Abstract
Background
This pooled analysis of MONALEESA trials evaluated the safety of ribociclib plus endocrine therapy (RIB + ET) with a focus on dose reductions in first-line patients.
Methods
In the dose reduction analysis, data were pooled from MONALEESA-2 (all patients), MONALEESA-3 (patients receiving treatment as first-line ET) and MONALEESA-7 (patients receiving combination therapy with an NSAI as initial ET). Efficacy was analysed by ribociclib relative dose intensity (DI). Safety was analysed in all patients in the trials (except those receiving tamoxifen in MONALEESA-7) and those with/without ≥1 ribociclib dose reduction.
Results
Of 818 women who received first-line RIB + ET, 41.8% required ≥1 dose reduction due to AEs (most commonly, neutropenia). Median RIB relative DI in patients without and with dose reductions was 99.3% and 65.6% in MONALEESA-2, 98.4% and 67.8% in MONALEESA-3 and 98·0% and 66·3% in MONALEESA-7. Median PFS was 24.8, 24.9 and 29.6 months for patients who received ≤71% (30th percentile), 72–96% (60th percentile) and 97–100% (90th percentile) RIB relative DI, respectively. No new safety signals emerged in the pooled safety analysis.
Conclusions
This analysis provides reassuring data showing that the clinical benefit of RIB is preserved when dose modifications are undertaken to manage AEs.
Trial registration
MONALEESA-2 (NCT01958021) first posted October 8, 2013; MONALEESA-3 (NCT02422615) first posted April 21, 2015; MONALEESA-7 (NCT02278120) first posted October 29, 2014.
Subject terms: Breast cancer, Cancer
Background
The majority of BC cases (~70%) are positive for oestrogen receptor and/or progesterone receptor (i.e., hormone receptor-positive (HR + )) and negative for human epidermal growth receptor 2 (HER2–).1,2 Although blockade of ER signalling is the basis of treatment for HR+/HER2– BC, many patients develop resistance to endocrine therapy (ET), which is challenging to manage and associated with poor prognosis.3–5
A variety of molecular mechanisms can contribute to ET resistance, including cell-cycle checkpoint alterations. For example, D–cyclin-dependent kinase (CDK) 4/6 inhibitor of CDK4 (INK4)–retinoblastoma (Rb) pathway, which regulates cellular proliferation, is frequently dysregulated in HR + BC and other types of cancer.6–8 Persistent cyclin D1 expression and Rb phosphorylation support the use of CDK4/6 inhibitors in HR + BC.9 Inhibition of the CDK4/6-INK4-Rb pathway alongside ET can be an effective strategy for patients with HR+/HER2– BC compared with ET alone. Three selective CDK4/6 inhibitors are currently approved by the US Food and Drug Administration: ribociclib, palbociclib and abemaciclib.10 The differences among these CDK4/6 inhibitors include, but are not limited to, administration schedules (ribociclib and palbociclib: once daily 3 weeks on, 1 week off; abemaciclib: twice daily) and varying selectivity against CDK4 versus CDK6 in preclinical studies.11–18 In addition, in a preclinical pharmacokinetic analysis, ribociclib was reported to have a higher amount of free drug available for binding compared with palbociclib and abemaciclib.18
Approval of ribociclib for women with HR+/HER2– advanced BC (ABC) was based on data from the Phase 3 MONALEESA trials in which ribociclib plus ET significantly extended progression-free survival (PFS) compared with placebo plus ET in pre-, peri- and postmenopausal patients who were treatment-naive or had received one prior therapy in this setting.12,14,19,20 Specifically, in postmenopausal patients, median PFS with ribociclib plus letrozole versus placebo plus letrozole was 25.3 versus 16.0 months (HR 0.568, 95% CI 0.457–0.704, P < 0.001) in MONALEESA-2 and 20.5 versus 12·8 months (HR 0.593, 95% CI 0.480–0.732, P < 0·001) with ribociclib plus fulvestrant versus placebo plus fulvestrant in MONALESSA-3.12,20 Similarly, PFS in the subset of pre- and perimenopausal patients receiving ribociclib plus a non-steroidal aromatase inhibitor (NSAI) versus placebo plus NSAI in MONALEESA-7 was 27.5 versus 13.8 months (HR 0.57, 95% CI 0.44–0.74).19 Data on overall survival (OS) have been reported from two of the MONALEESA trials; in MONALEESA-7, OS was significantly longer with ribociclib plus ET versus placebo plus ET (HR 0.71; 95% CI 0.54–0.95, P = 0.00973), and in MONALEESA-3, OS was significantly longer with ribociclib plus fulvestrant versus placebo plus fulvestrant (HR 0.72; 95% CI 0.57–0.92; P = 0.00455).15,16 Ribociclib has also been found to have a predictable and manageable safety profile when given in combination with ET. Neutropenia, leukopenia and elevated liver function test results were the most common grade 3/4 adverse events (AEs) observed in MONALEESA-2, -3 and -7.12,14,19,20 These Phase 3 studies demonstrated that ribociclib-associated AEs could be managed with dose reductions.12,14–16,19–21 However, data detailing the impact of ribociclib relative dose intensity (DI) on efficacy outcomes are needed to further inform treatment decisions.
The aims of this analysis were to further evaluate the safety of ribociclib plus ET and to gain insight into the efficacy of ribociclib in patients as a function of relative DI using pooled data from the MONALEESA-2, -3 and -7 trials.
Methods
The designs of the Phase 3 MONALEESA-2, -3 and -7 trials have been previously reported in detail.12,14,19,20 In brief, these randomised, double-blind, placebo-controlled studies investigated the use of oral ribociclib 600 mg/day (3 weeks on, 1 week off, in 28-day cycles) in combination with ET in patients with HR+/HER2– ABC. MONALEESA-2 (NCT01958021) was conducted in 223 centres in 29 countries and investigated ribociclib plus oral letrozole (2.5 mg/day continuous) in 668 postmenopausal women with previously untreated disease randomised 1:1.14 MONALEESA-3 (NCT02422615) was conducted in 174 centres in 30 countries and investigated ribociclib plus fulvestrant (500 mg intramuscularly cycle 1 day 1, cycle 1 day 15 and day 1 of every 28-day cycle thereafter) in 726 postmenopausal women randomised 2:1.12 MONALEESA-7 (NCT02278120) was conducted in 188 centres in 30 countries and investigated ribociclib plus an oral NSAI (anastrozole 1 mg/day or letrozole 2.5 mg/day) or oral tamoxifen (20 mg/day), both with goserelin (subcutaneously 3.6 mg every 28 days), in 672 peri- or premenopausal women randomised 1:1.19
Key inclusion criteria for the MONALEESA trials included histologically confirmed HR+/HER2– ABC, Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, adequate organ and bone marrow function, ≥1 measurable lesion (Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1) or ≥1 predominantly lytic bone lesion, no prior ET (MONALEESA-2 and −7) or ≤1 line of prior ET (MONALEESA-3) for ABC and no prior chemotherapy (MONALEESA-2 and −3) or ≤1 line of chemotherapy (MONALEESA-7) for ABC.12,14,19 Key exclusion criteria for the MONALEESA trials were prior treatment with a CDK4/6 inhibitor, inflammatory BC and clinically significant cardiac disease or history of cardiac dysfunction (including Fridericia’s corrected QT interval (QTcF) >450 ms).12,14,19 Investigator-assessed PFS was the primary endpoint in MONALEESA-2, -3 and -7 (tumour response was assessed locally per RECIST v1.1).12,14,19 Secondary endpoints included OS, overall response rate (ORR (partial or complete response)), clinical benefit rate (CBR (overall response plus stable disease lasting ≥24 weeks)) and safety (AEs were graded according to Common Terminology Criteria for Adverse Events (CTCAEs) version 4.03).12,14,19 All studies were conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. The institutional review board at each participating centre reviewed the protocol and any amendments, and all patients provided written informed consent.
The safety analysis reports outcomes from the following patient groups: all patients in MONALEESA-2 (data cut-off: January 4, 2017; median follow-up 26.5 months), all patients in MONALEESA-3 (data cut-off: November 3, 2017; includes patients who may have received ET in the adjuvant setting; median follow-up 20.5 months) and patients who received an NSAI with or without ribociclib as initial endocrine-based treatment in MONALEESA-7 (data cut-off: August 20, 2017; median follow-up 19·2 months). Patients in MONALEESA-7 who were treated with tamoxifen were not included in any of the analyses reported here because tamoxifen is not indicated for use in combination with ribociclib. The dose reduction analysis included the same population as above, with the exception of patients receiving ≥1 prior line of ET in MONALEESA-3. Investigator-assessed PFS (defined as the time from randomisation to either first documented disease progression per RECIST v1.122 or death from any cause), ORR and CBR data (tumour response was assessed locally per RECIST v1.1) were pooled across the three studies. OS was not yet mature for MONALEESA-2; therefore, an analysis of OS was not performed at this time. Because this was an exploratory analysis, no formal statistical analyses comparing patient groups were performed. Data were analysed according to ribociclib relative DI and grouped by percentile (30th, 60th and 90th corresponding to ≤71%, 72–96% and 97–100% relative DI, respectively). DI (mg/day) for patients with non-zero duration of exposure to ribociclib or placebo was defined as cumulative dose (mg)/adjusted duration of exposure to ribociclib or placebo (days), where adjusted duration of exposure (days) to ribociclib or placebo was the number of ribociclib or placebo dosing days a patient would be expected to have received per protocol, given their duration of exposure. Ribociclib or placebo relative DI was defined as DI (dosing unit/unit of time)/planned DI (dosing unit/unit of time). Relative DI was defined as DI (dosing unit/unit of time)/planned DI (dosing unit/unit of time). For patients who did not receive any drug, the DI was set to zero. Additional details on DI calculations for ET partners are included in the supplemental methods. Analyses were performed in the full analysis set comprising all randomised patients.
Safety (safety set, comprising patients who received ≥1 dose of any study treatment) and baseline characteristics (full analysis set) are summarised for all patients in the MONALEESA-2, -3 and -7 trials (pooled data) according to treatment arm (ribociclib plus ET or placebo plus ET, regardless of line of treatment). AEs and baseline characteristics are also summarised in these patients with and without ≥1 ribociclib dose reduction. No formal statistical analyses were performed.
Role of the funding source
MONALEESA-2, -3 and -7 were supported by Novartis Pharmaceuticals Corporation. The sponsor and investigators were involved in the study design. The sponsor was involved in data analysis. Investigators contributed to data collection and interpretation. All authors had full access to the data and were involved in the interpretation of the data, writing and review of all drafts of the paper and in the decision to submit the paper for publication. Medical writing support was provided by John McGuire at MediTech Media, funded by Novartis Pharmaceuticals Corporation.
Results
Baseline demographic and disease characteristics
This pooled analysis of the safety set included 1066 and 823 women with HR+/HER2– ABC who received ribociclib plus ET or placebo plus ET, respectively, across the MONALEESA-2, -3 and -7 studies. Baseline demographic and disease characteristics were generally well balanced between the ribociclib plus ET and placebo plus ET groups; median age was 58 and 57 years, respectively, and approximately half the patients in both the ribociclib and placebo arms had lung or liver metastases (Table 1). The median duration of exposure to study treatment was 14.6 months (range 0–34.4 months) for ribociclib and 16.5 months (range 0–34.4 months) for the ET partner in the ribociclib plus ET group and 12.9 months (range 0.5–32.4 months) in the placebo plus ET group.
Table 1.
Demographic and baseline disease characteristics.
| MONALEESA-2 | MONALEESA-3 | MONALEESA-7 | Pooled data | |||||
|---|---|---|---|---|---|---|---|---|
| Ribociclib + letrozolea, n = 334 | Placebo + letrozole, n = 334 | Ribociclib + fulvestranta, n = 484 | Placebo + fulvestrant, n = 242 | Ribociclib + endocrine therapya, n = 248 | Placebo + endocrine therapy, n = 247 | Ribociclib + endocrine therapya, n = 1066 | Placebo + endocrine therapy, n = 823 | |
| Age, median (range), years | 61 (23–91) | 62 (29–88) | 63 (31–89) | 63 (34–86) | 42 (25–58) | 44 (29–58) | 58 (23–91) | 57 (29–88) |
| Race, n (%)b | ||||||||
| Caucasian | 269 (81) | 280 (84) | 406 (84) | 213 (88) | 139 (56) | 136 (55) | 814 (76) | 629 (76) |
| Asian | 28 (8) | 23 (7) | 45 (9) | 18 (7) | 82 (33) | 84 (34) | 155 (15) | 125 (15) |
| Otherc | 37 (11) | 31 (9) | 33 (7) | 11 (5) | 27 (11) | 27 (11) | 97 (9) | 69 (8) |
| Weight, mean (SD), kg | 72 (16) | 72 (16) | 71 (15) | 72 (17) | 66 (16) | 68 (18) | 70 (16) | 71 (17) |
| ECOG performance status, n (%)d | ||||||||
| 0 | 204 (61) | 202 (61) | 310 (64) | 158 (65) | 183 (74) | 191 (77) | 697 (65) | 551 (67) |
| 1 | 130 (39) | 132 (40) | 173 (36) | 83 (34) | 63 (25) | 55 (22) | 366 (34) | 270 (33) |
| Metastatic disease status, n (%) | ||||||||
| De novoe | 114 (34) | 113 (34) | 95 (20) | 42 (17) | 72 (29) | 70 (28) | 281 (26) | 225 (27) |
| Non-de novo (relapsed) | 220 (66) | 221 (66) | 280 (58) | 158 (65) | 176 (71) | 177 (72) | 676 (63) | 556 (68) |
| Disease-free interval, n (%)f | ||||||||
| ≤12 months | 4 (1) | 10 (3) | 9 (2) | 5 (2) | 17 (7) | 9 (4) | 30 (3) | 24 (3) |
| >12 and ≤24 months | 14 (4) | 15 (4) | 25 (5) | 9 (4) | 31 (13) | 29 (12) | 70 (7) | 53 (6) |
| >24 months | 202 (60) | 195 (58) | 246 (51) | 144 (60) | 128 (52) | 139 (56) | 576 (54) | 478 (58) |
| Selected metastatic sites, n (%) | ||||||||
| Bone | 246 (74) | 244 (73) | 276 (57) | 145 (60) | 178 (72) | 178 (72) | 700 (66) | 567 (69) |
| Bone only | 69 (21) | 78 (23) | 86 (18) | 41 (17) | 57 (23) | 54 (22) | 212 (20) | 173 (21) |
| Lung or liver | 182 (54) | 191 (57) | 177 (37) | 100 (41) | 136 (55) | 130 (53) | 495 (46) | 421 (51) |
ECOG Eastern Cooperative Oncology Group.
aPatients from MONALEESA-2 (ribociclib plus letrozole group), MONALEESA-3 (ribociclib plus fulvestrant first-line group), and MONALEESA-7 (ribociclib plus non-steroidal aromatase inhibitor group).
bUnknown race was reported for n = 42 (ribociclib plus endocrine therapy arm) and n = 35 (placebo plus endocrine therapy arm).
cOther race includes Black, Native American, Pacific Islander and other/unknown.
dECOG performance status data missing for n = 3 (ribociclib plus endocrine therapy arm) and n = 2 (placebo plus endocrine therapy arm).
eDe novo disease in the MONALEESA-2 study referred to there being no date of first recurrence/progression or the first recurrence/progression occurring within 90 days of initial diagnosis with no prior antineoplastic therapy received, including medication and radiation. De novo disease in the MONALEESA-3 and -7 studies followed the same definition as MONALEESA-2, except the criterion of no prior antineoplastic therapy was restricted to medication only.
fDisease-free interval in patients with non-de novo metastatic disease was defined as the time from the initial diagnosis to first recurrence/progression; n = 1 (placebo plus endocrine therapy arm) had an unknown disease-free interval.
Safety of ribociclib plus ET
All-causality AEs occurring in patients receiving ribociclib plus ET and placebo plus ET across the MONALEESA-2, -3 and -7 trials are summarised in Table 2. Most non-haematologic AEs were mild to moderate in intensity (grade 1/2). Grade 3/4 all-causality AEs reported in ≥10% of patients in either arm (ribociclib plus ET vs placebo plus ET) were neutropenia (60% vs 2%) and leukopenia (18% vs 1%). All-grade AEs regardless of causality observed at ≥10% greater incidence in the ribociclib plus ET arm (vs the placebo plus ET arm) were neutropenia (74% vs 5%), nausea (45% vs 27%), leukopenia (31% vs 4%), vomiting (27% vs 16%), alopecia (24% vs 12%), anaemia (19% vs 6%), rash (18% vs 8%) and pruritus (16% vs 6%).
Table 2.
Common all-causality AES ( ≥ 15% in either arm) in MONALEESA-2, -3 and -7 (pooled data, safety set).
| n (%) | Ribociclib + endocrine therapya, n = 1065 | Placebo + endocrine therapy, n = 818 | ||||
|---|---|---|---|---|---|---|
| Grade (CTCAE v4.03) | All | 3 | 4 | All | 3 | 4 |
| Any AE | 1052 (99) | 699 (66) | 158 (15) | 782 (96) | 245 (30) | 26 (3) |
| Haematologic AEs | ||||||
| Neutropeniab | 788 (74) | 550 (52) | 91 (9) | 43 (5) | 12 (1) | 1 (<1) |
| Leukopeniac | 330 (31) | 180 (17) | 13 (1) | 36 (4) | 7 (1) | 0 |
| Anaemiad | 200 (19) | 28 (3) | 2 (<1) | 51 (6) | 12 (1) | 0 |
| Non-haematologic AEs | ||||||
| Nausea | 475 (45) | 15 (1) | 0 | 219 (27) | 4 (<1) | 0 |
| Fatigue | 348 (33) | 19 (2) | 1 (<1) | 249 (30) | 4 (<1) | 0 |
| Diarrhoea | 317 (30) | 16 (2) | 0 | 176 (22) | 5 (1) | 0 |
| Arthralgia | 310 (29) | 7 (1) | 1 (<1) | 243 (30) | 8 (1) | 0 |
| Vomiting | 284 (27) | 21 (2) | 0 | 128 (16) | 3 (<1) | 0 |
| Alopecia | 256 (24) | 0 | 0 | 97 (12) | 0 | 0 |
| Constipation | 253 (24) | 8 (1) | 0 | 129 (16) | 0 | 0 |
| Headache | 252 (24) | 5 (<1) | 0 | 177 (22) | 4 (<1) | 0 |
| Hot flush | 223 (21) | 2 (<1) | 0 | 200 (24) | 0 | 0 |
| Cough | 218 (21) | 0 | 0 | 132 (16) | 0 | 0 |
| Back pain | 211 (20) | 20 (2) | 0 | 153 (19) | 7 (1) | 0 |
| Rash | 187 (18) | 6 (1) | 0 | 63 (8) | 0 | 0 |
| Pruritus | 169 (16) | 3 (<1) | 0 | 47 (6) | 0 | 0 |
| Decreased appetite | 163 (15) | 6 (1) | 0 | 101 (12) | 1 (<1) | 0 |
| Increased ALT | 161 (15) | 71 (7) | 15 (1) | 48 (6) | 8 (1) | 0 |
AE adverse event, ALT alanine transferase, CTCAE Common Terminology Criteria for Adverse Events.
aPatients from MONALEESA-2 (ribociclib plus letrozole group), MONALEESA-3 (ribociclib plus fulvestrant group) and MONALEESA-7 (ribociclib plus non-steroidal aromatase inhibitor group).
b“Neutropenia” includes “neutropenia,” “decreased neutrophil count,” “febrile neutropenia,” “granulocytopenia” and “neutropenia sepsis.”
c“Leukopenia” includes “leukopenia,” “decreased white blood cell count,” “lymphopenia” and “decreased lymphocyte count.”
d“Anaemia” includes “anaemia,” “decreased haemoglobin,” “macrocytic anaemia” and “decreased red blood cell count.”
Serious all-grade adverse events (preferred term, irrespective of causality) reported in >10 patients in either arm (ribociclib plus ET vs placebo plus ET) were pneumonia (1% vs 1%), dyspnoea (1% vs 1%), abdominal pain (1% vs <1%), vomiting (1% vs <1%), anaemia (1% vs <1%), febrile neutropenia (1% vs <1%), nausea (1% vs <1%) and pleural effusion (1% vs 1%).
All-grade AEs of special interest (grouped AE terms) reported in the ribociclib plus ET vs placebo plus ET arms included infections and infestations (52.4% vs 42.5%), electrocardiogram QT interval prolongation (all grade: 6.5% vs 1.6%; grade 3/4: 1.2% vs 0.2%), pulmonary embolism (all grade: 3.5% vs 2.3%; grade 3/4: 1.0% vs 0.9%) and interstitial lung disease (standardised MedDRA query broad term; all grade: 1.5% vs 0.6%; grade 3/4: 0.3% vs 0%).
On-treatment deaths (occurring ≤30 days after the last day of study treatment) were reported for 2% of patients in each treatment arm.
Ribociclib dose reductions
Ribociclib dose reductions were investigated in a total of 818 patients who received ribociclib in combination with ET as first-line therapy only. Across MONALEESA-2, -3 and -7, 45.8% of patients (range 37.0–57.5%) required ≥1 ribociclib dose reduction, and 41.8% of dose reductions were due to AEs (most commonly, neutropenia, Table 3). Across each study, baseline demographic characteristics, including age and ECOG performance status, were generally similar in patients with and without ribociclib dose reductions. One notable imbalance was among Asian patients; the proportions of Asian patients with dose reductions versus no dose reduction were higher in MONALEESA-3 and -7 (MONALEESA-3: dose reduction, 16.3%; no dose reduction, 7.5%; MONALEESA-7: dose reduction, 40.7%; no dose reduction, 28.4%) but were similar for MONALEESA-2 (dose reduction, 9.5%; no dose reduction, 7.3%; Supplementary Table 1). Of the patients who received ribociclib dose reductions, most needed only a single reduction (257 of 375 (68.5%)). The median time to the first ribociclib dose reduction from the start of study treatment was between 2 and 3 months and was broadly consistent across the three studies (MONALEESA-2: 3.0 months; MONALEESA-3: 2.8 months; MONALEESA-7: 2.2 months). All-grade neutropenia (grouped AE of special interest term) was the most common AE leading to dose reduction (MONALEESA-2: 33.2%; MONALEESA-3: 19.7%; MONALEESA-7: 23.4%). Dose reductions due to all-grade QT interval prolongation were infrequent (MONALEESA-2: 0%; MONALEESA-3: 2.5%; MONALEESA-7: 2%). Relative median DI (range) in patients without versus with ribociclib dose reductions was MONALEESA-2: 99.3% (50.0–111.9%) versus 65.6% (31.4–99.8%); MONALEESA-3: 98.4% (65.9–131.8%) versus 67.8% (34.7–99.7%) and MONALEESA-7: 98.0% (57.1–104.8%) versus 66.3% (27.9–98.6%).
Table 3.
Ribociclib dose reductions in patients receiving first-line endocrine therapy (safety set).
| MONALEESA-2 | MONALEESA-3 | MONALEESA-7 | ||||
|---|---|---|---|---|---|---|
| Ribociclib 600 mg + letrozole, n = 334 | Ribociclib 600 mg + fulvestrant, n = 238 | Ribociclib 600 mg + NSAI, n = 246 | ||||
| n | % | n | % | n | % | |
| Dose reductions | ||||||
| 0 | 142a | 42.5 | 146 | 61.3 | 155 | 63.0 |
| 1 | 115 | 34.4 | 76 | 31.9 | 66 | 26.8 |
| 2 | 70 | 21.0 | 15 | 6.3 | 23 | 9.3 |
| ≥3 | 7 | 2.1 | 1 | 0.4 | 2 | 0.8 |
| Reason for dose reductionb | ||||||
| AE | 182 | 54.5 | 78 | 32.8 | 82 | 33.3 |
| Dosing error | 8 | 2.4 | 7 | 2.9 | 6 | 2.4 |
| Lack of efficacy | 1 | 0.3 | 0 | 0 | 0 | 0 |
| Physician decision | 7 | 2.1 | 5 | 2.1 | 4 | 1.6 |
| Patient/guardian decision | 8 | 2.4 | 1 | 0.4 | 1 | 0.4 |
| Technical issue | 0 | 0 | 1 | 0.4 | 0 | 0 |
| Most common all-grade AEs for dose reduction | ||||||
| Neutropenia | 111 | 33.2 | 47 | 19.7 | 58 | 23.4 |
AE adverse event, NSAI non-steroidal aromatase inhibitor.
an = 142 patients with no dose reduction. For the efficacy evaluations data from 165 patients (61.2%) were used. This included 23 patients who had dose interruptions where the dose was reduced from 600 to 0 mg.
bIn MONALEESA-2, more than one reason for dose reduction may have been reported.
Discontinuations due to AEs were reported in 155 patients (14.6%) who received ribociclib plus ET and 36 (4.4%) who received placebo plus ET. The most common AEs that led to discontinuation were increased levels of alanine aminotransferase (4.0% vs 0.4%) or aspartate aminotransferase (2.4% vs 0.6%), both of which required discontinuation per protocol because the recovery time was >28 days, and vomiting (1.2% vs 0). Less than 1% of patients in each arm discontinued treatment due to all-grade QT interval prolongation, and one patient in each arm discontinued due to grade 3 QT interval prolongation.
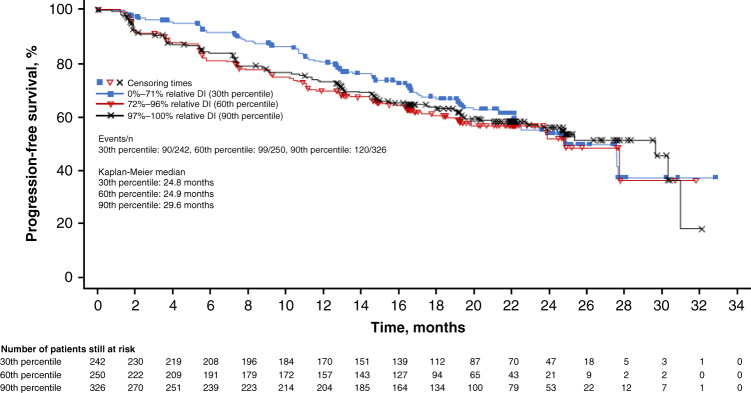
Pooled data for patients receiving first-line ribociclib plus ET in MONALEESA-2, -3 and -7 indicated that median PFS was consistent across patients grouped by ribociclib relative DI: ≤71% relative DI (30th percentile): 24.8 months, 72–96% relative DI (60th percentile): 24.9 months and 97–100% relative DI (90th percentile): 29.6 months (Fig. 1). The pooled data also revealed that ORR and CBR in patients receiving ribociclib plus ET were not compromised in patients who received dose reductions: for relative DI ≤ 71%, ORR was 47.5%, and CBR was 87.6%; for relative DI 72–96%, ORR was 38.0%, and CBR was 76.8%; for relative DI 97–100%, ORR was 37.7%, and CBR was 73.6% (Table 4).
Fig. 1. Progression-free survival according to ribociclib relative DI in patients receiving ribociclib with first-line endocrine treatment (FAS; investigator assessment)a.
DI dose intensity, FAS full analysis set. aPooled data: patients from MONALEESA-2 (ribociclib plus letrozole group), MONALEESA-3 (ribociclib plus fulvestrant group) and MONALEESA-7 (ribociclib plus non-steroidal aromatase inhibitor group).
Table 4.
RECIST response according to ribociclib relative DI in patients receiving ribociclib with first-line endocrine treatment (FAS; investigator assessment).a,b
| Ribociclib relative DI | ||||||
|---|---|---|---|---|---|---|
| 0–71% relative DI (30th percentile) | 72–96% relative DI (60th percentile) | 97–100% relative DI (90th percentile) | ||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
| Best overall response | ||||||
| Complete response | 7 | 2.9 | 5 | 2.0 | 14 | 4.3 |
| Partial response | 108 | 44.6 | 90 | 36.0 | 109 | 33.4 |
| Stable disease | 68 | 28.1 | 84 | 33.6 | 89 | 27.3 |
| Neither complete response nor progressive disease | 49 | 20.2 | 44 | 17.6 | 64 | 19.6 |
| Progressive disease | 7 | 2.9 | 22 | 8.8 | 20 | 6.1 |
| Unknown | 3 | 1.2 | 5 | 2.0 | 30 | 9.2 |
| Overall responsec | 115 | 47.5 (41.2–53.8) | 95 | 38.0 (32.0–44.0) | 123 | 37.7 (32.5–43.0) |
| Clinical benefitd | 212 | 87.6 (83.5–91.8) | 192 | 76.8 (71.6–82.0) | 240 | 73.6 (68.8–78.4) |
CI confidence interval, DI dose intensity, FAS full analysis set, RECIST Response Evaluation Criteria in Solid Tumours.
aPooled data: patients from MONALEESA-2 (ribociclib plus letrozole group), MONALEESA-3 (ribociclib plus fulvestrant group), and MONALEESA-7 (ribociclib plus non-steroidal aromatase inhibitor group).
bRelative DI = DI (mg/day)/planned DI (mg/day) × 100.
cIncludes complete and partial responses.
dIncludes complete and partial responses, stable disease lasting ≥24 weeks, and neither complete response nor progressive disease lasting ≥24 weeks.
Neutropenia was the most common all-grade, all-causality AE reported in patients receiving first-line ribociclib plus ET who received at least one ribociclib dose reduction (MONALEESA-2: 73.4%; MONALEESA-3: 76.1%; MONALEESA-7: 65.9%) and was observed at a higher frequency than in patients with no ribociclib dose reductions (MONALEESA-2: 54.5%; MONALEESA-3: 45.9%; MONALEESA-7: 51.6%). Nausea, diarrhoea, fatigue and vomiting were also commonly reported by patients with and without ribociclib dose reductions (Supplementary Table 2).
Discussion
The Phase 3, randomised, placebo-controlled MONALEESA-2, -3 and -7 studies demonstrated that ribociclib plus ET prolongs the duration of PFS compared with ET alone in pre-, peri- and postmenopausal women with HR+/HER2– ABC. This pooled analysis of data from these studies provides further insight into the safety profile of ribociclib from more than 1000 patients with HR+/HER2– disease who received ribociclib plus ET as initial endocrine-based treatment for ABC across the MONALEESA programme.
Importantly, no unanticipated AEs associated with ribociclib or ETs (letrozole, fulvestrant and NSAI) were identified in this large, pooled data set. The majority of non-haematologic AEs were of mild-to-moderate intensity (CTCAE grade 1 or 2), with all-grade nausea (45% vs 27%), vomiting (27% vs 16%), rash (18% vs 8%), alopecia (24% vs 12%) and pruritus (16% vs 6%) occurring more frequently with ribociclib plus ET vs placebo plus ET. Haematologic AEs were more frequent in the ribociclib vs placebo arm, including neutropenia (all-grade: 74% vs 5%; grade 3/4: 60% vs 2%), leukopenia (all-grade: 31% vs 4%; grade 3/4: 18% vs 1%) and anaemia (all-grade: 19% vs 6%; grade 3/4: 3% vs 1%). These observations are consistent with the known safety profile of ribociclib and that of other CDK4/6 inhibitors.21,23,24 For example, in PALOMA-2, grade 3/4 neutropenia (66% vs 1%) and grade 3/4 leukopenia (25% vs 0%) were more frequent in patients receiving first-line palbociclib plus letrozole compared with placebo plus letrozole.23 In MONARCH 3, grade 3/4 neutropenia (21% vs 1%) and grade 3/4 leukopenia (8% vs <1%) were more frequent in patients receiving first-line abemaciclib plus NSAI compared with placebo plus NSAI.24 Although the incidence of haematologic events reported with abemaciclib was lower than observed in the ribociclib and palbociclib studies, other AEs such as all-grade diarrhoea were more frequent with abemaciclib (abemaciclib plus NSAI vs placebo plus NSAI: 81% vs 30%) than ribociclib (ribociclib plus ET vs placebo plus ET: 30% vs 22%) or palbociclib (palbociclib plus ET vs placebo plus ET: 26% vs 19%), likely reflecting structural differences and differences in the spectrum of kinase inhibition among these agents.23–25 It must be noted that cross-trial comparisons should be interpreted with caution.
It is important that healthcare providers are familiar with guidance regarding dose reductions to manage moderate-to-severe or serious AEs that may occur with CDK4/6 inhibitor treatment. The prescribing information for ribociclib recommends two levels of dose reduction (to 400 and then 200 mg/day) as needed to manage AEs.21 Specifically, for neutropenia (the most common AE with ribociclib), it is recommended that ribociclib treatment is interrupted in patients experiencing CTCAE grade 3/4 events until recovery to grade ≤2, and treatment is then resumed at the same dose level for an uncomplicated first grade 3 event. Reduction by 200 mg/day is recommended for grade 4 neutropenia, grade 3 febrile neutropenia or recurring grade 3 neutropenia.21 The ribociclib prescribing information also offers specific dose modification guidance for hepatobiliary toxicities. In this analysis, QT interval prolongations occurred at a rate of only 6.5% (grade 3/4, 1.2%) with ribociclib treatment, dose reductions due to QT interval prolongation were infrequent (≤2.5%) and <1% of patients discontinued treatment due to QT interval prolongation events. However, healthcare providers should remain aware of the possibility of these events. Per the ribociclib-prescribing information, if a QTcF of >480 ms is observed, treatment should be interrupted until it resolves to <481 ms, and then treatment can be resumed at the next lower dose level. If a QTcF ≥481 ms recurs, treatments should be interrupted until resolution to <481 ms and then resumed at the next lower dose level. If a QTcF of >500 ms is observed, treatment should be interrupted until it resolves to <481 ms, and then treatment can be resumed at the next lower dose level; however, treatment should be permanently discontinued if either a QTcF of >500 ms or a change from baseline of >60 ms is observed and is associated with torsades de pointes, polymorphic ventricular tachycardia, unexplained syncope or signs/symptoms of serious arrhythmia.
This pooled analysis demonstrated that dose reductions, largely due to AE management, were required by fewer than half of patients (45.8%) receiving ribociclib in the first-line setting. Furthermore, of the patients who received ribociclib dose reductions, approximately two-thirds (68.5%) required only a single reduction. There were no patient characteristics that obviously influenced the requirement for ribociclib dose reduction across the studies in this analysis. The mean ages of patients who had a dose reduction versus no dose reduction were comparable within the studies, suggesting that there was no increased risk of dose reduction in older patients. Notably, the rates of dose reductions, including those due to neutropenia or any AEs, were lower in the MONALEESA-3 and –7 trials compared with the earlier MONALEESA-2 trial, possibly because greater experience with ribociclib improved the comfort level of physicians regarding the management of AEs. An important observation from this analysis is that the efficacy of ribociclib plus first-line ET was maintained in patients who received decreased relative DI compared with patients who remained on full-dose regimens. For context, the addition of ribociclib (with varying dose intensities observed) to ET in the first-line setting approximately doubled median PFS, based on median PFSs reported with placebo plus letrozole in MONALEESA-2 (16.0 months), first-line placebo plus fulvestrant in MONALEESA-3 (19.2 months) and placebo plus NSAI in MONALEESA-7 (13.8 months).12,19,20 Furthermore, ORR and CBR outcomes were not impaired in patients whose ribociclib dose was reduced compared with patients who received the full-dose regimen across the MONALEESA programme. Interestingly, there was a small but numerically greater improvement in the ORR in the lowest relative DI group (30th percentile (≤71% relative DI)) compared with the other relative DI groups; it should be noted that statistical comparisons were not performed. Combined with the improvements in PFS for this group, it could be postulated that, as seen with agents that target vascular endothelial growth factor and epidermal growth factor receptor,25 variations in drug metabolism and pharmacodynamic effects lead to variable drug exposure/activity such that patients with more on-target AEs, e.g., neutropenia, may receive greater drug exposure and therapeutic effect.
In summary, data from this pooled analysis of >1000 patients who received ribociclib plus ET in the MONALEESA programme confirm the safety profile of this CDK4/6 inhibitor in pre-, peri- and postmenopausal women with HR+/HER2– ABC. These pooled data also demonstrate that the clinical benefit of ribociclib is preserved when dose modifications are undertaken to manage AEs in accordance with the prescribing information.
Supplementary information
Acknowledgements
The authors thank the patients who participated in these trials, their families and the staff members at each study site.
Author contributions
H.A.B., A.C., A.B., J.T.B., J.S., P.N., D.T., S.-A.I., S.C., F.J.E., L.H., J.P.Z., A.R., K.R.L. and D.A.Y., including investigators and representatives of the sponsor, had access to the data and were involved in the interpretation of the data, writing and review of all drafts of the paper and in the decision to submit the paper for publication. Data were collected by H.A.B., A.C., A.B., J.T.B., J.S., P.N., D.T., S.-A.I., S.C., F.J.E., L.H. and D.A.Y. Data generation, statistical analyses and validation were performed by A.R. Medical writing support was provided by John McGuire at MediTech Media, funded by Novartis Pharmaceuticals Corporation.
Ethics approval and consent to participate
All studies were conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. The institutional review board at each participating centre reviewed the protocol and any amendments, and all patients provided written informed consent. Please see Institutional Review Board lists that approved the studies for MONALEESA-2, -3 and -7 in Supplementary Information.
Consent to publish
Not applicable.
Data availability
Novartis made the study protocols available for the MONALEESA-2, -3 and -7 studies at the time of primary publications in their respective journals. Individual participant data and data from this analysis will not be made available.
Competing interests
H.A.B. reports consulting or advisory for AstraZeneca, Bristol-Myers Squibb, FORMA Therapeutics, Janssen, MedImmune, Mersana, Novartis, Roche/Genentech, TG Therapeutics; grants from AbbVie, Agios, AstraZeneva, Bayer, BioMed Valley Discoveries, Boehringer Ingleheim, Bristol-Myers Squibb, Celgene, Celdex, CytomX Therapeutics, eFFECTOR Therapeutics, Gilead Sciences, GlaxoSmithKline, Immunocore, Incyte, Janssen, Jounce Therapeutics, Lilly, Loxo, Macrogenics, MedImmune, Merck, Mersana, Milennium, Moderna Therapeutics, Novartis, Pfizer, PTC Therapeutics, Roche/Genentech, Seattle Genetics, Takeda, Tarveda Therapeutics, Tesaro, TG Therapeutics, Valent Technologies, Vasastern, Vertex; A.C. has nothing to disclose; A.B. reports consulting or advisory for Immunomedics, Pfizer, Novartis, Genentech/Roche, Merck, Radius Health, Spectrum Pharma, Taiho Pharma, Biothernostics Inc., Sanofi, Daichi Pharma, Puma; personal fees from Biothernostics Inc., Pfizer, Novartis, Genentech/Roche, Merck, Radius Health, Immunomedics, Spectrum Pharma, Taiho Pharma, Sanofi, Daiichi Pharma, Puma; grants from Genentech/Roche, Novartis, Pfizer, Merck, Sanofi, Radius Health, Immunomedics, Mersana, Innocrin, Biothernostics Inc.; J.T.B. has nothing to disclose; J.S. has nothing to disclose; P.N. has nothing to disclose; D.T. reports consulting or advisory for Novartis, Pfizer, Genomic Health, GlaxoSmithKline; personal fees from Novartis, Pfizer, Genomic Health, GlaxoSmithKline; research grant from Novartis; S.-A.I. reports consulting or advisory for AstraZeneca, Novartis, Hanmi, Pfizer, Eisai, Amgen, MediPacto, Genentech/Roche, Lilly; personal fees from AstraZeneca, Novartis, Hanmi, Pfizer, Eisai, Amgen, MediPacto, Genentech/Roche, Lilly; grants from AstraZeneca, Pfizer, Genentech/Roche; S.C. reports payment to his institution from Novartis for conduct of the clinical trial during the conduct of this study; advisory board: his institution received funds for participation in clinical trials by Novartis, Pfizer, Hoffman LaRoche, Eli Lilly; he is a subject editor for the British Journal of Cancer. F.J.E. reports consulting or advisory for Novartis, Pfizer, Genentech/Roche, Celltrion Healthcare, Seattle Genetics; personal fees from Novartis, Pfizer, Genentech/Roche, Celltrion Healthcare, Seattle Genetics; grants from Novartis, Pfizer, GlaxoSmithKline, Genentech/Roche; L.H. reports consulting or advisory for Novartis; personal fees from Novartis; grants from Novartis; J.P.Z. reports employment and stock ownership from Novartis; A.R. reports employment and stock ownership from Novartis; K.R.L. reports employment and stock ownership from Novartis; D.A.Y. reports consulting or advisory for Biothernostics Inc., Bristol Myers Squibb, Celgene, Daiichi Sankyo, Lilly, Eisai, Genentech/Roche, NanoString Technologies, Novartis; personal fees from Biothernostics Inc., Bristol Myers Squibb, Celgene, Daiichi Sankyo, Lilly, Eisai, Genentech/Roche, NanoString Technologies, Novartis; grants from Daiichi Sankyo, Lilly, Eisai, Novartis, Abbvie, AstraZeneca, Clovis Oncology, Immunomedics, InventisBio, Lilly, MedImmune, Medivation, Merck, Oncothyreon, Pfizer, Syndax, Tesaro.
Funding information
These trials were funded by Novartis Pharmaceuticals Corporation. Medical writing assistance was provided by John McGuire, PhD at MediTech Media, funded by Novartis Pharmaceuticals Corporation.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01415-9.
References
- 1.Hwang KT, Kim J, Jung J, Chang JH, Chai YJ, Oh SW, et al. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: a population-based study using SEER database. Clin. Cancer Res. 2019;25:1970–1979. doi: 10.1158/1078-0432.CCR-18-2782. [DOI] [PubMed] [Google Scholar]
- 2.Howlader, N., Altekruse, S. F., Li, C. I., Chen, V. W., Clarke, C. A., Ries, L. A. et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl Cancer Inst. 106, dju055 (2014). [DOI] [PMC free article] [PubMed]
- 3.Szostakowska M, Trebinska-Stryjewska A, Grzybowska EA, Fabisiewicz A. Resistance to endocrine therapy in breast cancer: molecular mechanisms and future goals. Breast Cancer Res. Treat. 2019;173:489–497. doi: 10.1007/s10549-018-5023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy CG, Dickler MN. Endocrine resistance in hormone-responsive breast cancer: mechanisms and therapeutic strategies. Endocr. Relat. Cancer. 2016;23:R337–R352. doi: 10.1530/ERC-16-0121. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, et al. 4th ESO-ESMO International Consensus Guidelines for advanced breast cancer (ABC 4) Ann. Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr. Relat. Cancer. 2011;18:C19–C24. doi: 10.1530/ERC-11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016;45:129–138. doi: 10.1016/j.ctrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers. Med. 2014;7:203–215. doi: 10.2147/PGPM.S52762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, et al. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr. Relat. Cancer. 2011;18:333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes MT, Adashek JJ, Barreto CMN, Spinosa ACB, de Souza Gutierres B, Lopes G, et al. A paradigm shift for the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer: a review of CDK inhibitors. Drugs Context. 2018;7:212555. doi: 10.7573/dic.212555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018 doi: 10.1016/s1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 13.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018 doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 14.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 15.Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N. Engl. J. Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 17.Kim S, Tiedt R, Loo A, Horn T, Delach S, Kovats S, et al. The potent and selective cyclin-dependent kinases 4 and 6 inhibitor ribociclib (LEE011) is a versatile combination partner in preclinical cancer models. Oncotarget. 2018;9:35226–35240. doi: 10.18632/oncotarget.26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, Lee NV, Hu W, Xu M, Ferre RA, Lam H, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol. Cancer Ther. 2016;15:2273–2281. doi: 10.1158/1535-7163.MCT-16-0300. [DOI] [PubMed] [Google Scholar]
- 19.Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 20.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018;29:1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 21.Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nat. Rev. Cancer. 2008;8:288. doi: 10.1038/nrc2349. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 24.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 25.Dienstmann R, Brana I, Rodon J, Tabernero J. Toxicity as a biomarker of efficacy of molecular targeted therapies: focus on EGFR and VEGF inhibiting anticancer drugs. Oncologist. 2011;16:1729–1740. doi: 10.1634/theoncologist.2011-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Novartis made the study protocols available for the MONALEESA-2, -3 and -7 studies at the time of primary publications in their respective journals. Individual participant data and data from this analysis will not be made available.