Abstract
Abstract
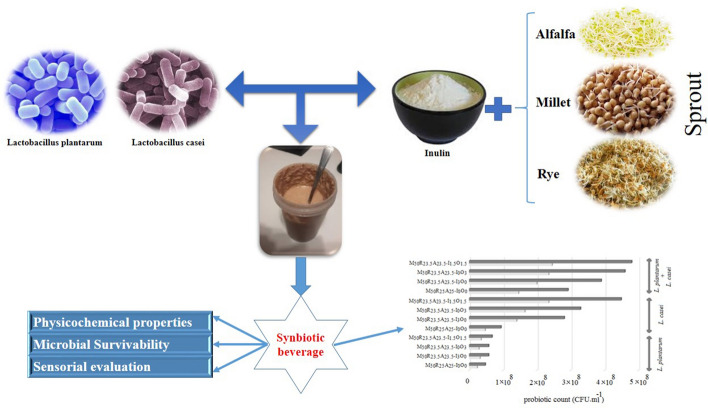
The study was devoted to developing a novel synbiotic beverage based on with millet, rye and alfalfa sprouts with a mixed culture of Lactobacillus casei and Lactobacillus plantarum. In this regard, the influences of incorporated prebiotics inulin and oligofructose on probiotics viability during the refrigerated storage (4 ± 1 °C, 28 days) as well as under the simulated gastric condition were investigated. The characteristics such as microbial viability, physicochemical properties (viscosity, pH, titrable acidity and radical scavenging activity) and sensorial evaluation were assessed. The synbiotic beverage produced contained 108 CFU ml−1 for L. casei, with a good survival throughout the storage period (108 CFU ml−1) and L. plantarum at sufficient levels (106 CFU ml−1) after about 21 days. Inulin and oligofructose promoted the growth of the strains and their viability under cold storage while conferring higher sensory scores. In this context, the beverages demonstrated acceptable sensory attributes. The viability (bacterial survival) of over 55% for all the strains was achieved under simulated gastric condition. Therefore, the introduced fermented beverage was a good food matrix from the viability of probiotics as well as under the gastric condition and sensory characteristics
Graphic abstract
Keywords: Millet, Prebiotic, Probiotic, Rye, Sprouts, Symbiotic, Healthy food
Introduction
Recently, functional foods are occupying an important place among both academic and industrial trends as they improve the quality of dietary intake by providing the required nutritional components for the human organism (Jafari et al. 2017). On the other hand, beverages are one of the most accessible food which could provide nutrients such as vitamins, mineral substances, antioxidants, organic acids and other active biological substances for the body (Mohammadi and Mortazavian 2011). In this regard, there are huge demands for the development of new non-dairy based probiotic beverages to the functional food market by using controlled fermentation by probiotics and incorporation of prebiotics.
Probiotics are live microorganisms which, upon consumption in sufficient quantities, provide health benefits higher than the intake of the inherent nutrition to the host, by improving the microbial balance of the intestine (Jafari et al. 2017). Lactic acid bacteria (LAB) mainly lactobacilli and bifidobacteria as the most widely used strains of probiotic can survive in the intestine system. LAB improve the host immune system and show anti-cancer and anti-tumor activity. Also, they play an important roles in colonization resistance in the intestinal, respiratory, and urogenital tracts, cholesterol metabolism, lactose metabolism, absorption of calcium, and synthesis of vitamins (Fuller 2012).
The term "prebiotic" is defined as non-digestible food that selectively stimulates the growth and/or activity of one or a few bacteria in the intestine and further improvements in the health of the host (Gibson and Roberfroid 1995). Among the prebiotics, inulin and oligofructose have shown convincing evidence of health-promoting effects (Afshari et al. 2015; Sarteshnizi et al. 2017). Moreover, by combining probiotics and prebiotics (synbiotic), synergistic benefits may be observed (Fernandez and Marette 2017).
Sprouted cereals have higher enzymatic activity, total protein, sugars, crude fat and fiber, vitamins and minerals (Lorenz and D’Appolonia 2009), total phenolic, flavonol, tannin, phytic acid, tocopherol and antioxidant activity and lower total dry matter and starch compared to unsprouted cereals (Kaur et al. 2017).
Sprouts are rich in digestible energy, bioavailable vitamins, minerals, amino acids, proteins, and phytochemicals, are necessary for a germinating of the plants. However, the nutritional content of sprouted seeds can be varied due to differences in species, they contain remarkable amounts protein, fiber, vitamin C, B vitamins, and small amounts of calcium, iron, magnesium, phosphorus, potassium, and zinc (El-Adawy 2002). Alfalfa also called lucerne and called Medicago sativa is a perennial flowering plant in the legume family Fabaceae, has been used traditionally as a liver protectant, antioxidant agent and also for the treatment of bleeding and digestive problems (Cornara et al. 2016). Their sprouts contain high amounts of vitamins A, and C (Hong et al. 2010).
Probiotic fermentation of bran such as rye not only aid to the proliferation of targeted microorganism in higher values but also improve the healthy composition. In this regard, the improvements in bioactivity as well as technical characteristics of rye bran was investigated by (Lamsal and Faubion 2009). Moreover, according to Morah 2017, the incorporation of rye sprouting improved the nutritional value (i.e., folate content by 1.7–3.8 fold) of millet and makes it safer for human consumption. According to researchers sprouting increases its (Morah 2017).
Therefore, the current investigation was aimed to produce a novel cereal sprout-based synbiotic beverage fermented by a mixed culture of Lactobacillus casei and Lactobacillus plantarum, with appropriate quality attributes.
Materials and methods
Chemical reagents and microbial strains
The inulin, oligofructose, MRS agar culture medium, MacConkey agar, potato dextrose agar sodium chloride and hydrochloric acid were supplied by Merck (Darmstadt, Germany). L. plantarum and L. caesi were obtained from the Iranian Research Organization for Science and Technology, Iran with an initial viable cell count of 108 CFU ml−1
Preparation of cereal sprouts
Two hundred g of each cereal, including alfalfa, millet, and rye (provided from the local market, Tehran, Iran) were soaked in 1000 mg of sodium hypochlorite (Merck, Germany) for 30 min. The seeds were then washed with distilled water to reach the neutral pH. The seeds were placed in germination machine at 20 °C with a relative humidity of 99% for 5 days (Fernandez-Orozco et al. 2006).
Preparation of synbiotic beverage
The sprouts were shredded and compressed before the green leaf emerged and after pasteurization were kept in a cool place until incorporation in the proposed formulations. The various proportions of obtained sprouts’ juices were inoculated with L. plantarum and L. caesi and supplemented with inulin or oligofructose according to Table 1. The mixture of suspension was incubated at 30 °C until reaching pH 4.8. The fermented beverages were kept at 4 ± 1 °C and used for analysis per 7-day intervals till day 28.
Table 1.
The formulation characteristics of treatments
| Probiotic | Treatment symbol | Millet sprout (% m/m) | Rye sprout (% m/m) | Alfalfa sprout (% m/m) | Inulin (% m/m) | Oligofructose (% m/m) |
|---|---|---|---|---|---|---|
| L. plantarum | M50R25A25–I0O0 | 50 | 25 | 25 | 0 | 0 |
| M50R23.5A23.5–I3O0 | 50 | 23.5 | 23.5 | 3 | 0 | |
| M50R23.5A23.5–I0O3 | 50 | 23.5 | 23.5 | 0 | 3 | |
| M50R23.5A23.5–I1.5O1.5 | 50 | 23.5 | 23.5 | 1.5 | 1.5 | |
| L. casei | M50R25A25–I0O0 | 50 | 25 | 25 | 0 | 0 |
| M50R23.5A23.5–I3O0 | 50 | 23.5 | 23.5 | 3 | 0 | |
| M50R23.5A23.5–I0O3 | 50 | 23.5 | 23.5 | 0 | 3 | |
| M50R23.5A23.5–I1.5O1.5 | 50 | 23.5 | 23.5 | 1.5 | 1.5 | |
| L. plantarum + L. casei | M50R25A25–I0O0 | 50 | 25 | 25 | 0 | 0 |
| M50R23.5A23.5–I3O0 | 50 | 23.5 | 23.5 | 3 | 0 | |
| M50R23.5A23.5–I0O3 | 50 | 23.5 | 23.5 | 0 | 3 | |
| M50R23.5A23.5–I1.5O1.5 | 50 | 23.5 | 23.5 | 1.5 | 1.5 | |
| Without probiotic | M50R25A25–I0O0 | 50 | 25 | 25 | 0 | 0 |
| M50R23.5A23.5–I3O0 | 50 | 23.5 | 23.5 | 3 | 0 | |
| M50R23.5A23.5–I0O3 | 50 | 23.5 | 23.5 | 0 | 3 | |
| M50R23.5A23.5–I1.5O1.5 | 50 | 23.5 | 23.5 | 1.5 | 1.5 |
Microbiological analysis
Viable counts of probiotics in beverages were measured using serial dilution technique and MRS agar culture medium (Tharmaraj and Shah 2003). Plates were incubated in anaerobiosis (37 °C, 72 h) using AnaeroPack system. Coliform and total yeast-mold counts were checked using MacConkey agar (37 °C, 48 h) and potato dextrose agar (25 °C, 72 h), respectively.
Determination of pH and titrable acidity
The pH was determined using a pH-meter (Model 691; Metrohm, Herisau, Switzerland) equipped to a food penetration probe (AOAC 2010). Also, the titrable acidity was determined by the titration method (10 g beverage with 90 ml distilled water) using a 0.1 N NaOH (Merck, Germany) solution (Coda et al. 2011).
Sensory evaluation
Sensory evaluation was performed using a descriptive test for overall quality (flavor, odor, color, and overall acceptability) of samples using a panel of 12 trained panelists using a hedonic scale (5 and 1 points showing like extremely and dislike extremely) (Stone 2012).
Probiotic survival under the simulated gastrointestinal condition In order to simulate the gastrointestinal condition, 3.2 g of pepsin (Sigma-Aldrich, USA) was mixed with 0.5% sodium chloride solution and 0.1 M hydrochloric acid to reach the pH 1.5 ± 0.02. The acidic solution was sterilized for 15 min at 121 °C (Jain et al. 2007).
To assess the survival of probiotics under acidic conditions, 0.5 g of each sample was incubated in a sterile flask containing 4.5 ml of acidic solution at 37 °C for 2 h; then centrifugation was performed at 11,952 g for 10 min. 0.5 ml of the supernatant solution after serial dilution was used for surface cultivation on MRS agar medium (Semyonov et al. 2012).
Statistical analysis
All experiments were performed in triplicate were carried out and the results presented as a mean of the three values with the standard deviation. Analysis of variance procedure followed by Duncan’s test using SPSS 13 (SPSS Inc., Chicago, IL, USA) software was applied to determine the significant differences (P < 0.05) among treatment means.
Results and discussion
pH and titrable acidity
Fermentation in samples although became limited but slowly continued by storing them under refrigerated conditions (4 ± 1 °C). Nighswonger et al. (1996) revealed that there was a slight fermentative activity by the probiotic even at 4 °C (Nighswonger et al. 1996). Changes in pH and titrable acidity in different treatments during the refrigerated period were demonstrated in Table 2. By increasing in the storage time up to 28 days, there is a significant decrease in pH among all the treatments except those not inoculated with probiotics. The highest reduction in pH and acidity augmentation was noted in samples inoculated with L. casei combined with inulin and oligofructose. The results showed that this combination increased the sensory acceptability of this functional synbiotic beverage. In general, fillers have a positive effect on reducing the unpleasant sensation of probiotic fermented beverages.
Table 2.
pH and titrable acidity in treatments during refrigerated storage
| Probiotic | Treatment symbol | pH | Titrable acidity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 0 | 7 | 14 | 21 | 28* | ||
| L. plantarum | M50R25A25–I0O0** | 4.40a*** | 3.62a | 3.32 c | 3.20c | 3.92c | 0.46 d | 0.43d | 0.61f | 0.66f | 0.66f |
| M50R23.5A23.5–I3O0 | 4.12a | 3.34a | 3.34c | 3.32c | 3.31c | 0.36d | 0.45d | 0.62f | 0.63f | 0.63f | |
| M50R23.5A23.5–I0O3 | 4.07a | 3.47a | 3.32c | 3.30c | 3.40c | 0.32d | 0.44d | 0.66f | 0.62f | 0.63f | |
| M50R23.5A23.5–I1.5O1.5 | 4.06a | 3.57a | 3.33c | 3.33c | 3.21c | 0.36d | 0.45d | 0.61f | 0.63f | 0.63f | |
| L. casei | M50R25A25–I0O0 | 4.09a | 4.79a | 4.72a | 4.62b | 3.23c | 0.36d | 0.45d | 0.63f | 0.64f | 0.63f |
| M50R23.5A23.5–I3O0 | 4.13a | 4.96a | 4.59a | 4.33b | 3.09c | 0.36d | 0.43d | 0.64f | 0.66f | 0.66f | |
| M50R23.5A23.5–I0O3 | 4.08a | 4.59a | 4.50a | 4.21b | 3.18c | 0.36d | 0.44d | 0.65f | 0.63f | 0.63f | |
| M50R23.5A23.5–I1.5O1.5 | 4.00a | 3.86a | 3.96a | 3.68b | 3.11c | 0.39d | 0.48d | 0.68f | 0.69f | 0.69f | |
| L. plantarum + L. casei | M50R25A25–I0O0 | 4.13a | 4.77a | 4.74b | 4.65b | 4.47b | 0.36d | 0.42d | 0.64f | 0.61f | 0.61f |
| M50R23.5A23.5–I3O0 | 4.94a | 3.57a | 3.59b | 3.33b | 4.81b | 0.36d | 0.44d | 0.63f | 0.64f | 0.64f | |
| M50R23.5A23.5–I0O3 | 4.22a | 4.12a | 4.27b | 4.02b | 4.98b | 0.36d | 0.45d | 0.67f | 0.62f | 0.62f | |
| M50R23.5A23.5–I1.5O1.5 | 4.19a | 4.46a | 3.56b | 3.42b | 4.18b | 0.36d | 0.40d | 0.62f | 0.61f | 0.61f | |
| Without probiotic | M50R25A25–I0O0 | 6.91b | 6.62b | 5.66b | 5.56b | 5.15 ab | 0.06 e | 0.08e | 0.09e | 0.09e | 0.09e |
| M50R23.5A23.5–I3O0 | 6.07b | 5.51b | 5.54b | 5.36b | 5.25ab | 0.06e | 0.07e | 0.07e | 0.08e | 0.08e | |
| M50R23.5A23.5–I0O3 | 5.94b | 5.87b | 5.77b | 5.62b | 5.29ab | 0.06e | 0.05e | 0.06e | 0.06e | 0.06e | |
| M50R23.5A23.5–I1.5O1.5 | 6.22b | 6.05b | 5.69b | 5.58b | 5.32ab | 0.06e | 0.05e | 0.06e | 0.06e | 0.06e | |
*(4 °C, 28 day)
**Letters ‘M, R, A, I and O’ represents millet, rye, alfalfa, inulin and oligofructose, respectively
***Means in a column shown with different English letters are significantly different (p < 0.5)
Similar findings were reported by Garro et al. (1998), who observed a more pronounced drop in pH and an increase in acidity in a synbiotic beverage fermented with L. casei (Garro et al. 1998). On the other hand, inulin and oligofructose stimulated the metabolic activity of probiotics which led to further increments in the acidity and decrement in pH (Cardarelli et al. 2008). The production of acidic metabolites by carbohydrates fermentation by the bacteria led to a decrease in pH and an increase in acidity.
In fermented beverages, the pH plays an important role in the microbiological durability of the product against foodborne pathogens. Generally, pH < 3.8 makes a rough condition for pathogens (Jayamanne and Adams 2009).
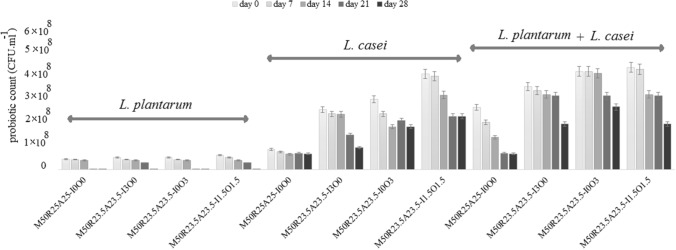
The viability of probiotics during storage
In Fig. 1, the survivability of probiotics during storage for 28 days in a refrigerated condition was demonstrated. L. casei survived well throughout the storage with the corresponded values for population ranged from 7.39 × 107 (d28) to 4.49 × 108 CFU ml−1 (d0). However, the population of L. plantarum was reported as 6.77 × 107 (d0) CFU ml−1 before storage and decreased during the storage to 2.32 × 106 (d28). The reduction of viability of L. casei and L. plantarum during refrigerated storage were in good agreement by the findings of Jayamanne and Adams (2009) reporting the reduction in viable cells of Bifidobacterium lactis in products over cold storage (Jayamanne and Adams 2009). The loss of viability of probiotic organisms can be associated with decreasing in the pH of the medium and accumulation of organic acid as a result of growth and fermentation of bacteria (Mohammadi et al. 2017; Ebrahimi et al. 2018). Addition of inulin and oligofructose had significant similar (P > 0.05) effect on L. casei and L. plantarum viability in synbiotic beverages. The stimulating effect of probiotics in the product was in accordance with previous studies (Khanniri et al. 2018; Heydari et al. 2018).
Fig. 1.
Viability of probiotic bacteria in beverages during storage (letters ‘M, R, A, I and O’ represents millet, rye, alfalfa, inulin and oligofructose, respectively)
The prebiotic inulin and oligofructose stimulated the growth and viability of L. casei and L. plantarum, in the product, therefore, can promote intestinal health (De Souza Oliveira et al. 2011). Treatments containing the mixture of the strains showed significantly higher viable cells for each strain compared to those with single strains which can be associated with the symbiotic effect of probiotic bacteria and the production of required compounds and vitamins B in the environment in the presence of each other (Hong et al. 2010). Investigated the mixture of probiotics in formulating a probiotic yogurt and found the synergistic effects of the mixed strains on their growth and viability. Noori et al. (2017) declared that rye sprout extract could enhance the growth and survival of probiotic bacteria and showed prebiotic activity, while sprout extracts used in this study did not significantly affect the growth of probiotic bacteria (Noori et al. 2017). Generally speaking, the designed matrix in this study met the requirement of being a probiotic food from a viability standpoint.
Sensory evaluation
The sensory evaluation scores for the 16 treatments during 21 days of storage were presented in Table 3. The samples with no added inoculation showed evidence of mold and yeast contamination. Hence, they were not presented for the panelists. Panelists did not declare a significant sensorial change in beverages during 21 days of storage. However, the inulin and oligofructose besides inoculation with L. casei and L. plantarum significantly increased the acceptability of beverage as higher proportions of inulin and oligofructose in formulations resulted in the higher acceptance. The positive effect of fillers in recovering the grittiness sense of the fermented beverage with probiotics was previously documented (Rouhi et al. 2015). Color is one of the main factors affecting beverage early acceptance or rejection for consumers, which influences the purchase and the general consumption of products (Tárrega and Costell 2007). According to results, no significant differences (P < 0.05) in the accepted values of formulations were observed, and the light color of the beverages was satisfactory in the point of panelists. The odor did not please the panelist, probably because of the acidic smell of formulations.
Table 3.
Sensory assessment of treatments during the storage
| Probiotic | Treatment symbol | Taste | Odor | Color | Overall acceptability | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21 | 0 | 7 | 14 | 21* | ||
| L. plantarum | M50R25A25–I0O0** | 2.01Aa*** | 3.12Bb | 2.00Aa | 3.20Bb | 2.98Aa | 2.99Aa | 3.00Bb | 3.01Bb | 3.53Bb | 3.97Bb | 3.66Bb | 3.65Bb | 4.56Cc | 4.58Cc | 4.25Cc | 4.32Cc |
| M50R23.5A23.5–I3O0 | 2.65Aa | 2.41Aa | 2.25Aa | 4.62Cc | 3.42Bb | 3.33Bb | 3.25Bb | 3.00Bb | 3.50Bb | 3.60Bb | 3.54Bb | 3.69Bb | 4.50Cc | 5.00Dd | 4.00Cc | 4.00Cc | |
| M50R23.5A23.5–I0O3 | 2.00Aa | 2.74Aa | 2.32Aa | 4.36Cc | 3.30Bb | 3.21Bb | 3.35Bb | 3.36Bb | 3.05Bb | 3.50Bb | 3.05Bb | 3.98Bb | 3.50Bb | 5.00Dd | 4.00Cc | 4.00Cc | |
| M50R23.5A23.5–I1.5O1.5 | 2.52Aa | 3.45Bb | 3.36Bb | 5.00Dd | 3.37Bb | 3.32Bb | 3.23Bb | 3.00Bb | 3.62Bb | 3.53Bb | 3.28Bb | 3.67Bb | 5.00Dd | 5.23Dd | 4.33Cc | 4.20Cc | |
| L. casei | M50R25A25–I0O0 | 2.05Aa | 2.00Aa | 2.02Aa | 4.01Cc | 2.00Aa | 2.12Aa | 2.03Aa | 2.30Aa | 3.85Bb | 3.98Bb | 3.31Bb | 3.68Bb | 3.32Bb | 4.25Cc | 3.31Bb | 4.30Cc |
| M50R23.5A23.5–I3O0 | 2.12Aa | 2.15Aa | 2.33Aa | 4.00Cc | 3.11Bb | 3.31Bb | 3.12Bb | 3.22Bb | 3.54Bb | 3.58Bb | 3.50Bb | 3.51Bb | 4.30Cc | 5.32Dd | 4.00Cc | 4.31Cc | |
| M50R23.5A23.5–I0O3 | 2.42Aa | 2.12Aa | 2.36Aa | 4.30Cc | 3.64Bb | 3.35Bb | 3.32Bb | 3.34Bb | 3.44Bb | 3.91Bb | 3.95Bb | 3.99Bb | 4.28Cc | 4.00Cc | 4.08Cc | 5.00Dd | |
| M50R23.5A23.5–I1.5O1.5 | 2.86Aa | 3.39Bb | 3.48Bb | 5.00Dd | 3.76Bb | 3.62Bb | 3.64Bb | 3.48Bb | 3.52Bb | 3.56Bb | 3.77Bb | 3.58Bb | 4.42Cc | 4.00Cc | 4.55Cc | 4.42Cc | |
| L. plantarum + L. casei | M50R25A25–I0O0 | 2.02Aa | 2.00Aa | 2.30Aa | 3.22Bb | 2.30Aa | 2.41Aa | 2.12Aa | 2.33Aa | 3.50Bb | 3.59Bb | 3.68Bb | 3.32Bb | 4.68Cc | 4.54Cc | 4.51Cc | 4.52Cc |
| M50R23.5A23.5–I3O0 | 2.21Aa | 2.03Aa | 2.25Aa | 4.30Cc | 3.37Bb | 3.31Bb | 3.21Bb | 3.00Bb | 3.53Bb | 3.95Bb | 3.58Bb | 3.94Bb | 4.30Cc | 4.21Cc | 4.20Cc | 4.22Cc | |
| M50R23.5A23.5–I0O3 | 2.35Aa | 2.36Aa | 2.21Aa | 4.23Cc | 3.69Bb | 3.65Bb | 3.41Bb | 3.47Bb | 3.58Bb | 3.69Bb | 3.98Bb | 3.50Bb | 4.36Cc | 4.00Cc | 4.12Cc | 4.84Cc | |
| M50R23.5A23.5–I1.5O1.5 | 3.62Bb | 3.67Bb | 3.35Bb | 5.00Dd | 3.56Bb | 3.48Bb | 3.14Bb | 3.27Bb | 3.65Bb | 3.53Bb | 3.35Bb | 3.58Bb | 4.68Cc | 4.89Cc | 4.64Cc | 4.57Cc | |
| Without probiotic | M50R25A25–I0O0 | 2.00Aa | 2.01Aa | – | – | 3.00Bb | 3.21Bb | 3.00Bb | 3.12Bb | 3.98Bb | 3.55Bb | 3.85Bb | 3.69Bb | – | – | – | – |
| M50R23.5A23.5–I3O0 | 2.21Aa | 2.28Aa | – | – | 3.54Bb | 3.90Bb | 3.34Bb | 3.65Bb | 4.02Cc | 3.94Bb | 3.71Bb | 3.98Bb | – | – | – | – | |
| M50R23.5A23.5–I0O3 | 2.50Aa | 2.34Aa | – | – | 3.51Bb | 3.90Bb | 3.33Bb | 3.98Bb | 3.85Bb | 3.50Bb | 3.59Bb | 3.65Bb | – | – | – | – | |
| M50R23.5A23.5–I1.5O1.5 | 2.52Aa | 2.27Aa | – | – | 3.21Bb | 3.67Bb | 3.36Bb | 3.85Bb | 3.65Bb | 3.99Bb | 3.55Bb | 3.38Bb | – | – | – | – | |
*(4 °C, 21 day)
**Letters ‘M, R, A, I and O’ represents millet, rye, alfalfa, inulin and oligofructose, respectively
***Means in a column or row shown respectively with different small or capital English letters, are significantly (p < 0.05) different
According to the sensorial analysis, although slight acidification of the beverage was detected by panelists, the beverage was acceptable for 21 days at 4 °C. All the beverages introduced in this study were in their natural form, without any additives or flavorings. The use of flavorings would considerably improve the sensorial characteristics of beverages.
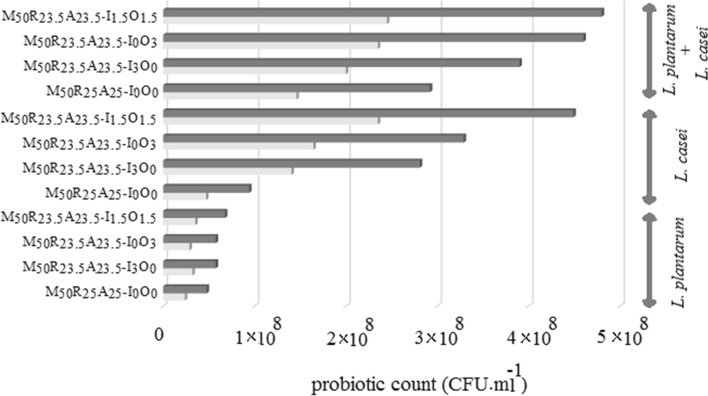
Probiotic survival under the simulated gastric condition
The resistance between the strains was noted as significant, while L. plantarum showed better resistance (Fig. 2) while based on the previous investigations, the highest resistance to simulated gastric condition was nominated for L. plantarum strains (Khalil et al. 2007; Pan et al. 2009; Kirtzalidou et al. 2011). It should be noted that the combination of strains had no significant effect on increasing the chance of survival of bacteria. The presence of oligofructose or inulin preserved the two strains in the simulated gastric condition. In this regard, Nazzaro et al. also declared inulin protected L. acidophilus against gastrointestinal juices (Nazzaro et al. 2012).
Fig. 2.
Probiotic cell survival under simulated gastric condition (letters ‘M, R, A, I and O’ represents millet, rye, alfalfa, inulin and oligofructose, respectively)
Probiotic cell survival in the digestive tract is the most important factor for selecting probiotic strains and for their efficiency (FAO/WHO 2006). However, various mechanisms are involved in surviving the bacteria under the gastric environment. The two bacteria evaluated in this study had suffered significantly under gastric conditions, although the number of viable cells was still more than 55%. High bacterial survival under simulated gastric condition increases the possibility of the viability of these cultures in the digestive tract.
Conclusion
For the first time, a synbiotic cereal sprout-based beverage was developed to evaluate the combined benefits of the probiotics L. casei and L. plantarum, and the prebiotic property of inulin and oligofructose besides nutritional profits of millet, rye and alfalfa sprouts. L. casei survived well (>107 CFU ml−1) throughout the refrigerated storage (4 °C, 28 days), while L. plantarum only survived at sufficient levels until day 21. Inulin and oligofructose positively affected the viability and sensory properties of beverage throughout the storage. Evaluation of bacterial survival under simulated gastric condition declared viability of over 55% of the strains. Moreover. The final product had acceptable sensory attributes. Therefore, the developed functional fermented beverage could be deemed as a standard healthy ready-to-drink product from functional, sensory and economical points of view. Further investigations might focus on finalizing and optimizing the sensory characteristics of the mentioned product using adequate flavorings and stabilizers.
Acknowledgements
This study was part of a Ph.D. thesis Mohsen Mohammadi in Food Science.
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest concerning the research, authorship, and publication of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afshari R, Hosseini H, Khaksar R, Mohammadifar MA, Amiri Z, Komeili R, Khaneghah AM. Investigation of the effects of inulin and β-glucan on the physical and sensory properties of low-fat beef burgers containing vegetable oils: optimisation of the formulation using D-optimal mixture design. Food Technol Biotechnol. 2015;53:436–445. doi: 10.17113/ftb.53.04.15.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis of the association official analytical chemists. Arlington: Association of Official Analytical Chemists; 2010. [Google Scholar]
- Cardarelli HR, Buriti FC, Castro IA, Saad SM. Inulin and oligofructose improve sensory quality and increase the probiotic viable count in potentially synbiotic petit-suisse cheese. LWT-Food Sci Technol. 2008;41:1037–1046. doi: 10.1016/j.lwt.2007.07.001. [DOI] [Google Scholar]
- Coda R, Rizzello CG, Trani A, Gobbetti M. Manufacture and characterization of functional emmer beverages fermented by selected lactic acid bacteria. Food Microbiol. 2011;28:526–536. doi: 10.1016/j.fm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Cornara L, Xiao J, Burlando B. Therapeutic potential of temperate forage legumes: a review. Crit Rev Food Sci Nutr. 2016;56:S149–S161. doi: 10.1080/10408398.2015.1038378. [DOI] [PubMed] [Google Scholar]
- De Souza Oliveira RP, Rodrigues Florence AC, Perego P, De Oliveira MN, Converti A. Use of lactulose as prebiotic and its influence on the growth, acidification profile and viable counts of different probiotics in fermented skim milk. Int J Food Microbiol. 2011;145:22–27. doi: 10.1016/j.ijfoodmicro.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Ebrahimi B, Mohammadi R, Rouhi M, Mortazavian AM, Shojaee-Aliabadi S, Koushki MR. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT. 2018;87:54–60. doi: 10.1016/j.lwt.2017.08.066. [DOI] [Google Scholar]
- El-Adawy TA. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Hum Nutr. 2002;57:83–97. doi: 10.1023/A:1013189620528. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (2006) Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO Food and Nutrition Paper, Roma, p 85
- Fernandez MA, Marette A. Potential health benefits of combining yogurt and fruits based on their probiotic and prebiotic properties. Adv Nutr. 2017;8:155S–164S. doi: 10.3945/an.115.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Orozco R, Piskula MK, Zielinski H, Kozlowska H, Frias J, Vidal-Valverde C. Germination as a process to improve the antioxidant capacity of Lupinus angustifolius L. var. Zapaton. Eur Food Res Technol. 2006;223:495. doi: 10.1007/s00217-005-0229-1. [DOI] [Google Scholar]
- Fuller R (2012) Probiotics: the scientific basis. Springer Science & Business Media
- Garro MS, de Valdez GF, Oliver G, de Giori GS. Growth characteristics and fermentation products of Streptococcus salivarius subsp. thermophilus, Lactobacillus casei and L. fermentum in soymilk. Z Lebensmittelunters Forsch A. 1998;206:72–75. doi: 10.1007/s002170050217. [DOI] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Heydari S, Amiri-Rigi A, Ehsani MR, Mohammadifar MA, Khorshidian N, Koushki MR, Mortazavian AM. Rheological behaviour, sensory properties and syneresis of probiotic yoghurt supplemented with various prebiotics. Int J Dairy Technol. 2018;71:175–184. doi: 10.1111/1471-0307.12491. [DOI] [Google Scholar]
- Hong Y-H, Wang S-C, Hsu C, Lin B-F, Kuo Y-H, Huang C-J. Phytoestrogenic compounds in alfalfa sprout (Medicago sativa) beyond coumestrol. J Agric Food Chem. 2010;59:131–137. doi: 10.1021/jf102997p. [DOI] [PubMed] [Google Scholar]
- Jafari M, Mortazavian AM, Hosseini H, Safaei F, Khaneghah AM, Sant'Ana AS. Probiotic bacillus: fate during sausage processing and storage and influence of different culturing conditions on recovery of their spores. Food Res Int. 2017;95:46–51. doi: 10.1016/j.foodres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Jain SK, Jain A, Gupta Y, Ahirwar M. Design and development of hydrogel beads for targeted drug delivery to the colon. AAPS PharmSciTech. 2007;8:E34–E41. doi: 10.1208/pt0803056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayamanne VS, Adams MR. Modelling the effects of pH, storage temperature and redox potential (Eh) on the survival of bifidobacteria in fermented milk. Int J Food Sci Technol. 2009;44:1131–1138. doi: 10.1111/j.1365-2621.2009.01931.x. [DOI] [Google Scholar]
- Kaur M, Asthir B, Mahajan G. Variation in antioxidants, bioactive compounds and antioxidant capacity in germinated and ungerminated grains of ten rice cultivars. Rice Sci. 2017;24:349–359. doi: 10.1016/j.rsci.2017.08.002. [DOI] [Google Scholar]
- Khalil R, El-Halafawy K, Mahrous H, Kamaly K, Frank J, El Soda M. Evaluation of the probiotic potential of lactic acid bacteria isolated from faeces of breast-fed infants in Egypt. Afr J Biotechnol. 2007;6:939–949. [Google Scholar]
- Khanniri E, Sohrabvandi S, Mortazavian AM, Khorshidian N, Malganji S. Effect of fermentation, cold storage and carbonation on the antioxidant activity of probiotic grape beverage. Curr Nutr Food Sci. 2018;14:335–340. doi: 10.2174/1573401313666170614100418. [DOI] [Google Scholar]
- Kirtzalidou E, Pramateftaki P, Kotsou M, Kyriacou A. Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe. 2011;17:440–443. doi: 10.1016/j.anaerobe.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Lamsal B, Faubion J. The beneficial use of cereal and cereal components in probiotic foods. Food Rev Int. 2009;25:103–114. doi: 10.1080/87559120802682573. [DOI] [Google Scholar]
- Lorenz K, D'Appolonia B. Cereal sprouts: composition, nutritive value, food applications. CRC Crit Rev Food Sci Nutr. 2009;13:353–385. doi: 10.1080/10408398009527295. [DOI] [PubMed] [Google Scholar]
- Mohammadi R, Mortazavian A. Technological aspects of prebiotics in probiotic fermented milks. Food Rev Int. 2011;27:192–212. doi: 10.1080/87559129.2010.535235. [DOI] [Google Scholar]
- Mohammadi R, Yousefi M, Sarlak Z, Shah NP, Mortazavian AM, Sadeghi E, Khajavi MZ. Influence of commercial culture composition and cow milk to soy milk ratio on the biochemical, microbiological, and sensory characteristics of a probiotic fermented composite drink. Food Sci Biotechnol. 2017;26:749–757. doi: 10.1007/s10068-017-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morah F. Effect of sprouting on nutritional value of Panicium miliaceum (proso millet) Edorium J Nutr Diet. 2017;4:1–4. [Google Scholar]
- Nazzaro F, Fratianni F, Nicolaus B, Poli A, Orlando P. The prebiotic source influences the growth, biochemical features and survival under simulated gastrointestinal conditions of the probiotic Lactobacillus acidophilus. Anaerobe. 2012;18:280–285. doi: 10.1016/j.anaerobe.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Nighswonger BD, Brashears MM, Gilliland SE. Viability of Lactobacillus acidophilus and Lactobacillus casei in fermented milk products during refrigerated storage1. J Dairy Sci. 1996;79:212–219. doi: 10.3168/jds.S0022-0302(96)76353-1. [DOI] [PubMed] [Google Scholar]
- Noori N, Hamedi H, Kargozari M, Shotorbani PM. Investigation of potential prebiotic activity of rye sprout extract. Food Biosci. 2017;19:121–127. doi: 10.1016/j.fbio.2017.07.001. [DOI] [Google Scholar]
- Pan X-D, Chen F-Q, Wu T-X, Tang H-G, Zhao Z-Y. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B. 2009;10:258–263. doi: 10.1631/jzus.B0820261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhi M, Mohammadi R, Mortazavian AM, Sarlak Z. Combined effects of replacement of sucrose with d-tagatose and addition of different probiotic strains on quality characteristics of chocolate milk. Dairy Sci Technol. 2015;95:115–133. doi: 10.1007/s13594-014-0189-y. [DOI] [Google Scholar]
- Sarteshnizi RA, Hosseini H, Khosroshahi NK, Shahraz F, Khaneghah AM, Kamran M, Komeili R, Chiavaro E. Effect of resistant starch and β-glucan combination on oxidative stability, frying performance, microbial count and shelf life of prebiotic sausage during refrigerated storage. Food Technol Biotechnol. 2017;55:475. doi: 10.17113/ftb.55.04.17.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyonov D, Ramon O, Kovacs A, Friedlander L, Shimoni E. Air-suspension fluidized-bed microencapsulation of probiotics. Dry Technol. 2012;30:1918–1930. doi: 10.1080/07373937.2012.708692. [DOI] [Google Scholar]
- Stone H (2012) Sensory evaluation practices. Academic Press
- Tárrega A, Costell E. Colour and consistency of semi-solid dairy desserts: instrumental and sensory measurements. J Food Eng. 2007;78:655–661. doi: 10.1016/j.jfoodeng.2005.11.003. [DOI] [Google Scholar]
- Tharmaraj N, Shah N. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J Dairy Sci. 2003;86:2288–2296. doi: 10.3168/jds.S0022-0302(03)73821-1. [DOI] [PubMed] [Google Scholar]