Abstract
Agarwood, one of the precious woods in the globe, is produced by Aquilaria plant species during an upshot of wounding and infection. Produced as a defence response, the dark, fragrant resin gets secreted in the plant’s duramen, which is impregnated with fragrant molecules with the due course. Agarwood has gained worldwide popularity due to its high aromatic oil, fragrance, and pharmaceutical value, which makes it highly solicited by numerous industries. Predominant chemical constituents of agarwood, sesquiterpenoids, and 2-(2-phenylethyl) chromones have been scrutinized to comprehend the scientific nature of the fragrant wood and develop novel products. However, the genes involved in the biosynthesis of these aromatic compounds are still not comprehensively studied in Aquilaria. In this study, publicly available genomic and transcriptomics data of Aquilaria agallochum were integrated to identify putative functional terpene synthase genes (TPSs). The in silico study enabled us to identify ninety-six TPSs, of which thirty-nine full-length genes were systematically classified into TPS-a, TPS-b, TPS-c, TPS-e, TPS-f, and TPS-g subfamilies based on their gene structure, conserve motif, and phylogenetic comparison with TPSs from other plant species. Analysis of the cis-regulatory elements present upstream of AaTPSs revealed their association with hormone, stress and light responses. In silico expression studies detected their up-regulation in stress induced tissue. This study provides a basic understanding of terpene synthase gene repertoire in Aquilaria agallochum and unlatches opportunities for the biochemical characterization and biotechnological exploration of these genes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-01040-z.
Keywords: Terpene synthase, Aquilaria agallochum, Classification, Phylogenetic analysis and functional prediction
Introduction
Terpenes belong to a class of secondary metabolites produced by bacteria, fungus, or plants as defence and adaptation against biotic and abiotic factors (Tholl 2015). Plants are specialized in the production of terpenes, which they utilize not only for physiological and biochemical processes (Pichersky et al. 2018) but also for interorganismal interaction such as antagonism against herbivores, pests, and harmful microbes (Schmelz et al. 2014; Vaughan et al. 2013; Keeling et al. 2006) and synergism towards pollinators and useful microbes (Pichersky et al. 2002; Gershenzon et al. 2007). Evolution and selective pressure on plant species have resulted in complex, diverse, and more classified terpenoid metabolism (Tholl 2015). Cytosolic mevalonate (MVA) pathway and plastidal methylerythritol phosphate (MEP) pathway produce the five-carbon precursor isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which form the building blocks of plant terpenoids. IPP and DMAPP are later converted into polyprenyl pyrophosphates, viz., geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP) by prenyltransferase (Sapir-Mir et al. 2008; Lichtenthaler 1999). These prenyl pyrophosphates are utilized as substrates by terpene synthases or cyclases to produce diverse terpenoids (Huang et al. 2017; Sharkey et al. 2013). Monoterpene synthase converts GPP into monoterpene (C10), sesquiterpene synthase converts FPP into sesquiterpene (C15), and diterpene synthase converts GGPP into diterpenes (C20) (Bohlmann et al. 1998).
Plant terpenoids have been extensively used in flavor and fragrances (Celedon et al. 2016), biopolymers (Bohlmann et al. 2008), natural rubber (Qu et al. 2015), and biofuels (D’espaux et al. 2015). Plants species are exploited from the wild for the extraction of fragrant compounds, and currently due to low yield, industries fall shortage of raw materials resulting in supply–demand imbalance and products being expensive (Caputi et al. 2011). An alternative to address the present situation would be microbial production of these chemicals in heterologous hosts such as bacteria or yeast by expression of plant metabolic pathway (Kulkarni, 2016; Gomes et al. 2016). Identification and functional characterization of the genes involved in the terpene biosynthesis is thus necessary for the biotechnological utilization of these important phytochemicals.
Genome-wide identification of the terpene synthase (TPS) family gene has revealed many novel and notable features that form the basis for understanding their functional biology and catalytic mechanism (Wang et al. 2019). Terpene synthases (TPSs) are classified into two classes viz. Type I and Type II based on structure and catalytic nature (Gao et al. 2012; Chen et al. 2011), where the Type I class of enzymes possess a highly conserved DDXXD motif (α domain) and a less conserved NSE/DTE motif metal (Mg2+or Mn2+) binding domain required for the catalysis (Christianson et al. 2018; Gao et al. 2012; Chen et al. 2011). Type II class of enzyme possess the DXDD motif (β domain) close to N-terminus, which is vital for protonation, and cyclization of geranylgeranyl pyrophosphate to convert into copalyl diphosphate/cyclic diterpene diphosphate (Zerbe et al. 2015). Another conserve motif R(R)X8W, was found to be located downstream to the N-terminal of many TPS genes (Gao et al. 2012; Thoma et al. 2004). The N terminal domain (ig. 3PF01397) of terpene synthases are conserved for lyase activity (GO: 0016829) and terpene synthase activity (GO: 0010333) and C terminal metal binding domain (PF03936) for magnesium ion binding (GO: 0000287), lyase activity (GO: 0016829), and terpene synthase activity (GO: 0010333) (Finn et al. 2011). Terpene synthases are also classified based on their gene structures, where Class I contains 12–14 introns, Class II contains nine introns, and Class III contains six introns (Trapp et al. 2001; Bohlmann et al. 1998).
According to a recent classification based on sequence similarities and functional features, TPS gene families are classified into eight subfamilies, namely TPS-a, TPS-b, TPS-c, TPS-d (found in gymnosperms only), TPS-e, TPS-f, TPS-g, and TPS-h (in Selaginella spp.), where TPS-c belongs to Type II TPS and others belongs to Type I (Wang et al. 2019). Terpene synthase family members have been detected and studied in a variety of plants, including Camellia sinensis (Zhou et al. 2020), Dendrobium officinale (Yu et al. 2020), Ocimum sanctum (Kumar et al. 2018), Ananas comusus (Chen et al. 2017), Daucus carota (Keilwagen et al. 2017), Populus trichocarpa (Irmisch et al. 2014), Malus domestica (Nieuwenhuizen et al. 2013), Solanum lycopersicum (Falara et al. 2011), Vitis vinifera (Martin et al. 2010), Oryza sativa (Harris et al. 2005), Arabidopsis thaliana (Aubourg et al. 2002), and Eucalyptus grandis (Trapp et al. 2001). In the well-known model plant Arabidaopsis thaliana (32 functional and eight pseudogenes) (Huang et al. 2017; Chen et al. 2011), Vitis vinifera (69 putative TPS genes and 63 pseudogenes) (Trapp et al. 2001), Solanum lycopersicum (29 functional and 15 mutated) (Martin et al. 2010), Oryza sativa (40 putative functional) (Harris et al. 2005), Eucalyptus grandis (103 functional putative) (Irmisch et al. 2014), and Populus trichocarpa (38 functional putative) (Falara et al. 2011) have been identified and listed. Besides these, genome-wide detection and analysis of TPS subfamily in few angiosperms (Alquézar et al. 2017; Booth et al. 2017) and in lower plant species including Marchantia polymorpha (Kumar et al. 2016), Selaginella moellendorffii (Li et al. 2012), and Physcomitrella patens (Hayashi et al. 2006) has also been carried out.
Aquilaria species are known for the accumulation of resinous fragrant agarwood in response to biotic and abiotic stress (Mohamed et al. 2010, 2014), which are loaded with terpenoids and phenylethyl chromones (Chen et al. 2012; Yagura et al. 2003; Naef 2011). Metabolite analysis of wood chips, leaf, stem, oil, and treated callus has also revealed the presence of similar phytochemicals (Kristanti et al. 2018; Wang et al. 2018; Sen et al. 2017, 2015). In contrast to these numerous reports on the phytochemical composition, strikingly scarce studies are available on the biosynthesis of sesquiterpenoid and phenyl ethyl chromones in Aquilaria. In Aquilaria malaccensis, AmSesTPS1 and AmGuaiS1 were identified, which show similarity with sesquiterpene synthase and δ-guaiene synthase, respectively (Azzarina et al. 2016). Delta-guaiene synthase was also identified and functionally characterized from cultured cells of Aquilaria crassna (Kumeta et al. 2010). Expression and biochemical studies of sesquiterpene synthases (ASS1, ASS2, and ASS3) of Aquilaria sinensis detected the production of δ-guaiene (Xu et al. 2013). Interestingly, different classes of δ-guaiene synthase producing δ-guaiene and α-guaiene were identified and characterized in Aquilaria plants, where ASS1, ASS2, and ASS3 from Aquilaria sinensis and GS-1 and GS-2 from Aquilaria microcarpa generate germacrene A as minor product. AcC2, AcC3, and AcC4 from Aquilaria crassna generates α-humulene, while GS-3 and GS-4 from Aquilaria microcarpa produces both germacrene A and α-humulene (Li et al. 2021). The key enzyme involved in terpenoid biosynthesis, 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR), was characterized from Aquilaria sinensis stem tissue (Liu et al. 2015). As evident, efforts have been made to comprehend agarwood mainly through transcriptomics, and metabolomics approaches and genomic sequence data have not been explored in terms of mining and mapping for genes involved in terpenoid biosynthesis (Tan et al. 2019).
Methods and materials
Genome refinement and candidate TPSs identification
The genome scaffolds of Aquilaria agallochum were retrieved from the NCBI genome database (https://www.ncbi.nlm.nih.gov/genome/, Accession: PRJNA240626) (Chen et al. 2014a). Since the annotation data was not available in the public database, genome annotation was carried out as per the method described by Chen et al. 2014a with few modifications. To the genome sequences, repeat masker coupled with repeat modeller was employed to create a repeat masked consensus sequence (http://www.repeatmasker.org). The repeat masked genome was then aligned (blastn and blastx) to the similar sequences of NCBI Refseq plant database to find the regions of local similarity. BLAT tool was used to obtain the sequence location and align exons structures (Kent 2002). To create maximal assemblies based on cDNA alignments, “Program to Assemble Spliced Alignments” (PASA) was utilized for alignments of expressed transcript sequences against the NCBI Refseq plant database (Haas et al. 2003). RNA-seq data (SRR8863602, SRR8238769, SRR7244139, and SRR1283218) were retrieved, and HISAT2 alignment was carried out with the genome (Kim et al. 2015). The alignment evidence were used to train multiple gene prediction tools viz. Augustus (Stanke et al. 2005), BRAKER (Hoff et al. 2019), GeneMarkES (Lomsadze et al. 2005), and SNAP (Johnson et al. 2008) for gene prediction. A set of consensus gene models was then executed along with translated predicted protein sequences (proteome) considering the alignments and Ab-initio predictions followed by BUSCO completeness measurement against the OrthoDB v9 eukaryota dataset. Transcripts of predicted gene models were BLAST searched against the NCBI Refseq plant database and the best hit was considered as annotation. For identification of putative terpene synthases (AaTPS), a methodology was followed as described by Chen et al. 2014b for which, terpene synthase N and C terminal domain models (PF01397 and PF03936) were retrieved from Pfam (Finn et al. 2011) to create a local protein database, and the proteome data of Aquilaria agallochum was searched as query (e value < 10–5) using NCBI BLAST + 2.10.1. To confirm candidate TPSs, sequences were searched for conserved domains and motifs against the Pfam database, ScanProsite tool (https://prosite.expasy.org/scanprosite/), and phmmer search tool integrated in the HMMER web server (https://www.ebi.ac.uk/Tools/hmmer/search/phmmer). Predicted TPS genes were then analysed for the presence of an uninterrupted coding sequence and were considered full-length. TPSs with very short sequence length i.e. 70–200 amino acid with missing N or C terminal domains were considered partial (Singh and Sharma 2015), and TPSs which are very similar to full-length were considered possible pseudogenes. The possible pseudogenes and partial sequences with missing structures were excluded. Full-length genes and few genes close to full length opted for downstream analysis.
Gene organization and phylogenetic analysis
The putative AaTPS genes were subjected to “gene structure display server 2.0” to present exon–intron boundaries (Hu et al. 2015). The protein sequences were aligned, and conserved residues (R(R)X8R, DDXXD, DXDD, RDR, and NSE) were analysed. For phylogenetic analysis, annotated TPS gene from Arabidopsis thaliana (http://arabidopsis.org/), Vitis vinifera (http://www.plantgdb.org/VvGDB), Sorghum bicolor (http://www.plantgdb.org/SbGDB/), Oryza sativa (http://www.plantgdb.org/OsGDB/), and Eucalyptus grandis (Külheim et al. 2015) were retrieved. Complete reading frames of AaTPS genes were translated into protein sequences and classified into the TPS subfamily based on similarity and terzyme database (Priya et al. 2018), and all the sequences were manually re-annotated for convenience (Supplementary file 1). MEGA X integrated muscle alignment was performed considering the normal parameters for obtaining sequence alignments (Kumar et al. 2018). A neighbor-joining type phylogenetic tree with 1000 bootstrap replications was then constructed.
Sequence annotation and prediction of secondary metabolite pathway
AaTPS protein sequences were blast searched against the UniProt database (https://www.uniprot.org/), and for Gene Ontology terms, sequences were deposited in InterPro, and Gene Ontology (GO), and terms were assigned (Mitchell et al. 2019). For the prediction of functional genes in secondary metabolite biosynthesis, the AaTPSs were mapped against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.kegg.jp/blastkoala/) and annotated.
Prediction of Cis-elements (CREs) and in silico expression analysis of AaTPSs
For prediction of the CREs, 1500 bp upstream sequences of AaTPS were downloaded and deposited to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) for detection of elements involved in stress and defence response (Zhao et al. 2018; Li et al. 2017; Rajsz et al. 2016; Kaur et al. 2014; Qi et al. 2013). To confirm the prediction of CREs, in silico expression study of AaTPSs in cultured cells/tissues exposed to hormone stress and light stress was carried out. RNA-seq data under accession SRP041869 were retrieved, pulled and assembled into transcripts using Trinity to obtain a reference transcriptome (Grabherr et al. 2011). In order to annotate the transcripts with gene model IDs, NCBI BLAST + blastn was carried out as query sequences to A. agallochum annotated transcripts. For determining the transcript abundance from RNA-seq reads, SRAs for each stress and normal conditions were individually mapped to the reference transcriptome and transcripts per million (TPM) were calculated using salmon (Srivastava et al. 2019; Patro et al. 2017). The TPM for each transcripts that corresponds to AaTPSs were retrieved and the log fold change (stress/normal) were calculated. To calculate the log fold change at gene level, average of the log fold change of transcripts that corresponds to a AaTPS gene was considered. Log fold change > 1, and < − 1 was considered as differentially up-regulated and down-regulated respectively. For the In silico expression study of AaTPSs, transcriptome data of stress-induced and healthy Aquilaria plant (wood tissue) were retrieved from the database (https://www.ncbi.nlm.nih.gov/sra), Accession: PRJNA449813 ID: 449813, SRX4184708-SRX4184710, and SRX4149019-SRX4149021). HISAT2 was used for the short reads alignment to the genome (Kim et al. 2015), followed by StringTie to assemble and quantify the reads (Kovaka et al. 2019). Expression analysis was carried out using edgeR, where the p-value threshold was adjusted to 0.05, and library size was normalized based on the Trimmed Mean of M-values (TMM) (Robinson et al. 2009). The expression data of AaTPS genes were retrieved and a bar graph of significantly expressed TPS genes was generated.
Homology modelling and docking
Protein sequences of terpene synthases of A. agallochum were searched for the best template in the Protein Data Bank (PDB) database, and AaTPS19 showed the best match (> 44% identity; and > 95% coverage) with 5-epi-aristolochene synthase from Nicotiana tabacum (Rising et al. 2015). The homology model was generated with the SWISS-MODEL server (Biasini et al. 2014). For docking, the ligand (farnesyl pyrophosphate) was obtained from the PubChem database, and preparation of the ligand, and model protein were carried out with AutoDock tools and AutoDock Vina (Trott et al. 2010). The structures were visualized using PyMol.
Results
Identification of candidate terpene synthases in Aquilaria agallochum
A total of 96 putative terpene synthase genes in A. agallochum (AaTPSs) were identified based on a similarity search of proteome data to terpene synthase domain models PF01397 and PF03936. Of these, 46 were predicted to be partial based on missing motifs and domains in their translated protein sequence, and eleven as possible pseudogenes due to their close similarity to full-length genes. Thus, the remaining full-length 39 genes with intact conserved amino acid residues in their predicted protein sequences were considered for subfamily classification and downstream analysis. The sequences and information regarding the total number of full, partial, and pseudogenes are provided in Supplementary files 2 and 3.
Classification of AaTPSs based on conserved motifs and gene structure
The AaTPS protein sequences were analysed for conserved residues i.e., R(R)X8W, DDXXD, DXDD, RDR, and NSE, and the number of exons and introns. Based on the conserved motif and similarity search with terzyme database of already reported terpene synthases (Priya et al. 2018), 21 AaTPS were included in TPS-a subfamily, 11 in the TPS-b subfamily, one each in TPS-c and TPS-e subfamily, and three in TPS-f. In AaTPS-a, RDR and DDXXD motifs were present, while R(R)X8W and DTE were only detected in few AaTPS (Fig. 1). In the TPS-b subfamily, DDXXD motifs were present, while no R(R)X8W motifs were observed except in AaTPS28 and AaTPS29. In the TPS-c subfamily, DXDD motifs were found, while no other motifs were detected, suggesting their role as copalyl diphosphate synthase. Similarly, TPS-e and TPS-f showed no R(R)X8W, but DDXXD motifs were present. DDXXD i.e., the magnesium binding motif was found to be conserved in all AaTPS members.
Fig. 1.
Conserved motifs in the putative terpene synthases identified in the genome of Aquilaria agallochum. In the TPS-a subfamily, RDR and DDXXD motifs were conserved, while R(R)X8W and DTE were not conserved. In the TPS-b subfamily, the DDXXD motif was conserved, and R(R)X8W motif was conserved only in AaTPS28 and AaTPS29. In the TPS-c subfamily, the DXDD motif was present, and in TPS-e and f, R(R)X8W was not seen but, DDXXD was found to be conserved
The AaTPS genes of TPS-a and TPS-g subfamily members contain 6–8 exons and 5–7 intron, and thereby were included in class III TPSs except for AaTPS11 and AaTPS21 which possess four exon and three introns respectively (Fig. 2). Genes of TPS-b subfamily contains 6–8 exons and 5–7 introns and were considered in class II TPSs except for AaTPS23, AaTPS25, and AaTPS26 that contain only 4 exons and 3 introns. AaTPS genes of TPS-c, TPS-e, and TPS-f members comprises of 13–15 exons and 12–14 introns and was considered in class I TPSs but, AaTPS33 and AaTPS34 contained fewer exon and intron number. The missing intron-exons structures were probably due to the genome assembly error, which may require further refinement.
Fig. 2.
Overview of the gene structure of 39 putative terpene synthase genes in Aquilaria agallochum. Intron–exon boundaries are presented where the black rectangular box depicts the exon, and the thin line represents the intron. Genes are presented in the different boxes based on the subfamily
Phylogenetic analysis between A. agallochum terpene synthases and TPSs of other plants
Phylogenetic analysis between the thirty-nine AaTPSs and TPSs of other plants (Vitis vinifera, Sorghum bicolor, Eucalyptus grandis, Arabidopsis thaliana and Oryza sativa) was carried out to decipher the orthologous groups and explore evolutionary relations. A phylogenetic tree was constructed between AaTPS, as presented in Fig. 3. One cluster was observed each for TPS-a (AaTPS1-AaTPS21), TPS-b (AaTPS22-AaTPS31), TPS-c (AaTPS33), TPS-g (AaTPS38 and AaTPS39), and a single cluster for TPS-e (AaTPS35) and within it a sub-cluster for TPS-f (AaTPS34, AaTPS36, and AaTPS37) family members. In the tree constructed with TPSs of other plants (Fig. 4), TPS-a members of A. agallochum appeared as two distinct clusters, where one appeared orthologous to Vitis vinifera and Eucalyptus grandis TPS-a members and the other as equidistant to the rest of the plant members. The seven AaTPS-b members (AaTPS22, AaTPS23, AaTPS25, AaTPS26, AaTPS27, AaTPS30, and AaTPS31) appeared close to TPSb3 of Vitis vinifera and the rest appeared equidistant to other plants. The two AaTPS-g members appeared ortholog to Vitis venifera and Eucalyptus grandis TPS-g. Similarly, AaTPS-c, AaTPS-f, and AaTPS-e subfamily members also appeared equidistant to all other plant group members. Interestingly, TPSs of Sorghum bicolor and Arabidopsis thaliana showed longer branches than AaTPS-a subfamily genes that suggest longer gene differentiation events. Moreover, AaTPS29 and AaTPS25 of TPS-b subfamily, AaTPS34 of TPS-f subfamily showed longer branches compared to the other family members of A. agallochum.
Fig. 3.
Neighbor-joining tree constructed between 39 AaTPS using 1000 bootstrap replications with substitution and p-distance model. Subfamily based classification is shown with labelling and different branch shade
Fig. 4.
Neighbor-joining tree constructed between other plants terpene synthases and 39 AaTPS using 1000 bootstrap replications with substitution and p-distance model
Functional annotation and prediction of secondary metabolite pathway
The thirty-nine protein sequences (AaTPS) were blastp searched to find the homologous sequence from UniProt database and the best matched reviewed sequences were considered as annotation. The protein sequences of A. agallochum were found homologous to functionally characterized terpene synthases of Vitis vinifera; Ricinus communis, Aquilaria crassna, Cucumis melo, Quercus ilex, Salvia miltiorrhiza, Arabidopsis thaliana, Fragaria ananassa, and Clarkia breweri (Table 1). Interestingly, AaTPS3 showed highest similarity (99%) with characterized Delta-guaine synthase 1 isolated from cultured cells of Aquilaria crassna (Xu et al. 2013; Kumeta et al. 2010). Full annotations of the AaTPSs and basic properties retrieved from https://web.expasy.org/protparam/ are presented in Supplementary file 4. Gene Ontology (GO) based functional categorization of AaTPS genes detected their participation in various biological processes (isoprenoid biosynthetic process, terpenoid biosynthetic process, lipid biosynthetic process, hormone regulation); molecular functions (magnesium binding, catalytic activity, isomerase activity, lyase activity, terpene synthase activity, and cyclase activity); and cellular components (intracellular organelle, plastid, cytoplasm, chloroplast, membrane-bound organelle) (Fig. 5). Pathway mapping to the KEGG database revealed that 24 AaTPS genes were associated with the sesquiterpenoid biosynthesis pathway, and they belong to TPS-a and TPS-g subfamilies. In the monoterpenoid biosynthetic pathway, eight AaTPS genes were predicted to be associated, which majorly belong to the TPS-b subfamily. AaTPS-c, e, and f subfamily genes belong to both monoterpenoid and diterpenoid biosynthesis. Gene AaTPS28 was predicted to be an isoprene synthase. Proposed pathways of Aquilaria agallochum’s terpene synthase genes (AaTPSs) participating in various terpenoid biosynthesis are summarized in Fig. 6.
Table 1.
Basic characteristics of predicted terpene synthases in A. agallochum and their closest characterized homologs from other plants
| Sl. no | Protein | Sub family | Length (aa) | pI | MW (kDa) | Best hit | Similarity (%) | UniProtKB accession number | Plant species |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AaTPS1 | a | 448 | 5.30 | 51.76 | (−)-germacrene D synthase | 69.27 | Q6Q3H3 | Vitis vinifera |
| 2 | AaTPS2 | a | 532 | 5.64 | 61.75 | (−)-germacrene D synthase | 68.04 | Q6Q3H3 | Vitis vinifera |
| 3 | AaTPS3 | a | 430 | 5.01 | 50.02 | δ-guaiene synthase 1 | 99.86 | D0VMR6 | Aquilaria crassna |
| 4 | AaTPS4 | a | 475 | 5.24 | 55.05 | (−)-germacrene D synthase | 68.35 | A0A4U5PZF0* | Populus alba |
| 5 | AaTPS5 | a | 540 | 4.84 | 62.10 | (−)-germacrene D synthase | 71.24 | Q6Q3H3 | Vitis vinifera |
| 6 | AaTPS6 | a | 517 | 5.33 | 59.65 | Valencene synthase | 65.25 | Q6Q3H2 | Vitis vinifera |
| 7 | AaTPS7 | a | 565 | 5.34 | 65.54 | (−)-germacrene D synthase | 63.98 | Q6Q3H3 | Vitis vinifera |
| 8 | AaTPS8 | a | 368 | 4.94 | 42.55 | (−)-germacrene D synthase | 59.00 | A0A4U5NNE0* | Populus alba |
| 9 | AaTPS9 | a | 336 | 5.55 | 38.87 | (+)-γ-cadinene synthase | 68.25 | B2KSJ5 | Cucumis melo |
| 10 | AaTPS10 | a | 543 | 5.41 | 62.65 | (−)-germacrene D synthase | 68.12 | Q6Q3H3 | Vitis vinifera |
| 11 | AaTPS11 | a | 375 | 6.65 | 43.24 | (-)-germacrene D synthase | 69.91 | Q6Q3H3 | Vitis vinifera |
| 12 | AaTPS12 | a | 539 | 5.38 | 62.22 | (+)-γ-cadinene synthase | 67.66 | B2KSJ5 | Cucumis melo |
| 13 | AaTPS13 | a | 570 | 5.77 | 65.74 | (−)-germacrene D synthase | 69.70 | Q6Q3H3 | Vitis vinifera |
| 14 | AaTPS14 | a | 532 | 5.35 | 60.69 | δ-guaiene synthase 3 | 70.57 | D0VMR8 | Aquilaria crassna |
| 15 | AaTPS15 | a | 557 | 5.05 | 63.80 | δ-guaiene synthase 3 | 74.31 | D0VMR8 | Aquilaria crassna |
| 16 | AaTPS16 | a | 556 | 5.17 | 64.06 | δ-guaiene synthase 3 | 75.22 | D0VMR8 | Aquilaria crassna |
| 17 | AaTPS17 | a | 422 | 5.13 | 48.50 | (−)-germacrene D synthase | 65.42 | Q6Q3H3 | Vitis vinifera |
| 18 | AaTPS18 | a | 518 | 5.21 | 59.80 | Valencene synthase | 65.85 | Q6Q3H2 | Vitis vinifera |
| 19 | AaTPS19 | a | 555 | 5.14 | 63.54 | δ-guaiene synthase 3 | 74.03 | D0VMR8 | Aquilaria crassna |
| 20 | AaTPS20 | a | 454 | 6.13 | 52.21 | (−)-germacrene D synthase | 63.81 | Q6Q3H3 | Vitis vinifera |
| 21 | AaTPS21 | a | 303 | 4.83 | 35.42 | δ -guaiene synthase 1 | 97.00 | D0VMR6 | Aquilaria crassna |
| 22 | AaTPS22 | b | 564 | 5.63 | 64.29 | Probable terpene synthase 9 | 60.02 | B9RPM3 | Ricinus communis |
| 23 | AaTPS23 | b | 449 | 5.49 | 51.79 | Probable terpene synthase 9 | 62.84 | B9RPM3 | Ricinus communis |
| 24 | AaTPS24 | b | 603 | 6.52 | 69.12 | Myrcene synthase | 70.92 | Q93X23 | Quercus ilex |
| 25 | AaTPS25 | b | 366 | 4.98 | 41.52 | Probable terpene synthase 9 | 57.21 | B9RPM3 | Ricinus communis |
| 26 | AaTPS26 | b | 356 | 5.13 | 41.50 | Probable terpene synthase 9 | 61.45 | B9RPM3 | Ricinus communis |
| 27 | AaTPS27 | b | 523 | 5.15 | 59.57 | Probable terpene synthase 9 | 59.26 | B9RPM3 | Ricinus communis |
| 28 | AaTPS28 | b | 577 | 6.21 | 66.33 | Isoprene synthase | 66.73 | Q50L36 | Populus alba |
| 29 | AaTPS29 | b | 580 | 5.98 | 66.89 | σ-farnesene synthase | 65.22 | B9RXW0 | Ricinus communis |
| 30 | AaTPS30 | b | 524 | 5.20 | 59.88 | Probable terpene synthase 9 | 59.71 | B9RPM3 | Ricinus communis |
| 31 | AaTPS31 | b | 458 | 5.21 | 52.85 | Probable terpene synthase 9 | 59.05 | B9RPM3 | Ricinus communis |
| 32 | AaTPS32 | b | 603 | 6.16 | 69.10 | Myrcene synthase | 71.00 | Q93X23 | Quercus ilex |
| 33 | AaTPS33 | c | 313 | 8.74 | 35.69 | Ent-copalyl diphosphate synthase | 85.00 | A0A0U3LQ20 | Salvia miltiorrhiza |
| 34 | AaTPS34 | f | 518 | 7.56 | 59.29 | (E, E)-geranyllinalool synthase | 56.49 | Q93YV0 | Arabidopsis thaliana |
| 35 | AaTPS35 | e | 785 | 5.64 | 88.75 | Ent-kaur-16-ene synthase | 73.30 | A0A0U4CDK4 | Salvia miltiorrhiza |
| 36 | AaTPS36 | f | 803 | 5.87 | 92.72 | S-linalool synthase | 61.00 | Q96376 | Clarkia breweri |
| 37 | AaTPS37 | f | 843 | 6.23 | 96.96 | (E, E)-geranyllinalool synthase | 60.72 | Q93YV0 | Arabidopsis thaliana |
| 38 | AaTPS38 | g | 572 | 5.99 | 65.51 | (3S, 6E)-nerolidol synthase 2 | 68.70 | P0CV95 | Fragaria ananassa |
| 39 | AaTPS39 | g | 572 | 5.90 | 65.54 | (3S, 6E)-nerolidol synthase 2 | 70.26 | P0CV95 | Fragaria ananassa |
*Enzymatic characterization not carried out
Fig. 5.
Functional classification of AaTPS based on Gene Ontology (GO). a. Percentage of AaTPS genes predicted to be involved in various Biological processes (most genes involved in terpenoid, isoprenoid, lipid biosynthetic and metabolic process). b. Percentage of AaTPS genes predicted to be involved in various molecular functions (highest number of genes were involved in ion binding, terpene synthase, and lyase activity, and fewer genes in isomerase and cyclase activity)
Fig. 6.
Overview of predicted secondary metabolic pathway of putative AaTPSs based on mapping with the KEGG database and characterized TPS genes in Aquilaria plant species a. Representation of the possible involvement of predicted terpene synthases in the biosynthesis of terpenes in A. agallochum. b. Characterized terpene synthases in Aquilaria plant species to date. AaTPSa3 showed the highest sequence similarity of 98.30% to delta-guaiene synthase characterized from cultured cells of Aquilaria crassna
Cis-regulatory elements and in silico expression analysis
Cis-regulatory elements (CRE) serve as the binding sites for various transcription factors to regulate gene expression (Wong et al. 2017). The predicted cis-regulatory elements involved majorly in stress, hormone and light responsiveness in the upstream promoter region of the AaTPS genes are presented in Fig. 7a. Seven stress-responsive elements viz. binding sites for WRKY transcription factors in stress responses (W box), low-temperature-responsive element (LTR), wound-responsive element (WUN-motif), MYB binding site involved in drought-inducibility (MBS), regulatory element essential for anaerobic induction (ARE), dehydration-responsive element (DRE), and stress-responsive element (TC-rich repeats) were detected upstream of AaTPS genes. Additionally, five hormone-responsive elements viz. abscisic acid-responsive element (ABRE), cis-acting regulatory element involved in the MeJA-responsiveness (TGACG-motif), cis-acting element involved in gibberellin-responsiveness (TATC-box), auxin-responsive element (TGA-element), and salicylic acid responsiveness element (TCA-element) were identified. Furthermore, light responsiveness elements viz., Box-4, GT-1 motif, L-box, GATA-motif, MRE, LAMP-element, ACE, AE-box, G-box, I-box, and TCT motifs were predicted. The expression level of AaTPSs in cultured cells exposed to hormone stress (0.5 mM methyl jasmonate) showed significant up-regulation of 10 AaTPSs, out of which 6 genes are members of TPS-b subfamily, 2 genes are of TPS-f, and 1 each from TPS-a and TPS-g subfamily (Fig. 7c). Interestingly, all the members possessed MeJA-responsive element (TGACG-motif) in their promoter regions, except for AaTPS28 and AaTPS39 indicating other transcription factors and CREs may involve for differential regulation of these two genes. Moreover, in light stress conditions, 4 AaTPS genes (TPS-a and TPS-g family members) were up-regulated in red-light conditions when compared to white light and no differential up-regulation of AaTPS members were observed in far-red light condition (Fig. 7d). The results not only confirm the predicted CREs and their involvement in regulation of AaTPSs in hormone and light stress conditions but also infer red-light as a better stress inducer for terpene synthases compared to normal white and far-red light. Furthermore, expression study of AaTPS in stress-induced Aquilaria plant (wood tissue) showed significant up-regulation of 13 AaTPS genes (members of TPS-a, TPS-b, and TPS-g) and down-regulation of one AaTPS genes (Fig. 7b). The genes AaTPS2, AaTPS10, AaTPS16, AaTPS17, AaTPS22, AaTPS24, and AaTPS27 showed enhanced expression up to 7–8 fold. AaTPS6, AaTPS13, AaTPS28, AaTPS32, AaTPS18, and AaTPS39 also up-regulated to 4–6 fold. However, AaTPS38 down-regulated by 1.7-fold.
Fig. 7.
Overview of the expression level of AaTPSs in stress induced tissue and their possible CREs in the upstream region. a. Predicted CREs in upstream regions of AaTPS genes. Stress-responsive elements include (W box: WRKY transcription factors in stress responses, LTR: Low-temperature-responsive element, WUN-motif: wound-responsive element, MBS: MYB binding site involved in drought-inducibility, ARE: regulatory elements essential for anaerobic induction, DRE dehydration-responsive elements, TC-rich repeats: stress-responsive elements); Hormone-responsive elements include (TCA-element salicylic acid responsiveness, TGA-element auxin-responsive element, TATC-box: gibberellin-responsiveness, ABRE: abscisic acid-responsive elements, TGACG-motif: MeJA-responsiveness); and light responsive elements (TCT-motif, L-box, G-box, GATA-motif, Box-4, 3-AF1, GT-1 motif, chs-CMA1a, MRE, ACE, TCCC-motif, ATCT-motif, ATC-motif, I-box, GA-motif, AE-box, LAMP-element, AAAC-motif, CAG-motif, Gap-box, Box II, Sp1, and sbp-CMA1c. b. Log fold change of the differentially regulated (p < 0.05) AaTPS genes in stress-induced tissues based on RNA-seq data retrieved from NCBI. Subfamily members are shown in a different coloured bar: TPS-a (green), TPS-b (red), TPS-g (violet). c. Differentially expressed genes (Log fold change > 1 or < − 1) in tissues exposed to 0.5 mM Methyl jasmonate (MeJ) when compared to control tissues (no treatment). Subfamily members are shown in a different coloured bar: TPS-a (green), TPS-b (red), TPS-f (blue), and TPS-g (violet). d. Differentially expressed AaTPS genes (*marked) (Log fold change > 1 or < 1) in tissues exposed to Far Red light compared to normal white light (FR/W), and Red light compared to white (R/W). Members are coloured coded based on subfamily
Functional prediction and expression studies of AaTPSs revealed that the members of the TPS-a subfamily are involved in the sesquiterpenoid biosynthesis pathway where AaTPS2 (Garmacradienol synthase), AaTPS10 (hedycaryol synthase), AaTPS16 (alfa-selinene synthase), AaTPS17 (germacrene D synthase), AaTPS6 (Valencene synthase), AaTPS13 (beta-caryophyllene synthase) showed up-regulation in stress condition. The majority of the TPS-b members are associated with monoterpenoid biosynthesis, where AaTPS22 (beta-ocimene synthase), AaTPS27 and AaTPS24 (myrcene synthase), and AaTPS32 (linalool synthase) showed up-regulation in stress conditions. Genes of three subfamilies viz. TPS-c (AaTPS33); TPS-e (AaTPS35); and TPS-f (AaTPS34, AaTPS36, and AaTPS37) were associated with diterpenoid and monoterpenoid biosynthesis. Significant down-regulation of AaTPS38 (nerolidol synthase) was observed 1.5-fold in stress conditions. However, AaTPS28, which was predicted as isoprene synthase showed significant up-regulation by 6-fold. Expression data are provided in detail in Supplementary file 5.
Homology modelling
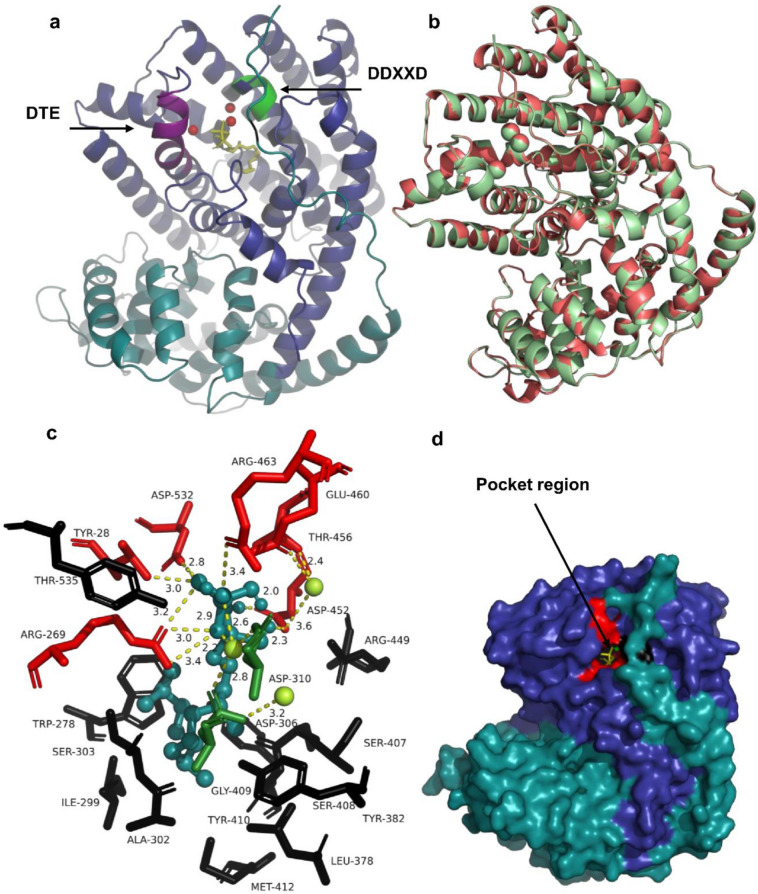
The protein AaTPS19 was modelled based on the crystal structure (X-RAY DIFFRACTION 2.47 Å) of 5-epi-aristolochene synthase from Nicotiana tabacum (PDB code 4RNQ) which depicted 44.94% identity with AaTPS19 (Fig. 8). The predicted model showed Q mean of − 2.39 and Global Model Quality Estimation (GMQE) of 0.77. Structure assessment was carried out with MolPobity (score 1.27) and the 99.44% of residues were Ramachandran favoured. The model fitted well with the crystallographic 3D structure and Docking of FPP to the modelled protein (binding affinity of -7.4 kcal/mol) revealed the DDXXD motif (ASP-310 and ASP-306) and DTE to be associated with the binding of magnesium ion in the pocket region. The possible polar and non-polar contacts of the amino acid residues with the ligand and metal ion were predicted. The amino acid residues actively participating in the polar interaction with the ligand and Mg2+ trio were revealed to be ARG-269, ASP-306, ASP-310, THR-535, ASP-532, ASP-452, THR-456, GLU-460, and ARG-463.
Fig. 8.
Homology model of A. agallochum AaTPSa19. a. Modelled structure of AaTPSa19 with substrate FPP along with Mg2+ trio. The N-terminal region is shown in cyan colour, and the C terminal region in pale blue with Mg2+ trio and the ligand FPP. The two conserved motif DTE (violet colour) and metal binding DDXXD motif (green colour) was found to be in close proximity to FPP and metal ions. b. Alignment of 5-epi-aristolochene synthase (red colour) from Nicotiana tabacum and AaTPSa19 (pale green) of A. agallochum (RMSD = 0.172). c. Probable polar (red stick) and non-polar (black stick) interaction (yellow colour dashes) with FPP (cyan deep teal colour) and Mg2+ trio in the pocket region. The residue ASP-306 and ASP-310 (green coloured stick) of metal binding motif are found to form polar interaction with two Mg2+. d. Surface view of the modelled protein where N terminal (pale cyan), C terminal (blue) and pocket region are shown. Residues involving in polar and non-polar interactions are shown in red and black colour respectively
Discussion
Agarwood accounts for an annual global market of approximately 6–8 billion US$ (Tan et al. 2019; Akter et al. 2013), and due to substantial utilization and exploitation, it has now been listed as an endangered species (Akter et al. 2013). Agarwood quality and oil grades are measured by its resin quantity and metabolite constituents, which has led to increased attention to the metabolomics studies to comprehend the pleasant aroma that agarwood exhibits (Pasaribu et al. 2005). The major constituents are sesquiterpenes and 2-(2-phenylethyl) chromones and a few other volatile metabolites which impart agarwood a unique fragrance property (Chen et al. 2012; Naef 2011). Despite holding a high economic value, not much attention has been given towards analysis of the genes involved in the production of these fragrant compounds, except for a few sesquiterpene synthases (Ye et al. 2018; Azzarina et al. 2016; Xu et al. 2013; Kumeta et al. 2010).
In the above study, refinement of the genomic and transcriptomics data, and evidence obtained from protein sequence alignment, and two Pfam TPS gene models helped identify thirty-nine putatively functional terpene synthases (AaTPSs) in Aquilaria agallochum. Based on KEGG pathway mapping, we propose their possible functional role in generating diverse terpenes in Aquilaria. The presence of stress, hormone and light response elements upstream of these genes suggests their regulation in various stress conditions by transcription factors. Reports also suggests that tissues exposed to red light condition and Methyl jasmonate (MeJ) were found to show high expression of terpene biosynthesis genes and endogenous accumulation of high amount of terpenes such as cucurbitacin (Kuo et al. 2015; Chen et al. 2014a). Additionally, literature on expression studies shows significant up-regulation of sesquiterpene biosynthesis genes in wound-inducing tissues in Aquilaria plant species and their possible involvement in the formation of agarwood sesquiterpenes (Islam et al. 2020; Xu et al. 2013; Azzarina et al. 2016). In our study, in silico expression analysis showed up-regulation of the most AaTPS genes in stress-induced wood tissue when compared to normal tissue in Aquilaria plant, suggesting their possible role in plant defence. Differential regulation of most AaTPSs in hormone (MeJ) and light stress condition indicates the involvement of predicted hormone and light CREs and confirms their presence. However, two genes AaTPS28 and AaTPS39, members of TPS-b and TPS-g subfamily respectively, up-regulated in MeJ treated cells despite absence of MeJ responsive element. It connects to the fact that jasmonic acid, which is a crucial signalling molecule may directly or indirectly induce the expression of genes involved in terpene biosynthesis (Xu et al. 2016). It also suggests that, it may activate other transcription factors which bind to CREs other than MeJ responsive element for regulating TPSs expression. These two genes would be an interesting candidate for further investigation. Our findings support the results presented by Kuo et al. 2015 and Chen et al. 2014a, where within they have stated that genes involved in terpene biosynthesis are up-regulated by hormone and red light stress. Thus, the presence of such CREs in the upstream regions of the identified AaTPSs may be crucial for their regulation. Additionally, 3D structural modelling of AaTPS19 and docking with FPP revealed the interaction of the DDXXD motif of the model with Mg ion and various polar and non-polar interactions of the key active site residues of the protein with the ligand. The first and the fifth Aspartic acid (Asp) of DDXXD motif was found highly conserved in all the TPS members of A. agallochum irrespective of their subfamily, thereby indicating the importance of the conserved metal binding domain necessary for catalysis of various substrates.
Various sesquiterpenoids and their derivatives including agarofurans, agarospiranes, eudesmanes, eremophilanes, guaianes, candinanes, and jinkohol-types have been extracted and identified through solvent extraction from agarwood and Aquilaria agallochum tress (Gao et al. 2019). But, the molecular mechanism for biosynthesis and generation of these diverse classes of aromatic molecules is poorly understood. The 39 terpene synthases identified in the present study are predicted to be involved in the generation of terpenes, sesquiterpenes such as garmacradienol, hedycaryol, alfa-selinene, germacrene D, Valencene, beta-caryophyllene, nerolidol, alfa-farnesene, delta-guaine, delta cadinene, alfa-copaene, kunzeaol, germacrene B, and hedycaryol; monoterpenes such as myrcene, limonene, ocimene, linalool, terpineol; and diterpene such as ent-copalyl, ent-kaurene, and casbane in Aquilaria agallochum. Similar to most of the terpene synthases reported in the literature, some of the terpene synthases from Aquilaria characterized earlier have also been reported to catalyse coincidental side reactions in addition to the main reaction possibly due to flexibility of amino acid residues in the pocket region. For example, Delta guaiene synthase isolated from A. microcarpa was able to produce multiple sesquiterpenes (d-guaiene, a-guaiene and b-elemene) from FPP (Lee et al. 2014) Fig. 4B. This can contribute to the diversity of the sesquiterpenes observed in Aquilaria. Furthermore, the core terpene skeleton biosynthesized by these TPSs may be further diversified by CYP450 class of enzymes through oxidation and creating a derivative profile corresponding to one obtained through extraction from agarwood (Li et al. 2021). Post-infection and stress expression studies of Aquilaria terpene synthases also describe their wound inducible nature (Azzarina et al. 2016), hence creating a scope for both in vitro and in vivo induction of tissues to identify and characterize TPS genes. Additionally, the current demand of terpenes and their derivatives for a wide and versatile usage such as for repellents and fragrance, treatment of migraines, microbial infection,; inflammation, and heart diseases, and; wound healing (Loreto et al. 2002, Chadwick et al. 2013, Zhang et al. 2012, Vasas et al. 2014), has necessitated their large scale production. Thus, advances in recombinant DNA technology and over expression of plant terpene synthases in heterologous host is expected to circumvent the need for fragrant compounds and make agarwood phytochemicals available at a reasonable price for various agarwood dependent industries more so the perfume industry.
Conclusion
In this study, genome-wide mining was opted to detect and analyse terpene synthases genes in Aquilaria agallochum genome, followed by systemic classification based on the conserved motif, gene structure, and evolutionary analysis with TPSs of other plants. This study presents the genome-wide detection and classification of TPS subfamily members in Aquilaria, where we identified 39 TPS genes that were distributed to specific six subfamilies of terpene synthases viz. TPS-a, TPS-b, TPS-c, TPS-e, TPS-f, and TPS-g. Seven stress, five hormone-responsive cis-elements, and twenty-three light-responsive elements in the upstream regions were predicted to be present and were confirmed by analysing their expression in hormone and light stressed cultured cells RNA-seq data. In silico expression analysis in stress-induced tissue detected the up regulation of most AaTPS genes. Their possible involvement in terpene biosynthesis in Aquilaria agallochum has been predicted through annotation and mapping. This information could serve in functional characterization of AaTPS to decipher their fundamental role in the biosynthesis of diverse and complex terpenoids and could also provide a biotechnological tool for the industrial production of these precious natural products.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are indebted to Gauhati University for providing the technical facility. The authors thank Chen and team for making the genomic data publicly available with no restriction to modify and reproduce. The authors also acknowledge Department of Biotechnology (DBT), Government of India for providing the financial aid.
Abbreviations
- TPS
Terpene synthase
- AaTPS
Aquilaria agallochum terpene synthase
- MVA
Mevalonate pathway
- MEP
Methylerythritol phosphate pathway
- IPP
Isopentenyl pyrophosphate
- DMAPP
Dimethylallyl pyrophosphate
- PASA
Program to assemble spliced alignments
- TMM
Trimmed mean of M-values
- GO
Gene ontology
- KEGG
Kyoto encyclopaedia of genes and genomes database
Funding
The authors acknowledge the financial aid received from Department of Biotechnology (DBT); Government of India under twinning scheme vide sanction number BT/PR24723/NER/95/832/2017 and BT/PR16867/NER/95/327/2015.
Data availability
The data supporting the finding of this study is provided in the manuscript and its supplementary material.
Declaration
Conflict of interest
The authors declares no conflict of interest exist.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akter S, Islam MT, Zulkefeli M, Khan SI. Agarwood production-a multidisciplinary field to be explored in Bangladesh. Int J Pharm Life Sci. 2013;2:22–32. doi: 10.3329/ijpls.v2i1.15132. [DOI] [Google Scholar]
- Alquézar B, Rodríguez A, de la Peña M, Peña L. Genomic analysis of terpene synthase family and functional characterization of seven sesquiterpene synthases from Citrus sinensis. Front Plant Sci. 2017;8:1481. doi: 10.3389/fpls.2017.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubourg S, Lecharny A, Bohlmann J. Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics. 2002;267:730–745. doi: 10.1007/s00438-002-0709-y. [DOI] [PubMed] [Google Scholar]
- Azzarina AB, Mohamed R, Lee SY, Nazre M. Temporal and spatial expression of terpene synthase genes associated with agarwood formation in Aquilaria malaccensis Lam. N Z J Sci. 2016;46:12. doi: 10.1186/s40490-016-0068-9. [DOI] [Google Scholar]
- Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Keeling CI. Terpenoid biomaterials. Plant J. 2008;54:656–669. doi: 10.1111/j.1365-313X.2008.03449.x. [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci. 1998;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JK, Page JE, Bohlmann J. Terpene synthases from Cannabis sativa. PLoS ONE. 2017;12:3. doi: 10.1371/journal.pone.0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputi L, Aprea E. Use of terpenoids as natural flavoring compounds in food industry. Recent Pat Food Nutr Agric. 2011;3:9–16. doi: 10.2174/2212798411103010009. [DOI] [PubMed] [Google Scholar]
- Celedon JM, Bohlmann J. Genomics-based discovery of plant genes for synthetic biology of terpenoid fragrances: a case study in sandalwood oil biosynthesis. Methods Enzymol. 2016;576:47–67. doi: 10.1016/bs.mie.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Sesquiterpenoids lactones: benefits to plants and people. Int J Mol Sci. 2013;14(6):12780–12805. doi: 10.3390/ijms140612780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- Chen HQ, Wei JH, Yang JS, Zhang Z, Yang Y. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem Biodivers. 2012;9:236–250. doi: 10.1002/cbdv.201100077. [DOI] [PubMed] [Google Scholar]
- Chen C, Kuo TC, Yang M, et al. Identification of cucurbitacins and assembly of a draft genome for Aquilaria agallocha. BMC Genomics. 2014;15:578. doi: 10.1186/1471-2164-15-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Wu B, Nelson DR, Wu K, Liu C. Computational identification and systematic classification of novel cytochrome P450 senes in salvia miltiorrhiza. PLoS ONE. 2014;9(12):e115149. doi: 10.1371/journal.pone.0115149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang W, Zhang L, Wu X, Cheng T, Li G. Genome-wide identification, functional and evolutionary analysis of terpene synthases in pineapple. Comput Biol Chem. 2017;70:40–48. doi: 10.1016/j.compbiolchem.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Christianson DW. Correction to structural and chemical biology of terpenoid cyclases. Chem Rev. 2018;118:11795. doi: 10.1021/acs.chemrev.8b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Espaux L, Mendez-Perez D, Li R, Keasling JD. Synthetic biology for microbial production of lipid-based biofuels. Curr Opin Chem Biol. 2015;29:58–65. doi: 10.1016/j.cbpa.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Falara V, Akhtar TA, Nguyen TTH, Spyropoulou EA, Bleeker PM, et al. The tomato terpene synthase gene family. Plant Physiol. 2011;157:770–789. doi: 10.1104/pp.111.179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Honzatko R. Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat Prod Rep. 2012;29:11531175. doi: 10.1039/c2np20059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Han X, Sun Y, Chen H, Yang Y, et al. Overview of sesquiterpenes and chromones of agarwood originating from four main species of the genus Aquilaria. RSC Adv. 2019;9:4113–4130. doi: 10.1039/C8RA09409H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- Gomes AR, Byregowda SM, Veeregowda BM, Balamurugan V. An overview of heterologous expression host systems for the production of recombinant proteins. Adv Anim Vet Sci. 2016;4(7):346–356. doi: 10.14737/journal.aavs/2016/4.7.346.356. [DOI] [Google Scholar]
- Grabherr M, Haas B, Yassour M, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo AY, Zhang H, Luo J. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BJ, Delcher AL, Mount SM, Wortman JR, Smith RK, Jr, et al. Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic Acids Res. 2003;31(19):5654–5666. doi: 10.1093/nar/gkg770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris LJ, Saparno A, Johnston A, Prisic S, Xu M, et al. The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol Biol. 2005;59:881–894. doi: 10.1007/s11103-005-1674-8. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Kawaide H, Notomi M, Sakigi Y, Matsuo A, Nozaki H. Identification and functional analysis of bifunctional ent-kaurene synthase from the moss Physcomitrella patens. FEBS Lett. 2006;580:6175–6181. doi: 10.1016/j.febslet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Hoff KJ, Lomsadze A, Borodovsky M, Stanke M. Whole-genome annotation with BRAKER. Methods Mol Biol. 2019;1962:65–95. doi: 10.1007/978-1-4939-9173-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Kautsar SA, Hong YJ, Medema MH, Bond AD, et al. Unearthing a sesterterpene biosynthetic repertoire in the Brassicaceae through genome mining reveals convergent evolution. Proc Natl Acad Sci USA. 2017;114:E6005–E6014. doi: 10.1073/pnas.1705567114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmisch S, Jiang Y, Chen F, Gershenzon J, Kollner TG. Terpene synthases and their contribution to herbivore-induced volatile emission in western balsam poplar (Populus trichocarpa) BMC Plant Biol. 2014;14:270. doi: 10.1186/s12870-014-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Bhau BS, Banu S. Gene expression analysis associated with agarwood formation in Aquilaria malaccensis. Plant Physiol Rep. 2020;25:304–314. doi: 10.1007/s40502-020-00505-9. [DOI] [Google Scholar]
- Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, et al. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24(24):2938–2939. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Mustafiz A, Sarkar AK, Ariyadasa TU, Singla-Pareek SL, et al. Expression of abiotic stress inducible ETHE1-like protein from rice is higher in roots and is regulated by calcium. Physiol Plant. 2014;152:1–16. doi: 10.1111/ppl.12147. [DOI] [PubMed] [Google Scholar]
- Keeling CI, Bohlmann J. Diterpene resin acids in conifers. Phytochem. 2006;67:2415–2423. doi: 10.1016/j.phytochem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Keilwagen J, Lehnert H, Berner T, Budahn H, Nothnagel T, et al. The terpene synthase gene family of carrot (Daucuscarota L.) Identification of QTLs and candidate genes associated with terpenoid volatile compounds. Front Plant Sci. 2017;8:1930. doi: 10.3389/fpls.2017.01930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ. BLAT–the BLAST-like alignment tool. Genome Res. 2002;2(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg S. HISAT: a fast-spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaka S, Zimin AV, Pertea GM, Razaghi R, Salzberg SL, et al. Transcriptome assembly from long-read RNA-seq alignments with StringTie2. Genome Biol. 2019;20:278. doi: 10.1186/s13059-019-1910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristanti AN, Tanjung M, Aminah NS. Review: secondary metabolites of Aquilaria, a Thymelaeaceae genus. Mini Rev Org Chem. 2018;15(1):36–55. doi: 10.2174/1570193X14666170721143041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külheim C, Padovan A, Hefer C, Krause ST, Kollner TG, et al. The Eucalyptus terpene synthase gene family. BMC Genomics. 2015;16(1):450. doi: 10.1186/s12864-015-1598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RS. Metabolic engineering: biological art of producing useful chemicals. Reson. 2016;21:233–237. doi: 10.1007/s12045-016-0318-4. [DOI] [Google Scholar]
- Kumar S, Kempinski C, Zhuang X, Norris A, Mafu S, et al. Molecular diversity of terpene synthases in the liverwort Marchantia polymorpha. Plant Cell. 2016;28:2632–2650. doi: 10.1105/tpc.16.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamuram K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Y, Khan F, Rastogi S, Shasany AK. Genome-wide detection of terpene synthase genes in holy basil (Ocimumsanctum L.) PLoS ONE. 2018;13(11):e0207097. doi: 10.1371/journal.pone.0207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumeta Y, Ito M. Characterization of delta-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiol. 2010;154(4):1998–2007. doi: 10.1104/pp.110.161828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo TCY, Chen CH, Chen SH, Lu IH, Chu MJ, et al. The effect of red light and far-red light conditions on secondary metabolism in Agarwood. BMC Plant Biol. 2015;15:139. doi: 10.1186/s12870-015-0537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Hirohashi S, Yamamura Y, Taura F, Kurosaki F. Induction, cloning and functional expression of a sesquiterpene biosynthetic enzyme, δ-guaiene synthase, of Aquilaria microcarpa cell cultures. Nat Prod Commun. 2014;9(9):1231–1235. [PubMed] [Google Scholar]
- Li G, Kollner TG, Yin Y, Jiang Y, Chen H, et al. Non seed plant Selaginella moellendorffii has both seed plant and microbial types of terpene synthases. Proc Natl Acad Sci USA. 2012;109:14711–14715. doi: 10.1073/pnas.1204300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang J, Sun Q, Li W, Yu Y, et al. Expression analysis of genes encoding double B-box zinc finger proteins in maize. Funct Integr Genom. 2017;17:1–14. doi: 10.1007/s10142-017-0562-z. [DOI] [PubMed] [Google Scholar]
- Li W, Chen QH, Wang H, Mei LW, Dai HF. Natural products in agarwood and Aquilaria plants: chemistry, biological activities and biosynthesis. Nat Prod Rep. 2021;38:528–556. doi: 10.1039/D0NP00042F. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu Y, Liang L, Wei J. Molecular cloning, characterization and expression analysis of the gene encoding 1-deoxy-D-xylulose 5-phosphate reductoisomerase from Aquilaria sinensis (Lour.) Gilg. J Genet. 2015;94:239–249. doi: 10.1007/s12041-015-0521-1. [DOI] [PubMed] [Google Scholar]
- Lomsadze A, Ter-Hovhannisyan V, Chernoff YO, Borodovsky M. Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 2005;33:6494–6506. doi: 10.1093/nar/gki937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Forster A, Durr M, Csiky O, Seufert G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of QuercusIlex L. fumigated with selected monoterpenes. Plant Cell Environ. 2002;21(1):101–107. doi: 10.1046/j.1365-3040.1998.00268.x. [DOI] [Google Scholar]
- Martin DM, Aubourg S, Schouwey MB, Daviet L, Schalk M, et al. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010;10:226. doi: 10.1186/1471-2229-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AL, Attwood TK, Babbitt PC, Blum M, Bork P, et al. InterPro in 2019: improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019;47(1):D351–D360. doi: 10.1093/nar/gky1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed R, Jong PL, Zali MS. Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers. 2010;43:67–74. doi: 10.1007/s13225-010-0039-z. [DOI] [Google Scholar]
- Mohamed R, Jong PL, Kamziah AK. Fungi inoculation induced agarwood in young Aquilaria malaccensis trees in the nursery. J for Res. 2014;25(1):201–204. doi: 10.1007/s11676-013-0395-0. [DOI] [Google Scholar]
- Naef R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: a review. Flavour Fragr J. 2011;26:73–87. doi: 10.1002/ffj.2034. [DOI] [Google Scholar]
- Nieuwenhuizen NJ, Green SA, Chen X, Bailleul EJD, Matich AJ, et al. Functional genomics reveals that a compact terpene synthase gene family can account for terpene volatile production in apple. Plant Physiol. 2013;161:787–804. doi: 10.1104/pp.112.208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasaribu GT, Waluyo TK, Pari G. Analysis of chemical compounds distinguisher for agarwood qualities. Indones J for Res. 2005;2:1–7. [Google Scholar]
- Patro R, Duggal G, Love M, et al. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5:237–243. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Pichersky E, Raguso RA. Why do plants produce so many terpenoid compounds? New Phytol. 2018;220:692–702. doi: 10.1111/nph.14178. [DOI] [PubMed] [Google Scholar]
- Priya P, Yadav A, Chand J, Yadav G. Terzyme: a tool for identification and analysis of the plant terpenome. Plant Methods. 2018;14:4. doi: 10.1186/s13007-017-0269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Xie S, Liu Y, Yi F, Yu J. Genome-wide annotation of genes and noncoding RNAs of foxtail millet in response to simulated drought stress by deep sequencing. Plant Mol Biol. 2013;83:459–473. doi: 10.1007/s11103-013-0104-6. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chakrabarty R, Tran HT, Kwon EJ, Kwon M, et al. A lettuce (Lactuca sativa) homolog of human Nogo-B receptor interacts with cis-prenyltransferase and is necessary for natural rubber biosynthesis. J Biol Chem. 2015;290:1898–1914. doi: 10.1074/jbc.M114.616920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajsz A, Warzybok A, Migocka M. Genes encoding cucumber full-size ABCG proteins show different responses to plant growth regulators and sclareolide. Plant Mol Biol Rep. 2016;34:720–736. doi: 10.1007/s11105-015-0956-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rising AK, Crenshaw CM, Koo HJ, Subramanian T, Chehade KAH, et al. Formation of a novel macrocyclic alkaloid from the unnatural farnesyl diphosphate analogue anilinogeranyl diphosphate by 5-epi-aristolochene synthase. ACS Chem Biol. 2015;10(7):1729–1736. doi: 10.1021/acschembio.5b00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir-Mir M, Mett A, Belausov E, Tal-Meshulam S, Frydman A, et al. Peroxisomal localization of Arabidopsis isopentenyl diphosphate isomerases suggest that part of the plant isoprenoid mevalonic acid pathway is Compartmentalized to Peroxisomes. Plant Physiol. 2008;148(3):1219–1228. doi: 10.1104/pp.108.127951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Huffaker A, Sims JW, Christensen SA, Lu X, et al. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014;79:659–678. doi: 10.1111/tpj.12436. [DOI] [PubMed] [Google Scholar]
- Sen S, Talukdar NC, Khan M. A simple metabolite profiling approach reveals critical biomolecular linkages in fragrant agarwood oil production from Aquilaria malaccensis—A traditional agro-based industry in North East India. Curr Sci. 2015;108(1):63–71. [Google Scholar]
- Sen S, Dehingia M, Talukdar NC, Khan M. Chemometric analysis reveals links in the formation of fragrant bio-molecules during agarwood (Aquilaria malaccensis) and fungal interactions. Sci Rep. 2017;7:44406. doi: 10.1038/srep44406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Gray DW, Pell HK, Breneman SR, Topper L. Isoprene synthase genes form a monophyletic clade of acyclic terpene synthases in the TPS-b terpene synthase family. Evolution. 2013;67:1026–1040. doi: 10.1111/evo.12013. [DOI] [PubMed] [Google Scholar]
- Singh B, Sharma RA. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech. 2015;5(2):129–151. doi: 10.1007/s13205-014-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, Hubley R (2008-2015) Repeatmodeler open-1.0. http://www.repeatmasker.org
- Smit AFA, Hubley R, Green P (1996-2010) Repeatmasker open-3.0. http://www.repeatmasker.org
- Srivastava A, Malik L, Smith T, Sudbery I, Patro R. Alevin efficiently estimates accurate gene abundances from dscRNA-seq data. Genome Biol. 2019;20:65. doi: 10.1186/s13059-019-1670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33:W465–W467. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CS, Isa NM, Ismail I, Zainal Z. Agarwood induction: current developments and future prespectives. Front Plant Sci. 2019;10:122. doi: 10.3389/fpls.2019.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(1D):506–515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D. Biosynthesis and biological functions of terpenoids in plants. Adv Biochem Eng Biotechnol. 2015;148:63–106. doi: 10.1007/10_2014_295. [DOI] [PubMed] [Google Scholar]
- Thoma R, Schulz-Gasch T, D’Arcy B, Benz J, Aebi J, et al. Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase. Nature. 2004;432:118–122. doi: 10.1038/nature02993. [DOI] [PubMed] [Google Scholar]
- Trapp SC, Croteau RB. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics. 2001;158:811–832. doi: 10.1093/genetics/158.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasas A, Hohmann J. Euphorbia Diterpenes: isolation, structure, biological activity, and synthesis. Chem Rev. 2014;114(17):8579–8612. doi: 10.1021/cr400541j. [DOI] [PubMed] [Google Scholar]
- Vaughan MM, Wang Q, Webster FX, Kiemle D, Hong YJ, et al. Formation of the unusual semivolatile diterpene rhizathalene by the Arabidopsis class I terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. Plant Cell. 2013;25:1108–1125. doi: 10.1105/tpc.112.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yu Z, Wang C, Wu C, Guo P, et al. Chemical constituents and pharmacological activity of agarwood and aquilaria plants. Molecules. 2018;23(2):342. doi: 10.3390/molecules23020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ouyang K, Wang K. Genome-wide identification, evolution, and expression analysis of TPS and TPP gene families in Brachypodium distachyon. Plants. 2019;8(10):362. doi: 10.3390/plants8100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DCJ, Gutierrez RL, Gambetta GA, Castellarin SD. Genome-wide analysis of cis-regulatory element structure and discovery of motif-driven gene co-expression networks in grapevine. DNA Res. 2017;24(3):311–326. doi: 10.1093/dnares/dsw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang Z, Wang M, Wei J, Chen H, et al. Identification of genes related to agarwood formation: transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genomics. 2013;14:227. doi: 10.1186/1471-2164-14-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Liao YC, Zhang Z, Liu J, Sun PW, et al. Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci Rep. 2016;6:21843. doi: 10.1038/srep21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagura T, Ito M, Kiuchi F, Honda G, Shimada Y. Four new 2-(2-phenylethyl) chromone derivatives from withered wood of Aquilaria sinensis. Chem Pharm Bull (tokyo) 2003;51(5):560–564. doi: 10.1248/cpb.51.560. [DOI] [PubMed] [Google Scholar]
- Ye W, He X, Wu H, Wang L, Zhang W, et al. Identification and characterization of a novel sesquiterpene synthase from Aquilaria sinensis: an important gene for agarwood formation. Int J Biol Macromol. 2018;108:884–892. doi: 10.1016/j.ijbiomac.2017.10.183. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhao C, Zhang G, Teixeira da Silva JA, Duan J. Genome-wide identification and expression profile of TPS gene family in Dendrobiumofficinale and the role of DoTPS10 in linalool biosynthesis. Int J Mol Sci. 2020;21(15):5419. doi: 10.3390/ijms21155419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbe P, Bohlmann J. Plant diterpene synthases: exploring modularity and metabolic diversity for bioengineering. Trends Biotechnol. 2015;33:419–428. doi: 10.1016/j.tibtech.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang P, Ye M, Kim SH, Jiang C, et al. Tanshinones: sources, pharmacokinetics and anti-cancer activities. Int J Mol Sci. 2012;13(10):13621–13666. doi: 10.3390/ijms131013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Wang D, Wang R, Kong N, Zhang C, et al. Genome-wide analysis of the potato Hsp20 gene family: identification, genomic organization and expression profiles in response to heat stress. BMC Genomics. 2018;19:61. doi: 10.1186/s12864-018-4443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Shamala LF, Yi X, Yan Z, Wei S. Analysis of terpene synthase family genes in Camelliasinensis with an emphasis on abiotic stress conditions. Sci Rep. 2020;10:933. doi: 10.1038/s41598-020-57805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the finding of this study is provided in the manuscript and its supplementary material.