Abstract
Background:
Chronic transportation noise exposure associates with cardiovascular events through a link involving heightened stress-associated neurobiological activity (as amygdalar metabolic activity, AmygA) on 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT). Increased AmygA also associates with greater visceral adipose tissue (VAT) and type 2 diabetes mellitus (DM). While relationships between noise exposure and VAT and DM have been reported, the underlying mechanisms remain incompletely understood. We tested whether: 1) transportation noise exposure associates with greater a) baseline and gains in VAT and b) DM risk, and 2) heightened AmygA partially mediates the link between noise exposure and these metabolic diseases.
Methods:
VAT was measured in a retrospective cohort (N=403) who underwent clinical 18F-FDG-PET/CT. AmygA was measured in those with brain imaging (N=238). Follow-up VAT was remeasured on available imaging (N=67). Among individuals (N=224) without baseline DM, incident DM was adjudicated over two years from clinical records. Noise (24-hour average) was modeled at each individual’s home address. Linear regression, survival, and mediation analyses were employed.
Results:
Higher noise exposure (upper tertile vs. others) associated with greater: baseline VAT (standardized β [95% confidence interval (CI)]=0.230 [0.021, 0.438], p=0.031), gains in VAT (0.686 [0.185, 1.187], p=0.008 adjusted for baseline VAT), and DM (hazard ratio [95% CI]=2.429 [1.031, 5.719], p=0.042). The paths of: ↑noise exposure→↑AmygA→↑baseline VAT and ↑noise exposure→↑AmygA→↑subsequent DM were significant (p<0.05).
Conclusions:
Increased transportation noise exposure associates with greater VAT and DM. This relationship is partially mediated by stress-associated neurobiological activity. These findings suggest altered neurobiology contributes to noise exposure’s link to metabolic diseases.
Keywords: amygdalar activity, diabetes mellitus, noise exposure, positron emission tomography, visceral adiposity
Graphical abstract
1. Introduction
Chronic noise exposure is an important contributor to human diseases, including metabolic diseases (i.e., obesity and diabetes mellitus (DM)) and cardiovascular disease (CVD).(Christensen et al., 2015; Christensen et al., 2016; Clark et al., 2017; Eze et al., 2017; Fritschi et al., 2011; Munzel et al., 2018; Osborne et al., 2020; Pyko et al., 2015; Pyko et al., 2017; Shin et al., 2020; Sorensen et al., 2013) The relationships between noise and anthropometric measures of adiposity as well as DM are known to be independent of key confounders in several populations (e.g., air pollution, socioeconomic factors, medical comorbidities, diseases, smoking, malignancy); however, no study has comprehensively evaluated these endpoints using objective imaging-based measurements.(Christensen et al., 2015; Christensen et al., 2016; Clark et al., 2017; Eze et al., 2017; Pyko et al., 2015; Pyko et al., 2017; Shin et al., 2020; Sorensen et al., 2013) Furthermore, the mechanisms linking noise exposure to these metabolic diseases remain incompletely understood.
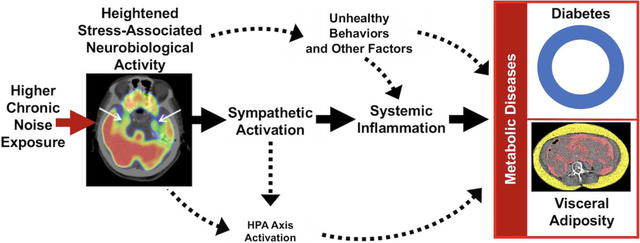
Chronic noise exposure generates a stress response that activates the sympathetic nervous system (SNS) leading to increased downstream inflammation and oxidative stress.(Munzel et al., 2018; Osborne et al., 2020; Schmidt et al., 2013) Noise exposure also stimulates the hypothalamic-pituitary-adrenal (HPA) axis and leads to impaired glucose metabolism and increased adiposity.(Munzel et al., 2018) These changes culminate in metabolic and cardiovascular diseases.(Munzel et al., 2017; Munzel et al., 2018; Osborne et al., 2020; Recio et al., 2016; Schmidt et al., 2013) High transportation noise exposure triggers increased stress-associated neural activity of the amygdala, a limbic brain center that participates in the response to psychosocial and environmental stressors.(Osborne et al., 2020; Tawakol et al., 2019; Zald and Pardo, 2002) 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) imaging provides a unique opportunity to detail neurobiological mechanisms by simultaneously evaluating regional brain activity (e.g., amygdalar metabolic activity, or AmygA) and the structures of extra-neural tissues (e.g., visceral adipose tissue, or VAT).(Ishai et al., 2019; Osborne et al., 2020; Tawakol et al., 2017) 18F-FDG-PET/CT imaging has previously been implemented to show that heightened stress-associated neurobiological activity (AmygA) associates with greater: VAT, type 2 DM, atherosclerotic inflammation, and CVD events.(Ishai et al., 2019; Osborne et al., 2018; Tawakol et al., 2017) Because higher transportation noise exposure associates with increased AmygA,(Osborne et al., 2020) we hypothesized that AmygA has an important role in linking noise exposure to metabolic diseases (e.g., increased VAT and DM).
Accordingly, we sought to investigate the independence of the associations between transportation noise exposure and: a) baseline and change in VAT and b) type 2 DM risk from key confounding factors. Further, we tested whether the relationship between higher noise exposure and these metabolic diseases is mediated by AmygA.
2. Methods
2.1. Study Samples
The study samples were derived from a retrospective cohort of 1,777 individuals without known CVD or active malignancy who underwent clinically indicated 18F-FDG-PET/CT imaging at Massachusetts General Hospital (Boston, MA) from 2005 to 2008 (largely for malignancy screening or surveillance). None received chemotherapies within one year before imaging or during follow-up, and none developed cancer during follow-up. Four hundred ninety-eight (498) individuals met additional inclusion criteria: 1) age >30 years, 2) no clinically diagnosed inflammatory disease, 3) ≥3 clinical notes to establish baseline health, 4) >1 follow-up note, 5) available 18F-FDG-PET/CT imaging, and 6) home address data to derive 24-hour average transportation noise levels. Of this group, 403 provided abdominal imaging data for the measurement of VAT and comprised the VAT sample. A subset of 238 individuals provided imaging data that allowed assessment of AmygA, and 67 individuals provided clinically indicated 18F-FDG-PET/CT imaging data approximately one year after baseline imaging that allowed measurement of follow-up VAT. Of the 498 total subjects, a separate group of 224 without baseline DM provided up to two years of follow-up data for the determination of incident type 2 DM. Complete details are provided in Figure 1. This retrospective study was approved by the Partners Institutional Review Board and was completed in compliance with the Declaration of Helsinki. Informed consent was not required for this retrospective study.
Figure 1:
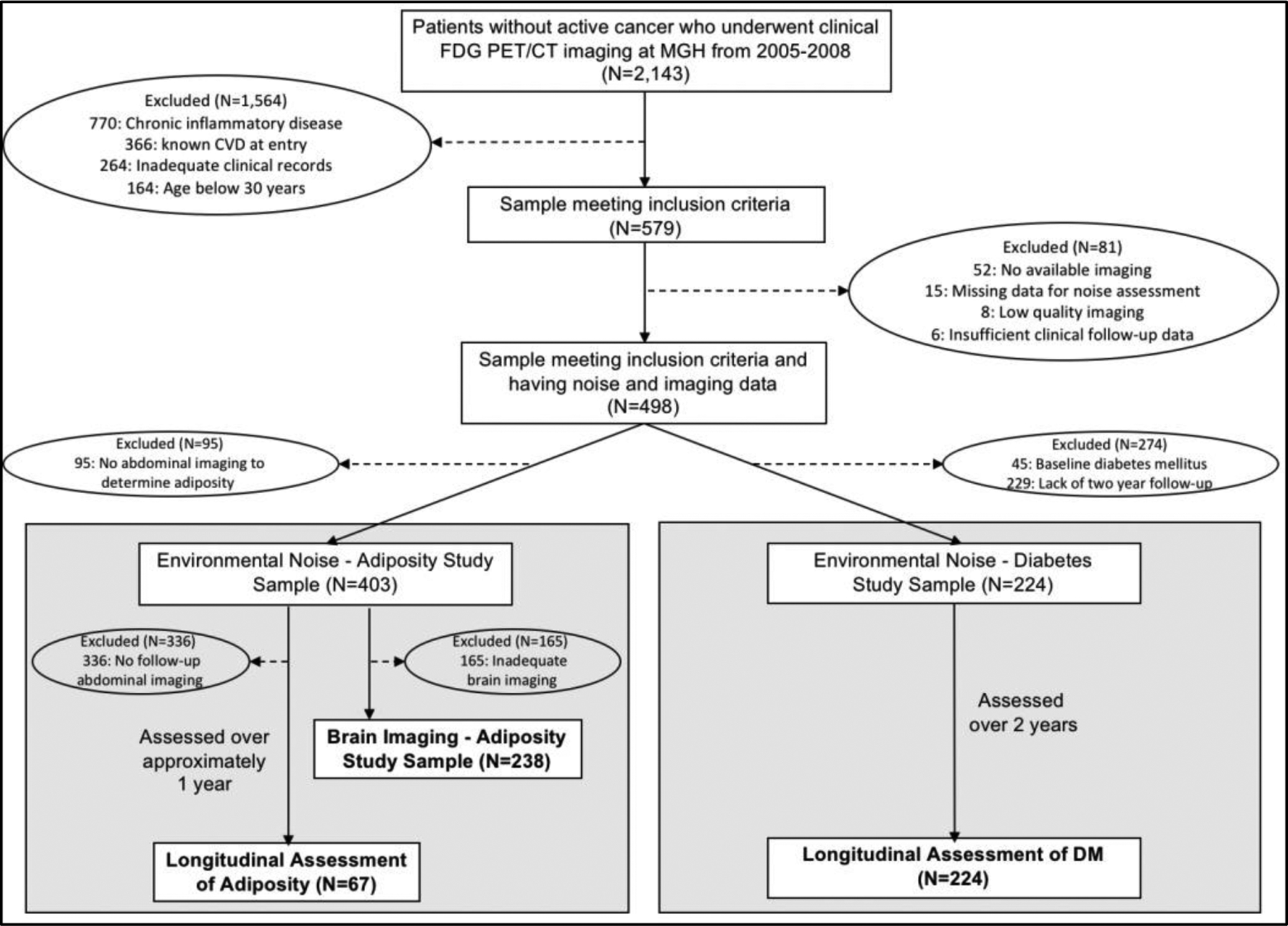
Patient selection for the adiposity and DM samples. Abbreviations: CVD-cardiovascular disease, DM-diabetes mellitus, FDG PET/CT-fluorodeoxyglucose positron emission tomography/computed tomography, HTN-hypertension, VAT-visceral adipose tissue.
2.2. 18F-FDG-PET/CT Imaging Acquisition
18F-FDG-PET/CT imaging was performed with a hybrid scanner (e.g., Biograph 64 Siemens Healthcare, Erlangen, Germany, or similar). Each individual was injected with intravenous 18F-FDG after an overnight fast. After an interval of 60 minutes in a quiet waiting area, resting state PET imaging was performed according to standard clinical protocol without programmed stimulation. A low-dose, non-contrast CT was performed for attenuation correction prior to PET imaging.
2.3. 18F-FDG-PET/CT Imaging Analysis
Imaging measurements were performed by trained and experienced investigators blinded to noise and clinical data. On baseline attenuation correction CT images, VAT was measured as the volume of adipose tissue with Hounsfield units from −195 to −45 using the abdominal, intercostal, and paraspinal musculature as boundaries on one 3 mm slice at the level of the umbilicus.(Ishai et al., 2019; Maurovich-Horvat et al., 2007) Subcutaneous adipose tissue (SAT) was measured as tissue of the same radiographic density within the subcutaneous space. VAT:SAT ratio, a known correlate of metabolic disease, was quantified.(Kaess et al., 2012)
Among the 238 individuals with brain imaging data, baseline AmygA was measured by a single reader. Using CT images and circular regions of interest with radii of approximately 15 mm, the mean bilateral amygdalar 18F-FDG uptake was measured as a standardized uptake value (SUV) and then averaged. This mean value was corrected for mean temporal lobe activity to obtain a ratio of amygdalar to regulatory brain tissue activity (AmygA).(Ishai et al., 2019; Osborne et al., 2018; Osborne et al., 2020; Tawakol et al., 2017) Among randomly selected subjects from the parent cohort from which the current study samples were derived, the inter- and intra-reader variability of the AmygA measurement were a mean (standard deviation) of 0.06 (0.16) or 5.77% (16.13%) among a sample of 265 individuals and 0.01 (0.13) or 1.16% (12.52%) among a sample of 50 subjects, respectively.(Tawakol et al., 2017)
2.4. Adjudication of Noise Exposure, DM Events, and Covariables
Clinical status was evaluated by a separate team of blinded and trained investigators. Average transportation noise exposure over a 24-hour period was evaluated in 5 A-weight decibel (dBA) increments from <35 dBA to 65–70 dBA at the street-facing façade of each individual’s home address using the United States Department of Transportation’s Road and Aviation Noise Map from 2014.(United States Department of Transportation, 2014) The model approximated average traffic and aircraft noise over 24-hours in dBA to adjust instrument-measured sound to reflect the human ear’s perception of relative loudness. Aircraft noise was quantified with the Aviation Environmental Design Tool version 2b service pack 2 and data on aircraft flight operation. Traffic noise was evaluated using roadway average annual traffic distributions, the Newly built National Transportation Noise Modeling Tool, and the Federal Highway Administration Traffic Noise Model version 2.5. For the DM sample, baseline type 2 DM was based on a clinical diagnosis, active medication, and existing laboratory values (i.e., two fasting blood glucose values >126 mg/dl or glycohemoglobin values >6.5%) applied to the American Diabetes Association (ADA) criteria.(American Diabetes Association, 2016) Individuals without baseline DM were evaluated over two years for the adjudication of incident type 2 DM using available notes and clinically acquired laboratory values and any of the same ADA criteria that were used to establishing a baseline diagnosis. For the DM sample, we additionally evaluated for the presence baseline of pre-DM (defined as a single fasting blood glucose ranging from 100–125 mg/dL or glycohemoglobin of 5.7–6.4%) with ADA criteria.(American Diabetes Association, 2016) For multivariable adjustment, clinically relevant covariables (e.g., age, sex, race, current smoking, hypertension, hyperlipidemia, malignancy and malignancy treatment history) were derived from the medical record. To assess and control for confounders that may be present in noisier neighborhoods, median neighborhood income was evaluated using the United States Census Bureau’s 2015 American Community Survey’s 5-Year Estimates.(United States Censusl Bureau, 2015) Crime rates were evaluated using the United States Federal Bureau of Investigation’s Massachusetts Offenses Known to Law Enforcement from 2015 for Massachusetts residents,(United States Federal Bureau of Investigation, 2015) and air pollution was assessed as the mean annual concentration of particulate matter with diameter <2.5 μm using 2017 United States Environmental Protection Agency data.(United States Environmental Protection Agency, 2017; McGuinn et al., 2017)
2.5. Statistical Analysis
For the primary prespecified analysis, noise exposure was divided into tertiles, after which those exposed to higher levels of noise (upper tertile, >45 dBA) were compared to those with lower exposure. Chi-square tests and t-tests (or Mann-Whitney U tests when skewed) were performed to assess for group differences between baseline categorical and continuous variables, respectively. Continuous variables were standardized for analyses. Linear regression was used to evaluate univariable and multivariable relationships with measures of adiposity and AmygA as standardized β and 95% confidence intervals (CIs). Cox proportional hazard ratios (HRs) were implemented to assess the relationship between noise exposure and subsequent type 2 DM. Kaplan-Meier curves were used to estimate DM-free survival. Individuals were censored at the date of DM diagnosis or last follow-up. Sensitivity analyses were performed to assess for the relationships between noise exposure in 5 dBA increments and >55 dBA (threshold established as unhealthy by the World Health Organization) with measures of visceral adiposity and incident type 2 DM.(Munzel et al., 2018) For multivariable analyses, covariables were selected a priori as those factors that were deemed to be potentially clinically relevant, including demographics (e.g., age, sex, race), markers of metabolic impairment (e.g., body mass index (BMI), adipose tissue measures, baseline DM, hypertension (HTN), hyperlipidemia, baseline pre-DM, and glucose measures), current smoking, prior malignancy and malignancy treatment, socioeconomic factors (e.g., neighborhood income and crime), air pollution exposure, and amygdalar activity. Combined models were implemented to assess the impact of higher transportation noise exposure on baseline VAT, change in VAT, and DM in models adjusted for combined demographic and clinical factors and combined socioeconomic and environmental factors. Backwards selection was employed in these combined models. Unadjusted mediation analysis was used to evaluate the prespecified single mediator paths:
↑noise exposure →↑AmygA→↑baseline VAT and
↑noise exposure →↑AmygA→↑DM risk.
The analyses estimate direct and indirect effects and CIs based upon 5,000 bias-corrected bootstrap samples by implementing an ordinary least squares or logistic regression framework.(Hayes, 2013) Given the use of bootstrapping, exact p-values for mediation analyses are not available; however, indirect pathways with CIs that do not cross zero are significant with p<0.05. Individuals with missing data were excluded from analysis. All analyses were performed using SPSS version 24 and the SPSS PROCESS macro version 3.4 Models 4 and 6 (IBM Corporation, Armonk, NY). A two-sided p-value <0.05 was determined to be significant for all analyses.
3. Results
3.1. Baseline Characteristics
The study cohorts were selected from an overall sample of 498 eligible individuals with a median age of 55 (interquartile range (IQR) 45–66) years, which was 42.0% male; 84.3% had prior malignancy. The VAT cohort of 403 eligible individuals (i.e., those in the overall sample with abdominal imaging to allow VAT measurement) had a median age of 55 (IQR 44–65) years and was 42.4% male; 86.4% had prior malignancy. Individuals with VAT > sample median (N=202) were older, had a higher BMI and glucose at the time of imaging, and were more likely to be male and have baseline HTN, hyperlipidemia, and DM. The DM cohort (i.e., those in the overall population without baseline type 2 DM and up to two years of clinical follow-up, N=224) had a median age of 54.5 (IQR 44–63) years and was 42.9% male; 89.3% had prior malignancy. Glucose at the time of imaging, maximum glucose within two years of imaging, pre-DM, amygdalar activity, and transportation noise exposure differed significantly between those who did and did not develop subsequent type 2 DM. Complete details are shown (Tables 1A & 1B). The distribution of transportation noise exposure in each of the three study samples is shown (Supplemental Tables 1A, 1B, and 1C).
Table 1A:
Visceral adiposity sample baseline characteristics.
| Overall Sample (N=403) | Baseline VAT Volume ≥ median (N=202) | Baseline VAT Volume < median (N=201) | p-value | |
|---|---|---|---|---|
| Median age, years (IQR) | 55.00 (44.00–65.00) | 60.00 (51.00–68.00) | 49.00 (39.00–60.00) | <0.001 |
| Male, N (%) | 171 (42.4%) | 117 (57.9%) | 54 (26.9%) | <0.001 |
| Caucasian, N (%) | 367 (91.1%) | 186 (92.1%) | 181 (90.0%) | 0.48 |
| Median baseline BMI, kg/m2 (IQR) | 26.31 (23.41–30.89) | 29.82 (26.63–32.72) | 23.75 (21.61–26.05) | <0.001 |
| Baseline DM, N (%) | 33 (8.2%) | 26 (12.9%) | 7 (3.5%) | 0.001 |
| Baseline HTN, N (%) | 136 (33.7%) | 99 (49.0%) | 37 (18.5%) | <0.001 |
| Baseline hyperlipidemia, N (%) | 110 (27.3%) | 65 (32.2%) | 35 (17.4%) | <0.001 |
| Median glucose at time of imaging, mg/dL (IQR) | 99.00 (90.00–108.00) | 96.00 (87.00–103.00) | 102.00 (94.00–114.25) | <0.001 |
| Current smoking, N (%) | 41 (10.2%) | 26 (12.9%) | 15 (7.5%) | 0.067 |
| History of cancer, N (%) | 348 (86.4%) | 171 (84.7%) | 177 (88.1%) | 0.32 |
| History of chemotherapy or radiation, N (%) | 317 (78.7%) | 155 (76.7%) | 162 (80.6%) | 0.34 |
| Median air pollution, particulate matter ≤2.5 μm, μg/m3 (IQR) | 5.23 (4.83–6.00) | 5.20 (4.83–6.00) | 5.61 (4.83–6.00) | 0.18 |
| Median neighborhood income, $ (IQR) | 79540 (61618–100286) | 77425.5 (61148.5–100286) | 80266 (63353.5–100604.5) | 0.28 |
| Median local crime rate (IQR) | 21.69 (13.98–36.96) | 22.46 (13.83–36.96) | 20.53 (13.98–34.22) | 0.74 |
| Median average transportation noise exposure, dBA (IQR) | 35–40 (<35–50) | 35–40 (<35–50) | 35–40 (<35–55) | 0.12 |
| Median amygdalar activity (IQR) | 0.80 (0.73–0.85) | 0.80 (0.72–0.84) | 0.79 (0.74–0.86) | 0.73 |
Median baseline VAT for population=48.38 cm3
Air pollution available in 384 subjects, crime in 304 subjects, and amygdalar activity in 238 subjects.
Bold type indicates p<0.05.
Abbreviations: BMI-body mass index, DM-diabetes mellitus, HTN-hypertension, IQR-interquartile range, VAT-visceral adipose tissue
Table 1B:
Diabetes sample baseline characteristics.
| Overall Sample (N=224) | DM Event (N=21) | No DM Event (N=203) | p-value | |
|---|---|---|---|---|
| Median age, years (IQR) | 54.50 (44.00–63.00) | 56.00 (46.00–67.50) | 54.00 (43.00–63.00) | 0.32 |
| Male, N (%) | 96 (42.9%) | 13 (61.9%) | 83 (40.9%) | 0.064 |
| Caucasian, N (%) | 210 (89.3%) | 19 (90.5%) | 181 (89.2%) | 0.85 |
| Median baseline BMI, kg/m2 (IQR) | 26.26 (23.02–30.84) | 28.45 (23.15–32.04) | 26.10 (23.01–30.68) | 0.21 |
| Median baseline VAT volume, cm3 (IQR) | 47.09 (26.00–72.51) | 66.34 (23.32–102.94) | 46.88 (26.27–71.25) | 0.23 |
| Baseline HTN, N (%) | 69 (30.8%) | 6 (28.6%) | 63 (31.0%) | 0.82 |
| Baseline hyperlipidemia, N (%) | 58 (25.9%) | 7 (33.3%) | 51 (25.1%) | 0.41 |
| Fasting glucose > 100 mg/dL within 2 years prior to imaging, N (%) | 109 (48.7%) | 8 (61.9%) | 96 (47.3%) | 0.20 |
| Median maximum fasting glucose < 2 years before imaging, mg/dL (IQR) | 102.00 (90.00–112.00) | 116.00 (98.00–128.00) | 101.00 (89.25–109.75) | 0.003 |
| Median fasting glucose at time of imaging, mg/dL (IQR) | 98.00 (90.00–107.00) | 104.50 (97.50–131.00) | 97.00 (90.00–106.00) | 0.006 |
| Pre-DM, N (%) | 113 (50.4%) | 15 (71.4%) | 6 (28.6%) | 0.043 |
| Current smoking, N (%) | 17 (7.6%) | 2 (9.5%) | 15 (7.4%) | 0.73 |
| History of cancer, N (%) | 200 (89.3%) | 20 (95.2%) | 180 (88.7%) | 0.35 |
| History of chemotherapy or radiation, N (%) | 186 (83.0%) | 20 (95.2%) | 166 (81.8%) | 0.12 |
| Median air pollution, particulate matter ≤2.5 μm, μg/m3 (IQR) | 5.61 (4.83–6.13) | 5.76 (4.83–6.06) | 5.61 (4.83–6.13) | 0.95 |
| Median neighborhood income, $ (IQR) | 80399 (61919–101597.75) | 92300 (71467.50–111612) | 79540 (61619–100286) | 0.084 |
| Median local crime rate (IQR) | 21.69 (14.16–34.22) | 16.50 (10.76–35.93) | 22.46 (14.50–34.22) | 0.51 |
| Median average transportation noise exposure, dBA (IQR) | 35–40 (<35–50) | 45–50 (35–55) | 35–40 (<35–50) | 0.019 |
| Median amygdalar activity (IQR) | 0.79 (0.73–0.85) | 0.84 (0.80–0.91) | 0.78 (0.72–0.84) | 0.005 |
Air pollution available in 384 subjects, crime in 304 subjects, and amygdalar activity in 238 subjects.
Abbreviations: BMI-body mass index, DM-diabetes mellitus, HTN-hypertension, IQR- interquartile range, VAT-visceral adipose tissue
3.2. Association between Noise Exposure and VAT
There were no significant relationships between higher transportation noise exposure and baseline: BMI, SAT, or VAT:SAT ratio (Table 2A). However, higher noise exposure associated with greater baseline VAT (standardized β [95% CI]: 0.230 [0.021, 0.438], unadjusted p=0.031). Furthermore, among the 67 individuals with follow-up VAT measured a median 358 (IQR 286–374) days after index scanning, noise exposure associated with greater gains in VAT (0.686 [0.185, 1.187], p=0.008) in a model adjusted for baseline VAT. Models for baseline VAT remained significant after adjustment for many covariables; however, the relationship was attenuated by adjustment for age, other baseline metabolic diseases (i.e., DM, HTN, and hyperlipidemia), and amygdalar activity. Further, the relationship remained in a model combining socioeconomic and environmental factors but was attenuated in a model combining demographic and clinical factors. Models for change in VAT remained robust to the addition of all covariables to baseline VAT in multivariable models and in both combined models (Table 2B). In sensitivity analyses, the relationship between noise exposure in 5 dBA increments and baseline (p=0.052) and change in VAT (p=0.066 when adjusted for baseline VAT) trended towards significance. The relationships with baseline and change in VAT were not significant when a threshold of >55 dBA was used (p=0.10 and p=0.53, respectively).
Table 2A:
Associations between higher transportation noise exposure (>45 dBA or upper tertile vs. others) and tissue measurements.
| Tissue Measurement | Standardized p (95% Cl) | p-value |
|---|---|---|
| Baseline VAT volume (cm3) | 0.230 [0.021, 0.438] | 0.031 |
| Baseline BMI (kg/m2) | 0.156 [−0.044, 0.356] | 0.13 |
| Baseline SAT volume (cm3) | 0.034 [−0.175, 0.243] | 0.75 |
| Baseline VAT:SAT ratio | 0.184 [−0.024, 0.392] | 0.082 |
| Subsequent change in VAT volume (cm3)a | 0.686 [0.185, 1.187] | 0.008 |
| Amygdalar activity | 0.572 [0.325, 0.820] | <0.001 |
Adjusted for baseline VAT volume (follow-up data available in 67 subjects). The remaining models are unadjusted.
Bold type indicates p<0.05.
Abbreviations: BMI-body mass index, SAT-subcutaneous adipose tissue, VAT-visceral adipose tissue
Table 2B:
Associations between higher transportation noise exposure (>45 dBA or upper tertile vs. others) and VAT volumes adjusted for covariables.
| Covariable | Baseline VAT (cm3) | Change in VAT (cm3)a | ||
|---|---|---|---|---|
| Standardized β [95% CI] | p-value | Standardized β [95% CI] | p-value | |
| Age | 0.149 [−0.051, 0.349] | 0.14 | 0.693 [0.188, 1.198] | 0.008 |
| Sex | 0.222 [0.025, 0.420] | 0.027 | 0.655 [0.153, 1.157] | 0.011 |
| Caucasian race | 0.239 [0.030, 0.448] | 0.025 | 0.684 [0.184, 1.185] | 0.008 |
| Baseline DM | 0.179 [−0.025, 0.382] | 0.086 | 0.673 [0.167, 1.178] | 0.010 |
| Baseline HTN | 0.129 [−0.071, 0.330] | 0.21 | 0.669 [0.161, 1.176] | 0.011 |
| Baseline hyperlipidemia | 0.170 [−0.035, 0.374] | 0.10 | 0.657 [0.152, 1.162] | 0.012 |
| Fasting glucose at time of imaging | 0.043 [−0.179, 0.265] | 0.71 | 0.790 [0.186, 1.394] | 0.011 |
| Current smoking | 0.212 [0.002, 0.423] | 0.048 | 0.706 [0.204, 1.207] | 0.007 |
| History of cancer | 0.230 [0.021, 0.440] | 0.031 | 0.686 [0.185, 1.187] | 0.008 |
| History of cancer treatment | 0.228 [0.019, 0.437] | 0.033 | 0.708 [0.201, 1.214] | 0.007 |
| Combined demographic and clinical factors | −0.061 [−0.261, 0.140] | 0.55 | 0.745 [0.189, 1.300] | 0.010 |
| Median neighborhood income | 0.216 [0.007, 0.426] | 0.043 | 0.684 [0.179, 1.190] | 0.009 |
| Neighborhood total crime rateb | 0.289 [0.053, 0.525] | 0.017 | 0.622 [0.078, 1.166] | 0.026 |
| Air pollutionc | 0.302 [0.082, 0.522] | 0.007 | 0.552 [0.070, 1.033] | 0.025 |
| Combined socioeconomic and environmental factorsc | 0.282 [0.068, 0.496] | 0.010 | 0.741 [0.222, 1.261] | 0.006 |
| Amygdalar activityd | 0.012 [−0.264, 0.288] | 0.93 | 0.591 [0.090, 1.093] | 0.022 |
Each row represents a separate model aside from the combined models: Combined demographic and clinical factors (age, sex, Caucasian race, baseline DM, baseline HTN, baseline hyperlipidemia, fasting glucose at time of imaging, current smoking, history of cancer, and history of cancer treatment) and combined socioeconomic and environmental factors (median neighborhood income and air pollution). Backwards selection was implemented for combined models.
Also adjusted for baseline VAT.
Crime data available in 304 subjects with baseline VAT and 50 patients with follow-up VAT.
Air pollution data available in 384 subjects with baseline VAT and 63 patients with follow-up VAT.
Amygdalar activity available in 238 patients with baseline VAT and all patients with follow-up VAT.
Bold type indicates p<0.05.
Abbreviations: CI-confidence interval, DM-diabetes mellitus, VAT-visceral adipose tissue
3.3. Association between Noise Exposure and DM Risk
During two years of follow-up, twenty-one individuals developed incident type 2 DM with confirmation by available laboratory values for all cases. Higher noise exposure predicted subsequent DM (HR [95% CI]: 2.429 [1.031, 5.719], p=0.042), and this remained significant after adjustment for age and sex (2.393 [1.015, 5.368], p=0.046) as well as additional adjustment for baseline adiposity measures, baseline HTN, baseline hyperlipidemia, glucose >100 mg/dL within two years before imaging, pre-DM, prior malignancy and malignancy treatment, or air pollution. The results were attenuated by addition of maximum glucose value within two years before imaging, glucose at the time of imaging, neighborhood crime rate, or amygdalar activity. The relationship remained significant in models adjusted for combined demographic and clinical factors and combined socioeconomic and environmental factors (Table 3). Furthermore, those with noise exposure >45 dBA had decreased DM-free survival (log-rank p=0.036, Figure 2). In sensitivity analyses, the relationship between transportation noise exposure in 5 dBA increments and incident type 2 DM remained significant (1.272 [1.031, 1.568], p=0.025), but the relationship was not significant when a threshold of >55 dBA was used (p=0.19).
Table 3:
Cox models assessing the unadjusted and adjusted relationships between higher transportation noise exposure (>45 dBA or upper tertile vs. others) and subsequent type 2 DM.
| Covariable | HR [95% CI] | p-value |
|---|---|---|
| None (univariable) | 2.429 [1.031, 5.719] | 0.042 |
| Age and sex | 2.393 [1.015, 5.638] | 0.046 |
| Caucasian race | 2.431 [1.022, 5.785] | 0.045 |
| Baseline BMI | 2.434 [1.033, 5.739] | 0.042 |
| Baseline VAT volume | 2.619 [1.032, 6.643] | 0.043 |
| Baseline VAT/SAT ratio | 2.666 [1.049, 6.774] | 0.039 |
| Baseline HTN | 2.438 [1.033, 5.755] | 0.042 |
| Baseline hyperlipidemia | 2.369 [1.003, 5.595] | 0.049 |
| Current smoking | 2.385 [1.011, 5.626] | 0.047 |
| Fasting glucose > 100 mg/dL within 2 years prior to imaging | 2.484 [1.050, 5.878] | 0.038 |
| Maximum fasting glucose < 2 years before imaging | 1.723 [0.680, 4.368] | 0.25 |
| Fasting glucose at time of imaging | 2.416 [0.989, 5.901] | 0.053 |
| Pre-DM | 2.472 [1.044, 5.849] | 0.040 |
| History of cancer | 2.424 [1.027, 5.721] | 0.043 |
| History of cancer treatment | 2.397 [1.016, 5.655] | 0.046 |
| Combined demographic and clinical factors | 2.658 [1.091, 6.475] | 0.036 |
| Median neighborhood income | 2.668 [1.112, 6.398] | 0.028 |
| Neighborhood total crime ratea | 2.258 [0.851, 5.993] | 0.10 |
| Air pollutionb | 2.601 [1.068, 6.334] | 0.035 |
| Combined Socioeconomic and environmental factors | 2.613 [1.102, 6.194] | 0.029 |
| Amygdalar activity | 1.968 [0.795, 4.873] | 0.14 |
Each row represents a separate model (all multivariable models additionally adjusted for age and sex) aside from combined models: Combined demographic and clinical factors (age, sex, Caucasian race, baseline BMI, baseline HTN, baseline hyperlipidemia, pre-DM, current smoking, history of cancer, and history of cancer treatment) and combined socioeconomic and environmental factors (median neighborhood income and air pollution). Backwards selection was implemented for combined models.
Crime data available in 171 subjects.
Air pollution data available in 212 subjects.
Bold type indicates p<0.05.
Abbreviations: BMI-body mass index, CI-confidence interval, HTN-hypertension, SAT-subcutaneous adipose tissue, VAT-visceral adipose tissue.
Figure 2:
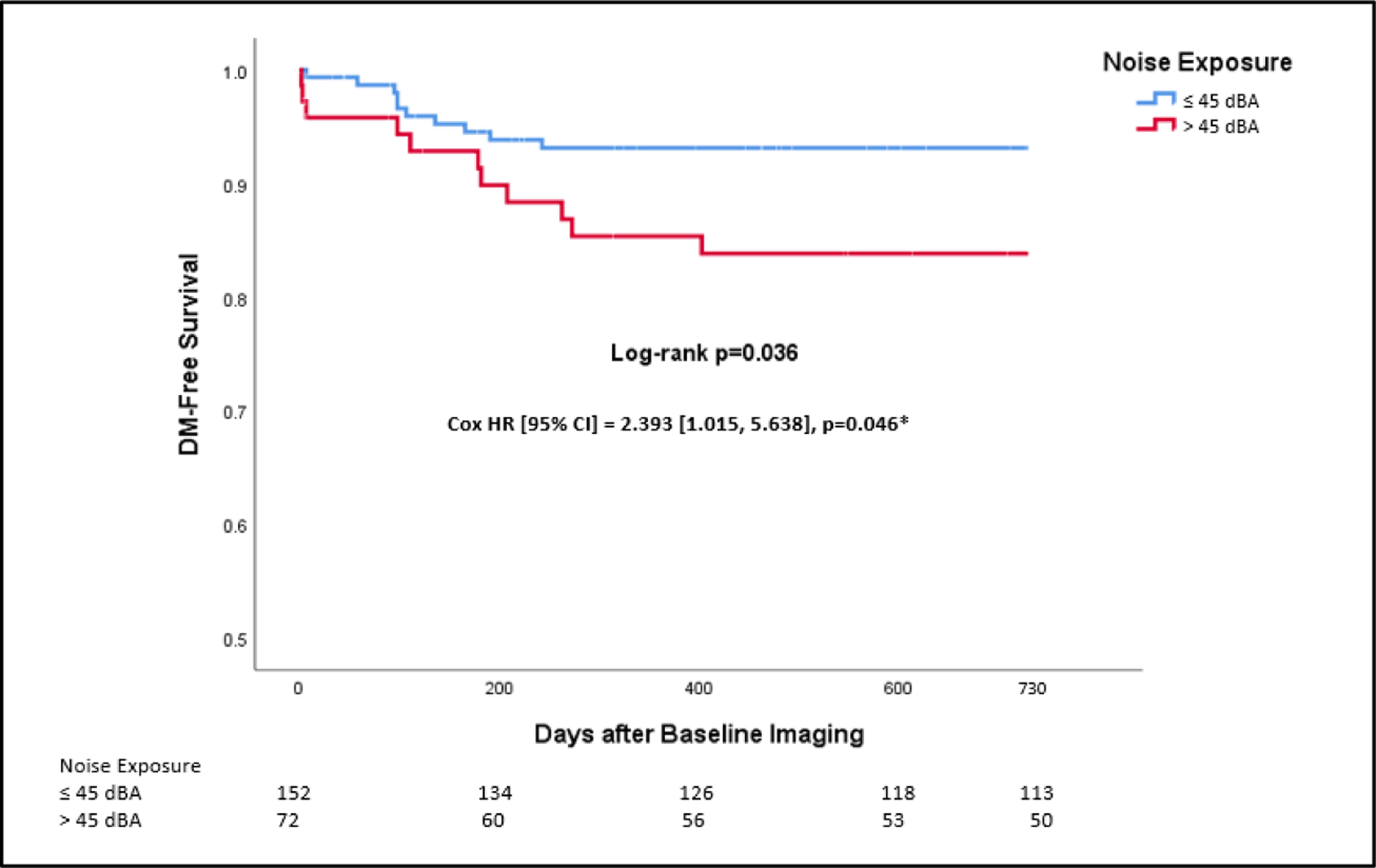
Kaplan-Meier plot of type 2 DM-free survival by transportation noise exposure (upper tertile or >45 dBA vs. others). Log-rank p-value and a multivariable Cox model adjusted for age and sex are shown. Abbreviations: CI-confidence interval, DM-diabetes mellitus, HR-hazard ratio.
3.4. The Link between Noise Exposure to Visceral Adiposity and Incident Diabetes Involves Increased Amygdalar Activity
Consistent with prior observations, heightened transportation noise exposure associated with higher AmygA (standardized β [95% CI]: 0.572 [0.325, 0.820], p<0.001, Table 2A).(Osborne et al., 2020) Moreover, in unadjusted path analysis, the indirect path of noise >45 dBA→↑AmygA→↑baseline VAT was significant (standardized β [95% CI]: 0.108 [0.005, 0.225], p<0.05, Figure 3A). Similarly, the indirect path of noise exposure >45 dBA→↑AmygA→↑incident DM was significant in an unadjusted model (standardized log odds ratio [95% CI]: 0.229 [0.036, 0.647], p<0.05, Figure 3B).
Figure 3:
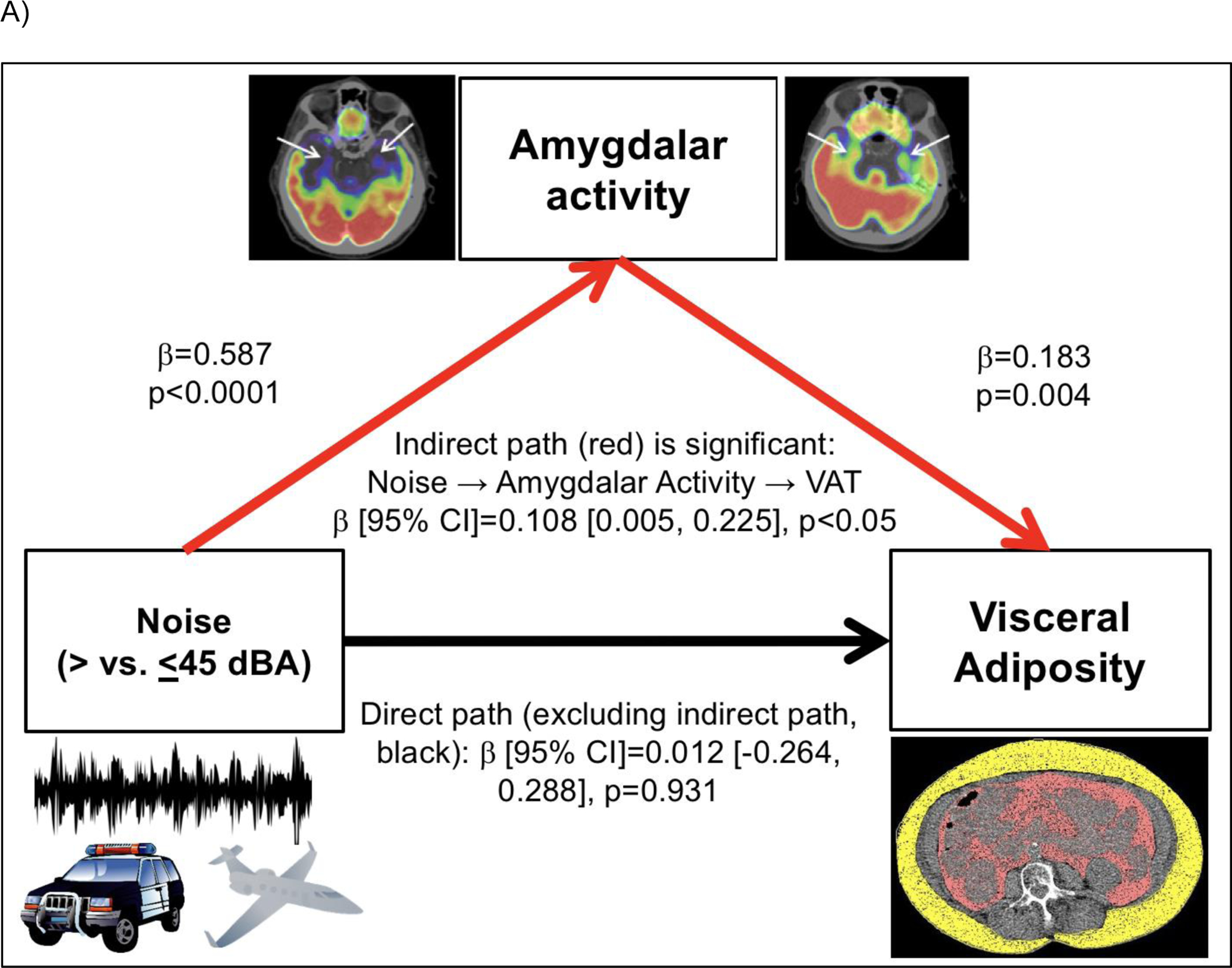
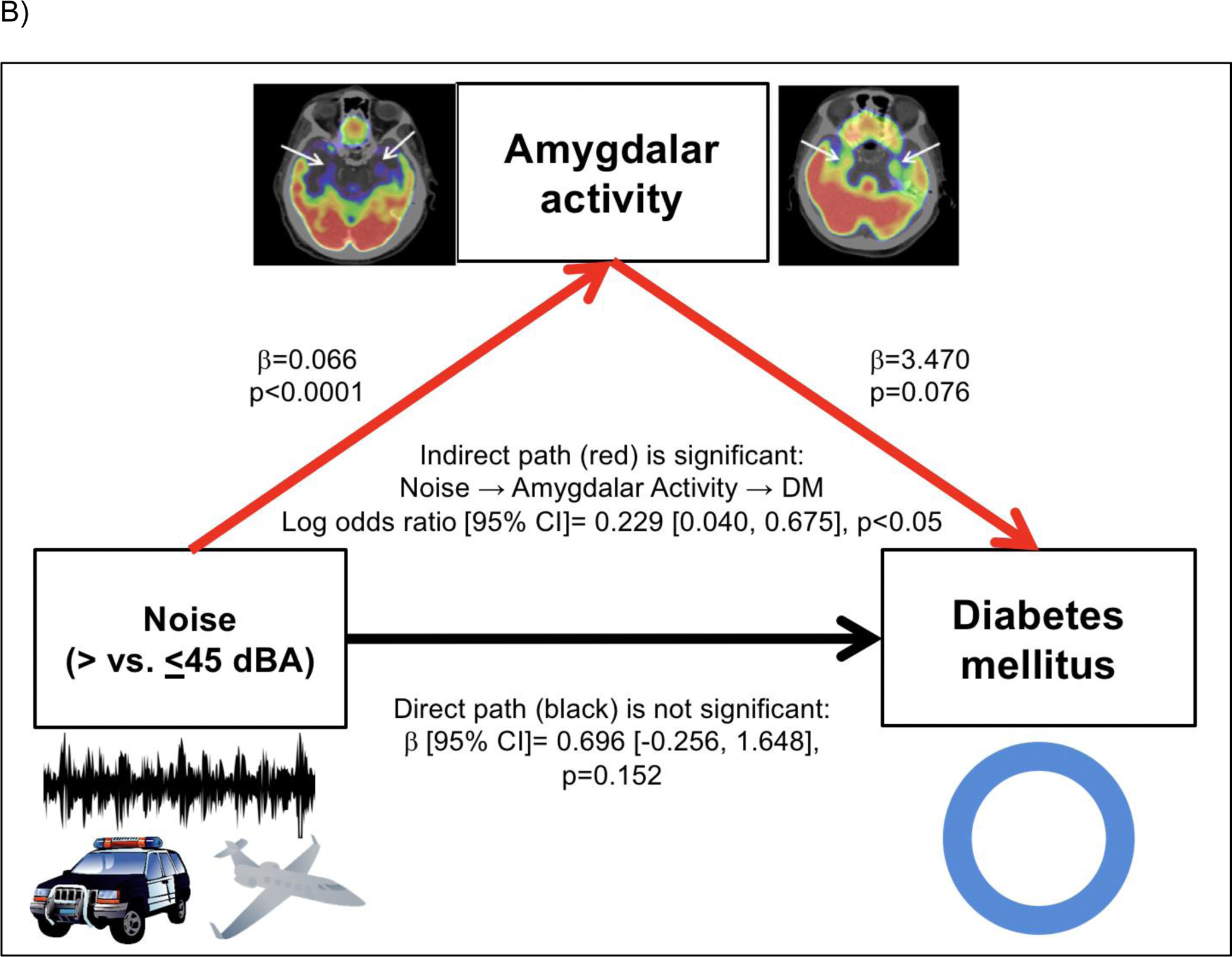
Mediation models evaluating the pathways linking increased transportation noise exposure (>45 dBA) to: A) increased VAT through increased amygdalar metabolic activity and B) increased type 2 DM risk through increased amygdalar metabolic activity. Models are unadjusted. Indirect pathways are shown in red and direct pathways are shown in black.
Abbreviations: AmygA-amygdalar metabolic activity, CI-confidence interval, VAT-visceral adipose tissue.
4. Discussion
This study leveraged multi-system imaging to show that increased chronic transportation noise exposure associates with increased baseline VAT and gains in VAT as well as type 2 DM risk independently of many important covariables. Additionally, heightened amygdalar metabolic activity on 18F-FDG-PET/CT imaging mediated the relationship between noise exposure and baseline VAT as well as incident type 2 DM. Although these pathways do not account for all the factors (e.g., health behaviors) that link noise exposure to metabolic diseases, the findings implicate stress-associated neural centers in participating in the initiation of this pathologic mechanism.
4.1. Relationship between Noise Exposure and Obesity and DM
Our results corroborate the known associations between noise exposure and obesity and DM.(Christensen et al., 2015; Christensen et al., 2016; Clark et al., 2017; Eze et al., 2017; Fritschi et al., 2011; Munzel et al., 2018; Pyko et al., 2015; Pyko et al., 2017; Shin et al., 2020; Sorensen et al., 2013) Notably, herein we extend prior findings by adjusting for a number of potential confounders in the same population, including radiographic adiposity measures and baseline blood glucose assessments, to show that there is a predominantly independent relationship between noise exposure and new onset type 2 DM within two years that persists even after accounting for baseline measures of metabolic impairment (e.g., pre-DM and baseline VAT). By using imaging to objectively quantify adipose tissue, we also build upon prior work by showing that individuals with higher noise exposure also have greater baseline VAT as well as gains in VAT over approximately one year. These results identify higher noise exposure as a largely independent and under-recognized risk factor for metabolic disease.
4.2. Mechanistic Insights
The amygdala is a limbic brain center that has a prominent role in responding to noise stress.(Osborne et al., 2020; Spreng, 2000; Zald and Pardo, 2002) When activated, amygdalar blood flow and metabolism increase, and its efferent neurons activate the SNS, leading to increased leukopoiesis, systemic inflammation, endothelial dysfunction, and oxidative stress.(Munzel et al., 2018; Osborne et al., 2020; Schmidt et al., 2015) Concurrently, HPA axis activity is increased, leading to increased cortisol with associated dysregulation of glucose metabolism and increased adiposity.(Munzel et al., 2018) Importantly, endothelial dysfunction, a known cardiovascular consequence of noise exposure, also predicts future prediabetes and DM and may directly contribute to the link between noise and metabolic disease.(Hahad et al., 2019; Munzel et al., 2017; Munzel et al., 2018) In addition, increased bone marrow activity, a marker of leukopoietic activity, is a known mediator of the relationship between increased AmygA and greater VAT as well as heightened atherosclerotic inflammation.(Ishai et al., 2019; Tawakol et al., 2017) Collectively, these changes ultimately lead to downstream cardiovascular and metabolic diseases, such as VAT, DM, and inflammatory atherosclerosis.(Heidt et al., 2014; Ishai et al., 2019; Munzel et al., 2017; Munzel et al., 2018; Osborne et al., 2018; Osborne et al., 2020; Spreng, 2000; Zald and Pardo, 2002) The current study expands upon the link from AmygA to VAT and type 2 DM to show that increased transportation noise exposure drives the front end of this pathway to incite these metabolic diseases, suggesting that the amygdala serves as an important conduit by which noise stress enters the body and triggers subsequent pathology.
Furthermore, these findings may provide further clarification of the complex interplay between noise exposure, AmygA, VAT, atherosclerotic inflammation, and CVD events.(Figueroa et al., 2016; Ishai et al., 2019; Osborne et al., 2020; Tawakol et al., 2017) Heightened AmygA leads to greater visceral adiposity. VAT itself has high levels of local inflammation that potentiate its own expansion and increase systemic inflammation.(Despres, 2012; Lau et al., 2005) Mechanistically, greater VAT volume further perpetuates the inflammatory milieu by augmenting monocytosis and priming local monocytes to produce cytokines, resulting in further peripheral monocyte migration and the development of inflammatory pathologies, including atherosclerosis.(Lau et al., 2005; Lumeng et al., 2007; Nagareddy et al., 2014) The current study suggests that noise-induced metabolic diseases likely participate in the multi-organ neuroinflammatory mechanism linking increased noise exposure to downstream CVD events.
Noise’s deleterious impact on sleep may play an important role in these findings. While nocturnal transportation noise could not be directly measured in this study that implemented mean 24-hour noise measurements, poor sleep has been linked to increased perceived stress, neurohormonal changes, and heightened inflammation and oxidative stress.(Killgore, 2013; Munzel et al., 2017; Schmidt et al., 2013) These sleep-induced changes culminate in metabolic and cardiovascular diseases.(Cappuccio et al., 2010; Schmidt et al., 2015) Furthermore, impaired sleep increases amygdalar activity and reactivity and negatively modifies its connectivity with the inhibitory prefrontal cortex and other regulatory regions.(Killgore, 2013; Motomura et al., 2014) The impact of nocturnal noise on sleep, stress-associated neural activity, and metabolic health should be simultaneously evaluated in subsequent research.
4.3. Clinical Implications
This study not only supports efforts to limit noise exposure in our communities but also identifies biological targets for possible therapeutic interventions in situations where noise mitigation alone is inadequate. The impact of behavioral therapies on the brain’s stress response and downstream systemic inflammation as well as the impact of anti-inflammatory therapies could be evaluated in studies of individuals with high noise exposure and increased metabolic risk.(Creswell et al., 2012; Holzel et al., 2010; Taren et al., 2015; Tawakol et al., 2013)
4.4. Limitations
This study has notable limitations that relate to the retrospective study design. The sample was largely derived from patients with prior or suspected malignancy and may only represent a portion of the broad population at risk for health complications from noise exposure. In addition, there were relatively few individuals (approximately 10–15%) in the study populations with noise exposure greater than the World Health Organization threshold (>55 dBA), limiting the statistical power to detect significant differences at this threshold. Clinical follow-up occurred in a non-uniform fashion at the discretion of individual physicians, which may have influenced the results. Transportation noise exposure was estimated as a 24-hour average at each individual’s home address; thus, nocturnal exposure and exposure at other locations (e.g., work) could not be assessed. In addition, measures of SNS and HPA axis activity (including cortisol) as well as those of systemic inflammation and oxidative stress, perceived stress, and health behaviors (e.g., sleep, diet, physical activity) were not available. The duration of time each individual lived at their home address prior to imaging was not known. Individual level socioeconomic factors were unavailable; thus, they were evaluated at the zip code or town level, a proxy which has been validated in prior studies.(Diez Roux, 2001) Future prospective studies that account for these limitations are warranted to further clarify the relationship between noise exposure and metabolic disease.
5. Conclusions
Increased transportation noise exposure associates with greater VAT and type 2 DM risk. Amygdalar metabolic activity mediates the relationships between higher noise exposure and VAT as well as DM. These findings suggest that altered stress-associated neurobiological activity partially mediates the relationship between noise exposure and metabolic diseases in humans.
Supplementary Material
Highlights.
Noise exposure associates with greater baseline and gains in visceral adiposity.
Noise exposure also associates with greater diabetes risk over two years.
Noise exposure’s link to these diseases involves altered neurobiological activity.
These findings suggest novel therapies for metabolic disease due to noise exposure.
Funding
The authors of this work were supported in part by the following grants: American Heart Association (Dallas, TX) #18CDA34110366, 2018 (MTO) and United States National Institutes of Health (Bethesda, MD) #KL2TR002542, 2018–2020 (MTO), #K23HL151909, 2021 (MTO), #P30DK040561, 2017–2021 (SKG), #P01HL131478, 2018–2021 (ZAF, LMS, and AT), #R01HL137913, 2017–2021 (AT), #R33HL141047, 2018–2021 (AT), #R01HL149516, 2020–2021 (AT, JL), and #R01HL152957, 2020–2021 (AT). None of the sponsors had any role in data collection, analysis, and interpretation or in manuscript preparation, review, and approval.
Abbreviations
- AmygA
amygdalar metabolic activity
- BMI
body mass index
- CI
confidence interval
- CVD
cardiovascular disease
- DM
diabetes mellitus
- 18F-FDG-PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- HPA
hypothalamic-pituitary-adrenal
- HR
hazard ratio
- HTN
hypertension
- IQR
interquartile range
- SAT
subcutaneous adipose tissue
- SNS
sympathetic nervous system
- SUV
standardized uptake value
- VAT
visceral adipose tissue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
Michael Osborne, MD received consulting fees from Intrinsic Imaging, LLC for unrelated work. Steven K. Grinspoon, MD received consulting fees from Viiv and Theratechnologies for unrelated work. Ahmed Tawakol, MD received institutional grants from Genentech and personal fees from Actelion, DalCor, Cor2ed, and Esperion for unrelated research. The remaining authors have no significant disclosures.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
The data for this study can be made available upon reasonable request.
References
- American Diabetes Association, 2016. 2. Classification and Diagnosis of Diabetes. Diabetes Care 39, S13–22. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA, 2010. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33, 414–420. 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JS, Raaschou-Nielsen O, Tjonneland A, Nordsborg RB, Jensen SS, Sorensen TI, Sorensen M, 2015. Long-term exposure to residential traffic noise and changes in body weight and waist circumference: A cohort study. Environ. Res 143, 154–161. 10.1016/j.envres.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Christensen JS, Raaschou-Nielsen O, Tjonneland A, Overvad K, Nordsborg RB, Ketzel M, Sorensen T, Sorensen M, 2016. Road Traffic and Railway Noise Exposures and Adiposity in Adults: A Cross-Sectional Analysis of the Danish Diet, Cancer, and Health Cohort. Environ. Health Perspect 124, 329–335. 10.1289/ehp.1409052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Sbihi H, Tamburic L, Brauer M, Frank LD, Davies HW, 2017. Association of Long-Term Exposure to Transportation Noise and Traffic-Related Air Pollution with the Incidence of Diabetes: A Prospective Cohort Study. Environ. Health Perspect 125, 087025. 10.1289/EHP1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD, Irwin MR, Burklund LJ, Lieberman MD, Arevalo JM, Ma J, Breen EC, Cole SW, 2012. Mindfulness-Based Stress Reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav. Immun 26, 1095–1101. 10.1016/j.bbi.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres JP, 2012. Body fat distribution and risk of cardiovascular disease: an update. Circulation 126, 1301–1313. 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, 2001. Investigating neighborhood and area effects on health. Am. J. Pub. Health 91, 1783–1789. 10.2105/ajph.91.11.1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze IC, Foraster M, Schaffner E, Vienneau D, Heritier H, Rudzik F, Thiesse L, Pieren R, Imboden M, von Eckardstein A, Schindler C, Brink M, Cajochen C, Wunderli JM, Roosli M, Probst-Hensch N, 2017. Long-term exposure to transportation noise and air pollution in relation to incident diabetes in the SAPALDIA study. Int. J. Epidemiol 46, 1115–1125. 10.1093/ije/dyx020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa AL, Takx RA, MacNabb MH, Abdelbaky A, Lavender ZR, Kaplan RS, Truong QA, Lo J, Ghoshhajra BB, Grinspoon SK, Hoffmann U, Tawakol A, 2016. Relationship Between Measures of Adiposity, Arterial Inflammation, and Subsequent Cardiovascular Events. Circ. Cardiovasc. Imaging 9, e004043. 10.1161/CIRCIMAGING.115.004043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi L, Brown AL, Kim R, Schwela DH, Kephalopoulos SE, 2011. Burden of Disease from Environmental Noise: Quantification of healthy life years lost in Europe World Health Organization, Regional Office for Europe, Bonn, and European Commission Joint Research Centre. [Google Scholar]
- Hahad O, Wild PS, Prochaska JH, Schulz A, Hermanns I, Lackner KL, Pfeiffer N, Schmidtmann I, Gori T, Deanfield JE, Munzel T, 2019. Endothelial Function Assessed by Digital Volume Plethysmography Predicts the Development and Progression of Type 2 Diabetes Mellitus. J. Am. Heart Assoc 15, e012509. 10.1161/JAHA.119.012509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A, 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach Guilford Press, New York. [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M, 2014. Chronic variable stress activates hematopoietic stem cells. Nat. Med 20, 754–758. 10.1038/nm.3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW, 2010. Stress reduction correlates with structural changes in the amygdala. Soc. Cog. Affect. Neurosci 5, 11–17. 10.1093/scan/nsp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Osborne MT, Tung B, Wang Y, Hammad B, Patrich T, Oberfeld B, Fayad ZA, Giles JT, Lo J, Shin LM, Grinspoon SK, Koenen KC, Pitman RK, Tawakol A, 2019. Amygdalar Metabolic Activity Independently Associates With Progression of Visceral Adiposity. J. Clin. Endocrinol. Metab 104, 1029–1038. 10.1210/jc.2018-01456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess BM, Pedley A, Massaro JM, Murabito J, Hoffmann U, Fox CS, 2012. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia 55, 2622–2630. 10.1007/s00125-012-2639-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, 2013. Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning. Sleep 36, 1597–1608. 10.5665/sleep.3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S, 2005. Adipokines: molecular links between obesity and atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol 288, H2031–2041. 10.1152/ajpheart.01058.2004 [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR, 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest 117, 175–184. 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U, 2007. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int. J. Obes 31, 500–506. 10.1038/sj.ijo.0803454 [DOI] [PubMed] [Google Scholar]
- McGuinn LA, Ward-Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, Schwartz J, Koutrakis P, Russell AG, Garcia V, Kraus WE, Hauser ER, Cascio W, Diaz-Sanchez D, Devlin RB, 2017. Fine particulate matter and cardiovascular disease: Comparison of assessment methods for long-term exposure. Environ. Res 159, 16–23. 10.1016/j.envres.2017.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura Y, Kitamura S, Oba K, Terasawa Y, Enomoto M, Katayose Y, Hida A, Moriguchi Y, Higuchi S, Mishima K, 2014. Sleepiness induced by sleep-debt enhanced amygdala activity for subliminal signals of fear. BMC Neurosci 15, 97. 10.1186/1471-2202-15-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sorensen M, 2018. Environmental Noise and the Cardiovascular System. J. Am. Coll. Cardiol 71, 688–697. 10.1016/j.jacc.2017.12.015 [DOI] [PubMed] [Google Scholar]
- Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S, 2017. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur. Heart J 38, 557–564. 10.1093/eurheartj/ehw294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ, 2014. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 19, 821–835. 10.1016/j.cmet.2014.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MT, Ishai A, Hammad B, Tung B, Wang Y, Baruch A, Fayad ZA, Giles JT, Lo J, Shin LM, Grinspoon SK, Koenen KC, Pitman RK, Tawakol A, 2018. Amygdalar activity predicts future incident diabetes independently of adiposity. Psychoneuroendocrinology 100, 32–40. 10.1016/j.psyneuen.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, Shin LM, Fayad ZA, Koenen KC, Rajagopalan S, Pitman RK, Tawakol A, 2020. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. Eur. Heart. J 41, 772–782. 10.1093/eurheartj/ehz820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyko A, Eriksson C, Lind T, Mitkovskaya N, Wallas A, Ogren M, Ostenson CG, Pershagen G, 2017. Long-Term Exposure to Transportation Noise in Relation to Development of Obesity-a Cohort Study. Environ. Health Perspect 125, 117005. 10.1289/EHP1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyko A, Eriksson C, Oftedal B, Hilding A, Ostenson CG, Krog NH, Julin B, Aasvang GM, Pershagen G, 2015. Exposure to traffic noise and markers of obesity. Occup. Environ. Med 72, 594–601. 10.1136/oemed-2014-102516 [DOI] [PubMed] [Google Scholar]
- Recio A, Linares C, Banegas JR, Diaz J, 2016. The short-term association of road traffic noise with cardiovascular, respiratory, and diabetes-related mortality. Environ. Res 150, 383–390. 10.1016/j.envres.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Munzel T, 2015. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin. Res. Cardiol 104, 23–30. 10.1007/s00392-014-0751-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Munzel T, 2013. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur. Heart J 34, 3508–3514a. 10.1093/eurheartj/eht269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Bai L, Oiamo TH, Burnett RT, Weichenthal S, Jerrett M, Kwong JC, Goldberg MS, Copes R, Kopp A, Chen H, 2020. Association Between Road Traffic Noise and Incidence of Diabetes Mellitus and Hypertension in Toronto, Canada: A Population-Based Cohort Study. J. Am. Heart Assoc 9, e013021. 10.1161/JAHA.119.013021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen M, Andersen ZJ, Nordsborg RB, Becker T, Tjonneland A, Overvad K, Raaschou-Nielsen O, 2013. Long-term exposure to road traffic noise and incident diabetes: a cohort study. Environ. Health Perspect 121, 217–222. 10.1289/ehp.1205503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng M, 2000. Central nervous system activation by noise. Noise Health 2, 49–58. [PubMed] [Google Scholar]
- Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, Rosen RK, Ferris JL, Julson E, Marsland AL, Bursley JK, Ramsburg J, Creswell JD, 2015. Mindfulness meditation training alters stress-related amygdala resting state functional connectivity: a randomized controlled trial. Soc. Cog. Affect. Neurosci 10, 1758–1768. 10.1093/scan/nsv066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, Nunes IO, Beals CR, Shankar SS, 2013. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. J. Am. Coll. Cardiol 62, 909–917. 10.1016/j.jacc.2013.04.066 [DOI] [PubMed] [Google Scholar]
- Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK, 2017. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 389, 834–845. 10.1016/S0140-6736(16)31714-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, Oberfeld B, Ishai A, Shin LM, Nahrendorf M, Warner ET, Wasfy J, Fayad ZA, Koenen K, Ridker PM, Pitman RK, Armstrong KA, 2019. Stress-Associated Neurobiological Pathway Linking Socioeconomic Disparities to Cardiovascular Disease. J. Am. Coll. Cardiol 73, 3243–3255. 10.1016/j.jacc.2019.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau, 2015. American Community Survey’s 5-Year Estimates https://factfinder.census.goc/faces/nav/jsf/pages/index.xhtml/ (accessed 15 December 2019).
- United States Department of Transportation, 2014. National Transportation Noise Map https://maps.bts.dot.gov/arcgis/apps/webappviewer/index.html?id=a303ff5924c9474790464cc0e9d5c9fb/ (accessed15 December 2019).
- United States Environmental Protection Agency, 2017. AQS Data Mart https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html/ (accessed15 December 2019).
- United States Federal Bureau of Investigation, 2015. Massachusetts Offenses Known to Law Enforcement by City https://ucr.fbi.gov/crime-in-the-u.s/2015/crime-in-the-u.s.−2015/tables/table-8/table-8-state-pieces/table_8_offenses_known_to_law_enforcement_massachusetts_by_city_2015.xls/ (accessed15 December 2019).
- Zald DH, Pardo JV, 2002. The neural correlates of aversive auditory stimulation. NeuroImage 16, 746–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study can be made available upon reasonable request.


