Abstract
Previous decades have witnessed a lot of challenges that have provoked a dire need of ensuring global food security. The process of augmenting food production has made the agricultural ecosystems to face a lot of challenges like the persistence of residual particles of different pesticides, accretion of heavy metals, and contamination with toxic elemental particles which have negatively influenced the agricultural environment. The entry of such toxic elements into the human body via agricultural products engenders numerous health effects such as nerve and bone marrow disorders, metabolic disorders, infertility, disruption of biological functions at the cellular level, and respiratory and immunological diseases. The exigency for monitoring the agroecosystems can be appreciated by contemplating the reported 220,000 annual deaths due to toxic effects of residual pesticidal particles. The present practices employed for monitoring agroecosystems rely on techniques like gas chromatography, high-performance liquid chromatography, mass spectroscopy, etc. which have multiple constraints, being expensive, tedious with cumbersome protocol, demanding sophisticated appliances along with skilled personnel. The past couple of decades have witnessed a great expansion of the science of nanotechnology and this development has largely facilitated the development of modest, quick, and economically viable bio and nanosensors for detecting different entities contaminating the natural agroecosystems with an advantage of being innocuous to human health. The growth of nanotechnology has offered rapid development of bio and nanosensors for the detection of several composites which range from several metal ions, proteins, pesticides, to the detection of complete microorganisms. Therefore, the present review focuses on different bio and nanosensors employed for monitoring agricultural ecosystems and also trying to highlight the factor affecting their implementation from proof-of-concept to the commercialization stage.
Keywords: Agroecosystems, Nanoparticles, Nanosensors, Biosensors, Pesticides, Heavy metals, Pathogens, Agricultural production
Introduction
The past several decades have witnessed a lot of challenges like perpetual demographic strain, unceasingly fluctuating climatic conditions, as well as the heightened sweepstakes for the resources, all of which have posed an egregious threat and thus provoked a dire need for guaranteeing global food security. The existing agricultural practices for fulfilling the food requirements include uncontrolled use of resources, sophisticated machinery as well as increasing and indiscriminate use of agrochemicals. These practices have led to significant deterioration of the soil, air, and water resources, thereby have expressively upturned the levels of pollution in the agricultural environments, which in turn has strongly affected human/animal health. The extent of health effects of pesticide use can be estimated from the information that 26 million people become victims of pesticide poisoning annually on a global basis which results in about 220,000 annual deaths [1]. Furthermore, due to their persistent nature, the residues of pesticides stay in the environment for a prolonged time period thereby contaminate the soil and thus raise concerns about the functioning of the soil, biodiversity, and food safety [2]. Moreover, there are many reports already available about the entry of pesticide residues in the food chain followed by their accumulation in the body of consumers which further results in severe health issues. The pesticides are also known to be cytotoxic and carcinogenic by nature [3–6]. They can also induce various nerve and bone marrow disorders, infertility, as well as respiratory and immunological diseases [7–10]. Therefore, the monitoring of pesticide residues in the environment becomes an imperative concern. Moreover, monitoring such residual pesticides regularly will also provide information about whether their occurrence is within or beyond the acceptable limits [11].
Another important challenge that is faced by the agroecosystems is the persistence of lethal heavy metals comprising cadmium, mercury, copper, zinc, nickel, lead, and chromium as they are held responsible for prolonged and significant damage to various biotic systems by disrupting biological actions at the cellular level [12, 13], for instance, via disruption of photosynthesis, disruption of mineral absorption, interruption of electron transport chain, induction of lipid peroxidation, disturbance in the metabolism of essential elements, induction of oxidative stress and by damaging the plant organs like root, leaves, and other cellular components [14–16]. Definitely, their natural occurrence in the earth’s crust is an undeniable fact but the uncontrolled anthropogenic activities have disturbed the geochemical cycling and biochemical balance of these elements to a remarkable extent. This has resulted in an increased prevalence of such metals in different plant parts. Together, all the risks posed by the presence and prevalence of heavy metals in various ecosystems emphasize the need to develop systems for sensing them even at low concentrations in environmental samples [17].
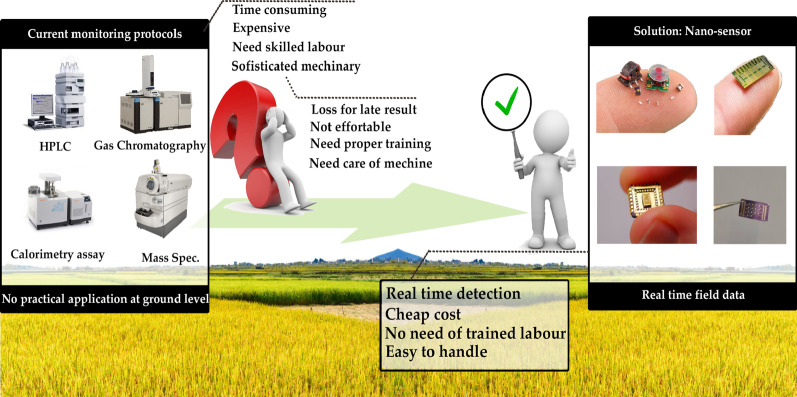
At present, various methods available for monitoring agroecosystems include gas chromatography, high-performance liquid chromatography, mass spectroscopy, and more (Fig. 1). All these techniques can easily detect and quantify contaminants in the environment as well as agricultural samples. On the contrary, the sensitivity, specificity, and reproducibility of such measurements are incontrovertible but the deployment of these methods is predominantly restricted by their time consumption, high cost, and requirement of sophisticated appliances along with skilled personnel [8]. Therefore, there is an impenetrable need for modest, quick, and economically viable methods for monitoring such agricultural contaminants [18–20]. Nanosensors are nanoscale element devices that are engineered to identify a particular molecule, biological component, or environmental circumstances. These sensors are highly specific, handy, cost-effective, and detect at a level much lower as compared to their macroscale analogs. A typical nanosensor device operation contains three basic components:
Sample preparation: It could be a homogenous or complex suspension of gas, liquid or solid-state. Sample preparation of agroecosystem is very challenging due to impurities and interferences. The sample contains specific molecules, functional groups of molecules or organisms, that the sensors can target. These targeted molecules/organisms known as the analyte and could be molecules (dyes/colors, toxicants, pesticides, hormones, antibiotics, vitamins, etc.), biomolecules (enzymes, DNA/RNA, allergens, etc.), ions (metals, halogens, surfactants, etc.), gas/vapor (oxygen, carbon dioxide, volatile compounds, water vapors, etc.), organisms (bacteria, fungi, viruses) and environment (humidity, temperature, light, pH, weather, etc.)
Recognition: Certain molecules/elements recognize the analytes within the sample. These recognition molecules are antibody, aptamer, chemical legends enzymes, etc., and having high affinity, specificity, selective characteristics to their analytes to quantify them to acceptance levels.
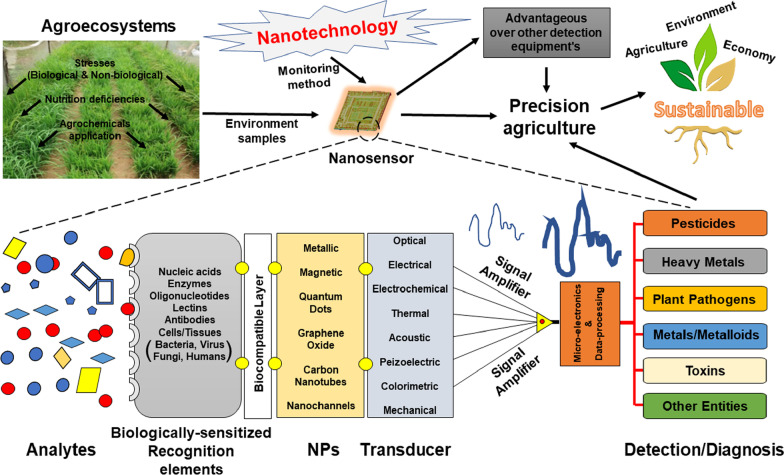
Signal transduction: Certain signal transduction methods have categorized these modest devices into different types such as optical, electrochemical, piezoelectric, pyroelectric, electronic, and gravimetric biosensors. They convert recognition events into computable signals that are further processed to produce the data (Fig. 2).
Fig. 1.
Schematic representation highlighting the differences between traditional and advanced monitoring technologies
Fig. 2.
Simplified representation illustrating the component of nanosensors to monitoring agroecosystems
The nano-technological interventions position the stimulus to transfigure the diverse zones of diagnostics like health, medication, food, environment, as well as the agriculture sector, thereby, transitioning the speculative characteristics into the practical output [21–28]. Nanotechnology plays a significant role in the advancement of numerous diagnostic methodologies by rendering mankind with contemporary tools comprising of sensors established on bio-techniques, nano-based medical facilities, along with bio-photonics which simplifies the detection of pesticides, drug residues, food-borne pathogenic microorganisms, toxin contaminants, and heavy metal ions [24, 29]. Fortunately, the arena of nanotechnology comprises an understanding coupled with governing material at the atomic or molecular scale where matter unveils distinctive attributes and performances when equated to the bulk form of similar matter [30]. Currently, among all the approaches, a biosensor is a modest and compacted investigative device that has the capability of producing definite systematic data either in a quantitative way or in a semi-quantitative form by employing a recognition component of biological origin which is joined to a signal transformation unit [31–33]. The type of employment of the signal transduction method has categorized these modest devices into different types such as optical, electrochemical, piezoelectric, pyroelectric, electronic, and gravimetric biosensors [34]. The recent advances in nanotechnology have opened various new ways for designing biosensors [29, 35]. The hybridization of nano-materials with different biosensing daises (nano-bio sensors) offers a great deal of conjoining and multipurpose approaches for enhanced sensitivity for detection [36] and thereby improves the capability in the monitoring of even a single molecule [32, 37, 38]. The nanoscale has been defined approximately as 1–100 nm, which is also equivalent to a billionth part of a meter. It can be easily understood by comparing it with the dimensions of an average bacterial cell which is around 1000 nm in diameter [39]. The nanomaterial that is employed in sensing is called a nanosensor which is constructed at the atomic scale for data collection. The nanomaterial is further reassigned into information which can be analyzed for several applications, for instance, to keep an eye on various physical and chemical portents in areas hard to approach, detect different chemicals of biological origin in various cellular organelles, and determine particles of nanoscale in the environment and the industry [40, 41]. The presence of even a single virus particle and substances present in very low concentrations can be detected using nanosensors. A nanosensor is comprised of a bio-sensitive layer that is attached covalently to another element called a transducer. The physiochemical change produced due to the interactions of the target analyte with the bioreceptor is converted into an electrical signal [40].
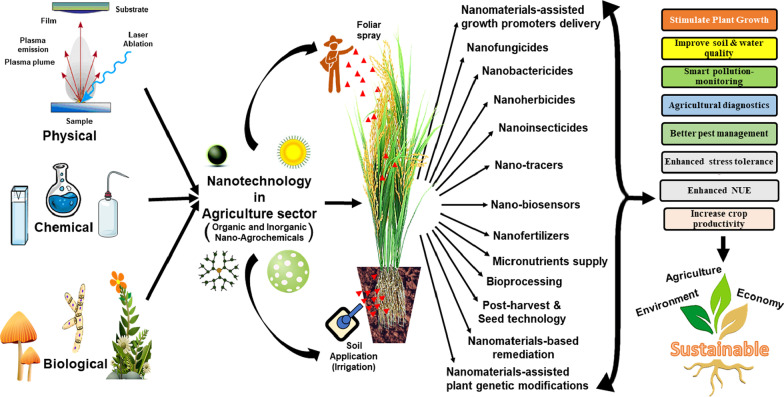
In recent years, a great deal of superior visual recognition bio and nanosensors have been employed for the detection of several composites from a vast array of samples. The range of composites covers several metal ions, proteins, pesticides, antibiotics to the detection of complete microorganisms, and nucleic acid amplification and sequencing [19, 33, 42, 43]. Apart from monitoring the agricultural-controlling process and residues, other potential applications of nanotechnology have also been surfaced in the last two decades [44–47]. The imperative benefits for engaging nanotechnology in the improvement of the agriculture sector include nanomaterials-assisted delivery of growth promoters [44, 48, 49], nutrition (especially micronutrients) [49, 50] as well as genetic modifications in plants [51, 52]. Additionally, various pesticides in form of nanofungicides, nanobacteriocides as well as nanoinsecticides have been also found to be employed [50, 53–55]. Furthermore, other benefits of nanotechnology include nanomaterials-based remediation [56], nanoherbicides [57] as well as uses in bioprocessing [58], aquaculture [59], post-harvest technology [60], veterinary care [61], fisheries [62], and seed-technology [63]. All these applications together show various advantages like reduced pollution (mainly soil and water), reduction in related costs of environmental protection, and enhanced nutrient use efficiency [45, 46, 50, 56, 64–68] (Fig. 3). Given the above-mentioned facts, the present review targets the employment of different kinds of nanosensors in different agroecosystems for revealing different components along with the detection of some foreign components intruding the natural agroecosystems.
Fig. 3.
Various applications of nanotechnology in the agriculture sector
Nanosensors for Pesticide Detection
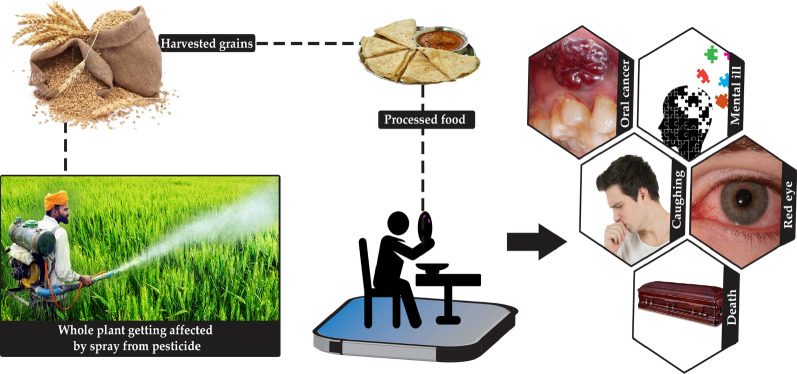
Pesticides find broad applications in agricultural systems for the avoidance, regulation, or abolition of pests, insects, weeds, and fungi to increase the productivity of agroecosystems [69]. The use of pesticides is on a perpetual increase and they might secure almost one-third share of the global agricultural products [70]. However, the indiscriminate usage of pesticides at field conditions has contaminated the groundwater and marked their accumulation in the food resources, thereby has also seriously affected non-target species like human beings and animals (Fig. 4). The exposure of humans to pesticides can affect health in diverse ways and the attendant health effects produced can range from mutagenicity, neurotoxicity, carcinogenicity to genotoxicity [71, 72]. Some pesticides like organophosphates accrue in the animal bodies even with their application in a small concentration and exposure to higher concentrations leads to the inhibition of enzymes like acetylcholinesterase that impart severe health risks to humans [73]. Therefore, to ensure food safety, the development of superior methods of detecting pesticide residues is very important.
Fig. 4.
Adverse effects of pesticides on human health
Although various approaches are being used from a very long time for the detection of pesticide residues like high-performance liquid chromatography, colorimetric assays, enzyme-linked immune sorbent assay, liquid/gas chromatography-mass spectrometry, electrophoresis, and fluorimetric assay procedures [8, 74–79]. Nevertheless, the majority of these techniques are single-signal assays that require costly apparatus, professional operators, and complex pretreatment of the samples whereas some are even prone to variations in the environmental conditions [80, 81]. Therefore, such detection measures are not suitable for the on-site detection of residual pesticides. Additionally, they are also not found to be appropriate for real-time detection which constraints their use in emergency cases [82]. Consequently, detection methods employing multiple signals enhance the reliability and convenience of the analysis. For instance, methods targeting a combination of a multi-signal fluorimetric method with colorimetric assays are capable of circumventing the influence of background in multifaceted structures and complement naked-eye sensing in different practical solicitations [83]. Therefore, concentrating more effort in evaluating different approaches for the detection of pesticides in a speedy, simplistic, selective, delicate, precise, and comprehensible means has led to the development of optical sensors for detecting pesticide residues [80].
Numerous optical strategies have already been recognized for pesticide detection which exploited recognition elements like enzymes, antibodies, molecularly imprinted polymers, aptamers, and host–guest recognizers. Such approaches can staunchly recognize and detect the particular pesticidal particle [81, 84–88]. Furthermore, the coupling of recognition components with the nanomaterials results in greater levels of sensitivity and tremendous specificity for instantaneous deployment, which is a principal requirement for expeditious and efficacious pesticide detection [82]. So the quest for a prompt, sensitive, specific, precise, and easy to operate method for detecting residual pesticides has resulted in the deployment of nanosensors as a pre-eminent substitute to conventional methods due to their cost effectiveness, compactness, ease of transportation, extraordinary sensitivity, and a lesser time of detection [89] (Fig. 1).
In general, an optical sensor is composed of a recognition element that is specific for the particular residual pesticidal particle and can network with the other constituent, the transducer, which is employed to produce the signal for the binding of a particular pesticide residue to the sensor. The recognition components which are comprised of enzymes, antibodies, molecularly-imprinted polymers, aptamers, and host–guest recognizers, are gripping the consideration of the scientific community for improving the diagnostic performance of any sensor. The prevailing entrenched optical probes could be categorized into four types based on signal output formats. These are fluorescence (FL), colorimetric (CL), surface-enhanced Raman scattering (SERS), and surface plasmon resonance (SPR) optical sensors [90].
Another kind of nanosensors widely known are immunochromatographic strip (ICTS) nanosensors that are broadly accredited in point-of-care analytical devices [91]. The immunochromatographic assays have also been reported for their involvement in monitoring agroecosystems owing to their point-of-care testing behavior. For instance, a visible colorimetric readout strategy was adopted in the reported immunochromatographic assay for the detection of GM crops, which only provided a yes/no response and often suffered from insufficient sensitivity [92–94]. Similarly, the gold nanoparticle-based ICTS sensors have also been reported to possess low detection sensitivity, owing to the production of relatively weaker color density, which limits their application [95, 96]. However, their sensitivity can be improved by several proposed amplification strategies like augmenting detection signal intensity, enhancing the affinity of the reagent, optimizing the labeling techniques, and amending the shapes of strip devices [96]. Therefore, the improved ICTS nanosensors can also prove to be an economically viable tool for pesticide residue detection in agroecosystems.
The amalgamation of nanotechnology with different electrochemical approaches compromises a superior operational surface area to the sensor along with a decent check on the electrode micro-environment. Nanoparticles owe divergent and numerous properties thereby possess the potential to play multiple purposes in the sensing structures grounded on electrochemical phenomena, for instance, catalyzing the electrochemical reactions, enhancing the transfer of electrons, tagging, and performing as a reactant [97]. Therefore, electrochemical nanosensors appear to be an effective tool meant for pesticide detection. Recently, electrochemical biosensors that were primarily grounded on the enzyme cholinesterase appeared as propitious devices meant for detecting residual pesticidal particles especially belonging to the class carbamates and organophosphates attributable to their great perceptiveness, choosiness, and painless methods of creation [98, 99]. Nevertheless, enzyme-based biosensors undergo quite a lot of restrictions comprising high price, diminished activity of the enzyme, and truncated reproducibility [100]. Moreover, enzymes seem to be inherently unstable and are also subject to denaturation in hostile environmental conditions which restricts the lifetime of biosensors thereby limiting their practical applications [101]. Additionally, a manifestation of several impurities such as the occurrence of different heavy metals in the samples of biological origin can also disturb the selectivity as well as the sensitivity of the enzyme during the detection that may produce false-positive results [102]. Therefore, it provokes the need for non-enzymatic electrochemical biosensors. Nanomaterials appear to be promising contestants to formulate non-enzymatic electrochemical sensors [103]. Various categories of nanomaterials comprising nanoparticles (e.g., CuO, CuO–TiO2, and ZrO2, NiO), nanocomposites (such as molybdenum nanocomposite), and nanotubes (e.g., peptide and carbon nanotubes) are widely found to be engaged in electrochemically determining the residual pesticidal particles [104–106]. The explicit and profound investigation of the residual pesticidal particles by such nanomaterials is attributable to their extremely small size, greater surface area, and the possession of inimitable electrical as well as chemical properties [70].
The sensitivity, as well as selectivity of various nanosensors for definite pesticides, has been reported in various studies (Table 1), for instance, the two different optical sensors grounded on silver nanodendrites and upconverting nanoparticles were found to detect the pesticides dimethoate and metribuzin at the levels of 0.002 ppm and 6.8 × 10−8 M, respectively [107, 108]. Similarly, the electrochemical nanosensor grounded using CuO nanoparticles decorated with 3D graphene nanocomposite detected malathion at the level of 0.01 nM [109] whereas the electrochemical aptasensor fabricated through chitosan-iron oxide nanocomposite detected malathion at a surprising sensitivity of 0.001 ng/mL [110].
Table 1.
Highlights of nanosensors for pesticide detection
| Nanosensor type | Used nanomaterial | Pesticide detected | Limit of detection | Method of nanosensor Formulation | Sensing mechanism | Observation | References |
|---|---|---|---|---|---|---|---|
| Fluorescent-nanosensor | 3-aminopropyl-triethoxysilane coated Yb2O3 | Imazapyr | 0.2 ppm | Hydrothermal method | Quenching of fluorescence intensity for APTES coated Yb2O3 NPs with the increasing concentration of imazapyr | Among the lanthanide oxide based nanomaterials, ytterbium (III) oxide (Yb2O3) NPs owes unique optical and luminescence properties with excellent efficiency for real field conditions | [173] |
| Surface plasmon resonance (SPR) based affinity sensor | Atrazine imprinted nanoparticles | Atrazine | 0.7134 ng/mL | Atrazine imprinted nanoparticles synthesis using emulsion polymerization method followed by their attachment on the gold surface of SPR | Increase in resonance frequency in proportion to the increment in atrazine concentration | The plastic antibody-based SPR nanosensor is an attractive recognition element for the detection of atrazine with high selectivity and sensitivity | [174] |
| Surface plasmon resonance based fiber–optic nanosensor | Tantalum(V) oxide nanoparticles | Fenitrothion | 38 nM | Chemical synthesis of Ta2O5 nanoparticles embedded in reduced graphene oxide matrix followed by its adhesion on silver-coated fiber optic probe | Change in refractive index due to the interaction of fenitrothion with the silver film | The sensor is selective, repeatable and works at ambient temperature with a response time of 23 s | [175] |
| Fluorescence sensor | Copper (II) oxide and multiwall carbon nanotubes (MWCNTs) | Glyphosate | 0.67 ppb | CuO/MWCNT were prepared by precipitating copper nitrate by the addition of aqueous NaOH solution | Inhibition of the catalytic activity of CuO/MWCNTs | A highly selective & promising approach for rapid screening of glyphosate | [176] |
| Electrochemical Luminescence sensor | Luminol-gold nanoparticles-L-cysteine-Cu(II) composites | Glyphosate | 0.5 nM | Layer-by-layer assembly of graphene-gold nanoparticle composite and Lu-Au-Lcys-Cu(II) composite | Decrease in electrochemical luminescence intensity with a respective increase in the glyphosate concentration | The sensor worked on dual inhibition strategy with excellent detection performance, high sensitivity, desirable reproducibility, stability, and accuracy | [177] |
| Electrochemical sensor | CuO-TiO2 hybrid nanocomposites | Methyl parathion | 1.21 ppb | CuO-TiO2 nanocomposites prepared by a facile liquid-control-precipitation method were decorated on the glass carbon electrode | Differential pulse voltammetry measurements assessed from decline in current density with increase in the methyl parathion concentration | A non-enzymatic sensor with good stability and excellent reproducibility | [103] |
| Electrochemical aptasensor | Chitosan-iron oxide nanocomposite | Malathion | 0.001 ng/mL | Iron Oxide nanoparticles synthesized using chemical co-precipitation method were deposited on fluorine tin Oxide followed by the immobilization of aptamer onto the iron oxide doped-chitosan/FTO electrode using streptavidin | Decline in the Differential Pulse Voltammetry peak current of the aptaelectrode with a corresponding increase in malathion concentration due to the formation of more 3D-complex between aptamer with malathion | A very attractive alternative to quantify and monitor malathion due to its sensitivity, stability, short analysis time and cost-effectiveness | [110] |
| Electrochemical nanosensor | CuO nanoparticles decorated 3D graphene nanocomposite | Malathion | 0.01 nM | Copper oxide nanoparticles electro-catalyst was prepared on 3D graphene synthesized using hydrothermal process | Decline in peak current with the increasing concentrations of malathion | Highly sensitive, reproducible and applicable in real field conditions | [109] |
| Optical nanosensor | Silver nanodendrites | Dimethoate | 0.002 ppm | Ag nanodendrites fabricated by laser-assisted photochemical method were immobilized on the surface of microsphere end-shape optical fibre | Increase in the intensity of the surface-enhanced Raman spectroscopy (SERS) signal with a proportionate increase in dimethoate concentration | A direct, rapid, real-time and non-destructive method of detecting pesticide residue in the outdoor fields | [107] |
| Optical sensor | Upconverting nanoparticles (UCNPs) of the NaYF4:Yb, Er type | Metribuzin | 6.8 × 10−8 M | Upconverting nanoparticles synthesized using the coprecipitation method of lanthanide metal-EDTA complexes were later used in the preparation of the sensor film by dissolving UCNPs in tetrahydrofuran along with the incorporation of NIR dye, PVC polymer, dioctyl phthalate | Metribuzin changes the color of sensor film from green to blue with a significant blue shift in the absorption peak | Highly sensitive sensor with unique luminescence properties of UCNPs and great recognition abilities within a very low detection limit | [108] |
Nanosensors for Detection of Heavy Metals
The existence of diverse heavy metal ions like Pb2+, Hg2+, Ag+ , Cd2+, and Cu2+ from different resources has a precarious influence on human beings as well as their surroundings. The accretion of heavy metals in different environments is supported by the uninterrupted boost in the agricultural and industrial accomplishments along with the inadequate discharge of heavy metal ions from wastewaters and domestic emissions [111]. Therefore, to assure the security of the environment along with the health analysis, the ferreting out of the trace heavy metal ions through proficient practices is extremely desired. The apprehension of heavy metals can be accomplished by exploring several analytical systems [112], for instance, X-ray fluorescence spectrometry (XRF), atomic absorption spectrometry (AAS), atomic emission spectrometry (AES), and inductively coupled plasma mass spectrometry (ICP-MS) but their application suffers a lot of limitations like lavishness of devices, time-consuming methods, and labor intensiveness. Therefore, to guide these restrictions, numerous types of optical, electrochemical, and colorimetric stratagems have been comprehensively scrutinized (Table 2) to contrive modest and lucrative daises for apprehending delicate, hasty, and discerning exploration of heavy metal ions [113, 114].
Table 2.
Recent developments in nanosensors for the detection of heavy metals
| Nanosensor type | Used nanomaterial | Detected heavy metal | Limit of detection | Method of nanosensor formulation | Sensing mechanism | Observation | References |
|---|---|---|---|---|---|---|---|
| ICTS Nanosensor | Au | Cadmium | 0.35 µg/L | The Cd(II)-EDTA-BSA antigen and goat anti-mouse IgG were dispersed on the Nitrocellulose (NC) membrane followed by the addition of concentrated colloidal gold probe on glass fiber membrane | Decline in color intensity with the increase in the concentration of Cd(II) | A highly sensitive sensor specific for the detection of cadmium | [178] |
| ICTS Nanosensor | Au | Lead | 0.19 ng/mL | Au nanoparticle conjugates were prepared using anti-Pb(II)-ITCBE monoclonal antibody and colloidal gold solution. The lateral flow assay strip for detecting lead ions was constructed using an NC membrane, absorbent pad, and two conjugate pads | Decline in color intensity with the increase in the concentration of Pb(II) | The detection method could be accomplished within 15 min | [179] |
| Optical | Nanohybrid CdSe QDs | Cadmium | 25 nM | Amino capped CdTe@SiO2 core–shell structured fluorescent silica NPs synthesized using a modified reverse microemulsion method. The green-emitting dual-stabilizers capped CdSe QDs were covalently linked to the silica surface to form CdTe@SiO2@CdSe ratiometric probes | On the cadmium introduction the green photoluminescence got gradually restored | An alternative sensing approach for highly sensitive and selective detection | [117] |
| Colorimetric nanosensor | Mesoporous silica nanoparticles (MSN) | Mercury | 60 pM | The nanodevice was fabricated by implanting new dithiocetal-grounded stimulus receptive molecular gates on MSN loaded with a reporter dye | Hg(II) has high affinity for sulfur, and can disrupt the linear dithioacetal linkages upon interaction with solid S2, yielding new Hg(S-R)2 leading to subsequent cargo release | The nanosensor displays a sensitivity of 29.9 a.u/μM | [180] |
| Colorimetric nanosensor | Au | Pd(II) | 4.23 µM | Gold nanoparticles were stabilized by cationic 1-(3-(acetylthio)propyl)pyrazin-1-iumligand to detect Pd(II) | Pd(II) selectively induced the aggregation of APP-AuNPs as compared to other metals, resulting into the complete significant disappearance of surface plasmon resonane | The nanosensors warrants naked eye detection | [181] |
| Colorimetric nanosensor | Silver-coated gold nanobipyramids | Mercury | 0.8 µM | The Au NBs were synthesized as per the seed-mediated growth method and later Au NBs@Ag nanoparticles were synthesized by adding different volumes of AgNO3 to Au NBs colloidal solution | Hg2+ detection is achieved by etching silver-coated gold nanobipyramids which brings a color change | The method is devoid of tedious procedures and is time-saving | [182] |
| Multimodal nanosensor | Superparamagnetic Fe2O3 nanoparticles | Mercury | 0.49 nM | Fe2O3 nanoparticles prepared by the chemical coprecipitation method were coated by silica and later electrostatically attached with the cysteamine capped CdTe QDs | Fluorescence quenching with increasing concentrations of Hg2+ | The detected analyte can be removed with the use of an external bar magnet leaving no residual pollution | [124] |
| Surface plasmon resonance | Epicatechin coated silver nanoparticles (ECAgNPs) | Lead | 1.52 μM | The ECAgNPs, were prepared by mixing various ratios of AgNO3 and epicatechin followed by magnetic stirring and was later used for lead detection | The metal exhibited hyperchromic shift upon binding with epicatechin based silver nanoparticles | ECAgNPs can selectively detect Pb2+ even in the presence of other interfering metal ions | [126] |
| Electrochemical sensor | Nano sheets of Fc-NH2-UiO-66, and thermally reduced graphene oxide (trGNO) | Cadmium, Lead and Copper | 8.5 nM for Cd2+, 0.6 nM for Pb2+ and 0.8 nM for Cu2+, respectively | NH2-UiO-66 was synthesized by hydrothermal method whereas N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) were used as crosslinking agents to prepare Fc-NH2-UiO-66 followed by the dispersion of Fc-NH 2—UiO-66 on the trGNO nanosheets | The peak current increases with the increasing concentrations of heavy metal | A very good platform for simultaneous detection of multiple heavy metal ions | [183] |
| Magnetic-fluorescent based nanosensor | Carboxymethyl chitosan-functionalized magnetic-fluorescent nanocomposites | Mercury | 9.1 × 10−8 mol/L | Carboxymethyl chitosan was used as encapsulation agent to package Fe3O4 nanoparticles and QDs, resulting in the multifunctional magnetic-fluorescent nanoparticle which were later used as nanosensors | Quenching of nanosensor’s fluorescence | The nanosensor shows a superior selectivity and sensitivity for Hg2+ ions | [128] |
Optical chemical sensors that are frequently targeted for heavy metal detection fit into a cluster of chemical sensors that primarily employ electromagnetic radiation for engendering a diagnostic signal in an element known as the transduction element. The interactions between the sample and the radiation change a specific optical consideration that can be interrelated to the concentration of an analyte [115, 116]. For instance, the optical nanosensor synthesized using nanohybrid CdSe quantum dots for the detection of cadmium restored its green photoluminescence on the sensation of cadmium metal [117]. The optical chemical sensors work on the principle of seemed variations in the optical possessions (emission, absorption, transmission, lifetime, etc.) which appear as a result of binding of the arrested indicator (organic dye) with the analyte [118]. The approach of enticing graphene-based nanotechnology embarks as an attributable tool that incapacitates such challenges and bequeaths the sensing platform with enhanced performance. The optical techniques predominantly grounded on nanomaterials of graphene-origin have been advanced in recent times as one of the rousing practices for detecting heavy metal ions owing to the probable eminences of their meek construction and sentient appreciation of some distinctive metal ions [116].
The noble nanoparticles like Ag, Au, Pd are endowed with a unique trait of mimicking peroxidase activity, and their congregation with graphene boosts their sturdiness along with superior catalytic performance. There is a diverse magnitude of sensors concerned with the detection of numerous heavy metal ions based on this feature. The hybridization of graphene oxide with silver nanoparticles resulted in nanohybrids mimicking the peroxidase enzyme activity and they were further found to be able to discriminate amid double-stranded and single-stranded DNA molecules. Therefore, making the calorimetric detection of Pb2+ and Hg2+ suitable based on the metal ion-provoked change in the DNA conformation because the conformation was altered into either a quadruplex arrangement or a hairpin-like assembly in their occurrence [119, 120]. Moreover, such colorimetric approaches are advantageous due to their simple operation, economically feasible, transportable instrumentation, and easy-to-use applications. The chemosensors for detecting heavy metals are found to be troublesome for the elimination of the objective species as they would result in secondary pollution. Therefore, the integration of fluorescent and magnetic functionality together in a sole nanocomposite particle seems to be a capable substitute [121]. Nevertheless, the manifestation of the magnetic nanoparticles strongly quenches the photoluminescence of the fluorescent moiety, thus ascend a staid challenge towards the development of such kinds of nanocomposites. Therefore, to steer this concern, numerous interactions happening at the molecular level, such as hydrophobic and electrostatic interactions, hydrogen bonding, and covalent bonding are often targeted for nanocomposite synthesis. For instance, the quantum dots placed on the shallow of polymer-layered Fe2O3 globules by employing the approaches of thiol chemistry. The gold nanoparticles arrested on the surface of several materials including Fe2O3 nanoparticles, and the silica microspheres employing electrostatic connections have also been synthesized [122, 123].
The approach of synthesizing multimodal nanosensors using principles of nano-chemistry is rather more appealing as it not only efficiently detects but also removes the heavy metal ions in the aqueous media. The multimodal nanosensor synthesized by Satapathi et al. [124] through multistep production practice, entailed a thin silica shell that encapsulated the magnetic (Fe2O3) nanoparticles, an immovable spacer arm, and a fluorescent quantum dot meant for the coinciding recognition as well as the elimination of the spotted mercury ion. The exceptional sensitivity of this nanosensor can be marked by its capability of detecting Hg2+ at the nanomolar level with a limit of detection of just 1 nm. The eco-friendly aspect of nanosensor can be advocated by the unique attribute of removing the detected analyte by using an external bar magnet thereby leaving no leftover as a pollutant. Several compounds are used for stabilizing nanosensors, such as polysaccharides citrates, different polymers, and proteins to improve the attributes of the nanosensors [125]. The silver nanoparticles stabilized with epicatechin can be used for discerning detection of Pb2+, that too, in the occurrence of different snooping metal ions. The low limit of detection, easy synthesis, admirable discernment, and economical production, make ECAgNPs, a potent sensor destined for repetitive checking of Pb2+ intensities in the ecological models [126]. The employment of quantum dots offers remarkable advantages in terms of their photophysical as well as chemical attributes, thereby, making fluorescent quantum dots-based sensors an efficient tool for sensing numerous metal ions [127, 128]. However, the major disadvantage with the employment of quantum dots is their separation and recovery in practical applications which happens to be an immoderate, laborious, and tedious task. Nevertheless, the introduction of magnetic nanomaterials (Fe3O4) into the quantum dot-based fluorescence sensors solves this problem and offers several additional advantages owing to their high specific surface area, special magnetic properties, magnetic operability, and low toxicity. Yang et al. [128] established multifunctional magnetic-fluorescent nanoparticles grounded on the carboxymethyl chitosan amalgamated with fluorescent quantum dots and magnetic nanomaterials which could detect and separate Hg2+ simultaneously along with a sensing level of 9.1 × 10−8 mol/L. Thus, the unpretentious and sophisticated methodology of nanotechnology offers a direction concerning field-based heavy metal sensory devices in the future which now appears to be a difficult task along with various limitations.
Nanosensors for Detecting Plant Pathogens
The ascertainment, recognition, and assessment of pathogens are vital for scientific elucidation, ecological surveillance, and governing food security. It is imperative for investigative outfits that the delicate element of biological origin, which is a constituent of biological provenance or biomimetic constituent, interacts with the analyte in the examination. There are numerous profound, trustworthy, and swift recognition components, for instance, lectin, phage, aptamers, antibody, bacterial imprint, or cell receptor, which have been described for exposure of bacteria [129]. The most widely used biosensing components for analyzing pathogens are bacterial receptors, antibodies, and lectins. These constituents find wide applications as biosensing components to scrutinize pathogens owing to their adaptability of amalgamation into biosensors [130, 131]. Aptamers, the nucleic acids having only a single strand, are economically feasible and chemically steady, as compared to the recognition elements which are based on the antibodies for detecting bacteria [132]. However, they also pose various disadvantages like batch-to-batch variations, sturdiness in complex materials and they are also comparatively complex to prepare. The approach pointing to ‘chemical nose’ is a recently established equipment for detecting pathogens. It appoints multifarious discriminatory receptors that generate a unique response configuration for every objective, thus permitting their ordering. It functions in a fashion analogous to the working of our intellect of smelling something [133]. This technique involves the training of sensors with competent bacterial samples to establish a reference database. The identification of bacterial pathogens is done by equating them with the reference catalog [134]. Usually, nanoparticle-centered “chemical nose” biosensors necessitate the amendment of the surface of the nanoparticle with several ligands where an individual ligand is liable for a distinctive communication with the objective [133]. The variance in the size, as well as the external make-up of the nanoparticles, is selected in a way that every single set of particles can retort to different classes of bacteria in an inimitable way thereby offers supplementary features to the absorption spectrum.
The addition of nanoparticles to the bacteria leads to the development of aggregates encompassing the bacteria as a result of electrostatic interfaces amid the anionic sections of the bacterial cell walls and cationic cetyltrimethylammonium bromide (CTBr). This process of aggregation promotes a change of color induced by a swing in localized surface plasmon resonance. The color variation is further denoted by procuring an absorption spectrum in the existence of several bacteria [135, 136]. The components of the bacterial cell wall which are responsible for this kind of aggregation are teichoic acids in Gram-positive and lipopolysaccharides and phospholipids in Gram-negative bacteria [137]. These aggregation patterns are unique and are motivated by the occurrence of extracellular polymeric substances on the bacterial surface. These varying aggregation patterns are accountable for offering discernable colorimetric responses. Therefore the “chemical nose” established on nanoparticles could be accomplished to sense blends of varying bacterial species. During infections the “chemical nose” is potent enough to differentiate amid polymicrobial and monomicrobial cases, which facilitates superior effectiveness along with prompting antimicrobial therapy, precluding the requirement of extensive and prolonged testing of the sample [133]. The multichannel nanosensors are highly sensitive and can detect bacterial species even strains present in biofilms within minutes. Li et al. [138] established a multichannel sensor based on gold nanoparticles (AuNPs) and used it to spot and recognize biofilms based on their physicochemical attributes. The sensitivity of the nanosensor can be well advocated by its ability to discriminate amongst six biofilms. Another sensor which was designed based on hydrophobically employed gold nanoparticles by Phillips et al. [139] rapidly recognized three different strains of E. coli. The conjugated polymers bearing negative charge in the sensor systems were eventually replaced by the pathogenic cells which differentially restored the polymer fluorescence.
Nanotechnology offers novel prospects for redefining the constraints of human discernment. In the course of evolution, the olfactory system of human beings has got the unique ability to detect volatile organic compounds present at tremendously low concentrations in different complex environments [140]. The great sensitivity and flexibility of human beings to differentiate more than a trillion olfactory stimuli marks olfaction as an encouraging dais for different biotechnological applications [141, 142]. Various effective sensors that primarily function based on olfaction have been proposed for unveiling bacteria. The system of such nanosensors is mainly encompassed of three different constituents: 1) surface-functionalized nanoparticles, 2) pro-smell fragments, and 3) enzymes that slice the pro-fragrances for generating the olfactory output. The fine-tuning of these three components offer a delicate sensory system, which allows the rapid detection of bacteria at levels as low as 102 CFU/ML [143]. The introduction of magnetic nanoparticles also enables the separation, purification, and recognition of pathogens under complex environments. The nanomaterial-grounded, ‘enzyme nose’ nanosensor is also a convenient investigative method meant for detecting toxicologically significant targets present in natural samples. Sun et al. [134]designed a unique enzyme nanosensor, which was grounded on the non-covalent centers, for detecting pathogens. The employment of magnetic nanoparticles–urease sensors permitted the profound recognition of bacteria with a precision of 90.7% at the concentration of 102 CFU/LL in a very small time of 30 min. Similarly, various other different types of optical, electrochemical, and immunosensors have also been developed for detecting diverse plant pathogenic microorganisms (Table 3). For instance, the optic particle plasmon resonance immunosensor synthesized using gold nanorods effectively detected Cymbidium mosaic virus (CymMV) or Odontoglossum ringspot virus at the concentrations of 48 and 42 pg/mL (Lin et al. 2014) whereas the Fe3O4/SiO2 based immunosensor revealed the presence of Tomato ringspot virus, Bean pod mottle virus and Arabis mosaic virus at the concentrations of 10−4 mg/mL [144]. Therefore, directing the performance of approachable nanomaterials at the molecular scale can be exploited to revise the annotations of humans regarding their environments in a fashion that seems otherwise unmanageable.
Table 3.
Review of literature illustrating the nanosensors for detecting other entities
| Nanosensor type | nanomaterial used | Entity detected/purpose | Limit of detection | Method of nanosensor formulation | Sensing mechanism | Observation | References |
|---|---|---|---|---|---|---|---|
| Optic particle plasmon resonance (FOPPR) immunosensor | Gold nanorods | Cymbidium mosaic virus (CymMV) or Odontoglossum ringspot virus (ORSV) | 48 and 42 pg/mL for CymMV and ORSV respectively | Gold nanorods were synthesized using the seed-mediated growth method followed by their immobilization on the fiber core surface. Afterward, the AuNR surface was functionalized by antibodies through sinking the fiber in a solution of CymMV or ORSV antibody | Detection strategy is based on the localized evanescent field absorption by the AuNRs upon biomolecular binding which results in decreased transmission intensity measured at the distal end of the fiber | This nanosensor solves the problem of color interference encountered by using AuNSs, provides faster analysis, better reproducibility, and lower detection limit | [184] |
| Nanoparticles based immunosensor | Fe3O4/SiO2 | Tomato ringspot virus (ToRSV), bean pod mottle virus (BPMV) and arabis mosaic virus (ArMV) | 10−4 mg/mL | Metal nanoparticles were surface modified to form amino-functionalized Fe3O4/SiO2 MNPs (NH2-Fe3O4/SiO2 MNPs) followed by the covalent immobilization of antibody | There is a good linear relationship between the enhanced fluorescence and the concentrations of viruses | The target viruses can be detected by a nongrowth step | [144] |
| Electrochemical sensor | Multiwalled carbon nanotube | Ganoderma boninense | 0.0414 mg/L | Gold (III) chloride trihydrate and sodium citrate dehydrate used for the synthesis of gold nanoparticles of different sizes and layer by layer assembly was used to modify the electrode | Electrocatalytic activities of a modified electrode towards oxidation of healthy and G. boninense-infected oil palm leaves | A sensitivity and reproducible method due to the unique characteristics of nanoparticles | [185] |
| Electrochemical nanosensor | TiO2 or SnO2 nanoparticles on screen-printed carbon (SP) electrodes | Phytophthora cactorum | 35–62 nM | SnO2 and TiO2 were used as electrochemical detection elements for amperometric sensing and Screen-printed carbon electrodes were modified with nanoparticles of SnO2 or TiO2 before their use in electrochemical detection | Detection of symbolic volatile compound p-ethyl guaiacol produced during infection | Electroanalytical data obtained using cyclic voltammetry and differential pulse voltammetry exhibited that both SnO2 and TiO2 displayed high sensitivity | [186] |
| Electrochemical Biosensor | Colloidal Gold Nanoparticles | Pseudomonas syringae | 214 pM | AuNPs were synthesized by the citrate reduction of HAuCl4. The AuNP-DNA probe was prepared by adding tris(2-carboxyethyl)phosphine to DNA mixture of Pseudomonas syringae | Assessment of electrochemical changes with differential pulse voltammetry | This method can readily identify P. syringae infected plant samples even before the appearance of disease symptoms | [187] |
| Optical nanosensors | Selective single-walled carbon nanotube | Wound signaling | - | Cy3-labeled G-SWNT was prepared by mixing 1 mg of Cy3-ss(GT)15 and 0.25 mg of HiPCO SWNT, followed by the purification | Selective single-walled carbon nanotubes are excellent fluorescent probes that have the capability for real-time monitoring of H2O2 produced due to mechanical injury in plants | This nanosensor probe is independent of species and capable of real-time, spatial and temporal biochemical measurements in plants | [188] |
| Oxygen nanosensors | Carbon-filled quartz micropipettes having platinum-coated tips | Oxygen concentration | - | The inert carbon surface of the electrode was functionalized for the detection of redox-active species. A nanocavity was created in a carbon electrode. The fabrication of platinum nanosensors was performed in two stages: etching in alkaline solution followed by the platinization | The platinized nanoelectrode displays enhanced catalytic activity for oxygen reduction and the current of the sensor electrode was recalculated to oxygen concentration | Such novel platinum nanoelectrodes are beneficial for understanding cell oxygen metabolism | [189] |
| Fluorescent nanosensor | Carbon dots (CDs) | Fe3+ | 6.4 nM | CDs were synthesized from Pseudo-stem of banana plant (as carbon source) by using hydrothermal method | Drastic decrease in the fluorescence intensity of CDs upon increase in the Fe3+ concentration | CDs are highly selective to Fe3 + ions even in the presence of other ions | [190] |
| SERS-barcoded nanosensor | Au | Bacillus thuringiensis (Bt) gene transformed rice expressing insecticidal proteins | 0.1 pg/mL | Encapsulation of gold nanoparticles with silica and conjugation of oligonucleotide strands for targeting DNA strands | DNA hybridization | The nanosensor provides precise detection of transgenic rice varieties | [163] |
| SPR nanosensor | Au | Aflatoxin B1 | 1.04 pg mL−1 | Aflatoxin and N-methacryloyl-L-phenylalanine were pre-complexed as a template molecule and functional monomer. Molecularly imprinted polymers with gold nanoparticles were coated onto surface plasmon resonance (SPR) gold chip surface | The reflectivity index in the gold electrode surface changed with the aflatoxin concentration | SPR nanosensors were have commendable selectivity and reusability | [162] |
Nanosensors for Detection of Other Entities
Amino acids are very crucial molecules required by the living systems as they play a pivotal role of building blocks in the process of protein synthesis [145], vital character for maintenance of redox environments in the cell and extenuating destruction from the toxin and free radicals [146]. The investigative methods for detecting amino acids have been reported, especially by chromatography, chemiluminescence, and electrochemistry [147]. However, the application of existing technologies is greatly restricted by the great expenses and time-consuming steps. Currently, nanomolecular sensors have been established for detecting such molecules owing to their chemical steadiness, bio-compatibility, and easy surface alteration [148, 149]. The employment of gold nanoparticles for biosensing solicitations has been reported in different biological environments. The amine side chain and sulfhydryl (thiol) group of amino acids may perhaps covalently bind with the gold nanoparticles, thereby inducing an accretion of these nanostructures which further results in a color alteration from red to blue on the aggregation of amino thiol molecules [150, 151]. Chaicham et al. [147] developed an optical nanosensor grounded on gold nanoparticles that could detect Cys and Lys at concentrations of 5.88 μM and 16.14 μM, respectively, along with an adequate percentage retrieval of 101–106 in actual samples.
Similarly, other metal ions that are required by living organisms for performing various metabolic functions can be detected by employing different nanosensors. A dual-emission fluorescent probe was developed by Lu et al. [152] for detecting Cu2+ ions by condensing hydrophobic carbon dots in micelles molded by the auto-assemblage of different amphiphilic polymers. A vigorous, self-accelerating, and magnetic electrochemiluminescence nanosensor which was established on the multi-functionalized CoFe2O4 MNPs was established for the foremost and later employed for the extremely sensitive as well as discriminating recognition of the target Cu2+ through click reaction in a quasi-homogeneous system [82]. Gold nanorods are also exploited for sensing Fe (III) ions. Thatai et al. [17] devised highly sensitive gold nanorods using cetyltrimethylammonium bromide as illustrative material for detecting ferric ions along with a surprising sensing level equivalent to 100 ppb. Zinc is another important element, and it occurs in a divalent cationic form as Zn2+ ions. Zn2+ ion has the capability of sustaining important activities counting synthesis of DNA and protein, RNA transcription, cell apoptosis, and metalloenzyme regulation [153, 154]. Usually, fluorescent probes are exploited for detecting the Zn2+ ions in biological systems. The pyridoxal-5′-phosphate (PLP) conjugated lysozyme cocooned gold nanoclusters (Lyso-AuNCs) can also be exploited for the selective and turn-on detection of divalent Zn2+ ions in the liquid environment. The yellow fluorescence of PLP Lyso-AuNCs displays noteworthy augmentation at 475 nm in the occurrence of Zn2+ generating bluish-green fluorescence which is accredited to the complexation-induced accretion of nanoclusters. The developed nanoprobe can detect Zn2+ ions in nanomolar concentrations (39.2 nM) [154]. The dual-emission carbon dots (DCDs) synthesized by Wang et al. [155] can also be exploited for revealing Zn2+ ions as well as iron ions (Fe3+) in different pH environments. The ferric ions could also be detected in an acidic environment along with an amazing sensation level equaling 0.8 µmol/L while Zn2+ ions could be detected in an alkaline environment along with a detection limit of 1.2 µmol/L.
These days groundwater is used for irrigation and it is also the solitary seedbed of potable water in numerous regions, exclusively in the isolated agronomic sections. The capricious expulsion of numerous contaminants into the environment has expressively deteriorated the eminence of groundwater, thus has significantly threatened environmental safety [156, 157]. Although there are numerous micropollutants, however, the rushing of fluoride in groundwater has stretched out accumulative civic consideration as a result of the grave fluorosis, severe abdominal and renal complications persuaded by the elevated intake of fluoride ion [158]. So, there is a quest to diagnose and unveil hardness as well as the presence of fluoride ions in the ground-water which has expected substantial considerations owing to their significant parts in the different ecological, biological, and chemical processes [157]. Although fluorescent probes which are considered as traditional methods, can be exploited for detecting F−, however, the employment of quantum dots, an inorganic nanomaterial, can grab extensive considerations on account of their distinctive optical possessions comprising size-oriented fluorescence, tapered and coherent emission peak with a wide exciting wavelength, and outstanding photo solidity [159, 160]. The creation of a fluorescence resonance energy transmission channel from the carbon dots and the gold nanoparticles appears to be a competent solution for detecting numerous analytes. Therefore, constructing a novel nanosensor via gold nanoparticles and carbon dots for detecting F− seems to be a proficient strategy. The hybrid nanosensor assorted with calcium ions has been reported to spot fluoride ions along with a subordinate recognition level parallel to 0.339 ppm [103]. Lu et al. [161] also developed another novel strategy for detecting fluoride, which was grounded on dual ligands coated with perovskite quantum dots, and the recognition level was found to be 3.2 μM.
The agricultural systems also necessitate the diagnosis of various other entities for the smooth functioning and enhanced productivity of the agroecosystems. The detection of other miscellaneous entities has also been facilitated by the employment of nanosensors (Table 3), for instance, the detection of transgenic plants, the presence of aflatoxins, and even the occurrence of wounds in plants. The SPR nanosensor developed using gold nanoparticles detected the Aflatoxin B1 at the concentration of 1.04 pg mL−1 [162] whereas the SERS-barcoded nanosensor fabricated using the encapsulation of gold nanoparticles with silica followed by the conjugation of oligonucleotide strands effectively detected the presence of Bacillus thuringiensis (Bt) gene-encoded insecticidal proteins in rice plants at 0.1 pg/mL, thereby, clearly advocating the transgenic nature of rice plants [163].
Nanosensors for Detection of Nanoparticles
Nanomaterials can also occur naturally, such as humic acids and clay minerals; extensive human activities can also lead to the incidental synthesis of various nanomaterials in the environment, for instance, diesel oil emanations or by the discharge of welding fumes; or they can also be explicitly concocted to unveil matchless electrical, optical, chemical or physical features [164]. These characteristics are exploited in plenty of consumable merchandise, for instance, medicines, food, cosmetics and suntan lotions, paints, and electronics, as well as processes that directly discharge nanomaterials into the surroundings, such as remediating contaminated environs [165, 166]. Furthermore, the rapid employment of metal nanoparticles in various systems has raised many concerns due to the potential environmental risks posed by them as they are unavoidably lost in the environment throughout the processes meant for their fabrication, conveyance, usage, and dumping [167]. Carbon-based nanomaterials are quite established against degradation and as a result, amass in the surroundings [168]. Nanoparticles, attributable to their greater surface area, find it much easier to bind and adsorb on the cellular surfaces. They harm the cell in several ways, such as, by hindering the protein transport pathway on the membrane, by destroying the permeability of the cell membrane, or by further inhibiting core components of the cell [169]. Currently, an overwhelming figure of the engineered nanoparticles engaged for different ecological and industrial solicitations or molded as by-products of different human deeds are ultimately discharged into soil systems. The usual nanoparticles employed comprise the metal engineered nanoparticles (elemental Fe, Au, Ag, etc.), metal oxides (SiO2, ZnO, FeO2, TiO2, CuO, Al2O3, etc.), composite compounds (Co–Zn–Fe oxide), fullerenes (grouping Buckminster fullerenes, nanocones, carbon nanotubes, etc.), quantum dots frequently encrusted with a polymer and other organic polymers (Dinesh et al. 2012). Different plant growth-promoting rhizobacteria (PGPR) like Bacillus subtilis, Pseudomonas aeruginosa, P. fluorescens, and P. putida, and different bacteria involved in soil nitrogen transformations are inhibited to varying degrees on exposure to nanoparticles in aqueous suspensions or pure culture conditions [170]. The nanoparticles grounded on metals copper and iron are alleged to interact with the peroxides existing in the environs thereby engender free radicals that are notorious for their high toxicity to microbes [171]. Therefore, there is a strong need to monitor the different nanoparticles which find an ultimate sink in the soils especially of agroecosystems.
Various techniques can be reconnoitered for sensing nanoparticles, one among them is the usage of microcavity sensors, which, in the form of whispering gallery resonators have acknowledged extensive consideration. Here, the particle binding on the exterior of the microcavity disturbs the optical possessions thereby instigating a resonant wavelength swing with magnitude reliant upon the polarizability of the particle. The measure of the change facilitates surveillance of the binding actions in real-time and is also used to evaluate the particle size [172]. Optical sensing empowered with the extreme sensitivity of single nanoscale entities is sturdily anticipated for solicitations in numerous arenas, for instance, in environmental checking, other than in homeland security. Split-mode microcavity Raman lasers are also highly sensitive optical sensors that can perceive the occurrence of even a single nanoparticle. The presence of nanoparticles is revealed by observing the distinct alterations in the beat frequency of the Raman lasers and the sensing level has been reported to be 20 nm radius of the nanoparticles [138].
Nanotechnology Implementation in an Agroecosystem: Proof-of-Concept to Commercialization
There are hundreds of research articles and studies that are being published every year on nanosensor's application in agriculture. However, very few nanosensors have yet been commercialized for the detection of heavy metals, pesticides, plant-pathogen, and other substances in an agroecosystem. Because these academic outputs are not properly converted/conveyed to commercial or other regulatory platforms. Certain scientific and non-scientific factors hinder these nanosensors from proof-of-concept to fully commercialized products. These factors are scale-up and real-use (technical), validation and compliances (regulatory), management priorities and decisions (political), standardization (legal), cost, demand and IPR protection (economic), safety and security (environmental health and safety) along with several ethical issues. It is necessary to support enthusiastic researchers and institutions for research and development to develop such nanosensors for agroecosystem, product validation, intellectual protection, and their social understanding and implementation. If we consider these factors strategically, it will help in nanosensor product betterment and implementation to agroecosystem. The US-based startup Razzberry developed portable chemical nanosensors to trace real-time chemical changes in water, soil, and the environment. Similarly, Italian startup Nasys invented a metal oxides-based nanosensor to detect air pollution. There are some other startups nGageIT and Tracense, implementing nanosensor technologies to detect biological and Hazardous contaminants in agriculture.
Perspectives and Conclusions
Since times immemorial, agriculture is the main source of food, income as well as employment for mankind around the globe. In the present era, due to upsurge of rapid urbanization and climate inconsistency, precision farming has been flocking significant attention worldwide. In agricultural system, this type of farming has the ability to maximize the crop’s productivity and improve soil quality along with the minimization of the agrochemicals input (such as fertilizers, herbicides, pesticides, etc.). Precision farming is possible through focused monitoring of environmental variables along with the application of the directed action. This type of farming system also employs computers, global satellite positioning systems, sensors, and remote sensing strategies. As a result, the monitoring of extremely confined environmental situations becomes easy. This monitoring even assists in defining the growth of crop plants by accurately ascertaining the nature and site of hitches. Eventually, it also employs smart sensors for providing exact data that grant enriched productivity by serving farmers to make recovery choices in a detailed manner. Among all the sensors, smart nanosensors are very sensitive and judiciously employed devices that have started proving to be an essential tool for advocating agricultural sustainability, in future.
It has been noticed that the use of nanosensors and or biosensors can accelerate agricultural productivity. These real-time sensors can physically monitor temperature, soil health, soil moisture content and even senses the soil microbiological/microenvironment and nutrient status of soils. Interestingly, these sensors have also been able to detect residual pesticides, heavy metals, monitor plant pathogens and quantify fertilizers and toxins. These nanosensors facilitate speedy, quick, reliable, and prior information that even aid in predicting as well as mitigating the crop losses in the agroecosystems. In addition, the use of nanotechnology-based biosensors also assists in accomplishing the concept of sustainable agriculture. It has been observed that the projection of nanosensors and or biosensors as plant diagnostic tools requires improvements regarding their sensitivity and specificity. Additionally, there is a need for quick, reliable, cheap, multiplexed screening to detect a wide range of plant-based bioproducts. Moreover, the development of broad-spectrum nanosensors that can detect multiple entities will also boost in mobilizing technology. It has been suggested that the biosensor efficiency can be improved further by developing super “novel nanomaterials” that will be available in near future. Perhaps in the coming years, the convergence among nanotechnology, agriculture sciences, rhizosphere engineering, and overall plant engineering will lead to the path towards accomplishment of all Sustainable Development Goals 2030 without incurring any fitness cost on mankind safety, economy, natural resources, and environment.
Acknowledgements
Not applicable.
Abbreviations
- AAS
Atomic absorption spectrometry
- AES
Atomic emission spectrometry
- Ag
Silver
- Al2O3
Aluminum oxide
- Au
Gold
- CdSe
Cadmium selenide
- CL
Colorimetric
- CoFe2O4
Cobalt iron oxide
- CTBr
Cationic cetyltrimethylammonium bromide
- CuO
Cupric oxide
- DCDs
Sual-emission carbon dots
- FeO2
Iron dioxide
- FL
Fluorescence
- ICP-MS
Inductively coupled plasma mass spectrometry
- ICTS
Immunochromatographic strip
- NiO
Nickel oxide
- Pd
Palladium
- PGPR
Plant growth-promoting rhizobacteria
- SERS
Surface-enhanced Raman scattering
- SiO2
Silicon dioxide
- SPR
Surface plasmon resonance
- TiO2
Titanium dioxide
- XRF
X-ray fluorescence spectrometry
- ZnO
Zinc oxide
- ZrO2
Zirconium dioxide
Authors' contributions
PS and VP conceived the concept and arranged the ideas into the content list, AP made and finalized the figures, PS and VP collected all the literature and initially drafted the raw manuscript, BS and MMMS together made all the tables. VP, SM, PS, and AH together edited the entire manuscript. All authors read and approved the final manuscript for submission.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pankaj Sharma, Email: psk14495@gmail.com.
Vimal Pandey, Email: vimal.bt04@gmail.com.
Mayur Mukut Murlidhar Sharma, Email: mayurmms0001@gmail.com.
Anupam Patra, Email: anupam.patra.003@gmail.com.
Baljinder Singh, Email: baljinder.yavanika@gmail.com.
Sahil Mehta, Email: sahilmehtasm21@gmail.com.
Azamal Husen, Email: adroot92@yahoo.co.in.
References
- 1.Thundiyil JG, Stober J, Besbelli N, Pronczuk J. Acute pesticide poisoning: a proposed classification tool. Bull World Health Organ. 2008;86:205–209. doi: 10.2471/BLT.07.041814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carvalho FP. Pesticides, environment, and food safety. Food Energy Secur. 2017;6:48–60. doi: 10.1002/fes3.108. [DOI] [Google Scholar]
- 3.FAO WHO (2018) Pesticide residues in food 2018‐Report 2018‐Joint FAO/WHO Meeting on Pesticide Residues
- 4.Dhouib IB, Annabi A, Jallouli M, et al. Carbamates pesticides induced immunotoxicity and carcinogenicity in human: a review. J Appl Biomed. 2016;14:85–90. doi: 10.1016/j.jab.2016.01.001. [DOI] [Google Scholar]
- 5.Akoto O, Oppong-Otoo J, Osei-Fosu P. Carcinogenic and non-carcinogenic risk of organochlorine pesticide residues in processed cereal-based complementary foods for infants and young children in Ghana. Chemosphere. 2015;132:193–199. doi: 10.1016/j.chemosphere.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 6.Saad-Hussein A, Beshir S, Taha MM, et al. Early prediction of liver carcinogenicity due to occupational exposure to pesticides. Mutat Res Toxicol Environ Mutagen. 2019;838:46–53. doi: 10.1016/j.mrgentox.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 7.FAO I. Global assessment of the impact of plant protection products on soil functions and soil ecosystems. Rome: FAO; 2017. [Google Scholar]
- 8.Chawla P, Kaushik R, Shiva Swaraj VJ, Kumar N. Organophosphorus pesticides residues in food and their colorimetric detection. Environ Nanotechnol Monit Manag. 2018;10:292–307. doi: 10.1016/j.enmm.2018.07.013. [DOI] [Google Scholar]
- 9.Pérez AP, Eugenio NR (2018) Status of local soil contamination in Europe
- 10.Silva V, Mol HGJ, Zomer P, et al. Pesticide residues in European agricultural soils–a hidden reality unfolded. Sci Total Environ. 2019;653:1532–1545. doi: 10.1016/j.scitotenv.2018.10.441. [DOI] [PubMed] [Google Scholar]
- 11.Giannoulis KM, Giokas DL, Tsogas GZ, Vlessidis AG. Ligand-free gold nanoparticles as colorimetric probes for the non-destructive determination of total dithiocarbamate pesticides after solid phase extraction. Talanta. 2014;119:276–283. doi: 10.1016/j.talanta.2013.10.063. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 13.Singh R, Gautam N, Mishra A, Gupta R. Heavy metals and living systems: an overview. Indian J Pharmacol. 2011;43:246. doi: 10.4103/0253-7613.81505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav SK. Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot. 2010;76:167–179. doi: 10.1016/j.sajb.2009.10.007. [DOI] [Google Scholar]
- 15.Diaconu M, Pavel LV, Hlihor R-M, et al. Characterization of heavy metal toxicity in some plants and microorganisms—a preliminary approach for environmental bioremediation. N Biotechnol. 2020;56:130–139. doi: 10.1016/j.nbt.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Rehman AU, Nazir S, Irshad R, et al (2020) Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J Mol Liq 114455
- 17.Thatai S, Khurana P, Prasad S, Kumar D. A new way in nanosensors: gold nanorods for sensing of Fe (III) ions in aqueous media. Microchem J. 2014;113:77–82. doi: 10.1016/j.microc.2013.11.004. [DOI] [Google Scholar]
- 18.Li H, Guo J, Ping H, et al. Visual detection of organophosphorus pesticides represented by mathamidophos using Au nanoparticles as colorimetric probe. Talanta. 2011;87:93–99. doi: 10.1016/j.talanta.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Kumar N, Kumar H, Mann B, Seth R. Colorimetric determination of melamine in milk using unmodified silver nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc. 2016;156:89–97. doi: 10.1016/j.saa.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Bala R, Dhingra S, Kumar M, et al. Detection of organophosphorus pesticide—Malathion in environmental samples using peptide and aptamer based nanoprobes. Chem Eng J. 2017;311:111–116. doi: 10.1016/j.cej.2016.11.070. [DOI] [Google Scholar]
- 21.Srivastava AK, Dev A, Karmakar S. Nanosensors and nanobiosensors in food and agriculture. Environ Chem Lett. 2018;16:161–182. doi: 10.1007/s10311-017-0674-7. [DOI] [Google Scholar]
- 22.Doroudian M, O’Neill A, Mac Loughlin R, et al. Nanotechnology in pulmonary medicine. Curr Opin Pharmacol. 2021;56:85–92. doi: 10.1016/j.coph.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahani S, Sharma YC (2020) Advancements in applications of nanotechnology in global food industry. Food Chem 128318 [DOI] [PubMed]
- 24.Acharya A, Pal PK. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact. 2020;19:100232. doi: 10.1016/j.impact.2020.100232. [DOI] [Google Scholar]
- 25.Dutta D, Das BM (2020) Scope of green nanotechnology towards amalgamation of green chemistry for cleaner environment: a review on synthesis and applications of green nanoparticles. Environ Nanotechnology, Monit Manag 100418
- 26.Usman M, Farooq M, Wakeel A, et al. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci Total Environ. 2020;721:137778. doi: 10.1016/j.scitotenv.2020.137778. [DOI] [PubMed] [Google Scholar]
- 27.Nagraik R, Sharma A, Kumar D, et al (2021) Amalgamation of biosensors and nanotechnology in disease diagnosis: mini-review. Sens Int 100089
- 28.Singh S, Sangwan S, Sharma P, et al. Nanotechnology for sustainable agriculture: an emerging perspective. J Nanosci Nanotechnol. 2021;21:3453–3465. doi: 10.1166/jnn.2021.19012. [DOI] [PubMed] [Google Scholar]
- 29.Shabaninejad Z, Yousefi F, Movahedpour A, et al. Electrochemical-based biosensors for microRNA detection: Nanotechnology comes into view. Anal Biochem. 2019;581:113349. doi: 10.1016/j.ab.2019.113349. [DOI] [PubMed] [Google Scholar]
- 30.USEPA (2007) Treatment technologies for site cleanup: annual status report. United States Environ Prot Agency, Washingt DC
- 31.Lu Y, Yang Q, Wu J (2020) Recent advances in biosensor-integrated enrichment methods for preconcentrating and detecting the low-abundant analytes in agriculture and food samples. TrAC Trends Anal Chem 115914
- 32.Mokhtarzadeh A, Dolatabadi JEN, Abnous K, et al. Nanomaterial-based cocaine aptasensors. Biosens Bioelectron. 2015;68:95–106. doi: 10.1016/j.bios.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 33.Nosrati R, Golichenari B, Nezami A, et al. Helicobacter pylori point-of-care diagnosis: nano-scale biosensors and microfluidic systems. TrAC Trends Anal Chem. 2017;97:428–444. doi: 10.1016/j.trac.2017.10.013. [DOI] [Google Scholar]
- 34.Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays Biochem. 2016;60:91–100. doi: 10.1042/EBC20150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dehghani S, Nosrati R, Yousefi M, et al. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): a review. Biosens Bioelectron. 2018;110:23–37. doi: 10.1016/j.bios.2018.03.037. [DOI] [PubMed] [Google Scholar]
- 36.Vigneshvar S, Sudhakumari CC, Senthilkumaran B, Prakash H. Recent advances in biosensor technology for potential applications–an overview. Front Bioeng Biotechnol. 2016;4:11. doi: 10.3389/fbioe.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner APF. Biosensors: sense and sensibility. Chem Soc Rev. 2013;42:3184–3196. doi: 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- 38.Charbgoo F, Nejabat M, Abnous K, et al. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J Control Release. 2018;272:39–53. doi: 10.1016/j.jconrel.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W. Nanoscale iron particles for environmental remediation: an overview. J Nanoparticle Res. 2003;5:323–332. doi: 10.1023/A:1025520116015. [DOI] [Google Scholar]
- 40.Saini RK, Bagri LP, Bajpai AK. New pesticides and soil sensors. Netherlands: Elsevier; 2017. [Google Scholar]
- 41.Singh S, Sharma MP, Ahmad A. Construction and characterization of protein-based cysteine nanosensor for the real time measurement of cysteine level in living cells. Int J Biol Macromol. 2020;143:273–284. doi: 10.1016/j.ijbiomac.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Rastogi L, Dash K, Ballal A. Selective colorimetric/visual detection of Al3+ in ground water using ascorbic acid capped gold nanoparticles. Sens Actuators B Chem. 2017;248:124–132. doi: 10.1016/j.snb.2017.03.138. [DOI] [Google Scholar]
- 43.Nosrati R, Dehghani S, Karimi B, et al. Siderophore-based biosensors and nanosensors; new approach on the development of diagnostic systems. Biosens Bioelectron. 2018;117:1–14. doi: 10.1016/j.bios.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 44.Manjunatha RL, Naik D, Usharani KV. Nanotechnology application in agriculture: A review. J Pharmacogn Phytochem. 2019;8:1073–1083. [Google Scholar]
- 45.Singh SK, Kasana RC, Yadav RS, Pathak R (2020) Current status of biologically produced nanoparticles in agriculture. In: Biogenic nano-particles and their use in agro-ecosystems. Springer, pp 393–406
- 46.Marchiol L, Iafisco M, Fellet G, Adamiano A. Nanotechnology support the next agricultural revolution: perspectives to enhancement of nutrient use efficiency. Adv Agron. 2020;161:27–116. doi: 10.1016/bs.agron.2019.12.001. [DOI] [Google Scholar]
- 47.Seleiman MF, Almutairi KF, Alotaibi M, et al. Nano-fertilization as an emerging fertilization technique: Why can modern agriculture benefit from its use? Plants. 2021;10:2. doi: 10.3390/plants10010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghormade V, Deshpande MV, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv. 2011;29:792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Rios JJ, Yepes-Molina L, Martinez-Alonso A, Carvajal M. Nanobiofertilization as a novel technology for highly efficient foliar application of Fe and B in almond trees. R Soc open Sci. 2020;7:200905. doi: 10.1098/rsos.200905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chhipa H, Joshi P (2016) Nanofertilisers, nanopesticides and nanosensors in agriculture. In: Nanoscience in food and agriculture 1. Springer, pp 247–282
- 51.Jat SK, Bhattacharya J, Sharma MK. Nanomaterial based gene delivery: a promising method for plant genome engineering. J Mater Chem B. 2020;8:4165–4175. doi: 10.1039/D0TB00217H. [DOI] [PubMed] [Google Scholar]
- 52.Chandrasekaran R, Rajiv P, Abd-Elsalam KA (2020) Carbon nanotubes: plant gene delivery and genome editing. In: Carbon nanomaterials for agri-food and environmental applications. Elsevier, pp 279–296
- 53.Alshehri MA, Panneerselvam C, Murugan K, et al. The desert wormwood (Artemisia herba-alba)–From Arabian folk medicine to a source of green and effective nanoinsecticides against mosquito vectors. J Photochem Photobiol B Biol. 2018;180:225–234. doi: 10.1016/j.jphotobiol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Baker S, Perianova OV. Bio-nanobactericides: an emanating class of nanoparticles towards combating multi-drug resistant pathogens. SN Appl Sci. 2019;1:1–9. doi: 10.1007/s42452-019-0715-x. [DOI] [Google Scholar]
- 55.Siddhartha, Verma A, Bashyal BM, et al (2020) New nano-fungicides for the management of sheath blight disease (Rhizoctonia solani) in rice. Int J Pest Manag 1–10
- 56.Latif A, Sheng D, Sun K, et al (2020) Remediation of heavy metals polluted environment using Fe-based nanoparticles: mechanisms, influencing factors, and environmental implications. Environ Pollut 114728 [DOI] [PubMed]
- 57.Moreno A, Jordana A, Grillo R, et al. A study on the molecular existing interactions in nanoherbicides: a chitooligosaccharide/tripolyphosphate loaded with paraquat case. Colloids Surf A Physicochem Eng Asp. 2019;562:220–228. doi: 10.1016/j.colsurfa.2018.11.033. [DOI] [Google Scholar]
- 58.Neethirajan S, Jayas DS. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2011;4:39–47. doi: 10.1007/s11947-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Márquez JCM, Partida AH, del Carmen M, et al. Silver nanoparticles applications (AgNPS) in aquaculture. Int J Fish Aquat Stud. 2018;6:5–11. [Google Scholar]
- 60.Esyanti RR, Zaskia H, Amalia A (2019) Chitosan nanoparticle-based coating as post-harvest technology in banana. In: Journal of physics: conference series. IOP Publishing, p 12109
- 61.Youssef FS, El-Banna HA, Elzorba HY, Galal AM. Application of some nanoparticles in the field of veterinary medicine. Int J Vet Sci Med. 2019;7:78–93. doi: 10.1080/23144599.2019.1691379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah BR, Mraz J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev Aquac. 2020;12:925–942. doi: 10.1111/raq.12356. [DOI] [Google Scholar]
- 63.Afzal I, Javed T, Amirkhani M, Taylor AG. Modern seed technology: seed coating delivery systems for enhancing seed and crop performance. Agriculture. 2020;10:526. doi: 10.3390/agriculture10110526. [DOI] [Google Scholar]
- 64.Husen A, Siddiqi KS. Phytosynthesis of nanoparticles: concept, controversy and application. Nanoscale Res Lett. 2014;9:1–24. doi: 10.1186/1556-276X-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Husen A, Siddiqi KS. Carbon and fullerene nanomaterials in plant system. J Nanobiotechnol. 2014;12:1–10. doi: 10.1186/1477-3155-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Husen A, Iqbal M (2019) Nanomaterials and plant potential: an overview. In: Nanomaterials and Plant Potential (Eds. Husen A, Iqbal M) Springer International Publishing AG, Gewerbestrasse 11, 6330 Cham, pp 3–29
- 67.Husen A (2020) Interactions of metal and metal-oxide nanomaterials with agricultural crops: an overview. In: Nanomaterials for Agriculture and Forestry Applications (Eds. Husen, A, Jawaid M) Elsevier Inc. 50 Hampshire St., 5th Floor, Cambridge, MA 02139, USA, pp 167–197
- 68.Pasinszki T, Krebsz M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials. 2020;10:917. doi: 10.3390/nano10050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma A, Shukla A, Attri K, et al. Global trends in pesticides: a looming threat and viable alternatives. Ecotoxicol Environ Saf. 2020;201:110. doi: 10.1016/j.ecoenv.2020.110812. [DOI] [PubMed] [Google Scholar]
- 70.Rhouati A, Majdinasab M, Hayat A. A perspective on non-enzymatic electrochemical nanosensors for direct detection of pesticides. Curr Opin Electrochem. 2018;11:12–18. doi: 10.1016/j.coelec.2018.06.013. [DOI] [Google Scholar]
- 71.Nsibande SA, Forbes PBC. Fluorescence detection of pesticides using quantum dot materials—a review. Anal Chim Acta. 2016;945:9–22. doi: 10.1016/j.aca.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Madianos L, Skotadis E, Tsekenis G, et al. Ιmpedimetric nanoparticle aptasensor for selective and label free pesticide detection. Microelectron Eng. 2018;189:39–45. doi: 10.1016/j.mee.2017.12.016. [DOI] [Google Scholar]
- 73.Liang M, Fan K, Pan Y, et al. Fe3O4 magnetic nanoparticle peroxidase mimetic-based colorimetric assay for the rapid detection of organophosphorus pesticide and nerve agent. Anal Chem. 2013;85:308–312. doi: 10.1021/ac302781r. [DOI] [PubMed] [Google Scholar]
- 74.Yetim NK, Özkan EH, Özcan C, Sarı N. Preparation of AChE immobilized microspheres containing thiophene and furan for the determination of pesticides by the HPLC-DAD method. J Mol Struct. 2020;1222:128931. doi: 10.1016/j.molstruc.2020.128931. [DOI] [Google Scholar]
- 75.Gascon J, Oubiña A, Barceló D. Detection of endocrine-disrupting pesticides by enzyme-linked immunosorbent assay (ELISA): application to atrazine. TrAC Trends Anal Chem. 1997;16:554–562. doi: 10.1016/S0165-9936(97)00051-4. [DOI] [Google Scholar]
- 76.Biparva P, Gorji S, Hedayati E. Promoted reaction microextraction for determining pesticide residues in environmental water samples using gas chromatography-mass spectrometry. J Chromatogr A. 2020;1612:460639. doi: 10.1016/j.chroma.2019.460639. [DOI] [PubMed] [Google Scholar]
- 77.López MG, Fussell RJ, Stead SL, et al. Evaluation and validation of an accurate mass screening method for the analysis of pesticides in fruits and vegetables using liquid chromatography–quadrupole-time of flight–mass spectrometry with automated detection. J Chromatogr A. 2014;1373:40–50. doi: 10.1016/j.chroma.2014.10.099. [DOI] [PubMed] [Google Scholar]
- 78.Cheng X, Wang Q, Zhang S, et al. Determination of four kinds of carbamate pesticides by capillary zone electrophoresis with amperometric detection at a polyamide-modified carbon paste electrode. Talanta. 2007;71:1083–1087. doi: 10.1016/j.talanta.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Guan J, Yang J, Zhang Y, et al. Employing a fluorescent and colorimetric picolyl-functionalized rhodamine for the detection of glyphosate pesticide. Talanta. 2021;224:121834. doi: 10.1016/j.talanta.2020.121834. [DOI] [PubMed] [Google Scholar]
- 80.Verdian A. Apta-nanosensors for detection and quantitative determination of acetamiprid—a pesticide residue in food and environment. Talanta. 2018;176:456–464. doi: 10.1016/j.talanta.2017.08.070. [DOI] [PubMed] [Google Scholar]
- 81.Christopher FC, Kumar PS, Christopher FJ, et al. Recent advancements in rapid analysis of pesticides using nano biosensors: a present and future perspective. J Clean Prod. 2020;269:122356. doi: 10.1016/j.jclepro.2020.122356. [DOI] [Google Scholar]
- 82.Lei Y-M, Xiao B-Q, Liang W-B, et al. A robust, magnetic, and self-accelerated electrochemiluminescent nanosensor for ultrasensitive detection of copper ion. Biosens Bioelectron. 2018;109:109–115. doi: 10.1016/j.bios.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Xie H, Bei F, Hou J, Ai S. A highly sensitive dual-signaling assay via inner filter effect between g-C3N4 and gold nanoparticles for organophosphorus pesticides. Sens Actuators B Chem. 2018;255:2232–2239. doi: 10.1016/j.snb.2017.09.024. [DOI] [Google Scholar]
- 84.Li H, Yan X, Qiao S, et al. Yellow-emissive carbon dot-based optical sensing platforms: cell imaging and analytical applications for biocatalytic reactions. ACS Appl Mater Interfaces. 2018;10:7737–7744. doi: 10.1021/acsami.7b17619. [DOI] [PubMed] [Google Scholar]
- 85.Yan X, Shi H, Wang M. Development of an enzyme-linked immunosorbent assay for the simultaneous determination of parathion and imidacloprid. Anal Methods. 2012;4:4053–4057. doi: 10.1039/C2AY25760B. [DOI] [Google Scholar]
- 86.Zhang C, Cui H, Cai J, et al. Development of fluorescence sensing material based on CdSe/ZnS quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and Chinese cabbage. J Agric Food Chem. 2015;63:4966–4972. doi: 10.1021/acs.jafc.5b01072. [DOI] [PubMed] [Google Scholar]
- 87.Qu F, Zhou X, Xu J, et al. Luminescence switching of CdTe quantum dots in presence of p-sulfonatocalix [4] arene to detect pesticides in aqueous solution. Talanta. 2009;78:1359–1363. doi: 10.1016/j.talanta.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 88.Lin B, Yu Y, Li R, et al. Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sens Actuators B Chem. 2016;229:100–109. doi: 10.1016/j.snb.2016.01.114. [DOI] [Google Scholar]
- 89.Majdinasab M, Yaqub M, Rahim A, et al. An overview on recent progress in electrochemical biosensors for antimicrobial drug residues in animal-derived food. Sensors. 2017;17:1947. doi: 10.3390/s17091947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan X, Li H, Su X. Review of optical sensors for pesticides. TrAC Trends Anal Chem. 2018;103:1–20. doi: 10.1016/j.trac.2018.03.004. [DOI] [Google Scholar]
- 91.Huang X, Aguilar ZP, Xu H, et al. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: a review. Biosens Bioelectron. 2016;75:166–180. doi: 10.1016/j.bios.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 92.Emslie KR, Whaites L, Griffiths KR, Murby EJ. Sampling plan and test protocol for the semiquantitative detection of genetically modified canola (Brassica napus) seed in bulk canola seed. J Agric Food Chem. 2007;55:4414–4421. doi: 10.1021/jf070267i. [DOI] [PubMed] [Google Scholar]
- 93.Santos VO, Pelegrini PB, Mulinari F, et al. A novel immunochromatographic strip test for rapid detection of Cry1Ac and Cry8Ka5 proteins in genetically modified crops. Anal Methods. 2015;7:9331–9339. doi: 10.1039/C5AY02051D. [DOI] [Google Scholar]
- 94.Zhou X, Hui E, Yu X-L, et al. Development of a rapid immunochromatographic lateral flow device capable of differentiating phytase expressed from recombinant aspergillus niger phy A2 and genetically modified corn. J Agric Food Chem. 2015;63:4320–4326. doi: 10.1021/acs.jafc.5b00188. [DOI] [PubMed] [Google Scholar]
- 95.Wang J, Zhang L, Huang Y, et al. Hollow Au-Ag nanoparticles labeled immunochromatography strip for highly sensitive detection of clenbuterol. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Y, Ding L, Wu Y, et al. Emerging strategies to develop sensitive AuNP-based ICTS nanosensors. TrAC Trends Anal Chem. 2019;112:147–160. doi: 10.1016/j.trac.2019.01.006. [DOI] [Google Scholar]
- 97.Luo X, Morrin A, Killard AJ, Smyth MR. Application of nanoparticles in electrochemical sensors and biosensors. Electroanal Int J Devoted Fundam Pract Asp Electroanal. 2006;18:319–326. [Google Scholar]
- 98.Cesarino I, Moraes FC, Lanza MRV, Machado SAS. Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline–carbon nanotubes. Food Chem. 2012;135:873–879. doi: 10.1016/j.foodchem.2012.04.147. [DOI] [PubMed] [Google Scholar]
- 99.Zhou L, Zhang X, Ma L, et al. Acetylcholinesterase/chitosan-transition metal carbides nanocomposites-based biosensor for the organophosphate pesticides detection. Biochem Eng J. 2017;128:243–249. doi: 10.1016/j.bej.2017.10.008. [DOI] [Google Scholar]
- 100.Ahmad R, Tripathy N, Ahn M-S, et al. Highly efficient non-enzymatic glucose sensor based on CuO modified vertically-grown ZnO nanorods on electrode. Sci Rep. 2017;7:5715. doi: 10.1038/s41598-017-06064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou C, Xu L, Song J, et al. Ultrasensitive non-enzymatic glucose sensor based on three-dimensional network of ZnO–CuO hierarchical nanocomposites by electrospinning. Sci Rep. 2014;4:1–9. doi: 10.1038/srep07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khairy M, Ayoub HA, Banks CE. Non-enzymatic electrochemical platform for parathion pesticide sensing based on nanometer-sized nickel oxide modified screen-printed electrodes. Food Chem. 2018;255:104–111. doi: 10.1016/j.foodchem.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Tian X, Liu L, Li Y, et al. Nonenzymatic electrochemical sensor based on CuO–TiO2 for sensitive and selective detection of methyl parathion pesticide in ground water. Sens Actuators B Chem. 2018;256:135–142. doi: 10.1016/j.snb.2017.10.066. [DOI] [Google Scholar]
- 104.Du D, Ye X, Zhang J, et al. Stripping voltammetric analysis of organophosphate pesticides based on solid-phase extraction at zirconia nanoparticles modified electrode. Electrochem Commun. 2008;10:686–690. doi: 10.1016/j.elecom.2008.02.019. [DOI] [Google Scholar]
- 105.Qu Y, Min H, Wei Y, et al. Au–TiO2/Chit modified sensor for electrochemical detection of trace organophosphates insecticides. Talanta. 2008;76:758–762. doi: 10.1016/j.talanta.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 106.Wang M, Huang J, Wang M, et al. Electrochemical nonenzymatic sensor based on CoO decorated reduced graphene oxide for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Food Chem. 2014;151:191–197. doi: 10.1016/j.foodchem.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 107.Pham TB, Bui H, Pham VH, Do TC. Surface-enhanced Raman spectroscopy based on Silver nano-dendrites on microsphere end-shape optical fibre for pesticide residue detection. Optik (Stuttg) 2020;219:165172. doi: 10.1016/j.ijleo.2020.165172. [DOI] [Google Scholar]
- 108.Saleh SM, Alminderej FM, Ali R, Abdallah OI. Optical sensor film for metribuzin pesticide detection. Spectrochim Acta Part A Mol Biomol Spectrosc. 2020;229:117971. doi: 10.1016/j.saa.2019.117971. [DOI] [PubMed] [Google Scholar]
- 109.Xie Y, Yu Y, Lu L, et al. CuO nanoparticles decorated 3D graphene nanocomposite as non-enzymatic electrochemical sensing platform for malathion detection. J Electroanal Chem. 2018;812:82–89. doi: 10.1016/j.jelechem.2018.01.043. [DOI] [Google Scholar]
- 110.Prabhakar N, Thakur H, Bharti A, Kaur N. Chitosan-iron oxide nanocomposite based electrochemical aptasensor for determination of malathion. Anal Chim Acta. 2016;939:108–116. doi: 10.1016/j.aca.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 111.Li M, Gou H, Al-Ogaidi I, Wu N. Nanostructured sensors for detection of heavy metals: a review. ACS Sustain Chem Eng. 2013;1:713–723. doi: 10.1021/sc400019a. [DOI] [Google Scholar]
- 112.Aragay G, Pons J, Merkoçi A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev. 2011;111:3433–3458. doi: 10.1021/cr100383r. [DOI] [PubMed] [Google Scholar]
- 113.Quang DT, Kim JS. Fluoro-and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem Rev. 2010;110:6280–6301. doi: 10.1021/cr100154p. [DOI] [PubMed] [Google Scholar]
- 114.Carter KP, Young AM, Palmer AE. Fluorescent sensors for measuring metal ions in living systems. Chem Rev. 2014;114:4564–4601. doi: 10.1021/cr400546e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ullah N, Mansha M, Khan I, Qurashi A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: recent advances and challenges. TrAC Trends Anal Chem. 2018;100:155–166. doi: 10.1016/j.trac.2018.01.002. [DOI] [Google Scholar]
- 116.Zhang L, Peng D, Liang R-P, Qiu J-D. Graphene-based optical nanosensors for detection of heavy metal ions. TrAC Trends Anal Chem. 2018;102:280–289. doi: 10.1016/j.trac.2018.02.010. [DOI] [Google Scholar]
- 117.Wang J, Jiang C, Wang X, et al. Fabrication of an “ion-imprinting” dual-emission quantum dot nanohybrid for selective fluorescence turn-on and ratiometric detection of cadmium ions. Analyst. 2016;141:5886–5892. doi: 10.1039/C6AN00868B. [DOI] [PubMed] [Google Scholar]
- 118.Gruber P, Marques MPC, Szita N, Mayr T. Integration and application of optical chemical sensors in microbioreactors. Lab Chip. 2017;17:2693–2712. doi: 10.1039/C7LC00538E. [DOI] [PubMed] [Google Scholar]
- 119.Chen X, Zhai N, Snyder JH, et al. Colorimetric detection of Hg2+ and Pb2+ based on peroxidase-like activity of graphene oxide–gold nanohybrids. Anal Methods. 2015;7:1951–1957. doi: 10.1039/C4AY02801E. [DOI] [Google Scholar]
- 120.Xia N, Feng F, Liu C, et al. The detection of mercury ion using DNA as sensors based on fluorescence resonance energy transfer. Talanta. 2019;192:500–507. doi: 10.1016/j.talanta.2018.08.086. [DOI] [PubMed] [Google Scholar]
- 121.LaConte L, Nitin N, Bao G. Magnetic nanoparticle probes. Mater today. 2005;8:32–38. doi: 10.1016/S1369-7021(05)00893-X. [DOI] [Google Scholar]
- 122.Neuberger T, Schöpf B, Hofmann H, et al. Superparamagnetic nanoparticles for biomedical applications: possibilities and limitations of a new drug delivery system. J Magn Magn Mater. 2005;293:483–496. doi: 10.1016/j.jmmm.2005.01.064. [DOI] [Google Scholar]
- 123.Yong K-T, Roy I, Swihart MT, Prasad PN. Multifunctional nanoparticles as biocompatible targeted probes for human cancer diagnosis and therapy. J Mater Chem. 2009;19:4655–4672. doi: 10.1039/b817667c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Satapathi S, Kumar V, Chini MK, et al. Highly sensitive detection and removal of mercury ion using a multimodal nanosensor. Nano-Struct Nano-Objects. 2018;16:120–126. doi: 10.1016/j.nanoso.2018.05.006. [DOI] [Google Scholar]
- 125.McGillicuddy E, Murray I, Kavanagh S, et al. Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci Total Environ. 2017;575:231–246. doi: 10.1016/j.scitotenv.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 126.Ikram F, Qayoom A, Aslam Z, Shah MR. Epicatechin coated silver nanoparticles as highly selective nanosensor for the detection of Pb2+ in environmental samples. J Mol Liq. 2019;277:649–655. doi: 10.1016/j.molliq.2018.12.146. [DOI] [Google Scholar]
- 127.Mancini MC, Kairdolf BA, Smith AM, Nie S. Oxidative quenching and degradation of polymer-encapsulated quantum dots: new insights into the long-term fate and toxicity of nanocrystals in vivo. J Am Chem Soc. 2008;130:10836–10837. doi: 10.1021/ja8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang CH, Ding YL, Qian J. Design of magnetic-fluorescent based nanosensor for highly sensitive determination and removal of HG2+ Ceram Int. 2018;44:9746–9752. doi: 10.1016/j.ceramint.2018.02.209. [DOI] [Google Scholar]
- 129.Chen J, Andler SM, Goddard JM, et al. Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev. 2017;46:1272–1283. doi: 10.1039/C6CS00313C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Ichi S, Leon F, Vossier L, et al. Microconductometric immunosensor for label-free and sensitive detection of Gram-negative bacteria. Biosens Bioelectron. 2014;54:378–384. doi: 10.1016/j.bios.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 131.Liu X, Lei Z, Liu F, et al. Fabricating three-dimensional carbohydrate hydrogel microarray for lectin-mediated bacterium capturing. Biosens Bioelectron. 2014;58:92–100. doi: 10.1016/j.bios.2014.02.056. [DOI] [PubMed] [Google Scholar]
- 132.Kim YS, Chung J, Song MY, et al. Aptamer cocktails: enhancement of sensing signals compared to single use of aptamers for detection of bacteria. Biosens Bioelectron. 2014;54:195–198. doi: 10.1016/j.bios.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Verma MS, Wei S-C, Rogowski JL, et al. Interactions between bacterial surface and nanoparticles govern the performance of “chemical nose” biosensors. Biosens Bioelectron. 2016;83:115–125. doi: 10.1016/j.bios.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 134.Sun Y, Fang L, Wan Y, Gu Z. Pathogenic detection and phenotype using magnetic nanoparticle-urease nanosensor. Sens Actuators B Chem. 2018;259:428–432. doi: 10.1016/j.snb.2017.12.095. [DOI] [Google Scholar]
- 135.Verma MS, Chen PZ, Jones L, Gu FX. “Chemical nose” for the visual identification of emerging ocular pathogens using gold nanostars. Biosens Bioelectron. 2014;61:386–390. doi: 10.1016/j.bios.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 136.Verma MS, Chen PZ, Jones L, Gu FX. Branching and size of CTAB-coated gold nanostars control the colorimetric detection of bacteria. Rsc Adv. 2014;4:10660–10668. doi: 10.1039/C3RA46194G. [DOI] [Google Scholar]
- 137.Sun J, Ge J, Liu W, et al. A facile assay for direct colorimetric visualization of lipopolysaccharides at low nanomolar level. Nano Res. 2012;5:486–493. doi: 10.1007/s12274-012-0234-1. [DOI] [Google Scholar]
- 138.Li B-B, Clements WR, Yu X-C, et al. Single nanoparticle detection using split-mode microcavity Raman lasers. Proc Natl Acad Sci. 2014;111:14657–14662. doi: 10.1073/pnas.1408453111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Phillips RL, Miranda OR, You C, et al. Rapid and efficient identification of bacteria using gold-nanoparticle–poly (para-phenyleneethynylene) constructs. Angew Chemie Int Ed. 2008;47:2590–2594. doi: 10.1002/anie.200703369. [DOI] [PubMed] [Google Scholar]
- 140.Sela L, Sobel N. Human olfaction: a constant state of change-blindness. Exp brain Res. 2010;205:13–29. doi: 10.1007/s00221-010-2348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bushdid C, Magnasco MO, Vosshall LB, Keller A. Humans can discriminate more than 1 trillion olfactory stimuli. Science (80-) 2014;343:1370–1372. doi: 10.1126/science.1249168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hellwig M, Henle T. Baking, ageing, diabetes: a short history of the Maillard reaction. Angew Chemie Int Ed. 2014;53:10316–10329. doi: 10.1002/anie.201308808. [DOI] [PubMed] [Google Scholar]
- 143.Duncan B, Le NDB, Alexander C, et al. Sensing by smell: nanoparticle-enzyme sensors for rapid and sensitive detection of bacteria with olfactory output. ACS Nano. 2017;11:5339–5343. doi: 10.1021/acsnano.7b00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang M, Chen W, Chen X, et al (2013) Multiplex immunoassays of plant viruses based on functionalized upconversion nanoparticles coupled with immunomagnetic separation. J Nanomater 2013
- 145.Ibba M, Stathopoulos C, Söll D. Protein synthesis: twenty three amino acids and counting. Curr Biol. 2001;11:R563–R565. doi: 10.1016/S0960-9822(01)00344-X. [DOI] [PubMed] [Google Scholar]
- 146.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219. [PMC free article] [PubMed] [Google Scholar]
- 147.Chaicham C, Tuntulani T, Promarak V, Tomapatanaget B. Effective GQD/AuNPs nanosensors for selectively bifunctional detection of lysine and cysteine under different photophysical properties. Sens Actuators B Chem. 2019;282:936–944. doi: 10.1016/j.snb.2018.11.150. [DOI] [Google Scholar]
- 148.Nurunnabi M, Khatun Z, Huh KM, et al. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano. 2013;7:6858–6867. doi: 10.1021/nn402043c. [DOI] [PubMed] [Google Scholar]
- 149.Yang S-T, Cao L, Luo PG, et al. Carbon dots for optical imaging in vivo. J Am Chem Soc. 2009;131:11308–11309. doi: 10.1021/ja904843x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lou T, Chen Z, Wang Y, Chen L. Blue-to-red colorimetric sensing strategy for Hg2+ and Ag+ via redox-regulated surface chemistry of gold nanoparticles. ACS Appl Mater Interfaces. 2011;3:1568–1573. doi: 10.1021/am200130e. [DOI] [PubMed] [Google Scholar]
- 151.Zhang L, Zhang Z-Y, Liang R-P, et al. Boron-doped graphene quantum dots for selective glucose sensing based on the “abnormal” aggregation-induced photoluminescence enhancement. Anal Chem. 2014;86:4423–4430. doi: 10.1021/ac500289c. [DOI] [PubMed] [Google Scholar]
- 152.Lu L, Feng C, Xu J, et al. Hydrophobic-carbon-dot-based dual-emission micelle for ratiometric fluorescence biosensing and imaging of Cu2+ in liver cells. Biosens Bioelectron. 2017;92:101–108. doi: 10.1016/j.bios.2017.01.066. [DOI] [PubMed] [Google Scholar]
- 153.Kim JH, Hwang IH, Jang SP, et al. Zinc sensors with lower binding affinities for cellular imaging. Dalt Trans. 2013;42:5500–5507. doi: 10.1039/c3dt33024a. [DOI] [PubMed] [Google Scholar]
- 154.Bothra S, Babu LT, Paira P, et al. A biomimetic approach to conjugate vitamin B6 cofactor with the lysozyme cocooned fluorescent AuNCs and its application in turn-on sensing of zinc(II) in environmental and biological samples. Anal Bioanal Chem. 2018;410:201–210. doi: 10.1007/s00216-017-0710-2. [DOI] [PubMed] [Google Scholar]
- 155.Wang Y, Lao S, Ding W, et al. A novel ratiometric fluorescent probe for detection of iron ions and zinc ions based on dual-emission carbon dots. Sens Actuators B Chem. 2019;284:186–192. doi: 10.1016/j.snb.2018.12.139. [DOI] [Google Scholar]
- 156.Thebo AL, Drechsel P, Lambin EF, Nelson KL. A global, spatially-explicit assessment of irrigated croplands influenced by urban wastewater flows. Environ Res Lett. 2017;12:74008. doi: 10.1088/1748-9326/aa75d1. [DOI] [Google Scholar]
- 157.Tian X, Wang J, Li Y, et al. Sensitive determination of hardness and fluoride in ground water by a hybrid nanosensor based on aggregation induced FRET on and off mechanism. Sens Actuators B Chem. 2018;262:522–530. doi: 10.1016/j.snb.2018.02.020. [DOI] [Google Scholar]
- 158.Susheela AK, Das TK, Gupta IP, et al. Fluoride ingestion and its correlation with gastrointestinal discomfort. Fluoride. 1992;25:5–22. [Google Scholar]
- 159.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, et al. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 160.Schreiber SL. Organic synthesis toward small-molecule probes and drugs. Proc Natl Acad Sci. 2011;108:6699–6702. doi: 10.1073/pnas.1103205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lu L-Q, Ma M-Y, Tan T, et al. Novel dual ligands capped perovskite quantum dots for fluoride detection. Sens Actuators B Chem. 2018;270:291–297. doi: 10.1016/j.snb.2018.05.038. [DOI] [Google Scholar]
- 162.Akgönüllü S, Yavuz H, Denizli A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta. 2020;219:121219. doi: 10.1016/j.talanta.2020.121219. [DOI] [PubMed] [Google Scholar]
- 163.Chen K, Han H, Luo Z, et al. A practicable detection system for genetically modified rice by SERS-barcoded nanosensors. Biosens Bioelectron. 2012;34:118–124. doi: 10.1016/j.bios.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 164.Picó Y, Andreu V (2014) Nanosensors and other techniques for detecting nanoparticles in the environment. In: Nanosensors for chemical and biological applications. Elsevier, pp 295–338
- 165.O’Brien N, Cummins E. Recent developments in nanotechnology and risk assessment strategies for addressing public and environmental health concerns. Hum Ecol Risk Assess. 2008;14:568–592. doi: 10.1080/10807030802074261. [DOI] [Google Scholar]
- 166.O’Brien N, Cummins E. Ranking initial environmental and human health risk resulting from environmentally relevant nanomaterials. J Environ Sci Heal Part A. 2010;45:992–1007. doi: 10.1080/10934521003772410. [DOI] [PubMed] [Google Scholar]
- 167.Hou J, Wu Y, Li X, et al. Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere. 2018;193:852–860. doi: 10.1016/j.chemosphere.2017.11.077. [DOI] [PubMed] [Google Scholar]
- 168.Farré M, Gajda-Schrantz K, Kantiani L, Barceló D. Ecotoxicity and analysis of nanomaterials in the aquatic environment. Anal Bioanal Chem. 2009;393:81–95. doi: 10.1007/s00216-008-2458-1. [DOI] [PubMed] [Google Scholar]
- 169.Kumar A, Pandey AK, Singh SS, et al. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic Biol Med. 2011;51:1872–1881. doi: 10.1016/j.freeradbiomed.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 170.Mishra VK, Kumar A. Impact of metal nanoparticles on the plant growth promoting rhizobacteria. Dig J Nanomater Biostruct. 2009;4:587–592. [Google Scholar]
- 171.Saliba AM, De Assis M-C, Nishi R, et al. Implications of oxidative stress in the cytotoxicity of Pseudomonas aeruginosa ExoU. Microb Infect. 2006;8:450–459. doi: 10.1016/j.micinf.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 172.Lu T, Lee H, Chen T, et al. High sensitivity nanoparticle detection using optical microcavities. Proc Natl Acad Sci. 2011;108:5976–5979. doi: 10.1073/pnas.1017962108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kumar S, Sachdeva S, Chaudhary S, Chaudhary GR. Assessing the potential application of bio-compatibly tuned nanosensor of Yb2O3 for selective detection of imazapyr in real samples. Colloids Surf A Physicochem Eng Asp. 2020;593:124612. doi: 10.1016/j.colsurfa.2020.124612. [DOI] [Google Scholar]
- 174.Yılmaz E, Özgür E, Bereli N, et al. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater Sci Eng C. 2017;73:603–610. doi: 10.1016/j.msec.2016.12.090. [DOI] [PubMed] [Google Scholar]
- 175.Kant R. Surface plasmon resonance based fiber–optic nanosensor for the pesticide fenitrothion utilizing Ta2O5 nanostructures sequestered onto a reduced graphene oxide matrix. Microchim Acta. 2020;187:1–11. doi: 10.1007/s00604-019-4002-8. [DOI] [PubMed] [Google Scholar]
- 176.Chang Y-C, Lin Y-S, Xiao G-T, et al. A highly selective and sensitive nanosensor for the detection of glyphosate. Talanta. 2016;161:94–98. doi: 10.1016/j.talanta.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 177.Liu H, Chen P, Liu Z, et al. Electrochemical luminescence sensor based on double suppression for highly sensitive detection of glyphosate. Sens Actuators B Chem. 2020;304:127364. doi: 10.1016/j.snb.2019.127364. [DOI] [Google Scholar]
- 178.Xing C, Kuang H, Hao C, et al. A silver enhanced and sensitive strip sensor for Cadmium detection. Food Agric Immunol. 2014;25:287–300. doi: 10.1080/09540105.2013.781140. [DOI] [Google Scholar]
- 179.Kuang H, Xing C, Hao C, et al. Rapid and highly sensitive detection of lead ions in drinking water based on a strip immunosensor. Sensors. 2013;13:4214–4224. doi: 10.3390/s130404214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jimenez-Falcao S, Villalonga A, Parra-Nieto J, et al. Dithioacetal-mechanized mesoporous nanosensor for Hg (II) determination. Microporous Mesoporous Mater. 2020;297:110054. doi: 10.1016/j.micromeso.2020.110054. [DOI] [Google Scholar]
- 181.Anwar A, Minhaz A, Khan NA, et al. Synthesis of gold nanoparticles stabilized by a pyrazinium thioacetate ligand: A new colorimetric nanosensor for detection of heavy metal Pd(II) Sens Actuators B Chem. 2018;257:875–881. doi: 10.1016/j.snb.2017.11.040. [DOI] [Google Scholar]
- 182.Qi Y, Zhao J, Weng G, et al. Modification-free colorimetric and visual detection of Hg2+ based on the etching from core-shell structural Au-Ag nanorods to nanorices. Sens Actuators B Chem. 2018;267:181–190. doi: 10.1016/j.snb.2018.04.042. [DOI] [Google Scholar]
- 183.Wang X, Qi Y, Shen Y, et al. A ratiometric electrochemical sensor for simultaneous detection of multiple heavy metal ions based on ferrocene-functionalized metal-organic framework. Sens Actuators B Chem. 2020;310:127756. doi: 10.1016/j.snb.2020.127756. [DOI] [Google Scholar]
- 184.Lin H-Y, Huang C-H, Lu S-H, et al. Direct detection of orchid viruses using nanorod-based fiber optic particle plasmon resonance immunosensor. Biosens Bioelectron. 2014;51:371–378. doi: 10.1016/j.bios.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 185.Isha A, Akanbi FS, Yusof NA, et al (2019) An NMR metabolomics approach and detection of ganoderma boninense-infected oil palm leaves using MWCNT-based electrochemical sensor. J Nanomater 2019
- 186.Fang Y, Umasankar Y, Ramasamy RP. Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analyst. 2014;139:3804–3810. doi: 10.1039/C4AN00384E. [DOI] [PubMed] [Google Scholar]
- 187.Lau HY, Wu H, Wee EJH, et al. Specific and sensitive isothermal electrochemical biosensor for plant pathogen DNA detection with colloidal gold nanoparticles as probes. Sci Rep. 2017;7:1–7. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lew TTS, Koman VB, Silmore KS, et al. Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat Plants. 2020;6:404–415. doi: 10.1038/s41477-020-0632-4. [DOI] [PubMed] [Google Scholar]
- 189.Alova A, Erofeev A, Gorelkin P, et al. Prolonged oxygen depletion in microwounded cells of Chara corallina detected with novel oxygen nanosensors. J Exp Bot. 2020;71:386–398. doi: 10.1093/jxb/erz433. [DOI] [PubMed] [Google Scholar]
- 190.Vandarkuzhali SAA, Jeyalakshmi V, Sivaraman G, et al. Highly fluorescent carbon dots from pseudo-stem of banana plant: applications as nanosensor and bio-imaging agents. Sens Actuators B Chem. 2017;252:894–900. doi: 10.1016/j.snb.2017.06.088. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.