Abstract
Despite the critical role played by carbon monoxide (CO) in physiological and pathological signaling events, current approaches to deliver this messenger molecule are often accompanied by off-target effects and offer limited control over release kinetics. To address these challenges, we developed an electrochemical approach that affords on-demand release of CO through reduction of carbon dioxide (CO2) dissolved in the extracellular space. Electrocatalytic generation of CO by cobalt phthalocyanine molecular catalysts modulates signaling pathways mediated by a CO receptor, soluble guanylyl cyclase. Furthermore, by tuning the applied voltage during electrocatalysis, we explore the effect of the CO release kinetics on CO-dependent neuronal signaling. Finally, we integrate components of our electrochemical platform into microscale fibers to produce CO in a spatially-restricted manner and to activate signaling cascades in the targeted cells. By offering on-demand local synthesis of CO, our approach may facilitate the studies of physiological processes affected by this gaseous molecular messenger.
Keywords: carbon monoxide, cell signaling, electrochemistry, fiber drawing, receptors
Graphical Abstract
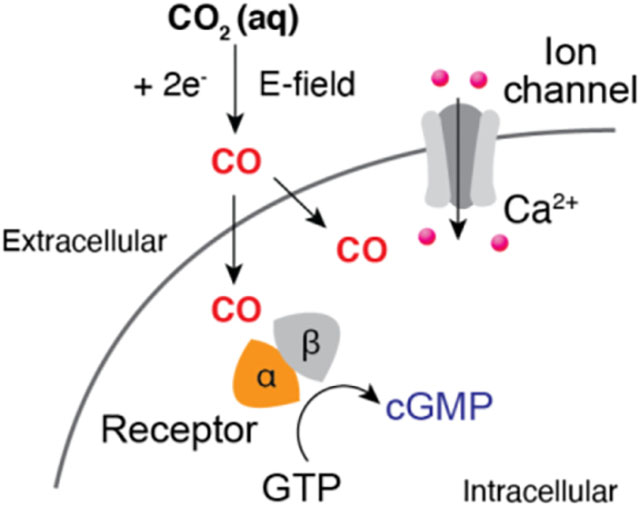
We devise an electrochemical strategy to locally synthesize carbon monoxide (CO), a gaseous messenger molecule, with tunable kinetics. By integrating this strategy with fiber-based fabrication, diverse CO-dependent signaling pathways in genetically engineered cells or neurons can be modulated at the microscale.
Introduction
Carbon monoxide (CO) is a gaseous and transmembrane diffusible messenger affecting numerous physiological and pathological processes, including vasoactive response, neurotransmission, and inflammation[1–5]. To understand the physiological effects of CO and potentially harness them in a therapeutic context, prior work has explored direct delivery of CO gas via respiratory administration[2,6]. However, this approach does not permit targeting of CO to specific tissues or organs and poses a risk of a global increase in carboxyhemoglobin levels manifesting in CO poisoning[2,7–9]. Consequently, CO-releasing molecules (CORMs), which release CO as a free gas or transfer it to biological molecules, have been designed for targeted delivery of CO[10–12]. However, it remains challenging to tune CO-release kinetics of CORMs without modifying their molecular structures, and thus multiple CORMs are necessary for applications demanding variable CO release kinetics[2,11,13,14]. Moreover, degradation of inherently unstable CORMs during delivery limits precision of CO dosing often leading to off-target release beyond tissues of interest[7].
To circumvent these challenges, we developed a system that enables on-demand synthesis of CO through electrochemical CO2 reduction reaction (Figure 1a). By leveraging a selective catalyst cobalt phthalocyanine (CoPc)[15–17], CO2 dissolved in the extracellular solution can be reduced to CO at the cathode (Figure 1b). Due to its high solubility (~ 34 mM) in water, dissolved CO2, which exists in equilibrium with bicarbonate buffer[16,18,19], serves as a precursor for electrochemical formation of CO. Electrochemically produced CO is shown to modulate diverse CO-dependent cell signaling processes in vitro. Furthermore, CO release kinetics can be controlled by varying the cathode voltage. This tunability of CO release kinetics enabled systematic investigation of neuronal signaling mediated by this molecule. Finally, we demonstrate microscale, CO-releasing electrocatalytic fibers as tools to locally activate cell signaling.
Figure 1.
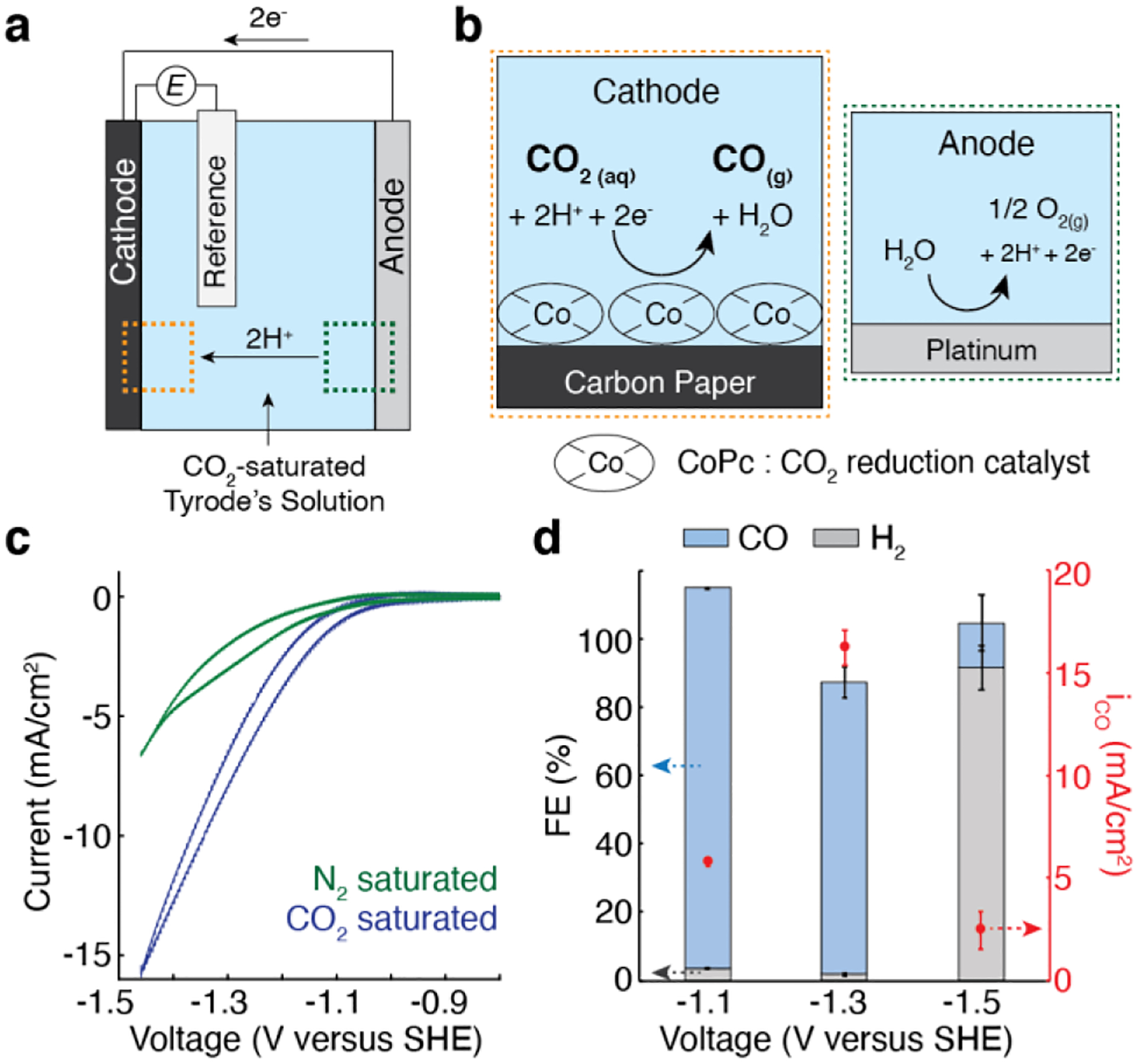
a, A schematic illustrating the electrochemical system for in situ CO delivery. b, An illustration of the electrochemical reactions at the CoPc/OxCP cathode and Pt anode. c, CV curves of CoPc/OxCP electrodes in CO2− (blue) or N2− (green) saturated Tyrode’s solution at pH 7.4 (scan rate, 100 mV/s). d, The Faradaic efficiency (FE) for CO and H2, and partial current density of CO (iCO) (mean ± standard error of the mean (s.e.m.), n = 3) at various applied voltages.
Results and Discussion
CoPc loaded cathodes were prepared by drop-casting CoPc ink (~ 1 mg/ml) onto the oxygen-functionalized carbon paper (OxCP). The electrocatalytic activities of CoPc/OxCP cathodes were evaluated in a three-compartment electrochemical cell containing physiological solution (Tyrode’s) saturated with CO2 at pH 7.4. Pt and Ag/AgCl electrodes were employed as anode and reference electrodes, respectively (Figure 1a, b). Cyclic voltammetry (CV) profiles of CoPc/OxCP in CO2-saturated Tyrode’s solution showed higher reductive currents as compared with those recorded in N2-saturated solution (Figure 1c).
The cathodic products in CO2-saturated Tyrode’s solution were analyzed by chronoamperometry across a range of applied voltages. The cathodes exhibited high selectivity toward CO up to −1.3 V versus standard hydrogen electrode (SHE), and partial current density of CO increased as we applied more reductive potentials to the cathodes at this voltage range. Negligible amounts of H2, a side product at the cathodes[15], were generated at identical reaction conditions with the Faradaic Efficiencies (FE) in the range of 1.8–3.4 % (Figure 1d). At higher negative voltages (≤ −1.5 V), hydrogen evolution reaction dominated over CO2 reduction reaction (Figure 1d and Figure S1). At Pt anodes, oxygen evolution was predominately found with a minor chlorine evolution (Figure S2). Together, these data indicated that CO can be electrochemically generated from CO2 dissolved in Tyrode’s solution with high selectivity, and CO release kinetics can be controlled via applied voltage.
To illustrate the utility of our electrochemical system for modulating CO-dependent signaling, we first applied it to activate soluble guanylyl cyclase (sGC), one of the well-characterized receptors of CO[1–3]. It was previously shown that CO can bind to the heme moiety in sGC, activating the catalytic conversion of guanosine 5’ triphosphate (GTP) to the second messenger cyclic guanosine 3’,5’-monophosphate (cGMP)[20–22]. To elicit robustly measurable cGMP formation, human embryonic kidney (HEK) 293 FT cells were co-transfected with two plasmids carrying DDK-tagged α- and DDK-tagged β-subunits of human sGC under the broad mammalian cytomegalovirus (CMV) promoter, respectively. The expression of these subunits in the cells was confirmed by immunostaining with anti-DDK antibodies (Figure 2a). Consistent with previous reports[23,24], cells overexpressing both subunits (sGC+ cells) exhibited substantially higher basal cGMP levels (~300 fold) as compared to unmodified cells only expressing low levels of endogenous sGC. In contrast, no noticeable increases in the cGMP levels were found after transfection of cells with either α-subunit or β-subunit alone (Figure 2b). When exposed to nitric oxide (NO), a highly potent activator of sGC[3,25], robust increase in cGMP levels (~20 fold) was found in sGC+ cells, confirming the functionality of the genetically introduced sGC (Figure S3).
Figure 2.
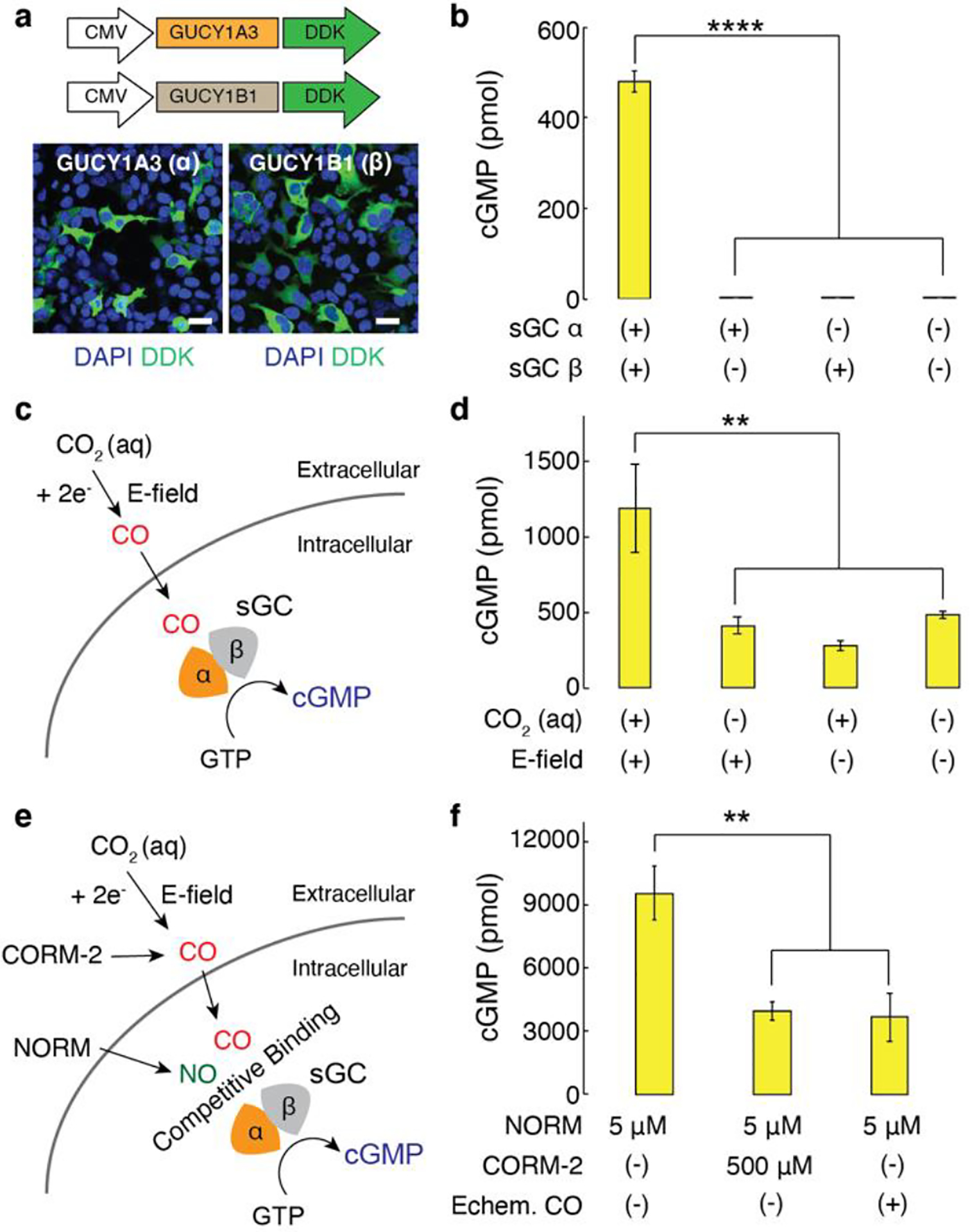
a, Representative confocal images of HEK cells transfected with DDK-tagged α-subunit of sGC or DDK-tagged β-subunit of sGC under the CMV promoter (scale bar, 50 μm). b, Intracellular cGMP levels (mean ± s.e.m.) in 106 HEK cells 48 h after the transfection (n = 6, one-way analysis of variance (ANOVA) and Tukey’s multiple comparison test, F3,20 = 392.2, **** p = 1.1 × 10−16 < 0.0001). c, A schematic illustrating activation of sGC mediated by electrochemically produced CO. GTP, guanosine 5’ triphosphate. d, Intracellular cGMP levels (mean ± s.e.m.) in 106 sGC+ cells following CO delivery driven by CoPc/OxCP cathodes at −1.3 V versus SHE for 10 min. The statistical significance of an increase in cGMP levels after electrochemical CO delivery as compared with controls was assessed by one-way ANOVA and Tukey’s multiple comparison test (n = 6, F3,20 = 7.4, ** p = 0.0016 < 0.01). e, An illustration of NO-sGC-cGMP signaling pathways modulated by CO. NORM, nitric oxide releasing molecule. f, Intracellular cGMP levels (mean ± s.e.m.) in 106 sGC+ cells following NO delivery in the presence or absence of CO (n = 5, one-way ANOVA and Tukey’s multiple comparison test, F2,12 = 10.9, ** p = 0.002 < 0.01).
We next investigated whether electrochemically synthesized CO could activate sGC-cGMP signaling pathways (Figure 2c). We first confirmed that CoPc catalysts did not elicit any cytotoxic responses in HEK cells. Similarly, negligible cytotoxic responses were found in cells following acute exposure to CO2-saturated Tyrode’s solution (Figure S4). Additionally, we confirmed that the concentration of dissolved CO2 at the bottom of the well plate, where sGC+ cells were located, remained largely constant over 30 min in the absence of continuous CO2 purging (Figure S5). CO produced at the CoPc/OxCP cathodes at −1.3 V versus SHE in CO2-saturated Tyrode’s solution at pH 7.4 led to a moderate increase in cGMP levels (~150 %) in sGC+ cells. To investigate whether the observed increase in cGMP is attributable to electrochemically formed CO, we recorded the cGMP levels in control groups, which included sGC+ cells immersed in CO2-saturated Tyrode’s solution in the absence of an applied voltage and in the cells immersed in a solution not saturated in CO2 in the presence of an applied voltage (−1.3 V versus SHE). Consistent with prior studies that found no changes in cGMP levels upon CO2 delivery[26], no substantial increases in cGMP levels were observed in our control groups, indicating that electrochemically formed CO was responsible for sGC activation (Figure 2d).
Furthermore, electrochemically synthesized CO was sufficient to modulate the NO-mediated sGC-cGMP signaling in sGC+ cells (Figure 2e). It has been previously proposed that CO, a weaker activator of sGC as compared to NO, could attenuate NO-mediated cGMP increases by competing for the same binding site (heme moiety of sGC) with NO[3,27]. Indeed, inhibitory effects of CO on NO-mediated sGC activation were found in a number of organs, including cerebellar cortex, retina, and resistance arteries[28–30]. Consistent with these prior studies[3,27], we observed noticeable decreases in NO-stimulated cGMP levels (~60 %) in sGC+ cells following addition of 500 μM CORM-2 (tricarbonyldichlororuthenium(II) dimer), a molecular CO donor. Similarly, CO synthesized at CoPc/OxCP cathodes in CO2-saturated Tyrode’s solution led to attenuation in NO-mediated cGMP production (~70 %) (Figure 2f). These results suggested that the electrocatalytic CO-delivery approach could be extended to regulate diverse sGC-mediated signaling pathways.
We next evaluated whether our electrochemical system can be applied to interrogate CO-dependent signaling in neurons. Among the diversity of brain regions, hippocampus neurons have been shown to express high levels of heme oxygenase, which produces endogenous CO, implying that CO could play a critical role in hippocampal physiology[1,31]. Indeed, it has been reported that both endogenous and exogenous CO can trigger a myriad of processes in the hippocampus, including apoptosis, long-term potentiation, and expression of immediate early genes[32–34]. Thus we adopted hippocampal neurons as test beds for the CO-dependent signaling in neurons, and CO-triggered neuronal activity was recorded using an intracellular Ca2+ indicator fluo-4 as a proxy for neuronal membrane depolarization[35].
The effects of exogenous CO on hippocampal neurons were first investigated using CORM-2. When exposed to CORM-2 (50 μM), the normalized fluo-4 fluorescence (ΔF/F0) in hippocampal neurons gradually increased over time indicating Ca2+ influx into these cells, whereas the addition of an inactive form of CORM-2, RuCl3, did not evoke any comparable fluorescence changes in neurons (Figure 3a, b). Addition of lower concentration of CORM-2 (from 1 to 10 μM) led to smaller Ca2+ influxes in neurons, indicating that observed Ca2+ signaling were predominantly triggered by CORM-2 (Figure 3c).
Figure 3.
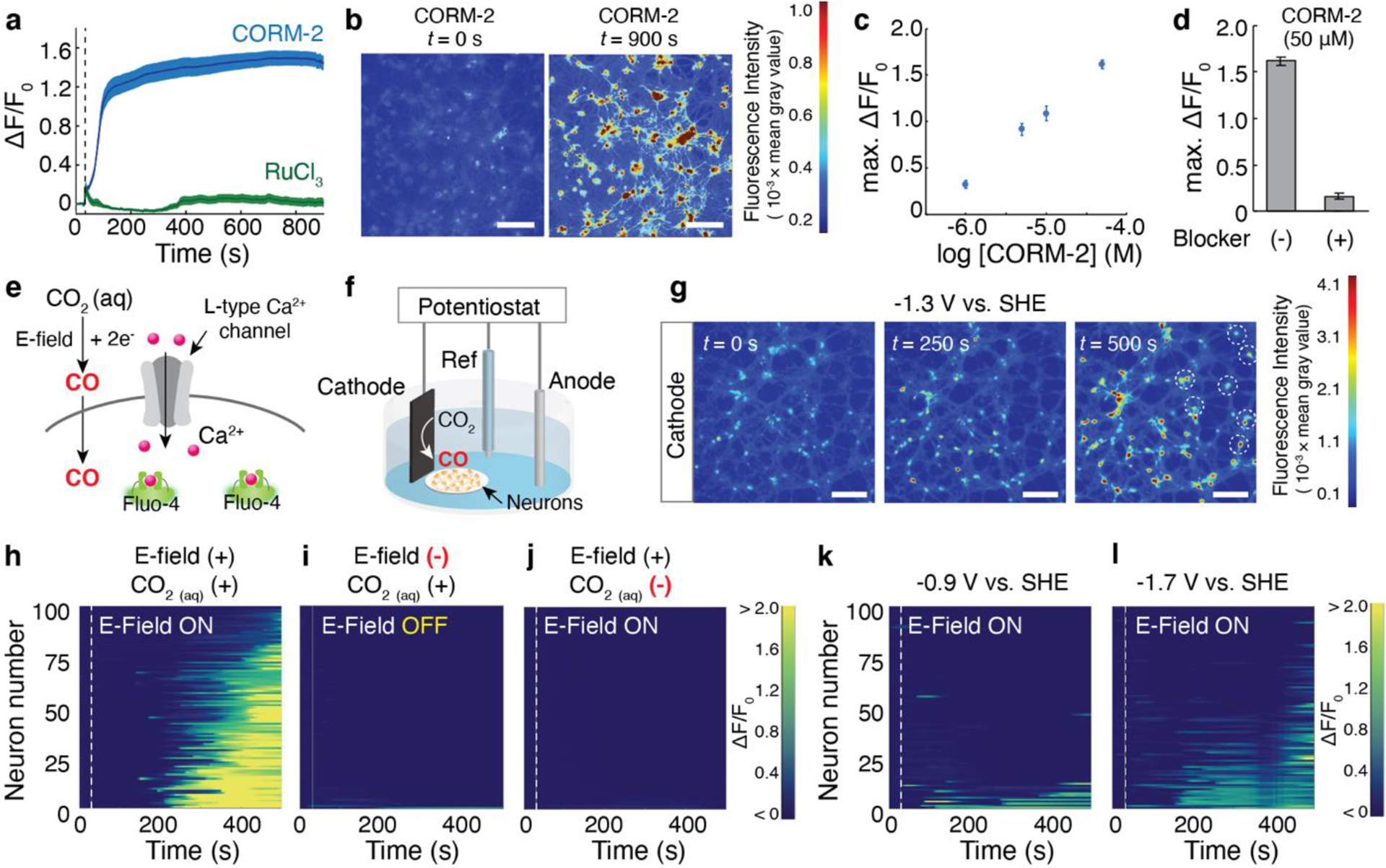
a, Averaged fluo-4 fluorescence traces for hippocampal neurons (n = 100 neurons for each trace) following 50 μM CORM-2 (blue) or 50 μM RuCl3 (green) infusion at 30 s (dashed lines). The solid lines and shaded areas represent the mean and s.e.m., respectively. b, Time-lapse images of Ca2+ responses in response to CORM-2 infusion (scale bar, 50 μm). c, CORM-2 concentration-dependent maximum of normalized fluo-4 fluorescence change averaged across 100 neurons. d, Maximum of normalized fluo-4 fluorescence increases in 100 neurons following the infusion of 50 μM CORM-2 in the presence or absence of L-type Ca2+ channel blocker nitrendipine. e, A schematic illustrating a potential mechanism of CO-mediated Ca2+ responses in neurons through L-type Ca2+ channel. f, Experimental scheme for electrochemical CO delivery to neurons. g, Time-lapse images of Ca2+ increases in neurons triggered by CO produced from CoPc/OxCP cathodes, which were positioned at the left edge in all three images, at −1.3 V versus SHE (scale bar, 50 μm). Neurons located at greater distances from the cathode responded over time (white dotted circles). h-j, Individual fluo-4 fluorescence traces for 100 neurons at different experimental conditions. E-field (–) in (i) represents neurons immersed in CO2-saturated solution in the absence of an applied voltage. CO2(aq) (–) in (j) represents neurons immersed in Tyrode’s not saturated in CO2 in the presence of an applied voltage (j). Voltage of −1.3 V were turned on at 30 s (dashed lines) in h and j. k-l, Individual fluo-4 fluorescence traces for 100 neurons after electrochemical CO generation at −0.9 V (k) and −1.7 V versus SHE (l). Voltages were turned on at 30 s (dashed lines).
To investigate the biological mechanisms underlying the effects of CO-mediated Ca2+ influxes in neurons, we employed blockers (or inhibitors) of ion channels and receptors that have been previously proposed as molecular targets of CO[2,36–38]. The latter included large-conductance, voltage- and Ca2+-activated K+-channel (BKCa), L-type Ca2+-channel, hyperpolarization-activated cyclic nucleotide-gated channel (HCN), and sGC. Among blockers (or inhibitors) of the putative CO targets, L-type Ca2+ channel blocker nitrendipine[39] significantly attenuated Ca2+ responses driven by CORM-2 (Figure 3d and Figure S6). Although the understanding of the mechanisms contributing to the CO-mediated Ca2+ increases in neurons continues to evolve[36], our findings suggest that observed Ca2+ responses are at least partially related to the interactions between CO and L-type Ca2+ channels.
We then applied our electrochemical CO-delivery system to similarly evoke Ca2+ responses in hippocampal neurons (Figure 3e). Here, CoPc/OxCP cathode, Pt anode, and Ag/AgCl reference electrode were utilized to deliver CO to neurons in CO2-saturated Tyrode’s solution at pH 7.4. Because the local CO concentration is calculated to be much lower at greater distances from the cathode (Figure S5), we positioned the CoPc/OxCP cathode in the immediate vicinity of the neurons (Figure 3f). Robust Ca2+ responses (as marked by ΔF/F0 ≥ 25%) in neurons were observed 250 s after application of −1.3 V versus SHE, and continued application of −1.3 V to the cathode led to Ca2+ influxes in neurons located at greater distances from the cathode (Figure 3g, h). To assess whether observed Ca2+ responses were attributable to CO released from the cathodes, we recorded Ca2+ changes in control experiments, which included neurons immersed in CO2-saturated Tyrode’s solution with no voltage applied (Figure 3i) and in neurons immersed in Tyrode’s not saturated in CO2 in the presence of a cathodic bias (−1.3 V versus SHE, Figure 3j). The extent of Ca2+ responses found in the control experiments was significantly lower than that triggered by electrochemically formed CO (Figure 3h). Moreover, Ca2+ increases in response to CO released from the cathode were largely inhibited by the addition of L-type channel blocker nitrendipine (Figure S7), akin to our observations with CORM-2 (Figure 3d). These results suggested that Ca2+ responses observed in neurons are predominately mediated by electrochemically produced CO.
Our electrochemical approach further enabled an investigation of CO-mediated neuronal signaling at different CO release kinetics. When we applied −0.9 V versus SHE to the cathodes in CO2-saturated Tyrode’s solution, only 13% of neurons exhibited robust Ca2+ responses (as marked by ΔF/F0 ≥ 50%) (Figure 3k). In contrast, 91% of neurons responded at −1.3 V (Figure 3h) due to faster CO release kinetics at this voltage as compared to −0.9 V (Figure 1c, d). Despite the higher Faradaic current, a smaller fraction of neurons showed Ca2+ responses at −1.7 V as compared to −1.3 V (Figure 3l). This observation is consistent with the extremely low CO selectivity of CoPc/OxCP catalysts at this reductive voltage (Figure 1d and Figure S1). By controlling CO generation kinetics with applied voltage, we found that CO release kinetics, not Faradic current, is the key factor affecting CO-mediated Ca2+ responses in neurons.
Finally, we miniaturized our electrochemical system by leveraging fiber drawing[25,40]. Fiber-based fabrication allowed us to scale down macroscale components of an electrochemical cell into a microscopic electrocatalytic fiber (Figure 4a–d). A polycarbonate-based macroscopic preform, which contained two grooves on its surface for placing microelectrodes and one hollow channel for delivering CO2-saturated solution, was fabricated through macroscale machining (Figure 4b). During drawing, the preform was heated and stretched into a microscale fiber (Figure 4c, d). Carbon nanotube (CNT) and Pt microwires (50 μm in diameter) were then placed into grooves on the surface of the fiber. The microwires extended from the fiber tips by approximately 300 μm, and CoPc ink was deposited onto the CNT microwires. The non-exposed region of the microwires was then fully coated with epoxy that provided electrical insulation and mechanical stability, and the microwires and the microfluidic channel were interfaced with electrical pin connectors and tubing inlets, respectively (Figure 4e, f). We confirmed that CO2-saturated Tyrode’s solution can be delivered through the microfluidic channel integrated within the electrocatalytic fiber (Figure 4g). CoPc/CNT microwires, which served as cathodes integrated within the fibers, catalyzed CO2 reduction reaction, as confirmed by chronoamperometry analyses (Figure 4h). Integrated Pt microwires served as anodes during the reaction.
Figure 4.
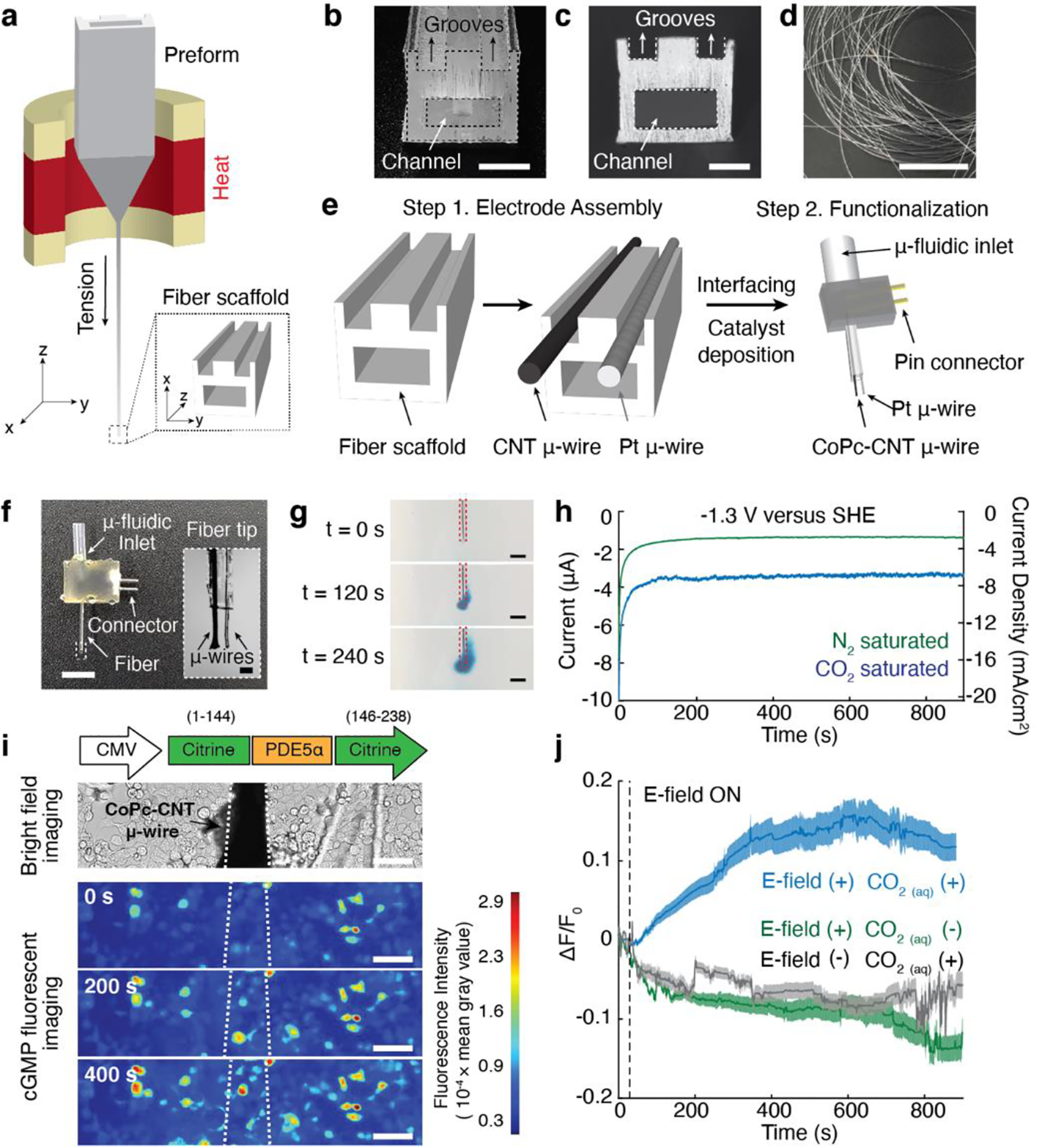
a, A schematic illustrating the fiber drawing procedure. b-c, Cross-sectional images of the preform (b, scale bar, 5 mm) and the fiber scaffold after the drawing process (c, scale bar, 100 μm). Two microscale grooves and one hollow microchannel are visible in c. d, A photograph of a bundle of fiber scaffolds after the drawing process (scale bar, 5 cm). e, A schematic demonstrating microelectrode assembly on the fiber scaffold, followed by fiber connectorization and functionalization of the CNT microwires with CoPc catalyst. f, A photograph of the resulting CO delivery fiber (scale bar, 5 mm) and a microscope image of the fiber tip (inset, scale bar, 100 μm). g, Delivery of CO2-saturated Tyrode’s solution with a dye (BlueJuice) into a brain phantom (0.6% agarose gel) through the microfludic channel within the fiber at an infusion rate of 100 nL/min (scale bar, 500 μm). h, Chronoamperometry measurements conducted with the electrocatalytic fiber in CO2-saturated (blue) or N2-saturated (green) Tyrode’s solution at pH 7.4. i, An optical image of a CoPc-CNT microwire of the CO-delivery fiber positioned above Green cGull-expressing cells (top). Time-lapse images of local cGMP dynamics in Green cGull-expressing cells in response to electrochemically synthesized CO from the CoPc-CNT microwire (white dotted lines) at −1.3 V versus SHE (scale bar, 50 μm) (bottom). j, Averaged Green cGull fluorescence traces of cells (n = 100 cells for each trace) at different experimental conditions. The solid lines and shaded areas indicate the mean and s.e.m., respectively. E-field (–) and CO2(aq) (–) indicate cells after delivery of CO2-saturated solution in the absence of an applied voltage and cells subjected to cathodic voltage without CO2 saturation, respectively. Voltages of −1.3 V were turned on at 30 s (dashed lines).
The electrocatalytic fibers were then applied to locally deliver CO and evoke CO-mediated sGC-cGMP signaling cascades in the targeted cells. To monitor local cGMP dynamics, HEK cells were transfected with a plasmid carrying a genetically encoded fluorescent cGMP sensor, Green cGull[41], under the CMV promoter. The functionality of Green cGull expressed in cells was first confirmed with a NO releasing molecule (NORM) (Figure S8). After delivery of CO2-saturated Tyrode’s solution, CO was generated from the CoPc-CNT microwires at −1.3 V versus SHE. A gradual increase in Green cGull fluorescence (a marker of cGMP accumulation) was found in cells located in the proximity of the microwires (Figure 4i, j). In contrast, no noticeable Green cGull fluorescence changes were found in cells after delivery of CO2-saturated solution in the absence of an applied voltage or in cells subjected to electric stimulation in the absence of CO2 precursor (Figure 4j).
Conclusion
By leveraging electrocatalytic activity and selectivity of CoPc catalysts toward reduction of CO2 into CO at modest voltages, we have developed an electrochemical approach for in situ targeted delivery of this molecule to physiological environments. Electrochemically released CO from CoPc-functionalized cathodes was shown to modulate several sGC-mediated signaling cascades in cells. Furthermore, facile control over CO release kinetics in our platform enabled a systematic investigation of CO-mediated signaling in neurons. This electrochemical system was further implemented in microscale fibers produced via thermal drawing. CO generated from the electrocatalytic fibers evoked local cGMP accumulation in the targeted cells. We envision that our electrochemical approach can be extended to explore CO-mediated cellular signaling in diverse systems, including the peripheral nervous system (Figure S9), offering addition insights onto its role as a messenger molecule.
Supplementary Material
Acknowledgements
We would like to thank T. Kitaguchi, and F. Zhang for the generous gifts of the plasmids and cell lines. The authors are also grateful to T. Khudiyev, S. Rao, and D. Rosenfeld for their technical advice on the experiments. This work was funded in part by the National Institutes of Health (NIH) BRAIN Initiative (5-R01-MH111872, 1-R01-NS115576), the National Institute of Neurological Disorders and Stroke (1-R01-NS115025), the National Science Foundation (NSF) Center for Neurotechnology (EEC-1028725), the Hock E. Tan and K. Lisa Yang Center for Autism Research, and the McGovern Institute for Brain Research. Work by K.M. and J.S.Z. was supported by the NSF under grant no. 1955628. This work made use of the MIT MRSEC Shared Experimental Facilities under award number DMR-14-19807 from the NSF. J.P. is a recipient of scholarship from the Kwanjeong Educational Foundation. A.S. is a recipient of the Lore Harp McGovern graduate student fellowship.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Verma A, Hirsch DJ, Glatt CE, V Ronnett G, Snyder SH, Science (80-.) 1993, 259, 381–384. [DOI] [PubMed] [Google Scholar]
- [2].Motterlini R, Otterbein LE, Nat. Rev. Drug Discov 2010, 9, 728. [DOI] [PubMed] [Google Scholar]
- [3].Ingi T, Cheng J, V Ronnett G, Neuron 1996, 16, 835–842. [DOI] [PubMed] [Google Scholar]
- [4].Romão CC, Blättler WA, Seixas JD, Bernardes GJL, Chem. Soc. Rev 2012, 41, 3571–3583. [DOI] [PubMed] [Google Scholar]
- [5].Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AMK, Nat. Med 2000, 6, 422–428. [DOI] [PubMed] [Google Scholar]
- [6].Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J, Engl N. J. Med 1989, 321, 1426–1432. [DOI] [PubMed] [Google Scholar]
- [7].Meng J, Jin Z, Zhao P, Zhao B, Fan M, He Q, Sci. Adv 2020, 6, DOI 10.1126/sciadv.aba1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ernst A, Zibrak JD, Engl N. J. Med 1998, 339, 1603–1608. [DOI] [PubMed] [Google Scholar]
- [9].Heinemann SH, Hoshi T, Westerhausen M, Schiller A, Chem. Commun 2014, 50, 3644–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].García-Gallego S, Bernardes GJL, Angew. Chemie Int. Ed 2014, 53, 9712–9721. [DOI] [PubMed] [Google Scholar]
- [11].Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ, Circ. Res 2002, 90, e17–e24. [DOI] [PubMed] [Google Scholar]
- [12].Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R, Circ. Res 2003, 93, e2–e8. [DOI] [PubMed] [Google Scholar]
- [13].Motterlini R, Sawle P, Bains S, Hammad J, Alberto R, Foresti R, Green CJ, FASEB J. 2005, 19, 1–24. [DOI] [PubMed] [Google Scholar]
- [14].Foresti R, Hammad J, Clark JE, Johnson TR, Mann BE, Friebe A, Green CJ, Motterlini R, Br. J. Pharmacol 2004, 142, 453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang X, Wu Z, Zhang X, Li L, Li Y, Xu H, Li X, Yu X, Zhang Z, Liang Y, Wang H, Nat. Commun 2017, 8, 14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zeng JS, Corbin N, Williams K, Manthiram K, ACS Catal. 2020, 10, 4326–4336. [Google Scholar]
- [17].Zhu M, Ye R, Jin K, Lazouski N, Manthiram K, ACS Energy Lett. 2018, 3, 1381–1386. [Google Scholar]
- [18].Dunwell M, Lu Q, Heyes JM, Rosen J, Chen JG, Yan Y, Jiao F, Xu B, J. Am. Chem. Soc 2017, 139, 3774–3783. [DOI] [PubMed] [Google Scholar]
- [19].Wuttig A, Yoon Y, Ryu J, Surendranath Y, J. Am. Chem. Soc 2017, 139, 17109–17113. [DOI] [PubMed] [Google Scholar]
- [20].Ingi T, Chiang G, Ronnett GV, J. Neurosci 1996, 16, 5621–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH, Proc. Natl. Acad. Sci 1997, 94, 14848–14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kharitonov VG, Sharma VS, Pilz RB, Magde D, Koesling D, Proc. Natl. Acad. Sci 1995, 92, 2568–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Parkinson SJ, Jovanovic A, Jovanovic S, Wagner F, Terzic A, Waldman SA, Biochemistry 1999, 38, 6441–6448. [DOI] [PubMed] [Google Scholar]
- [24].Buechler WA, Nakane M, Murad F, Biochem. Biophys. Res. Commun 1991, 174, 351–357. [DOI] [PubMed] [Google Scholar]
- [25].Park J, Jin K, Sahasrabudhe A, Chiang P-H, Maalouf JH, Koehler F, Rosenfeld D, Rao S, Tanaka T, Khudiyev T, Schiffer ZJ, Fink Y, Yizhar O, Manthiram K, Anikeeva P, Nat. Nanotechnol 2020, 15, 690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].YOU JP, WANG Q, ZHANG W, JANSEN-OLESEN I, PAULSON OB, LASSEN NA, EDVINSSON L, Acta Physiol. Scand 1994, 152, 391–397. [DOI] [PubMed] [Google Scholar]
- [27].Marazioti A, Bucci M, Coletta C, Vellecco V, Baskaran P, Szabó C, Cirino G, Marques AR, Guerreiro B, Gonçalves AML, Seixas JD, Beuve A, Romão CC, Papapetropoulos A, Arterioscler. Thromb. Vasc. Biol 2011, 31, 2570–2576. [DOI] [PubMed] [Google Scholar]
- [28].Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M, Antioxid. Redox Signal 2010, 13, 157–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kajimura M, Shimoyama M, Tsuyama S, Suzuki T, Kozaki S, Takenaka S, Tsubota K, Oguchi Y, Suematsu M, FASEB J. 2003, 17, 1–23. [DOI] [PubMed] [Google Scholar]
- [30].Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS, Am. J. Physiol. Physiol 1999, 277, F882–F889. [DOI] [PubMed] [Google Scholar]
- [31].Scapagnini G, D’Agata V, Calabrese V, Pascale A, Colombrita C, Alkon D, Cavallaro S, Brain Res. 2002, 954, 51–59. [DOI] [PubMed] [Google Scholar]
- [32].Piantadosi CA, Zhang J, Levin ED, Folz RJ, Schmechel DE, Exp. Neurol 1997, 147, 103–114. [DOI] [PubMed] [Google Scholar]
- [33].Tang Y-P, Murata Y, Nagaya T, Noda Y, Seo H, Nabeshima T, Cereb J. Blood Flow Metab. 1997, 17, 771–780. [DOI] [PubMed] [Google Scholar]
- [34].Zhuo M, Small SA, Kandel ER, Hawkins RD, Science (80-.). 1993, 260, 1946–1950. [DOI] [PubMed] [Google Scholar]
- [35].Gee KR, Brown KA, Chen W-NU, Bishop-Stewart J, Gray D, Johnson I, Cell Calcium 2000, 27, 97–106. [DOI] [PubMed] [Google Scholar]
- [36].Wilkinson WJ, Kemp PJ, J. Physiol 2011, 589, 3055–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Peers C, Boyle JP, Scragg JL, Dallas ML, Al-Owais MM, Hettiarachichi NT, Elies J, Johnson E, Gamper N, Steele DS, Br. J. Pharmacol 2015, 172, 1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lim I, Gibbons SJ, Lyford GL, Miller SM, Strege PR, Sarr MG, Chatterjee S, Szurszewski JH, Shah VH, Farrugia G, Am. J. Physiol. Liver Physiol 2005, 288, G7–G14. [DOI] [PubMed] [Google Scholar]
- [39].Ambrósio AF, Silva AP, Malva JO, Soares-da-Silva P, Carvalho AP, Carvalho CM, Neuropharmacology 1999, 38, 1349–1359. [DOI] [PubMed] [Google Scholar]
- [40].Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P, Nat. Biotechnol 2015, 33, 277. [DOI] [PubMed] [Google Scholar]
- [41].Matsuda S, Harada K, Ito M, Takizawa M, Wongso D, Tsuboi T, Kitaguchi T, ACS Sensors 2017, 2, 46–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


