Abstract
Anosognosia, or general lack of self-awareness, is often present following neurological injury and can result in poor functional outcomes. The specific phenomenon of intellectual awareness, the knowledge that a function is impaired in oneself, has not been widely studied in post-stroke aphasia. We aim to identify behavioral and neural correlates of intellectual awareness by comparing stroke survivors’ self-reports of anomia to objective naming performance and examining lesion sites. Fifty-three participants with chronic aphasia without severe comprehension deficits rated their naming ability and completed a battery of behavioral tests. We calculated the reliability and accuracy of participant self-ratings, then examined the relationship of poor intellectual awareness to speech, language, and cognitive measures. We used support vector regression lesion-symptom mapping (SVR-LSM) to determine lesion locations associated with impaired and preserved intellectual awareness. Reliability and accuracy of self-ratings varied across the participants. Poor intellectual awareness was associated with reduced performance on tasks that rely on semantics. Our SVR-LSM results demonstrated that anterior inferior frontal lesions were associated with poor awareness, while mid-superior temporal lesions were associated with preserved awareness. An anterior-posterior gradient was evident in the unthresholded lesion-symptom maps. While many people with chronic aphasia and relatively intact comprehension can accurately and reliably report the severity of their anomia, others overestimate, underestimate, or inconsistently estimate their naming abilities. Clinicians should consider this when administering self-rating scales, particularly when semantic deficits or anterior inferior frontal lesions are present. Administering self-ratings on multiple days may be useful to check the reliability of patient perceptions.
Keywords: anomia, aphasia, awareness, anosognosia, self-rating, voxel-based lesion symptom mapping
1. Introduction
Many people with impairments caused by brain injury lack awareness of the presence, extent, and/or consequences of their deficits. This phenomenon, called anosognosia, is most typically associated with dementia, traumatic brain injury, and right hemisphere stroke, and has been observed for impairments in motor, cognitive, language, affective and sensory systems (Shany-Ur et al., 2014; Tate et al., 2014; Vocat et al., 2010). Anosognosia can be present for all deficits, or can be deficit-specific (Cocchini et al., 2012; Hibbard et al., 1992). Although anosognosia for language deficits is classically associated with acute Wernicke’s Aphasia, reduced awareness of language impairments can occur in both fluent and non-fluent aphasia (Kertesz, 2010; Kinsbourne & Warrington, 1963; Rubens & Garrett, 1991). Anosognosia in aphasia may result in denial of language deficits, failure to repair errors, and/or poor compliance with treatment recommendations, such as using communication strategies. Moreover, poor awareness of deficits could lead to erroneous rating on self-reported measures of health-related quality of life used in aphasia research and in clinical care settings to determine patient needs. Understanding the nature of reduced awareness in aphasia is thus potentially clinically important, but at present, we have limited understanding of anosognosia in people living with aphasia.
Three types of self-awareness have been described in the literature, including: 1) intellectual awareness, the general knowledge that a physical, behavioral, or cognitive function is impaired in oneself, as well as the extent and implications of the impairment, 2) emergent awareness, the knowledge that an error is being made while it is occurring, and 3) anticipatory awareness, the foreknowledge that a mistake might be made and the ability to take steps to prevent it (Crosson et al., 1989). In this paper, we will operate from these definitions of awareness. Multiple theories on the cognitive processing of awareness exist. The Pyramid Model of Self Awareness proposes that the three aforementioned types of awareness are hierarchical, with intellectual awareness being a prerequisite to emergent awareness, and at least a degree of emergent awareness being necessary for anticipatory awareness to occur (Crosson et al., 1989). The Dynamic Comprehensive Model of Awareness suggests that the types of awareness are dynamic and influence each other (Toglia & Kirk, 2000). Recent research comparing intellectual and emergent awareness in clinical populations with varied neurological conditions supports the two relying on distinct processes (Dean et al., 2017; Goverover et al., 2014; Hoerold et al., 2013; O’Keeffe et al., 2007; but see Dockree et al., 2015). Other theories propose a more integrated whole-brain system in which awareness is supported by lower cognitive processes such as memory and executive functions (McGlynn & Schacter 1989; Stuss 1991b; see Sansonetti et al., 2021 for a review). The majority of research on self-awareness in aphasia has focused on emergent awareness, with minimal work on intellectual awareness, and none, to our knowledge, on anticipatory awareness.
Research on emergent awareness in aphasia has largely focused on detection of item-specific errors committed during speech, often referred to as error detection (Maher et al., 1994; Marshall et al., 1998; Oomen et al., 2001; Schuchard et al., 2017; Shuren et al., 1996). This work has demonstrated that people with aphasia can have impaired emergent awareness of their language skills, including speech (e.g. naming and repetition), reading, and writing. Debate remains over what causes impairments in emergent awareness of speech. Two main theories exist, one comprehension-based (Levelt et al., 1999; Roelofs, 2020) and the other production-based (Nozari, 2020; Nozari et al., 2011). The idea that impairments in emergent awareness of speech result from poor auditory comprehension is based on the model of speech production proposed by Levelt (Levelt, 1983; Levelt et al., 1991; Levelt et al., 1999; Roelofs, 2020) and was originally supported by case studies of persons with aphasia who had impaired auditory comprehension and impaired emergent awareness (Alajouanine, 1956; Alajouanine et al., 1964; Kinsbourne & Warrington, 1963; Maher et al., 1994; Shuren et al., 1996). However, several researchers (e.g., Nickels & Howard, 1995; Nozari et al., 2011) have noted a double dissociation between emergent awareness and auditory comprehension, including an individual with impaired auditory comprehension and intact emergent awareness (Marshall et al., 1985), as well as individuals with intact auditory comprehension and impaired emergent awareness (Butterworth & Howard, 1987; Marshall et al., 1998). As a result of this double dissociation and theoretical issues concerning the speed and capacity of comprehension processing (Blacfkmer & Mitton, 1991; Vigliocco & Hartsuiker, 2002; for a review see Nozari et al., 2011), some researchers have suggested that deficits in speech production better account for the deficits in emergent awareness of speech (Nozari, 2020; Nozari et al., 2011). Other researchers posit that somatosensory processes involved in motor speech aid in emergent awareness of speech through feedback on internal models for speech production (Guenther, 1994; Hickok, 2012; Tourville & Guenther, 2011). Notably, none of these proposals are mutually exclusive and impairment of more than one speech/language process may account for impairments of emergent awareness of speech (Postma, 2000). Indeed, poor performance on measures of speech production and speech comprehension have both been shown to systematically relate to impaired emergent awareness of speech in people with aphasia (Dean et al., 2017; Mandal et al., 2020; Nozari et al., 2011).
Few studies have examined intellectual awareness in aphasia, perhaps due to concerns about reliable measurement. Intellectual awareness in clinical populations is most often assessed via questionnaires that request patients to make self-judgments about their behavioral functioning. Most often, a discrepancy score between caregiver ratings and patient ratings is used to determine patient awareness. Some of these measures incorporate healthcare professionals’ ratings, either separately from caregiver ratings or in addition to them (Anderson & Tranel, 1989; Bivona et al., 2020; Kolakowsky-Hayner, 2010; Malouf et al., 2014; Sherer et al., 1998; see Mahoney et al., 2019 for a review). While multiple measures have been developed to assess intellectual awareness in acquired brain injury and dementia (Hallam et al., 2020; Mahoney et al., 2019), many studies exclude people with aphasia, possibly due to concerns that people with language impairments may not have the ability to understand the questions or provide responses on traditional measures of intellectual awareness (Bivona et al., 2020; Hoerold et. al, 2013; Orfei et al., 2009).
To our knowledge, only two studies have been published on intellectual awareness of language impairments in aphasia. Cocchini and colleagues (2010) developed the Visual-Analogue Test Assessing Anosognosia for Language Impairment (VATA-L) using visuals and comprehension check questions to allow people with aphasia to self-report directly on their perception of their deficits. Only 14% of stroke participants were excluded based on inaccurate responses to “check questions” implemented to ensure understanding of the questionnaire. The researchers found a highly significant correlation for test-retest measures in their stroke participant group, although they did not test reliability of self-report via an agreement metric. Approximately twenty percent of participants misestimated their language abilities, using caregiver ratings as the comparison metric. Dean and colleagues (2017) compared intellectual awareness (via the VATA-L) and emergent awareness (via online error correction), and also examined the relationship between awareness and specific language skills. Twenty-three percent of participants were unaware of their language deficits as measured by the VATA-L using caregiver ratings as the validating metric. Intellectual awareness related to production performance in this study, while emergent awareness related to comprehension skills.
In these two existing studies, intellectual awareness was assessed by comparing patient self-ratings with caregiver ratings. This approach is useful only to the extent that caregivers understand the impairment accurately. When comparing results of caregiver ratings to expert ratings, mixed results have been found. Cocchini and colleagues (2010) found high agreement between professional caregivers (i.e., therapists, nurses, medical doctors, etc.) and personal caregivers, but the professional caregivers were not limited to speech-language experts who have training on understanding language impairments. In a study comparing caregiver ratings of their family member’s language abilities to speech-language pathologists’ evaluations, personal caregivers rated their family member more positively than the functional language outcomes as assessed by speech-language pathologists (de Jong-Hagelstein et al., 2012). Furthermore, using caregiver ratings is only useful when a caregiver is available and active in a patient’s care, which is not a possibility for all patients. Subjective professional assessments of awareness can also pose limitations as they require the healthcare provider to acquire sufficient exposure to the patient in functional contexts in order to make an accurate judgment of self-awareness.
An alternative to using patient-carer or patient-professional discrepancy scores to assess intellectual awareness is to compare patient self-ratings to their behavioral performance. This method is utilized in the Assessment of Awareness of Disability (Tham et al., 1999), and has been used in some studies using performance on neuropsychological tests (Anderson & Tranel 1989; Wagner et al., 1997). The strength of this approach is that it requires no subjective ratings from a caregiver or healthcare professional. To our knowledge, no study has examined intellectual awareness in people with aphasia by comparing self-ratings of people with aphasia to their performance on quantitative measures of language impairment. This method may be useful in providing an accurate representation of the patient’s abilities for comparison to self-ratings to determine self-awareness of an impairment in aphasia.
Only a few studies have systematically investigated the neural basis of awareness in aphasia, and these have focused solely on emergent awareness. Mandal and colleagues (2020) conducted a support vector regression-based lesion-symptom mapping (SVR-LSM) analysis for spontaneous online error detection, a measure of emergent awareness. They found that impaired emergent awareness was associated with damage to frontal white matter tracts and the dorsolateral prefrontal cortex. Another study used electroencephalographic (EEG) to examine the neural basis of error monitoring in aphasia, although it did not investigate conscious awareness of errors. Riès and colleagues analyzed error-related negativities, a known EEG correlate of errors that is centered in the medial prefrontal cortex (Riès et al., 2013). They found that error related negativities were intact during naming in their cohort of individuals who had damage to the lateral prefrontal cortex. However, error-related negativities have not been consistently linked to conscious awareness of errors (see Wessel, 2012 for a review). To our knowledge, no studies have examined the neural correlates of intellectual awareness in aphasia.
In this study, we assess intellectual awareness by comparing participant self-ratings of anomia to actual confrontation naming performance. We focus here on awareness of anomia because anomia is the most commonly reported symptom in aphasia (Laine & Martin, 2006). Additionally, it is an easily quantifiable language measure, making it a viable skill to estimate for participants and to measure for researchers. In addition to accuracy of awareness, we investigate reliability of self-awareness between two separate days of testing to ensure consistency of self-reports. We then explore relationships of reduced awareness to speech, language, and cognitive abilities. We also test the relationship between spontaneous online error detection, a measure of emergent awareness, and intellectual awareness. In line with prior literature, we hypothesize that these two metacognitive abilities are dissociable. Finally, we utilize SVR-LSM to examine neural correlates of poor intellectual awareness.
2. Methods
2.1. Participants
Participants for this study were native English speakers with aphasia due to left-hemisphere stroke that occurred at least 6 months prior to enrollment into a larger study (Fama, et al., 2019a; Fama, et al., 2019b; Mandal et al., 2020, cohort 2). All participants provided informed consent as approved by the Institutional Review Board at Georgetown University. Sixty-five participants were initially enrolled. Twelve were excluded from the final study group, with two unable to complete testing, nine who did not meet the comprehension criterion of 48/60 on the Yes/No Questions subtest of the Western Aphasia Battery-Revised (Kertesz, 2007), and one who had near-floor performance on most tasks in the language battery despite meeting the comprehension criterion. The remaining 53 participants (22 female, 31 male) had an average age of 60.2 years (SD=9.8, range 40–80), average education of 16 years (SD=2.8, range 12–24), and mean time-since-onset of 5 years (SD=4.8, range 0.5–22.9). Forty-six participants were right-handed, six were left-handed, and one was ambidextrous. In addition to a left hemisphere stroke, six participants had evidence of a prior small, incidental stroke that was asymptomatic (i.e., one in the right putamen, two in the right cerebellum, one in the left cerebellum, and two in right hemisphere cortical areas). All participants presented with good single-word intelligibility as assessed by two certified speech-language pathologists (MEF, SFS) which was important in order to easily determine participants accuracy on naming items. Three participants did not complete the MRI portion of the study due to claustrophobia, one due to a metal implant, and one due to scheduling issues, resulting in forty-eight participants completing the MRI portion of the study.
2.2. Assessment Procedures
2.2.1. Structured Interview
As part of a larger study on inner speech in aphasia, participants were interviewed regarding their experiences with aphasia. Subjective experiences of anomia were measured via a continuous scale from “never” (0) to “every time” (10) accompanied by visuals to support auditory comprehension. To ensure understanding of the scale, the interview began with a yes/no question with the correct answer known to the tester (i.e., Do you drive?) and follow-up questions that required the scale for response (e.g., If I asked you, when you go somewhere with your family, how often are you the driver? You would say [gesture to indicate response via scale]). If a participant used the scale incorrectly by giving incongruent responses (e.g., saying “no” they don’t drive and saying they drive “every time”), the clinician provided direct cueing regarding how to use the scale. This process was followed until the participant provided congruent responses. Then, anomia was explained and auditory comprehension was supported using slowing verbal pacing, visuals, and gestures. Participants were asked if they experience anomia via the question, “Do you ever know what you want to say but you can’t say it out loud?” (Figure 1). All participants responded “yes”, affirming that they experience anomia. Participants were asked to describe the feeling of anomia to confirm their understanding of the question, using verbal and nonverbal communication as able. Then participants were asked a question requiring intellectual awareness, again using visual supports to support auditory comprehension of the question, “Of all the times you want to say a word out loud, how often can you say the right word out loud?”. Participants provided their responses by pointing to a location on the visual scale or providing a verbal response (Figure 2). When participants chose to respond via verbal response, but the response did not clearly align with the options on the scale (e.g., “not too often”), the participant was directed to indicate a specific location on the scale. Ratings were converted to percentages (e.g., a rating of at “none of the time” indicated 0% and “every time” indicated 100%). Participants completed this interview on two separate days approximately 2 weeks apart (mean = 11 days), resulting in two self-ratings of their anomia severity. The same interview questions were utilized during both sessions.
Figure 1. Visual aid used during interview.
This illustration was used to introduce the concept of anomia and support the question, “Do you ever know what you want to say but you can’t say it out loud?” All participants reported “yes”, affirming that they experience anomia and described the feeling of anomia.
Figure 2. Intellectual awareness question.
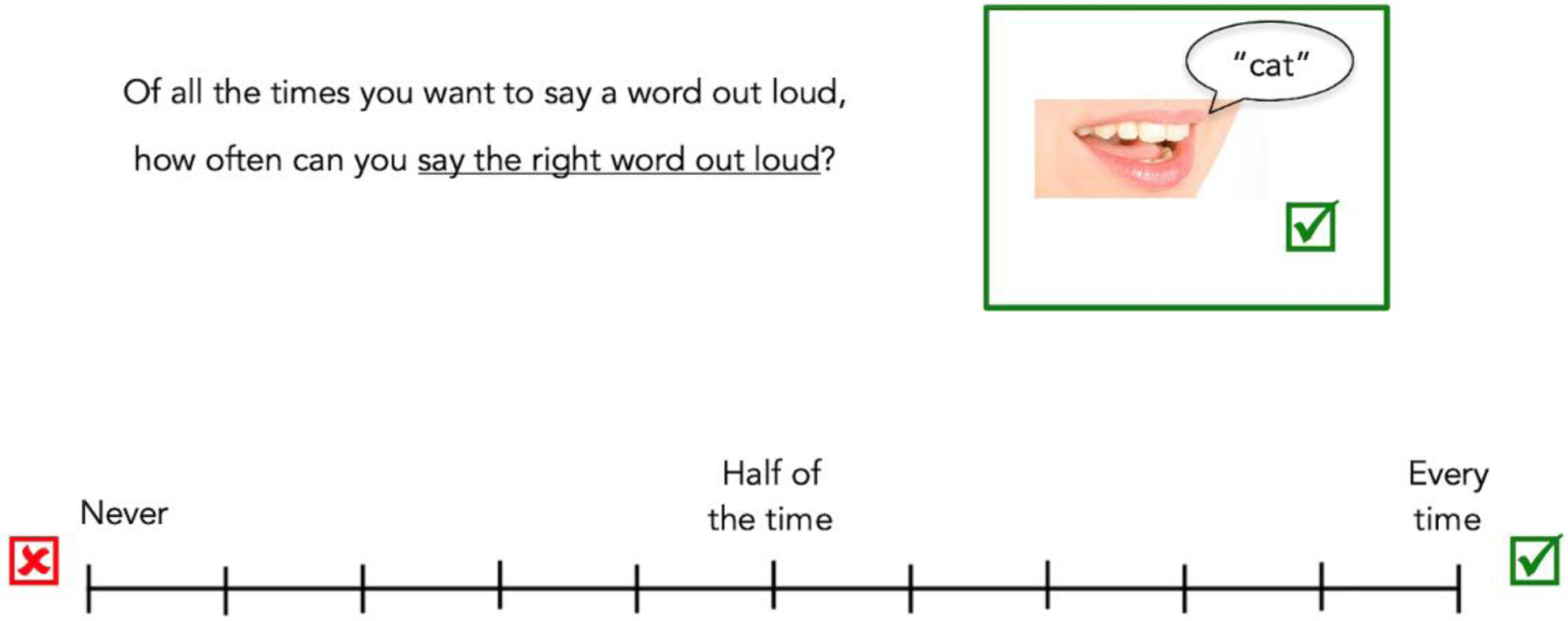
The question, scale, and visual supports used to assess intellectual awareness of anomia during a larger interview regarding self-perceptions of aphasia are shown. Participants indicated a location on the scale to indicate the frequency with which they can say words aloud correctly. Responses were converted to a percentage: “Never” indicates 0% of the time, “Half of the time” indicates 50% of the time, and “Every time” indicates 100% of the time.
2.2.2. Speech, Language, and Cognitive Measures
During each session following the interview, participants completed a battery of speech, language, and cognitive tasks administered by a certified speech-language pathologist (MEF). Anomia was assessed using two confrontation naming assessments. Each test included 30 items from the Philadelphia Naming Test - Short Form (Walker & Schwartz, 2012) and 30 items from a set of in-house naming stimuli, resulting in two 60-item naming tests with no repeating items. The items of the two tests were matched to each other on frequency, length, age of acquisition, and articulatory complexity (see Fama et al., 2019b; naming stimuli available at https://www.cognitiverecoverylab.com/researchers). One naming test was administered at each of the two testing sessions, with the order counterbalanced across participants. The other speech, language, and cognitive tests described below were split between the two interview days so that participants completed each assessment once. Motor speech metrics were derived from Haley and colleagues (2012). Language and cognitive subtests from the Philadelphia Naming Test - Short Form (PNT; Walker & Schwartz, 2012), Temple Assessment of Language and (Verbal) Short-term Memory in Aphasia (TALSA; Martin et al., 2018), Western Aphasia Battery-Revised (WAB-R; Kertesz, 2007), and Boston Diagnostic Aphasia Examination (BDAE; Goodglass et al., 2001). See a full list of measures and descriptions in Table 1. The naming tests and TALSA subtests were administered on a laptop computer via PsychoPy to implement a 20-second response time limit.
Table 1.
Speech, language and cognitive measures.
| Construct Measured | Assessment Measure | Task Description | Score Metric |
|---|---|---|---|
| Lexical Retrieval | PNT Short-Form + 60 in-house items | Naming pictures of objects (nouns) | Percent Correct |
| Semantic Judgments | TALSA Category Judgment Test | Judging whether two pictures belong to the same category or not | Percent Correct |
| Auditory Comprehension | TALSA Lexical Comprehension Test | Selecting a picture that matches an auditory word from four pictures (semantic foils) | Percent Correct |
| WAB-R Yes/No Questions | Answering yes or no questions with increasingly complex, abstract content | Percent Correct | |
| Speech Fluency | BDAE Cookie Theft Picture Description | Describing what you see in a visual picture scene | Words per utterance |
| Motor Speech | Alternating Motion Rates (AMRs) | Producing puh, tuh, and kuh as fast and steadily as possible | Syllables per second |
| Sequential Motion Rates (SMRs) | Producing puh-tuh-kuh as fast and steadily as possible | 3 correct syllable triads or not | |
| Speech Perception | TALSA Phoneme Discrimination: Real Words | Judging whether two auditorily presented words are exactly the same or not | Percent Correct |
| TALSA Phoneme Discrimination: Pseudowords | Judging whether two auditorily presented non-words are exactly the same or not | Percent Correct | |
| Verbal Working Memory | Digit Span: Forward | Repeating digit strings of increasing length in the same order as they were presented | Span Length |
| Digit Span: Backward | Repeating digit strings of increasing length in reverse order as they were presented | Span Length | |
| Nonverbal working Memory | Spatial Span: Forward | Selecting sequences of blocks of increasing length in the same order in which they were | Span Length |
| Spatial Span: Backward | Selecting sequences of blocks of increasing in the reverse order in which they were presented | Span Length |
2.3. Measurement of Awareness
2.3.1. Intellectual Awareness
We measured intellectual awareness by examining the reliability of participants’ self-rating and assessing the accuracy of their self-rating as compared to their objective naming performance (Table 2).
Reliability of self-rating was measured via a change score between the two days of self-rating, calculated as: Absolute Value (Day 1 Self-Rating - Day 2 Self-Rating) = Reliability Score. We determined a cutoff for reliability by examining the actual change in naming performance between the two days of testing. Participants averaged 6.1% change (SD = 5.2) between their actual naming scores on day 1 and day 2, indicating that a reasonable change in rating from day-to-day would be close to this number. Given that the self-rating scale increments were even tens from 0–100, participants with a Reliability Score of more than 10% were considered unreliable raters (i.e., greater score indicates larger change between self-ratings on the two days).
Accuracy of participant self-rating as compared to their naming performance was measured via a difference score, calculated as: (Average Self-Rating - Average Naming Accuracy) = Agreement Score. Twenty-percent was established as the cut-off score for accuracy, with 10% accounting for reasonable change in ratings as mentioned above for the Reliability Score and an additional 10% to allow for a margin of error for accuracy estimation since no person can be expected to estimate with exact accuracy. Therefore, participants with an Agreement Score of 1) greater than or equal to 20% or 2) less than or equal to −20% were labeled as inaccurate estimators. A difference of ≥ 20% indicates the participant’s rating was higher than their naming performance (i.e., overestimating their ability), while a difference score of ≤ −20% indicates the participant’s rating was lower than their naming performance (i.e., underestimating their ability).
Table 2.
Measurement of awareness.
| Constructs Measured | Definition | Formula | |
|---|---|---|---|
| Intellectual Awareness | Reliability | Consistency of self-rating between Day 1 and Day 2 | Reliability Score = Absolute Value (Day 1 Self-Rating − Day 2 Self-Rating) |
| Accuracy | Agreement between participants’ average self-rating and their objective naming performance | Agreement Score = (Average Self-Rating − Average Naming Accuracy) | |
| Emergent Awareness | Spontaneous online error detection | Percentage of errors detected during confrontation naming tasks | Percentage of Errors Detected = Number of Errors Detected / (Total Number of Errors − No Responses) |
2.3.2. Subgrouping based on Intellectual Awareness
Participants were first grouped into “Aware” and “Unaware” groups based on their Reliability and Agreement Scores. Participants who had self-ratings that were both reliable (Reliability Score ≤10%) and accurate (Agreement Score >−20 and <20) were considered “Aware” of their naming ability. All other participants were considered “Unaware.” The Unaware group was then further divided into three subgroups: 1) Unreliable raters (Reliability Score >10%), 2) Reliable Overestimators (Reliability Score ≤ 10%, Agreement Score ≥ 20%), and Reliable Underestimators (Reliability Score ≤ 10%, Agreement Score ≤ −20%).
2.3.3. Emergent Awareness
The participant’s first response was used to calculate accuracy for the two confrontation naming tests. However, participants were not limited in the number of naming attempts within the 20-second time limit per item, providing them the opportunity to detect and correct their errors online. Participants were not provided with feedback on the accuracy of their responses. To include a metric of emergent awareness, spontaneous online error detection was measured via participants’ direct negation of their naming attempt (e.g., “no, that’s not it”) or a repair attempt (i.e., changing their response) (Schuchard et al., 2017). Spontaneous error detection was calculated for each participant as: Number of Errors Detected / (Total Number of Errors – No Responses) = Percentage of Errors Detected (see Mandal et al., 2020 for full details). Items in which no verbal response was provided (i.e., No Responses) were excluded from the emergent awareness calculation because there was no opportunity to detect an error on these items. Furthermore, since error commission is necessary to assess emergent awareness, only participants who made five or more errors on the naming tests were included in the analyses for self-detection of naming errors (N=48). See Table 2 for a summary of the intellectual and emergent awareness measures.
2.4. Statistical Methods
2.4.1. Reliability and Accuracy in the Entire Group
We first examined session-to-session reliability of both self-ratings and naming performance across the entire group. A two-way mixed effects, absolute agreement intraclass correlation coefficient was calculated to establish group reliability across the two days of self-rating, as well as the consistency of their naming accuracy on two separate days of testing. Next, we examined agreement between self-ratings and naming performance for the entire group using a Pearson correlation and paired-samples t-test to calculate overall group accuracy for their estimation of anomia severity (i.e., average self-rating across the two interviews vs. average naming accuracy across the two naming tests), as well as accuracy of Day 1 and Day 2 self-ratings separately. The sample size of the study provides 80% power to detect an effect of d=.39 in a paired t-test and an effect of d=.37 in a Pearson’s Correlation with an alpha of .05.
2.4.2. Relationship of Awareness with Speech/Language/Cognitive Abilities
We then compared the intellectual awareness subgroups to investigate the relationship between intellectual awareness and speech, language, and cognitive abilities. We performed independent samples t-tests comparing the Aware and Unaware groups on the speech, language, and cognitive test scores listed in Table 2. The sample size in this study provides 80% power to detect an effect of d= .79 with an alpha of .05 in an unpaired t-test. We used a one-way ANOVA to investigate differences between the Aware Group and subgroups of Unaware raters (i.e., Unreliable raters, Overestimators, and Underestimators) for online spontaneous error detection.
2.5. Neuroimaging Methods
2.5.1. MRI Acquisition and Pre-Processing
We collected high-resolution T1-weighted MRIs on a 3.0 T Siemens Trio scanner for 45 of the participants (MPRAGE, TR = 1900 ms; TE = 2.56 ms; flip angle = 9°; 160 contiguous 1 mm sagittal slices; field of view (FOV) = 250 × 250 mm; matrix size = 246 × 256, voxel size 1 mm3). Twenty-two participants also had T2-weighted sampling perfection with application optimized contrasts using different flip angle evolution (SPACE) sequences (176 sagittal slices; slice thickness = 1.25 mm, FOV = 240 × 240 mm; matrix size = 384 × 384; TR = 3200 ms; echo train length = 145, variable TE; variable flip angle, voxel size = 0.625 × 0.625 × 1.25 mm) available from a prior study. A board-certified neurologist (P.E.T.) manually traced lesions on T1-weighted images in native space, using co-registered T2-weighted images as well when available, in ITK-SNAP 3.6 (http://www.itksnap.org).
We then warped native space MPRAGEs and lesion tracings to MNI space using the Clinical Toolbox Older Adult Template as the target template (Rorden et al., 2012) via a custom pipeline described in detail in Mandal et al., (2020). Briefly, we segmented the brain from surrounding tissue and then normalized the extracted brain image using an iterative approach in Advanced Normalization Tools software (ANTs; available at http://picsl.upenn.edu/software/ants/; (Avants et al., 2009)). After we applied bias field correction, normalization proceeded using a typical ANTs procedure, including a rigid transform step, an affine transform step, and a nonlinear SyN step. Next, we submitted the output of this initial ANTs warp recursively to three additional applications of the SyN step. Finally, we concatenated the resulting linear (rigid and affine) and four nonlinear warp fields to transform the original native space MPRAGE and lesion tracings to the template space using BSpline interpolation. Lesion masking was implemented at each step of the ANTs process. This iterative nonlinear process helps to improve normalization of expanded ventricles and displaced deep structures in individuals with large lesions. The normalized lesion tracings were then downsampled to 2.5 mm cubic voxels.
2.5.2. Lesion-Symptom Mapping
We conducted SVR-LSM analyses (Zhang et al., 2014) using a MATLAB-based toolbox (Demarco & Turkeltaub, 2018) running under MATLAB R2019a (The MathWorks, Inc., Natick, Massachusetts, United States). SVR-LSM was used to identify areas in the left-hemisphere associated with either intact or impaired intellectual awareness. This method applies a machine learning based algorithm to find lesion-symptom relationships, addressing limitations of traditional mass-univariate lesion-symptom mapping approaches related to lesion covariance (Mah et al., 2014). Only voxels damaged in at least five participants in the study (greater than 10%) were considered for each analysis. We controlled lesion volume confounds in all analyses by regressing the lesion volume out of behavioral scores and lesion masks. This method provides rigorous control of lesion volume and is more sensitive than alternative approaches (Demarco & Turkeltaub, 2018). We assessed significance using 10,000 permutations of the behavioral scores to generate voxelwise null distributions of SVR beta values. To correct for multiple comparisons, we applied a voxel-level threshold determined from the 10,000 permutation maps to control the continuous family-wise error rate (CFWER) at .05 (v = 100 voxels) (Mirman et al., 2018). Given the possibility of false positives, we then tested for cluster-level significance using a threshold of p < .05 using the same 10,000 permutations. Three subjects were excluded from the SVR-LSM analysis, two due to lesions outside the left hemisphere and one due to severe leukoaraiosis affecting both hemispheres. Two additional participants did not contribute to the analysis because they did not have any lesioned voxels within the minimum lesion cutoff mask, giving an effective sample size for the SVR-LSM analysis of n = 43 total, with 21 in the Unaware group, and 22 in the Aware group.
3. Results
3.1. Group Reliability and Accuracy
Before dividing participants into intellectual awareness subgroups, we assessed reliability and accuracy of anomia awareness across the entire group. There was a moderate two-day test-retest reliability (Koo & Li, 2016) in self-rating their naming abilities, ICC=.60 with 95% CI=.38–.75 (F(52,52)=4.29, p<.001), while the reliability of their naming performance between testing days was excellent, ICC=.98 with 95% CI=.97–.99 (F(52,52)=65.49, p<.001). The group’s average self-rating score and average naming accuracy were positively correlated (r(51)=.61, p < .001; d=.37) (Figure 3a). A paired t-test demonstrated no significant difference between average self-rating and average naming accuracy across the group, (t(52)=.413, p = .681; d=.05). Participants more accurately estimated their average naming performance on day 1 (r(51) =.65, p <.001; d=.42) compared to day 2 (r(51)=.43, p=.001; d=.19; comparison between days: z = 2.21, p=.014) (Figure 3, b and c).
Figure 3. Participant self-ratings reliability and accuracy (n = 53).
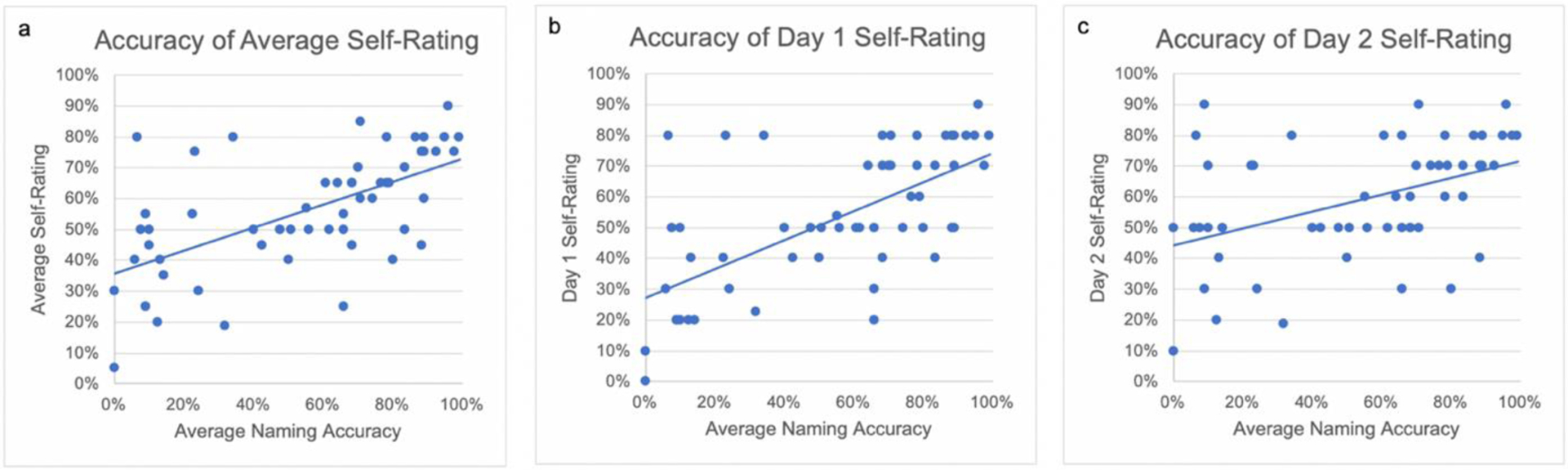
(a) Correlation of participant average self-ratings with their average naming scores. (b) Correlation of day 1 self-ratings with average naming scores. (c) Correlation of day 2 self-ratings with average naming scores.
3.2. Subgroup Analyses
3.2.1. Aware and Unaware Groups
Twenty-nine of the 53 participants were identified as Aware of their naming abilities, and the remaining twenty-four were identified as Unaware of their naming abilities based on our criteria. Fourteen members of the Unaware group were subgrouped as Unreliable self-raters who inconsistently rated their naming abilities between the two interviews. The remaining 10 Unaware participants were reliable but inaccurate at estimating their naming abilities on both days. Six of these participants overestimated their ability (Reliable Overestimators), and four underestimated their ability (Reliable Underestimators) (Table 3, Figure 4). Although we did not further subdivide the Unreliable group based on the agreement of self-ratings and naming performance, it is worth noting that 9 of the fourteen Unreliable raters would also have qualified as inaccurate raters based on their Agreement Score. Unaware participants ranged from underestimating their ability by 43% to overestimating their ability by 73%.
Table 3. Naming performance, self-reports, and intellectual awareness scores.
Means are listed with standard deviation in parentheses for all participants. The Reliability Score is the absolute value of Day 1 Self-Rating minus Day 2 Self-Rating, where high values represent larger discrepancy between the two self-ratings. The Agreement Score is Average Self-rating minus Average Naming Score, where values closest to 0 represent the most agreement between self-ratings and actual naming ability.
| Total Sample (n = 53) | Aware Group (n = 29) | Unaware Group | ||||
|---|---|---|---|---|---|---|
| Unreliable Raters (n = 14) | Reliable Overestimators (n = 6) | Reliable Underestimators (n = 4) | Total Unaware (n = 24) | |||
| Naming Accuracy (percent) | 55.4 (31.7) | 64.31 (27.23) | 46.73 (32.60) | 15.83 (10.83) | 80.0 (15.41) | 44.55 (33.76) |
| Range of Self-Reports | 0–90% | 0–90% | 10–90% | 40–80% | 20–80% | 10–90% |
| Mean Reliability Score | 11.70 (15.16) | 4.14 (5.01) | 32.14 (14.73) | 1.67 (4.08) | 10.0 (.00) | 20.83 (18.15) |
| Mean Agreement Score | 1.43 (25.19) | 4.66 (9.85) | 4.35 (27.57) | 46.67 (15.48) | −32.5 (11.12) | 8.78 (34.84) |
Figure 4. Box plot with individual data points for Agreement Scores in each group.
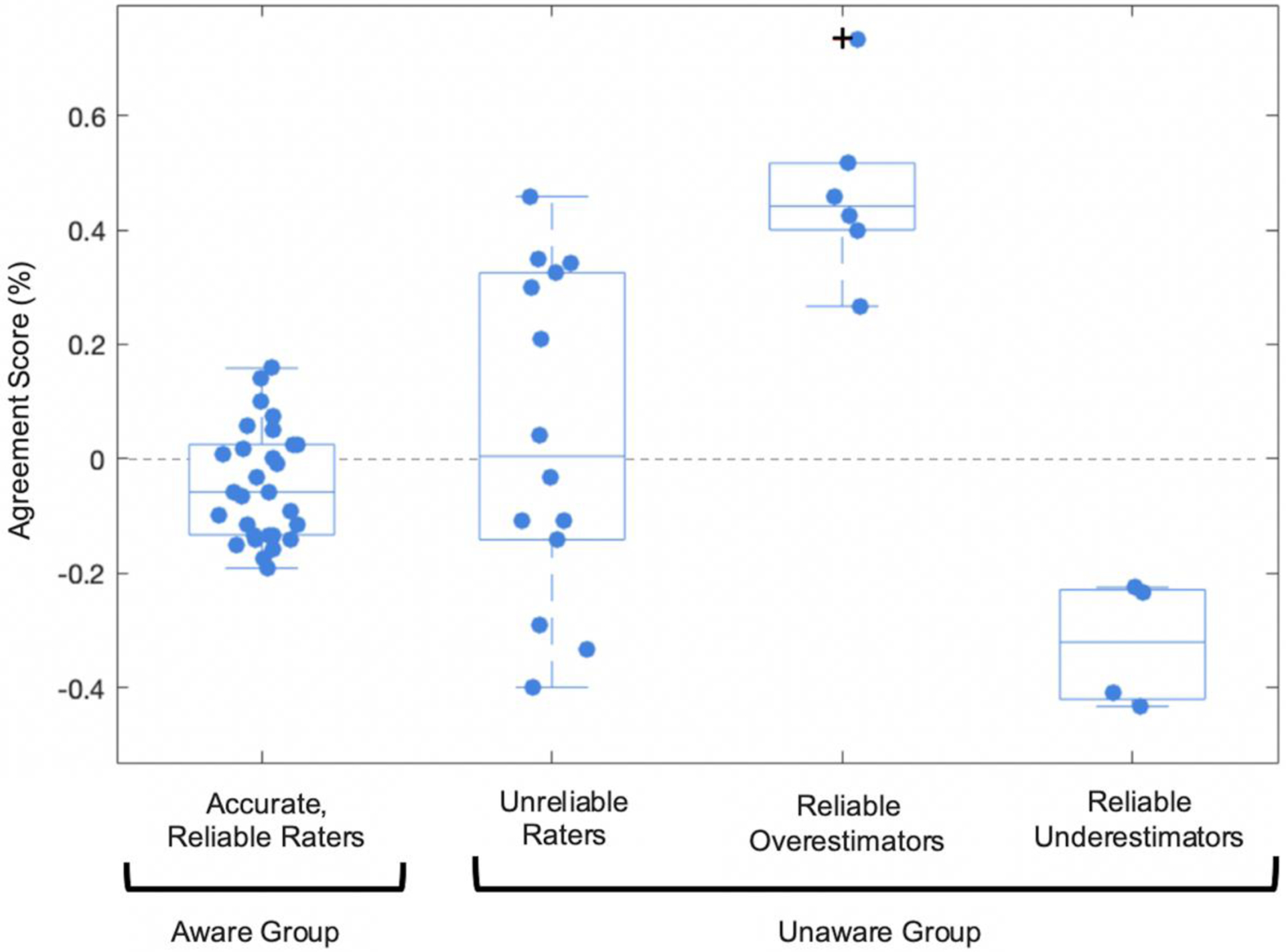
The Aware Group had both reliable (Reliability Score ≤10%) and accurate anomia ratings (Agreement Score >−20 and <20). Within the Unaware Group, the Unreliable raters had discrepant self-ratings between the two interviews (Reliability Score >10%), the Reliable Overestimators consistently overrated their naming ability (Reliability Score ≤ 10%, Agreement Score ≥ 20%), and the Reliable Underestimators consistently underrated their naming ability (Reliability Score ≤ 10%, Agreement Score ≤ −20%). Outliers indicated with +.
Overall from day 1 to day 2, 11 of the 14 Unreliable raters gained confidence in their abilities and 3 lost confidence in their naming abilities. Four of the unreliable raters’ self-rating improved from day 1 of evaluation to day 2 to more accurately agree with their naming performance. The remaining ten unreliable raters were less accurate on day 2 than on day 1.
3.2.2. Relationship of intellectual awareness with Speech, Language, and Cognitive Abilities
No significant differences were present between the Aware and Unaware groups in age (t(51)=.357, p = .72), education (t(51)=.393, p = .70), or months post-stroke (t(51)=.840, p = .41). Therefore, these factors were not controlled for in our group comparisons on the behavioral measures. The results of independent samples t-tests demonstrate that the Unaware group had significantly reduced confrontation naming ability (t(44)=2.312, p = .03; d = .65) category judgment (t(51)=2.354, p = .02; d = .65), word-level comprehension with semantic foils (t(29)=2.760, p = .01; = .82), and phoneme discrimination in real words (t(27)=2.069, p = .05; d = .62,). The Unaware group did not differ from the Aware group on measures of sentence-level auditory comprehension, production of AMRs and SMRs, phoneme discrimination in pseudowords, average words per utterance, digit spans, or spatial spans (see Table 4 for full results).
Table 4. Relationship of intellectual awareness with speech, language, and cognitive skills.
All analyses had n = 53 except Error Detection with n = 48. All tests had 51 degrees of freedom except Confrontation Naming (43.97), Single Word Auditory Comprehension (28.97), WAB Yes/No Questions (40.68), Phoneme Discrimination on Real Words (26.99), and Error Detection (46). Significant results indicated with an asterisk (p < .05*). Moderate effect sizes indicated with † (d = .50–.79), and large effects size with †† (d ≥ .80).
| Task | Aware Group Mean (SD) | Unaware Group Mean (SD) | Mean Difference (95% CI) | t-values | p-values | Cohen’s d |
|---|---|---|---|---|---|---|
| Confrontation Naming | 64.3 (27.2) | 44.5 (33.8) | 19.7 (2.5, 37.0) | 2.31 | .026* | .65† |
| Category Judgment | 91.1 (10.1) | 84.3 (11.0) | 6.8 (1.0, 12.7) | 2.35 | .022* | .65† |
| Word-Level Auditory Comprehension | 96.8 (5.3) | 88.7 (13.4) | 8.1 (2.1, 14.0) | 2.76 | .010* | .82†† |
| WAB Yes/No | 94.7 (4.8) | 91.7 (6.7) | 3.0 (−0.2, 6.2) | 1.83 | .075 | .52† |
| Average Words per Utterance | 4.68 (2.4) | 4.35 (2.1) | 0.3 (−0.9, 1.6) | .536 | .594 | .15 |
| AMR (syllables per second) | 3.35 (2.0) | 3.20 (2.4) | 0.2 (−1.1, 1.4) | .263 | .794 | .07 |
| Adequate SMR (≥3 repetitions) | .46 (.51) | .59 (.50) | 0.1 (−0.2, 0.4) | .918 | .363 | .25 |
| Phoneme Discrimination - Real Words | 95.4 (4.6) | 89.1 (14.2) | 6.3 (0.0, 12.5) | 2.07 | .048* | .62† |
| Phoneme Discrimination - Pseudowords | 90.8 (10.6) | 87.6 (11.5) | 3.2 (−3.0, 9.3) | 1.04 | .309 | .29 |
| Digit Span Forward | 5.52 (3.0) | 4.54 (2.9) | 1.0 (−0.7, 2.6) | 1.19 | .241 | .33 |
| Digit Span Backward | 2.83 (2.3) | 2.13 (1.8) | 0.7 (−0.5, 1.9) | 1.20 | .237 | .33 |
| Spatial Span Forward | 6.97 (2.3) | 6.29 (2.1) | 0.7 (−0.5, 1.9) | 1.11 | .271 | .31 |
| Spatial Span Backward | 5.55 (2.7) | 5.29 (2.0) | 0.3 (−1.1, 1.6) | .396 | .693 | .12 |
| Error Detection | 41.0 (25.7) | 39.6 (20.8) | 1.4 (−12.4, 15.2) | .207 | .837 | .06 |
3.2.3. Relationship of Intellectual Awareness and Emergent Awareness
As a group, people with aphasia detected an average of 40.3% of their errors (SD = 23.4%). The Aware group (n = 26) detected 41.0% (SD = 25.7), while the Unaware group (n = 22) detected 39.6% (SD = 20.8). No significant difference between the two groups was observed for overall self-detection of errors (t(46)=.207, p = .837; d=.06). We also examined error detection rates of semantic and phonological errors specifically. For these analyses, only participants who made at least five errors of each type were included in the respective analyses. No significant difference was observed between groups for semantic error detection (t(23) = .346, p = .732; d=.14) or phonological error detection (t(35) = .116, p = .908; d=.04). Because some models predict a relationship between intellectual and emergent awareness, we assessed the level of evidence for and against an effect using Bayes Factors. There was moderate evidence for absence of effect of intellectual awareness group on error detection rates (BFoverall = 4.55, BFsemantic= 3.35, BFphonological = 4.14) (Lee & Wagenmakers, 2014).
It may be expected that different unaware behaviors (i.e., overestimation vs. underestimation) would lead to varied error detection behavior, such as the Overestimators detecting fewer errors than the Aware group and the Underestimators detecting more errors than the Aware group. To test this, we used a one-way ANOVA to compare self-detection frequency between Overestimators, Underestimators, Unreliable raters, and the Aware group. The results indicated no significant difference between groups for overall error detection (F(3,44) = .596, p = .621), semantic error detection (F(3,21) = .992, p = .416), or phonological error detection (F(3,33) = .415, p = .743). However, it is important to note that after removing participants who didn’t produce at least five errors of each type, some subgroups had a very small sample size, so these results should be interpreted with caution (see Table 5).
Table 5. Percent spontaneous error detection by subgroup.
Only participants who made 5 or more errors were included in the overall error detection analysis. Only participants who made 5 or more semantic or phonological errors were included in the respective analysis.
| Error Detection Type | Aware Group Mean (SD) | Unaware Group | |||
|---|---|---|---|---|---|
| Unreliable Raters Mean (SD) | Reliable Overestimators Mean (SD) | Reliable Underestimators Mean (SD) | Total Unaware Mean (SD) | ||
| N | 26 | 13 | 6 | 3 | 22 |
| Overall Error Detection % | 41.0 (25.7) | 35.1 (15.3) | 50.4 (23.9) | 37.5 (34.8) | 39.6 (20.8) |
| N | 14 | 5 | 5 | 1 | 11 |
| Semantic Error Detection % | 42.1 (20.9) | 28.2 (19.0) | 44.0 (30.6) | 66.7 (n/a) | 38.9 (25.8) |
| N | 19 | 11 | 5 | 2 | 18 |
| Phonological Error Detection % | 41.3 (32.7) | 34.9 (24.5) | 53.0 (30.0) | 36.7 (39.0) | 40.1 (32.7) |
3.3. Lesion-Symptom Mapping
A lesion overlap map demonstrated adequate lesion coverage across the majority of the left middle cerebral artery territory (Figure 5). Lesion volume was not significantly different between the Aware and Unaware groups (t(41)= 1.077, p = .288). The SVR-LSM maps examining lesions associated with the Unaware Group and the Aware Group both surpassed the CFWER threshold of 100 voxels (107 voxels and 116 voxels respectively). This whole-brain test indicates that there is a greater than 95% chance that the lesion-symptom associations were non-random. Given that the CFWER method allows for the possibility of false positives at the voxel level, we next applied a cluster-level corrected threshold (familywise error rate <.05) to the voxels that survived the CFWER thresholding. One significant cluster in the anterior orbital part of the inferior frontal gyrus was associated with the Unaware Group (p = .03, 1172 mm3 centered at MNI coordinates −40.9, 44.5, −8.5), and one significant cluster in the mid-portion of the superior temporal gyrus was associated with the Aware group (p = .03, 1203 mm3 centered at−58, −13.4, −2.2) (Figure 6). Given this frontal-temporal dichotomy between lesions associated Unaware and Aware Groups, we next examined the unthresholded Z-map to assess this pattern more globally. This revealed a clear anterior to posterior gradient with poor awareness mapping onto lesions in the frontal lobe and good awareness mapping onto lesions in the temporal and parietal lobes (Figure 7).
Figure 5. Lesion overlap map (n = 43).
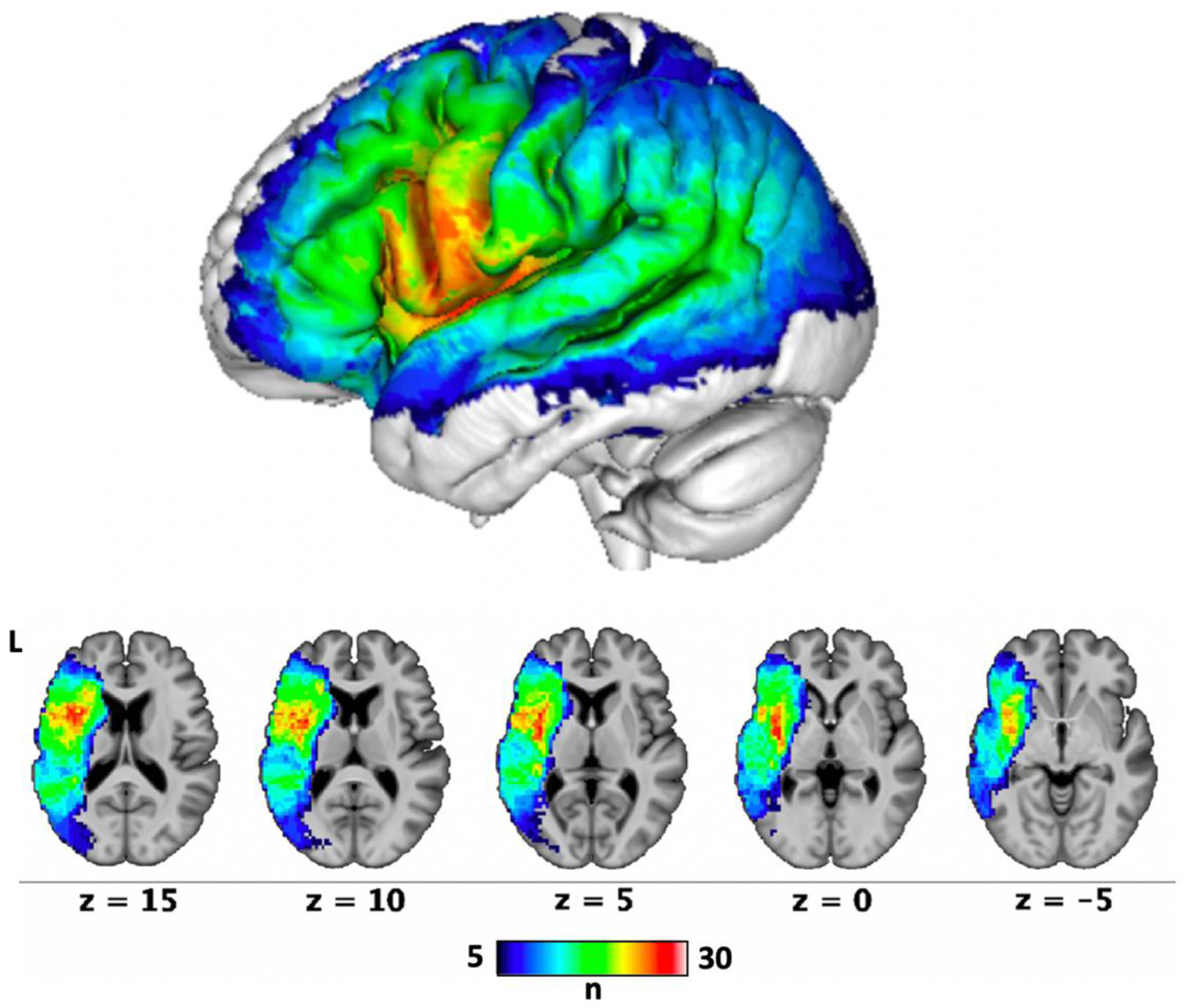
Each voxel is colored based on the number of people with aphasia whose lesions involved that voxel. SVR-LSM analyses were limited to voxels that were lesioned in at least 10% of the participants (n = 5).
Figure 6. SVR-LSM results for intellectual awareness.
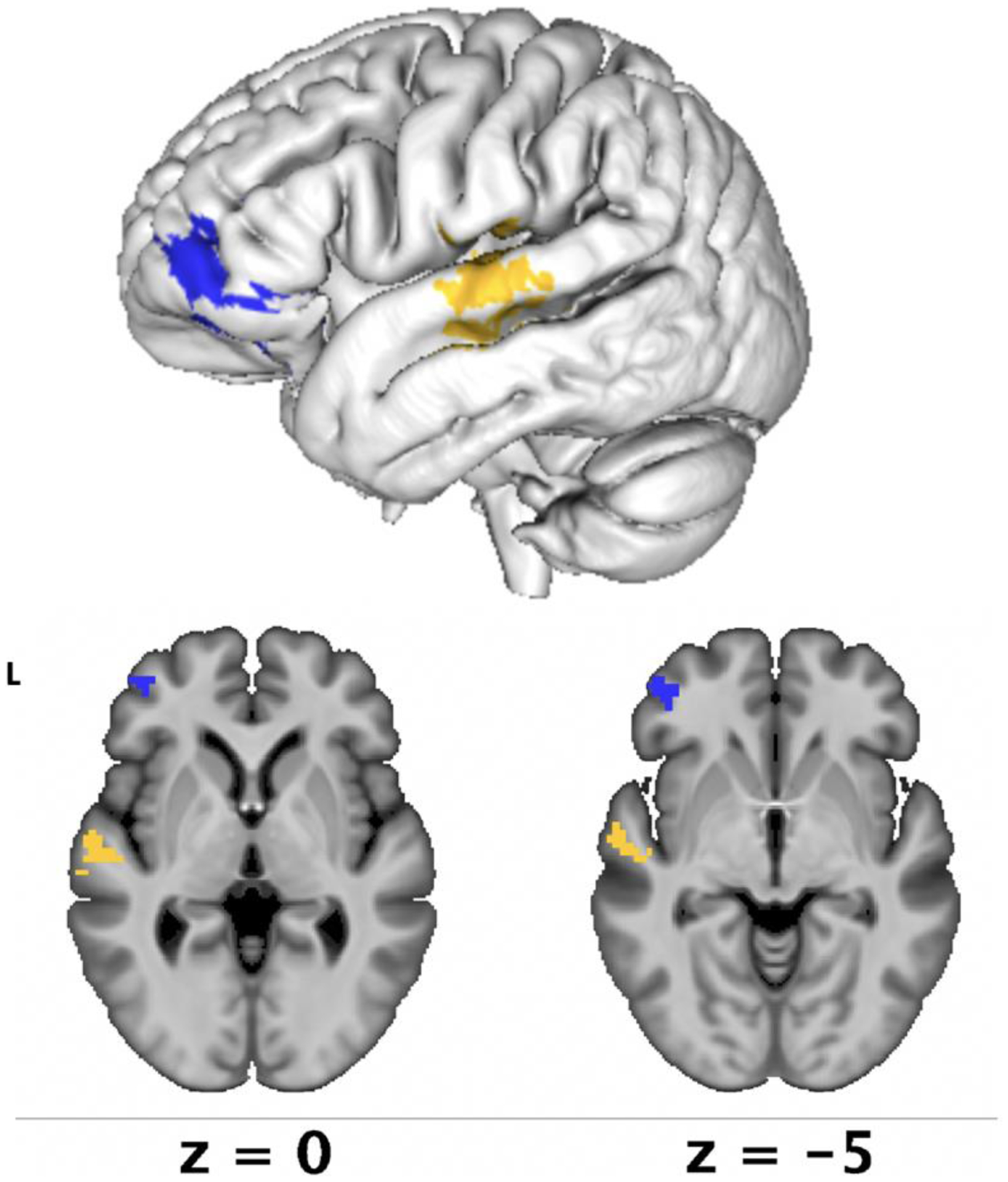
Lesion-symptom mapping results demonstrated that reduced intellectual awareness was associated with lesions in the anterior inferior frontal gyrus, primarily in the pars orbitalis (in blue, p = .03) and preserved intellectual awareness was associated with lesions in the mid-superior temporal gyrus (in yellow, p = .03).
Figure 7. Unthresholded SVR-LSM results for unaware and aware participant groups.
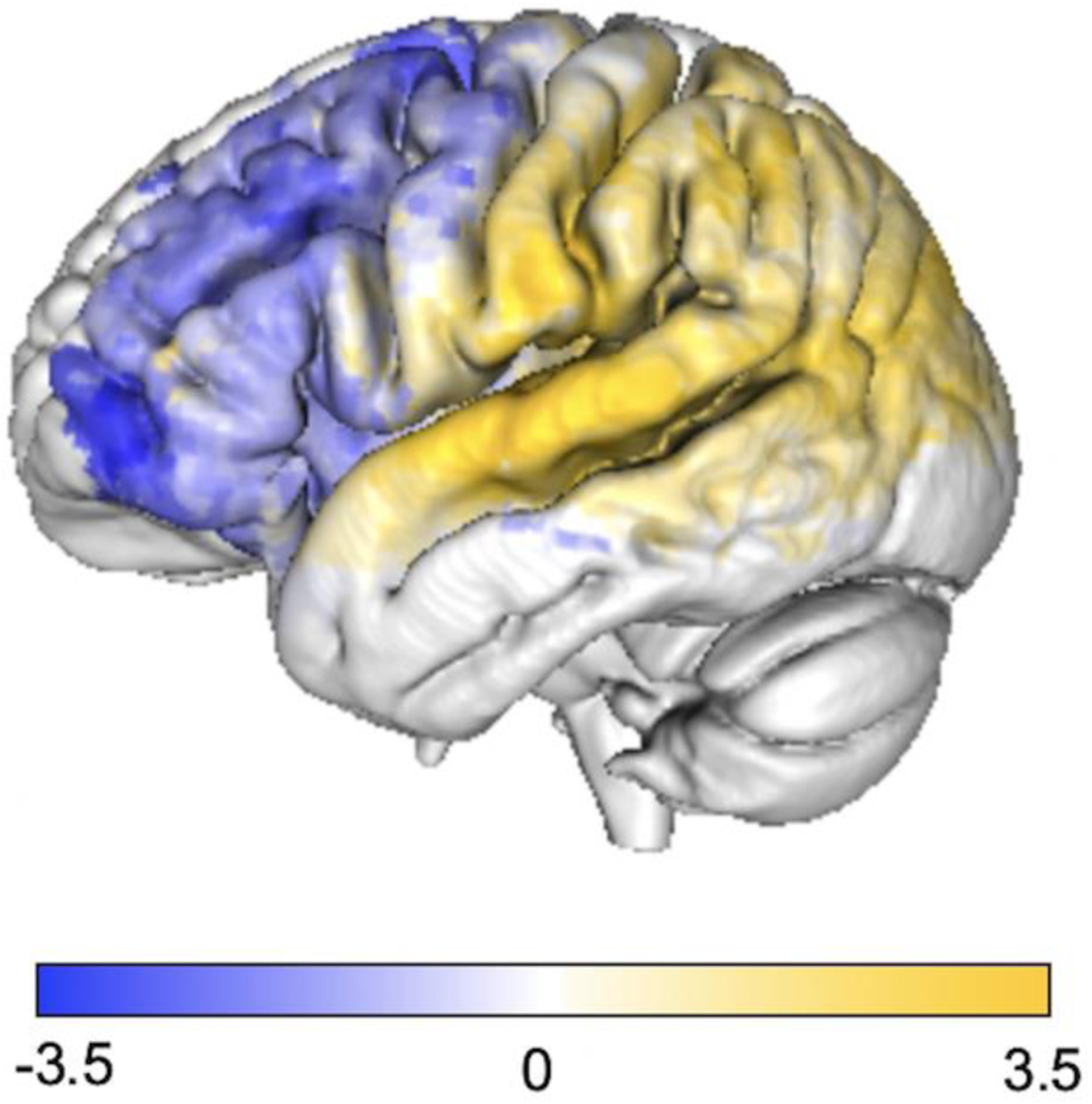
An anterior to posterior gradient was observed with lesions associated with poor awareness in the frontal lobe (in blue) and lesions in the temporal and parietal lobes being associated with preserved awareness (in yellow). Uncorrected voxelwise p-values were converted to Z-scores for visualization.
4. Discussion
Our findings demonstrate that many people with chronic aphasia with relatively intact comprehension can be accurate self-reporters of their anomia severity. All participants acknowledged the presence of anomia. By our criteria, slightly more than half of people with aphasia in our sample were able to reliably and accurately estimate their anomia severity. Participants’ estimates were generally more accurate on the first day of rating compared to the second day. People with aphasia who gave unreliable or inaccurate ratings of their naming ability demonstrated greater impairment on tasks that rely to some extent on semantics (i.e., single word comprehension with semantic foils, category judgment, confrontation naming, and phoneme discrimination in real words but not pseudowords). Anterior lesions were associated with reduced awareness and posterior lesions with preserved awareness. Our results highlight the importance of conducting two self-ratings to check the reliability of a patient’s self-awareness, and being cognizant of possible inaccurate self-ratings, particularly when semantic deficits and anterior lesions are present.
4.1. Relationship of Self-Awareness to Speech, Language, and Cognitive Functions
The prior literature examining the relationship of self-awareness to language and cognitive functions in people with aphasia has focused on error detection as a measure of emergent awareness. Here, we explored relationships of language and cognitive functions, including emergent awareness, with intellectual awareness. Of the two prior studies on intellectual awareness in aphasia, one found that poor intellectual awareness related to both low comprehension and low verbal production ability (Cocchini et al., 2010), while the other found that poor intellectual awareness was associated with low verbal production, but not low comprehension ability (Dean et al., 2017). Our results do not support a conclusion that intellectual awareness of one’s naming deficits is modulated specifically by comprehension or production systems. Rather, our findings suggest an underlying relationship of intellectual awareness to an individual’s semantic ability, which manifested in tasks involving comprehension (i.e., single-word auditory recognition with semantic foils), speech production (i.e., confrontation naming), and silent, non-lexical semantic processing (i.e., category judgement of pictures). Additionally, Unaware participants had more difficulty discriminating subtle phonemic differences in real words but performed similarly to the Aware group discriminating differences in pseudowords. This result is consistent with the presence of semantic deficits in the Unaware group because real words engage the semantic system, allowing for discrimination between words based on meaning, while pseudowords isolate the phonological system. We did not correct for multiple comparisons given the exploratory nature of these analyses, so some caution is warranted in interpreting the relationships observed between intellectual awareness and individual test scores. However, the consistent pattern of results across tests, implicating only those that involve word-level semantics, suggests that the results are unlikely to have occurred by chance alone.
Semantics is the conceptual knowledge of word meanings, including their features, categories, relationships, and symbols (Helm-Estabrooks et al., 2014). An impairment in the semantic system may be related to intellectual awareness in at least two ways. First, semantic deficits could lead to an inability to identify errors when they occur (i.e., poor emergent awareness), which could in turn lead to poor intellectual awareness. In an overlapping cohort with the individuals examined in the current study, Mandal et al. (2020) recently demonstrated a relationship between a single spoken word-to-picture matching comprehension task and online detection of semantic errors. Similarly, Dean et al. (2017) found a relationship between single word comprehension and online error detection. If intellectual awareness arises from an accumulation of individual experiences of online error awareness, then factors leading to poor emergent awareness might also ultimately lead to poor intellectual awareness. A significant caveat to this interpretation is that we found no direct relationship between emergent and intellectual awareness in the current study, and even more specifically, no difference between Aware and Unaware groups in detection of semantic errors (with moderate evidence against these relationships). Thus, our data do not support this explanation. A second explanation for the relationship between intellectual awareness and performance on semantic tasks is that the domain-general executive control demands required for these two skills might overlap. In semantic tasks, one must use cognitive control and mental flexibility to compare and contrast semantic representations to reach decisions. Indeed, semantic processing tasks have been observed to be correlated with executive functioning in patients with aphasia (Allen et al., 2012), and often group with executive measures in factor analyses (Butler, et al., 2014; Lacey et al., 2017). Similarly, intellectual awareness is a metacognitive skill that requires one to compare and contrast their own behavior over time and to integrate how their abilities compare with task demands (Toglia & Kirk, 2000). A deficit in these executive skills used for comparing two or more pieces of information and integrating the evidence to reach decisions may impact both semantic tasks and intellectual awareness. Zimmerman and colleagues (2017) observed a similar phenomenon in which semantic verbal fluency, a task requiring both semantic knowledge and executive function skills, was correlated with reduced awareness in the TBI population. In our study, intellectual awareness did not correlate with our direct measures of cognition, however, these were limited to digit and spatial span tasks which are measures of working memory rather than comparing/contrasting and decision making. Our tasks involving semantics may have required executive skills more closely related to those used when making self-judgments. This explanation would be in line with theoretical models of self-awareness in which selfrepresentations occur at multiple hierarchical levels of cognition (i.e., arousal, sensory-perceptual knowledge, executive function skills, and self-reflectiveness/metacognition) and a breakdown in a lower cognitive mechanism can result in self-awareness deficits (Stuss 1991b; Stuss & Anderson 2004). Here, our results may suggest that executive functions support both language processes as well as metacognition. Future studies may consider incorporating more tasks requiring executive control skills without semantic content, and vice versa, in order to better understand the relationship between semantics and intellectual awareness observed here.
4.2. Relationship of Intellectual Awareness and Emergent Awareness
We did not find a significant relationship between intellectual awareness and emergent awareness as measured through spontaneous online error detection, and indeed provide moderate evidence against a relationship. These results support our hypothesis that these two metacognitive skills are dissociable, and are consistent with previous studies indicating that intellectual awareness and emergent awareness are distinct processes (Dean et al., 2017). If intellectual awareness is a foundational skill for emergent awareness as the Pyramid Model proposes (Crosson et al., 1989), we would expect to see lower levels of online error detection for participants who had poor intellectual awareness of their anomia severity. We consider three explanations for the lack of relationship observed in our results. First, intellectual awareness may be partly related to past awareness of errors, but this relationship may be masked by other factors that also contribute to intellectual awareness, such as feedback from others and one’s overall sense of self-efficacy. Since these other factors likely vary over time depending on recent events, this theory is consistent with our observation that awareness varies from one day to another for some participants. Secondly, types of awareness may have a more dynamic relationship as suggested by Toglia and Kirk (2000), with multiple types of awareness developing simultaneously and these types of awareness mutually influencing each other. Finally, emergent and intellectual awareness may be supported by different cognitive mechanisms (Stuss 1991b), resulting in difficulty with one type of awareness but not another.
It is also important to note that our findings should be interpreted with reference to the specific measure of emergent awareness used, spontaneous error detection. While participants had the opportunity to detect and correct their errors (20 seconds given for each trial), they were not explicitly directed to judge the accuracy of their response on each trial. The alternative would be to ask participants to make an accuracy judgement after each naming trial. We chose the former method of measurement for two reasons. First, there is evidence that explicitly requesting participants to make accuracy judgements alters the way they detect their errors, and perhaps also their approach to and processing of the task itself (Grützmann et al. 2014). Because we were interested in gaining a naturalistic measure of the participants’ naming performance, we did not want to alter their approach to the naming task (e.g., unintentionally cueing strategies not typically used by the participant). Secondly, much of the prior research on error-monitoring in aphasia, particularly including research on the relation between error monitoring and clinical outcomes, uses the same method of measurement we used here (Marshall 1985; Marshall 1994; Nozari et al., 2011, Schwartz et al., 2016, Schuchard et al., 2017, Mandal et al., 2020). Despite these reasons for using spontaneous error detection as a measure of emergent awareness, the possibility remains that some participants were aware of their errors, but chose not to display overt recognition of them or to attempt self-corrections. Further research with additional measures of both emergent and intellectual awareness would be useful to confirm the dissociation observed here.
4.3. Relationship of Awareness to Lesion Location
While there is little research on the neural underpinnings of awareness in aphasia, there are numerous imaging studies examining awareness in people with Alzheimer’s Disease and other dementias. In a systematic review that included people with Alzheimer’s Disease and frontotemporal dementia, studies found neural correlates associated with dementia across the frontal, temporal, and parietal lobes, as well as the hippocampus (Zamboni & Wilcock, 2011). More recently, in a review of studies examining the neural correlates of anosognosia in Alzheimer’s Disease, Hallam and colleagues (2020) found eight specific brain regions associated with reduced awareness, including the superior, medial, and inferior frontal gyri; the orbitofrontal cortex; the anterior and posterior cingulate cortices; medial temporal lobe; and the insula. The authors of both of these reviews noted significant differences in methods for measuring dementia, including patient-carer discrepancy scores, examiner’s scores, and patient’s own assessment of their behavior. It is reasonable to assume a complex phenomenon, such as awareness, has a neural network with multiple brain regions contributing to different aspects of awareness. Therefore, the varied neural findings across studies may be due to the method used to measure awareness. One study has examined neural correlates for two different measures of intellectual awareness in the same population (Salmon et al, 2006). The authors found that awareness metrics that involved patients making self-judgments involved the left orbitofrontal lobe and right parahippocampal regions, while reduced awareness associated with patient-carer discrepancy was associated with the left temporoparietal junction and the inferior frontal gyrus. The frontal lobe has also been indicated in studies on awareness in TBI and right-hemisphere stroke (Schmitz et al., 2006; Spikman & van der Naalt, 2010; Stuss, 1991a;). This is consistent with clinical documentation and neuroimaging studies of the prefrontal cortex’s role in metacognition and self-reflective thought (Johnson et al., 2002).
Despite literature linking self-awareness to frontal regions in dementia and acquired brain injury, clinically lack of awareness in aphasia has canonically been associated with Wernicke’s aphasia (Hécaen & Albert 1978; Heilman 1991; as cited in Stuss 2005) in which large lesions in the temporal and parietal lobes are present. Consistent with studies on broader clinical populations, our lesion analysis indicates that lesions in the frontal lobe are associated with reduced intellectual awareness, whereas lesions in the temporal and parietal lobes are associated with preserved awareness in individuals with chronic aphasia with relatively intact comprehension. This anterior-posterior distinction has been observed in mild Alzheimer’s disease and in persons with chronic, focal brain lesions (Fujimoto et al., 2017; Hoerold et al., 2013). Our results also correspond with the prior finding that poor emergent awareness related to frontal lesions in an overlapping cohort of participants with aphasia (Mandal et al., 2020). Importantly, the dorsomedial frontal white matter and dorsolateral prefrontal cortex were implicated in emergent awareness in this prior study, whereas the current study found the strongest relationship between lesions and poor intellectual awareness more anteriorly and ventrally, in the orbital part of the inferior frontal gyrus. This difference is consistent with the separability of emergent and intellectual awareness observed in our behavioral analyses. Furthermore, the region associated with the Unaware group, the pars orbitalis, is canonically associated with semantic associations (Price, 2012), which is consistent with our behavioral results demonstrating that these participants also had reduced performance on several tasks involving semantic skills. The discrepancy between our lesion findings and the classical association of poor awareness with Wernicke’s aphasia may be due to the evolving nature of awareness over the course of recovery in aphasia because our study focused on individuals with chronic aphasia with relatively intact comprehension. Wernicke’s aphasia in the acute stage often evolves into conduction aphasia in the chronic stage (Kertesz & Mccabe, 1977). Conduction aphasia is typically associated with good awareness of errors and multiple attempts to self-correct (Goodglass, 1992). Therefore, the lesions associated with poor awareness may not be the same in the acute and chronic stages of recovery due to neuroplasticity related to evaluation, treatment, feedback from communication partners, and experiences living with aphasia (i.e., natural consequences due to communication breakdowns).
It is important to note that our exclusion criteria for poor comprehension skills (i.e., below 48/60 on the WAB-R Yes/No Questions subtest) may have precluded some participants with large posterior temporoparietal lesions from participating in the study. Excluding participants with severe comprehension deficits is a limitation that exists for all research on aphasia and awareness because metacognition is a complex, abstract topic that may not be easily understood by individuals with severe comprehension deficits. Although our lesion overlap map demonstrates adequate lesion coverage in most of the temporal lobe, including participants with severe comprehension deficits in the sample could theoretically reduce the observed anterior-posterior gradient observed in the current study; however, it would be difficult to know if discrepancies in self-ratings reflected true unawareness or poor comprehension of the task in these individuals. Nevertheless, our findings do suggest that the frontal lobe is involved in the neural network for intellectual awareness, which may relate to the executive control skills, perhaps specific to semantics, required for accurate self-assessment. Importantly, this finding highlights the clinical need to consider the possibility of poor intellectual awareness beyond cases of Wernicke’s aphasia, including individuals with frontal lesions.
4.4. Clinical Considerations
In the traumatic brain injury population, extensive research has demonstrated an association between intellectual awareness and positive functional outcomes, including psychosocial functioning and returning to paid work and education. (Geytenbeek et al., 2017; Sherer et al., 1998; Sherer et al., 2003). However, it is unknown how intellectual awareness impacts functional outcomes for individuals living with aphasia. Decreased awareness in people with aphasia may impact a person’s understanding of the rationale for use of compensatory strategies resulting in reduced interest in and compliance with treatment recommendations, thereby negatively impacting communication effectiveness. Researching and evaluating intellectual awareness in persons with aphasia may eventually lead to interventions that improve functional outcomes by increasing the individual’s understanding of their impairments and how treatment recommendations (e.g., communication strategies) enhance their overall communication effectiveness.
Intellectual awareness may also be important for the validity of self-reported measures of health-related quality of life used in aphasia research and in clinical care settings to determine patient needs. The Stroke and Aphasia Quality of Life Scale (SAQOL-39) is a self-report questionnaire (Hilari et al., 2003) identified by aphasia experts as a core outcome measure to be used in aphasia research (Wallace et al., 2019). The questionnaire includes 39 items, 21 of which ask the participant to rate their difficulty performing different activities. However, poor intellectual awareness could inflate or deflate the overall score if a patient highly overestimates or underestimates their abilities. The use of self-reported measures to assess aphasia outcomes in both research and clinical contexts necessitates further research on intellectual awareness in aphasia.
While slightly fewer than 50% of our participants were considered “Unaware,” this number is not an indication of prevalence of anosognosia in aphasia because the frequency will change depending on how intellectual awareness is assessed, how the criteria for “aware” and “unaware” are defined, which language function is being assessed, how that language function is measured, and who is included/excluded in the sample. Our results do suggest, however, that some people with aphasia are not as accurate or reliable in estimating their anomia severity compared with their peers. Importantly, since the accuracy of the unreliable raters’ self-ratings differed on the two days of testing, intellectual awareness may be variable in some people. Good awareness on one day may not indicate that good awareness is present all of the time, and poor awareness on one day may not indicate poor awareness all of the time. Thus, multiple administrations of self-assessments may be useful for clinicians to check reliability of a patient’s intellectual awareness of their communication skills.
Interestingly, in the current study, participants’ awareness as a group worsened from day 1 to day 2 while completing evaluation tasks in between without feedback. This finding indicates that clinicians cannot expect the evaluation process alone to improve patient awareness, and suggests instead that testing without providing feedback may actually distort awareness. Although this finding requires replication and further examination, distortion of awareness could occur as individuals inaccurately assess their performance on tests and then incorporate these self-evaluations into their overall sense of ability. This finding supports the need for patient education on the impairment throughout evaluation and incorporation of awareness activities into treatment programs.
Caregiver ratings have often been used as a point of comparison for patient self-ratings, and may be similarly used as a validation metric to assess intellectual awareness in cases when the caregiver is present and active in the patient’s recovery process and demonstrates understanding of the patient’s language impairments. However, there are many clinical cases in which caregivers may not be able to provide a valid measure of patient performance, e.g., due to limited involvement, lack of education regarding the patient’s impairments, personal biases, or other factors. In these cases, use of objective test results may offer an alternative for assessing patients’ intellectual awareness. One challenge with this approach is that individuals are likely to judge their abilities based on real-world functioning in natural environments, which may not correspond with their ability as measured the test chosen for comparison to their self-rating. For example, the interview question measuring intellectual awareness in our study inquired about one’s ability to name words aloud in general, while the available behavioral comparison was limited to confrontation naming. Given the typical nature of everyday language use, it is likely that participants were also considering the ability to name words beyond an isolated noun, such as saying a specific word in connected speech. For this reason, our results should be considered preliminary. Although our findings do show significant relationships between the self-ratings and objective performance, it would be useful to confirm these results in future studies including a quantitative metric of anomia in more naturalistic connected speech samples to account for both spontaneously generated words and contexts beyond a structured naming task. The challenge of this approach, however, is that individual anomic events can be difficult to quantify in naturalistic connected speech as they may be masked by compensatory strategies (e.g., circumlocution). Comparing self-ratings to multiple complementary objective measures of anomia, as well as caregiver and clinician ratings, would be ideal in order to understand the relationships among these different methods of assessing ability.
4.5. Limitations
This study was limited to investigating impaired self-awareness of anomia, although aphasia often encompasses impairments across multiple language functions. Since impaired self-awareness can be modality specific (Cocchini et al., 2012), further research is needed on self-awareness of other language functions (e.g., reading, writing) using patient performance on objective tests as the validating metric, ideally alongside caregiver and clinician ratings as discussed above. We also acknowledge that some healthy individuals may over- or underestimate themselves (Harty et al., 2013; Sakurai et al., 2013); therefore, some of the variability in stroke participants’ awareness may be due to premorbid self-perceptions. Nevertheless, the relationships between intellectual awareness and both lesion location and semantic deficits in our results suggest that the stroke also contributes to the observed variability in awareness.
4.6. Conclusions
Intellectual awareness is a fundamental cognitive ability, yet it has been widely overlooked in the evaluation and treatment of people living with aphasia after stroke. Understanding intellectual awareness in persons with aphasia may allow clinicians to better determine their patients’ self-perceptions of their abilities and deficits and guide decisions in treatment. Our results indicate that semantic deficits may relate to reduced intellectual awareness of naming abilities in people with aphasia with relatively intact comprehension. Finally, we observe intellectual awareness and emergent awareness to be two distinct abilities behaviorally, each negatively impacted by lesions to different regions of the left frontal lobe.
Highlights.
Some people with aphasia are aware of their naming abilities, while others are not
People with reduced awareness also demonstrated semantic deficits
Frontal lesions, particularly of pars orbitalis, related to reduced awareness
Intellectual awareness did not correlate with emergent awareness
The results inform current neurocognitive theories of awareness
Acknowledgements
This study was funded by the NIH/NIDCD [grant numbers R03DC014310, F31DC014875, R01DC014960]. Financial sponsors did not have a role in conducting the research or preparing the manuscript. We express the deepest gratitude to our participants who made this research possible. We thank Zainab Anbari, Maryam Ghaleh, Katherine Spiegel, Mary Henderson, and Harshini Pyata for contributing to data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: none.
References
- Alajouanine T (1956). Verbal realization in aphasia. Brain, 79(1), 1–28. 10.1093/brain/79.1.1 [DOI] [PubMed] [Google Scholar]
- Alajouanine T, Lhermitte F, Ledoux M, Renaud D, & Vignolo L (1964). Les composantes phonémiques et sémantiques de la jargonaphasie. Revue Neurologique, 5–20. [PubMed] [Google Scholar]
- Allen CM, Martin RC, & Martin N (2012). Relations between short-term memory deficits, semantic processing, and executive function. Aphasiology, 26(3–4), 428–461. 10.1080/02687038.2011.617436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, & Tranel D (1989). Awareness of disease states following cerebral infarction, dementia, and head trauma: Standardized assessment. Clinical Neuropsychologist, 3(4), 327–339. 10.1080/13854048908401482 [DOI] [Google Scholar]
- Avants BB, Tustison N, & Song G (2009). Advanced Normalization Tools (ANTS). Insight Journal, 1–35. http://hdl.handle.net/10380/3113 [Google Scholar]
- Bivona U, Ciurli P, Ferri G, Fontanelli T, Lucatello S, Donvito T, Villalobos D, Cellupica L, Mungiello F, Lo Sterzo P, Ferraro A, Giandotti E, Lombardi G, Azicnuda E, Caltagirone C, Formisano R, & Costa A (2020). The Self-Awareness Multilevel Assessment Scale, a new tool for the assessment of self-awareness after severe acquired brain injury: Preliminary findings. Frontiers in Psychology, 11. 10.3389/fpsyg.2020.01732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacfkmer ER, & Mitton JL (1991). Theories of monitoring and the timing of repairs in spontaneous speech. Cognition, 39(3), 173–194. 10.1016/0010-0277(91)90052-6 [DOI] [PubMed] [Google Scholar]
- Butler RA, Lambon-Ralph MA, & Woollams AM (2014). Capturing multidimensionality in stroke aphasia: Mapping principal behavioural components to neural structures. Brain: A Journal of Neurology, 137(12), 3248–3266. 10.1093/brain/awu286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B, & Howard D (1987). Paragrammatisms. Cognition, 26(1), 1–37. 10.1016/0010-0277(87)90012-6 [DOI] [PubMed] [Google Scholar]
- Cocchini G, Beschin N, & Sala SD (2012). Assessing anosognosia: A critical review. ACTA Neuropsychologica, 10(3), 419–443. [Google Scholar]
- Cocchini G, Gregg N, Beschin N, Dean M, & Sala SD (2010). Vata-L: Visual-Analogue Test Assessing Anosognosia for Language Impairment. The Clinical Neuropsychologist, 24(8), 1379–1399. 10.1080/13854046.2010.524167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Barco PP, Velozo CA, Bolesta MM, Cooper PV, Werts D, & Brobeck TC (1989). Awareness and compensation in postacute head injury rehabilitation. The Journal of Head Trauma Rehabilitation, 4(3), 46–54. 10.1097/00001199-198909000-00008 [DOI] [Google Scholar]
- de Jong-Hagelstein M, Kros L, Lingsma HF, Dippel DWJ, Koudstaal PJ, & VischBrink EG (2012). Expert versus proxy rating of verbal communicative ability of people with aphasia after stroke. Journal of the International Neuropsychological Society, 18(6), 1064–1070. 10.1017/S1355617712000811 [DOI] [PubMed] [Google Scholar]
- Dean MP, Della Sala S, Beschin N, & Cocchini G (2017). Anosognosia and self-correction of naming errors in aphasia. Aphasiology, 31(7), 725–740. 10.1080/02687038.2016.1239014 [DOI] [Google Scholar]
- Demarco AT, & Turkeltaub PE (2018). A multivariate lesion symptom mapping toolbox and examination of lesion-volume biases and correction methods in lesion-symptom mapping. Human Brain Mapping. 10.1002/hbm.24289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockree PM, Tarleton YM, Carton S, & FitzGerald MCC (2015). Connecting self-awareness and error-awareness in patients with traumatic brain injury. Journal of the International Neuropsychological Society, 21(7), 473–482. 10.1017/S1355617715000594 [DOI] [PubMed] [Google Scholar]
- Fama ME, Henderson MP, Snider SF, Hayward W, Friedman RB, & Turkeltaub PE (2019a). Self-reported inner speech relates to phonological retrieval ability in people with aphasia. Consciousness and Cognition, 71, 18–29. 10.1016/j.concog.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama ME, Snider SF, Henderson MP, Hayward W, Friedman RB, & Turkeltaub PE (2019b). The subjective experience of inner speech in aphasia is a meaningful reflection of lexical retrieval. Journal of Speech, Language, and Hearing Research, 62(1), 106–122. 10.1044/2018_JSLHR-L-18-0222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Matsuoka T, Kato Y, Shibata K, Nakamura K, Yamada K, & Narumoto J (2017). Brain regions associated with anosognosia for memory disturbance in Alzheimer’s disease: A magnetic resonance imaging study. Neuropsychiatric Disease and Treatment, 13, 1753–1759. 10.2147/NDT.S139177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geytenbeek M, Fleming J, Doig E, & Ownsworth T (2017). The occurrence of early impaired self-awareness after traumatic brain injury and its relationship with emotional distress and psychosocial functioning. Brain Injury, 31(13–14), 1791–1798. 10.1080/02699052.2017.1346297 [DOI] [PubMed] [Google Scholar]
- Goodglass H (1992). Diagnosis of conduction aphasia. In Kohn SE (Ed.), Conduction Aphasia (pp. 39–49). Lawrence Erlbaum Associates. [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2001). BDAE: The Boston Diagnostic Aphasia Examination (Third). Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Goverover Y, Genova H, Griswold H, Chiaravalloti N, & DeLuca J (2014). Metacognitive knowledge and online awareness in persons with multiple sclerosis. NeuroRehabilitation, 35(2), 315–323. 10.3233/NRE-141113 [DOI] [PubMed] [Google Scholar]
- Grützmann R, Endrass T, Klawohn J, & Kathmann N (2014). Response accuracy rating modulates ERN and Pe amplitudes. Biological Psychology, 96, 1–7. 10.1016/j.biopsycho.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Guenther FH (1994). A neural network model of speech acquisition and motor equivalent speech production. Biological Cybernetics, 72(1), 43–53. 10.1007/bf00206237 [DOI] [PubMed] [Google Scholar]
- Haley KL, Jacks A, de Riesthal M, Abou-Khalil R, & Roth HL (2012). Toward a quantitative basis for assessment and diagnosis of apraxia of speech. Journal of Speech, Language, and Hearing Research, 55(5). 10.1044/1092-4388(2012/11-0318) [DOI] [PubMed] [Google Scholar]
- Hallam B, Chan J, Gonzalez Costafreda S, Bhome R, & Huntley J (2020). What are the neural correlates of meta-cognition and anosognosia in Alzheimer’s disease? A systematic review. Neurobiology of Aging, 94, 250–264. 10.1016/j.neurobiolaging.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty S, O’Connell RG, Hester R, & Robertson IH (2013). Older adults have diminished awareness of errors in the laboratory and daily life. Psychology and Aging, 28(4), 1032–1041. 10.1037/a0033567 [DOI] [PubMed] [Google Scholar]
- Hécaen H, & Albert M (1978). Human Neuropsychology. Wiley. [Google Scholar]
- Heilman KM (1991). Anosognosia: Possible neuropsychological mechanisms. In Awareness of deficit after brain injury: Clinical and theoretical issues (pp. 53–62). Oxford University Press. [Google Scholar]
- Helm-Estabrooks N, Albert ML, & Nicholas M (2014). Manual of aphasia and aphasia therapy. PRO-ED. [Google Scholar]
- Hibbard MR, Gordon WA, Stein PN, Grober S, & Sliwinski M (1992). Awareness of Disability in Patients Following Stroke. Rehabilitation Psychology, 37(2), 18. [Google Scholar]
- Hickok G (2012). Computational neuroanatomy of speech production. Nature Reviews. Neuroscience, 13(2), 135–145. 10.1038/nrn3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilari K, Byng S, Lamping DL, & Smith SC (2003). Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39): Evaluation of acceptability, reliability, and validity. Stroke, 34(8), 1944–1950. 10.1161/01.STR.0000081987.46660.ED [DOI] [PubMed] [Google Scholar]
- Hoerold D, Pender NP, & Robertson IH (2013). Metacognitive and online error awareness deficits after prefrontal cortex lesions. Neuropsychologia, 51(3), 385–391. 10.1016/j.neuropsychologia.2012.11.019 [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, & Prigatano GP (2002). Neural correlates of self-reflection. Brain: A Journal of Neurology, 125(Pt 8), 1808–1814. 10.1093/brain/awf181 [DOI] [PubMed] [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery - Revised. San Antonio, TX: Pearson. [Google Scholar]
- Kertesz A (2010). Anosognosia in aphasia. In Prigatano GP (Ed.), The study of anosognosia (pp. 113–122). Oxford University Press. [Google Scholar]
- Kertesz A, & Mccabe P (1977). Recovery patterns and prognosis in aphasia. Brain, 100(1), 1–18. 10.1093/brain/100.1.1 [DOI] [PubMed] [Google Scholar]
- Kinsbourne M, & Warrington EK (1963). Jargon aphasia. Neuropsychologia, 1(1), 27–37. 10.1016/0028-3932(63)90010-1 [DOI] [Google Scholar]
- Kolakowsky-Hayner S (2010). Introduction to the patient competency rating scale. The Center for Outcome Measurement in Brain Injury. http://www.tbims.org/combi/pcrs [Google Scholar]
- Koo TK, & Li MY (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15(2), 155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey EH, Skipper-Kallal LM, Xing S, Fama ME, & Turkeltaub PE (2017). Mapping common aphasia assessments to underlying cognitive processes and their neural substrates. Neurorehabilitation and Neural Repair, 31(5), 442–450. 10.1177/1545968316688797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine M, & Martin N (2006). Anomia: Theoretical and clinical aspects. New York, NY: Psychology Press. [Google Scholar]
- Lee MD, & Wagenmakers EJ (2013). Bayesian Cognitive Modeling: A Practical Course. Cambridge University Press. [Google Scholar]
- Levelt WJ (1983). Monitoring and self-repair in speech. Cognition, 14(1), 41–104. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22(01), Article 01. 10.1017/S0140525X99001776 [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Schriefers H, Vorberg D, Meyer AS, Pechmann T, & Havinga J (1991). The time course of lexical access in speech production: A study of picture naming. Psychological Review, 98(1), 122–142. 10.1037/0033-295X.98.1.122 [DOI] [Google Scholar]
- Mah YH, Husain M, Rees G, & Nachev P (2014). Human brain lesion-deficit inference remapped. Brain, 137(9), 2522–2531. 10.1093/brain/awu164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher LM, Rothi LJ, & Heilman KM (1994). Lack of error awareness in an aphasic patient with relatively preserved auditory comprehension. Brain and Language, 46(3), 402–418. 10.1006/brln.1994.1022 [DOI] [PubMed] [Google Scholar]
- Mahoney D, Gutman S, & Gillen G (2019). A scoping review of self-awareness instruments for acquired brain injury. The Open Journal of Occupational Therapy, 7(2). 10.15453/2168-6408.1529 [DOI] [Google Scholar]
- Malouf T, Langdon R, & Taylor A (2014). The insight interview: A new tool for measuring deficits in awareness after traumatic brain injury. Brain Injury, 28(12), 1523–1541. 10.3109/02699052.2014.922700 [DOI] [PubMed] [Google Scholar]
- Mandal AS, Fama ME, Skipper-Kallal LM, DeMarco AT, Lacey EH, & Turkeltaub PE (2020). Brain structures and cognitive abilities important for the self-monitoring of speech errors. Neurobiology of Language, 1–52. 10.1162/nol_a_00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J, Robson J, Pring T, & Chiat S (1998). Why does monitoring fail in jargon aphasia? Comprehension, judgment, and therapy evidence. Brain and Language, 63(1), 79–107. 10.1006/brln.1997.1936 [DOI] [PubMed] [Google Scholar]
- Marshall RC, Rappaport BZ, & Garcia-Bunuel L (1985). Self-monitoring behavior in a case of severe auditory agnosia with aphasia. Brain and Language, 24(2), 297–313. 10.1016/0093-934x(85)90137-3 [DOI] [PubMed] [Google Scholar]
- Marshall Robert C., Neuburger SI, & Phillips DS (1994). Verbal self-correction and improvement in treated aphasic clients. Aphasiology, 8(6), 535–547. 10.1080/02687039408248680 [DOI] [Google Scholar]
- Martin N, Minkina I, Kohen FP, & Kalinyak-Fliszar M (2018). Assessment of linguistic and verbal short-term memory components of language abilities in aphasia. Journal of Neurolinguistics, 48, 199–225. 10.1016/j.jneuroling.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn SM, & Schacter DL (1989). Unawareness of deficits in neuropsychological syndromes. Journal of Clinical and Experimental Neuropsychology, 11(2), 143–205. 10.1080/01688638908400882 [DOI] [PubMed] [Google Scholar]
- Mirman D, Landrigan JF, Kokolis S, Verillo S, Ferrara C, & Pustina D (2018). Corrections for multiple comparisons in voxel-based lesion-symptom mapping. Neuropsychologia, 115, 112–123. 10.1016/j.neuropsychologia.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels L, & Howard D (1995). Phonological errors in aphasic naming: Comprehension, monitoring and lexicality. Cortex, 31(2), 209–237. [DOI] [PubMed] [Google Scholar]
- Nozari N (2020). A comprehension- or a production-based monitor? Response to Roelofs (2020). Journal of Cognition, 3(1), 19. 10.5334/joc.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Dell GS, & Schwartz MF (2011). Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cognitive Psychology, 63(1), 1–33. 10.1016/j.cogpsych.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe F, Dockree P, Moloney P, Carton S, & Robertson IH (2007). Awareness of deficits in traumatic brain injury: A multidimensional approach to assessing metacognitive knowledge and online-awareness. Journal of the International Neuropsychological Society, 13(01). 10.1017/S1355617707070075 [DOI] [PubMed] [Google Scholar]
- Oomen CC, Postma A, & Kolk HH (2001). Prearticulatory and postarticulatory self-monitoring in Broca’s aphasia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 37(5), 627–641. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Caltagirone C, & Spalletta G (2009). The evaluation of anosognosia in stroke patients. Cerebrovascular Diseases, 27(3), 280–289. 10.1159/000199466 [DOI] [PubMed] [Google Scholar]
- Postma A (2000). Detection of errors during speech production: A review of speech monitoring models. Cognition, 77(2), 97–132. [DOI] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riès SK, Xie K, Haaland KY, Dronkers NF, & Knight RT (2013). Role of the lateral prefrontal cortex in speech monitoring. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs A (2020). Self-monitoring in speaking: In defense of a comprehension-based account. Journal of Cognition, 3(1), 18. 10.5334/joc.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Bonilha L, Fridriksson J, Bender B, & Karnath HO (2012). Age-specific CT and MRI templates for spatial normalization. NeuroImage, 61(4), 957–965. 10.1016/j.neuroimage.2012.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens AB, & Garrett MF (1991). Anosognosia of linguistic deficits in patients with neurological deficits. In Schacter DL & Prigatano GP (Eds.), Awareness of deficit after brain injury: Clinical and theoretical issues (pp. 40–52). Oxford University Press. [Google Scholar]
- Sakurai R, Fujiwara Y, Ishihara M, Higuchi T, Uchida H, & Imanaka K (2013). Age-related self-overestimation of step-over ability in healthy older adults and its relationship to fall risk. BMC Geriatrics, 13(1), 44. 10.1186/1471-2318-13-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E, Perani D, Herholz K, Marique P, Kalbe E, Holthoff V, Delbeuck X, Beuthien-Baumann B, Pelati O, Lespagnard S, Collette F, & Garraux G (2006). Neural correlates of anosognosia for cognitive impairment in Alzheimer’s disease. Human Brain Mapping, 27(7), 588–597. 10.1002/hbm.20203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti D, Fleming J, Patterson F, & Lannin NA (2021). Conceptualization of self-awareness in adults with acquired brain injury: A qualitative systematic review. Neuropsychological Rehabilitation, 0(0), 1–48. 10.1080/09602011.2021.1924794 [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Rowley HA, Kawahara TN, & Johnson SC (2006). Neural correlates of self-evaluative accuracy after traumatic brain injury. Neuropsychologia, 44(5), 762–773. 10.1016/j.neuropsychologia.2005.07.012 [DOI] [PubMed] [Google Scholar]
- Schuchard J, Middleton EL, & Schwartz MF (2017). The timing of spontaneous detection and repair of naming errors in aphasia. Cortex, 93, 79–91. 10.1016/j.cortex.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Middleton EL, Brecher A, Gagliardi M, & Garvey K (2016). Does naming accuracy improve through self-monitoring of errors? Neuropsychologia, 84, 272–281. 10.1016/j.neuropsychologia.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, & Rankin KP (2014). Self-awareness in neurodegenerative disease relies on neural structures mediating rewarddriven attention. Brain, 137(8), 2368–2381. 10.1093/brain/awu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer M, Bergloff P, Levin E, High WM, Oden KE, & Nick TG (1998). Impaired awareness and employment outcome after traumatic brain injury. The Journal of Head Trauma Rehabilitation, 13(5), 52–61. 10.1097/00001199-199810000-00007 [DOI] [PubMed] [Google Scholar]
- Sherer Mark, Hart T, Nick TG, Whyte J, Thompson RN, & Yablon SA (2003). Early impaired self-awareness after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 84(2), 168–176. 10.1053/apmr.2003.50045 [DOI] [PubMed] [Google Scholar]
- Shuren JE, Greenwald M, & Heilman KM (1996). Spontaneous grammatical corrections in an anomic aphasic. Neurology, 47(3), 845–846. 10.1212/WNL.47.3.845 [DOI] [PubMed] [Google Scholar]
- Spikman JM, & van der Naalt J (2010). Indices of impaired self-awareness in traumatic brain injury patients with focal frontal lesions and executive deficits: Implications for outcome measurement. Journal of Neurotrauma, 27(7), 1195–1202. 10.1089/neu.2010.1277 [DOI] [PubMed] [Google Scholar]
- Stuss DT (1991a). Disturbance of self-awareness after frontal system damage. In Awareness of deficit after brain injury: Clinical and theoretical issues (pp. 63–83). Oxford University Press. [Google Scholar]
- Stuss DT (1991b). Self, Awareness, and the Frontal Lobes: A Neuropsychological Perspective. In Strauss J & Goethals GR (Eds.), The Self: Interdisciplinary Approaches (pp. 255–278). Springer-Verlag New York, Inc. [Google Scholar]
- Stuss DT, & Anderson V (2004). The frontal lobes and theory of mind: Developmental concepts from adult focal lesion research. Brain and Cognition, 55(1), 69–83. 10.1016/S0278-2626(03)00271-9 [DOI] [PubMed] [Google Scholar]
- Stuss DT, Rosenbaum RS, Malcolm S, Christiana W, & Keenan JP (2005). The frontal lobes and self-awareness. In Feinberg TE & Keenan JP (Eds.), The lost self (pp. 50–64). Oxford University Press. [Google Scholar]
- Tate R, Kennedy M, Ponsford J, Douglas J, Velikonja D, Bayley M, & Stergiou-Kita M (2014). INCOG recommendations for management of cognition following traumatic brain injury, part III: Executive function and self-awareness. Journal of Head Trauma Rehabilitation, 29(4), 338–352. 10.1097/HTR.0000000000000068 [DOI] [PubMed] [Google Scholar]
- Tham K, Bernspang B, & Fisher AG (1999). Development of the assessment of awareness of disability. Scandinavian Journal of Occupational Therapy, 6(4), 184–190. 10.1080/110381299443663 [DOI] [Google Scholar]
- Toglia J, & Kirk U (2000). Understanding awareness deficits following brain injury. NeuroRehabilitation, 15(1), 57–70. 10.3233/NRE-2000-15104 [DOI] [PubMed] [Google Scholar]
- Tourville JA, & Guenther FH (2011). The DIVA model: A neural theory of speech acquisition and production. Language and Cognitive Processes, 26(7), 952–981. 10.1080/01690960903498424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigliocco G, & Hartsuiker RJ (2002). The interplay of meaning, sound, and syntax in sentence production. Psychological Bulletin, 128(3), 442–472. 10.1037/0033-2909.128.3.442 [DOI] [PubMed] [Google Scholar]
- Vocat R, Staub F, Stroppini T, & Vuilleumier P (2010). Anosognosia for hemiplegia: A clinical-anatomical prospective study. Brain, 133(12), 3578–3597. 10.1093/brain/awq297 [DOI] [PubMed] [Google Scholar]
- Wagner MT, Spangenberg KB, Bachman DL, & O’Connell P (1997). Unawareness of cognitive deficit in Alzheimer disease and related dementias. Alzheimer Disease & Associated Disorders, 11(3), 125–131. [DOI] [PubMed] [Google Scholar]
- Walker GM, & Schwartz MF (2012). Short-Form Philadelphia Naming Test: Rationale and empirical evaluation. American Journal of Speech-Language Pathology, 21(2). 10.1044/1058-0360(2012/11-0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace SJ, Worrall L, Rose T, Le Dorze G, Breitenstein C, Hilari K, Babbitt E, Bose A, Brady M, Cherney LR, Copland D, Cruice M, Enderby P, Hersh D, Howe T, Kelly H, Kiran S, Laska A-C, Marshall J, … Webster J (2019). A core outcome set for aphasia treatment research: The ROMA consensus statement. International Journal of Stroke, 14(2), 180–185. 10.1177/1747493018806200 [DOI] [PubMed] [Google Scholar]
- Wessel JR (2012). Error awareness and the error-related negativity: Evaluating the first decade of evidence. Frontiers in Human Neuroscience, 6. 10.3389/fnhum.2012.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G, & Wilcock G (2011). Lack of awareness of symptoms in people with dementia: The structural and functional basis. International Journal of Geriatric Psychiatry, 26(8), 783–792. 10.1002/gps.2620 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kimberg DY, Coslett HB, Schwartz MF, & Wang Z (2014). Multivariate lesion-symptom mapping using support vector regression. Human Brain Mapping, 35(12), 5861–5876. 10.1002/hbm.22590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N, Mograbi DC, Hermes-Pereira A, Fonseca RP, & Prigatano GP (2017). Memory and executive functions correlates of self-awareness in traumatic brain injury. Cognitive Neuropsychiatry, 22(4), 346–360. 10.1080/13546805.2017.1330191 [DOI] [PubMed] [Google Scholar]


