memory deficits (MCI - EF; n=15) and those with memory and executive function deficits (MCI + EF; n=11). The MCI groups were divided based on their performance on the Wisconsin Card Sorting task. Scores less than 1.0 standard deviations below a normative sample were considered indicative of impaired executive functioning.
All participants were originally identified from registries that allowed for pre-selection based on our inclusion and exclusion criteria. The NC group was recruited from the subject pool of the Claude Pepper Older Adults Independence Center, while the patient groups were recruited from the registry of the MADRC. Both the NC and MCI groups scored 24 or above on the Mini Mental State Examination (MMSE; 23) to rule out generalized cognitive impairment. AD patients were classified as having mild dementia (MMSE range 18-23). Subjects underwent history and medical examination by a nurse practitioner and had to ambulate without assistance, have no extrapyramidal signs, no significant musculoskeletal symptoms or limitations, visual disease or visual field defects, history of head injury with loss of consciousness over five minutes, seizures, transient ischemic attack, cardiac arrhythmias, diabetes or peripheral neuropathy, or medications with known deleterious effects on cognitive functioning. In addition, all patients were screened for other possible neurological disorders or extrapyramidal signs using the Unified Parkinson's Disease Rating Scale by an MADRC neurologist investigator as part of their original MADRC research evaluation, using a cut-off score of less than 5 for inclusion. Participants also were excluded if there was evidence of significant depressive symptoms (24). Three AD subjects were excluded from analyses due to problems understanding walkway instructions. Further, one MCI- EF and one MCI+ EF were excluded due to missing computer data. All participants were consented prior to the start of the study. This study was approved by the University of Michigan Medical Center Institutional Review Board.
Neuropsychological Measures
Neuropsychological tests were chosen to assess cognitive domains expected to play a role in gait performance. Executive functioning was assessed with Map Planning and Paper Folding (25). Map Planning assesses problem solving and action planning while Paper Folding measures aspects of spatial planning and mental flexibility that do not require a motor response. The more common executive measure, WCST, could not be used as an outcome measure, because group designations were made based on WCST performance. Significant correlations were found between WCST performance and both Map Planning (r = −.62) and Paper Folding (r = −.52). Visual short-term attention skills were assessed with the Corsi Block Forward Span (25). Benton Visual Form Discrimination (BVFD; 27) gave a measure of non-motor general spatial ability. Block Design gave a measure of visual motor performance (28). Efficiency in a motor-based dual task situation was assessed with the Bead Tapper (29) comparison of simultaneous tapping and sorting ability. Finally, memory functions were assessed with the delayed recall from the Word List Learning Test of the Wechsler Memory Scale-III (28). For all measures, higher scores reflected better performance.
Walkways
Five meter-long walkways, each equipped with 33 stepping targets, were used. Placement of the forefoot on a target with an accuracy of better than 2 cm was deemed ‘successful’ and activated an electrical circuit monitored by a computer's parallel port. Participants wore standardized flat-soled walking shoes. Subjects were instructed to successfully step on sequential targets with the requisite accuracy. For safety, all subjects wore a gait belt that a spotter could grab in the case of a stumble or fall. Subjects were told not to use the handrails provided alongside the walkway unless they felt unsteady and were in need of support. For a more detailed description of the walkways refer to Alexander et al. (20).
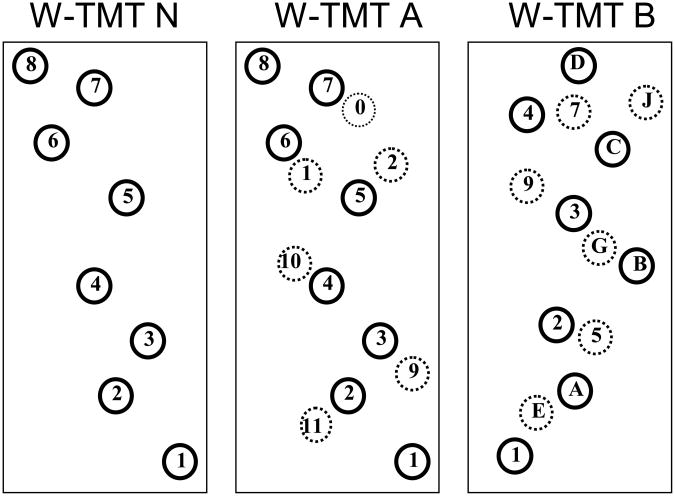
Three walkways of increasing complexity were used (Figure 1). Walking Trail Making Test-Numbers Only (W-TMT N) consisted of sequentially numbered circles (i.e. 1-2-3 etc.). W-TMT A was similar to W-TMT N with the exception of additional numbered distracters that required the participant to select the necessary path to walk. W-TMT B consisted of alternating number and letter targets incremented sequentially with similarly marked distracters. Participants were told to choose a path by alternating between the numbers and letters in order (i.e. 1-A-2-B-3-C etc.). In order to ensure an adequate data collection and minimize practice effects, two versions of W-TMT A and W-TMT B were used that differed in the pattern of placement of the numbers and letters (diagonal- and chevron-shaped configurations).
Figure 1.
Example Pathways of the Walking Trail Making Test.
Design
Baseline walking speed was first assessed by asking participants to walk up and down a walkway without any targets at a comfortable walking speed. Participants then were required to complete three trails on each of the W-TMT walkways. To ensure that participants understood basic task requirements, all groups first completed the W-TMT N trials. This was followed by randomization of the W-TMT A and W-TMT B walkways. Subjects were told to imagine that the walkway is an icy sidewalk and the numbered white dots are dry spots on which they can safely step without slipping. Consistent with the paper-and-pencil version of the TMT, accuracy was emphasized and errors were corrected by having participants go back and resume walking from the last correct response. In this way the time measure reflects errors. Neuropsychological measures were administered either on the same day or on a separate day following completion of the walkways.
Statistical Analyses
Repeated measures ANOVA (SAS Version 9.1) was used to examine performance differences among the four groups for the three walkways, with individual group comparisons completed by least squares means. Age and comfortable walking speed were used as covariates. Data analyses were repeated using a logarithmic transformation in order to address concerns about possible skewness in the distribution of the timed data. As these results were consistent with the initial analyses, only raw data are presented for ease of interpretation. In order to isolate the increased complexity of completing the W-TMT B as compared to W-TMT A walkways, a percent difference score was calculated using the formula : [(W-TMT B) – (W-TMT A)] / (W-TMT A) * 100. The relationship between specific aspects of cognitive functioning and walkway performance was examined by performing linear regression analyses using the neuropsychological measures as independent predictors and both the W-TMT-N condition and the percent difference score as the dependent variable with age and walking speed as fixed covariates. Age and walking speed were first forced into the regression model, followed by the neuropsychological variables. A p level of 0.05 was considered significant after Bonferroni corrections.
Results
Participant characteristics and neuropsychological function
Significant differences were found between the groups for age (see Table 1). Post hoc analyses showed that the AD group was significantly older than the NC and MCI- EF groups. As expected MMSE scores were significantly different with the AD group scoring lower than the three other groups, and the MCI+ EF group scoring significantly lower than the NC and MCI- EF groups. No education differences were found. Results of the neuropsychological variables are shown in Table 2. As expected the NC group performed better than the other groups for memory, while MCI+ EF and AD groups were worse on Map Planning compared to the NC and MCI- EF groups . AD patients scored lower than the other three groups for the BVFD.
Table 1.
Demographic Information for the four groups.
| NC (n=12, 7M, 5F) | MCI-EF (n=14, 10M, 4 F) | MCI+EF (n=10, 6M, 4 F) | AD (n=12, 9M, 3 F) | F, p value | |
|---|---|---|---|---|---|
| Age (years) | 70.0 ± 5.8 | 72.5 ± 4.6 | 75.1 ± 6.9 | 77.5 ± 5.3 | 4.0, p<.01* |
| Education (years) | 17.0 ± 2.6 | 16.57 ±3.2 | 15.8 ± 3.2 | 14.8 ± 2.9 | 1.2, n.s. |
| Mini Mental State Examination (MMSE) | 27.8 ± 2.2 | 26.6 ± 2.1 | 25.8 ± 2.0 | 22.6 ± 2.3 | 13.1. p<0.000† |
| Time for 10 m Walk (s) | 11.0 ± 2.0 | 11.7 ± 2.4 | 14.1 ± 2.5 | 14.2 ± 4.3 | 3.8 p<0.02‡ |
NC= Normal Controls
MCI-EF = Mild Cognitive Impairment with Isolated Memory problems
MCI+ EF= Mild Cognitive Impairment with Memory and Executive function deficits
AD = Alzheimer's Disease
AD >NC
AD < MCI+EF, MCI-EF, NC
AD, MCI+ EF< MCI-EF, NC
Table 2.
Means and standard deviations of the neuropsychological data for the four patient groups.
| NC | MCI -EF | MCI +EF | AD | F, p value | |
|---|---|---|---|---|---|
| Bead Tapper Percent Taps | 82.6±20.1 | 73.5±30.4 | 63.7±29.5 | 55.0±16.8 | 2.8, p<.06 |
| Paper Folding | 2.4±3.0 | 1.9±1.9 | 0.5±1.9 | 0.3±1.7 | 2.6, p<.07 |
| Map Planning | 7.2±2.5 | 6.5±2.5 | 2.9±1.9 | 2.7±1.2 | 13.4, p<.0001* |
| Block Design | 9.7±2.1 | 11.3±2.8 | 9.3±3.1 | 10.2±6.6 | 0.6, p = n.s. |
| Word List Delayed Recall | 7.6±2.1 | 2.3±2.0 | 2.3±3.2 | 0.3±0.5 | 25.0, p<.0001† |
| Corsi Block Forward Span | 6.2±2.3 | 5.8±2.0 | 6.1±2.9 | 4.8±1.8 | 0.9, p=n.s. |
| Benton Visual Form score | 29.5±2.9 | 29.1±2.3 | 28.2±4.9 | 22.9±5.7 | 6.1, p<.002‡ |
NC= Normal Controls
MCI-EF = Mild Cognitive Impairment with Isolated Memory problems
MCI+EF= Mild Cognitive Impairment with Memory and additional Executive function deficits
AD = Alzheimer's Disease
AD, MCI+EF < NC, MCI-EF
AD, MCI+EF, MCI-EF < NC; AD < MCI+EF, MCI-EF
AD < MCI+EF, MCI-EF, NC
Participant walking and stepping performance
Comfortable baseline walking speed showed that the AD and MCI+ groups walked significantly more slowly than the NC and MCI- EF subjects (Table 1). Comfortable walking speed was significantly correlated with the two measures of executive functioning: paper folding (r=−0.37, p<0.01) and map planning (r=−0.44, p<0.002).
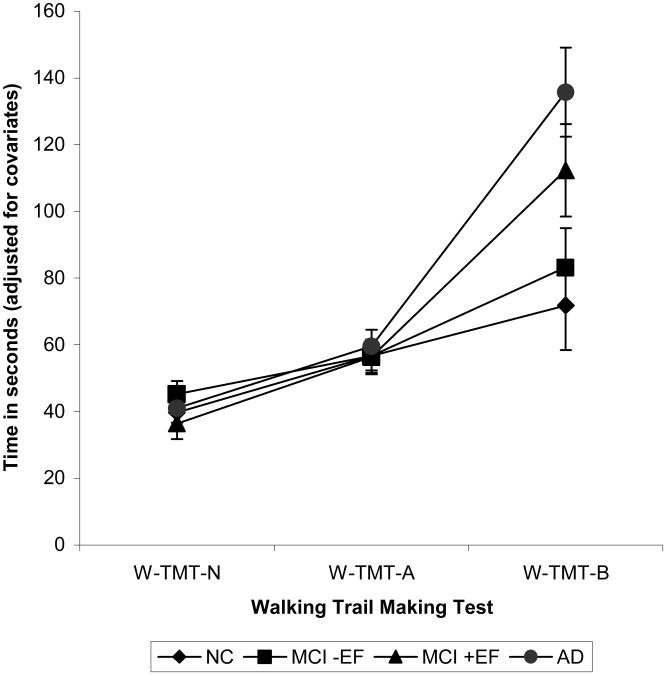
Times to complete the alternate versions for the W-TMT A and W-TMT B walkways were compared. As no significant differences were found between trials, data were averaged across all trials for each condition for use in the subsequent analyses. After covarying out comfortable walking speed and age, a significant main effect for walkway condition (F=106.16, p<0.0001) as well as a significant interaction between patient group and walkway type were found (F=6.78, p<0.0001; Figure 2). Post hoc comparisons, after controlling for the covariates such as comfortable walking speed, found no differences between the groups on average time to complete W-TMT N and W-TMT A. However, significant group differences were found for W-TMT B. Specifically, the AD group took longer in completing W-TMT B than both the NC (t=5.28, p<0.0001) and MCI- EF groups (t=4.66, p<0.0001). The MCI+ EF group was not significantly different from the AD group (t=1.8, p<0.08), but was significantly different from the MCI- EF group (t=−2.7, p<0.008) and the NC group (t=3.5, p<0.000). No differences were found between the NC and MCI- EF groups (t=1.07, p=0.29).
Figure 2.
Estimated Marginal Means and Standard Errors for the Walking Trail Making Test (W-TMT).
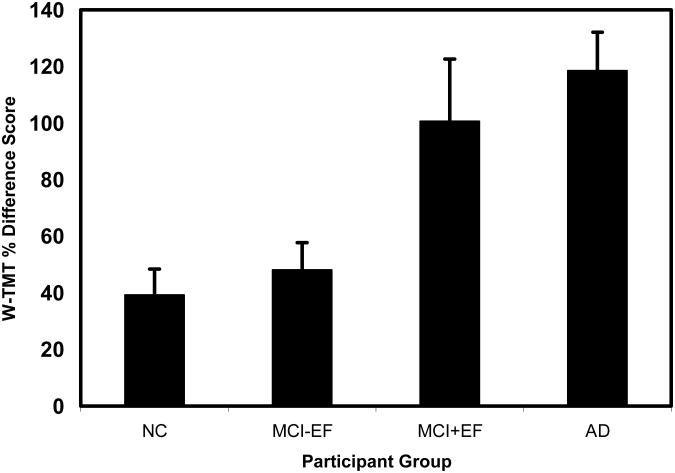
W-TMT percent different scores shown in Figure 3. Regression analyses using the neuropsychological measures as predictors of performance on the W-TMT-N did not find any relationship between cognitive functioning and walking performance on the simple walkway. When the executive component of the W-TMT B walkway (percent difference score) was used as a dependent variable in the regression analysis, results showed that only executive function tasks with a strong visual spatial planning component [map planning, t= −3.35, β= −0.584, p< .002 and paper folding, t= −2.18, β= 0.378, p< .04] were significantly associated with the percent difference score (adjusted R square=0.42). There was, however, a trend for basic visuospatial skills [BVFD, t= −1.87, β= −0.297, p< .08].
Figure 3.
Percent Difference Score for the four participant groups.
Discussion
Consistent with earlier findings, these results demonstrate the importance of executive functioning to successful balance and gait performance in both healthy and cognitively impaired older adults. The fact that the amnestic subtype of MCI (MCI- EF) did not differ in performance on any of the walkways from the healthy, cognitively intact individuals, suggests that memory functions do not play a significant role in successful gait performance in older adults in certain situations. In contrast, those MCI patients with additional executive dysfunction (MCI+ EF) performed quite similarly to the AD group. The difference in performance of the two MCI groups, which differ only in executive impairment, suggests that the increased fall risk in patients with cognitive impairment may be attributable more to executive dysfunction, than to memory deficits, at least early on in the disease process. Although the MCI+ EF and AD groups did not differ significantly in their times to complete W-TMT B, the AD group did take noticeably longer. This most likely reflects the more global nature of the AD participants' deficits, though they represented a relatively mildly impaired group. Indeed, the only area other than memory and executive functioning that the AD patients were found to differ significantly from the others was in their performance on a basic measure of visual spatial ability (BVFD). Given that performance on this task also was marginally related to overall performance on the W-TMT B, the addition of increased inefficiency in visual spatial ability for the AD patients may have contributed to their longer mean times on W-TMT B.
Consistent with earlier findings, executive functioning is associated with gait disturbances in older adults. This was seen for both comfortable walking speed as well as performance on the more complex walkway. After controlling for simple walking speed differences, the lack of group differences under any but the most complex walkway condition highlights the need to consider the interaction between individual cognitive functions and environmental demands in understanding the role of cognition in fall risk. Based on our data, when individuals with executive deficits are placed in complex situations that require some decision-making or mental flexibility, they may be at greater risk of a misstep from choosing the incorrect motor response. One may speculate that this places them at greater risk for a trip and fall. Understanding this relationship also will be important for the development of rehabilitation and other training programs to address ambulation under conditions requiring more cognitive control.
This study highlights two important points in the study of cognition and gait. First, specific cognitive factors can be evaluated by other means than the typical divided attention paradigm, namely by increasing the complexity of the gait task in measurable ways. Second, this study demonstrates the utility of testing patient groups with different cognitive profiles as another means of studying the relative role of specific cognitive abilities to mobility performance.
In summary, our findings highlight the critical importance of specific cognitive factors, and particularly executive functioning as opposed to global cognitive skills or other cognitive factors such as memory to mobility performance in older individuals even in patients with clinically documented cognitive concerns. The ability to predict which individuals may have more difficulty when confronted with complex walking tasks is relevant because the risk of falling is likely greater in unfamiliar conditions. Identifying patients earlier in the course of disease, such as MCI+ EF, would be crucial as these patients could potentially benefit more from intervention strategies designed to reduce falls. The findings from this study will need to be replicated with other patient populations that have different cognitive deficit profiles to further elucidate the role of executive functioning to mobility. In addition, although performance on our walkways may represent how individuals perform in increasingly more cognitively demanding situations, they are not representative of a typical daily ambulatory setting.
Acknowledgments
This research was supported in part by NIA R03 AG023239 (Giordani), the Michigan Alzheimer's Disease Research Center (MADRC) NIA P50-AG08671 (Gilman), a Claude Pepper Research Career Development Award (C. Persad) NIA AG024824, the Biomechanics Core of the Claude Pepper Geriatrics Center NIA AG024824 (Halter), NIA (AG109675) K24 Mid-Career Investigator Award in Patient-Oriented Research (Alexander), and the Department of Veterans Affairs Research and Development (Alexander). Partial results of this study were presented at the 1st Gait and Mental Function Conference in Madrid, Spain 2005. The authors thank Diane Scarpace for help in subject screening, Benjamin Long, Rebecca Reiten, and Stephanie Smith in data collection and Janet Kemp for technical assistance.
References
- 1.Morris JC, Rubin EH, Morris EJ, Mandel SA. Senile dementia of the Alzheimer's type: An important risk factor for serious falls. J Gerontol. 1987;42:412–417. doi: 10.1093/geronj/42.4.412. [DOI] [PubMed] [Google Scholar]
- 2.Walsh JS, Welch HG, Larson EB. Survival of outpatients with Alzheimer-type dementia. Ann Intern Med. 1990;113:200–204. doi: 10.7326/0003-4819-113-6-429. [DOI] [PubMed] [Google Scholar]
- 3.Brody EM, Kleban MH, Moss MS, Kleban F. Predictors of falls among institutionalized women with Alzheimer's disease. JAGS. 1984;32:877–882. doi: 10.1111/j.1532-5415.1984.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Kijk PTM, Meulenberg OGRM, van de Sande HJ, et al. Falls in dementia patients. Gerontologist. 1993;33:200–204. doi: 10.1093/geront/33.2.200. [DOI] [PubMed] [Google Scholar]
- 5.Giordani B, Persad C. Neuropsychological influences on gait in the elderly. In: Hausdorff J, Alexander N, editors. Gait Disorders – Evaluation and Management. Florida: Taylor & Francis Group; 2005. pp. 227–142. [Google Scholar]
- 6.Persad CC, Giordani B, Chen H, Ashton-Miller JA, Alexander NB, Wilson CS, Berent S, Guire K, Schultz AB. Neuropsychological predictors of complex obstacle avoidance in healthy older adults. J Gerontol Psy Sci. 1995;50:272–277. doi: 10.1093/geronb/50b.5.p272. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in he elderly as a complex cognitive task. Experimental Brain Research. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 8.Holtzer R, Friedman R, Lipton RB, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer's disease. Neurology. 1997;48:955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: Divided attention increased gait variability in Alzheimer's Disease. JAGS. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 11.Pettersson AF, Olsson E, Wahlund L. Effects of divided attention on gait in subjects with and without cognitive impairment. J Geriatr Psychiatry Neurol. 2007;20:58–62. doi: 10.1177/0891988706293528. [DOI] [PubMed] [Google Scholar]
- 12.Hauer K, Pfisterer M, Weber C, Wezler N, Kliegel M, Oster P. Cognitive impairment decreases postural control during dual tasks in geriatric patients with a history of severe falls. JAGS. 2003;51:1638–1644. doi: 10.1046/j.1532-5415.2003.51517.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer's disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- 14.Berg WP, Alessio HM, Mills EM, et al. Circumstances and consequences of falls in dependent community-dwelling older adults. Age Ageing. 1997;26:261–268. doi: 10.1093/ageing/26.4.261. [DOI] [PubMed] [Google Scholar]
- 15.Alexander NB, Mollo JM, Giordani B, Ashton-Miller JA, Schultz AB, Grunawalt JA, Foster NL. Maintenance of balance, gait patterns, and obstacle clearance in Alzheimer's disease. Neurology. 1995;45:908–914. doi: 10.1212/wnl.45.5.908. [DOI] [PubMed] [Google Scholar]
- 16.Chen HC, Schultz AB, Ashton-Miller JA, Giordani B, Alexander NB, Guire K. Stepping over obstacles: dividing attention impairs performance of old more than young adults. J Gerontol. 1996;51A:M116–M122. doi: 10.1093/gerona/51a.3.m116. [DOI] [PubMed] [Google Scholar]
- 17.Springer S, Giladi N, Peretz C, et al. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Movement Dis. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 18.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Movement Dis. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: The InCHIANTI Study. J Am Geriatri Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 20.Alexander NB, Ashton-Miller JA, Giordani B, Guire K, Schultz AB. Age differences in timed accurate stepping with increasing cognitive and visual demand: A walking trail making test. J Gerontol Med Sci. 2005;60:1558–1562. doi: 10.1093/gerona/60.12.1558. [DOI] [PubMed] [Google Scholar]
- 21.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: A prospective study. J Gerontol. 1991;5:M164–170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 22.National Alzheimer's Coordinating Center (NACC) Uniform Date Set (UDS) Appendix Version 1.2. Seattle: Dept of Epidemiology, School of Public Health and Community Medicine, University of Washington; 2006. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state. ” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Rose TL, Lapp D. Validity of the Geriatric Depression Scale in subjects with senile dementia. Palo Alto, CA: Clinical Diagnostic and Rehabilitation Unit, Veterans Administration Medical Center; 1981. [Google Scholar]
- 25.Ekstrom RB, French JW, Harman HH, Dermen D. Manual for Kit of Factor-Referenced Cognitive Tests. Princeton; New Jersey: Educational Testing Services; 1976. [Google Scholar]
- 26.Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 27.Benton AL, Hamsher KdeS, Varney NR, Spreen O. Contributions to Neuropsychological Assessment. New York: Oxford University Press; 1983. [Google Scholar]
- 28.Wechsler D. MS-III, Wechsler Memory Scale-Third Edition. New York: Psychological Corporation; 1997. [Google Scholar]
- 29.Talland G, Schwab RS. Performance with multiple sets in Parkinson's disease. Neuropsychologia. 1964;2:45–53. [Google Scholar]