Abstract
Excessive generation of oxygen free radicals plays a pivotal role in destruction of biological molecules like DNA, proteins, lipids, carbohydrates and results in various pathologies including neuronal disorders. Antioxidant molecules from natural products are reported to have ability to mitigate their production or at least halt their progression and metastasis in the system. Different studies have been performed to spot antioxidants from natural sources and attempts have been made to integrate them in conventional therapy. In our present study, food grade Phycocyanin, a nutraceutical isolated from Spirulina platensis, has been evaluated for its in vitro and in vivo antioxidant potential using a battery of antioxidant assays viz. DPPH, TAC, FRAP, hydroxyl radical, hydrogen peroxide scavenging, SOD, GSH, and LPO assays. Reducing properties of Phycocyanin were also assessed by FRAC assay. For in vivo evaluation of antioxidant profile, animal model of intracerebroventricular administration of streptozotocin was employed. Levels of oxidative stress biomarkers were measured in cortex and hippocampal parts of brain. Results obtained depicted that Phycocyanin demonstrated a dose-dependent pattern in its efficacy, which indicates the presence of free radical scavenger moieties and possible role as a neuroprotective agent.
Keywords: Antioxidant, Food grade, In vitro, In vivo, Phycocyanin
Introduction
Ironically Oxygen, an element vital for life, at some points becomes destructive for human body and the most deleterious effects are owing to the production of free radicals and reactive oxygen species (ROS), which have a disposition to donate oxygen to other elements (Lobo et al. 2010). Free radical is depicted as any chemical moiety, which has an unpaired electron in its orbital and thus has the tendency to donate/accept an electron from other molecules (Galano 2015). The most significant oxygen-containing free radicals contributing towards many pathophysiological problems are hydroxyl radical, superoxide anion radical, hydrogen peroxide, nitric oxide radical, and peroxynitrite radical (Birben et al. 2012). These highly reactive and unstable species are capable of doing damages and their most vulnerable targets are biologically important molecules such as DNA, proteins, carbohydrates, and lipids. Apart from physical damages this leads to havoc in the homeostasis of body. Moreover, role of free radicals and oxidative stress has long been linked to the neuronal cell damage that is implicated in various neurodegenerative situations. Taking this into consideration targeting free radicals may embody a promising treatment option to attenuate neurodegeneration and lessen allied symptoms.
Spirulina platensis, a filamentous cyanobacterium and nutritionally enriched algae, owes versatile biological and nutritional profile (Karkos et al. 2010). It has been produced in several countries for its health befitting properties due to its rich armory of proteins and vitamins. Spirulina is a good source of the phycobilliproteins and Phycocyanin is one of them with many beneficial effects being a nutraceutical as well as therapeutic agent (Agrawal et al. 2017). As it has fluorescent properties, it is commercially utilized as fluorescent probe or fluorescent tracer that is beneficial in medical diagnosis and other research fields. Phycocyanin is a nontoxic photosensitizer that can be used as an adjuvant in the photodynamic therapy (PDT) of tumors (Agrawal et al. 2017). Besides this, Phycocyanin is reported to have potent therapeutic regimen in having strong anticancer, antidiabetic, hepatoprotective and neuroprotective effects to list a few and that to be principally due to its strong antioxidant activity (Bertolin et al. 2011). It scavenges free radicals, protects against lipid peroxidation and has been studied for its anti-cancer effect on malignant solid tumors (Jiang et al. 2017). Phycocyanin is documented to protect against diabetic as well as cisplatin-induced nephrotoxicity by inhibiting oxidative stress and activation of antioxidant enzymes (Fernández-Rojas 2014). Protective effects of phycocyanin on ischemia/reperfusion cardiac dysfunctions and liver injuries have also been demonstrated through its potent antioxidant profile along with antiapoptotic activities (Khan et al. 2006; Gdara et al. 2018). Through its combined effects of enhanced antioxidative, neurotrophic, and anti-inflammatory mechanisms, phycocyanin has been known to stimulate oxidized astrocytes to protect and repair the ischemic brain (Min et al. 2015).
Considering these merits, objective of the present study was to investigate the free radical scavenging properties of Phycocyanin of food grade status in vitro and later on to evaluate its effect on oxidative stress induced by streptozotocin in animal model of neurodegenration.
Materials and methods
Chemicals and reagents
Food grade Phycocyanin (Pc) was procured as gift sample from EID Parry Nutraceuticals, India. Ascorbic acid was procured from Sigma Aldrich chemical Co. 2,2-Diphenyl-1-picrylhydrazyl (DPPH), potassium ferricyanide, FeCl3, Nitroblue tetrazolium, 2-Deoxy-d-ribose, trichloroacetic acid (TCA), thiobarbituric acids (TBA) were procured from Himedia laboratories and all other chemicals including, solvents were of analytical grade and obtained from SD fine chemicals Ltd., India.
In vitro antioxidant assay
Several different in vitro methods were employed to ensure the free radical scavenging activities and reducing power of Phycocyanin. Pc was dissolved in water at a stock concentration of 10 mg/ml and serially diluted at required concentration of 5–1000 µg/ml. All the assays were carried out in triplicate, and mean values were considered for final evaluation. IC50 value was calculated by Graphpad prism 5.0 (Graphpad Software Inc, CA, USA).
DPPH (2, 2′-diphenyl-1-picrylhydrazyl) method
DPPH radical-scavenging activity was determined according to the procedure described by Dontha (2016) with some modifications. The assay is based on the principle of bleaching of DPPH violet colour and then its reaction with a hydrogen donor. Briefly, 100 µl of each concentration (50–1000 µg/ml) was added to 400 µL of 100 mM TRIS-HCl buffer (pH 7.4) and 500 µL of 100 µM of DPPH. The reaction mixture was kept for incubation for 20 min in the dark at 37 °C. The absorbance was recorded at 517 nm. Ascorbic acid was used as reference standard which is a water soluble vitamin C analog and known to have powerful oxygen radical inhibition. Percentage scavenging of the DPPH radical was calculated using the below equation.
Ferric ion reducing antioxidant power (FRAP)
FRAP assay is based upon the principle of reducing tri pyridyltriazine complex to the ferrous tri pyridyltriazine, an intensive blue colour complex by a reductant at low pH (Sirawdink Fikreyesus Forsido et al. 2013). Briefly, the FRAP reagent consists of 25 ml acetate buffer, 2.5 ml TPTZ solution (in 40 mM HCl), and 2.5 ml FeCl3.6H2O solution. Each concentration (5–1000 µg/ml) in distilled water was mixed with freshly prepared FRAP reagent and incubated at 37 °C for 5 min. Absorbance of resultant coloured product was recorded at 593 nm. All solutions were freshly prepared on the day of experiment. Results were calculated as ascorbic acid equivalent (µg/ml).
Hydroxyl radical antioxidant capacity (HORAC)
Hydroxyl radical scavenging activity of Pc was analysed by the method described by Dontha (2016). The assay is based on the principle of measuring the quantity of 2-deoxy-d-ribose degradation product, which forms a pink chromogen upon heating with TBA at low pH. Briefly, the reaction mixture for the assay consisted of 0.2 M sodium phosphate buffer (pH 7.4), 10 mM 2-deoxyribose, 10 mM FeSO4-EDTA, 10 mM H2O2 and required concentrations (5–1000 µg/ml) of sample solution. Immediately after addition of H2O2, reaction mixture was kept for incubation of 4 h at 37 °C. The reaction was stopped by adding 2.8% trichloroacetic acid and 1% TBA in 50 mM NaOH, followed by boiling for 10 min and then cooling in water bath. The absorbance of the solution was recorded at 520 nm. Ascorbic acid (50–1000 µg/ml) was used as positive control. The ability to scavenge the hydroxyl radical was calculated using the following equation.
Hydrogen peroxide scavenging activity
The scavenging of hydrogen peroxide radicals by Pc were established according to the method of Nagonda (2013). Required concentrations (5–1000 µg/ml) of the Pc was combined with hydrogen peroxide solution prepared in phosphate buffer at pH 7.4 and absorbance was recorded at 560 nm using UV spectrophotometer against a blank solution containing phosphate buffer, without hydrogen peroxide. Ascorbic acid was used as reference standard. The percentage amount of hydrogen peroxide scavenged by Pc was calculated using the given formula.
Total antioxidant capacity (TAC)
Total antioxidant capacity of Pc was determined by Phosphomolybdenum assay as described by Rahman et al. (2015). Briefly, 100 µl of each dilution (5–1000 µg/ml) was taken in a vial and 900 µl of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) was added to each of them. Reaction was kept for incubation in a water bath at 95 °C for 90 min. After the incubation, samples were brought to room temperature, and the absorbance of the reaction mixture was recorded at 695 nm against a blank. TAC value was calculated by following equation.
Reducing power assay
The reducing power of Pc at all the concentrations (5–1000 µg/ml) was assessed by the method of Rahman et al. (2015). Briefly, 250 µL of (0.2 M) phosphate buffer (pH 6.6) was added to 250 µL of (1%) K4FeCN6 and incubated for 20 min at 50 °C, followed by precipitation with 10% TCA. This supernatant was diluted with equal volume of distilled water. Ferric reducing capacity of Pc was checked by adding 0.10% FeCl3 to this solution. The absorbance was recorded at 700 nm against a reagent blank. The blank was prepared by adding every other solution but without Pc and ferric chloride (0.1%) and the control was prepared by adding all other solution but without Pc. The absorbance measured is a direct reflection of the reducing power of the test samples.
Animals
As a part of current study, thirty two female Wistar albino rats weighing between 200 and 250 g bred in Central Animal House of Panjab University, Chandigarh, India were used. Animals were acclimatized for 2 weeks before the initiation of experiment. They were maintained in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals, Government of India, on animal experimentation. The experiment was approved by the University Animal Ethics Committee. We selected the doses of phycocyanin (50 mg/kg and 100 mg/kg) to study based on previous literature and both of the doses exerted no toxic effects in rats (Romay et al. 1998; Salama et al. 2016). Twenty-four hours after the last treatment, animals were sacrificed to harvest brain. Rivastigmine (RIV) was used as reference standard. Rats were randomly divided into five groups. Group 1 served as the sham (n = 6), Group 2 served as diseased i.e. Intracerebroventricular-Streptozotocin (ICV-STZ) induced (n = 8), Group 3 received ICV-STZ + 2mg/kg (i.p.) of RIV, Group 4 received ICV-STZ + 50 mg/kg (i.p.) of the Pc (n = 6), and Group 5 received 100 mg/kg (i.p.) of the Pc (n = 6).
Surgical procedures: Intracerebroventricular (ICV) injection of Streptozotocin (STZ)
ICV injection of STZ was given according to the procedure of Mehala et al. (2013). Rats were anesthetized with Ketamine (60–100 mg/kg BW)/Xylazine (5–13 mg/kg BW), (i.p.). The scalp was shaved, cleaned and cut to expose the skull. The head was positioned in a stereotaxic frame and a midline sagittal incision was made in the scalp. Burr holes were drilled in the skull on both sides over the lateral ventricles by using the following coordinates: 0.8 mm posterior to bregma; 1.5 mm lateral to sagittal suture and 3.6 mm beneath the surface of the brain (Kumar et al. 2017). STZ (3 mg/kg, ICV) was injected bilaterally in two divided doses on first and third day making the dose of 1.5 mg/kg each day. The concentration of STZ in artificial cerebrospinal fluid (ACSF) was adjusted so as to deliver 6 μl of the solution. Sham animals received ICV injection of the same volume of ACSF on the first and third day. The skin was sutured after second injection followed by daily application of antiseptic powder. Postoperatively, the rats were fed with oral glucose and normal pellet diet for 4 days, followed by normal pellet diet alone. The animals were dosed for 28 days and were observed daily for changes and other signs of toxicity and death throughout the period of study. To measure plasma nitrite level, before sacrificing, blood was taken retro orbitally.
In vivo antioxidant activity
Determination of Lipid peroxidation levels
The extent of lipid peroxidation was determined quantitatively in the form of thiobarbituric acid-reactive substances by the method described by Wills (1965). Briefly, 0.5 ml of Tris–HCl was added to 0.5 ml of supernatant and the mixture was incubated at 37 °C for 2 h. After incubation, 1 ml of 10% ice-cold trichloroacetic acid was added to it and mixture was centrifuged at 1000 g for 10 min. Then, 1 ml of 0.67% thiobarbituric acid was added to the tubes containing 1 ml of supernatant and the tubes were kept in boiling water for 10 min. After cooling, 1 ml of double distilled water was added and absorbance was measured at 532 nm (Perkin Elmer UV/VIS Spectrophotometer, Lamda 20). The amount of malondialdehyde (MDA) was calculated using molar extinction coefficient of 1.56 x 105 M-1 cm-1 and expressed as nanomoles (nmol) of malondialdehyde equivalents per mg protein (mgpr).
Plasma nitrite estimation
Total nitrite was estimated using the Greiss reagent which served as an indicator of nitric oxide (NO) production. The nitric oxide spontaneously oxidizes to nitrite and nitrate. A measure of 500 µL of Griess reagent (1:1 solution of 1% sulphanilamide in 5% phosphoric acid and 0.1% Naphthyl ethylene diamine dihydrochloride in water) was added to the 100 µL of plasma and 400 µL of distilled water was added to it. The mixture was kept at room temperature for 5 min. Absorbance was taken at 546 nm. Sodium nitrite (5.0 µmol/L) was taken as standard. Nitrite concentration was calculated using a standard curve for sodium nitrite. Nitrite levels were expressed as percentage of control and expressed as µg/ml (Green et al. 1982).
Determination of superoxide dismutase (SOD) activity
Superoxide dismutase is an important antioxidant defense in cells exposed to oxygen. It converts superoxide (O2−) radical into molecular oxygen (O2)/hydrogen peroxide (H2O2). Superoxide dismutase activity was assayed according to the method of Kono (1978). The assay system consisted of 96 mM of nitro blue tetrazolium (NBT) was dissolved in Solution A [50 mM sodium carbonate in 0.1 mM EDTA (pH 10.8)]. In the cuvette, 2 ml of NBT solution along with 0.1 ml of brain homogenate and 0.5 ml of hydroxylamine hydrochloride (adjusted to pH 6.0 with NaOH) was added as reaction mixture. The auto-oxidation of hydroxylamine was observed by measuring the change in optical density at 560 nm for 2 min at 30/60s intervals. SOD activity was calculated in terms of units per mg protein (Kono 1978).
Determination of reduced glutathione activity
Glutathione (GSH) is an antioxidant that prevents cellular damage associated with reactive oxygen species (ROS). Reduced glutathione in the brain was estimated using Ellman et al. (1961). Briefly, equal volumes of homogenate and 5% sulphosalicylic acid were mixed and kept for cold digestion at 4 °C for 1 h. The supernatant was separated by centrifugation at 4500 g for 10 min at 4 °C. 50 μl of the clear supernatant was mixed with 450 μL of 0.01 M 5, 5-dithiobis-(2-nitrobenzoic acid) (DTNB) (0.4% in 0.1 M phosphate buffer, (pH 7.4)). Reaction mixture was incubated for 10 min at 37 °C and colour developed was measured at 412 nm. Results were expressed as µM GSH per mg protein.
Result and discussion
In the present study, food grade Pc was evaluated against a panel of in vitro and in vivo anti oxidant assays. Previous studies suggested that the tetra chromophore in the Phycobiliprotein accounts for the blue color as well as powerful antioxidant activity of Pc. Several other studies have claimed that the antioxidant activity is related not only to the tetra chromophore but also to the protein backbone (Garcia et al. 2016). However no study was found where a series of in vitro tests along with in vivo assays on ICV-STZ rat model was used to evaluate its antioxidant efficacy.
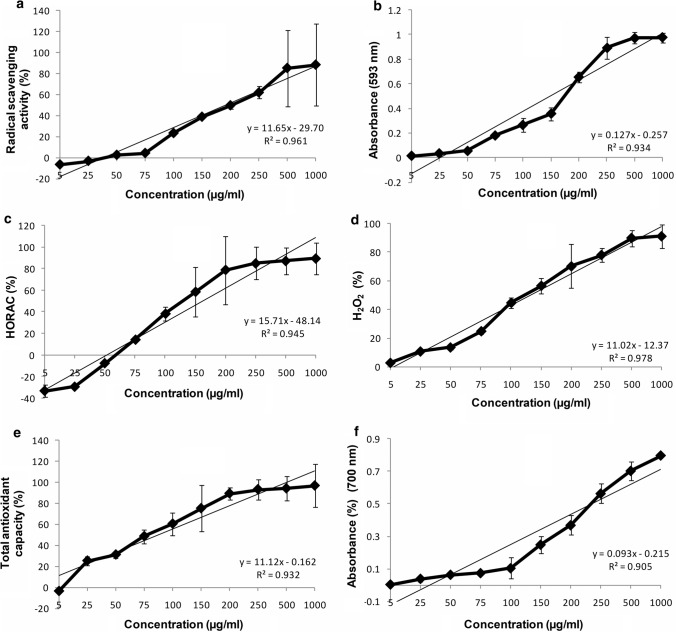
DPPH radical is commonly used as a marker in evaluating the hydrogen donating capacity and thus the anti oxidant activity of any molecule (Cerretani and Bendini 2010). DPPH radical scavenging activity is visually evident as the purple colour of the solution changed into yellow on scavenging of radicals. This change in colour resulted in decrease of absorbance and is inversely proportional to the extent of scavenging. In this assay, aqueous solution of Pc at different concentrations (5–1000 µg/ml) was evaluated for its free radical scavenging activity compared with ascorbic acid as standard. It was observed that the radical scavenging activity of Pc increased with increasing concentrations respectively (R2 = 0.961, p <0.01). The IC50 value for Pc was 158.3 µg/ml which was slightly higher as compared to the IC50 (112.9 µg/ml) of ascorbic acid. These results depicted that aqueous solution of food grade Pc displayed the ability to quench the DPPH radical which further pointed out that solution was antioxidant with radical scavenging activity (Fig 1a). The FRAP assay is also regarded as an imperative marker of the antioxidant potential of any test sample. It bestowed a direct inference of the antioxidants or redunctants available in the sample. In FRAP assay reduction of ferric (Fe3+)/(Fe2+) ferrous couple takes place. In this assay, blue-colored ferrous tripyridyltriazine complex at acidic pH were monitored spectrophotometrically at 593 nm. The activity was concentration dependant, as the aqueous solution of Pc displayed the highest antioxidant capacity at the highest concentration (R2 = 0.934, p <0.05) (Fig 1b). The IC50 value for Pc and ascorbic acid were 152.7 µg/ml and 91.47 µg/ml respectively. Hydroxyl radical is a strong oxidizing molecule having the potency to react unselectively and immediately. It targets proteins, DNA, PUFA in membranes, and most of the biological molecule in its adjacent area and aids to peroxidic reaction of lipids (Phaniendra et al. 2014). Pc displayed concentration dependent scavenging activity against hydroxyl radical generated in the Fenton reaction system (R2 = 0.945, p <0.01) (Fig 1c). The IC50 value for Pc and ascorbic acid were 88.67 µg/ml and 57.78 µg/ml respectively. Pc was capable of scavenging hydrogen peroxide in a concentration-dependent manner (R2 = 0.985, p <0.05) (Fig 1d). The radical scavenging activity of Pc increased with increase in concentrations and the IC50 value for Pc and ascorbic acid were 110.9 µg/ml and 44.63 µg/ml respectively. Total antioxidant capacity (TAC) of Pc was determined using phosphomolybdate assay. The assay is based on the reduction of Mo (VI) to Mo (V) forming a green phosphomolybdate (V) complex, which can be estimated spectrophotometrically at 695 nm (Ahmed et al. 2015). The results indicated a concentration dependent total antioxidant capacity (R2 = 0.932, p <0.001) (Fig 1e). The IC50 value for Pc and ascorbic acid were 164.78 µg/ml and 26.76 µg/ml respectively. These results indicate that aqueous solution of Pc exhibited the effective in vitro antioxidant activity. Generally the reducing ability of any compound is demonstrated by the presence of reductants that break the free radical chain by donating a hydrogen atom and depict their antioxidant potential (Adebiyi et al. 2017). In line with some previous study by Mahfooz et al. (2017), here too, Pc caused the reduction of the Fe3+/ferricyanide complex to the ferrous form and hence the Fe2+ can be observed by analyzing the formation of Perl's Prussian blue at 700 nm. Also the reducing power of Pc was increased with increase in quantity of sample (R2 = 0.905, p <0.001) (Fig 1f). The IC50 value for Pc was 198.9 µg/ml, which was comparable to the value of ascorbic acid i.e. 176.5 µg/ml.
Fig. 1.
a Percent free radical inhibition of Pc as per DPPH assay; b Ferric reducing antioxidant power of Pc expressed as absorbance at 593 nm; c Percent free radical inhibition of Pc as per Hydroxyl radical antioxidant assay; d Percent free radical inhibition of Pc as per Hydrogen per oxide antioxidant assay; e Total antioxidant capacity (TAC) of Pc; f Reducing power assay of Pc expressed as absorbance at 700 nm. Values are given as mean ± S. D. (n = 3)
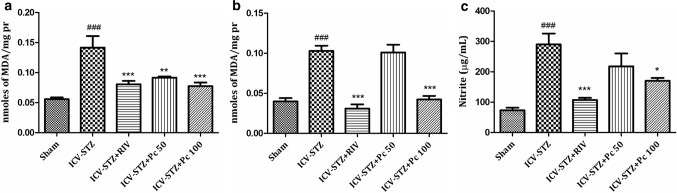
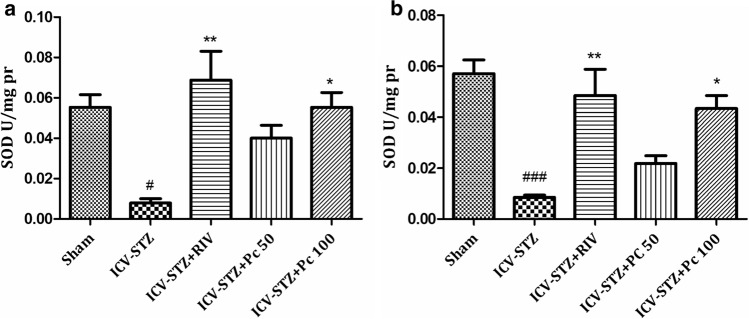
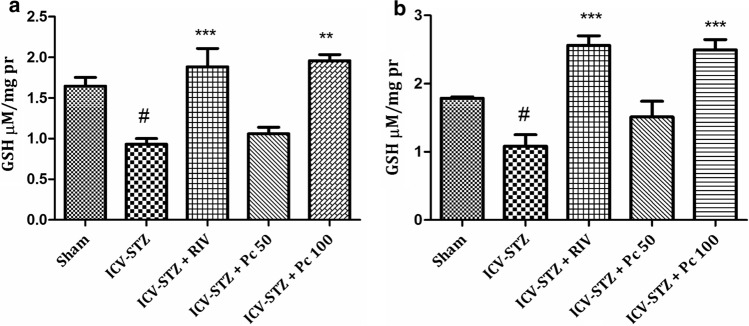
Disparity in reactive oxygen species (ROS) and antioxidative protection can lead to oxidative stress and has been established to play a decisive role in pathogenesis of several neurodegenerative diseases (Birben et al. 2012). Despaired antioxidative systems comprising antioxidant enzymes and antioxidants like super oxide dismutase and reduced glutathione respectively encourage the augmented free radical generation. Owing to the presence of unpaired electron in the outer orbit, these free radicals are highly unstable and have greater affinity towards macromolecules such as proteins, sugars, lipids and nucleic acids, leading to the alterations in structural and functional integrity of brain cells (Lobo et al. 2010), eventually leading to destruction, abnormal functioning and cell death. The laboratory rats as a disease model, has provided hugely to neuroscience research all over the years and wistar rats has been a popular and potent animal model in this field (Benedikz et al. 2009). Further, as ICV-STZ administration in rats has been well established to induce oxidative stress and increased cognitive dysfunction thereafter (Perumal et al. 2018); we assessed several oxidative stress markers in rat’s brain pre and post treatment. Lipid peroxidation (LPO) is the oxidative destruction of lipid molecule and is considered as a critical step in the pathogenesis of various diseases. Nitrosative stress, via increased production of nitric oxide and peroxynitrite, further destructs protein structure (Dalle-Donne et al. 2005). Thus oxidative damage to lipids (lipid peroxidation) and proteins initially results in a change in cell structure, enzyamatic inactivation, followed by loss of normal physiological cell function and eventually neuronal cell death (Barrere 2012). Increased malondialdehyde (MDA) levels depict the enhanced lipid peroxidative damage in the brain (Ayala et al. 2014). In the present study MDA levels were significantly increased in both cortex (p <0.001) (Fig 2a) and hippocampus (p <0.001) (Fig 2b) in the ICV-STZ group as compared to sham. Treatment with Pc significantly lowered the MDA levels in cortex (p <0.01) at both the concentrations, whereas in hippocampus significant activity (p <0.01) was observed only at higher concentration of 100 mg/kg. Further, rapid oxidation of nitric oxide converts it into nitrite which is responsible for nitrosative stress in brain. Nitrite levels were also significantly increased (p <0.001) in plasma of ICV-STZ treated rats as compared to sham group. Results indicated that chronic treatment with Pc (50, 100 mg/kg) produced reduction in nitrite levels, but could not achieve statistical significance, when compared to ICV-STZ animals (Fig 2c). The super oxide dismutase (SODs) is a group of universal enzymes that constitutes first line of defense against ROS (Wang et al. 2018). They catalyze the conversion of superoxide radical into hydrogen peroxide (H2O2). Also, reduced glutathione, a prevalent tripeptide thiol, is an important intracellular and extracellular protective antioxidant (Lushchak 2012). In the present study STZ significantly reduced the levels of SOD both in cortex (p <0.001) and hippocampus (p <0.001) of ICV-STZ group (Fig 3), whereas treatment with Pc significantly elevated the levels in cortex at both the concentrations, where effect was more pronounced at higher concentration (p <0.001). However, Pc demonstrated significant increase only at 100 mg/kg (p <0.01) in hippocampus. Further, significant decrease in glutathione levels was also observed in ICV-STZ animals compared to sham animals in cortex (p < 0.01), and hippocampus (p <0.05). Treatment with Pc displayed increase in the enzymatic activities of GSH significantly in both cortex (p <0.001) and hippocampus (p <0.001) at 100 mg/kg concentration (Fig 4).
Fig. 2.
Effect of treatment with Pc (50, 100 mg/kg) on lipid peroxides levels in (a) cerebral cortex; ###p <0.001—sham versus ICV-STZ, ***p <0.001–ICV-STZ versus RIV, **p <0.01—ICV-STZ versus Pc 50, ***p <0.001—ICV-STZ versus Pc 100, (b) hippocampus; ###p <0.001—sham versus ICV-STZ, ***p <0.001—ICV-STZ versus RIV, ***p <0.001—ICV-STZ versus Pc 100 and (c) nitrite levels in plasma of ICV-STZ administered rats; ###p <0.001—sham versus ICV-STZ, ***p <0.001—ICV-STZ versus RIV, *p <0.05—ICV-STZ versus Pc 100. Values were expressed as mean ± SEM. The intergroup variation was measured by one way ANOVA followed by Tukey’s test using Graphpad prism 5.0 (Graphpad Software Inc, CA, USA)
Fig. 3.
Effect of treatment with Pc (50, 100 mg/kg) on superoxide dismutase (SOD) levels in (a) cerebral cortex; #p <0.05—sham versus ICV-STZ, **p <0.01—ICV-STZ versus RIV, *p <0.05—ICV-STZ versus Pc 100 and (b) hippocampus of ICV-STZ administered rats; ###p <0.001—sham versus ICV-STZ, **p <0.01—ICV-STZ versus RIV, *p <0.05—ICV-STZ versus Pc 100. Values were expressed as mean ± SEM. The intergroup variation was measured by one way ANOVA followed by Tukey’s test using Graphpad prism 5.0 (Graphpad Software Inc, CA, USA)
Fig. 4.
Effect of treatment with Pc (50, 100 mg/kg) on glutathione levels in (a) cerebral cortex; #p <0.05—sham versus ICV-STZ, ***p <0.001—ICV-STZ versus RIV, **p <0.01—ICV-STZ versus Pc 100 and (b) hippocampus of ICV-STZ administered rats; #p <0.05—sham versus ICV-STZ, ***p <0.001—ICV-STZ versus RIV,***p <0.001—ICV-STZ versus Pc 100. Values were expressed as mean ± SEM. The intergroup variation was measured by one way ANOVA followed by Tukey’s test using Graphpad prism 5.0 (Graphpad Software Inc, CA, USA)
In line with the previous reports our results indicated strong antioxidant profile of Pc, as depicted by several in vitro assays performed in the study. Further, in case of animal model, ICV-STZ administration has led to significant increase in cortical and hippocampal oxidative stress. Treatment with Pc has demonstrated the alleviation of the STZ-induced lipid peroxidation and nitrite levels. Furthermore, it also helped in restoration of antioxidant enzymatic armory like reduced glutathione and superoxide dismutase levels.
Conclusion
Present study has displayed that the food grade Phycocyanin possesses the significant antioxidant activity both in vitro and in vivo. This antioxidant potential may be attributed to the presence of amino acids in it, along with its chromophore part. Amino acid often referred to as synergetic oxidant agent due to their capability of chelation of pro-oxidative metal traces and regeneration of intracellular antioxidative system and Phycocyanin has a rich amount of them in it. Study suggested that Phycocyanin is a promising source of natural antioxidants that could have great importance as nutritional as well as therapeutic supplement in preventing or slowing the progress of ageing and age associated oxidative stress related degenerative disorders.
Acknowledgement
Acknowledgements are due to Council of Scientific and Industrial Research (CSIR), New Delhi for Research Associateship.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adebiyi OE, Funsho O, Olayemi FO, Ning-Hua T, Guang-Zhi Z. In vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. J Basic Appl Sci. 2017;6(1):10–14. [Google Scholar]
- Agrawal M, Yadav SK, Agrawal SK, Karmakar S. Nutraceutical phycocyanin nanoformulation for efficient drug delivery of paclitaxel in human glioblastoma U87MG cell line. J Nanoparticle Res. 2017;19:272. doi: 10.1007/s11051-017-3972-x. [DOI] [Google Scholar]
- Ahmed D, Khan MM, Saeed R. Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic and aqueous extracts from Adiantum caudatum leaves. Antioxidants. 2015;4:394–409. doi: 10.3390/antiox4020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A, Mario F, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedikz E, Kloskowska E, Winblad B. The rat as an animal model of Alzheimer’s disease. J Cell Mol Med. 2009;13(6):1034–1042. doi: 10.1111/j.1582-4934.2009.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolin TE, Farias D, Guarienti CP, Fernanda TS, Colla LM, Costa JAV. Antioxidant effect of phycocyanin on oxidative stress induced with monosodium glutamate in rats. Brazilian Arch Biol Technol. 2011;54(4):733–737. doi: 10.1590/S1516-89132011000400012. [DOI] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretani L, Bendini A. Rapid assays to evaluate the antioxidant capacity of phenols in virgin olive oil. In: Preedy PR, Watson RR, editors. Olives and olive oil in health and disease prevention. USA: Academic Press; 2010. pp. 625–635. [Google Scholar]
- Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redoxproteomics. Mass Spectrom Rev. 2005;24(1):55–99. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- Dontha S. A review on antioxidant methods. Asian J Pharm Clin Res. 2016;9(2):14–32. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fernández-Rojas B, Omar Noel Medina-Campos ON, Hernández-Pando R, Negrette-Guzmán M, Huerta-Yepez S, Pedraza-Chaverri J. C-Phycocyanin prevents cisplatin-induced nephrotoxicity through inhibition of oxidative stress. Food Func. 2014;5:480–490. doi: 10.1039/C3FO60501A. [DOI] [PubMed] [Google Scholar]
- Galano A. Free radicals induced oxidative stress at a molecular level: the current status, challenges and perspectives of computational chemistry based protocols. J Mex Chem Soc. 2015;59(4):231–262. [Google Scholar]
- García IIS, Jaritz NBM, Ramírez RO. Antioxidant effect of phycobiliproteins of the cyanobacteria Arthrospira maxima on growth of Saccharomyces cerevisiae under oxidative stress. Int J Curr Microbiol Appl Sci. 2016;5(10):233–239. doi: 10.20546/ijcmas.2016.510.025. [DOI] [Google Scholar]
- Gdara NB, Belgacem A, Khemiri I, Mannai S, Bitri L. Protective effects of phycocyanin on ischemia/reperfusion liver injuries. Biomed Pharmacother. 2018;102:196–202. doi: 10.1016/j.biopha.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaumm SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Jiang L, Wang Y, Yin Q, Liu G, Liu H, Huang Y, Li B. Phycocyanin: a potential drug for cancer treatment. J Cancer. 2017;8(17):3416–3429. doi: 10.7150/jca.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkos PD, Leong SC, Karkos CD, Sivaji N, Assimakopoulos DA. Spirulina in clinical practice: Evidence-based human applications. Evid Based Complement Alternat Med. 2010;2011:1–4. doi: 10.1093/ecam/nen058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Varadharaj S, Ganesan LP, Shobha JC, Naidu MU, Parinandi NL, Tridandapani S, Kutala VK, Kuppusamy P. C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am J Physiol Heart Circ Physiol. 2006;290(5):H2136–45. doi: 10.1152/ajpheart.01072.2005. [DOI] [PubMed] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophy. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- Kumar M, Kaur D, Bansal N. Caffeic acid phenethyl ester (cape) prevents development of stz-icv induced dementia in rats. Pharmacog Mag. 2017;13(Suppl 1):S10–S15. doi: 10.4103/0973-1296.203974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacog Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:1–26. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfooz S, Bano S, Shamim A, Husain A, Farooqui A. Partial purification, characterization and bioactive potential of c-phycocyanin from Cyanobacterium plectonema boryanum. Biochem Cell Biol. 2017;17(1):57–64. [Google Scholar]
- Mehla J, Pahuja M, Gupta YK. Streptozotocin-induced sporadic Alzheimer's disease: selection of appropriate dose. J Alzheimers Dis. 2013;33(1):17–21. doi: 10.3233/JAD-2012-120958. [DOI] [PubMed] [Google Scholar]
- Min S, Park J, Luo L, Kwon YS, Lee HC, Shim HJ, Kim ID, Lee JK, Shin HS. Assessment of C-phycocyanin effect on astrocytes-mediated neuroprotection against oxidative brain injury using 2D and 3D astrocyte tissue model. Sci Rep. 2015;5:14418. doi: 10.1038/srep14418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngonda F. In- vitro anti-oxidant activity and free radical scavenging potential of roots of Malawian Trichodesma zeylanicumm (burm. F.) Asian J Biomed Pharm Sci. 2013;3(20):21–25. [Google Scholar]
- Perumal Y, Ray RS, Chopra K. Vitamin D3 attenuates cognitive deficits and neuroinflammatory responses in ICV-STZ induced sporadic Alzheimer's disease. Inflammopharmacol. 2018;26(11):39–55. doi: 10.1007/s10787-017-0372-x. [DOI] [PubMed] [Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clinical Biochem. 2014;30(1):11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Islam MB, Biswas M, Khurshid Alam AMH. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015;8:621. doi: 10.1186/s13104-015-1618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romay C, Ledon N, Gonzalez R. Further studies on anti-inflammatory activity of phycocyanin in some animal models of inflammation. Inflamm res. 1998;47:334–338. doi: 10.1007/s000110050338. [DOI] [PubMed] [Google Scholar]
- Salama ASA, Osman AOM, Abdel Ghany AA, Sitohy MZ. Hematological and histopathological evaluation of anabaena oryzae sos13 phycocyanin in wistar albino rats. Zagazig J Agric Res. 2016;43(3):1–14. [Google Scholar]
- Sirawdink Fikreyesus Forsido HP, Rupasinghe V, Astatkie T. Antioxidant capacity, total phenolics and nutritional content in selected ethiopian staple food ingredients. Int J Food Sci Nutr. 2013;64(8):915–920. doi: 10.3109/09637486.2013.806448. [DOI] [PubMed] [Google Scholar]
- Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol. 2018;8(17):3416–3429. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]