Abstract
Little is known about the impact of coronavirus disease 2019 (COVID-19) pandemic to the care of patients with systemic lupus erythematosus (SLE) in the long-term. By crossing population data with the results of a web-based survey focused on the timeframes January–April and May–December 2020, we found that among 334/518 responders, 28 had COVID-19 in 2020. Seventeen cases occurred in May–December, in parallel with trends in the general population and loosening of containment policy strength. Age > 40 years (p = 0.026), prednisone escalation (p = 0.008) and infected relatives (p < 0.001) were most significantly associated with COVID-19. Weaker associations were found with asthma, lymphadenopathy and azathioprine or cyclosporine treatment. Only 31% of patients with infected relatives developed COVID-19. Healthcare service disruptions were not associated with rising hospitalisations. Vaccination prospects were generally welcomed. Our data suggest that COVID-19 has a moderate impact on patients with SLE, which might be significantly modulated by public health policies, including vaccination.
Keywords: Coronavirus, COVID-19, Systemic lupus erythematosus, Public health, Vaccination, Treatment, Prednisone, Lockdown, Containment
Abbreviations: ACE, angiotensin converting enzyme; COVID-19, SARS-CoV-2-related disease; cCOVID, COVID-19 cases confirmed by reverse transcriptase polymerase chain reaction; IFN, interferon; IQR, interquartile range; noCOVID, patients who were not diagnosed with COVID-19; NRS, numerical rating scale; pCOVID, presumed COVID-19 cases, based on symptoms, serological or imaging findings; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SLE, systemic lupus erythematosus; totCOVID, cCOVID + pCOVID cases
1. Introduction
Cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related disease (COVID-19) are accumulating around the world with dramatic consequences in terms of life expectancy, biological and psychological morbidity as well as social and economic stability [[1], [2], [3]]. Public health policies, including containment measures and, more recently, vaccination campaigns have become crucial to contrast the effects of the pandemic [4]. In parallel, an unprecedented international effort has led to significant advancement in understanding COVID-19 pathophysiology. In this context, the differential performance of interferon (IFN)-driven and antibody-mediated immune responses among individuals emerged as a pivotal mechanism in determining the clinical course of the disease [5]. Curiously, this landmark discovery disclosed an unexpected pathophysiological similarity between acute COVID-19-related inflammatory events and chronic aberration in the deployment of the immune response as observed in patients with systemic lupus erythematosus (SLE) [[6], [7], [8]]. Moreover, evidence of a potential association between SLE-related pre-pandemic anti-IFN antibodies and COVID-19 has been provided by some authors [9]. These data further stimulated a general interest in studying the impact of SARS-CoV-2 infection in patients with SLE, who bear a unique combination of potential protective and detrimental factors for COVID-19 and might also constitute a paradigm to assess the wider social impact of the pandemic in persons with chronic diseases [3,[10], [11], [12]]. In fact, while favourable demographics (SLE is more frequent in young women, who have lower risk of severe COVID-19), hyperactive antiviral-like IFN-responses and potential prevention of COVID-related cytokine storm manifestations by chronic immunosuppression/immunomodulation could have constituted potential protective factors for patients with SLE, dysregulation of IFN responses together with altered epigenetics of angiotensin converting enzyme 2 (ACE2, the human target of SARS-CoV-2) and impairment of physiological immune responses by immunodepression might have behaved in the opposite way [9,[13], [14], [15]].
At a clinical level, a slightly increased COVID-19 morbidity has been reported in patients with SLE and other rheumatic diseases in comparison to the general population [11,12,16]. Nonetheless, a significant geographical variability is consistently detectable among different studies [[17], [18], [19]], possibly suggesting that local factors including population-level containment strategies [10,11,20,21] might affect the global impact of COVID-19 pandemic on patients with SLE. In addition, experimental strategies also varied among studies. Multi-national registries focused on clinical features and disease course of patients with rheumatic diseases and COVID-19 and continuously provide data of increasing robustness about potential risk factors for severe or fatal outcomes, beside potential limitations due to the lack of non-COVID control groups and risk of overreporting of more severe cases [[22], [23], [24]]. Other researchers analysed population-based data and public health databases, thus complementing registry information with a bird's-eye view of contagion dynamics in patients with rheumatic diseases in the frame of the general population [18,19,25]. Complementary to these approaches, cohort studies based on questionnaires or chart review were also frequently reported [3,10,[26], [27], [28], [29], [30]] and provided a deeper insight into the multifaceted clinical, social and psychological impact of the pandemic in the specific settings of patients with selected rheumatic diseases or general inflammatory disorder cohorts, despite potential limitations in capturing more severe cases [11,12].
Similar to other Countries in Europe and around the world, Italy was severely hit by the pandemic, with a first surge of cases in February–April 2020 and a second peak in November 2020 [31]. Of note, public health measures implemented during these two timeframes differed significantly, with looser rules prescribed during the second contagion wave when a three-colour regional risk classification was applied (high-risk = “red”, intermediate-risk = “orange”, and low-risk = “yellow”) as compared to a homogenous country-wide “red”-zone instituted during the first wave [32]. While data about the early impact of COVID-19 in patients with SLE are accumulating, little is known about the global effects of the pandemic in the medium/long-term, also in light of emerging topics, such as the ongoing vaccination campaigns. To address this issue, we performed a web-based survey in a multicentre cohort of patients with SLE referring to three tertiary care hospitals in the Milan metropolitan area.
2. Methods
2.1. Questionnaire
From January 4th to 14th 2021, 518 patients routinely followed up at IRCCS Ospedale San Raffaele, ASST Pini-CTO and Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (all members of SMILE, Milan Lupus Consortium) were invited to take a web-based anonymous [33] survey (Supplementary Material) hosted on the surveymonkey.com platform and covering the clinical events of the entire year 2020. Patients were instructed to avoid multiple registrations. Face and content validity of the questionnaire were assessed by direct interview of five randomly selected patients and by consensus among three expert rheumatologists (LB, EPB, MG) and a specialist in Infectious diseases, respectively. The questionnaire encompassed general demographics and clinical features, exposure to subjects with definite COVID-19 and occurrence of COVID-19 in patients. Numerical rating scales (NRS) ranging from zero (no compliance) to ten (strict compliance) were included to assess patients' and patients' family members' attitudes towards behavioural measures across the two pandemic waves. As data from the first pandemic wave have already been reported, we focused some additional questions, including prevalence of potential COVID-19 symptoms, hospitalisations and cases of COVID-19 among patients' family members/cohabitants, on the second observation period only. Data were also acquired about patients' attitude towards COVID-19 vaccination and about their perception of the impact of the pandemic on SLE course and management. The questionnaire was built in compliance with the European Guidelines for anonymisation [33] and after confirmation by the Ethics Committee and Data Protection Officer of IRCCS Ospedale San Raffaele, Milan, Italy that no further approval was required for this study.
2.2. Definitions of COVID-19 cases and timeframes
We defined cases diagnosed through reverse-transcriptase polymerase chain reaction as confirmed cases (cCOVID). Cases of COVID-19 diagnosed by a Physician on the basis of clinical features, radiological findings or a positive serology were labelled as presumptive cases (pCOVID). Total cases with COVID-19 (totCOVID) identified patients with either cCOVID or pCOVID in contrast to patients without COVID-19 (noCOVID) [21,34]. We identified two timeframes of interest: January to April 2020 (first period) and May to December 2020 (second period), based on trends of infection curves in Italy [35].
2.3. Validation
In order to test the consistency of responders' features with the general demographics and clinical features of the reference cohort, we analysed a representative [36] random sample of 75 patients routinely followed up in one of our three reference centres. Furthermore, to validate the reliability of our dichotomic observation timeframe for epidemiological analyses, we screened all visits performed from January to May 2021 for COVID-19 cases having occurred in 2020. New patients were excluded. This section of the study involved patients enrolled upon informed consent in a larger observational protocol (Pan-immuno), approved by the Institutional Review Board of IRCCS San Raffaele Hospital, Milan, Italy under registry number 22/INT/2018.
2.4. Population data
General demographic, epidemiological, and public security data were retrieved from publicly accessible databases under the Italian National Institute of Statistics [37], National Emergency Agency (Protezione Civile) [35], National Institute of Health (Istituto Superiore di Sanità) [31] and Ministry of Internal Affairs [38] and from the Official Gazette of the Italian Republic (https://www.gazzettaufficiale.it/).
2.5. Statistical analysis
The chi-squared test with Fisher's exact correction was used to compare the relative frequency of categorical variables among groups. The Mann-Whitney's U test or the Student's t-test were used to assess differences in quantitative variables among two groups under non-normally or normally distribution settings, respectively. Kruskal-Wallis' or ANOVA tests were employed in the same way for comparisons involving more than two groups. Microsoft Excel® 2019 and Statacorp STATA® version 15.0 were used for data elaboration and statistical analysis. Data are expressed as median (interquartile range, IQR) unless otherwise specified.
3. Results
3.1. Demographics, general clinical and treatment features
A total of 334 patients (out of 518 invited, 64%), responded to the survey. Two-hundred-seventy-one (81%) participated to a previous survey addressing the impact of COVID-19 during the first pandemic wave [21]. Most patients were women (301/334, 90%), older than 40 years of age (209/334, 63%) with disease duration exceeding 10 years (205/334, 61%). Joint and skin involvement along with fatigue were the most frequent SLE features, while hypertension and allergy represented the most frequent comorbidities (Table 1). Demographics and general clinical features were consistent with those of a representative random sample of patients from the same cohort and with data from the previous survey [21], except for slightly different frequencies of joint involvement and constitutional symptoms (Supplementary Table 1). Patient self-reported global health status on a 0–10 NRS (with 10 representing the optimal status) was 7 (6–8) at time of taking the survey. The majority of patients reported being on hydroxychloroquine (235/334, 70%); 211/334 (63%) were on one or more immunosuppressants; 233/334 were on prednisone and 128 of them (55%) were taking less than 5 mg/day prednisone equivalents. Ninety-seven patients (29%) reported treatment escalation at least once during the course of 2020. Sixty patients (62%) required increased prednisone dose, 49 (51%) new or potentiated immunosuppressants, 23 (7%) addition of belimumab. Conversely, 114 patients (34%) reported that during 2020 their disease was enough controlled to allow tapering of prednisone (78/114, 68%) and/or immunosuppressants (40/114, 35%); in three cases belimumab could also be withdrawn (Table 1). Two-hundred-twenty-eight patients (68%) reported having missed one or more scheduled appointments due to healthcare service disruption secondary to the pandemic. For 179/329 responders (54%) the pandemic had no significant impact on the course of their disease; 56 (17%) reported a worsening impact; 12 (4%) felt that the pandemic had somehow been beneficial for their disease; 82 (25%) were uncertain about how the pandemic had possibly affected the course of SLE.
Table 1.
Clinical features of responders to the survey by COVID-19 diagnosis and timing of infection.
| Patients with SLE (total) | noCOVID | totCOVID |
cCOVID |
pCOVID |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | First phase | Second phase | All | First phase | Second phase | All | First phase | Second phase | |||
| N | 334 | 306 | 28 | 12 | 17 | 19 | 4 | 16 | 9 | 8 | 1 |
| Females: n (%) | 301 (90) | 274 (90) | 27 (96) | 12 (100) | 16 (94) | 18 (95) | 4 (100) | 15 (94) | 9 (100) | 8 (100) | 1 (100) |
| Age groups (years): n (%) | |||||||||||
| 18–25 | 12 (4) | 12 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 26–30 | 26 (8) | 23 (8) | 3 (11) | 2 (17) | 1 (6) | 1 (5) | 0 (0) | 1 (6) | 2 (22) | 2 (25) | 0 (0) |
| 31–35 | 33 (10) | 33 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 36–40 | 54 (16) | 52 (17) | 2 (7) | 1 (8) | 1 (6) | 2 (11) | 1 (25) | 1 (6) | 0 (0) | 0 (0) | 0 (0) |
| 41–45 | 28 (8) | 21 (7) | 7 (25) | 2 (17) | 5 (29) | 5 (26) | 1 (25) | 4 (25) | 2 (22) | 1 (13) | 1 (100) |
| 46–50 | 43 (13) | 38 (12) | 5 (18) | 2 (17) | 3 (18) | 3 (16) | 0 (0) | 3 (19) | 2 (22) | 2 (25) | 0 (0) |
| >50 | 138 (41) | 127 (42) | 11 (39) | 5 (42) | 7 (41) | 8 (42) | 2 (50) | 7 (44) | 3 (33) | 3 (38) | 0 (0) |
| Disease duration (years): n (%) | |||||||||||
| <2 | 19 (6) | 18 (6) | 1 (4) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) | 1 (100) |
| 2 to 10 | 110 (33) | 100 (33) | 10 (36) | 5 (42) | 5 (29) | 6 (32) | 1 (25) | 5 (31) | 4 (44) | 4 (50) | 0 (0) |
| >10 | 205 (61) | 188 (61) | 17 (61) | 7 (58) | 11 (65) | 13 (68) | 3 (75) | 11 (69) | 4 (44) | 4 (50) | 0 (0) |
| General clinical features: n (%) | |||||||||||
| Skin involvement | 185 (55) | 174 (57) | 11 (39) | 7 (58) | 5 (29) | 7 (2) | 3 (1) | 4 (25) | 4 (44) | 4 (50) | 0 (0) |
| Joint involvement | 243 (73) | 222 (73) | 21 (75) | 9 (75) | 13 (76) | 14 (74) | 3 (1) | 12 (75) | 7 (78) | 6 (75) | 1 (100) |
| Haematological disease | 180 (54) | 164 (54) | 16 (57) | 5 (42) | 11 (65) | 12 (63) | 1 (25) | 11 (69) | 4 (44) | 4 (50) | 0 (0) |
| Leukopenia | 105 (31) | 94 (31) | 11 (39) | 4 (33) | 7 (41) | 7 (37) | 0 (0) | 7 (44) | 4 (44) | 4 (50) | 0 (0) |
| Thrombocytopenia | 95 (28) | 88 (29) | 7 (25) | 1 (8) | 6 (35) | 7 (37) | 1 (25) | 6 (38) | 0 (0) | 0 (0) | 0 (0) |
| Anaemia | 84 (25) | 78 (25) | 6 (21) | 1 (8) | 5 (29) | 5 (26) | 0 (0) | 5 (31) | 1 (11) | 1 (13) | 0 (0) |
| Nephritis | 123 (37) | 115 (38) | 8 (29) | 3 (25) | 5 (29) | 6 (32) | 1 (25) | 5 (31) | 2 (22) | 2 (25) | 0 (0) |
| NPSLE | 52 (16) | 49 (16) | 3 (11) | 3 (25) | 0 (0) | 1 (5) | 1 (25) | 0 (0) | 2 (22) | 2 (25) | 0 (0) |
| Serositis | 93 (28) | 83 (27) | 10 (36) | 4 (33) | 7 (41) | 7 (37) | 1 (25) | 7 (44) | 3 (33) | 3 (38) | 0 (0) |
| Constitutional symptoms | 317 (95) | 292 (95) | 25 (89) | 11 (92) | 15 (88) | 18 (95) | 4 (100) | 15 (94) | 7 (78) | 7 (88) | 0 (0) |
| Fever | 172 (51) | 162 (53) | 10 (36) | 4 (33) | 7 (41) | 7 (37) | 1 (25) | 7 (44) | 3 (33) | 3 (38) | 0 (0) |
| Lymph-node enlargement | 138 (41) | 121 (40) | 17 (61)⁎ | 8 (67) | 10 (59) | 11 (58) | 2 (50) | 10 (63) | 6 (67) | 6 (75) | 0 (0) |
| Weight loss | 107 (32) | 95 (31) | 12 (43) | 7 (58) | 6 (35) | 7 (37) | 2 (50) | 6 (38) | 5 (56) | 5 (63) | 0 (0) |
| Fatigue | 283 (85) | 259 (85) | 24 (86) | 10 (83) | 15 (88) | 17 (89) | 3 (75) | 15 (94) | 7 (78) | 7 (88) | 0 (0) |
| Comorbidities: n (%) | |||||||||||
| None | 3 (1) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Arterial hypertension | 111 (33) | 105 (34) | 6 (21) | 3 (25) | 3 (18) | 3 (16) | 0 (0) | 3 (19) | 3 (33) | 3 (38) | 0 (0) |
| Myocardial infarction | 11 (3) | 10 (3) | 1 (4) | 0 (0) | 1 (6) | 1 (5) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) |
| Chronic heart failure | 4 (1) | 4 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Stroke | 10 (3) | 8 (3) | 2 (7) | 2 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22) | 2 (25) | 0 (0) |
| Diabetes | 6 (2) | 4 (1) | 2 (7) | 1 (8) | 1 (6) | 1 (5) | 0 (0) | 1 (6) | 1 (11) | 1 (13) | 0 (0) |
| COPD | 6 (2) | 5 (2) | 1 (4) | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 1 (13) | 0 (0) |
| Malignancy | 22 (7) | 20 (7) | 2 (7) | 2 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22) | 2 (25) | 0 (0) |
| Haematological malignancy | 1 (0) | 1 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Asthma | 22 (7) | 17 (6) | 5 (18)⁎ | 1 (8) | 5 (29) | 5 (26) | 1 (25) | 5 (31) | 0 (0) | 0 (0) | 0 (0) |
| Any allergy | 130 (39) | 117 (38) | 13 (46) | 6 (50) | 8 (47) | 11 (58) | 4 (100) | 8 (50) | 2 (22) | 2 (25) | 0 (0) |
| Drug allergy | 89 (27) | 81 (26) | 8 (29) | 4 (33) | 5 (29) | 7 (37) | 3 (75) | 5 (31) | 1 (11) | 1 (13) | 0 (0) |
| Allergy to food, inhalants (pollens, grasses, dustmites…), insect venom | 70 (21) | 64 (21) | 6 (21) | 3 (25) | 3 (18) | 4 (21) | 1 (25) | 3 (19) | 2 (22) | 2 (25) | 0 (0) |
| Other | 90 (27) | 84 (27) | 6 (21) | 3 (25) | 3 (18) | 3 (16) | 0 (0) | 3 (19) | 3 (33) | 3 (38) | 0 (0) |
| Treatment | |||||||||||
| Hydroxychloroquine: n (%) | 235 (70) | 215 (70) | 20 (71) | 6 (50) | 14 (82) | 15 (79) | 2 (50) | 13 (81) | 5 (56) | 4 (50) | 1 (100) |
| Prednisone: n(%) | 233 (70) | 209 (68) | 24 (86) | 11 (92) | 14 (82) | 16 (84) | 4 (100) | 13 (81) | 8 (89) | 7 (88) | 1 (100) |
| <5 mg/day: n(%) | 128 (38) | 115 (38) | 13 (46) | 4 (33) | 9 (53) | 9 (47) | 1 (25) | 8 (50) | 4 (44) | 3 (38) | 1 (100) |
| 5 mg/day: n(%) | 55 (16) | 51 (17) | 4 (14) | 3 (25) | 1 (6) | 2 (11) | 1 (25) | 1 (6) | 2 (22) | 2 (25) | 0 (0) |
| 5–7.5 mg/day: n(%) | 32 (10) | 28 (9) | 4 (14) | 2 (17) | 2 (12) | 3 (16) | 1 (25) | 2 (13) | 1 (11) | 1 (13) | 0 (0) |
| 7.5–10 mg/day: n(%) | 9 (3) | 8 (3) | 1 (4) | 0 (0) | 1 (6) | 1 (5) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 0 (0) |
| 10–25 mg/day: n(%) | 4 (1) | 2 (1) | 2 (7) | 2 (17) | 1 (6) | 1 (5) | 1 (25) | 1 (6) | 1 (11) | 1 (13) | 0 (0) |
| >25 mg/day: n(%) | 5 (1) | 5 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Immunosuppressants: n(%) | 211 (63) | 189 (62) | 22 (79) | 10 (83) | 13 (76) | 15 (79) | 3 (75) | 13 (81) | 7 (78) | 7 (88) | 0 (0) |
| Azathioprine | 45 (13) | 37 (12) | 8 (29)⁎ | 4 (33) | 4 (24) | 6 (32) | 2 (50) | 4 (25) | 2 (22) | 2 (25) | 0 (0) |
| Methotrexate | 30 (9) | 27 (9) | 3 (11) | 2 (17) | 1 (6) | 1 (5) | 0 (0) | 1 (6) | 2 (22) | 2 (25) | 0 (0) |
| Mycophenolate mofetil | 82 (25) | 77 (25) | 5 (18) | 2 (17) | 3 (18) | 3 (16) | 0 (0) | 3 (19) | 2 (22) | 2 (25) | 0 (0) |
| Cyclosporine A | 12 (4) | 7 (2) | 5 (18)⁎⁎ | 4 (33) | 2 (12) | 2 (11) | 1 (25) | 2 (13) | 3 (33) | 3 (38) | 0 (0) |
| Belimumab | 52 (16) | 46 (15) | 6 (21) | 3 (25) | 3 (18) | 3 (16) | 0 (0) | 3 (19) | 3 (33) | 3 (38) | 0 (0) |
| Other | 95 (28) | 87 (28) | 8 (29) | 2 (17) | 6 (35) | 6 (32) | 0 (0) | 6 (38) | 2 (22) | 2 (25) | 0 (0) |
| Off prednisone and immunosuppressants: n (%) | 54 (16) | 52 (17) | 2 (7) | 1 (8) | 1 (6) | 1 (5) | 0 (0) | 1 (6) | 1 (11) | 1 (13) | 0 (0) |
| Treatment changes | |||||||||||
| Any escalation: n(%) | 97 (29) | 84 (27) | 13 (46)⁎ | 6 (50) | 8 (47) | 9 (47) | 2 (50) | 8 (50) | 4 (44) | 4 (50) | 0 (0) |
| Immunosuppressant | 49 (15) | 46 (15) | 3 (11) | 1 (8) | 3 (18) | 3 (16) | 1 (25) | 3 (19) | 0 (0) | 0 (0) | 0 (0) |
| Prednisone | 60 (18) | 49 (16) | 11 (39)⁎ | 6 (50) | 6 (35) | 7 (37) | 2 (50) | 6 (38) | 4 (44) | 4 (50) | 0 (0) |
| Belimumab | 23 (7) | 21 (7) | 2 (7) | 2 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (22) | 2 (25) | 0 (0) |
| Any de-escalation: n(%) | 114 (34) | 103 (34) | 11 (39) | 4 (33) | 7 (41) | 8 (42) | 1 (25) | 7 (44) | 3 (33) | 3 (38) | 0 (0) |
| Immunosuppressant | 40 (12) | 35 (11) | 5 (18) | 1 (8) | 4 (24) | 4 (21) | 0 (0) | 4 (25) | 1 (11) | 1 (13) | 0 (0) |
| Prednisone | 78 (23) | 72 (24) | 6 (21) | 3 (25) | 3 (18) | 4 (21) | 1 (25) | 3 (19) | 2 (22) | 2 (25) | 0 (0) |
| Belimumab | 3 (1) | 3 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
: p < 0.05.
: p < 0.010.
3.2. COVID-19 cases in 2020
Twenty-eight patients (8%) reported a diagnosis of COVID-19 during 2020. Eleven totCOVID cases (three cCOVID, eight pCOVID) reportedly occurred before May 2020 (first period), and 16 (15 cCOVID, one pCOVID) from May to December 2020 (second period). One patient reported to have repeatedly been classified as cCOVID both in the first and second period. The total annual incidence of cCOVID among responders to the survey was 19/334 (6%). Consistently, among 217 patients seen during routine outpatients visits from January to May 2021, 15 (7%) had a history of cCOVID (n = 12) or pCOVID (n = 3) during 2020: five cases occurred between February and April 2020, one in September and nine in November 2020. Compared to survey responders with noCOVID, responders with totCOVID were more frequently older than 40 years of age (23/28 vs 186/306; p = 0.026), had more frequently a history of SLE-related lymphadenopathy (17/28 vs 121/306; p = 0.043) and of asthma (5/28 vs 17/306, p = 0.028). Patients who needed treatment escalation throughout 2020 (13/28 vs 84/306; p = 0.048), especially with prednisone (11/28 vs 49/306; p = 0.008) were more frequent in the totCOVID than in the noCOVID subgroup. Therapy with prednisone (24/28 vs 209/306; p = 0.056), azathioprine (8/28 vs 37/306; p = 0.037) and cyclosporine A (5/28 vs 7/306; p = 0.002) were also relatively more frequent in patients with totCOVID than in patients with noCOVID (Table 1).
3.3. Comparisons among the two phases
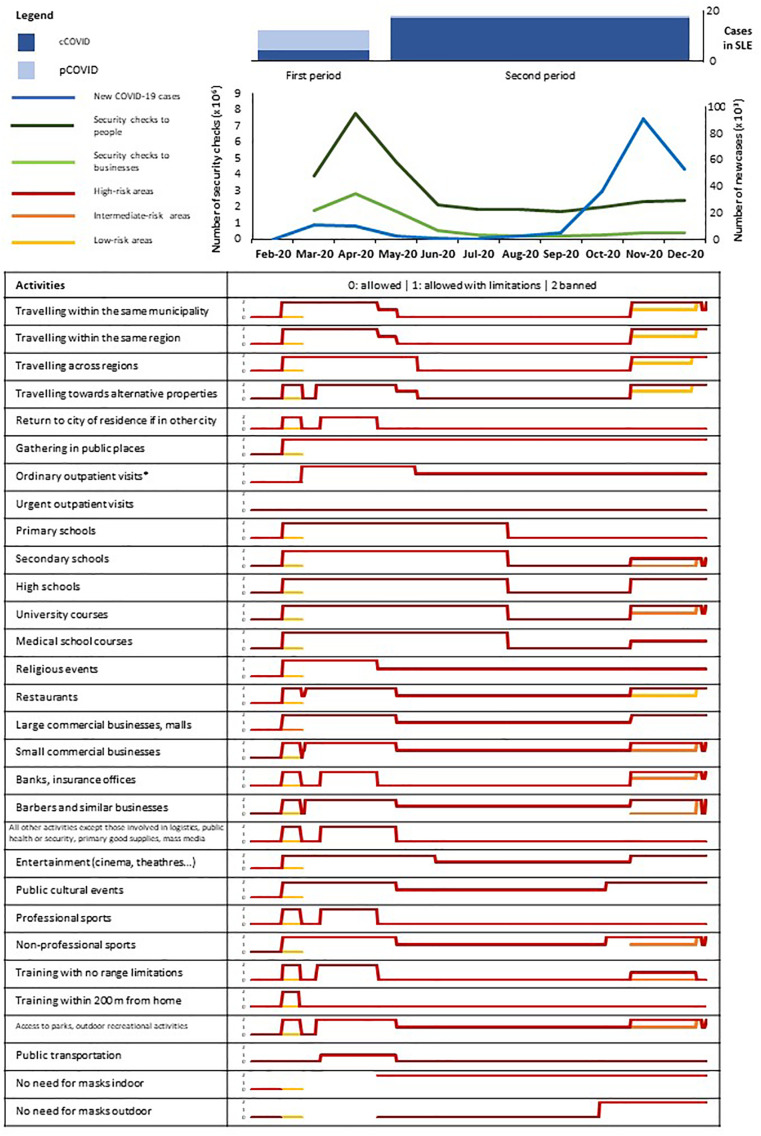
During the two observation timeframes a total of 204,335 and 1,905,198 cases of COVID-19 were respectively recorded in Italy, yielding a crude annual incidence rate of 4%. To contrast the spread of the contagion, multiple activities were banned or subject to limitations, with looser rules in the second period. Individual or business compliance to behavioural rules was checked regularly. However, the median number of checks/month to persons and businesses in the second observation period was significantly lower than in the first period [2.08 (1.82–2.36) x 106 vs 5.83 (3.88–7.77) x 106, p = 0.068 for individuals; 0.36 (0.25–0.47) x106 vs 2.27 (1.75–2.80) x106, p = 0.037 for businesses; Fig. 1 ).
Fig. 1.
Population context.
In this figure, the number of cases of confirmed (cCOVID, dark blue) and presumed (pCOVID, pale blue) COVID-19 are depicted in their temporal relation with general population variables, including a) trends of COVID-19 cases in Italy (light blue line); b) trends of security checks to people (dark green line) and businesses (light green line) upon the application of the laws prescribing limitations to gatherings and movements; and c) type and validity of such limitations (table). For each task subject to regulation, a colour-code is applied to distinguish among high- (red), intermediate- (orange) or low-risk (yellow) areas in Italy (the whole Country was homogeneously considered high-risk for almost all the first observation period). Higher-risk colours overlap lower-risk colours where the strictness of a given rule is the same into different risk areas. In addition, rule strictness is graded from 0 (complete freedom) to 2 (complete ban) to generate sparkline graphs for each task. Containment policies were significantly looser in the second observation period. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Regarding patients taking the survey and their families, there were no differences in self-reported compliance to the use of gloves or masks between the first and the second period. Patients' compliance to the prescription to adopt smart-working measures did also not change among the two observation timeframes, in contrast to patients' family members/cohabitants, who almost invariably returned to their usual work practices [compliance NRS = 0 (0–8) in the second period vs 3 (0−10) in the first period; p = 0.032]. Both patients and patients' family members/cohabitants showed lower compliance to lockdown measures in the second period than in the first period [compliance NRS = 7 (5–9) vs 8 (5–10); p < 0.001 for patients; 7 (3–9) vs 8 (5–10); p < 0.001 for patients' family members/cohabitants]. Exposure to confirmed COVID-19 cases was reported by 23 patients in the first period, 63 patients in the second period and 22 in both periods. Nineteen of these 108 patients with at least one contact with COVID-19 (18%) eventually had COVID-19, in contrast to 9/226 totCOVID cases among patients with no contact with other confirmed cases of COVID-19 (p < 0.001).
3.4. Specific features of the second observation timeframe
During the second observation period, 36 patients reported to have had at least one family member/cohabitant with COVID-19. Of these, only 11 (31%) were eventually classified as totCOVID (compared to 6/288, 2% totCOVID among patients with no family/member cohabitants with COVID-19; p < 0.001). Compared to the other 25 patients with potential family contacts with COVID-19, these 11 patients were more frequently older than 40 years (10/11 vs 11/25; p = 0.011) and tended to be more frequently on immunosuppression (10/11 vs 16/25; p = 0.127), while no difference was observed in terms of immunomodulation with hydroxychloroquine (8/11 vs 18/25) or in terms of other clinical or treatment features.
Twenty-one hospitalisations were reported over 35 weeks by 325 patients, yielding a hospitalisation rate of 9.6/100 person-years. Five patients (24%) required oxygen support during hospitalisation and two of them received intensive care. COVID-19 was the reason of admission in none of the 21 hospitalised patients. However, 3/21 hospitalised patients also had COVID-19 during the second observation timeframe (compared to 14/304 among non-hospitalised; p = 0.088).
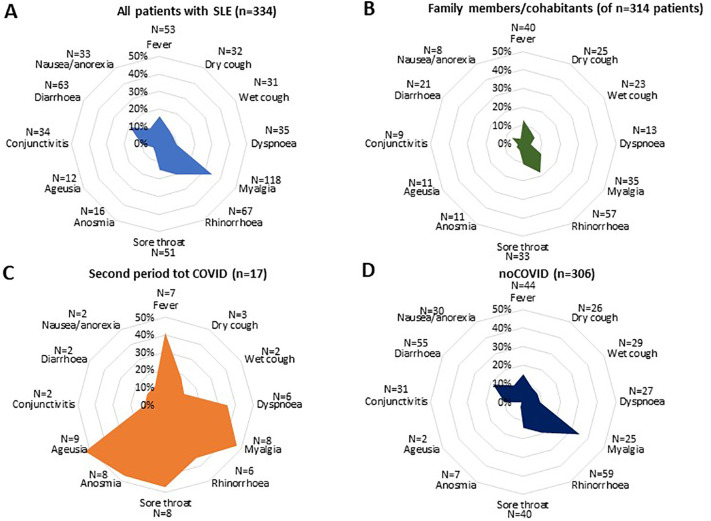
During the second period, one or more symptoms potentially attributable to COVID-19 were experienced by 63% of patients with SLE and 48% of patients' family members/cohabitants, independent of COVID-19 diagnosis. Dry cough, dyspnoea, sore throat, and anosmia or ageusia were significantly more frequent in patients with totCOVID than in patients with noCOVID. Among patients' family members/cohabitants a reported history of COVID-19 (either confirmed by polymerase chain reaction or presumed based on clinical features) was instead significantly associated with fever, dyspnoea, myalgia, anosmia and ageusia (Fig. 2 ).
Fig. 2.
Symptoms among patients with SLE and their relatives during the second period.
Radar graphs showing the prevalence of COVID-19 related symptoms in patients with SLE (blue) with (totCOVID, orange) or without (noCOVID, dark blue) COVID-19 and their family members/cohabitants. The percentage of symptoms was significantly higher in patients with totCOVID than in patients with noCOVID and in patients with SLE compared to their family members/cohabitants. Dry cough, dyspnoea, sore throat, and anosmia or ageusia were significantly more specifically represented in patients with totCOVID, while myalgia and fever were frequent also in patients with noCOVID. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Vaccination attitudes
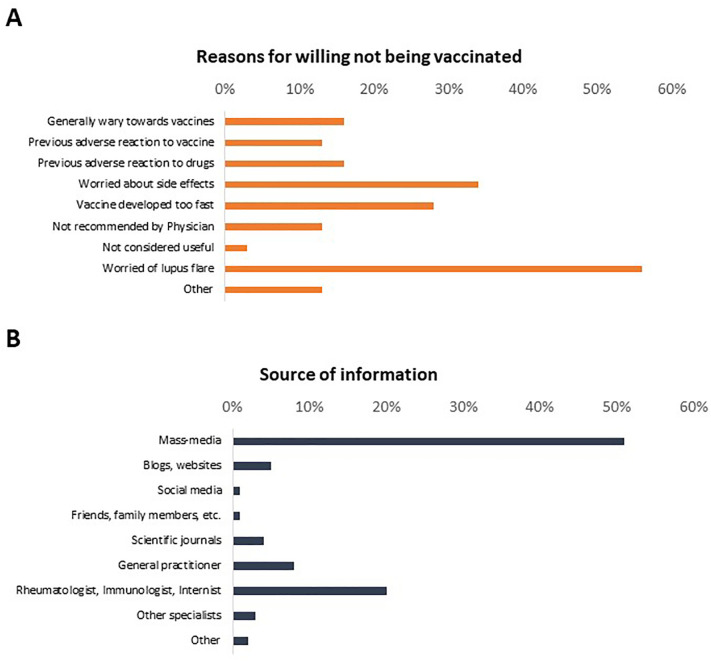
The median NRS for the estimated likelihood of receiving COVID-19 vaccination was 8 (5–10) out of 10. Consistently, 246 patients (76%) were generally in favour of being vaccinated, although 179 only if prescribed by a Physician and two only if compelled by law. Eighteen of 32 patients not willing to be vaccinated (56%) reported to be worried of experience a lupus flare due to vaccination, while 11/32 expressed concern about potential side-effects. Five and four patients reported previous adverse reactions to drugs or vaccines respectively as reasons to refuse COVID-19 vaccination. Mass-media were the most frequent source of information about vaccines in responders to the survey (Fig. 3 ).
Fig. 3.
Reasons for vaccine hesitancy and source of information on vaccines.
This figure summarises the reasons for vaccine hesitancy in 32/325 reporting to be not in favour of being vaccinated (A) and the main sources of information about vaccinations against SARS-CoV-2 among 325 responders to the survey (B).
4. Discussion
We performed a web-based survey investigating multiple aspects of the ongoing COVID-19 pandemic in a multicentre cohort of patients with SLE, integrated the results with information about the course of the contagion in Italy and validated their reliability against data from patients' charts. Our results show that COVID-19 cases accumulated among patients with SLE throughout 2020, yielding an annual incidence possibly exceeding the one of the general population [12,16,18,21], also in light of underestimation of the impact of fatal or very severe cases (which are reportedly frequent in SLE [22,39]) with our research strategy.
Analysis of individual features associating with the development of COVID-19 showed that having a family member/cohabitant with COVID-19 and being older were major risk factors for infection in patients with SLE, in line with the literature [16]. Furthermore, SARS-CoV-2 infection was associated with unstable disease (either consequent or preceding COVID-19), requiring treatment escalation. Our results also confirm that COVID-like symptoms are frequent in patients with SLE, which might further support the hypothesis of shared mechanisms of aberrant inflammation between COVID-19 and SLE [9].
At a cohort level, we observed a slightly higher number of totCOVID and especially of cCOVID cases in the second half of the year, which included the second pandemic wave. During this second time interval, a higher number of cases was recorded throughout the Country, in parallel with a lower number of checks for the effective application of public health measures. Consistently, relatively looser compliance to behavioural measures was reported by patients and their families along with higher rates of exposure to confirmed COVID-19 cases in the second observation period. Beside infections, the pandemic also affected the routine clinical management of SLE. More than two thirds of patients reported at least one cancelled appointment due to the contagion. Despite this, only 17% of patients felt that the pandemic had a detrimental impact on their disease. Indeed, the reported annual rate of patients needing treatment escalation at least once was 29%, which is stable compared to the pre-pandemic setting [40]. The all-cause annual hospitalisation rate in this study was 9.6/100 person-years, which is also consistent with our preliminary observations during the first pandemic wave [21] and previous evidence in the literature from non-pandemic settings [41]. Constant engagement of patients in a multi-centre clinical and research network dedicated to patients with SLE might have contributed to mitigate the detrimental effect of the pandemic [10], besides other potential structural advantages such as relatively easy access to health care, thanks to a public national health system [42]. Efficient individual and population containment measures (with adequate monitoring), such as social distancing, timely lockdowns and extensive vaccination campaigns [43] might further act in synergy [44] and be more relevant than clinical variables such as disease extent and comorbidities in modulating the risk of COVID-19 and its complications in patients with SLE [[45], [46], [47]]. Consistently and in line with previous observations [21,48], patients with SLE tended to be more adherent than their family members/cohabitants to the adoption of behavioural measures. Furthermore, although family members/cohabitants constituted a fundamental source of infection, 68% of patients with COVID-19-positive family members/cohabitants did not develop COVID-19, possibly suggesting that correct awareness of potential risky behaviours positively affects the contagion risk. Data from this study also show that most patients with SLE are favourable to engage in public efforts including vaccination campaigns [49,50] and suggests that cases of vaccine hesitancy might possibly be overcome with adequate counselling.
For a comprehensive interpretation of our results, multiple limitations should be considered. The use of a patient-centred anonymous web-based questionnaire, excluded severely ill or deceased subjects, prevented a full validation of patient-reported information with patients' clinical records and introduced patient's subjectivity as a potential bias to the collection of data from patients' relatives. Furthermore, data from the general population were generated with different methods, warranting caution in comparing them to those of our dataset. On the other hand, our research strategy enjoys the strengths of web-based surveys [3,21,51,52], such as minimising potential biases in the interpretation of patient-reported data and promoting patient engagement in research. In addition, our study is not affected by reporting biases due to the potential selection of most severe cases or by delay in case identification secondary to cancellation of routine outpatient visits and thus possibly provides complementary information to works based on registries or hospitalisation records [21].
In conclusion, our results suggest that the impact of COVID-19 pandemic on patients with SLE is non-negligible and is significantly modulated by the course of the contagion in the general population. Consistently, similar to the general population, older age and contact with COVID-19 cases within the family setting were major risk factors for infection in our study, although a significant proportion of patients did not develop COVID-19 despite infected subjects in their family. Treatment escalation, especially with corticosteroids, constitutes an additional factor associating with COVID-19 in patients with SLE. Maintenance of strict public health policies to prevent SARS-CoV-2 spread in the population, along with evidence-based, patient-tailored approaches for safe and effective vaccination, might be particularly important to overcome the potential threats posed by the pandemic to the health status of patients with SLE.
The following are the supplementary data related to this article.
Comparison of general clinical features among responders to the survey, a sample of patients from the same reference cohort and responders to a previous survey
Supplementary material
Authors' contributions
All authors contributed to the general design of the study and of the questionnaire. GAR analysed the data and drafted the paper. All authors contributed to the critical analysis of the results and to revise the manuscript draft. All authors approved the final version of the article and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work will appropriately be investigated and resolved.
Funding statement
The authors received no specific funding for this research.
Conflict of interest statement
The authors declare no conflict of interest in connection with this paper.
Acknowledgements
The authors gratefully acknowledge the support of Dr. Andrea Lombardi (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico) in building up the questionnaire used in this study.
References
- 1.Marois G., Muttarak R., Scherbov S. Assessing the potential impact of COVID-19 on life expectancy. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0238678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rovere Querini P., De Lorenzo R., Conte C., et al. Post-COVID-19 follow-up clinic: depicting chronicity of a new disease. Acta Biomed. 2020;91(9–S):22–28. doi: 10.23750/abm.v91i9-S.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sloan M., Gordon C., Harwood R., et al. 2020. The Impact of the COVID-19 Pandemic on the Medical Care and Healthcare-Behaviour of Patients with Lupus and Other Systemic Autoimmune Diseases: A Mixed Methods Longitudinal Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilinski A., Emanuel E.J. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 2020;324(20):2100–2102. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastard P., Rosen L.B., Zhang Q., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515) doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salloum R., Niewold T.B. Interferon regulatory factors in human lupus pathogenesis. Transla. Res. 2011;157(6):326–331. doi: 10.1016/j.trsl.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Romo G.S., Caielli S., Vega B., et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3(73) doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morawski P.A., Bolland S. Expanding the B cell-centric view of systemic lupus Erythematosus. Trends Immunol. 2017;38(5):373–382. doi: 10.1016/j.it.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S., Nakabo S., Chu J., Hasni S., Kaplan M.J. 2021. Correspondence on ‘Clinical Course of Coronavirus Disease 2019 (COVID-19) in a Series of 17 Patients with Systemic Lupus Erythematosus under Long-Term Treatment with Hydroxychloroquine’. annrheumdis-2020-219648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathi M., Singh P., Bi H.P., et al. Impact of the COVID-19 pandemic on patients with systemic lupus erythematosus: observations from an Indian inception cohort. Lupus. 2020;961203320962855 doi: 10.1177/0961203320962855. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Ruiz R., Paredes J.L., Niewold T.B. COVID-19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease. Transl. Res. 2021;232:13–36. doi: 10.1016/j.trsl.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez G.A., Moroni L., Della-Torre E., et al. Systemic lupus erythematosus and COVID-19: what we know so far. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218601. In press. [DOI] [PubMed] [Google Scholar]
- 13.Sawalha A.H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin. Immunol. 2020;215:108410. doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danza A., Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 15.Rahman A., Isenberg D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008;358(9):929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama S., Hamdeh S., Micic D., Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-218946. In press. [DOI] [PubMed] [Google Scholar]
- 17.Cho J., Kandane-Rathnayake R., Louthrenoo W., et al. COVID-19 infection in patients with systemic lupus erythematosus: data from the Asia Pacific lupus collaboration. Int. J. Rheum. Dis. 2020;23(9):1255–1257. doi: 10.1111/1756-185X.13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pablos J.L., Abasolo L., Alvaro-Gracia J.M., et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann. Rheum. Dis. 2020;79(9):1170–1173. doi: 10.1136/annrheumdis-2020-217763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.So H., Mak J.W.Y., Tam L.S. No systemic lupus Erythematosus with COVID-19 in Hong Kong: the effect of masking? J. Rheumatol. 2020;47(10):1591. doi: 10.3899/jrheum.200605. [DOI] [PubMed] [Google Scholar]
- 20.Favalli E.G., Monti S., Ingegnoli F., Balduzzi S., Caporali R., Montecucco C. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheum. 2020;72(10):1600–1606. doi: 10.1002/art.41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez G.A., Gerosa M., Beretta L., et al. COVID-19 in systemic lupus erythematosus: data from a survey on 417 patients. Semin. Arthritis Rheum. 2020;50(5):1150–1157. doi: 10.1016/j.semarthrit.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strangfeld A., Schafer M., Gianfrancesco M.A., et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2021;80(7):930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianfrancesco M.A., Hyrich K.L., Gossec L., et al. Rheumatic disease and COVID-19: initial data from the COVID-19 global rheumatology alliance provider registries. Lancet Rheumatol. 2020;2(5) doi: 10.1016/S2665-9913(20)30095-3. e250-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scire C.A., Carrara G., Zanetti A., et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19) Clin. Exp. Rheumatol. 2020;38(4):748–753. [PubMed] [Google Scholar]
- 25.Fernandez-Ruiz R., Masson M., Kim M.Y., et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheum. 2020;72(12):1971–1980. doi: 10.1002/art.41450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favalli E.G., Gerosa M., Murgo A., Caporali R. Are patients with systemic lupus erythematosus at increased risk for COVID-19? Ann. Rheum. Dis. 2021;80(2) doi: 10.1136/annrheumdis-2020-217787. [DOI] [PubMed] [Google Scholar]
- 27.Fredi M., Cavazzana I., Moschetti L., et al. COVID-19 in patients with rheumatic diseases in northern Italy: a single-Centre observational and case–control study. Lancet Rheumatol. 2020;2(9) doi: 10.1016/S2665-9913(20)30169-7. e549-e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zen M., Fuzzi E., Astorri D., et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in Northeast Italy: a cross-sectional study on 916 patients. J. Autoimmun. 2020;112:102502. doi: 10.1016/j.jaut.2020.102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendebien Z., von Frenckell C., Ribbens C., et al. 2020. Systematic analysis of COVID-19 infection and symptoms in a systemic lupus erythematosus population: correlation with disease characteristics, hydroxychloroquine use and immunosuppressive treatments. annrheumdis-2020-218244. [DOI] [PubMed] [Google Scholar]
- 30.Goyal M., Patil P., Pathak H., et al. Impact of COVID-19 pandemic on patients with SLE: results of a large multicentric survey from India. Ann. Rheum. Dis. 2021;80(5):e71. doi: 10.1136/annrheumdis-2020-218013. [DOI] [PubMed] [Google Scholar]
- 31.Task-force-COVID-19-del-Dipartimento-Malattie-Infettive-e-Servizio-di-Informatica-dell’Istituto-Superiore-di-Sanità-(Task-force-COVID-19-of-the-Department-of-Infectious-Diseases-and-the-Department-of-Informatics-of-the-Italian-National-Institute-of-Health), Riccardo F., Andrianou X., et al. 2021. [Epidemia COVID-19 Aggiornamento Nazionale 20 Gennaio 2021] COVID-19 Epidemic - Nationwide update 20th January 2021. In. [Google Scholar]
- 32.Signorelli C., Scognamiglio T., Odone A. COVID-19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. 2020;91(3–S):175–179. doi: 10.23750/abm.v91i3-S.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European-Commission . 2014. ARTICLE 29 DATA PROTECTION WORKING PARTY (0829/14/EN WP216). In: European-Commission, ed. 0829/14/EN WP216. [Google Scholar]
- 34.Bozzalla Cassione E., Zanframundo G., Biglia A., Codullo V., Montecucco C., Cavagna L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann. Rheum. Dis. 2020;79(10):1382–1383. doi: 10.1136/annrheumdis-2020-217717. [DOI] [PubMed] [Google Scholar]
- 35.Protezione-Civile-Italiana-(Italian-National-Emergency-Agency) [COVID-19 Italia] COVID-19 Italy. 2021. https://github.com/pcm-dpc/COVID-19;
- 36.Naing L., Winn T., Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch. Orofac. Sci. 2006;1:9–14. [Google Scholar]
- 37.ISTAT-Istituto-Nazionale-di-Statistica-(Italian-National-Institute-of-Statistics) Population by Region. 2020. http://dati.istat.it
- 38.Ministero-Dell'Interno-(Italian-Ministry-of-Internal-Affairs) Coronavirus, i dati dei servizi di controllo. 2021. https://www.interno.gov.it/it/coronavirus-i-dati-dei-servizi-controllo;
- 39.Serling-Boyd N., D’Silva K.M., Hsu T.Y., et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann. Rheum. Dis. 2020;80(5):660–666. doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramirez G.A., Canti V., Moiola L., et al. Performance of SLE responder index and lupus low disease activity state in real life: a prospective cohort study. Int. J. Rheum. Dis. 2019;22(9):1752–1761. doi: 10.1111/1756-185X.13663. [DOI] [PubMed] [Google Scholar]
- 41.Petri M., Genovese M. Incidence of and risk factors for hospitalizations in systemic lupus erythematosus: a prospective study of the Hopkins lupus cohort. J. Rheumatol. 1992;19(10):1559–1565. [PubMed] [Google Scholar]
- 42.Pons-Estel G.J., Catoggio L.J., Cardiel M.H., et al. Lupus in Latin-American patients: lessons from the GLADEL cohort. Lupus. 2015;24(6):536–545. doi: 10.1177/0961203314567753. [DOI] [PubMed] [Google Scholar]
- 43.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vinceti M., Filippini T., Rothman K.J., et al. Lockdown timing and efficacy in controlling COVID-19 using mobile phone tracking. EClinicalMedicine. 2020;25:100457. doi: 10.1016/j.eclinm.2020.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang C.C., Chang Y.S., Chen W.S., Chen Y.H., Chen J.H. Effects of annual influenza vaccination on morbidity and mortality in patients with systemic lupus erythematosus: a Nationwide cohort study. Sci. Rep. 2016;6:37817. doi: 10.1038/srep37817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2021-220272. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramirez G.A., Della-Torre E., Moroni L., Yacoub M.R., Dagna L. Correspondence on “Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort”. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2021-220539. In press. [DOI] [PubMed] [Google Scholar]
- 48.Hooijberg F., Boekel L., Vogelzang E.H., et al. Patients with rheumatic diseases adhere to COVID-19 isolation measures more strictly than the general population. Lancet Rheumatol. 2020;2(10) doi: 10.1016/S2665-9913(20)30286-1. e583-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lupus_Research_Alliance . 2021. Two Thirds of People with Lupus Would Take COVID-19 Vaccine, Shows LRA Survey. [Google Scholar]
- 50.Campochiaro C., Trignani G., Tomelleri A., Cascinu S., Dagna L., Group C-VS Potential acceptance of COVID-19 vaccine in rheumatological patients: a monocentric comparative survey. Ann. Rheum. Dis. 2021 doi: 10.1136/annrheumdis-2020-219811. In press. [DOI] [PubMed] [Google Scholar]
- 51.Pierantoni L., Lenzi J., Lanari M., et al. Nationwide COVID-19 survey of Italian parents reveals useful information on attitudes to school attendance, medical support, vaccines and drug trials. Acta Paediatr. 2021;110(3):942–943. doi: 10.1111/apa.15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seyahi E., Poyraz B.C., Sut N., Akdogan S., Hamuryudan V. The psychological state and changes in the routine of the patients with rheumatic diseases during the coronavirus disease (COVID-19) outbreak in Turkey: a web-based cross-sectional survey. Rheumatol. Int. 2020;40(8):1229–1238. doi: 10.1007/s00296-020-04626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of general clinical features among responders to the survey, a sample of patients from the same reference cohort and responders to a previous survey
Supplementary material