Abstract
The practice of using nutritional knowledge to enhance the general health of the populace forms the basis of food fortification. In this study, an antioxidant-rich Curculigo pilosa is substituted (1, 3, 5 and 10%) into yam tuber flour with the aim of improving the antidiabetic and antioxidant properties of the yam tuber flour. Antioxidant property was evaluated by polyphenol contents, ascorbic acid content, reducing effects, scavenging activity and inhibition of linoleic acid peroxidation. Antidiabetic activity was assessed by inhibition of α- amylase and α- glucosidase enzymes. The antioxidant property was significantly (p < 0.05) enhanced; also, the ability of the sample to inhibit the activity of α- amylase and α- glucosidase enzymes were significantly (p < 0.05) enhanced by supplementation with Curculigo pilosa. The profiling of the samples by High Performance Liquid Chromatography revealed some proven antioxidant and antidiabetic agents which were enhanced in supplemented yam flour. It can be concluded from the results obtained in this study that Curculigo pilosa powder could be a promising functional ingredient for yam flour in the management of diabetes and other oxidant-related diseases.
Keywords: Fortification, Antidiabetic, Antioxidant, HPLC-DAD
Introduction
The association between food intake and prevention or management of diseases has an increasing impact on food innovation due to popularity of the concept of functional foods. Such foods are rich in phytochemicals which modulate the risk of disease development (Olaiya et al. 2016); hence the practice of food fortification through proper utilization of nutritional knowledge to improve man’s health has gained increasing acceptance worldwide (IFPRI 2016).
Food supplementation can be defined as deliberate addition or inclusion of a food component into a food, so as to improve the nutritional and health benefits of such foods (Caballero 2003). Originally this concept was well used to curtain the problem of micronutrient deficiency in infants by introduction of essential vitamins and minerals into their diets. But, recently due to popularity and acceptance of herbal products, the concept has been extended to inclusion of medicinal plants and spicy to staple food in order to increase or enhance the health functionality of such foods (Alashi et al. 2018).
Diabetes mellitus (DM) has become a global burden. According to International Diabetes Federation (IDF), in 2019, 48 million suffered the disease in North America, 59 million in Europe, 19 million in Africa and 88 million in South East Asia (IDF 2019). According to the recent IDF atlas, two-thirds of people living with diabetes globally live in urban areas (IDF 2019). This dense population of diabetic patients in urban areas might be attributed to many factors, namely structural change from agriculture to industrialization which has reduced the cost of calories through agricultural innovation and more importantly, the high cost of fruit and vegetables in the urban area which is due to the limited supply (Zakariah et al. 2017).
DM is characterized by elevated blood glucose which induced oxidative stress on body’s tissues thereby causing its complications. Also postprandial hyperglycemia plays an important role in the development of type 2 diabetes and its complications, and this phenomenon has been attributed to excessive activity of two carbohydrate-hydrolyzing enzymes; α-amylase and α-glucosidase (Phan et al. 2013). Therefore inhibiting these enzymes can be a therapeutic approach in the management of diabetes mellitus.
Yams, Dioscorea (spp.), are prominent source of carbohydrate in the sub-Sahara regions (Akissoe et al. 2001). They are grown in tropical regions and mostly in an area where rainfalls are divided into dry and wet seasons (FAO 1997). Nigeria is the largest producer of yams in the world, followed by Ghana (Verter and Beerarova, 2015). The utilization of yams include: boiling/roasting them as food, pounding them into a thick paste (pounded yam) after boiling and processing into flour, which is used in the preparation of the paste (Amala) (Kordylas, 1990). Amala has become so popular and acceptable recently that its market has increased considerably (Mestres et al. 2004). It is more consumed in urban areas than the traditional pounded yam because of its relative ease of preparation.
Curculigo pilosa (CP), a member of the family Hypoxidaceae was the first African species to be described of the Curculigo genus. The rhizomes of this plant possess medicinal properties and are also used as food. Traditionally, it is used in the management of diabetes mellitus. It is also used in the manufacture of infant food and sorghum beer. Its use in manufacture of infant food is due to presence of its high amylolytic activity. The CP rhizome flour has been reported to contain high level of dietary fiber, protein, essential minerals, polyphenols and possessed antioxidant abilities (Adefegha et al. 2018). Apart from its richness in polyphenols, it has been reported to possess antimicrobial and antidiabetic activities and ability to inhibit carbohydrate hydrolyzing enzymes (Gbadamosi and Egunyomi 2010; Karigidi and Olaiya 2019).
Due to the various nutritional, antioxidative and medicinal properties of Curculigo pilosa, the interest to use it as a functional ingredient in producing pro-health foods especially against non communicable and oxidant-related diseases is increasing. Therefore the objective of this present study is to evaluate the influence of Curculigo pilosa as a fortificant on the invitro antidiabetic and antioxidant activities of yam flour and profiling of the composite yam flour using High Performance Liquid Chromatography (HPLC-DAD).
Materials and methods
Plant materials
Healthy yam tubers of Dioscorea mangenotiana Miege and Curculigo pilosa rhizomes used in this work were obtained from a local market (Bodija), Ibadan. Identification and authentication were done at Herbarium of Department of Botany, University of Ibadan. Vouchers for Dioscorea mangenotiana Miege (UIH-22954) and Curculigo pilosa (UIH-22759) were deposited at their herbarium. They were washed under running tap water to remove dirts.
Preparation of Curculigo pilosa powder
Curculigo pilosa was sliced and shade dried in the laboratory. The shade dried sample was grinded into powdery form, sieved and packaged in an airtight container and stored in a freezer prior to use.
Preparation of yam powder
The yam flour was prepared by the method of (Mestres et al. 2004). Fresh tubers were peeled, sliced into 1.5–2.0 cm thickness and immediately transferred into a cooking pot containing cold water. The pot was heated from ambient temperature to 65 ± 2 °C and heating was stopped after 25 min. Boiled yams were left overnight in the heated water, thereafter, dried in an oven at 50 °C for 5 days. The dried flakes were crushed and grinded into flour, sieved and used for the preparation of yam- Curculigo pilosa composite flour.
Formulation of different blend of yam tuber and Curculigo pilosa flours
Different blends of yam tuber flour and Curculigo pilosa were prepared as follows:
Y0CP: Yam flour; Y1CP: 1% of yam flour was substituted by Curculigo pilosa; Y3CP: 3% of yam flour was substituted by Curculigo pilosa; Y5CP: 5% of yam flour was substituted by Curculigo pilosa; Y10CP: 10% of yam flour was substituted by Curculigo pilosa.
The different blend samples were packaged in airtight containers and stored in a freezer.
Extraction of samples for antioxidant and antidiabetic assays
Fifty grams (50 g) of the different blend of yam tuber flour and Curculigo pilosa was extracted with 500 ml of distilled water on orbital shaker for 4 h at 250 rpm. The slurry obtained was centrifuged at 3000 rpm (988 × g) for 10 min. The supernatant was used for the chemical assays immediately.
Determination of total phenolic content (TPC)
TPC of the flour samples was done using the (Kim et al. 2003) method as modified by (Karigidi et al. 2018). Briefly 1.0 ml of the sample was mixed with 1.0 ml (10%) of Folin-Ciocalteu phenol reagent. After 5 min, 5.0 ml of 7% Na2CO3 was added followed immediately with by addition of 5.0 ml of distilled water and shaken thoroughly. The mixture was kept in the dark for 90 min at room temperature. The absorbance was read at 750 nm and the TPC was evaluated from Gallic acid standard curve and expressed as Gallic acid equivalent (mg GAE/100 g).
Determination of total flavonoid content (TFC)
TFC of the flour samples was done using the (Park et al. 2008) method. Briefly 0.3 ml of the sample was mixed with 3.4 ml (30%) of methanol, 0.15 ml (0.5 M) of NaNO2 and 0.15 ml (0.3 M) of AlCl36H20 successively. After 5 min, 1 ml of 1 M NaOH was added and mixed well. The absorbance was read at 506 nm and the flavonoid content evaluated from Quercetin standard curve and expressed as Quercetin equivalent (mg QUE/100 g).
Total antioxidant capacity phosphomolybdate assay (TAC)
TAC of the flour samples was determined using the method of (Prieto et al. 1999). Sample (0.4 ml) and 4.0 ml of phosphomolybdate reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was thoroughly mixed together. The mixture was capped and incubated in a boiling water bath at 95 °C for 90 min. The mixture was cooled to room temperature and the absorbance of the samples was measured at 695 nm. The total antioxidant capacity was calculated from Ascorbic acid calibration curve and expressed as ascorbic acid equivalent (mg AAE/100 g).
Determination of reducing power (RP)
RP of the flour samples was done using the method of Oyaizu (1986). Appropriate dilutions of sample, 1.0 ml (0.1–0.4 mg/ml) were mixed with 1.0 ml phosphate buffer (0.2 M, pH 6.6) followed by 1 ml of potassium ferricyanide (1%) and incubated for 20 min at 50 °C. The reaction was terminated by 1 ml trichloroacetic acid (10%). One millitre (1.0 ml) of the upper portion was taken, mixed with 1 ml of distilled water and followed by 1 ml ferric chloride (0.1%). The reaction mixture was thoroughly mixed and the absorbance was read at 700 nm. Reducing power was evaluated from ascorbic acid calibration curve and expressed as ascorbic acid equivalent (mg AAE/100 g).
Determination of ferric reducing antioxidant potential (FRAP)
FRAP of the flour samples was done using the method of Benzie and strain 1996. The FRAP working reagent was freshly prepared by mixing solutions of 25 ml acetate buffer, 2.5 ml TPTZ solution, and ferric chloride in ratio 10:1:1 and warmed at 37 °C before use. Samples (0.2 ml) were mixed with 2.80 ml of the FRAP reagent and the mixtures were kept in the dark for 30 min at room temperature. The absorbance was read at 593 nm and FRAP was evaluated from ferrous sulphate standard curve and expressed as (mg Fe2+E/100 g).
Determination of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) scavenging activity
DPPH radical scavenging activity of the flour samples was done using the (Gyamfi, et al. 1999) method with slight modification. Appropriate dilutions of 1.0 ml (0.1–0.4 mg/ml) sample were added to 4 ml of DPPH solution (30 mg/l) prepared in methanol. The samples were mixed thoroughly and left in the dark for 30 min. The absorbance was read at 520 nm. The inhibition percentage was calculated as.
Determination of ascorbic acid (AsA)
AsA of the flour samples was done using the method AOAC (2014). Briefly, 5 g of the sample was extracted with 50 ml (3%) metaphosphoric acid, the extract was filtered using filter paper and the filtrate was using for determination of ascorbic acid immediately. 10 ml of meta-phosphoric acid extracted sample in 50 ml conical flask was titrated against 0.25% of 2, 6-dichloro indophenol dye solution (DPIP) dye to faint pink colour which should persist for 15 s. The ascorbic acid content was calculated as follows:
Where CA = Concentration of the Standard Vitamin C (mg/ml).
VD = Titre value for the sample (ml).
VA = Titre value for Standard Vitamin C (ml).
VB = Volume of the sample used (ml).
W = Weight of the sample (mg).
Inhibition of linoleic acid peroxidation (ILP)
ILP of the flour samples was determined according to the method of (Kuo et al. 1999) with some modifications. After appropriate reconstitution of the sample extracts, 10 µl of sample was mixed with 0.37 mL (0.05 M; pH 7) phosphate buffer containing 0.05% Tween 20 and 4.0 mmol linoleic acid and then equilibrated at 37 °C for 3 min. The linoleic acid peroxidation in this reaction mixture was initiated by 0.02 ml (10 mmol) ferrous chloride, followed by incubation in a water bath at 37 °C for 10 min. The peroxidation was halted by addition of 5.0 ml 0.6% HCl in ethanol. The hydroxyperoxide formed was assayed by addition of 0.1 ml (0.02 mol) ferrous chloride and 0.1 ml (30%) ammonium thiocyanate. The absorbance was read at 480 nm and percentage inhibition was calculated as follows:
Reaction mixture without sample was used as a control.
Determination of amylase inhibition assay (AI)
AI of the flour samples was determined using the method of Worthington (1993). After appropriate dilution, 500 µl (0.2–1.0 mg/ml) of sample and 500 µl (0.02 M) of sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing 0.5 mg/ml α-amylase solution were incubated at room temperature for 10 min. Thereafter, 500 µl (1%) of starch solution prepared with 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M sodium chloride) was added. The reaction mixture was then incubated at 25 °C for 10 min. The reaction was halted with 1.0 ml (96 mM) of dinitrosalicylic acid. The test tubes were then incubated in a boiling water bath for 5 min and allowed to cool. The absorbance was read at 540 nm.The inhibition percentage was calculated:
Reaction mixture without sample was used as a control.
Determination of glucosidase inhibition assay (GI)
GI of the flour samples was done using the method of Apostolidis et al. 2007. After appropriate dilution, 500 µl (0.2–1.0 mg/ml) of sample was suspended in 1000 μl α-glucosidase solution (1.0 U/L) prepared in of 0.1 M phosphate buffer (pH 6.9) and pre-incubated for 10 min at 25 °C. After pre-incubation, 500 μl of 5 mM nitrophenyl-glucopyranoside solution prepared in 0.1 M phosphate buffer (pH 6.9) was added. The reaction mixture was incubated at 25 °C for 5 min. The absorbance of the reaction mixture was measured at 405 nm. The percentage inhibition was calculated using the formula:
Reaction mixture without sample was used as a control.
Quantification of compounds by HPLC-DAD
This was determined by the earlier method of (Boligon et al. 2013) with slight modifications. Ten grammes of flour samples were extracted with acetonitrile, stabilized with ethyl acetate and made up to 25 ml. Standard form of analytes profile were first injected into the HPLC. This generated a chromatogram of given peak area and peak profile that was used to create a window in the HPLC for the test sample analysis. Briefly, an aliquot of the extracted yam sample was injected into the HPLC to obtain a corresponding peak area and peak profile. The peak area of the sample is compared with that of the standard relative to the concentration of the standard. The concentration of the sample was calculated using the formula:
Statistical analysis
Data are expressed as the mean ± SD of triplicate measurements. The significance between means of the samples were established by the analysis of variance using least significant difference (LSD) P < 0.05, charts were drawn with graph pad prism 5 and Pearson correlation test was conducted to determine the correlation among polyphenols content, antioxidant activities and enzymes inhibition. Significant levels were established using p < 0.05.
Results and discussion
The effects of Curculigo pilosa powder (CPP) supplementation on the total phenolic and total flavonoid (polyphenols) content of yam flour is presented in Table 1. The polyphenols content increased significantly with increasing CPP in the composite flour. Polyphenols are secondary metabolites of immerse physiological roles in food. They are distributed into several classes which include phenolic and flavonoids. The increase in the polyphenols content of the composite flour might not be unconnected with the high phenolic content of Curculigo pilosa (Adefegha et al. 2018). Also, the ability of the composite yam flour to reduce the transition metal Fe3+ to Fe2+ by electron transfer (FRAP and RP)were also reported in Table 1. The result showed that the ability of samples was significantly increased upon supplementation. The results of DPPH scavenging and inhibition of linoleic acid peroxidation is presented in Fig. 1. The DPPH scavenging and inhibition of linoleic acid peroxidation of the composite flours were increased significantly (p < 0.05) by increasing the CPP content in the composite flours (Table 2). DPPH assay measures the ability of the sample to donate hydrogen atom to DPPH salts thereby converting it to its reduced form, diphenylpicrylhydrazine with the loss of its violet colour (Alam et al. 2013). The increase in DPPH scavenging activity could be attributed to enhanced polyphenols content of the composite flours which is further proven by strong correlation between polyphenols and DPPH (Table 3). Also, Curculigo pilosa extracts have been reported to exhibit strong DPPH scavenging activities (Adefegha et al. 2018; Karigidi et al. 2019). Lipid peroxidation can arise from the reaction of metmyoglobin (MbFe3+–OH), the pigment of red meat, dietary iron, hydrogen peroxide and poly unsaturated fatty acids in the stomach (Achat et al. 2016). In this study, the reaction was mimicked invitro using linoleic acid and the rate of inhibition of the reaction was significantly increased with supplementation of yam flour with CPP. This enhanced inhibition might not be unconnected with increase polyphenols content of the composite flours as most of antioxidant potential of foods are due to their polyphenols content which includes reducing agents, free radical scavengers and hydrogen donors (Gawlik-Dziki et al. 2013).
Table 1.
Antioxidant activity of Yam-Curculigo pilosa composite flours
| Y0CP | Y1CP | Y3CP | Y5CP | Y10CP | |
|---|---|---|---|---|---|
| TPC | 51.48 ± 0.62a | 66.48 ± 1.48b | 109.29 ± 0.47c | 119.60 ± 1.04c | 128.14 ± 3.80d |
| TFC | 6.69 ± 0.27a | 10.11 ± 0.31b | 20.37 ± 1.79c | 26.70 ± 1.81c | 40.38 ± 1.57d |
| TAC | 28.88 ± 2.60a | 37.11 ± 5.35b | 53.20 ± 6.28c | 54.70 ± 5.60c | 74.44 ± 2.06d |
| RP | 13.90 ± 0.31a | 15.53 ± 0.41a | 20.30 ± 0.31b | 21.45 ± 0.27b | 30.35 ± 1.25c |
| FRAP | 26.30 ± 0.55a | 71.30 ± 0.38b | 97.24 ± 1.31c | 106.04 ± 0.80d | 110.12 ± 0.07d |
| AsA | 3.37 ± 0.25a | 4.30 ± 0.22b | 4.95 ± 0.22b | 5.52 ± 0.45c | 6.60 ± 0.33d |
Values were represented as Mean ± SD. Values with different letter across the same row are significantly (P < 0.05) different. TPC, mg GAE/g; TFC, mg QUE/g; TAC, mg AAE/g; RP, mg AAE/g; FRAP, mg Fe2+E/g; AsA, mg/g; Y0CP, yam flour; Y1CP, 1% of yam flour was substituted by Curculigo pilosa; Y3CP, 3% of yam flour was substituted by Curculigo pilosa; Y5CP, 5% of yam flour was substituted by Curculigo pilosa; Y10CP, 10% of yam flour was substituted by Curculigo pilosa
Fig. 1.
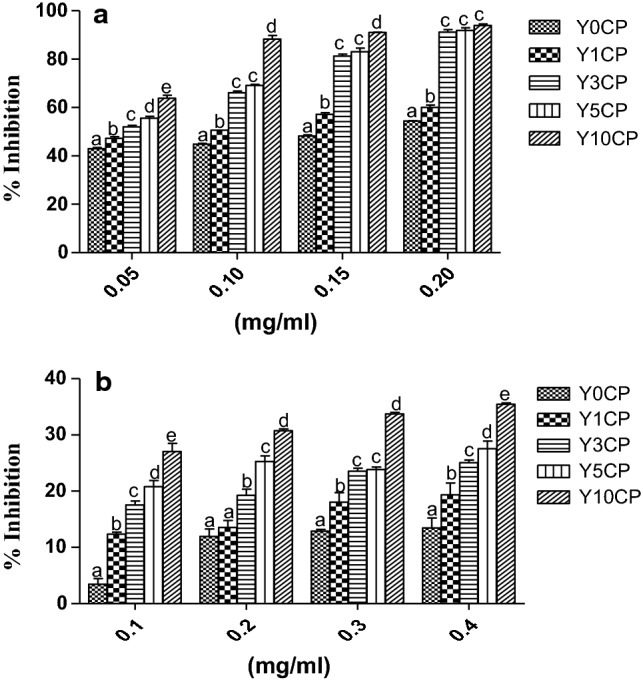
DPPH scavenging (a) and inhibition of linoleic acid peroxidation (b) of Yam-Curculigo pilosa composite flours. Y0CP, Control; Y1CP, 1% of yam flour was substituted by Curculigo pilosa; Y3CP, 3% of yam flour was substituted by Curculigo pilosa; Y5CP, 5% of yam flour was substituted by Curculigo pilosa; Y10CP, 10% of yam flour was substituted by Curculigo pilosa
Table 2.
IC50 (mg/mL) of yam-Curculigo pilosa on DPPH, α-amylase, α-glucosidase and ILP
| Y0CP | Y1CP | Y3CP | Y5CP | Y10CP | |
|---|---|---|---|---|---|
| DPPH | 0.33 ± 0.02a | 0.15 ± 0.01b | 0.10 ± 0.01c | 0.08 ± 0.01d | 0.07 ± 0.01d |
| α-amylase | 1.82 ± 0.12a | 1.70 ± 0.15a | 1.63 ± 0.20a | 1.52 ± 0.11b | 1.22 ± 0.06c |
| α-glucosidase | 1.78 ± 0.09a | 1.68 ± 0.11a | 1.58 ± 0.07b | 1.36 ± 0.10c | 1.30 ± 0.10c |
| ILP | 0.94 ± 0.03a | 0.86 ± 0.04a | 0.75 ± 0.03b | 0.69 ± 0.01b | 0.58 ± 0.01c |
Values were represented as Mean ± SD. Values with different letter across the same row are significantly (P < 0.05) different
Table 3.
Correlations between polyphenols, antioxidant activities and inhibition of enzymes linked to diabetes mellitus
| TPC | TFC | TAC | DPPH | FRAP | RP | ILP | AMY | GLU | |
|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | ||||||||
| TFC | 0.92 | 1 | |||||||
| TAC | 0.91 | 0.96 | 1 | ||||||
| DPPH | 0.99 | 0.91 | 0.91 | 1 | |||||
| FRAP | 0.95 | 0.84 | 0.86 | 0.94 | 1 | ||||
| RP | 0.88 | 0.96 | 0.96 | 0.86 | 0.80 | 1 | |||
| ILP | 0.93 | 0.96 | 0.93 | 0.91 | 0.89 | 0.93 | 1 | ||
| AMY | 0.84 | 0.96 | 0.94 | 0.82 | 0.78 | 0.98 | 0.92 | 1 | |
| GLU | 0.90 | 0.98 | 0.93 | 0.89 | 0.86 | 0.96 | 0.96 | 0.95 | 1 |
TPC, Total phenolic content; TFC, Total Flavonoid content; TAC, Total antioxidant capacity; DPPH, 1, 1-diphenyl-2-picrylhydrazyl scavenging activity; FRAP, Ferric reducing antioxidant power; RP, Reducing power; ILP, Inhibition of linoleic acid peroxidation; AMY, α- amylase inhibition; GLU = α- glucosidase inhibition
Ascorbic acid, popularly known as Vitamin C is found in food and often used as dietary supplement. It is an essential nutrient involved in the repair of tissue and the enzymatic production of certain neurotransmitters. It has also been reported to possess strong reducing ability (Badejo et al. 2019). Supplementation of yam flour with CPP led to increase in ascorbic acid content of the composite flours. The yam flour supplemented with 10% of CPP has almost 2-folds content of ascorbic acid when compared with unsupplemented yam flour. The enhanced ascorbic acid of the composite yam flour might be attributed to higher content of ascorbic acid in CPP. This present study is in agreement with the work of Badejo et al. (2019) where inclusion of tigernut enhanced the ascorbic acid content of Kunnu.
Supplementation of yam flour with CPP also significantly increased the abilities of the composite flours to inhibit digestive enzymes linked to diabetes mellitus; α-amylase and α-glucosidase enzymes (Fig. 2). These enzymes are responsible for breakdown of large carbohydrates into simple absorbable glucose in the body. Excessive activity of the enzymes has been reported in diabetes mellitus (Tysoe et al. 2016). Therefore, their inhibition has been used as a therapeutic target in the treatment of diabetes (Gajera et al. 2017). In the study, the ability to inhibit these enzymes significantly increased as the inclusion of CPP increased. This enhanced ability might not be unconnected with increased polyphenols content of the composite flours as many researchers have shown positive correlation between polyphenols content and ability to inhibit these enzymes (Nowicka and Wojdylo 2019). Also, it might be due to the presence of antidiabetic phytochemicals in CPP as we have previously reported its ability to inhibit α-amylase and α-glucosidase enzymes (Karigidi and Olaiya 2019).
Fig. 2.
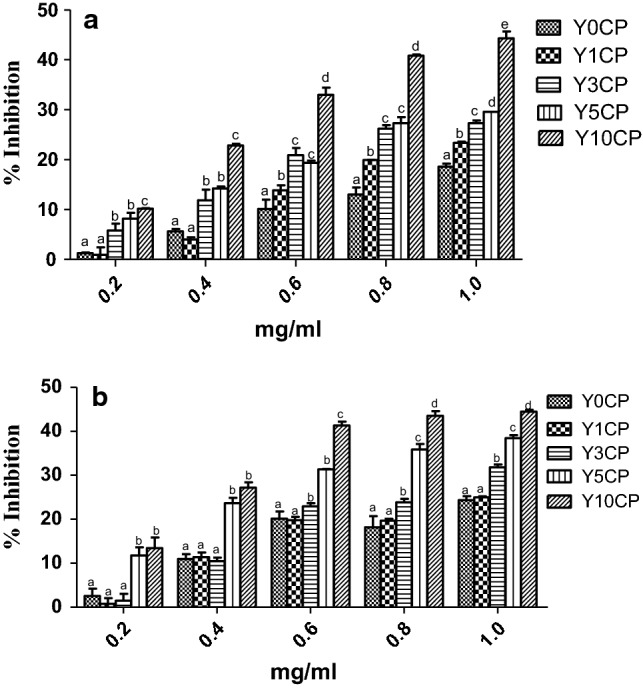
α-amylase (a) and α-glucosidase (b) inhibitory capacities of Yam-Curculigo pilosa composite flours. Y0CP, Control; Y1CP, 1% of yam flour was substituted by Curculigo pilosa; Y3CP, 3% of yam flour was substituted by Curculigo pilosa; Y5CP, 5% of yam flour was substituted by Curculigo pilosa; Y10CP, 10% of yam flour was substituted by Curculigo pilosa
The result of phytochemical (HPLC) fingerprinting of the yam and composite yam flours is presented in Table 4. The result shown that substitution of yam flour with CPP led to enhancement and introduction of some phytochemicals which were not present in yam flour before. For example, Sitosterol and Stigmasterol which are present in yam flour were enhanced with substitution of 5% and 10% CPP. Also, phytochemicals like Curculin, Curculigol, Curculigenin, Piloside and Curculigoside were not present in yam flour but introduced by inclusion of CPP. Except Piloside, the content of these phytochemicals increased with increase CPP. Many of these phytochemicals have been reported with antioxidant and antidiabetic properties; therefore the enhanced antioxidant and antioxidant activities of the composite yam flours might be due to their increase. (Wu et al. 2005; Ward et al. 2017; Chang et al. 2018).
Table 4.
Phytochemical profiling (mg/g) of Yam-Curculigo pilosa composite Flour
| Y0CP | Y1CP | Y3CP | Y5CP | Y10CP | |
|---|---|---|---|---|---|
| Sitosterol | 3.01 ± 0.08c | 2.73 ± 0.05a | 2.95 ± 0.07a | 3.07 ± 0.12a | 3.22 ± 0.07b |
| Curculin | ND | 1.37 ± 0.02a | 1.60 ± 0.04a | 2.10 ± 0.05b | 2.10 ± 0.17b |
| Curculigol | ND | 0.84 ± 0.08a | 1.18 ± 0.11b | 1.28 ± 0.06b | 1.39 ± 0.04c |
| Curculigenin A | ND | 0.71 ± 0.04a | 0.81 ± 0.02a | 1.11 ± 0.10b | 1.14 ± 0.07b |
| Curculigenin B | ND | 0.60 ± 0.05a | 0.97 ± 0.04b | 1.61 ± 0.05c | 1.74 ± 0.02c |
| Poliside A | ND | 0.21 ± 0.00a | 0.21 ± 0.00a | 0.21 ± 0.00a | 0.21 ± 0.00a |
| Curculigoside | ND | 0.65 ± 0.02a | 1.07 ± 0.10b | 1.53 ± 0.15c | 1.61 ± 0.02c |
| Stigmasterol | 4.59 ± 0.15d | 3.63 ± 0.04a | 4.01 ± 0.05a | 4.72 ± 0.03b | 5.46 ± 0.10c |
Values were represented as Mean ± SD. Values with different letter across the same row are significantly (P < 0.05) different. ND = not detected. Y0CP, Control; Y1CP, 1% of yam flour was substituted by Curculigo pilosa; Y3CP, 3% of yam flour was substituted by Curculigo pilosa; Y5CP, 5% of yam flour was substituted by Curculigo pilosa; Y10CP, 10% of yam flour was substituted by Curculigo pilosa
Conclusion
Supplementation of Curculigo pilosa powder with yam flour was significantly able to increase the phenolic contents and antioxidant activities of the composite flour. Also the abilities to inhibit digestive enzymes; (α-amylase and α-glucosidase) and induced linoleic acid peroxidation was enhanced. Therefore, Curculigo pilosa powder can be a promising functional ingredient for yam flour in the management of diabetes and other oxidant-related diseases.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Achat S, Rakotomanomana N, Madani K, Dangles O. Antioxidant activity of olive phenols and other dietary phenols in model gastric conditions: scavenging of the free radical DPPH and inhibition of the haem-induced peroxidation of linoleic acid. Food Chem. 2016;213:135–142. doi: 10.1016/j.foodchem.2016.06.076. [DOI] [PubMed] [Google Scholar]
- Adefegha SA, Oyeleye SI, Oboh G. African crocus (Curculigo pilosa) and wonderful kola (Buchholziacoriacea) seeds modulate critical enzymes relevant to erectile dysfunction and oxidative stress. J Complemen Integrat Med. 2018 doi: 10.1515/jcim-2016-0159. [DOI] [PubMed] [Google Scholar]
- Akissoe NH, Hounhouigan JD, Bricas N, Vernier P, Olorunda NMC, OA, Physical, chemical and sensory evaluation of dried yam (Dioscorea rotundata) tubers, flour and amala—a flour-derived product. Trop Sci. 2001;41:151–156. [Google Scholar]
- Alam MN, Bristi NJ, Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 2013;21:143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alashi AM, Taiwo KA, Oyedele DJ, Adebooye OC, Aluko RE. Polyphenol composition and antioxidant properties of vegetable leaf-fortified bread. J Food Biochem. 2018;43(6):e12625. doi: 10.1111/jfbc.12625. [DOI] [PubMed] [Google Scholar]
- AOAC (2014) Official methods of analysis. Aromatic intermediates and derivatives. Association of Official Analytical Chemists, Washington USA, Paris, 22nd edn. pp. A.IV.1–A.IV.17
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit and fungal enriched cheese against key enzymes linked to type-2 diabetes and hypertension. Innov Food Sci Technol. 2007;8:46–54. doi: 10.1016/j.ifset.2006.06.001. [DOI] [Google Scholar]
- Badejo AA, Nwachukwu U, Ayo-Omogie HN, Fasuhanmi OS. Enhancing the antioxidative capacity and acceptability of Kunnu beverage from gluten-free pearl millet (Pennisetum glaucum) through fortification with tigernut sedge (Cyperus esculentus) and coconut (Cocos nucifera) extracts. Food Measure. 2019;14:438–445. doi: 10.1007/s11694-019-00305-2. [DOI] [Google Scholar]
- Benzie F, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Boligon AA, Kubica TF, Mario DN, Brum TF, Piana M, Weiblen R, et al. Antimicrobial and antiviral activity-guided fractionation from Scutiabuxifolia Reissek extracts. Acta Physiol Plant. 2013;35:2229–2239. doi: 10.1007/s11738-013-1259-0. [DOI] [Google Scholar]
- Caballero B. Fortification, supplementation, and nutrient balance. Eur J Clin Nutr. 2003;57:S76–S78. doi: 10.1038/sj.ejcn.1601803. [DOI] [PubMed] [Google Scholar]
- Chang H, Jan C, Liang W. Protective effects of a phenolic glycoside compound curculigoside on H2O2-induced oxidative stress and cytotoxicity in normal human breast epithelial cells. J Funct Foods. 2018;41(2018):171–182. doi: 10.1016/j.jff.2017.12.009. [DOI] [Google Scholar]
- FAO . Report on the inter-center review of root and tuber crops research in the CGIAR. Rome: Food and Agricultural Organisation; 1997. [Google Scholar]
- Gajera HP, Gevariya SN, Hirpara DG, Patel SV, Golakiya BA. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruits parts of indigenous black jamun (Syzygium cumini L.) landraces. J Food Sci Technol. 2017 doi: 10.1007/s13197-017-2756-8(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik-Dziki U, Swieca M, Dziki D, Baraniak B, Tomiło J, Czyz J. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 2013;138:1621–1628. doi: 10.1016/j.foodchem.2012.09.151. [DOI] [PubMed] [Google Scholar]
- Gbadamosi IT, Egunyomi A. Phytochemical screening and invitro anti-candidal activity of extracts and essential oil of Curculigo pilosa (Schum and Thonn) Engl. Hypoxidaceae Afr J Biotechnol. 2010;9(8):1236–1240. doi: 10.5897/AJB09.1207. [DOI] [Google Scholar]
- Gyamfi M, Yonamine M, Aniya Y. Free radical scavenging action of medicinal herbs from Ghana: thonningia sanguine on experimentally induced liver injuries. Gen Pharmacol. 1999;32(6):661–667. doi: 10.1016/S0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation the IDF Diabetes Atlas, 9th edn, Belgium, (2019)
- IFPRI (2016) Global nutrition report (2016): from promise to impact: ending malnutrition by 2030, Washington DC
- Karigidi KO, Ojebode ME, Olaiya CO. Effect of cooking on antioxidant and enzymes activity linked to carbohydrate metabolism and lipid peroxidation of eggplant (Solanum melongena) Pertanika J Trop Agricul Sci. 2018;41(4):1717–1730. [Google Scholar]
- Karigidi KO, Olaiya CO. In vitro antidiabetic, antioxidant and anti-lipid peroxidative activities of corn steep liquor extracts of Curculigo pilosa and its solvent fractions. J Herbs Spices Med Plants. 2019;25(4):377–388. doi: 10.1080/10496475.2019.1635549. [DOI] [Google Scholar]
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. doi: 10.1016/S0308-8146(02)00423-5. [DOI] [Google Scholar]
- Kordylas JM (1990) Processing and preservation of tropical and subtropical foods, Macmillan Publishers Ltd., London and Basingstoke, U.K, pp 49–71, 128–136, 324–340, 350–374
- Kuo JM, Yeh DB, Pan BS. Rapid photometric assay evaluating antioxidative activity in edible plant material. J Agric Food Chem. 1999;47:3206–3209. doi: 10.1021/jf981351o. [DOI] [PubMed] [Google Scholar]
- Mestres C, Dorthe S, Akissoé N, Hounhouigan JD. Prediction of sensorial properties (color and taste) of amala, a paste from yam chips flour of West Africa, through flour biochemical properties. Plant Foods Hum Nutr. 2004;59(3):93–99. doi: 10.1007/s11130-004-0028-z. [DOI] [PubMed] [Google Scholar]
- Nowicka P, Wojdyło A. Anti-hyperglycemic and anticholinergic effects of natural antioxidant contents in edible flowers. Antioxidants. 2019;8:308. doi: 10.3390/antiox8080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaiya CO, Esan SKO, AM The role of nutraceuticals, functional foods and value added food products in the prevention and treatment of chronic diseases. African J Food Sci. 2016;10(10):185–193. doi: 10.5897/AJFS2015.1402. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Park Y-S, Jung S-T, Kang S-G, Heo BK, Arancibia-Avila P, Toledo F, Gorinstein S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–648. doi: 10.1016/j.foodchem.2007.08.070. [DOI] [Google Scholar]
- Phan MAT, Wang J, Tang J, Lee YZ, Ng K. Evaluation of a glucosidase inhibition potential of some flavonoids from Epimedium brevicornum. LWT Food Sci Technol. 2013;53:492–498. doi: 10.1016/j.lwt.2013.04.002. [DOI] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;26(9):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Tysoe C, Leslie KW, Robert K, Nham TN, Chris T, Jacqueline W, Ethan DG, Adeleke HA, Suzanne P, Leonard JF, Raymond JA, Gary DB, Withers SG. Potent human α-amylase inhibition by the β-defensin-like protein helianthamide. ACS Cent Sci. 2016 doi: 10.1021/acscentsci.5b00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verter N, Beerarova V. An analysis of yam production in Nigeria. Acta Univ Agric Silvic Mendelianae Brun. 2015;63:599–665. doi: 10.11118/actaun201563020659. [DOI] [Google Scholar]
- Ward MG, Li G, Barbosa-Lorenzi V, Hao M. Stigmasterol prevents glucolipotoxicity induced defects in glucose-stimulated insulin secretion. Sci Rep. 2017;7:9536. doi: 10.1038/s41598-017-10209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington V. Alpha amylase. Worthington enzyme manual. New Jersey, United States: Worthington Biochemical Corporation; 1993. pp. 36–41. [Google Scholar]
- Wu Q, Fu D, Hou A, Lei G, Liu Z, Chen J, Zhou T. Antioxidative phenols and phenolic glycosides from Curculigo orchioides Chem. Pharm Bull. 2005;53(8):1065–1067. doi: 10.1248/cpb.53.1065. [DOI] [PubMed] [Google Scholar]
- Zakariah G, Dianna S, Sarah F, Valentina G. Association between urbanisation and type 2 diabetes: an ecological study. BMJ Glob Health. 2017;2:e000473. doi: 10.1136/bmjgh-2017-000473. [DOI] [PMC free article] [PubMed] [Google Scholar]


