Abstract
Amphibian chytridiomycosis, caused by the fungus Batrachochytrium dendrobatidis (Bd), has caused the greatest known loss of biodiversity due to an infectious disease. We used Bd infection data from quantitative real-time PCR (qPCR) assays of amphibian skin swabs collected across Chile during 2008–2018 to model Bd occurrence with the aim to determine bioclimatic and anthropogenic variables associated with Bd infection. Also, we used Bd presence/absence records to identify geographical Bd high-risk areas and compare Bd prevalence and infection loads between amphibian families, ecoregions, and host ecology. Data comprised 4155 Bd-specific qPCR assays from 162 locations across a latitudinal gradient of 3700 km (18º to 51ºS). Results showed a significant clustering of Bd associated with urban centres and anthropogenically highly disturbed ecosystems in central-south Chile. Both Bd prevalence and Bd infection loads were higher in aquatic than terrestrial amphibian species. Our model indicated positive associations of Bd prevalence with altitude, temperature, precipitation and human-modified landscapes. Also, we found that macroscale drivers, such as land use change and climate, shape the occurrence of Bd at the landscape level. Our study provides with new evidence that can improve the effectiveness of strategies to mitigate biodiversity loss due to amphibian chytridiomycosis.
Subject terms: Biodiversity, Conservation biology, Fungal infection
Introduction
In recent years, amphibians have declined dramatically in many regions of the world1 with approximely 50% of amphibian species under risk of global extinction2,3. The causes behind these declines are multiple and complex, and they include well-established factors such as habitat loss and invasive species and, more recently, infectious diseases4,5. The discovery of the emerging disease amphibian chytridiomycosis6, caused by the chytrid fungus Batrachochytrium dendrobatidis (hereafter Bd)7, and its role in the decline and extinction of numerous amphibian species has led to a paradigm shift towards wildlife diseases as a conservation issue8. Recently, another species, B. salamandrivorans, has been found causing severe mortality and population declines of fire salamanders (Salamandra salamandra) in Europe9. Thus far, Bd has been associated with the decline of over 500 amphibian species, including the presumed extinction of 90 species10 (e.g., but see11,12), and is linked with the collapse of amphibian communities in eastern Australia6, Costa Rica13,14, Panama4,15,16 and Peru17. The fungus infects amphibian skin, leading to epidermal hyperplasia and hyperkeratosis, resulting in death in susceptible individuals due to electrolyte loss and osmotic imbalance18. The impacts of Bd on amphibian populations can be attributed to the introduction of this pathogen into naïve host populations, the persistence of Bd in the environment, the existence of a free-living infective stage, and the presence of amphibian reservoir hosts8,19,20.
Studies analysing the distribution patterns of Bd at the large scale show a broad spatial distribution, high environmental tolerance, and a wide range of host species, indicating that Bd is a generalist pathogen21–27. Several factors related to host28,29, pathogen30, and environment21 have been shown to interact, facilitating the emergence of Bd and increasing the severity of its impacts. However, many aspects of the landscape epidemiology of Bd (i.e., studies comprising different ecoregions) remain unknown. These include pathogen distribution in under-surveyed areas (e.g., parts of Africa and South America), mechanisms for local or regional spread and identification of reservoir hosts (amphibian and non-amphibian)8,14,31–33. These can be relevant for Bd mitigation, for example helping to predict the potential impacts of chytridiomycosis in understudied areas or following Bd introduction into naïve amphibian populations. Also, Bd landscape epidemiology is crucial to inform biosecurity recommendations at the country level, particularly given the potential for strain recombination to result in increased virulence8. Among climatic factors, temperature and humidity have been reported to be important determinants of Bd occurrence, influencing the survival and infection rate of the pathogen19,24,25,34–36. Chytridiomycosis outbreaks generally have been associated with cooler months and higher altitudes4,23,37,38. Seasonal climatic variation can affect the occurrence of the pathogen and the timing of chytridiomycosis outbreaks in the wild13,35; for example, infection prevalence in robber frogs (Eleutherodactylus spp.) in Montserrat is higher in the cool, dry season than in the warmer, wetter months39. This is also of interest in temperate areas where climate has a strong seasonality, which is also influenced by latitude and altitude40–42. In addition, urbanization has been proposed as a factor associated with high Bd occurrence24,36. Nevertheless, we believe that understanding of the factors driving the occurrence of Bd infection have been overlooked, despite the fundamental answers that such insight may provide, particularly focused on disease mitigation strategies43. Taking a regional perspective (in contrast to a global or local one) might allow the use of high-resolution data over a large spatial scale (such as the case of Chile) to evaluate potential interactions among different factors involved in the epidemiology of Bd.
South America is one the regions most impacted by amphibian chytridiomycosis7,44,45, and Bd has been detected in wild amphibians in virtually all countries in this region25. Here, amphibian population declines, and extinctions have been reported since the 1970s5. A likely recent introduction of the hypervirulent Global Panzootic Lineage of Bd (BdGPL) into South America46,47 coincides with the onset of these enigmatic amphibian declines5,19,48, but the presence in a restricted area of the Atlantic forests of Brazil of the more ancient lineage BdAsia-2/BdBrazil could make the evolutionary history of Bd in in this region more complex47. Native Chilean amphibians consist of 63 anuran species, of which 45 (72%) are endemic, and adapted to a range of different ecosystems, from dry desert and altiplano in the North, to subpolar forest and cold steppe in the south49. In addition, feral populations of the African clawed frog (Xenopus laevis) have been established in central Chile since the 1970s50. Batrachochytrium dendrobatidis infection is widespread in Chile20,36,43,51,52, and chytridiomycosis has been associated with the population decline and extinction of Darwin’s frogs (Rhinoderma rufum and R. darwinii)52,53.
Here, we use Bd occurrence data of amphibians from across Chile collected over 11 years to assess the epidemiology of Bd over time and across a large latitudinal, altitudinal and taxonomic range. Based on the results of quantitative real-time PCR (qPCR) assays, we modelled Bd prevalence to determine bioclimatic and anthropogenic variables associated with the distribution of infection. Using Bd presence/absence records we followed a spatial scan statistics approach to identify geographical Bd-infection clusters (or Bd high-risk areas). Finally, we compare variation in Bd prevalence and infection loads among taxonomic families, ecoregions and host ecology (aquatic vs terrestrial, see14,54) to further complement our epidemiological study. Although similar studies have been conducted previously40,43,55,56, our multi-approach analyses using different methods over such a large latitudinal gradient has not been done before, and thus can provide new insights into the landscape epidemiology of Bd. Understanding macroscale drivers of Bd and their interactions is critical to the conservation management of amphibians through Bd prevention and mitigation strategies31.
Results
Bd prevalence patterns
In total, we processed swab samples from 4155 wild anurans collected over 11 years (2008–2018) from 40 species across Chile (Fig. 1, Table 1). Infection with Bd was detected across a broad geographical range (97 out of 162 sites were infected) with an overall prevalence of 19.1% (95% CI 17.8–20.3). The prevalence of Bd infection varied throughout the 11 years of the study (GLM, d.f. = 47, P < 0.05), with the odds ratio analysis showing that anurans are more likely to get infected by Bd over time (OR = 1.03, 95% CI 1.01–1.07). The Chilean Matorral ecoregion had the highest prevalence of Bd infection (26.2% [95% CI 23.9–28.7%], GLM, d.f. = 47, OR = 6.2, 95% CI 4.9–7.7, P < 0.05) in comparison with the other studied ecoregions (Fig. 1).
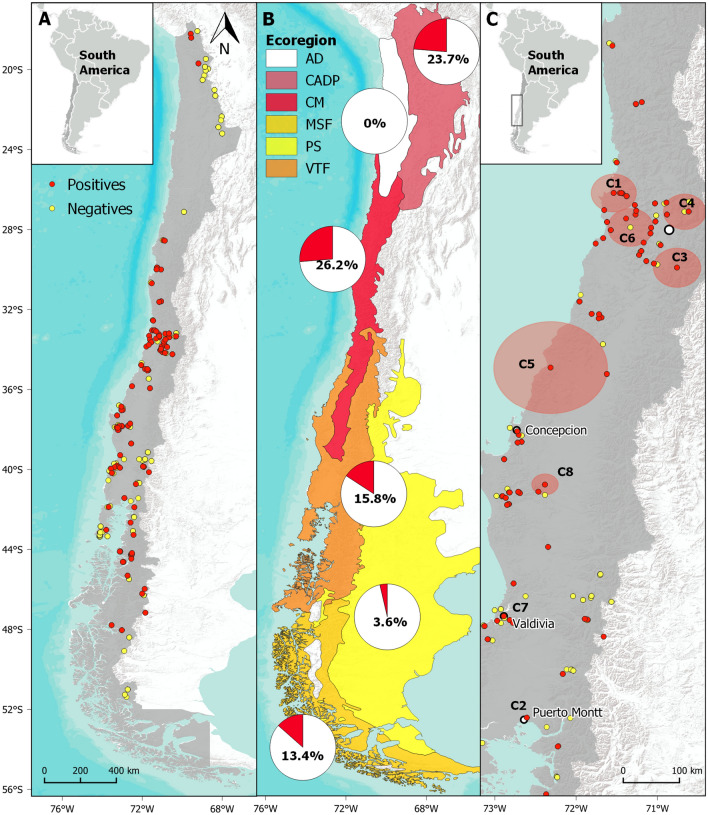
Figure 1.
Spatial distribution of Batrachochytrium dendrobatidis (Bd) infection in amphibian populations in Chile studied from 2008 to 2018. (A) Bd-positive (red dots) and Bd-negative (yellow dots) sites. (B) Proportion of Bd-positive amphibians (red portion of pie chart) according to ecoregions: Chilean Andean Dry Puna (CADP), Atacama Desert (AD), Chilean Matorral (CM), Valdivian Temperate Forest (VTF), Patagonian Steppe (PS) and Magellanic Subpolar Forest (MSF). (C) Eight statistically significant (P < 0.05) spatial clusters of Bd high-risk areas (C1 to C8) obtained from scan statistics analysis. All clusters were located in central and south Chile. Most clusters are represented by red shaded circles, apart from C2 and C7 which were smaller in size. Maps and rasters were generated using QuantumGIS v.3.8.2 (QGIS Geographic Information System). Open Source Geospatial Foundation Project. http://qgis.osgeo.org (2018).
Table 1.
Chilean anurans studied for Batrachochytrium dendrobatidis (Bd) infection from 2008 to 2018.
| Species | Ecology | Negative | Positive | Sample size | Proportion of infection (%) | 95% CI | Median ZE | IUCN |
|---|---|---|---|---|---|---|---|---|
| Alsodes australis | T | 13 | 0 | 13 | 0 | 0–22.8 | 0 | DD |
| Alsodes barrioi | T | 27 | 0 | 27 | 0 | 0–12.5 | 0 | EN |
| Alsodes coppingeri | T | 2 | 0 | 2 | 0 | 0–65.8 | 0 | DD |
| Alsodes nodosus | A | 27 | 5 | 32 | 15.6 | 6.9–31.8 | 166 | NT |
| Alsodes tumultuosus | A | 47 | 21 | 68 | 30.9 | 21.2–42.6 | 1302 | VU |
| Alsodes valdiviensis | T | 11 | 9 | 20 | 45.0 | 25.8–65.8 | 13 | EN |
| Alsodes verrucosus | T | 3 | 0 | 3 | 0 | 0–56.2 | 0 | EN |
| Atelognathus salai | A | 0 | 4 | 4 | 100 | 51.0–100 | 419 | LC |
| Batrachyla antartandica | A | 143 | 21 | 164 | 12.8 | 8.5–18.8 | 62 | LC |
| Batrachyla leptopus | A | 66 | 2 | 68 | 2.9 | 0.8–10.1 | 1.6 | LC |
| Batrachyla taeniata | A | 83 | 5 | 88 | 5.7 | 2.5–12.6 | 318 | LC |
| Calyptocephalella gayi | A | 281 | 150 | 431 | 34.8 | 30.5–39.4 | 436 | VU |
| Chaltenobatrachus grandisonae | A | 22 | 8 | 30 | 26.7 | 14.2–44.5 | 621 | LC |
| Eupsophus altor | T | 5 | 0 | 5 | 0 | 0–43.5 | 0 | NE |
| Eupsophus calcaratus | T | 87 | 12 | 99 | 12.1 | 7.1–20.0 | 53 | LC |
| Eupsophus contulmoensis | T | 30 | 7 | 37 | 18.9 | 9.5–34.2 | 30 | EN |
| Eupsophus emiliopugini | A | 17 | 1 | 18 | 5.6 | 0.3–25.8 | 6 | LC |
| Eupsophus migueli | T | 1 | 0 | 1 | 0 | 0–94.9 | 0 | EN |
| Eupsophus nahuelbutensis | T | 104 | 5 | 109 | 4.6 | 2.0–10.3 | 55 | EN |
| Eupsophus roseus | A | 35 | 4 | 39 | 10.3 | 4.1–23.6 | 86 | LC |
| Eupsophus septentrionalis | A | 10 | 2 | 12 | 16.7 | 4.7–44.8 | 33 | DD |
| Eupsophus vertebralis | T | 9 | 0 | 9 | 0 | 0–29.9 | 0 | LC |
| Hylorina sylvatica | T | 6 | 3 | 9 | 33.3 | 12.1–64.6 | 222 | LC |
| Nannophryne variegata | T | 3 | 0 | 3 | 0 | 0–56.2 | 0 | LC |
| Pleurodema bufonina | A | 98 | 6 | 104 | 5.8 | 2.7–12 | 154 | LC |
| Pleurodema thaul | A | 737 | 266 | 1003 | 26.5 | 23.9–29.3 | 115 | LC |
| Rhinella arunco | T | 4 | 11 | 15 | 73.3 | 48.1–89.1 | 61 | NT |
| Rhinella atacamensis | T | 26 | 0 | 26 | 0 | 0–12.9 | 0 | VU |
| Rhinella spinulosa | T | 45 | 0 | 45 | 0 | 0–7.9 | 0 | LC |
| Rhinoderma darwinii | T | 788 | 14 | 802 | 1.8 | 1.0–2.9 | 1170 | EN |
| Telmatobius chusmisensis | A | 11 | 24 | 35 | 68.6 | 52.0–81.5 | 1830 | EN |
| Telmatobius dankoi | A | 49 | 0 | 49 | 0 | 0–7.3 | 0 | CR |
| Telmatobius fronteriensis | A | 14 | 0 | 14 | 0 | 0–21.5 | 0 | CR |
| Telmatobius marmoratus | A | 31 | 0 | 31 | 0 | 0–11.0 | 0 | EN |
| Telmatobius pefauri | A | 19 | 16 | 35 | 45.7 | 30.5–61.8 | 28 | CR |
| Telmatobius peruvianus | A | 5 | 6 | 11 | 54.6 | 28.0–78.7 | 2 | VU |
| Telmatobius halli | A | 7 | 0 | 7 | 0 | 0–35.4 | 0 | DD |
| Telmatobius vilamensis | A | 13 | 0 | 13 | 0 | 0–22.8 | 0 | CR |
| Telmatobufo bullocki | A | 3 | 0 | 3 | 0 | 0–56.2 | 0 | EN |
| Xenopus laevis | A | 388 | 172 | 560 | 30.7 | 27.0–34.7 | 400 | LC |
Host ecology (aquatic = A, or terrestrial = T), positive, negative, sample size, proportion of Bd infection, and 95% binomial confidence intervals (CI). Bd infection loads are shown as median zoospore equivalents per swab (ZE). Conservation status for each species from the IUCN redlist is also showed.
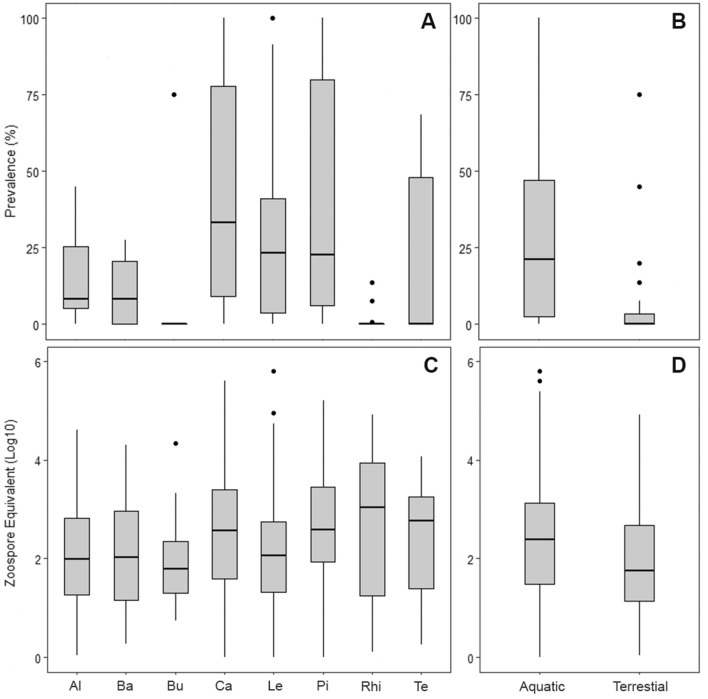
Among the eight anuran families present in Chile, Calyptocephalellidae showed the highest Bd infection prevalence with 34.5% (95% CI 30.2–39.1, GLM, d.f. = 47, OR = 1.13, 95% CI 0.7–1.8, P = 0.6), followed by Pipidae with 30.7% (CI 27.4–34.6, GLM, d.f. = 47, OR = 1.6, 95% CI 1–1.8, P < 0.05) and Leptodactylidae with 24.5% (95% CI 22.1–27.1; Fig. 2A). Sixty percent (24 out of 40) of the sampled anuran species had at least one individual positive for Bd infection (Table 1). The species with the highest prevalence were Telmatobius chusmisensis with 68.6% (95% CI 52–81.5), T. pefauri with 45.7% (95% CI 30.5–61.8) and C. gayi with 34.8% (95% CI 30.4–39.4). Aquatic anurans exhibited a significantly higher prevalence of 25.3% (95% CI 23.7–26.9) than terrestrial anurans with 4.9% (95% CI 3.9–6.34; GLM, d.f. = 47, OR = 4.3, 95% CI 2.5–7.5, P < 0.05; Fig. 2B).
Figure 2.
Boxplots showing Batrachochytrium dendrobatidis (Bd) prevalence (%) by (A) anuran family and (B) host ecology (aquatic vs. terrestrial), and Bd zoospore equivalent (Log10) by (C) anuran family and (D) host ecology, for amphibians in Chile collected from 2008 to 2018. Anuran family abbreviation: Al (Alsodidae), Ba (Batrachylidae), Bu (Bufonidae), Ca (Calyptocephalellidae), Le (Leptodactylidae), Pi (Pipidae), Rhi (Rhinodermatidae) and Te (Telmatobiidae). The centre line in each box indicates the median, the upper and lower box sides represent the interquartile range, the whiskers extend to the 5th and 95th percentiles and dots represent outliers.
For each species sampled, the proportion of swabbed animals that were Bd-positive and the median infection load are summarized in Table 1. The infection load in Bd-positive amphibians ranged from 0.1 to 630,720 ZE (Zoospore equivalents; median = 234; the highest load was obtained from a P. thaul individual). Despite the presence of some high infection loads, swabbed animals did not exhibit clinical signs consistent with chytridiomycosis. Of the total number of infected frogs, only 6.5% (774 individuals) had more than 10,000 ZE/swab. There were no significant differences in Bd infection load across ecoregions (GLM, d.f. = 47, P = 0.7), but at the family level, Rhinodermatidae, Calyptocephalellidae, and Telmatobiidae had higher burdens of infection compared with other families (GLM, d.f. = 47, P < 0.01; Fig. 2C). Overall, aquatic anurans had greater Bd infection loads (median = 259.2; range: 0.1–630,720) compared with terrestrial species (median = 56.4; range: 0.1–84,709; GLM, d.f. = 47, P < 0.01; Fig. 2D).
Bd modelling
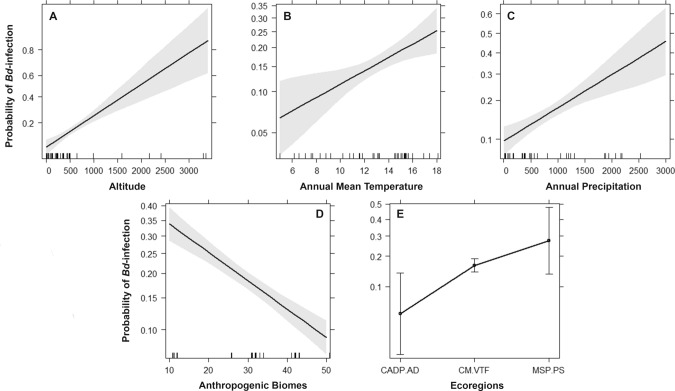
From the models tested to evaluate Bd infection risk against bioclimatic and anthropogenic factors (Table S2), the best model included the variables: altitude, annual mean temperature, annual precipitation, anthropogenic biomes and ecoregions (AICw = 0.88, AICc = 424.3, Z = − 4.9; d.f. = 42, Table S3) Batrachochytrium dendrobatidis infection probability was positively correlated with altitude (95% CI 1–1.01), annual mean temperature (95% CI 1.03–1.22), and annual precipitation (95% CI 1–1.01; Fig. 3A–C). Also, Bd infection was positively associated with anthropogenic biomes (95% CI 0.9–1; Fig. 3D), which means a higher Bd prevalence was observed in highly anthropogenic impacted ecosystems. Using model averaging, the anthropogenic biomes variable better explained Bd prevalence, and it was present in almost all the candidate models with low AICc scores (delta AICc < 4; Fig. 3D; Table S2). Also, the effect of ecoregions was significant, explaining differences in Bd prevalence (OR = 3, 95% CI 1.1–11.4, Fig. 3E, Table S3). Other factors, such as amphibian species richness and human footprint were not predictive for Bd prevalence.
Figure 3.
Best fit generalized linear model for Batrachochytrium dendrobatidis (Bd) prevalence in Chile: Bd prevalence ~ altitude + annual mean temperature + annual precipitation + anthropogenic biomes + ecoregion. Separate effects of (A) altitude, (B) annual mean temperature, (C) annual precipitation, (D) anthropogenic biomes and (E) ecoregions are shown. Line was draw using estimates from best supported generalized linear model. Grey shading is 95% confidence intervals. Hash marks in the x-axis represents measured values. Abbreviations in (E), CADP.AD = Chilean Andean dry puna + Atacama Desert, CM.VTF = Chilean matorral + Valdivian temperate forest, MSP.PS = Magellanic subpolar forest + Patagonian steppe.
Spatial distribution of Bd clusters
Our spatial analysis detected eight statistically significant clusters of Bd-infection, all located in central-south Chile within the Chilean Matorral and Valdivian Temperate Forest ecoregions (Fig. 1C, Table 2). Half of the clusters, all of similar size, were located in the contiguous Metropolitan and Valparaíso administrative regions, the most densely populated area in the country. A large single cluster was identified further south, covering an area highly impacted by land-use change to exotic pine and eucalypt monocultures. The remaining three clusters were smaller in size and located near the cities of Angol, Valdivia and Puerto Montt, all in southern Chile. In the four spatial clusters in central Chile, the observed Bd prevalence was almost three times higher than would be expected by chance (Loglikelihood ratio range = 16.31–117.73 and relative risk range = 2–3.7). The Global Moran’s I index was statistically non-significant (p = 0.2).
Table 2.
Spatial clusters of Batrachochytrium dendrobatidis infection in anurans from Chile studied between 2008 and 2018 ordered by significance.
| Cluster | Latitude | Longitude | Radius (km) | O | E | O/E | RR | LLR |
|---|---|---|---|---|---|---|---|---|
| 1 | − 33.04 | − 71.5 | 33.5 | 153 | 47.4 | 3.22 | 3.75 | 117.73 |
| 2 | − 41.43 | − 72.89 | 0 | 45 | 14 | 3.22 | 3.36 | 32.54 |
| 3 | − 34.23 | − 70.48 | 34.7 | 34 | 8.99 | 3.78 | 3.91 | 31.77 |
| 4 | − 33.34 | − 70.36 | 31.3 | 64 | 32.7 | 1.96 | 2.04 | 16.31 |
| 5 | − 35.83 | − 72.51 | 81.9 | 26 | 8.8 | 2.95 | 3.02 | 15.99 |
| 6 | − 33.59 | − 71.23 | 33.4 | 74 | 41.1 | 1.8 | 1.88 | 14.81 |
| 7 | − 39.81 | − 73.26 | 0 | 10 | 2.3 | 4.36 | 4.4 | 11.60 |
| 8 | − 37.7 | − 72.59 | 18.5 | 33 | 15.3 | 2.16 | 2.21 | 10.58 |
For each cluster area, observed cases (O), expected cases (E), observed/expected (O/E), relative risk (RR) and log-likelihood ratio (LLR) are shown.
Discussion
Amphibian chytridiomycosis is well recognized as a main causative factor of the current global amphibian extinction crisis10,57. Therefore, it is essential to identify risk factors facilitating the occurrence of Bd, as well as high risk areas of infection, which can provide guidance for effective conservation management58. Our results show that, at the landscape level across a large latitudinal gradient in Chile, Bd occurrence is: (i) biased towards certain families and species that use aquatic habitats; (ii) largely determined by bioclimate and human associated risk factors such as altitude, annual mean temperature, annual precipitation and anthropogenic biomes; and (iii) grouped in spatial clusters associated with urban centres.
Overall, our data shows that Bd is widely distributed in Chile, infecting an extensive number of amphibian species and over broad altitudinal (0 to 3460 m) and latitudinal (18° to 48°S, comprising 3400 km) gradients. This included the finding of Bd infection in a Puerto Eden frog (Chaltenobatrachus grandisonae) near Villa O’Higgins (Fig. 1A), extending the previously known southernmost global record of Bd by 588 km further south59,60. From the total of 40 sampled anuran species in Chile (64% of total richness)49, we found that 24 species showed evidence of Bd infection (see Table 1). All 11 species which had a Bd infection prevalence > 30% were exclusively aquatic amphibians, likely due to a higher contact rate with the infective stage of Bd in the aquatic environment53,61. Of these aquatic species, high Bd prevalences were found at the extreme north of the Andes in the altiplano frogs T. pefauri (3308 m) and T. chusmisensis (3365 m; see Table 1). Chytridiomycosis-related mortality has been described in the related species, T. pisanoi and T. atacamensis from northern Argentina62 and T. marmoratus from Peru17,63. In addition, Bd-implicated disappearances of Telmatobius populations have been described in Peru17 and Bolivia64.
The effects of Bd infection on anurans are highly variable and species-, population- or context-specific. For example, some species exhibit high disease-induced mortality, while others experience no detrimental individual or population effects of disease while maintaining enzootic infections57,65. In some cases, populations with cryptic, enzootic infections can experience chytridiomycosis-related mortality under certain environmental conditions, such as drought41. Vredenburg et al.65 proposed a 10,000 ZE infection load rule, above which lethal disease invariably occurs. In our study, the species with highest Bd loads were C. gayi (median 436 ZE and a maximum of 409,440 ZE) and R. darwinii (median 1170 ZE and a maximum of 84,709 ZE; Table 1), possibly indicating impacts at the population level in these species. We recommend, therefore, that longitudinal population monitoring and Bd surveillance programmes be initiated or continued for these threatened species at Bd-positive sites (e.g., see Binational Conservation Strategy for Darwin's Frog66). We found statistical differences in ZE between aquatic and terrestrial species, which emphasizes the role of aquatic species and aquatic environments in the maintenance and spread of Bd. Population declines due to Bd infection can occur in the absence of evident mass mortality or other obvious signs of disease, as evidenced by the Bd-driven declines of R. darwinii in Chile53 and the possible extinction of its sister species, R. rufum, which has not been observed since 198148,52,66.
Our best ranked model included the effects of altitude, annual mean temperature, annual precipitation, anthropogenic biomes and ecoregions, as predictors of Bd infection (Fig. 3). Anthropogenically-disturbed ecosystems proved to be one of the most important predictors that explain Bd infection. Also, altitude, annual mean temperature and annual precipitation were positively associated with Bd prevalence. Amphibian chytridiomycosis has been reported at high elevations, for example in the Rocky Mountains67, the Sierra Nevada65, the Pyrenees68 and the high Andes63, suggesting that cold high-altitude environments do not necessary limit Bd spread and subsequent impacts on wild populations. The arrival of Bd in high altitude areas has been facilitated by human movement that has spread the fungus among isolated water bodies, but also climate change can facilitate such spread modifying the environment that anurans inhabit63 and further force the severity of infection35. In addition to altitude, temperature and precipitation appear to be relevant climatic variables shaping the occurrence of Bd in Chile, as previously reported for other world regions21,27,36,43,69–73. However, other studies have found sometimes different patterns (e.g.,39,41,74), suggesting that the mechanisms between these climatic factors and Bd occurrence are complex.
In our study, the highest prevalences of Bd infection were detected near to densely populated human settlements. Our results show that high human perturbation (anthropogenic biomes) is correlated with an increase in Bd infection probability, highlighting the importance of human activities on the epidemiology of Bd, possibly due to human-assisted pathogen introduction and spread (e.g., through the transport of Bd contaminated water and sediment) between anuran populations24,57,75. Additionally, human activities can spread Bd through the movement of infected amphibians, as has been shown to occur with amphibian trade, including the introduction of exotic amphibians24,46,47,76–78. Also, it has been proposed that the reduced connectivity among amphibian populations resulting from human perturbation of the environment might impact host skin microbiome, affecting the innate immunity in amphibian skin against pathogens79. Habitat fragmentation can also affect ecological (e.g., colonization/extinction, host physiology, etc.) and evolutionary (e.g., local adaptation, evolution of resistance/tolerance mechanisms, etc.) processes that can affect host–parasite interactions19,79. The development of increased susceptibility to infection through amphibian immunosuppression as a result of environmental contamination (e.g., pesticides) and habitat perturbation80 are also possible impacts of human activities that increase the persistence and spread of Bd.
Our models showed that anthropogenic impacts and climate variables could synergistically interact and exacerbate infection risk (Table S2). The mechanisms enabling such synergy remain unclear, but our results support anthropogenic disturbance as a driver of Bd infection risk. In this context, anthropogenic biomes are generalizations for the restructured terrestrial biosphere due to agriculture, forestry and urbanization81. Elevated risk of Bd infection in areas close to human activities and settlements has been described previously in both temperate and tropical regions23,25,36,43,75,82. Most studies based species distribution models of Bd in the Americas have found an association of Bd occurrence with several climatic variables, notably precipitation, temperature and seasonality13,21,32,33,44, although few incorporate explicitly the effect of human impact, such as urban centres, in the analyses43. Interestingly, Zumbado-Ulate et al.33 found a higher Bd occurrence in undisturbed ecosystems or protected areas, highlighting the fact that Bd occurrence is context specific as can be influenced by many factors including time of Bd introduction, species and population susceptibility, among others.
As with prevalence, the same association has been shown with intensity of infection, namely a positive association of Bd loads with anthropogenic disturbance71. The highest observed Bd prevalence was in the Chilean Matorral ecoregion, an area considered as a priority for global biodiversity conservation83. This region harbours a high level of anuran endemism49, yet contains the highest human population density in the country (almost 90% of the Chilean population). Consequently, increasing urbanization is resulting in deforestation and habitat loss which is negatively impacting amphibian populations84. Such environmental changes could favour the invasion of alien species, including Bd24.
All clusters of Bd-infection were located in central-south Chile, suggesting that amphibians in this region are at a higher risk of Bd-infection than elsewhere in the country. Although these findings are similar to those found by Bacigalupe et al.43, by using a different approach in a much wider area of Chile, this strengthens the hypothesis of urban centres playing an important role in the epidemiology of Bd. The four clusters located in the Metropolitan and Valparaíso regions could result from a potential initial introduction of Bd in this part of Chile and its subsequent spread46 with fewer clusters in the south of Chile. When Bd is introduced to a new geographic location, first foci of introductions represented by narrow spatiotemporal cluster(s) occur followed by subsequent spread over time68,85. This has been seen in Spain, where Bd infection shows a pattern of introduction and spread along the Pyrenees with narrow spatial clusters, indicating recent introductions into Iberian biomes35. Our results are consistent with such a pattern having occurred in our study area. An alternative hypothesis is that instead of high-risk areas we might be capturing oversampled regions86. Therefore, we recommend considering other methods such as species distribution models43 or kriging interpolation33 to have a more accurate picture of the identified high-risk areas.
Batrachochytrium dendrobatidis has been recognized as causing the greatest recorded loss of biodiversity due to a single pathogen10. Therefore, having a better understanding of the factors that shape the occurrence of Bd at the landscape level is valuable for conservation strategies and actions at the regional or country levels to halt the loss of biodiversity due to chytridiomycosis14,87,88. In this study, we provide evidence linking climate and anthropogenic factors as macroscale drivers of Bd occurrence at the landscape level. In particular the interaction of altitude, temperature, precipitation and human modified landscapes, appears to be the most relevant factors facilitating establishment and spread of Bd over large areas. Although we did not observe any mass mortalities or obvious signs of disease, we found high infection loads (> 10,000 ZE) which have been shown to lead to fatal chytridiomycosis in other amphibian species, with potential impacts at the population level. Although some species can coexist initially with high loads of Bd, host defence mechanisms such as anti-Bd skin microbiota, immunity, and skin peptides could reduce Bd infection loads to allow host–pathogen co-existence89,90. Strategies are required to prevent the further spread of Bd and to mitigate its impacts where it already occurs10. Our study provides valuable information for the design of such conservation strategies as long-term monitoring and control of biotic and abiotic factors (e.g., environmental disinfection, anti-Bd microbiome bioaugmentation or environmental management to reduce Bd exposure)91,92 in areas with high occurrence of Bd and especially in areas where amphibian populations are under a high degree of anthropogenic pressure.
Materials and methods
Ethic statement
This study was approved by Bioethics Committees of the Universidad Andres Bello (reference number 13/2015) and the Zoological Society of London’s Ethics Committee (WLE717), and followed the guidelines under permit from the Chilean Agriculture and Livestock Service (351/2015). All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Study area and sampled amphibians
From 2008 to 2018 we sampled amphibians at 162 sites from north (18°11′43″S, 69°34′6″W) to south (51°23′27″S, 72°46′59″W) Chile, covering a latitudinal gradient of 3700 km and an altitudinal range from sea level to 4434 m. We sampled recently metamorphosed, juvenile, and adult frogs of 40 species belonging to seven families from sites representatives of all the six ecoregions present in Chile: Andean Dry Puna, Atacama Desert, Chilean Matorral, Valdivian Temperate Forest, Patagonian Steppe, and Magellanic Subpolar Forest93. Since in Chile many amphibians are inactive during winter, for Bd prevalence study sites were surveyed only once in spring–summer and at each site a minimum of 23 amphibian samples were obtained. Minimum sample size was calculated assuming a test sensitivity of 100%, expected Bd prevalence of 12.5%52 and level of confidence of 95%.
Animal capture, biosecurity and sampling
Each amphibian was located through diurnal and nocturnal captures by direct observation and caught by hand or, in the case of aquatic species (i.e., Calyptocephalella gayi, Telmatobius spp. and X. laevis), caught using herpetology nets or funnel traps baited with chicken liver94,95. Following capture, each individual was handled for sampling with the use of clean disposable nitrile gloves and then released back to the exact point of capture. To minimize any false positive results and to avoid pathogen cross-contamination within or between study sites, a strict field sampling and disinfection protocol was followed20. For Bd detection, a non-invasive skin swab (MW100, Medical & Wire Equipment Co.) was obtained from each amphibian by firmly running it five times each over the ventral abdomen, the pelvis, both ventral hind limbs (femur and tibia), and the plantar surface of both hind feet, to complete a total of 35 strokes52. Swabs were kept in a refrigerated box until being stored frozen at – 80 °C once back at the laboratory until they were analysed.
Bd detection assay
Extraction of DNA from skin swabs and subsequent detection of Bd DNA using a specific real time qPCR assay was done20. For each sample, diagnostic assays were performed in duplicate, and standards of known zoospore concentration were included within each PCR plate as positive controls. We assumed that a Bd-positive swab indicated Bd infection. By including known concentrations of Bd DNA in serial diluted positive control (four standards of 100, 10, 1 and 0.1 Bd genomic equivalent) wells on each PCR plate, we were able to quantify infection intensity, which we defined as the number of zoospore equivalents/swab (ZE). To quantify and correct the infection intensity per swab, each genomic value was multiplied by 120 following Hudson et al.96.
Bd prevalence and infection loads by family, host ecology and ecoregion
We first calculated prevalence by counting the number of positive animals in a particular taxonomic family, host ecology (aquatic vs. terrestrial), or ecoregion divided by the total number of samples within that category. Host ecology was defined by considering whether the adult frogs of each species spent most of their time in or out of the water (see Table 1). We estimated 95% binomial confidence interval (95% CI) with a logistic (logit) parameterization for each category using the binom.confint function (R package ‘binom’) in the statistical software R v.3.1.397. We evaluated whether there was a trend in Bd prevalence over time using a binomial generalized linear model (GLM) using year as an explanatory variable. The deviance of a null GLM model was estimated to explore the contribution of time (year) as an explanatory variable. An autocorrelation function ‘acf’ was used to explore a potential temporal autocorrelation of the residuals. Finally, we applied odds ratio (OR) statistics to estimate the probability the amphibian to having Bd at each site in different years.
Modelling Bd prevalence across the landscape
We employed an information-theoretic modelling approach to contrast the adequacy of different working hypotheses explaining the geographic occurrence of Bd infection in our landscape gradient. In order to model Bd infection, we used bioclimatic and anthropogenic factors as explanatory variables and Bd prevalence from each of the 162 surveyed sites as response variable. Eight variables derived from landscape-scale geographic layers were used as predictors in the statistical modelling. Explanatory variables included annual mean temperature, temperature seasonality, annual precipitation, altitude, human footprint, anthropogenic biomes, ecoregions, and amphibian species richness (see Table S1 for a full description of each variable and data sources81,98–103. We extracted all data for each sampled anuran to GPS coordinates using raster layers of 30 s (~ 1 km2) spatial resolution with QuantumGIS v.3.8.2104. The assumption of normality of the data, the presence of outliers, the number of absolute zeros in the response variable and the collinearity between environmental variables and anthropogenic factors were explored. We only retained those variables with a correlation coefficient < 0.7 by the Pearson and Kendall tests. The homogeneity of variance was verified using the residuals of the model, plotting residuals vs. fitted values, and making a similar set of conditional boxplots for the residuals105.
Twenty-one GLMs were constructed based on biological hypotheses using a binomial error structure (link = logit) for Bd infection model to evaluate factors that influence the occurrence of Bd infection at the individual level (i.e., Bd prevalence as response variable by study site). Then, we evaluated the degree of support for each competing model based on Akaike information criterion (AIC), which takes into consideration the likelihood of each model while penalizing for the number of included parameters to obtain the best-fitted model, and we used Akaike weights (AICw) to quantify the relative support of each model in the set (R package ‘MuMIn’). As a result, a suitable model that describes the factors associated with Bd prevalence in anurans was obtained. To validate the GLM final model, the residuals were plotted against fitted values to assess homogeneity. In addition, we plotted a histogram and q-q plot of the residuals to verify normality of the data (Figure S1). The existence of patterns in the residuals due to the assumption of independence was verified with a plot of the residuals against each of the explanatory variables. Finally, the effect of each significant variable in the GLM was displayed with R package ‘effects’106.
Bd spatial cluster analysis
Each sampled site was categorized as Bd-positive or Bd-negative according to the results of the Bd qPCR assays: a site was considered positive if at least one individual swab sample tested positive. Visualization of the sample sites was carried out using QuantumGIS and projected for analysis using the WGS 1984 datum as a coordinate system. Spatial distribution was characterized by the Moran’s I spatial autocorrelation107, to identify spatial autocorrelation globally. We used Kulldorf´s clustering algorithm108 under Bernoulli probability model, using the software SatScan v.9.4.4109 to identify any cluster of Bd-positive samples across space with the proportion of infection at a given sample site. The model was run using Bd locations under the null hypothesis that cases were randomly distributed in space. The model was set to scan for areas with high Bd-positives numbers to test for clusters with a spatial occurrence higher than that outside the cluster. Briefly, the number of observed and expected Bd-infected amphibians is counted by a scanning window that moves across space for each location and variable window sizes108. Scan statistics allows the detection of the most “unusual” excess of observed Bd-positives and therefore provides georeferenced high-risk areas of Bd infection. Distributions of the likelihood ratio and its corresponding P-value were obtained using Monte Carlo simulation by generating 999 replications of the data set under the null hypothesis. The test statistics were computed for each replication and the test was deemed significant at P < 0.05.
Supplementary Information
Acknowledgements
We thank B. Molina-Burgos, M. Mora, R. Monsalve and S. Lebuy for their important fieldwork support. This work has been supported by Fondecyt Projects No. 1181758 and 1211587 (to C.A.), 3180107 (to A.V.S.) and 1150029 (to L.D.B.). This study was carried out as part fulfilment of the Conservation Medicine Ph.D. degree (by M.A.R.) at the Faculty of Life Sciences, Universidad Andres Bello, Chile.
Author contributions
M.A.R. and C.A. led the project, designed the work, collected data and performed the systematic review. M.A.R. and M.L. perform spatiotemporal analysis, modelling, visualization and plotting results were performed and described. M.A.R, C.A., A.V.S., C.V., A.P.R, made qPCR analyses. M.A.R. and C.A. wrote the paper and A.P.R, A.V.S., C.V., F.O.M, L.D.B., R.P. and A.A.C helped to improve the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-96535-w.
References
- 1.Collins, J. P., & Crump, M. L. Extinction in Our Times: Global Amphibian Decline. (2009).
- 2.Catenazzi A. State of the world's amphibians. Annu. Rev. Environ. Resour. 2015;40:91–119. doi: 10.1146/annurev-environ-102014-021358. [DOI] [Google Scholar]
- 3.González-del-Pliego P, et al. Phylogenetic and trait-based prediction of extinction risk for data deficient amphibians. Curr. Biol. 2019;29:1557–1563. doi: 10.1016/j.cub.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. PNAS. 2006;102:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:e72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. PNAS. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. doi: 10.1080/00275514.1999.12061011. [DOI] [Google Scholar]
- 8.Berger L, et al. History and recent progress on chytridiomycosis in amphibians. Fungal Ecol. 2016;19:89–99. doi: 10.1016/j.funeco.2015.09.007. [DOI] [Google Scholar]
- 9.Martel A, et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. PNAS. 2013;110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheele BC, et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science. 2019;363:1459–1463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 11.Lambert MR, et al. Comment on “Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity”. Science. 2020 doi: 10.1126/science.aay1838. [DOI] [PubMed] [Google Scholar]
- 12.Scheele BC, et al. Response to Comment on “Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity”. Science. 2020 doi: 10.1126/science.aay2905. [DOI] [PubMed] [Google Scholar]
- 13.Puschendorf R, et al. Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: Proposing climatic refuges as a conservation tool. Divers. Distrib. 2009;15:401–408. doi: 10.1111/j.1472-4642.2008.00548.x. [DOI] [Google Scholar]
- 14.Zumbado-Ulate H, et al. Endemic infection of Batrachochytrium dendrobatidis in Costa Rica: Implications for amphibian conservation at regional and species level. Diversity. 2019;11:129. doi: 10.3390/d11080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crawford AJ, Lips KR, Bermingham E. Epidemic disease decimates amphibian abundance, species diversity, and evolutionary history in the highlands of central Panama. PNAS. 2010;107:13777–13782. doi: 10.1073/pnas.0914115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodhams DC, et al. Chytridiomycosis and amphibian population declines continue to spread eastward in Panama. EcoHealth. 2008;5:268–274. doi: 10.1007/s10393-008-0190-0. [DOI] [PubMed] [Google Scholar]
- 17.Catenazzi A, Lehr E, Rodriguez L, Vredenburg V. Batrachochytrium dendrobatidis and the collapse of anuran species richness and abundance in the upper Manu National Park, southeastern Peru. Conserv. Biol. 2011;25:382–391. doi: 10.1111/j.1523-1739.2011.01667.x. [DOI] [PubMed] [Google Scholar]
- 18.Voyles J, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 19.James T, et al. Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: Lessons from the first 15 years of amphibian chytridiomycosis research. Ecol. Evol. 2015;5:4079–4097. doi: 10.1002/ece3.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto-Azat C, et al. Xenopus laevis and emerging amphibian pathogens in Chile. EcoHealth. 2016;13:775–783. doi: 10.1007/s10393-016-1186-9. [DOI] [PubMed] [Google Scholar]
- 21.Ron S. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the new world. Biotropica. 2005;37:209–221. doi: 10.1111/j.1744-7429.2005.00028.x. [DOI] [Google Scholar]
- 22.Rödder D, Kielgast J, Lötters S. Future potential distribution of the emerging amphibian chytrid fungus under anthropogenic climate change. Dis. Aquat. Org. 2010;92:201–207. doi: 10.3354/dao02197. [DOI] [PubMed] [Google Scholar]
- 23.Murray KA, et al. Assessing spatial patterns of disease risk to biodiversity: Implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. J. Appl. Ecol. 2011;48:163–173. doi: 10.1111/j.1365-2664.2010.01890.x. [DOI] [Google Scholar]
- 24.Liu X, Rohr J, Li Y. Climate, vegetation, introduced hosts and trade shape a global wildlife pandemic. Proc. Biol. Sci. 2013;280:20122506. doi: 10.1098/rspb.2012.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson DH, et al. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS ONE. 2013;8:e56802. doi: 10.1371/journal.pone.0056802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penner J, et al. West Africa—A safe haven for frogs? A sub-continental assessment of the chytrid fungus (Batrachochytrium dendrobatidis) PLoS ONE. 2013;8:e56236. doi: 10.1371/journal.pone.0056236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie GY, Olson DH, Blaustein AR. Projecting the global distribution of the emerging amphibian fungal pathogen, Batrachochytrium dendrobatidis, based on IPCC climate futures. PLoS ONE. 2016;11:e0160746. doi: 10.1371/journal.pone.0160746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RN, et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009;3:818–824. doi: 10.1038/ismej.2009.27. [DOI] [PubMed] [Google Scholar]
- 29.Searle CL, et al. Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv. Biol. 2011;25:965–974. doi: 10.1111/j.1523-1739.2011.01708.x. [DOI] [PubMed] [Google Scholar]
- 30.Farrer R, et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. PNAS. 2011;108:18732–18736. doi: 10.1073/pnas.1111915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lips KR. Overview of chytrid emergence and impacts on amphibians. Philos. Trans. R. Soc. B. 2016;371:20150465. doi: 10.1098/rstb.2015.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolom-Huet R, Pineda E, Díaz-Fleischer F, Muñoz-Alonso AL, Galindo-González J. Known and estimated distribution in Mexico of Batrachochytrium dendrobatidis, a pathogenic fungus of amphibians. Biotropica. 2019;51:731–746. doi: 10.1111/btp.12697. [DOI] [Google Scholar]
- 33.Zumbado-Ulate H, García-Rodríguez A, Searle CL. Species distribution models predict the geographic expansion of an enzootic amphibian pathogen. Biotropica. 2021;53:221–231. doi: 10.1111/btp.12863. [DOI] [Google Scholar]
- 34.Berger L, et al. Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust. Vet. J. 2004;82:434–439. doi: 10.1111/j.1751-0813.2004.tb11137.x. [DOI] [PubMed] [Google Scholar]
- 35.Clare FC, et al. Climate forcing of an emerging pathogenic fungus across a montane multi-host community. Philos. Trans. R. Soc. B. 2016;371:20150454. doi: 10.1098/rstb.2015.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacigalupe LD, Soto-Azat C, García-Vera C, Barría-Oyarzo I, Rezende EL. Effects of amphibian phylogeny, climate and human impact on the occurrence of the amphibian-killing chytrid fungus. Glob. Change Biol. 2017;23:3543–3553. doi: 10.1111/gcb.13610. [DOI] [PubMed] [Google Scholar]
- 37.Raffel T, Michel P, Sites W, Rohr J. What drives chytrid infections in newt populations? Associations with substrate, temperature, and shade. EcoHealth. 2010;7:526–536. doi: 10.1007/s10393-010-0358-2. [DOI] [PubMed] [Google Scholar]
- 38.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 39.Hudson M, et al. Reservoir frogs: Seasonality of Batrachochytrium dendrobatidis infection in robber frogs. PeerJ. 2019;7:e7021. doi: 10.7717/peerj.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kriger KM, Hero JM. Large-scale seasonal variation in the prevalence and severity of chytridiomycosis. J. Zool. 2007;271:352–359. [Google Scholar]
- 41.Longo AV, Burrowes PA, Joglar RL. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis. Aquat. Org. 2010;92:253–260. doi: 10.3354/dao02054. [DOI] [PubMed] [Google Scholar]
- 42.Zumbado-Ulate H, Bolaños F, Gutiérrez-Espeleta G, Puschendorf R. Extremely low prevalence of Batrachochytrium dendrobatidis in frog populations from Neotropical dry forest of Costa Rica supports the existence of a climatic refuge from disease. EcoHealth. 2014;11:593–602. doi: 10.1007/s10393-014-0967-2. [DOI] [PubMed] [Google Scholar]
- 43.Bacigalupe LD, et al. The amphibian-killing fungus in a biodiversity hotspot: Identifying and validating high-risk areas and refugia. Ecosphere. 2019;10:e02724. doi: 10.1002/ecs2.2724. [DOI] [Google Scholar]
- 44.Flechas SV, et al. Current and predicted distribution of the pathogenic fungus Batrachochytrium dendrobatidis in Colombia, a hotspot of amphibian biodiversity. Biotropica. 2017;49:685–694. doi: 10.1111/btp.12457. [DOI] [Google Scholar]
- 45.Lampo M, et al. Batrachochytrium dendrobatidis in Venezuela. Herpetol. Rev. 2008;39:449. [Google Scholar]
- 46.Valenzuela-Sánchez A, et al. Genomic epidemiology of the emerging pathogen Batrachochytrium dendrobatidis from native and invasive amphibian species in Chile. Transbound. Emerg. Dis. 2018;65:309–314. doi: 10.1111/tbed.12775. [DOI] [PubMed] [Google Scholar]
- 47.O’Hanlon S, et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science. 2018;360:621–627. doi: 10.1126/science.aar1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soto-Azat C, et al. The population decline and extinction of Darwin’s frogs. PLoS ONE. 2013;8:e66957. doi: 10.1371/journal.pone.0066957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soto-Azat C, et al. ASG Chile leads update of the extinction risk of Chilean amphibians for the IUCN red list of threatened speciesTM. FrogLog. 2015;23:6–7. [Google Scholar]
- 50.Mora M, et al. High abundance of invasive African clawed frog Xenopus laevis in Chile: Challenges for their control and updated invasive distribution. Manag. Biol. Invasions. 2019;10:377–388. doi: 10.3391/mbi.2019.10.2.11. [DOI] [Google Scholar]
- 51.Solís R, Penna M, De la Riva I, Fisher M, Bosch J. Presence of Batrachochytrium dendrobatdis in anurans from the Andes highlands of northern Chile. Herpetol. J. 2015;24:55–59. [Google Scholar]
- 52.Soto-Azat C, et al. Is Chytridiomycosis driving Darwin’s frogs to extinction? PLoS ONE. 2013;8:e79862. doi: 10.1371/journal.pone.0079862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valenzuela-Sánchez A, et al. Cryptic disease-induced mortality may cause host extinction in an apparently stable host–parasite system. Proc. Biol. Sci. 2017;284:20171176. doi: 10.1098/rspb.2017.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lips KR, Reeve JD, Witters LR. Ecological traits predicting amphibian population declines in Central America. Conserv. Biol. 2003;17:1078–1088. doi: 10.1046/j.1523-1739.2003.01623.x. [DOI] [Google Scholar]
- 55.Hero JM, Williams SE, Magnusson WE. Ecological traits of declining amphibians in upland areas of eastern Australia. J. Zool. 2005;267(3):221–232. doi: 10.1017/S0952836905007296. [DOI] [Google Scholar]
- 56.Kriger KM, Hero JM. Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Austral. Ecol. 2008;33(8):1022–1032. doi: 10.1111/j.1442-9993.2008.01872.x. [DOI] [Google Scholar]
- 57.Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125. doi: 10.1007/s10393-007-0093-5. [DOI] [Google Scholar]
- 58.Langwig K, et al. Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 2015;13:195–202. doi: 10.1890/140241. [DOI] [Google Scholar]
- 59.Shaw SD, et al. The distribution and host range of Batrachochytrium dendrobatidis in New Zealand, 1930–2010. Ecology. 2013;94:2108–2111. doi: 10.1890/12-1994.1. [DOI] [Google Scholar]
- 60.Ghirardi R, et al. Endangered amphibians infected with the chytrid fungus Batrachochytrium dendrobatidis in austral temperate wetlands from Argentina. Herpetol. J. 2014;24:129–133. [Google Scholar]
- 61.Bielby J, Cooper N, Cunningham A, Garner T, Purvis A. Predicting susceptibility to future declines in the world’s frogs. Conserv. Lett. 2008;1:82–90. doi: 10.1111/j.1755-263X.2008.00015.x. [DOI] [Google Scholar]
- 62.Barrionuevo S, Mangione S. Chytridiomycosis in two species of Telmatobius (Anura: Leptodactylidae) from Argentina. Dis. Aquat. Org. 2006;73:171–174. doi: 10.3354/dao073171. [DOI] [PubMed] [Google Scholar]
- 63.Seimon TA, et al. Upward range extension of Andean anurans and chytridiomycosis to extreme elevations in response to tropical deglaciation. Glob. Change Biol. 2007;13:288–299. doi: 10.1111/j.1365-2486.2006.01278.x. [DOI] [Google Scholar]
- 64.Burrowes PA, De la Riva I. Unraveling the historical prevalence of the invasive chytrid fungus in the Bolivian Andes: Implications in recent amphibian declines. Biol. Invasions. 2017;19:1781–1794. doi: 10.1007/s10530-017-1390-8. [DOI] [Google Scholar]
- 65.Vredenburg VT, Knapp R, Tunstall T, Briggs C. Dynamics of an emerging disease drive large-scale amphibian population extinctions. PNAS. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Azat C. A flagship for Austral temperate forest conservation: an action plan for Darwin’s frogs bringing together key stakeholders. Oryx. 2021;55:356–363. doi: 10.1017/S0030605319001236. [DOI] [Google Scholar]
- 67.Pilliod DS, et al. Effects of amphibian chytrid fungus on individual survival probability in wild boreal toads. Conserv. Biol. 2010;24:1259–1267. doi: 10.1111/j.1523-1739.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- 68.Walker SF, et al. Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol. Lett. 2010;13:372–382. doi: 10.1111/j.1461-0248.2009.01434.x. [DOI] [PubMed] [Google Scholar]
- 69.Kriger KM, Pereoglou F, Hero JM. Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conserv. Biol. 2007;21:1280–1290. doi: 10.1111/j.1523-1739.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 70.Petersen CE, Lovich RE, Phillips CA, Dreslik MJ, Lannoo MJ. Prevalence and seasonality of the amphibian chytrid fungus Batrachochytrium dendrobatidis along widely separated longitudes across the United States. EcoHealth. 2016;13:368–382. doi: 10.1007/s10393-016-1101-4. [DOI] [PubMed] [Google Scholar]
- 71.Thorpe CJ, et al. Climate structuring of Batrachochytrium dendrobatidis infection in the threatened amphibians of the northern Western Ghats, India. R. Soc. Open Sci. 2018;5:180211. doi: 10.1098/rsos.180211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonn JM, Utz RM, Richards-Zawacki CL. Effects of latitudinal, seasonal, and daily temperature variations on chytrid fungal infections in a North American frog. Ecosphere. 2019;10:e02892. doi: 10.1002/ecs2.2892. [DOI] [Google Scholar]
- 73.Raffel TR, Halstead NT, McMahon TA, Davis AK, Rohr JR. Temperature variability and moisture synergistically interact to exacerbate an epizootic disease. Proc. Biol. Sci. 2015;282:20142039. doi: 10.1098/rspb.2014.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodhams DC, Alford RA. Ecology of chytridiomycosis in rainforest stream frog assemblages of tropical Queensland. Conserv. Biol. 2005;19:1449–1459. doi: 10.1111/j.1523-1739.2005.004403.x. [DOI] [Google Scholar]
- 75.Adams MJ, et al. Using occupancy models to understand the distribution of an amphibian pathogen, Batrachochytrium dendrobatidis. Ecol. Appl. 2010;20:289–302. doi: 10.1890/08-2319.1. [DOI] [PubMed] [Google Scholar]
- 76.Fisher M, Garner T, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 2009;63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- 77.Schloegel LM, et al. Novel, panzootic and hybrid genotypes of amphibia chytridiomycosis associated with the bullfrog trade. Mol. Ecol. 2012;21:5162–5177. doi: 10.1111/j.1365-294X.2012.05710.x. [DOI] [PubMed] [Google Scholar]
- 78.Wilson EA, Briggs CJ, Dudley TL. Invasive African clawed frogs in California: A reservoir for or predator against the chytrid fungus? PLoS ONE. 2018;13:e0191537. doi: 10.1371/journal.pone.0191537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Becker CG, Longo AV, Haddad CF, Zamudio KR. Land cover and forest connectivity alter the interactions among host, pathogen and skin microbiome. Proc. Biol. Sci. 2017;284:20170582. doi: 10.1098/rspb.2017.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCoy KA, Peralta AL. Pesticides could alter amphibian skin microbiomes and the effects of Batrachochytrium dendrobatidis. Front. Microbiol. 2018;9:748. doi: 10.3389/fmicb.2018.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellis E, Ramankutty N. Putting people in the map: Anthropogenic biomes of the world. Front. Ecol. Environ. 2008;6:439–447. doi: 10.1890/070062. [DOI] [Google Scholar]
- 82.Rohr J, Halstead N, Raffel T. Modelling the future distribution of the amphibian chytrid fungus: The influence of climate and human-associated factors. J. Appl. Ecol. 2011;48:174–176. doi: 10.1111/j.1365-2664.2010.01891.x. [DOI] [Google Scholar]
- 83.Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 84.Echeverria C, Coomes D, Hall M, Newton A. Spatially explicit models to analyze forest loss and fragmentation between 1976 and 2020 in southern Chile. Ecol. Model. 2008;212:439–449. doi: 10.1016/j.ecolmodel.2007.10.045. [DOI] [Google Scholar]
- 85.Rodriguez D, Becker C, Pupin C, Haddad F, Zamudio K. Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol. Ecol. 2014;23:774–787. doi: 10.1111/mec.12615. [DOI] [PubMed] [Google Scholar]
- 86.Puschendorf R, Hodgson L, Alfors RA, Skerrat LF, VanDerWal J. Underestimated ranges and overlooked refuges from amphibian chytridiomycosis. Divers. Distrib. 2013;19:1313–1321. doi: 10.1111/ddi.12091. [DOI] [Google Scholar]
- 87.Scheele BC, et al. After the epidemic: Ongoing declines, stabilizations and recoveries in amphibians afflicted by chytridiomycosis. Biol. Conserv. 2017;206:37–46. doi: 10.1016/j.biocon.2016.12.010. [DOI] [Google Scholar]
- 88.Mendelson JR, III, Whitfield SM, Sredl MJ. A recovery engine strategy for amphibian conservation in the context of disease. Biol. Conserv. 2019;236:188–191. doi: 10.1016/j.biocon.2019.05.025. [DOI] [Google Scholar]
- 89.Van Rooij P, Martel A, Haesebrouck F, Pasmans F. Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Vet. Res. 2015;46:1–22. doi: 10.1186/s13567-014-0124-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christie MR, Searle CL. Evolutionary rescue in a host–pathogen system results in coexistence not clearance. Evol. Appl. 2018;11:681–693. doi: 10.1111/eva.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bletz MC, et al. Mitigating amphibian chytridiomycosis with bioaugmentation: Characteristics of effective probiotics and strategies for their selection and use. Ecol. Lett. 2013;16:807–820. doi: 10.1111/ele.12099. [DOI] [PubMed] [Google Scholar]
- 92.Bosch J, et al. Successful elimination of a lethal wildlife infectious disease in nature. Biol. Lett. 2015;11:20150874. doi: 10.1098/rsbl.2015.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olson DM, et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience. 2001;51:933–938. doi: 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2. [DOI] [Google Scholar]
- 94.Pellet J, Schmidt BR. Monitoring distributions using call surveys: Estimating site occupancy, detection probabilities and inferring absence. Biol. Conserv. 2005;123:27–35. doi: 10.1016/j.biocon.2004.10.005. [DOI] [Google Scholar]
- 95.Drechsler A, Bock D. Ortmann’s funnel trap—A highly efficient tool for monitoring amphibian species. Herpetol. Notes. 2010;3:13–21. [Google Scholar]
- 96.Hudson M, et al. Dynamics and genetics of a disease-driven species decline to near extinction: Lessons for conservation. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep30772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.R Development Core Team. R: A Language and Environment for Statistical Computing, https://www.R-project.org/ (2019).
- 98.Sanderson EW, et al. The human footprint and the last of the wild. Bioscience. 2002;52:891–904. doi: 10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2. [DOI] [Google Scholar]
- 99.Center for International Earth Science Information Network (CIESIN). Gridded Population of the World, Version 3 (GPWv3). 10.7927/H4639MPP (2005).
- 100.Booth TH, Nix HA, Busby JR, Hutchinson MF. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014;20:1–9. doi: 10.1111/ddi.12144. [DOI] [Google Scholar]
- 101.Center for International Earth Science Information Network (CIESIN). Gridded Species Distribution: Global Amphibian Richness Grids. 10.7927/H4RR1W66 (2015).
- 102.Fick S, Hijmans R. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017;37:4302–4315. doi: 10.1002/joc.5086. [DOI] [Google Scholar]
- 103.ASTER. ASTER global digital elevation model V003. 10.5067/ASTER/ASTGTM (2018).
- 104.QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org (2018).
- 105.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 106.Fox J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003;8:1–27. doi: 10.18637/jss.v008.i15. [DOI] [Google Scholar]
- 107.Carpenter TE. Methods to investigate spatial and temporal clustering in veterinary epidemiology. Prev. Vet. Med. 2001;48:303–320. doi: 10.1016/S0167-5877(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 108.Kulldorff M. A spatial scan statistic. Commun. Stat-Theor. M. 1997;26:1481–1496. doi: 10.1080/03610929708831995. [DOI] [Google Scholar]
- 109.Kulldorff, M. Information Management Services, Inc. SaTScanTM v.9.4.4: software for the spatial and space-time scan statistics. http://www.satscan.org (2009).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.