Abstract
The increase in the manufacture of craft beers follows the boost in the consumption of this beverage. Meanwhile, brewers face a drawback with beer aging, its flavor’s change. The addition of compounds that overcome this downside is one alternative used by brewers. Species from the Ocimum genus are known for having antioxidant properties. Therefore, this study aimed to manufacture craft Pilsner beers with increased shelf-life performance. We prepared craft beers adding in natura leaves or aqueous extract from the leaves of Ocimum selloi and determined volatiles, and total phenolic compounds content, pH, color, and antioxidant activity. We can assure that as the fermentation proceeded, there was an increasing at the content of volatile metabolites and the addition of O. selloi improved the shelf-life of the beverages and the antioxidant potential increased when the aqueous extract at 0.1% (m/v) was added after the fermentation step.
Keywords: Ocimum selloi, Craft beer, Beer staling, Antioxidant activity, Stability
Introduction
Beer is one of the most consumed alcoholic beverages worldwide. It is composed of water (95%), hop, malt, and yeast. Its production is based on the fermentation of sugar present at malt by the yeast, to produce ethanol, water, and CO2. There are many styles of beer (Pilsner, Bock, Malzbier, Stout, Weissbier) that are divided into two classifications, Ale or Lager, based on their fermentation procedure, body, color, and flavors. In Brazil, Lager Pilsner beers are the most sold because of their low fermentation, which makes it a less carbonated blond beer, easier to drink (de Keukeleire 2000). Comparing to other beverages, beer is unstable after bottled and stored, its flavor alters to an unpleasant one, and it changes with time (Vanderhaegen et al. 2007). This flavor staling with aging is considered the major drawback at the beer storage step, attributed to the oxidation of specific compounds, such as trans-2-nonenal (Zhao et al. 2010). Beer aging is difficult to control and predict, so a challenge for brewers to offer a product with reproducible quality (Vanderhaegen et al. 2007).
Craft beers are distributed regionally, which can have different ingredients, recipes, flavors, and are manufactured using creativity and innovation (da Costa Jardim et al. 2018). Given the current appeal for consumption of craft beers (de Oliveira and Falconi 2018; Garavaglia and Swinnen 2017), it is fundamental to improve their shelf-life.
One way to improve stability and add a flavor to beer (usually done to craft beers) is the addition of food, spice or vegetal species. The specie of the genus Ocimum (Lamiaceae) are known for being aromatic, having antioxidant properties and phenolic compounds (Hakkim et al. 2008). Ocimum selloi Benth (‘basil pepper’) is a native species from Southeast and South regions of Brazil, it is used in folk medicine to treat inflammation and stomachache (Vieira and Simon 2000). As far as we know only Hakkim and coworkers (2008) analyzed an extract of O. selloi, every other study were about the essential oil. They showed antioxidant activity of the methanolic extract from the seeds of O. selloi.
Considering the aforementioned, this study aimed to produce craft beers, adding leaves from O. selloi in two different manufacturing steps (before or after fermentation) seeking a stabler product.
Materials and methods
Plant samples and extraction
Leaves of O. selloi were collected at the Horto de Plantas Medicinais of the Federal University of Grande Dourados (UFGD). A voucher was identified and deposited in the UFGD herbarium under number #5689, and was registered at SisGen (A055721), the Brazilian genetic heritage control system. We prepared the aqueous extract by decoction (98 ± 2 °C for 10 minutes) using in natura crushed leaves (3–5 mm) at 10% (vegetable mass and the volume of water). After the mixture reached room temperature (about 30 minutes) it was filtered and lyophilized (Christ, Alpha 1-2 LD Plus). Extracts were prepared in triplicate and the yield (18.43 ± 1.02%) calculated using the masses from in natura leaves and the final extract.
Beer preparation
We prepared the beer samples employing the traditional method of production of Pilsner beer. Fifteen liters of non-chlorinated water from the artesian well of the State University of Mato Grosso do Sul (UEMS) were heated to 65 °C and mixed with 5 kg of malt previously ground in a disc mill. The mixture was kept at constant temperature (65 °C) for 90 minutes under stirring. 10 L of non-chlorinated water was separately heated to 78 °C for the clarification process.
The must was subjected to clarification process by filtration with a false bottom of the mustard boiler using water preheated at 78 °C (spray). Then the clarified must was warmed to the boiling point and after 5 min, 20 g of bitterness hops were added to it. After 45 min of vigorous boiling, 20 g of aroma hops were added. At the end of 60 minutes of boiling, the must was vigorously stirred counterclockwise. After having the wort cooled with the aid of a copper coil, it was unloaded into the fermentation tank (20 L), taking care not to drag the hops with the wort. The fermentation tank used was a tank manufactured by the engineers of the Federal University of Grande Dourados.
For the fermentation process, the yeast (Saccharomyces cerevisiae—0.5 g L-1) which was previously rehydrated in 10 mL of must, was added to the fermenters. The cooled must (20 °C) was divided into 9 fermenters, being added in two of them the dried aqueous extract of O. selloi in the concentrations of 0.05% (m/v) (E0.05B, B means ‘addition of the vegetal material before fermentation’) and 0.1% (m/v) (E0.1B) and in other two in natura leaves of O. selloi in the concentrations 0.1% (m/v) (L0.1B) and 0.5% (m/v) (L0.5B), the remaining fermenters were kept only with yeast addiction. Strong agitation was provided for wort aeration and it was brought to fermentation at constant temperature (12 °C) in BOD (SSBOD 342 L, SoildSteel, Piracicaba, SP, Brazil). The inner tube valve was fitted at the upper outlet of the fermentation tank at a constant temperature of 12 °C for 168 h (7 days). After this fermentation process was completed, the yeast was removed, transferring the must to another fermenter.
To start the maturation process, the must was cooled to 4 °C and the aqueous extract of O. selloi at 0.05% (E0.05A, A means ‘addition of the vegetal material after fermentation’) and 0.1% (E0.1A) and in natura leaves of O. selloi at concentrations of 0.1% (L0.1A) and 0.5% (L0.5A) were added separately to four fermenters which the vegetal material was not added before, leaving one fermenter without the addition of O. selloi (BLANK). The maturation step occurred for 10 days and later the beers were bottled up. For bottling up, a solution of 8 g L-1 of sugar was added to the raw beer, followed by homogenization, and sealing with a sealing cap. The beer remained at room temperature for 7 days in a dark room for the secondary carbonation process. Figure 1 describes the process of manufacture the craft beers with the addition of in natura leaves or aqueous extract of O. selloi.
Fig. 1.
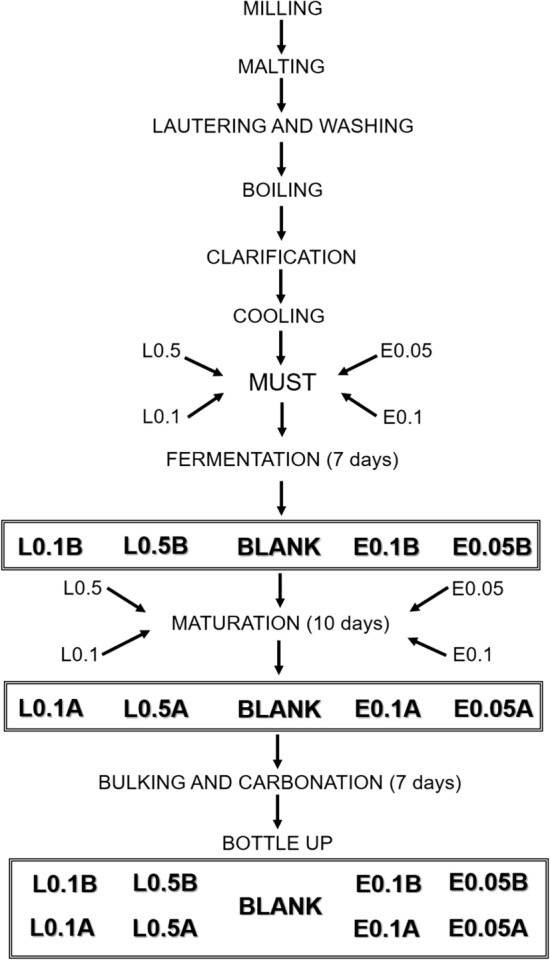
Production flowchart of Pilsner craft beer added with in natura leaves or aqueous extract from the leaves of Ocimum selloi
After the period of secondary carbonation, we opened the bottles and took an aliquot to analyze the samples by DPPH radical scavenging activity, total phenolic content, pH, color, and volatiles content. These samples were named as “recently prepared”. Right after that, bottles were closed over again.
Color determination
We used the method 8.5 spectrophotometric Analytica (A x 25 = color) from EBC (European Brewery Convention 2005). Samples were filtered in paper filter (0.45 μm of pore size) and then the absorbance of the samples was measured at 430 nm in a glass cuvette (10 mm) on a FEMTO 700 PLUS spectrometer.
pH determination
We measured employing the phmeter HACH PH31.
Determination of antioxidant activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was used according to the procedure employed by Kumaran and Karunakaran (2006). Absorbance was determined by spectrophotometry (λ = 517 nm) (FEMTO 700 PLUS) and used to calculate the percentage of inhibition. Analyses were performed in triplicate.
Determination of total phenolic content (TPC)
We determined TPC using Folin–Ciocalteu's reagent based on the procedure of Djeridane et al. (2006). Gallic acid (GA) was used to construct a standard curve, varying the concentration from 5 to 1000 μg mL-1 employing a spectrophotometer (λ = 756 nm) (FEMTO 700 PLUS). The result was expressed in µg of gallic acid equivalent (GAE) per mL of samples. Analyzes were performed in triplicate.
Volatiles
Sample preparation
Approximately 200 mg of anhydrous NaCl was added to 1000 µL of the samples until saturation followed by intense mixing using a vortex mixer (SCILOGEX D-160, Rocky Hill, Connecticut, USA) for 10–15 s and then 500 µL of ethyl acetate was added to the mixture and mixed vigorously for 1 min. The samples were then centrifuged for 2 min at 2000×g. The acetate phase (200 µL) was transferred to a vial for analysis.
GC-MS analysis
The samples were analyzed using a gas chromatograph (GC-2010 Plus, Shimadzu, Kyoto, Japan) coupled to a mass spectrometer (GC-MS Ultra 2010, Shimadzu, Kyoto, Japan). We used a DB-5 fused silica capillary column (J and W, Folsom, California, USA) (30 m long x 0.25 mm internal diameter x 0.25 μm film thickness) operating at 70 eV. Helium (99.999%) was the carrier gas (48.851 mL min-1) and injections (1 µL) were in 100:1 split mode. The temperature of the inlet was kept at 180 °C. The GC oven temperature was initially held at 50 °C for 1 min and then raised to 200 °C at 40 °C min-1. The interface and quadrupole temperatures were 230 °C and 150 °C respectively. The MS detector was turned off between 2.03 and 2.21 min to offload ethyl acetate peak and operated in scan mode with a mass range of m/z 30–250 (Pinu and Villas-Boas 2017). Seven volatile compounds were analyzed, among them methanol, ethanol, acetic acid, isoamylic alcohol, acetoin, ranging from 0.1 to 10000 mg L-1, and 2,3-butanediol, and phenylethyl alcohol in the range of 10–200 mg L-1. Samples were analyzed in triplicate.
Shelf-life
Shelf-life of beers was evaluated using the DPPH radical scavenging activity, pH value, total phenolic content, and volatile compounds after 90 days of storage. The samples were kept at 4 °C in the dark. The analyzes were performed as done previously for the recently prepared samples in triplicate.
Statistical analysis
Values for % of inhibition of the DPPH radical scavenging, pH, total phenolic content, volatile metabolites, and ethanol content were evaluated by Factorial Variance Analysis using the software Statistic 13.3 [TIBCO] in different beers recently prepared and for samples after 90 days of storage. p values less than 0.05 (p < 0.05) means that the treatments showed statistically significant differences. Using the same kind of analysis, we compared the differences between the groups of beers recently prepared and also for samples after 90 days of storage.
Results and discussion
We manufactured nine Pilsner craft beers, eight with O. selloi, to evaluate the effect of the specie at beer properties, mainly at the shelf-life performance. Craft beers prepared with in natura leaves (L0.1B, L0.1A, L0.5B, L0.5A) presented a slightly stronger color compared to samples prepared with the aqueous extract of Ocimum selloi (E0.05B, E0.05A, E0.1B, E0.1A) and the BLANK, which has the same color as the samples with the extract. The fragrance and appearance of all samples were remarkably similar. Ocimum species are known for their high antioxidant properties (Hakkim et al. 2008), thus it is expected that the craft beers prepared with in natura leaves or extract of leaves of this specie cause an improvement at the antioxidant potential compared to the BLANK.
The antioxidant activity of the craft beers are presented in Table 1, as the percentages of inhibition. The range of antioxidant activity was from 45.1 ± 0.2 to 83.5 ± 0.4% of inhibition. As can be seen at Table 1, all samples prepared with O. selloi presented a better antioxidant inhibition, furthermore, when the aqueous extract was employed, the percentages of inhibition were higher (69.0 ± 0.8–83.5 ± 0.4%), what means that the extract composition is improving the antioxidant performance of the beverages by around 14%. The DPPH radical scavenging activity of the samples with the extract at 0.1% was higher, thus an increasing amount of the extract results on a greater radical scavenging capacity. Besides that, the samples in which the vegetal material was added after the fermentation step presented a higher antioxidant performance. A higher antioxidant activity improves beer flavor stability, so the addition of O. selloi is increasing the stability of the craft beers. Pai et al. (2015) examined the antioxidant potential of 15 pale larger beers from different places around the world and they had values for percentage of inhibition close to ours (68.34 ± 0.85–89.90 ± 0.71).
Table 1.
Antioxidant activity and total phenolic content for craft beers with O. selloi recently prepared (fresh) and after 90 days of storage (shelf-life)
| Sample | DPPH radical scavenging activity (% inhibition) | Total phenolic content (μg GAE mL-1 sample) | ||
|---|---|---|---|---|
| Fresh | Shelf-life | Fresh | Shelf-life | |
| BLANK | 45.1 ± 0.2 | 30.9 ± 0.4 | 291.2 ± 4.0 | 252.6 ± 4.1 |
| L0.1B | 50.3 ± 0.5 | 38.5 ± 0.3 | 364.9 ± 2.7 | 299.0 ± 1.2 |
| L0.1A | 66.8 ± 1.2 | 52.3 ± 0.7 | 360.6 ± 3.1 | 333.9 ± 2.8 |
| L0.5B | 54.9 ± 0.4 | 34.0 ± 0.2 | 371.9 ± 1.9 | 308.8 ± 3.6 |
| L0.5A | 62.0 ± 0.7 | 46.7 ± 0.6 | 359.0 ± 2.8 | 314.8 ± 2.5 |
| E0.05B | 69.0 ± 0.3 | 59.8 ± 0.6 | 370.7 ± 1.6 | 341.6 ± 2.4 |
| E0.05A | 69.0 ± 0.4 | 60.8 ± 0.2 | 369.0 ± 1.9 | 339.8 ± 2.6 |
| E0.1B | 69.0 ± 0.8 | 60.8 ± 1.1 | 367.9 ± 2.1 | 349.8 ± 4.0 |
| E0.1A | 83.5 ± 0.4 | 69.9 ± 0.6 | 370.4 ± 3.2 | 350.4 ± 1.9 |
According to the results, the total phenolic content did not vary much between the different samples, it ranged from 359.0 ± 2.8 to 371.9 ± 1.9 μg GAE mL-1 sample (Table 1). Nardini and Garaguso (2020) characterized many beers from different countries, and the only lager type studied presented a TPC of 321 ± 9 μg GAE mL-1, a close value to ours, but smaller. Once we added O. selloi, it is expected that our values were higher. The aqueous extract from the leaves of O. selloi presented 23.9 ± 1.1 μg GAE mL-1, so comparing with the BLANK, the specie is contributing to the total phenolic content.
The pH values of the samples varied from 4.4 to 4.5. These values are in accordance with other studies in the literature (Marques et al. 2017; Nardini and Garaguso 2020). The craft beer produced with propolis extract, by Ulloa and coworkers (2017), presented a pH of around 4.04. Tozetto et al. (2019) produced a Pilsner craft beer with ginger and compared it to twenty-eight commercial samples, the pH values were between 3.47 and 4.98.
The color of the samples was determined applying the EBC method. The mean color value for all recently prepared samples was 14.07±0.33. According to the Brazilian legislation (Decree 6.871/09), our craft beers are considered blond beers once they present less than 20 units of EBC.
Considering the consumer and product quality, it is important to quantify the volatile compounds present at the final product. Volatile metabolites play especially important roles in sensory properties of beers.
The ethanol content of the craft beers prepared in this study was situated between 7.20 ± 0.08 and 8.50 ± 0.09 (% v/v) (Table 2). The samples that were prepared with in natura leaves of O. selloi were more alcoholic (7.78±–8.50%) and samples with a greater amount of vegetal material resulted in more alcoholic beers. Marques et al. (2017) prepared four different styles of beer (American Pale Ale, Brown Poter, Classic American Pilsner, and Irish Red Ale) and the ethanol content of them was between 5.00 and 5.88. According to the review done by Bamforth (2002), the alcohol content of beers ranges from < 0.05% to 10%, however, most beers have an alcohol content between 3 and 6% (v/v), thus, our craft beers have an alcohol content higher than the average. Therefore, there was no ethanol loss during the manufacturing process and the temperature of fermentation employed by us favored the formation of ethanol.
Table 2.
Quantification of volatile metabolites and alcohol content of craft beers with O. selloi recently prepared (fresh) and after 90 days of storage (shelf-life)
| Sample | Blank | L0.1B | L0.1A | L0.5B | L0.5A | E0.05B | E0.05A | E0.1B | E0.1A | |
|---|---|---|---|---|---|---|---|---|---|---|
| Methanol (mg L-1) | Fresh | 18.96±0.89 | 11.28±1.32 | 10.65±0.83 | 9.99±0.97 | 10.45±0.33 | 9.88±0.38 | 12.03±0.42 | 11.13±0.76 | 13.49±0.72 |
| Shelf-life | 19.01±0.22 | 11.40±0.48 | 10.71±0.61 | 10.03±0.45 | 10.49±0.21 | 9.93±0.17 | 12.07±0.21 | 11.15±0.28 | 13.52±0.25 | |
| Ethanol (g L-1) | Fresh | 59.77±0.11 | 61.38±0.92 | 66.20±2.13 | 67.10±0.57 | 64.80±0.14 | 56.80±1.83 | 57.33±1.22 | 57.13±0.29 | 59.60±0.89 |
| Shelf-life | 59.91±0.16 | 61.49±0.41 | 66.42±0.58 | 67.32±0.67 | 65.01±0.51 | 56.93±0.54 | 57.61±0.37 | 57.34±0.17 | 59.89±0.21 | |
| Acetic acid (mg L-1) | Fresh | 47.75±0.22 | 39.28±1.72 | 40.28±0.03 | 49.00±1.47 | 46.67±0.25 | 44.80±0.75 | 46.23±1.22 | 42.23±0.13 | 43.39±0.87 |
| Shelf-life | 47.82±0.28 | 39.33±0.65 | 40.36±0.14 | 49.11±0.61 | 46.81±0.12 | 44.87±0.29 | 46.28±0.71 | 42.29±0.27 | 43.45±0.46 | |
| Isoamylic alcohol (mg L-1) | Fresh | 1.12±0.01 | 1.38±0.12 | 0.91±0.03 | 1.60±0.17 | 1.66±0.07 | 0.68±0.04 | 0.97±0.02 | 1.08±0.10 | 1.31±0.08 |
| Shelf-life | 1.13±0.02 | 1.39±0.19 | 0.91±0.06 | 1.62±0.19 | 1.67±0.05 | 0.69±0.03 | 0.97±0.04 | 1.08±0.02 | 1.32±0.04 | |
| Acetoin (mg L-1) | Fresh | 77.65±2.27 | 78.65±3.29 | 79.65±4.33 | 81.54±2.24 | 83.65±5.66 | 81.65±2.82 | 83.43±5.03 | 78.41±2.99 | 79.98±6.01 |
| Shelf-life | 87.65±4.29 | 88.99±8.11 | 90.53±5.39 | 92.54±5.38 | 94.13±6.14 | 81.98±6.16 | 95.25±5.55 | 89.01±4.67 | 90.76±4.64 | |
| 2,3-butanediol (mg L-1) | Fresh | 87.65±7.23 | 89.72±5.44 | 92.37±4.45 | 87.98±5.89 | 91.93±8.02 | 87.99±6.14 | 89.42±5.29 | 88.93±4.66 | 89.96±7.76 |
| Shelf-life | 98.52±7.67 | 102.13±6.44 | 104.39±5.12 | 101.98±5.25 | 103.97±9.02 | 101.13±5.75 | 101.42±5.56 | 102.93±5.16 | 103.96±8.12 | |
| Phenylethyl alcohol (mg L-1) | Fresh | 81.12±2.11 | 88.38±5.67 | 97.87±6.03 | 91.60±4.34 | 95.66±9.54 | 86.87±5.45 | 87.98±6.11 | 91.08±7.10 | 94.39±4.83 |
| Shelf-life | 105.12±4.34 | 106.82±5.12 | 110.44±2.47 | 108.66±3.02 | 113.54±2.53 | 100.96±4.03 | 105.94±2.58 | 104.17±1.13 | 107.49±2.77 | |
| Alcohol content (%) | Fresh | 7.57±0.12 | 7.78±0.08 | 8.39±0.09 | 8.50±0.09 | 8.21±0.11 | 7.20±0.08 | 7.27±0.05 | 7.24±0.07 | 7.55±0.09 |
| Shelf-life | 7.62±0.40 | 7.83±0.31 | 8.44±0.34 | 8.55±0.35 | 8.24±0.29 | 7.23±0.32 | 13.81±0.09 | 7.29±0.35 | 7.58±0.28 | |
Acetic acid is the second most abundant volatile compound at craft beers, and it is, by Pinu and Vilas-Boas (2017), the second most common and abundant volatile metabolite in fermented food and beverages, considered an off-flavor. The content of acetic acid in our recently prepared samples ranged from 39.28 ± 1.72 to 49.00 ± 1.47 mg L-1 (Table 2). Analyzing all values, we infer that when in natura leaves of O. selloi where added at 0.5% the content of acetic acid was higher, and the concentration of acetic acid increases as the fermentation proceeded (Guerrini et al. 2018). Pinu and Vilas-Boas (2017) analyzed a beer sample with an acetic acid content of around 300 mg L-1.
Methanol is a byproduct of the fermentation which is toxic to human at an average dose estimated at 56.2 g/person for oral administration or 400–13000 mg/L for respiratory administration (Moon 2017) and its content should be determined for security to safe ingestion (Wang et al. 2004). The methanol content ranged from 9.88 ± 0.38 to 18.96 ± 0.89 mg L-1 on our recently manufactured samples. The BLANK presented the highest content of methanol; therefore, methanol is highly consumed as the fermentation proceeded at the manufacturing process, when the extract or leaves of O. selloi were added. Accordingly, the consumption of our craft beers is safe considering the content of methanol.
We also determined the content of isoamylic alcohol, acetoin, 2,3-butanediol and phenylethyl alcohol on our samples by GC-MS (Table 2). These compounds are abundant at fermented beverages (Pinu and Villas Boas 2017) and here for most of the samples, as the fermentation proceeded the content of volatile compounds increased. Meanwhile, there was no correlation between the amount of vegetal material (in natura leaves or extract) with the content of volatiles.
The antioxidant activity (Wilks’ lambda = 0.301; F = 2.034; p = 0.0011), total phenolic content (Wilks’ lambda = 0.289; F = 2.112; p = 0.0018), pH (Wilks’ lambda = 0.312; F = 2.998; p = 0.0021) and volatiles content (Wilks’ lambda = 0.299; F = 3.137; p = 0.035) of the different craft beers recently prepared showed statistically significant differences.
Shelf-life monitoring of a beer is extremely important once the oxidation process (inherent at the product) causes beer staling, which may reduce the quality and consequently the consumption of the product. For this reason, we analyzed the samples to check their shelf-life performance. After 90 days of storage at 4 °C in the dark, the DPPH radical scavenging activity, total phenolic content and volatile metabolites content, color, and pH were determined and compared to the data of the recently prepared samples (Tables 1 and 2). As can be checked, after 90 days the DPPH radical scavenging activity of the samples decreased by around 20%. For samples prepared with the extract the activity decreased by around 13%, compared to the ones prepared with in natura leaves (27%). The samples in which the vegetal material was added after the fermentation step, showed greater activity, the same was observed for the recently prepared samples. The total phenolic content followed the same pattern, the content decreased by 10.1% after the storage period of 90 days, ranging from 299.01 ± 1.21 to 350.36 ± 1.94 μg GAE mL-1 of sample. The samples manufactured with the extract of O. selloi did not changed much considering the content of total phenolic compounds, it reduced by around 6.5 μg GAE mL-1 of sample compared to the ones prepared with the leaves (13.7 μg GAE mL-1 sample). The color did not alter during this period (mean EBC 14.14 ± 0.32–blond beer), as the pH values (4.3–4.4).
Comparing the parameters individually (pH, volatiles content, antioxidant activity and total phenolic content) between the samples of recently prepared craft beers and after storage of 90 days, all showed statistical differences with p < 0.05. Despite that, the ones that were stored presented a slight increase in the alcohol content (0.04%), which can be because of residual yeast and sugar.
Hence, analyzing the properties of the craft beers after 90 days of storage, it is possible to conclude that the samples prepared with leaves or extract from O. selloi have a better shelf-life performance than the BLANK, in other words, their properties kept steady during this period, what is desirable considering later commercialization and consumption of these products.
Furthermore, it can be inferred that the samples that were prepared with the extract were more stable than the ones manufactured with in natura leaves; it can be because the samples with the extract have the compounds more available in a manner that they can improve the properties of the beers. Compounds from the samples prepared with the leaves of O. selloi are going to be extracted during the process of beer manufacture, what will probably be less effective. The characteristics of compounds and the amount of them will be different for the aqueous extract because the medium is not pure water and these could not be reproducible regarding the manufacturing procedure.
Conclusion
Nine different craft beers were satisfactorily prepared with the addition of in natura leaves or aqueous extract form leaves of Ocimum selloi. Color, pH, and ethanol content were in total accordance with other craft beers on the literature. Volatile metabolites quantification showed that beers are safe to be consumed. The DPPH radical scavenging activity showed that all samples prepared with O. selloi presented a better antioxidant activity compared to BLANK. Additionally, the use of the extract, a higher amount of vegetal material, and addition of it after the fermentation step, improved the antioxidant capacity. The total phenolic content did not differ significantly from one sample to another. Volatile compounds amount determined that samples prepared with in natura leaves were more alcoholic than the others, and this alcohol content is higher when compared to other craft Pilsner beers on the literature. The shelf-life study demonstrated that craft beers prepared with in natura leaves or aqueous extract of O. selloi kept their characteristics for at least 90 days of storage.
Acknowledgements
RCP, MHV, and MSMS thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship (Finance code 001) and CALC thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (concession number 311975/2018-6) and Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) (concession number 71/700.139/2018 ; 036/20108 and SIAFEM 028991).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raul Cremonezi Piva, Email: raul.c.piva@hotmail.com.
Maria Helena Verdan, Email: mhelenaverdan@gmail.com.
Maria do Socorro Mascarenhas Santos, Email: maria_mascarenhas@outlook.com.
Margareth Batistote, Email: margareth@uems.br.
Claudia Andrea Lima Cardoso, Email: claudiacardosouems1@gmail.com.
References
- Bamforth CW. Nutritional aspects of beer—a review. Nutr Res. 2002;22:227–237. doi: 10.1016/S0271-5317(01)00360-8. [DOI] [Google Scholar]
- da Costa Jardim C, de Souza D, Machado ICK, Pinto LMN, Ramos LCS, Garavaglia J. Sensory, profile, consumer preference and chemical composition of craft beers from Brazil. Beverages. 2018;4:106. doi: 10.3390/beverages4040106. [DOI] [Google Scholar]
- de Keukeleire D. Brazilian beers: blond is beautiful, dark is dreadful. An expert's view. Quím Nova São Paulo. 2000 doi: 10.1590/S0100-40422000000100025. [DOI] [Google Scholar]
- European Brewery Convention (2005) Fachverlag hans carl. In: Analytica-EBC. Nürnberg, Germany
- de Oliveira DM, Falconi D. The evolution of craft beer industry in Brazil. J Econ Bus. 2018;1:618–626. [Google Scholar]
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. doi: 10.1016/j.foodchem.2005.04.028. [DOI] [Google Scholar]
- Garavaglia C, Swinnen J (2017) The craft beer revolution: An international perspective. Choices 32:1-8, 3rd Quarter. http://www.jstor.org/stable/90015005
- Guerrini L, Angeloni G, Masella P, Calamai L, Parenti A. A technological solution to modulate the aroma profile during beer fermentation. Food Bioprocess Technol. 2018;11:1259–1266. doi: 10.1007/s11947-018-2099-0. [DOI] [Google Scholar]
- Hakkim FL, Arivazhagan G, Boopathy R. Antioxidant property of selected Ocimum species and their secondary metabolite content. J Med Plant Res. 2008;2(9):250–257. [Google Scholar]
- Kumaran A, Karunakaran RJ. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006;97:109–114. doi: 10.1016/j.foodchem.2005.03.032. [DOI] [Google Scholar]
- Marques DR, Cassis JOFQ, Bertozzi JC, Visentainer JV, Oliveira CC, Monteiro ARG. Characterization of craft beers and their bioactive compounds. Chem Eng Transactions. 2017;57:1747–1752. doi: 10.3303/CET1757292. [DOI] [Google Scholar]
- Moon C-S. Estimation of the lethal and exposure doses for representative methanol symptoms in humans. Ann Occup Environ Med. 2017;29:44. doi: 10.1186/s40557-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini M, Garaguso I. Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem. 2020;305:125437. doi: 10.1016/j.foodchem.2019.125437. [DOI] [PubMed] [Google Scholar]
- Pai TV, Sawant SY, Gathac AA, Chaturvedi PA, Gupte AM, Desai NS. Characterization of Indian beers: chemical composition and antioxidant potential. J Food Sci Technol. 2015;52:1414–1423. doi: 10.1007/s13197-013-1152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinu F, Villas-Boas SG. Rapid quantification of major volatile metabolites in fermented food and beverages using gas chromatography-mass spectrometry. Metabolites. 2017;7:37. doi: 10.3390/metabo7030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIBCO Software Inc. (2017) Statistica (data analysis software system), version 13. http://statistica.io
- Tozetto LM, Nascimento RF, Oliveira MH, van Beik J, Canteri MHG. Production and physicochemical characterization of craft beer with ginger (Zingiber officinale) Food Sci Technol. 2019;39:962–970. doi: 10.1590/fst.16518. [DOI] [Google Scholar]
- Ulloa PA, Vidal J, Ávila MI, Labbe M, Cohen S, Salazar FN. Effect of the addition of propolis extract on bioactive compounds and antioxidant activity of craft beer. J Chem ID. 2017 doi: 10.1155/2017/6716053. [DOI] [Google Scholar]
- Vanderhaegen B, Delvaux F, Daenen L, Verachtert H, Delvaux FR. Aging characteristics of different beer types. Food Chem. 2007;103:404–412. doi: 10.1016/j.foodchem.2006.07.062. [DOI] [Google Scholar]
- Vieira RF, Simon JE. Chemical characterization of basil (Ocimum Spp.) found in the markets and used in traditional medicine in Brazil. Econ Bot. 2000;54:207–216. doi: 10.1007/BF02907824. [DOI] [Google Scholar]
- Wang ML, Wang JT, Choong YM. Simultaneous quantification of methanol and ethanol in alcoholic beverage using a rapid gas chromatographic method coupling with dual internal standards. Food Chem. 2004;86:609–615. doi: 10.1016/j.foodchem.2003.10.029. [DOI] [Google Scholar]
- Zhao H, Chen W, Lu J, Zhao M. Phenolic profiles and antioxidant activities of commercial beers. Food Chem. 2010;119:1150–1158. doi: 10.1016/j.foodchem.2009.08.028. [DOI] [Google Scholar]


