Abstract
The present study investigated the effect of different wine strains and inoculum size on the physicochemical, bioactive, and sensorial attributes of wine prepared from beetroot with varying TSS content (18 and 20°Brix) and inoculum sizes (5% (v/v) and 10% (v/v)). The beetroot wine produced by fermenting the must for 0–14 days and standard protocols adopted to analyze the wine properties. It was noticed that the acidity of wine increased and pH was found to be decreased as the fermentation days increased. In addition, alcohol content was significantly enhanced (> 10% by volume) in T5, which was higher than the other trials. In general, results of sensory and physicochemical analysis of different trials showed that T5 (TSS 18°Brix and inoculum size of 5% (v/v)) produced the wine of acceptable quality using Saccharomyces cerevisiae. The color properties revealed that the L* value increased as fermentation progressed. Wine prepared from T5 possessed TSS of 6.55°Brix, 3.96 pH, 0.35% titratable acidity, reducing sugar of 26.75 µg ml−1, 30.03% of DPPH free radical scavenging activity, phenol content (104.20 µg ml−1) and betalain content of 10.85 mg 100 g−1. There were significant differences in the taste, flavor, and overall acceptability of beetroot wines fermented for 14 days.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-021-05136-y.
Keywords: Beetroot, Fermentation, Wine, Yeast strains, Sensory evaluation
Introduction
Wine is an alcoholic drink made from fermented grapes or other fruits and plants. Undistilled alcoholic beverages which were fermented and aged are known as fruit wines. They are nutritious and are a moderate stimulant (Swamy et al. 2014). The fruit-based fermented product contains significant nutrients and, as an effect of amino acids and yeast nutrients released during fermentation, is more nutritious than fresh juice (Swamy et al. 2014).
Fermentation is an economical processing method, used globally and is a useful tool in household fermented traditional foods that haphazardly employs huge quantities of microbial and raw inoculants (Ashaolu 2019). It is traditionally used to obtain various products from different cereals. Fermentation of foods offers many advantages including saving energy when processing the matrix, desired biochemical changes to improve nutrition, healthy food, product shelf life, and enhanced sensory characteristics (Guyot 2012; Luana et al. 2014). Numerous substrates from vegetables, cereals, milk and fruit, and starchy root crops have been fermented more easily due to their very low costs of fermentation and LAB cultures, so that their nutritional status could improve, the toxic and non-nutritional features are reduced and food shelf life and sensory properties are improved (Simatende et al. 2015).
Beetroot juice (BRJ) contains an appreciable amount of antioxidants and many other health-benefiting compounds like minerals such as calcium, magnesium, iron, potassium, phosphorus, sodium, and zinc whereas vitamins like biotin, folic acid, niacin, vitamin B6, besides soluble fiber (Wootton-Beard and Ryan 2011) and water-soluble pigments betalains like betacyanin and betaxanthins (Azeredo 2009). Previous reports depict that beetroot juice significantly decreased levels and activities of several hepatic and mammary gland carcinogen metabolizing enzymes in rats and its pigments have shown the anticancer property (Szaefer et al. 2014).
In the fermentation of beer, ales, sakes, and wines, the S. cerevisiae is the most used yeast. Some fermented drinks, however, are made at interspecies or interspecies levels from Saccharomyces hybrids. Among the plants commonly used as fermentation, yeast hybrids are S. mikatae, S. kudriavzevii, and S.uvarum. In the production of many wines and Belgian beers at low temperatures, for instance, hybrids of S. cerevisiae and S. kudriavzevii are genetically hybridized to make some fermented cider and white wine (Peris et al. 2018). S. cerevisiae yeast vigorous fermentation capacity in the presence and lack of oxygen has been identified as the key strategy in the fight against other microbial species present in must. S. cerevisiae consume sugar resources more quickly and its rivals may harm or less tolerate the ethanol and CO2 produced during fermentation. S. cerevisiae can then use accumulated ethanol as a substratum for aerobic respiration once the competitors are overcome (Thomson et al. 2005; Piškur et al. 2006). Fermentation process of wine can be extended for as long as possible, but a sweet wine is created by stopping the process before the complete conversion of all the sugar (Otegbayo et al. 2020).
Exotic vegetables such as passion fruit are used to make wine (Chowdhury and Ray 2007). Research on wine production in India shows that there has been remarkable progress in the creation of technologies for the preparation of different types of wines from different fruit species. Efficient marketing in India of grapes and apple wines is a sign that fruit wine could be expected from a potential Indian market. However, the preparation of wine with various vegetables in India and the world is still not very popular. Therefore, there is a need to develop value-added products to enhance beetroot utilization and minimize losses. This study aimed to develop beetroot wine using the three different commercial S. cerevisiae strains (MTCC 178, ATCC 9080, and NDRI) to know the most appropriate fermenting strains for good quality beetroot wine and also to evaluate the influence of their inoculum sizes with varying total soluble solids on the physicochemical compounds of the produced beetroot wine.
Materials and methods
Raw materials and chemicals
Beetroot (Var. Detroit Dark Red) was purchased from the local market of Varanasi, Uttar Pradesh, India. All the chemicals and reagents were of analytical grade and were procured from Merck-Sigma and Fisher Scientific, Mumbai, India.
Starter culture and maintenance media
The freeze-dried S. cerevisiae MTCC 178 strain was procured from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology, Chandigarh (India). S. uvarum ATCC 9080 and S. cerevisiae NDRI strain was received from the National Collection of Dairy Culture (NCDC), NDRI, Karnal, Haryana (India). The freeze-dried culture was revived in peptone water, followed by streaking on PDA plates, and incubated at 25 ± 0.2 °C for 48 h. After 48 h, pure colonies were re-cultured in the sterilized growth medium (Patel et al. 2020).
Inoculum preparation
For inoculum preparation, one inoculation loop (6 log CFU ml−1) of S. cerevisiae MTCC 178, S. uvarum ATCC 9080, and S. cerevisiae NDRI strain colonies were transferred in beetroot juice (100 ml) (Supplementary Fig. 1). The total soluble solids (TSS) were maintained at 18 and 20°Brix. The inoculated beetroot juice was then incubated at 25 ± 0.2 °C for 14 days. The appearance of bubbles and over-ripened beet odor in pulp showed completion of inoculum preparation (Buxaderas and López 2012).
Preparation of must for fermentation and fermentation process
The beetroot samples (1 kg) were sorted, washed, peeled, and cut into pieces (Supplementary Fig. 2). The pieces of beets were blanched using hot water for 2–3 min at 100 °C. The blanched beets were placed on cold water and allowed to cool for 5–10 min followed by pulp preparation in a pulper (Phillips, Netherlands). The extracted pulp was then homogenized in a laboratory blender (Bajaj, India). The pulp juice is collected through a muslin cloth by squeezing the pulp. The extracted beetroot juice was centrifuged (5430R, Eppendorf, Hamburg) at 10,000 rpm for 15 min to produce clear juice and stored at − 20 °C for further process and analysis. The juice was transferred into a 250 mL clean conical flask and mixed with distilled water. To maintain the initial TSS at 18 and 20°Brix, 0.105 and 0.116 kg of sugar added respectively in the must prepare from beetroot juice with vigorous stirring. Then, 0.05% (w/v) of potassium metabisulphite was added to each must sample before inoculation of yeast culture (Vigentini et al. 2016).
The batch fermentation was carried out in sterile conical flasks (500 ml) and each flask contained 100 ml of beetroot juice. Strain’s inoculum (2.5 ml, 6 log CFU ml−1) was added to the must samples and incubated at 25 °C for 14 days. After 14 days, the fermented juice was filtered through a 3-layered muslin cloth and clarified by bentonite (clarifying agent) in the concentration of 0.05%. Filtration was performed with muslin cloth and sieve after 14 days of clarification. All the wine samples were siphoned into the sieve containing four layers of muslin cloth. The residues were removed and the filtrates were collected for further physiochemical analysis.
Physicochemical analysis of juice/wine
Total soluble solids of juice or wine were estimated according to AOAC (2019) (AOAC 932.14). The color of beetroot wine was measured according to Das et al. (2020) using Colour flex (Hunter Associates Laboratory Inc., Reston, VA, USA). Betalain content of juice or wine was analyzed as per Stintzing, Schieber and Carle (2003) where the samples were homogenized in methanol (1:5 v/v) and stirred for 1 min at 500 rpm. The homogenate was filtered through a Whatman filter (0.45 µm). Betalain content in the sample was studied using UV–Vis spectrophotometer (Shimadzu UV Visible 1650 PC Shimadzu, Japan) and the quantification of betalains was carried out in triplicate applying the molar extinction coefficients of betacyanin (€ = 60,000 L mol−1 cm in H2O; λ = 532 nm; MW = 550 g mol−1 and betaxanthin (€ = 48,000 L mol−1 cm in H2O; λ = 482 nm; MW = 308 g mol−1). The betalain content (sum of betacyanin and betaxanthin) expressed as mg/L and was calculated as per the following equation:
| 1 |
where, BC: Betalain content (mg l−1), A: Absorption (nm), DF: Dilution factor, L: Path length, MW: Molecular weights, €: Extinction coefficient.
Sample preparation for ethanol estimation using GC–MS
For gas chromatographic analysis, 2 µL of samples was injected with the help of a micro-syringe (Hamilton, Germany). Nucon gas chromatograph instrument was used with 5% Carbowax 20 m glass column on Carbopack-B 80/120 mesh. Nitrogen is used as a carrier gas with a flow rate of 20 ml min−1. The eluted compounds were detected by the FID detector. The fuel gas was hydrogen with a flow rate of 40 ml min−1, and the oxidant was air, with a flow rate of 40 ml min−1. The analytes were then identified based on their retention time. The fermented samples were centrifuged at 5000 g for 10 min. The supernatant was used for ethanol analysis (Šrámková et al. 2014).
Sensory evaluation
Sensorial assessment of the acceptability of wine was conducted using in-house panelists to conduct an in-house customer acceptance test, as per Meilgaard et al. (2016). Twenty semi-trained panelists chosen based on availability, objectiveness, and wine tasting experience conducted a sensory assessment.
Statistical analysis
The data were analyzed statistically using package IBM SPSS Statistics Version 20.0, Armonk, NY: IBM Corporation. All the data were presented as the mean with the standard deviation.
Results and discussion
Physico-chemical properties of the juice
The beetroot juice used in the present investigation had the TSS of 8°Brix, moisture content of 86.50%, and 0.14% of titratable acidity (% tartaric acid). Besides, reducing sugars were found to be 4.10% whereas 7.73% of total sugars were observed. The content of betalain was 14.50 mg 100 g−1 (Supplementary Table 1).
Physico-chemical and phytochemical properties of wine
pH, TSS, and titratable acidity
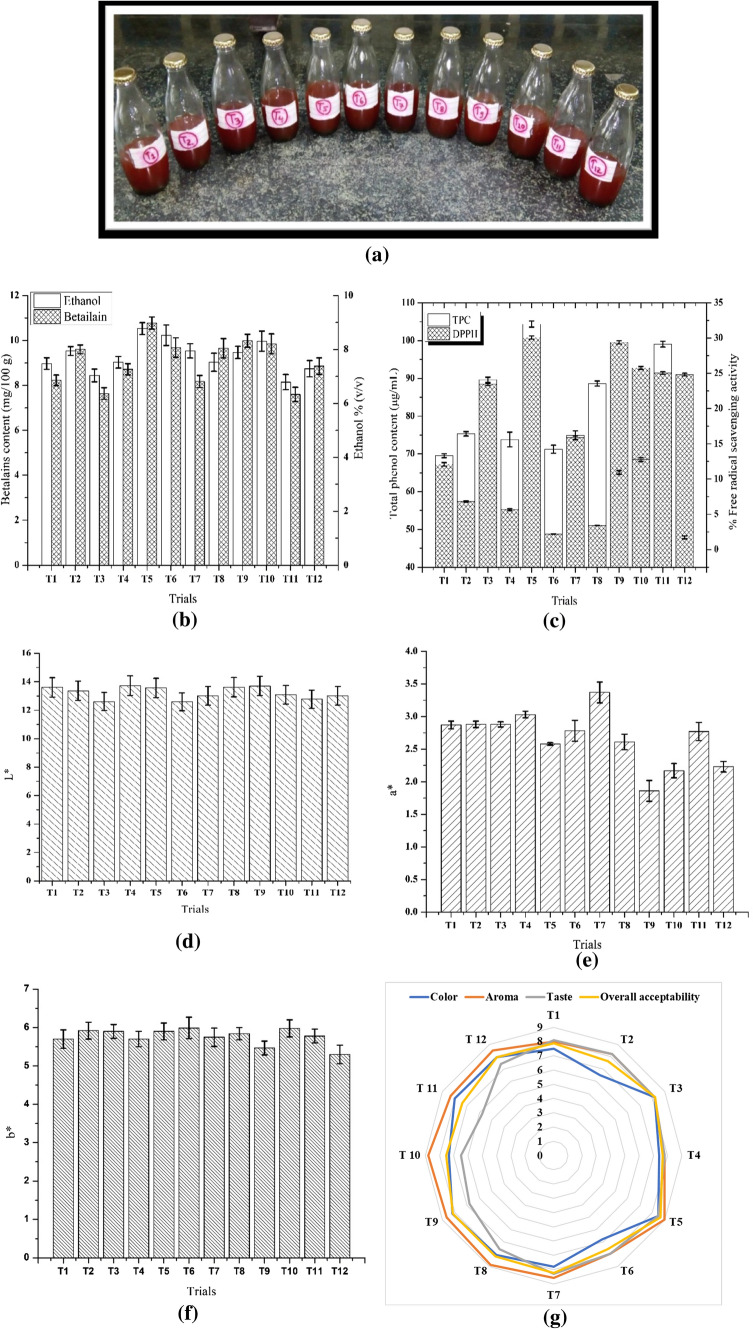
The wine obtained after the fermentation of beet juice is presented in Fig. 1a. The physicochemical properties of the wine are presented in Tables 1 and 2. At first, the pH was adjusted to 4, and after 14 days of fermentation, a drop in pH was observed as the fermentation progressed between 3.56 and 3.70. This showed that during fermentation days, the acidity of wine increased. The pH level was similar to the results of Haliu and Mekonnen (2017) who reported a pH of 2.89–3.53 for beetroot wine. Soibam et al. (2017) revealed that a pH of 3.45–3.8 affects the aroma, flavor, and mouthfeel of the wine. With different strains and inoculum sizes, the pH of wine was significantly affected. The maximum pH values were observed in T5 (3.70 ± 0.85) and the least pH value was seen in T12 (3.56 ± 0.61). The pH level of wine has an important effect on wine taste; as wine tastes are typically graded on the basis of their pH; low pH wines will taste smooth and crisp, and higher pH wines will be slightly flabby and more prone to develop microbial growth.
Fig. 1.
a Different trials of Beetroot wine prepared from different Saccharomyces strains b Ethanol and Betalain concentration c Phytochemical attributes d L* value e a* value f b* value g Sensory profile of the wine
Table 1.
Effect of different Saccharomyces strains and their inoculum sizes on TSS and pH of the wine
| Samples | TSS | pH | ||||
|---|---|---|---|---|---|---|
| 0 Day | 7 Day | 14 Day | 0 Day | 7 Day | 14 Day | |
| T1 | 18 | 5.70 ± 0.86d | 3.82 ± 0.60c | 4 | 3.93 ± 0.32a | 3.64 ± 0.58a |
| T2 | 18 | 6.05 ± 0.96d | 4.80 ± 0.99a | 4 | 3.97 ± 0.16a | 3.64 ± 0.63a |
| T3 | 20 | 7.25 ± 0.55b | 4.82 ± 0.68a | 4 | 3.92 ± 0.32a | 3.60 ± 0.89b |
| T4 | 20 | 6.72 ± 0.23c | 4.55 ± 0.82a | 4 | 3.99 ± 0.55a | 3.64 ± 0.61a |
| T5 | 18 | 6.55 ± 0.19c | 4.24 ± 0.72b | 4 | 3.96 ± 0.65a | 3.70 ± 0.85a |
| T6 | 18 | 6.00 ± 0.63d | 4.60 ± 0.92a | 4 | 3.97 ± 0.96a | 3.61 ± 0.49b |
| T7 | 20 | 7.25 ± 0.89b | 4.72 ± 0.52a | 4 | 3.91 ± 0.48a | 3.59 ± 0.68b |
| T8 | 20 | 6.12 ± 0.66c | 4.10 ± 0.64b | 4 | 3.96 ± 0.62a | 3.61 ± 0.99b |
| T9 | 18 | 5.55 ± 0.78d | 3.84 ± 0.52c | 4 | 3.96 ± 0.32a | 3.62 ± 0.94b |
| T10 | 18 | 5.72 ± 0.66d | 4.08 ± 0.33b | 4 | 4.03 ± 0.75a | 3.66 ± 0.37a |
| T11 | 20 | 8.10 ± 0.96a | 4.86 ± 0.66a | 4 | 3.91 ± 0.68a | 3.58 ± 0.78b |
| T12 | 20 | 7.92 ± 0.68b | 4.42 ± 0.48b | 4 | 3.89 ± 0.28ab | 3.56 ± 0.61b |
Values are means ± standard deviation of three determinations (n = 8). Values followed by a different superscript letter in a row are significantly different (p ≤ 0.05)
Table 2.
Titratable acidity and reducing sugars of beetroot wine
| Samples | Titratable acidity (% acidity) | Reducing sugars (µg ml−1) |
|---|---|---|
| T1 | 0.16 ± 0.04f | 24.91 ± 1.27e |
| T2 | 0.15 ± 0.06 g | 26.91 ± 0.59d |
| T3 | 0.19 ± 0.12e | 26.67 ± 1.83d |
| T4 | 0.22 ± 0.13c | 28.75 ± 0.96c |
| T5 | 0.35 ± 0.16a | 26.75 ± 1.10d |
| T6 | 0.16 ± 0.048f | 26.08 ± 0.76d |
| T7 | 0.25 ± 0.036b | 31.00 ± 0.95a |
| T8 | 0.23 ± 0.014c | 26.25 ± 0.88d |
| T9 | 0.22 ± 0.03c | 25.91 ± 0.69d |
| T 10 | 0.20 ± 0.05d | 25.67 ± 0.79d |
| T 11 | 0.26 ± 0.01b | 30.50 ± 2.63ab |
| T 12 | 0.19 ± 0.015e | 30.16 ± 2.19ab |
Values are means ± standard deviation of three determinations (n = 8). Values followed by a different superscript letter in a row are significantly different (p ≤ 0.05)
The TSS level was initially adjusted to 18 and 20°Brix but it varied significantly with different strains and their inoculum sizes within 14 days of fermentation. The maximum TSS was observed in T11 (4.86 ± 0.66) and T3 (4.82 ± 0.68) respectively while T1 (3.82 ± 0.60) and T9 (3.84 ± 0.52) showed the minimum TSS value.
The maximum and minimum titratable acidity was observed in T5 (0.35%) and T2 (0.15%) respectively (Table 2). Higher acidic conditions facilitate keeping the quality of the product as it limits the growth of harmful bacteria which ultimately helps in improved preservative properties. The production of succinic acid, produced as a significant non-volatile acid, has shown an increased acidity in wine during the fermentation process (Robinson 2006).
Sugar
To assess wine quality, the level of sugar fermentation is an important characteristic, as its content acts as an indicator of alcohol content. There is no substantial difference in the reduction in total sugar content between the different samples but the maximum reduction was found in T1 (Table 2). The higher the reduction of the total sugar content, the more the production of ethanol. The must is fermented under the conditions of anaerobicity and thus has used more possible alcohol than the available sugar. In the absence of oxygen, the wine sugar is converted by fermentation into alcohol and carbon dioxide. Similarly, S. cerevisiae, isolated from fermented grape pomace, is found for sugar fermentation (Asli 2010).
Ethanol concentration
Beetroot wine may be considered a natural wine because its alcohol content ranged from 9 to 14% (Swami et al. 2014) and with the majority of the natural wines ranging from 12.5 to 14.5%. In comparison to other fermented fruits beverage, considering the duration of fermentation (14 days), beetroot juice produced a wine with a higher content of alcohol than a banana (10%) and sugarcane-beetroot wine (8.2–11.7%) (Soibam et al. 2017). The maximum alcohol content was found to be 10.6% in T5 whereas 7.5% was observed as the minimum alcohol content in T3.
Betalain content of beetroot wine
The maximum content of betalain was observed in T5 (10.85 mg 100 ml−1) and the minimum amount was observed in T11 (7.65 mg 100 ml−1). The present study also shows the correlation between the amount of betalain content and alcohol content. Higher the betalain content more is the ethanol produced in the sample which can be seen in Fig. 1b. However, there is no previous such report for this finding but the present investigation suggests that sample T5 (S. cerevisiae NDRI strain having TSS 18° and 5% (v/v) inoculum size) has the highest betalain and alcohol content with respect to other samples. Results obtained are in accordance with the findings of Sawicki and Wiczkowski (2018).
DPPH free radical scavenging activity
The present study also revealed the effect of different Saccharomyces strains with varying inoculum sizes on the antioxidant potential of the prepared beetroot wine. The higher the value of the activity, the more the antioxidant property it possessed. The maximum activity was found to be 30% in T5 while T6 presented the minimum activity of 2.20% (Fig. 1c).
Total phenol content (TPC)
The phenolic content of wine consists of a wide group of chemical compounds, including phenolic acids, flavanols, dihydroflavanols, anthocyanins, monomers of flavanol (catechins) and polymers of flavanol (proanthocyanidins), quercetin, all of which contribute to color, mouthfeel, and taste (Kennedy et al. 2002). The maximum value of TPC was found in T5 (104.20 µg ml−1) whereas the minimum value of TPC was found in T12 (47.80 µg ml−1) (Fig. 1c). In comparison to the other sources that affected the development of polymeric pigments and hence the higher phenolic content of beetroot, the higher phenolic content of the root may be attributable to its higher content of anthocyanin expressed in the beet in its deep red color.
Effects of inoculum sizes and strain on color parameters of wine
Beetroot consists of a group of intensely bioactive pigments called betalains, the components of betacyanin which gives the beetroot its deep dark red color and have an exceptionally high free radical scavenging activity, Kapadia and Rao (2013). During the fermentation phase, L* value varies from black to white. Thus, the fermentation process has led to the increased lightness of the wine. Garcia-Estevez et al. (2017) reported a similar pattern of increased lightness over time for red wine, which may be attributed to a reduction in the overall level of pigment. The influence of various yeast strains of different sizes on the colors of beetroot wines can be easily observed from Fig. 1d–f. The a∗ value indicating the red-green (positive and negative values respectively) axis and the b∗ coordinate of the prepared beetroot wine also gave positive values, which showed that they tend towards yellow. The L* value ranges from 12.57 to 13.73, a∗ value from 1.86 to 3.37, and b∗ value varies from 5.30 to 5.99 for different trials. The tristimulus color values suggested that during fermentation the characteristics of dark red color shift slightly towards lighter shade, however, a* value found to increase in double fold whereas a slight shift was observed in b* values during fermentation of the juice.
Sensory properties of beetroot wine
The sensory evaluation results of the beetroot wines are presented in Fig. 1g. The maximum overall acceptability was found to be T5 (8.67) whereas the minimum score was reported in T11 (7.39). There was perceptible sweetness but not as much as of sweet wine. The good aroma obtained in the wines could be attributed to the high alcohol content in accordance with the report of Clement-Jimenez et al. (2005).
Microbial load
In all the trails, the initial microbial load was in the range of 5–6 log CFU ml−1 and observed to increase with the fermentation period and reached 8–9 log CFU ml−1 after 14 days of storage. The trend of microbial growth curve that was noticed during fermentation highlighted that alcoholic fermentation was taking place as the sugar was consumed and alcohol was produced throughout the process. The result of microbial load can easily be correlated with the physicochemical properties of wine where after 14 days of fermentation, sugar, TSS, and alcohol levels elevated to a certain level than the control.
Cost economics
From 1 kg of beetroots, approximately 400 ml of juice is produced, and takes 1500 ml of juice for making 1 L of wine. Hence 1 L of wine would cost approximately Rs 55.00 which is comparatively much less than other reported wines such as Mango wine (Patel et al. 2020). To evaluate the actual cost of beetroot wine production, however, scale-up studies are required.
Conclusion
The present study conducted to determine the efficiency of three strains of Saccharomyces as a starter culture for beetroot wine production and revealed that beetroot wine prepared with the Saccharomyces cerevisiae NDRI strain with 5% (v/v) inoculum size showed better potential in winemaking owing to its functional attributes and higher alcohol production efficiency. The study showed that fermenting beetroot Must for 14 days produced wine that exhibits appreciable physicochemical and bioactive properties and better acceptance by consumers. There is a limited study focusing on the effect of inoculum size on wine quality, but the results of the present study highlight the importance of defining this process parameter for key the development of certain bioactive and chemical compounds for discrimination and selection of wine yeasts.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge Mr. Abhinay Shashank, Department of Dairy Science and Food Technology, Institute of Agricultural Sciences, Banaras Hindu University, Varanasi-221005, U.P, India for their effort towards the proofread of the manuscript.
Author contribution
We author(s) of the above-titled paper hereby declare that the work included in the above paper is original and is an outcome of the research carried out by the authors indicated in it. SS: Visualization, Supervision, Investigation, Writing—Original Draft. ADT: Conceptualization, Supervision, Visualization, Writing-reviewing, and editing. AKC: Supervision, Writing-reviewing, and editing. AKG: Writing-reviewing and editing.
Funding
This work did not receive any external funding.
Declarations
Conflict of interest
The authors have no conflicts of interest to disclose with the present submission.
Consent for publication
All the authors have read the manuscript and approved it for possible publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . Official methods of analysis. 21. Washington: Association of Official Analytical Chemists AOAC; 2019. [Google Scholar]
- Ashaolu TJ. A review on selection of fermentative microorganisms for functional foods and beverages: the production and future perspectives. Int J Food Sci Tech. 2019;54(8):2511–2519. doi: 10.1111/ijfs.14181. [DOI] [Google Scholar]
- Asli MS. A study on some efficient parameters in batch fermentation of ethanol using Saccharomyces cerevisiae SC1 extracted from fermented Siahe Sardasht pomace. Afr J Biotechnol. 2010;9(20):2906–2912. [Google Scholar]
- Azeredo HM. Betalains: properties, sources, applications, and stability–a review. Int J Food Sci Tech. 2009;44(12):2365–2376. doi: 10.1111/j.1365-2621.2007.01668.x. [DOI] [Google Scholar]
- Buxaderas S, López-Tamames E. Sparkling wines: features and trends from tradition. Adv Food Nutr Res. 2012;66:1–45. doi: 10.1016/B978-0-12-394597-6.00001-X. [DOI] [PubMed] [Google Scholar]
- Chowdhury PRCR, Ray RC. Fermentation of Jamun (Syzgium cumini L.) fruits to form red wine. ASEAN Food J. 2007;14(1):15. doi: 10.20546/ijcmas.2020.901.109. [DOI] [Google Scholar]
- Clemente-Jimenez JM, Mingorance-Cazorla L, Martínez-Rodríguez S, Las Heras-Vázquez FJ, Rodríguez-Vico F. Influence of sequential yeast mixtures on wine fermentation. Int j Food Microbiol. 2005;98(3):301–308. doi: 10.1016/j.ijfoodmicro.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Das D, Das D, Gupta AK, Mishra P. Drying of citrus grandis (pomelo) fruit juice using block freeze concentration and spray drying. Acta Aliment Hung. 2020;49(3):295–306. doi: 10.1556/066.2020.49.3.8. [DOI] [Google Scholar]
- Garcia-Estevez I, Perez-Gregorio R, Soares S, Mateus N, de Freitas V. Oenological perspective of red wine astringency. Oeno One. 2017;51(3):237–249. [Google Scholar]
- Guyot JP. Cereal-based fermented foods in developing countries: ancient foods for modern research. Int J Food Sci Tech. 2012;47:1109–1114. doi: 10.1111/j.1365-2621.2012.02969.x. [DOI] [Google Scholar]
- Hailu Z, Mekonnen D. Effects of yeast and oxygen on quality attributes of wine produced from Ethiopian beetroot. GJRE. 2017 doi: 10.4172/2157-7048.1000329. [DOI] [Google Scholar]
- Kapadia GJ, Rao GS. Anticancer effects of red beet pigments. In: Neelwarne B, editor. Red beet biotechnology. Boston: Springer; 2013. pp. 125–154. [Google Scholar]
- Kennedy JA, Matthews MA, Waterhouse AL. Effect of maturity and vine water status on grape skin and wine flavonoids. Am J Enol Vitic. 2002;53(4):268–274. [Google Scholar]
- Luana N, Rossana C, Curiel JA, Kaisa P, Marco G, Rizzello CG. Manufacture and characterization of a yogurt-like beverage made with oat flakes fermented by selected lactic acid bacteria. Int j Food Microbiol. 2014;185:17–26. doi: 10.1016/j.ijfoodmicro.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Meilgaard MC, Civille GV, Carr BT (2016) Sensory Evaluation Techniques (5th edn), CRC Press Taylor & Francis group, US
- Otegbayo BO, Akwa IM, Tanimola AR. Physico-chemical properties of beetroot (Beta vulgaris L.) wine produced at varying fermentation days. Sci Afr. 2020;8:e00420. [Google Scholar]
- Patel V, Tripathi AD, Adhikari KS, Srivastava A. Screening of physicochemical and functional attributes of fermented beverage (wine) produced from local mango (Mangifera indica) varieties of Uttar Pradesh using novel saccharomyces strain. J Food Sci Technol. 2020;58(6):1–10. doi: 10.1007/s13197-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris D, Pérez-Torrado R, Hittinger CT, Barrio E, Querol A. On the origins and industrial applications of Saccharomyces cerevisiae× Saccharomyces kudriavzevii hybrids. Yeast. 2018;35(1):51–69. doi: 10.1002/yea.3283. [DOI] [PubMed] [Google Scholar]
- Piškur J, Rozpędowska E, Polakova S, Merico A, Compagno C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006;22(4):183–186. doi: 10.1016/j.tig.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Robinson J (2006) The Oxford companion to wine (3rd edn). Oxford University Press
- Sawicki T, Wiczkowski W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018;259:292–303. doi: 10.1016/j.foodchem.2018.03.143. [DOI] [PubMed] [Google Scholar]
- Simatende P, Gadaga TH, Nkambule SJ, Siwela M. Methods of preparation of Swazi traditional fermented foods. J Ethn Foods. 2015;2(3):119–125. doi: 10.1016/j.jef.2015.08.008. [DOI] [Google Scholar]
- Soibam H, Ayam VS, Chakraborty I. Preparation and evaluation of wine from sugarcane and beet juice. Adv Biores. 2017;8(4):216–219. doi: 10.5958/2395-146x.2017.00117.x. [DOI] [Google Scholar]
- Šrámková I, Horstkotte B, Solich P, Sklenářová H. Automated in-syringe single-drop head-space micro-extraction applied to the determination of ethanol in wine samples. Anal Chim Acta. 2014;828:53–60. doi: 10.1016/j.aca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Stintzing FC, Schieber A, Carle R. Evaluation of color properties and chemical quality parameters of cactus juices. Eur Food Res Technol. 2003;216(4):303–311. doi: 10.1007/s00217-002-0657-0. [DOI] [Google Scholar]
- Swami SB, Thakor NJ, Divate AD. Fruit wine production: a review. J Food Res Technol. 2014;2(3):93–100. [Google Scholar]
- Szaefer H, Krajka-Kuźniak V, Ignatowicz E, Adamska T, Baer-Dubowska W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female Sprague-Dawley rats. Phytother Res. 2014;28(1):55–61. doi: 10.1002/ptr.4951. [DOI] [PubMed] [Google Scholar]
- Thomson JM, Gaucher EA, Burgan MF, De Kee DW, Li T, Aris JP, Benner SA. Resurrecting ancestral alcohol dehydrogenases from yeast. Nat Genet. 2005;37(6):630–635. doi: 10.1038/ng1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigentini I, Maghradze D, Petrozziello M, Bonello F, Mezzapelle V, Valdetara F, Failla O, Foschino R. Indigenous Georgian wine-associated yeasts and grape cultivars to edit the wine quality in a precision enology perspective. Front Microbiol. 2016;7:352. doi: 10.3389/fmicb.2016.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS, and Folin-Ciocalteu methods. Food Res Int. 2011;44(1):217–224. doi: 10.1016/j.foodres.2010.10.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.