Abstract
KRAS mutations drive a wide variety of cancers. Drugs targeting the protein product of KRASG12C mutations are currently being evaluated show preliminary efficacy in clinical trials. A clinical trial of VS-6766, a dual RAF–MEK inhibitor, has reported early single agent activity in non-G12C mutated KRAS driven cancers.
Subject terms: Drug development, Medical research
Main
KRAS mutations are commonly associated with a range of solid tumours and haematological malignancies.1 Initial attempts to drug KRAS proved challenging due to the high affinity of KRAS to GTP and the relative abundance of GDP/GTP in human cellular tissue.2 This led a plethora of efforts to target downstream effectors such as RAF, MEK, PI3K, AKT, mTOR and the combination of these signalling nodes,1–3 but these approaches have not led to registration of a drug or drug combinations in the setting of KRAS mutant (KRASM) cancers. There has however been significant progress in developing drugs directly targeting protein products of specific subsets of KRAS mutations i.e. KRASG12C. These inhibitors (sotorasib and adagrasib) have shown promising activity in non-small cell lung cancer (NSCLC) with KRASG12C mutations4,5 and will no doubt be further explored in registration enabling studies.
Guo et al. reported a Phase 1b dose-escalation, basket expansion study of a dual RAF–MEK inhibitor, VS-6766 (previously known as CH5126766 and RO5126766), which showed promising anti-tumour activity in patients with solid tumours and multiple myeloma harbouring non-G12C-KRAS mutations.6 There are several interesting aspects to this study.
Firstly, the drug has interesting pharmacological properties. It is a MEK inhibitor but also blocks in-complex (CRAF–MEK) phosphorylation of MEK,7 leading to reduction of phosphorylation of both MEK and ERK as demonstrated in pre- and post-treatment biopsies. This is distinct to a reduction of phosphorylation of ERK but not MEK caused by currently licensed MEK inhibitors. The inhibition of signalling in two distinct nodes in the MEK–ERK signalling network by a single drug may explain the preliminary single agent efficacy of VS-6766 reported.
Secondly, the authors have exploited an unusually long half-life of the drug of approximately 50 hours (which is significantly longer than other RAF or MEK inhibitors) to run pharmacokinetic simulations to predict drug concentrations and design a twice a week dosing schedule. The highly intermittent twice a week schedule established in the trial enables patients to tread the fine line between efficacy and toxicity. Further, a dose modification strategy to drop to three-weeks on-one week off rather than reducing the dose of the drug is different from dose modification strategies commonly used in combination studies of targeted agents.8 This reflects previous preclinical studies by the group where they had studied the importance of maximal inhibition of MEK signalling in KRASM models.9 The intermittent schedule has significantly improved the therapeutic index of the drug compared with previous studies that explored continuous dosing schedules.10
Finally, the authors have demonstrated single agent activity in a variety of KRASM cancers in a cohort of heavily pre-treated patients. Of note, they have demonstrated partial responses in 3/10 patients with KRASM NSCLC as a single agent and interestingly two of the three patient who responded had KRASG12V mutations. Given there are no KRAS targeted therapies for KRASG12V mutations, VS-6766 could be explored further in this disease space both as a single agent or in combination. Of note, 3/5 patients with RAS/RAF mutations with gynaecological cancers responded to treatment. There is considerable excitement and activity of MEK inhibitors (trametinib and binimetinib) in low grade serous ovarian cancer (LGSOC).11,12 The combination study of VS-6766 with defactinib have shown promising activity in Phase 1 studies with expansions in LGSOC13 and randomised a Phase 2 trial is exploring the activity of the combination in LGSOC is ongoing (NCT04625270). Further, an interesting response was seen in patient with KRASG12V driven multiple myeloma. While anecdotal responses of MEK inhibitors have been noted in myeloma,14 this further confirms response of this agent in an independent cancer type driven by KRAS mutations.
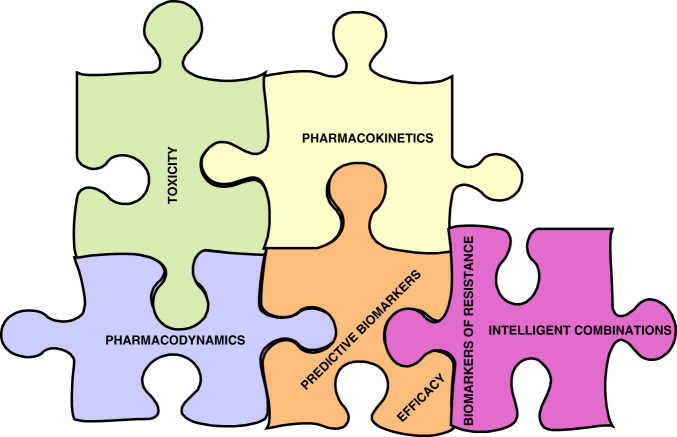
Attention to detail of pharmacokinetics, pharmacodynamics, toxicity and predictive biomarkers of efficacy are part of the pharmacological audit trail and remain critical to successful development of targeted therapy15 (Fig. 1). The trial by Guo et al.6 has defined a backbone of a highly intermittent schedule of novel RAF–MEK inhibitor VS-6766. Multiple early Phase clinical trials of combinations with this VS-6766 are currently ongoing with agents such as everolimus (NCT02407509) and defactinib (NCT03875820). Randomised studies of the combination of VS-6766 and defactinib to explore efficacy of the combination in KRASM LGSOC (NCT04625270) and NSCLC (NCT04620330) are currently ongoing.
Fig. 1. The Pharmacological Audit Trial.
Crucial elements in the pharmacological audit trail that are key to optimise dosing schedules and use predictive biomarkers to accelerate early clinical development.
Author contributions
C.G. and U.B. contributed to the writing of the manuscript.
Ethics approval and consent to participate
Not applicable.
Data availability
Not applicable.
Competing interests
U.B. has received funding for the investigator-initiated clinical trial discussed in the manuscript from Chugai Pharmaceuticals and Verastem Oncology. U.B. has also received funding from Verastem Oncology for preclinical research. U.B. is an employee of The Institute of Cancer Research that has commercial interests in the development of RAF inhibitors.
Funding information
The authors acknowledge funding from the Experimental Cancer Medicine Centre and Biomedical Research Centre to the Institute of Cancer Research and the Royal Marsden Hospital NHS foundation trust. U.B. also acknowledges funding from CRUK and the NIHR.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uprety D, Adjei AA. KRAS: from undruggable to a druggable cancer target. Cancer Treat. Rev. 2020;89:102070. doi: 10.1016/j.ctrv.2020.102070. [DOI] [PubMed] [Google Scholar]
- 2.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hymowitz, S. G. & Malek, S. Targeting the MAPK pathway in RAS mutant cancers. Cold Spring Harb. Perspect. Med. 8, a031492 (2018). [DOI] [PMC free article] [PubMed]
- 4.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 2020;383:1207–1217. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janne, P., Rybin, I., Sipra, A., Riely, G., Papadopoulous, K., Sabari J. et al. KRYSTAL-1: activity and safety of Adagrasib (MRTX849) in advanced/metastatic non–small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur. J. Cancer138, S1–S2 (2020).
- 6.Guo C, Chenard-Poirier M, Roda D, de Miguel M, Harris SJ, Candilejo IM, et al. Intermittent schedules of the oral RAF-MEK inhibitor CH5126766/VS-6766 in patients with RAS/RAF-mutant solid tumours and multiple myeloma: a single-centre, open-label, phase 1 dose-escalation and basket dose-expansion study. Lancet Oncol. 2020;21:1478–1488. doi: 10.1016/S1470-2045(20)30464-2. [DOI] [PubMed] [Google Scholar]
- 7.Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S, et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 2014;25:697–710. doi: 10.1016/j.ccr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat. Rev. Clin. Oncol. 2017;14:57–66. doi: 10.1038/nrclinonc.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart A, Thavasu P, de Bono JS, Banerji U. Titration of signalling output: insights into clinical combinations of MEK and AKT inhibitors. Ann. Oncol. 2015;26:1504–1510. doi: 10.1093/annonc/mdv188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Garcia M, Banerji U, Albanell J, Bahleda R, Dolly S, Kraeber-Bodere F, et al. First-in-human, phase I dose-escalation study of the safety, pharmacokinetics, and pharmacodynamics of RO5126766, a first-in-class dual MEK/RAF inhibitor in patients with solid tumors. Clin. Cancer Res. 2012;18:4806–4819. doi: 10.1158/1078-0432.CCR-12-0742. [DOI] [PubMed] [Google Scholar]
- 11.Gershenson, D., Brady, W., Paul, J., Carty, K., Rodgers, W., Millian, D. et al. A randomized phase II/III study to assess the efficacy of trametinib in patients with recurrent or progressive low-grade serous ovarian or peritoneal cancer. Ann. Oncol.30, v897–v898 (2019).
- 12.Monk BJ, Grisham RN, Banerjee S, Kalbacher E, Mirza MR, Romero I, et al. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J. Clin. Oncol. 2020;38:3753–3762. doi: 10.1200/JCO.20.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinde, R., Terbuch, A., Little, M., Caldwell, R., Kurup, R., Riisnaes, R. et al. Phase I study of the combination of a RAF-MEK inhibitor CH5126766 and FAK inhibitor defactinib in an intermittent dosing schedule with expansions in KRAS mutant cancers. Cancer Res.80(16 Suppl), Abstract CT143 (2020).
- 14.Heuck CJ, Jethava Y, Khan R, van Rhee F, Zangari M, Chavan S, et al. Inhibiting MEK in MAPK pathway-activated myeloma. Leukemia. 2016;30:976–980. doi: 10.1038/leu.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerji U, Workman P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin. Oncol. 2016;43:436–445. doi: 10.1053/j.seminoncol.2016.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.