Abstract
Down syndrome (DS) is caused by the trisomy of chromosome 21. Among the many disabilities found in individuals with DS is an increased risk of early-onset Alzheimer's disease (AD). Although higher oxidative stress and an upregulation of amyloid β (Aβ) peptides from an extra copy of the APP gene are attributed to the AD susceptibility, the relationship between the two factors is unclear. To address this issue, we established an in vitro cellular model using neurons differentiated from DS patient-derived induced pluripotent stem cells (iPSCs) and isogenic euploid iPSCs. Neurons differentiated from DS patient-derived iPSCs secreted more Aβ compared to those differentiated from the euploid iPSCs. Treatment of the neurons with an antioxidant, N-acetylcysteine, significantly suppressed the Aβ secretion. These findings suggest that oxidative stress has an important role in controlling the Aβ level in neurons differentiated from DS patient-derived iPSCs and that N-acetylcysteine can be a potential therapeutic option to ameliorate the Aβ secretion.
Subject terms: Dementia, Induced pluripotent stem cells, Developmental disorders
Introduction
Down syndrome (DS) is the chromosome abnormality defined by an extra copy of chromosome 21. DS develops various complications such as neurological, skeletal, cardiovascular, and immunological defects1, but is perhaps best known for being the most common genetic cause of mental retardation and intellectual disability2, occurring at a rate of 1 in 800 to 1000 births3,4. Chromosome 21 contains genes related to neurodegenerative diseases and oxidative stress5, and research on the involvement of these genes on the pathophysiology of DS is underway6.
Consistent with the neurological complications and intellectual disabilities is that individuals with DS have a higher susceptibility to early onset Alzheimer's disease (AD)7,8. One of the hypothesized reasons is the existence of an extra copy of amyloid precursor protein (APP) gene located on chromosome 21. APP is a precursor of amyloid-beta (Aβ), and the extra copy increases the expression of APP and subsequent production of Aβ9,10. Since AD-like pathological lesions such as Aβ deposition can be recognized in individuals with DS younger than 40 years old and symptoms of cognitive impairment due to AD exponentially increases from this age, DS has been regarded as a “young model” of AD1,6,11. Connecting AD and DS is Aβ42, which plays an important role in the pathogenesis of AD and may also affect the cognitive function seen in DS12,13.
It has been suggested that the brains of individuals with DS are exposed to oxidative stress14–16. Oxidative stress plays a central role in neurogenic changes in DS4,17. In animal studies, the administration of antioxidants from the fetal period increases the number of cells in the hippocampus18, indicating that early intervention to circumvent the effect of oxidative stress may be important to improve the neurological prognosis. Oxidative stress in DS is attributed to several genes on chromosome 21, including an extra copy of superoxide dismutase 1 (SOD1)19. Oxidative stress is enhanced because of the accumulation of H2O2. The overexpression of SOD1 produces a large amount of H2O2 that cannot be catalyzed because the copy number of catalase is normal19. However, in experiments using DS model mice, oxidative stress was increased even when only two copies of SOD1 existed20. Therefore, although oxidative stress is caused by the overexpression of SOD1, other factors are also involved.
The relationship between oxidative stress and Aβ is complicated. Oxidative stress may be involved in Aβ production21, and Aβ42 oligomers are known to produce reactive oxygen species (ROS) and have neurotoxicity22–24. Accurate evaluation of the regulatory role of oxidative stress on the production of Aβ production is therefore important for establishing an appropriate therapeutic strategy for DS-related neuronal degeneration.
In this study, we differentiated induced pluripotent stem cells (iPSCs) derived from individuals with DS (D-iPSCs) into neurons (D-iNs) and measured their Aβ production. Secreted Aβ was increased in D-iNs, and the amount of Aβ was reduced by a high-dose administration of the antioxidant N-acetylcysteine (NAC). Similar results were obtained from a human embryonic stem cell (ESC) line with trisomy 21. Although many animal models of DS have been constructed, no model animal can completely trace the symptoms occurring in humans. Our study succeeded in establishing an in vitro human model to evaluate the effect of oxidative stress on neurons with trisomy 21.
Results
Conversion of D-iPSCs into neurons
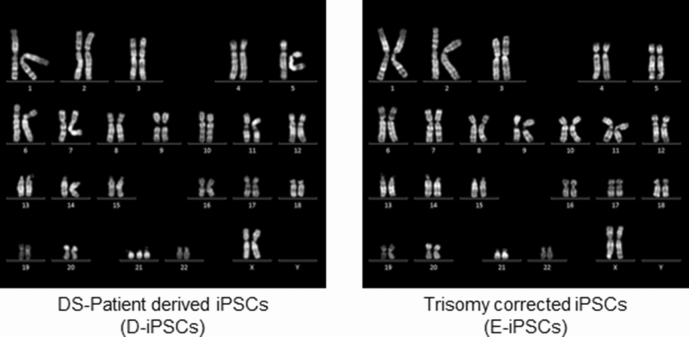
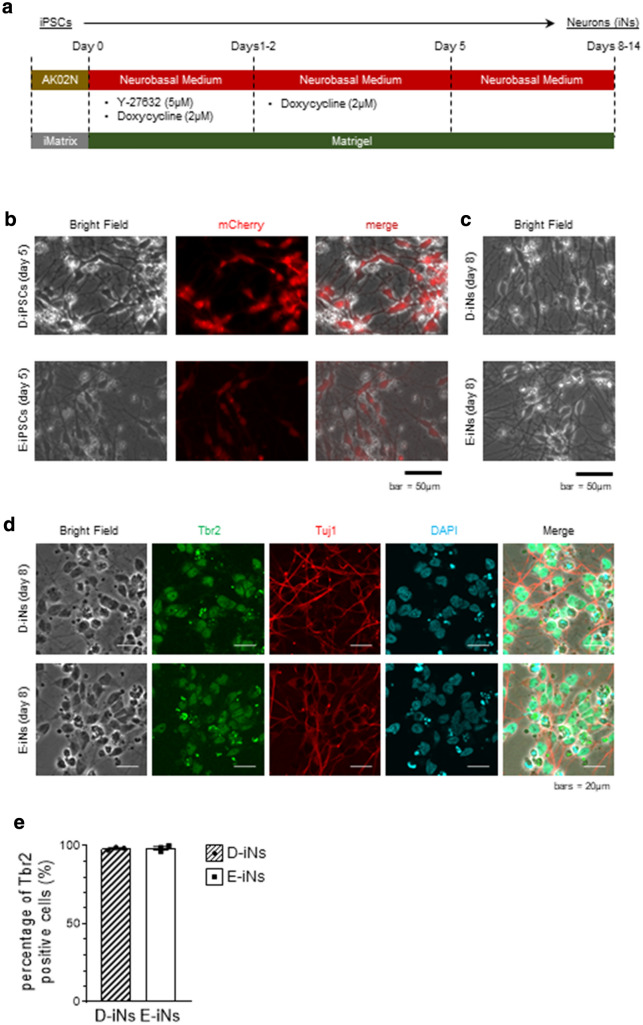
To precisely evaluate the phenotype of iNs, we used D-iPSCs and an isogenic euploid control clone established from the D-iPSCs (E-iPSCs)25 (Fig. 1). These iPSCs were then directly converted into neuronal lineage cells by the transient overexpression of Neurogenin 2 (NGN2) gene26,27 (Fig. 2a). For this, we incorporated a doxycycline-inducible expression vector encoding NGN2 and mCherry fluorescent protein into the iPSC clones and treated the clones with doxycycline for 5 days (Fig. 2a). At day 5, almost all cells were positive for mCherry (Fig. 2b) and showed a compatible morphological appearance with neurons, such as elongated neurites (Fig. 2b,c). At day 8, almost all cells expressed an intermediate neuronal progenitor cell, marker Tbr2, and were positive for a neuron-specific human tubulin protein, Tuj1 (Fig. 2d,e). These observations confirmed the neuronal differentiation of D-iPSCs and E-iPSCs.
Figure 1.
Karyotype analysis of the iPSC clones.
Figure 2.
Neuronal differentiation with over-expression of NGN2. (a) Schematic of the neuronal differentiation using the doxycycline-inducible NGN2 expression system. (b) mCherry expression at day 5. (c) Bright-field images of neuronal cells on day 8. (d) Immunostaining images of neuronal cells on day 8. (e) Quantification of the Tbr2-positive cells in (d). Data are means ± SEM from three independent experiments.
D-iNs secrete a larger amount of Aβ
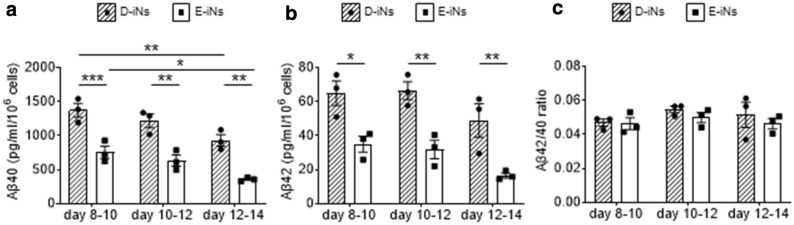
We next evaluated the secretion of Aβ peptides Aβ40 and Aβ42 from iNs. β-secretase-1 (beta-site APP cleaving enzyme; BACE1) and γ-secretase cleave APP to produce Aβ peptides, and Aβ peptides exert neurotoxicity through various proposed mechanisms28–31. In particular, Aβ42 plays an essential and important role in all AD. Aβ42 is highly hydrophobic and has high aggregation properties. Additionally, a high Aβ42/Aβ40 ratio is associated with the formation of amyloid plaques found in familial AD patients12,32. We therefore evaluated the amount of Aβ secretion from D-iNs and E-iNs, finding the amount of secreted Aβ40 and Aβ42 was higher in D-iNs at all times observed (Fig. 3a,b). However, the Aβ42/Aβ40 ratio was not different between the two iN types (Fig. 3c). These data indicated that D-iNs are predisposed to secrete more Aβ protein.
Figure 3.
Aβ secretion from iNs at different periods. (a,b) Amount of secreted Aβ40 (a) and Aβ42 (b) from iNs at the indicated periods. (c) The Aβ40/Aβ42 ratio at each period. ELISA results obtained from technical duplicates and biological triplicates are shown. Data are means ± SEM; ***, p < 0.001; **, p < 0.01; *, p < 0.05; two-way ANOVA test followed by Bonferroni’s multiple comparison test (a,b). Two-way ANOVA found no significance (interaction p value = 0.8188) in (c).
Oxidative stress affects the amount of secreted Aβ from D-iNs
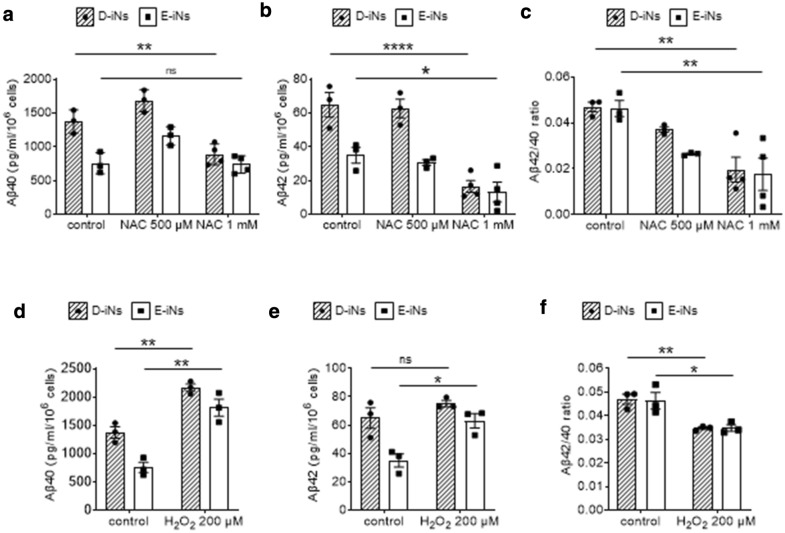
The brains of individuals with DS are exposed to high oxidative stress. The deposition of Aβ increases oxidative stress33–35, and the existence of an extra copy of superoxide dismutase 1 (SOD1) causes the overproduction of H2O2. Consistently, antioxidants and catalase show neuroprotective effects in individuals with DS and model mice14,18. Therefore, we investigated the effect of an antioxidant and an oxidant on the amount of Aβ secretion from iNs. NAC treatment decreased Aβ secretion at the highest dose (Fig. 4a,b). On the contrary, H2O2 treatment significantly increased the secretion of Aβ protein from both D-iNs and E-iNs (Fig. 4d,e). Interestingly, both NAC and H2O2 treatment decreased the Aβ42/Aβ40 ratio (Fig. 4c,f). These findings show oxidative stress positively correlates with the secretion of Aβ protein from iNs.
Figure 4.
Effects of an oxidant or anti-oxidant on Aβ secretion from iNs. (a–c) Amount of secreted Aβ40 (a) and Aβ42 (b) from iNs and the Aβ40/Aβ42 ratio (c) at day 10. NAC at the indicated concentrations was added on day 8. (d–f) Amount of secreted Aβ40 (d) and Aβ42 (e) from iNs and the Aβ40/Aβ42 ratio (f) at day 10. H2O2 (200 μM) was added on day 8. ELISA results obtained from technical duplicates and biological triplicates are shown. Data are means ± SEM; ***, p < 0.001; **, p < 0.01; *, p < 0.05; two-way ANOVA test followed by Bonferroni’s multiple comparison test (a–c) or Student’s t-test (d–f). Control data in (a,d), (b,e), and (c,f) are the same as the corresponding data for days 8–10 in Fig. 3a–c, respectively.
Effect of oxidative stress on the expression of APP
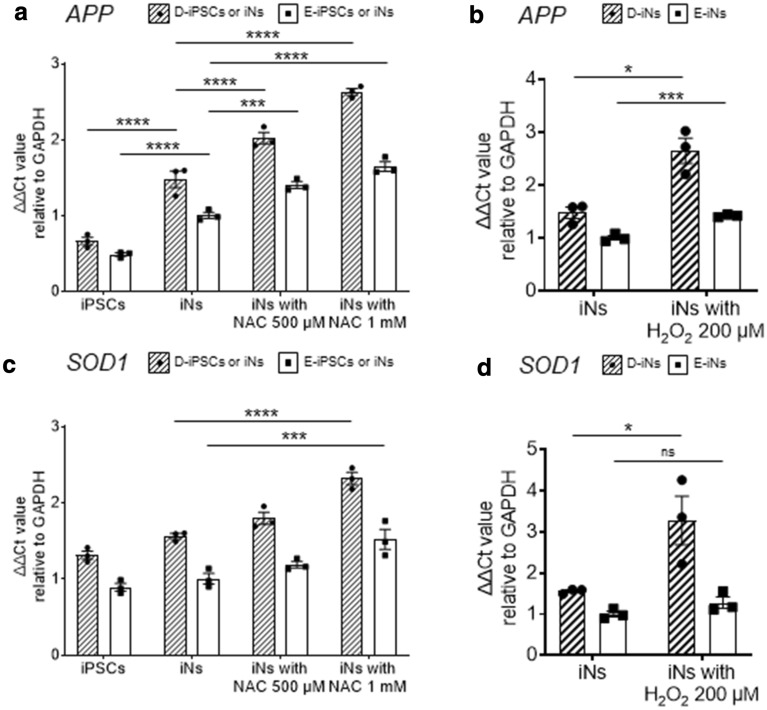
We next examined whether oxidative stress affects the expression of APP in iNs. APP was upregulated during the neuronal differentiation of D-iPSCs, as expected (Fig. 5a). H2O2 treatment upregulated the expression of APP gene both in D-iNs and E-iNs, but the effect was more prominent in D-iNs (Fig. 5b). NAC treatment also upregulated APP expression (Fig. 5a), indicating that the NAC effect is related to posttranscriptional modifications. The expression of SOD1 was upregulated by treatment with H2O2 or NAC (Fig. 5c,d).
Figure 5.
Effects of an oxidant or anti-oxidant on the expression of APP and SOD1. (a–d) Expression of APP (a,b) and SOD1 (c,d) evaluated by the ΔΔCt method relative to GAPDH. Data were converted into 2-ΔΔCt values and plotted. NAC or H2O2 at the indicated concentrations were added on day 8, and the samples were collected on day 10. Data are means ± SEM from three independent experiments; ****, p < 0.0001; ***, p < 0.001; **, p < 0.01; two-way ANOVA test followed by Dunnett’s multiple comparison test (a,c) or Student’s t-test (b,d). Data are normalized to the mean expression levels of control E-iNs. Data for iNs in (a) and (b) and in (c) and (d) are the same.
Verification with another isogenic PSC pair
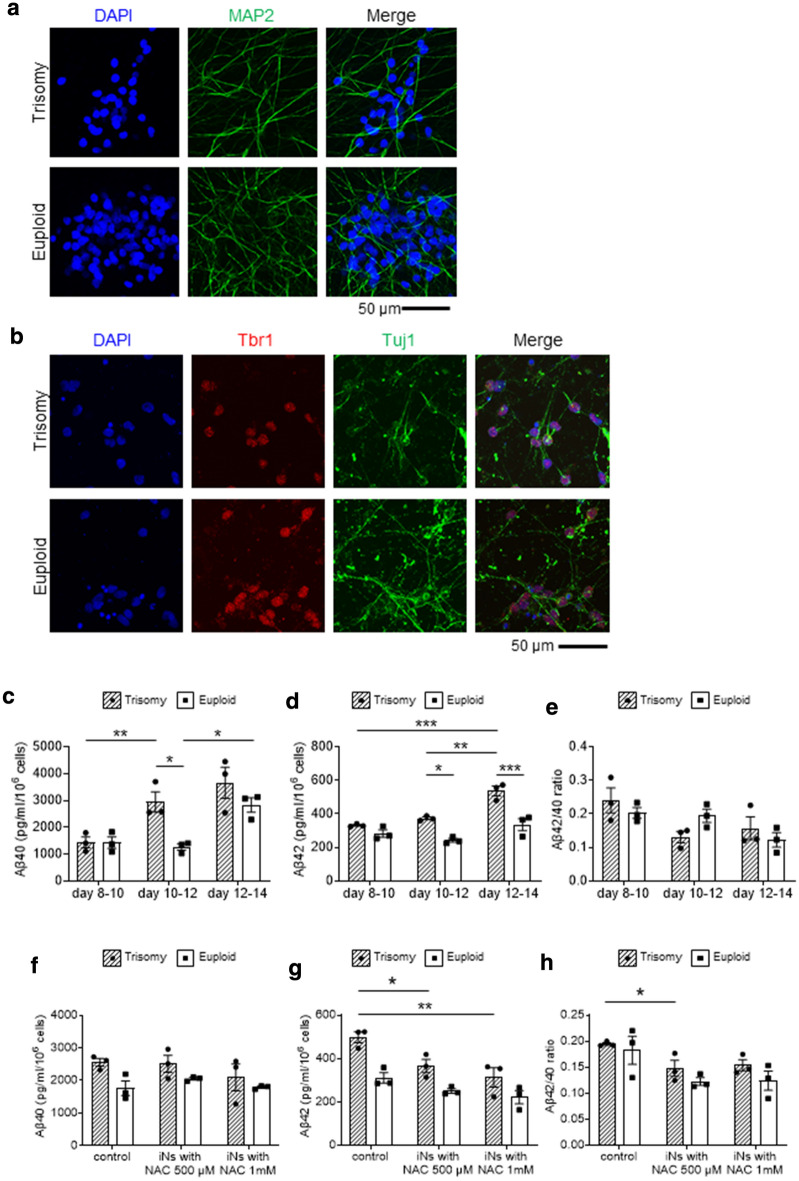
To confirm that the increased Aβ secretion and the inhibitory effect of high-dose NAC are not a clone-specific phenomenon, we tested the reproducibility of the results using another isogenic PSC pair. We used KhES1, a euploid human ESC line, and a subclone of KhES1 in which chromosome 21 was artificially inserted to make trisomy 2136. We introduced a doxycycline-inducible NGN2 expression vector into these clones and induced neuronal differentiation. Both clones showed good NGN2-dependent differentiation properties and differentiated into neurons expressing Tuj1, MAP2 and Tbr1 (Fig. 6a,b). As in the case with the DS-derived clones, Aβ secretion was increased in a time-dependent manner and higher in the trisomy clone after day 12 (Fig. 6c–e). NAC administration reduced the production of Aβ42 but had no significant effect on Aβ40 (Fig. 6f–h). These results indicate that the increased secretion of Aβ from trisomy 21 and the inhibitory effect of high-dose NAC are not clone- dependent.
Figure 6.
Verification with another isogenic PSC pair. (a,b) Immunostaining of ESC-derived neurons on day 14. (c,d) Amount of secreted Aβ40 (c) and Aβ42 (d) from ESC-derived neurons at the indicated periods. (e) The Aβ40/Aβ42 ratio at each period. (f–h) Amount of secreted Aβ40 (f) and Aβ42 (g) from iNs and the Aβ40/Aβ42 ratio (h) at day 10. NAC at the indicated concentrations was added on day 8. Data are means ± SEM from three independent experiments; ***, p < 0.001; **, p < 0.01; *, p < 0.05; two-way ANOVA test followed by Tukey’s Multiple Comparison Test. Two-way ANOVA found no significance in (e,f): interaction p values were 0.1242 (e) and 0.5453 (f).
Discussion
Here we investigated the effect of trisomy 21 on neuronal Aβ by using trisomy 21 iPSCs and their isogenic euploid control and differentiating them into neurons. Aβ was produced early after initiating the direct conversion and higher in D-iNs. In addition, the Aβ production was reduced by using an antioxidant, NAC.
The brains of individuals with DS show pathological changes similar to those of AD patients11,37. In familial AD, the proportion of Aβ42 increases from the early stage due to genetic abnormalities of PSEN1 or PSEN2. A relative increase of Aβ42 to Aβ40 has been considered a risk of synaptic dysfunction12,22,29. On the other hand, in DS, the total amount of both Aβ40 and Aβ42 increases due to the increased copy number of APP caused by extra chromosome 21. Therefore, the Aβ42/Aβ40 ratio is considered unchanged, especially in the early stage38, which is consistent with our findings.
Treatment with various antioxidants has been tried against the increased oxidative stress in DS. However, the effects were often partial or limited in animal models39–42. In the present study, we found that NAC treatment dose-dependently reduced the production of Aβ from iNs, which is consistent with previous studies on DS and AD. NAC is a precursor of glutathione peroxidase and is known as an antioxidant that can prevent the enhanced death due to oxidative stress of neurons derived from D-iPSCs17. NAC treatment was also seen to improve the cognitive memory behavior of AD model mice and rats43,44 and to suppress neuroinflammation45. Therefore, NAC itself can reduce oxidative stress in the whole brain and exert a neuronal cell protective effect. Adding our study gives further argument to NAC improving the prognosis of the cognitive function of DS.
Although the addition of NAC suppressed the secretion of Aβ, it did not down-regulate the expression of APP. This finding suggests that the anti-oxidative stress effect of NAC may affect the cleavage of APP protein post-transcriptionally. Aβ production is known to be affected by oxidative stress, and increased oxidative stress up-regulates BACE1 expression46 and PSEN1 expression in lipid rafts47,48. Since PSEN1 is an active center of γ-secretase, the activity of γ-secretase may also be increased by oxidative stress21. In addition, the β and γ secretase-dependent processing of APP is promoted by α-synuclein49. These reports and our data indicate that, in addition to an extra copy of APP, the brains of individuals with DS are exposed to an unfavorable environment where Aβ production is enhanced due to increased oxidative stress.
In anticipation of the antioxidant effect of NAC, clinical trials have been conducted on various neuropsychiatry diseases and neurodegenerative diseases50–52. In this study, we showed the effect of NAC in iNs, suggesting that the neuroprotective effect of NAC can be expanded to diseases associated with Aβ and oxidative stress. However, our study is based upon a relatively small number of control and DS cases and it will be important for this work to be replicated using larger sample sizes.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee and the recombinant DNA Experiments Safety Committee of Kyoto University. The use of human ESCs was approved by the Ministry of Education Culture, Sports, Science and Technology of Japan (MEXT). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from the legal guardians of the DS patient.
iPSC clones and introduction of the doxycycline-inducible NGN2 expression vector
We used an iPSC clone obtained from a DS patient and a trisomy-corrected isogenic clone generated from the trisomy clone as previously described25. The source fibroblasts of the iPSCs were obtained from the Coriell Institute for Medical Research (AG06892). As another isogenic pair, we used an euploid ESC line KhES1 and trisomy KhES1 subclone with chromosome 21 artificially introduced36,53. The original euploid KhES1 clone was kindly provided by Hirofumi Suemori (Institute for Frontier Medical Sciences, Kyoto University, Kyoto, Japan).
For the generation of iPSC-derived neurons, we took advantage of the doxycycline-induced NGN2 expression system26,27. We constructed a plasmid vector encoding human NGN2 cDNA under the tetracycline-inducible promoter (TetO::NGN2-IRES-mCherry). The piggyBac backbone vector, PB-TAC-ERN, was a gift from Dr. Knut Woltjen (Addgene plasmid #80475; http://n2t.net/addgene:80475; RRID: Addgene_80475)26. We selected subclones showing high differentiation ability after the antibiotic selection with G418 disulfate (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
Neuronal induction and sample taking
Neuronal induction by doxycycline-inducible NGN2 expression was performed as previously described27. Briefly, on day 0, iPSCs were dissociated with TrypLE Select (GIBCO, Thermo Fisher Scientific, MA, USA) and disseminated on a mixed coating of poly-l-lysine (final 0.0002% v/w, Merck, Darmstadt, Germany), and Matrigel (final 2% v/v, Corning, NY, USA). The disseminated iPSCs were cultured in Neurobasal Medium (GIBCO, Thermo Fisher Scientific, MA, USA) supplemented with 0.5% B27 without vitamin A (GIBCO, Thermo Fisher Scientific, MA, USA), 1 × Glutamax (GIBCO, Thermo Fisher Scientific, MA, USA), 2 mg/mL doxycycline hydrochloride (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and 5 mM Y-27632 (Nacalai-Tesque, Kyoto, Japan). Y-27632 was withdrawn on day 1 or 2. On day 5, we exchanged culture media for new media without doxycycline hydrochloride. To evaluate the amount of Aβ between timepoints, on day 8, all culture media were replaced with fresh medium. On days 10, 12 and 14, we recovered old media as samples for the ELISA analysis. To check the effect of an oxidant (H2O2) or anti-oxidant (NAC), all culture media were replaced with fresh medium containing H2O2 or NAC on day 8. The culture media were subjected to analysis on day 10.
Enzyme-linked immunosorbent assay (ELISA)
Aβ40 and Aβ42 peptides were quantified using human Aβ40 and Aβ42 commercially available ELISA kits from Immuno-Biological Laboratories (Gumma, Japan). ELISA measurements were performed according to the manufacturer's instructions. Biological triplicates were obtained from supernatants derived from separately differentiated neurons. Technical duplicates were obtained by separating supernatant samples into two wells of primary antibody-conjugated plates.
Immunofluorescent staining
Cells were washed in D-PBS and then fixed in 4% paraformaldehyde at 4 ºC for 15 min. After washing the cells twice in D-PBS, we incubated the cells for 30 min in 0.025% Triton-10 diluted with blocking reagent (Block Ace, KAC, Kyoto, Japan) at room temperature. Then, primary antibodies were applied overnight at 4 ºC after washing in D-PBS twice. The next day, cells were washed and incubated with secondary antibodies for 1 h at room temperature. Finally, the cells were counterstained with DAPI at room temperature.
The rate of Tbr2-positive cells was calculated using ImageJ software. Using the "analyze particle" function of Image J, we set DAPI positive areas as regions of interest (ROIs). For E-iNs and D-iNs, 69–80 and 69–88 ROIs were counted in each experiment, respectively. The ratio of Tbr2-positive cells to DAPI-positive cells was calculated.
Quantitative PCR
RNA samples were prepared with the RNeasy Mini Kit (Qiagen, Hilden, Germany) and subjected to reverse transcription with a PrimeScript RT Master Mix (Takara Bio, Shiga, Japan). All procedures were performed following the manufacturer's instructions. Quantitative PCR (qPCR) was performed on the StepOnePlus Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, MA, USA). For the detection of transgenes, DNA was subjected to qPCR, and SYBR Premix ExTaqII (Takara Bio, Shiga, Japan) was used for the detection. Data were processed using the ΔΔ cycle threshold method, converted 2-ΔΔCt values, and the relative quantities are shown in the figures. The primer sequences are shown below.
| SOD1 (f) | ACAAAGATGGTGTGGCCGAT |
| SOD1 (re) | AACGACTTCCAGCGTTTCCT |
| APP (f) | GACCACTCGACCAGGTTCTG |
| APP (re) | GCCCACCATGAGTCCAATGA |
| GAPDH (f) | AATCCCATCACCATCTTCCA |
| GAPDH (re) | TGGACTCCACGACGTACTCA |
Statistical analysis
GraphPad Prism8 (GraphPad Software, La Jolla, CA, USA) was used for the analysis. All results represent means ± SEM. “n” represents the number of independent cultures. Statistical analysis was performed using Student’s t-test, two-way ANOVA and post-hoc tests. A p value less than 0.05 was considered significant.
Acknowledgements
We thank Dr. Knut Woltjen (CiRA, Kyoto University, Kyoto, Japan) for providing the plasmid vector, Ms. Harumi Watanabe (CiRA, Kyoto University, Kyoto, Japan) for providing administrative assistance, and Dr. Peter Karagiannis (CiRA, Kyoto University, Kyoto, Japan) for proofreading the paper.
Author contributions
H.To. designed the study, performed almost all the experiments, and analyzed the data. A.I. performed revise experiments. Y.N-A. designed the study, performed some experiments, and analyzed the data. A.N., A.A., T.N., and H.Ta. analyzed and discussed the data. L.L. and D.W.R. constructed the trisomy and disomy iPSCs. Y.K. constructed the trisomy ESCs. M.K.S. managed the entire research. H.To. and M.K.S. wrote the manuscript. All authors read and accepted the content of the manuscript.
Funding
Funding was provided to M.K.S. by the Core Center for iPS Cell Research of Research Center Network for Realization of Regenerative Medicine [JP21bm0104001] from the Japan Agency for Medical Research and Development (AMED) and the Acceleration Program for Intractable Diseases Research utilizing Disease-specific iPS cells from AMED [17935423], and to Y.K. by JST CREST Grant Number JPMJCR18S4.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hiromitsu Toshikawa and Akihiro Ikenaka.
References
- 1.Barca D, et al. Intellectual disability and epilepsy in Down syndrome. Maedica. 2014;9:344–350. [PMC free article] [PubMed] [Google Scholar]
- 2.AgarwalGupta N, Kabra M. Diagnosis and management of Down syndrome. Indian J. Pediatr. 2014;81:560–567. doi: 10.1007/s12098-013-1249-7. [DOI] [PubMed] [Google Scholar]
- 3.Carmona-Iragui M, Videla L, Lleo A, Fortea J. Down syndrome, Alzheimer disease, and cerebral amyloid angiopathy: The complex triangle of brain amyloidosis. Dev. Neurobiol. 2019;79:716–737. doi: 10.1002/dneu.22709. [DOI] [PubMed] [Google Scholar]
- 4.Haydar TF, Reeves RH. Trisomy 21 and early brain development. Trends Neurosci. 2012;35:81–91. doi: 10.1016/j.tins.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lott IT, Head E, Doran E, Busciglio J. Beta-amyloid, oxidative stress and Down syndrome. Curr Alzheimer Res. 2006;3:521–528. doi: 10.2174/156720506779025305. [DOI] [PubMed] [Google Scholar]
- 6.Vacca RA, et al. Down syndrome: Neurobiological alterations and therapeutic targets. Neurosci. Biobehav. Rev. 2019;98:234–255. doi: 10.1016/j.neubiorev.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman FK, et al. A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat. Rev. Neurosci. 2015;16:564–574. doi: 10.1038/nrn3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartley D, et al. Down syndrome and Alzheimer's disease: Common pathways, common goals. Alzheimers Dement. 2015;11:700–709. doi: 10.1016/j.jalz.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol. 2010;9:623–633. doi: 10.1016/s1474-4422(10)70112-5. [DOI] [PubMed] [Google Scholar]
- 10.Head E, Helman AM, Powell D, Schmitt FA. Down syndrome, beta-amyloid and neuroimaging. Free Radic. Biol. Med. 2018;114:102–109. doi: 10.1016/j.freeradbiomed.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, et al. A human stem cell model of early Alzheimer's disease pathology in Down syndrome. Sci. Transl. Med. 2012;4:124ra129. doi: 10.1126/scitranslmed.3003771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citron M. Alzheimer's disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010;9:387–398. doi: 10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- 13.Hartley SL, et al. Cognitive decline and brain amyloid-beta accumulation across 3 years in adults with Down syndrome. Neurobiol. Aging. 2017;58:68–76. doi: 10.1016/j.neurobiolaging.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 15.Tramutola A, et al. Activation of p53 in Down syndrome and in the Ts65Dn mouse brain is associated with a pro-apoptotic phenotype. J. Alzheimers Dis. 2016;52:359–371. doi: 10.3233/jad-151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perluigi M, Butterfield DA. Oxidative stress and Down syndrome: A route toward Alzheimer-like dementia. Curr. Gerontol. Geriatr. Res. 2012;2012:724904. doi: 10.1155/2012/724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briggs JA, et al. Integration-free induced pluripotent stem cells model genetic and neural developmental features of Down syndrome etiology. Stem Cells. 2013;31:467–478. doi: 10.1002/stem.1297. [DOI] [PubMed] [Google Scholar]
- 18.Shichiri M, et al. Alpha-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic. Biol. Med. 2011;50:1801–1811. doi: 10.1016/j.freeradbiomed.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Barone E, Arena A, Head E, Butterfield DA, Perluigi M. Disturbance of redox homeostasis in Down syndrome: Role of iron dysmetabolism. Free Radic. Biol. Med. 2018;114:84–93. doi: 10.1016/j.freeradbiomed.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara K, et al. Increased lipid peroxidation in Down's syndrome mouse models. J. Neurochem. 2009;110:1965–1976. doi: 10.1111/j.1471-4159.2009.06294.x. [DOI] [PubMed] [Google Scholar]
- 21.Tamagno E, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J. Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellani RJ, Plascencia-Villa G, Perry G. The amyloid cascade and Alzheimer's disease therapeutics: Theory versus observation. Lab Invest. 2019;99:958–970. doi: 10.1038/s41374-019-0231-z. [DOI] [PubMed] [Google Scholar]
- 23.Sanmartin CD, Adasme T, Hidalgo C, Paula-Lima AC. The antioxidant N-acetylcysteine prevents the mitochondrial fragmentation induced by soluble amyloid-beta peptide oligomers. Neurodegener. Dis. 2012;10:34–37. doi: 10.1159/000334901. [DOI] [PubMed] [Google Scholar]
- 24.Cenini G, et al. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim. Biophys. Acta. 1822;130–138:2012. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li LB, et al. Trisomy correction in Down syndrome induced pluripotent stem cells. Cell Stem Cell. 2012;11:615–619. doi: 10.1016/j.stem.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SI, et al. Inducible transgene expression in human iPS cells using versatile all-in-one piggyBac transposons. Methods Mol. Biol. (Clifton, NJ). 2016;1357:111–131. doi: 10.1007/7651_2015_251. [DOI] [PubMed] [Google Scholar]
- 27.Kondo T, et al. iPSC-based compound screening and in vitro trials identify a synergistic anti-amyloid beta combination for Alzheimer's disease. Cell Rep. 2017;21:2304–2312. doi: 10.1016/j.celrep.2017.10.109. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed M, et al. Structural conversion of neurotoxic amyloid-beta(1–42) oligomers to fibrils. Nat. Struct. Mol. Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 30.Huynh TV, Davis AA, Ulrich JD, Holtzman DM. Apolipoprotein E and Alzheimer's disease: The influence of apolipoprotein E on amyloid-beta and other amyloidogenic proteins. J. Lipid Res. 2017;58:824–836. doi: 10.1194/jlr.R075481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE. A new era for understanding amyloid structures and disease. Nat. Rev. Mol. Cell Biol. 2018;19:755–773. doi: 10.1038/s41580-018-0060-8. [DOI] [PubMed] [Google Scholar]
- 32.Page RM, et al. Generation of Abeta38 and Abeta42 is independently and differentially affected by familial Alzheimer disease-associated presenilin mutations and gamma-secretase modulation. J. Biol. Chem. 2008;283:677–683. doi: 10.1074/jbc.M708754200. [DOI] [PubMed] [Google Scholar]
- 33.Butterfield DA, Boyd-Kimball D. Redox proteomics and amyloid beta-peptide: Insights into Alzheimer disease. J. Neurochem. 2018;151:459–487. doi: 10.1111/jnc.14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelat PB, et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 35.Mao P, Reddy PH. Aging and amyloid beta-induced oxidative DNA damage and mitochondrial dysfunction in Alzheimer's disease: Implications for early intervention and therapeutics. Biochim. Biophys. Acta. 1812;1359–1370:2011. doi: 10.1016/j.bbadis.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazuki Y, et al. Down syndrome-associated haematopoiesis abnormalities created by chromosome transfer and genome editing technologies. Sci. Rep. 2014;4:6136. doi: 10.1038/srep06136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez C, et al. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol. Psychiatry. 2018;23:2363–2374. doi: 10.1038/s41380-018-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Head E, et al. Plasma amyloid-β as a function of age, level of intellectual disability, and presence of dementia in Down syndrome. J. Alzheimers Dis. 2011;23:399–409. doi: 10.3233/jad-2010-101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sano M, et al. Vitamin E in aging persons with Down syndrome: A randomized, placebo-controlled clinical trial. Neurology. 2016;86:2071–2076. doi: 10.1212/wnl.0000000000002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shichiri M. The role of lipid peroxidation in neurological disorders. J. Clin. Biochem. Nutr. 2014;54:151–160. doi: 10.3164/jcbn.14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis JM, et al. Supplementation with antioxidants and folinic acid for children with Down's syndrome: Randomised controlled trial. BMJ. 2008;336:594–597. doi: 10.1136/bmj.39465.544028.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lott IT. Antioxidants in Down syndrome. Biochim. Biophys. Acta. 1822;657–663:2012. doi: 10.1016/j.bbadis.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A. Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer's disease in a rat model: A behavioral and electrophysiological study. Brain Res. Bull. 2017;131:142–149. doi: 10.1016/j.brainresbull.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Costa M, et al. N-acetylcysteine protects memory decline induced by streptozotocin in mice. Chem. Biol. Interact. 2016;253:10–17. doi: 10.1016/j.cbi.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes J, Gupta GL. N-acetylcysteine attenuates neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Behav. Brain Res. 2019;364:356–365. doi: 10.1016/j.bbr.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 46.Mouton-Liger F, et al. Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway. Biochim. Biophys. Acta. 1822;885–896:2012. doi: 10.1016/j.bbadis.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Cordy JM, Hooper NM, Turner AJ. The involvement of lipid rafts in Alzheimer's disease. Mol. Membr. Biol. 2006;23:111–122. doi: 10.1080/09687860500496417. [DOI] [PubMed] [Google Scholar]
- 48.Oda A, Tamaoka A, Araki W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J. Neurosci. Res. 2010;88:1137–1145. doi: 10.1002/jnr.22271. [DOI] [PubMed] [Google Scholar]
- 49.Roberts HL, Schneider BL, Brown DR. Alpha-Synuclein increases beta-amyloid secretion by promoting beta-/gamma-secretase processing of APP. PLoS ONE. 2017;12:e0171925. doi: 10.1371/journal.pone.0171925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Tardiolo G, Bramanti P, Mazzon E. Overview on the effects of N-acetylcysteine in neurodegenerative diseases. Molecules. 2018 doi: 10.3390/molecules23123305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deepmala, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015;55:294–321. doi: 10.1016/j.neubiorev.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Nishinaka-Arai Y, et al. Down syndrome-related transient abnormal myelopoiesis is attributed to a specific erythro-megakaryocytic subpopulation with GATA1 mutation. Haematologica. 2021;106:635–640. doi: 10.3324/haematol.2019.242693. [DOI] [PMC free article] [PubMed] [Google Scholar]