Highlights
-
•
Boys had higher impulsivity scores than girls with externalizing disorders.
-
•
Boys showed greater ventromedial prefrontal-ventral striatal connectivity than girls.
-
•
Boys showed weaker medial-lateral prefrontal (mPFC-lPFC) connectivity than girls.
-
•
The mPFC-lPFC connectivity may represent a male-specific biomarker of impulsivity.
Keywords: Externalizing disorders, Impulsivity, Adolescence, Sex Differences, Resting-state Functional Connectivity, Frontal-striatal Connectivity
Abstract
Background
Sex-specific neurobiological underpinnings of impulsivity in youth with externalizing disorders have not been well studied. The only report of functional connectivity (FC) findings in this area demonstrated sex differences in fronto-subcortical connectivity in youth with attention-deficit/hyperactivity disorder (ADHD).
Methods
The current study used functional magnetic resonance imaging (fMRI) to examine sex differences in resting-state seed-based FC, self-rated impulsivity, and their interactions in 11-12-year-old boys (n = 43) and girls (n = 43) with externalizing disorders. Generalized linear models controlling for pubertal development were used. Seeds were chosen in the ventral striatum, medial prefrontal cortex, middle frontal gyrus and amygdala.
Results
Impulsivity scores were greater in boys than girls (p < 0.05). Boys showed greater positive connectivity within a ventromedial prefrontal-ventral striatal network. In addition, boys demonstrated weaker connectivity than girls within two medial–lateral prefrontal cortical networks. However, only boys showed greater medial–lateral prefrontal connectivity correlated with greater impulsivity.
Conclusions
The findings provide evidence supporting sex differences in both ventral striatal-ventromedial prefrontal and medial–lateral prefrontal functional networks in youth with externalizing disorders. These important networks are thought to be implicated in impulse control. Medial-lateral prefrontal connectivity may represent a male-specific biomarker of impulsivity.
1. Introduction
Sex differences in impulsivity are well established, although underlying neurobiological mechanisms, particularly in adolescents, are less clear. Numerous studies have demonstrated sex differences in impulsivity across the age-span, though the direction of the differences is mixed, depending on the assessment modality being used. For instance, in self-report assessment studies, higher sensation seeking and lower impulse control have been found in boys (Dekkers et al., 2019, Jensen et al., 2011, Martin et al., 2002), with only a few exceptions (Gökçe et al., 2017). With studies involving self-reported impulsivity subtypes as measured by the UPPS impulsive behavior scale (Whiteside and Lynam, 2001), sensation seeking, positive urgency and lack of perseverance were higher in adult males relative to females (Cyders, 2013, Navas et al., 2019). Only one study examined sex-specific impulsivity subtypes in youth, with boys reporting greater sensation seeking and girls greater negative urgency (d'Acremont and Linden, 2005).
Using tasks which measure impulsive action in the laboratory, boys displayed poorer performance on go/no-go (Liu et al., 2013, O'Brien et al., 2010), continuous performance (Hasson and Fine, 2012) and probabilistic balloon analogue risk (Cross et al., 2011) tasks, whereas girls displayed poorer performance on stop signal (Crosbie et al., 2013) and delay discounting tasks (Patros et al., 2018, Rosch and Mostofsky, 2016). Task-based functional magnetic resonance imaging (fMRI) studies have demonstrated sex differences in corticolimbic activation during performance of decision making/inhibition tasks in youth/adolescents. Specifically, adolescent boys (ages 13–17; n = 10) showed greater neuronal activation than adolescent girls (n = 9) in orbitofrontal cortex on a go/no-go task. Female adolescents (ages 13–17; n = 9) showed greater activation in medial prefrontal cortex (mPFC) in response to threats than female children/adults (ages 6–12/>18; n = 8/10), while adolescent males (ages 13–17; n = 10) did not significantly differ from children or adults (ages 6–12/>18; n = 10/10) in this region (Dreyfuss et al., 2014). During the performance of reward-related decision making tasks, adolescent boys (ages 13–17; n = 724) showed greater activation in anterior cingulate cortex (ACC), putamen, precuneus, middle temporal gyrus, and cerebellum than girls (n = 805) (Barkley-Levenson et al., 2013, Cao et al., 2019). Of note, preadolescent boys (ages 11–12; n = 33) with externalizing disorders had greater activation in the middle frontal gyrus (MFG) than girls (n = 13) when making safer choices during the probabilistic balloon analogue risk task (Dir et al., 2019). Overall, the direction of the sex differences on impulsivity relevant tasks depends, in part, on tasks administrated (Weafer and de Wit, 2014), given that impulsivity is a multidimensional construct (Cross et al., 2011, Whelan et al., 2012).
To date, only one functional connectivity (FC) neuroimaging study has examined sex differences in youth with externalizing/impulse control disorders (Rosch et al., 2018). This study found that resting-state FC within the fronto-subcortical circuitry differed between 8–12-year-old girls (n = 20) and boys (n = 52) with attention-deficit/hyperactivity disorder (ADHD) compared to same-sex typically developing controls (n = 75; 21 girls); FC was also correlated with delay discounting behavior. Stronger positive ventromedial prefrontal cortex (vmPFC)-striatal connectivity and weaker negative vmPFC-amygdala connectivity were found in children with ADHD. Girls, but not boys, with ADHD showed stronger positive and negative striatal connectivity with ACC and dorsolateral prefontal cortex (dlPFC) respectively. Real-time discounting was differentially related to dlPFC-amygdala connectivity and ACC-amygdala connectivity among girls and boys with and without ADHD. In studies with typically developing youth (Alarcón et al., 2015, Satterthwaite et al., 2015), sex differences in FC suggest that females experience greater connectivity between frontal, limbic and striatal regions, compared to boys.
The present study used resting-state fMRI to characterize resting-state FC differences and associations with self-reported impulsivity between boys and girls with externalizing disorders. We hypothesized that boys would rate themselves as more impulsive than girls (Dekkers et al., 2019, Navas et al., 2019, Shulman et al., 2015). Based on research reviewed above, we focused on four seed regions (i.e., ventral striatum, amygdala, medial prefrontal cortex (mPFC), and middle frontal gyrus). We hypothesized that relative to boys, girls would show stronger ventral striatal connectivity with frontal regions including mPFC and ACC, frontal connectivity with subcortical regions including striatum and amygdala, and amygdala connectivity with frontal regions, based on extrapolations from previous neuroimaging studies (Costa Dias et al., 2013, Diekhof and Gruber, 2010, Diekhof et al., 2012, Ma et al., 2016, Rosch et al., 2018).
2. Methods and materials
2.1. Participants
Eighty-six 11-12-year-old drug-naive youth (n = 43 girls) with externalizing psychopathology participated in the present study. Drug-naïve youth in the current study refers to those reporting not having used drugs of abuse. “Externalizing” is operationalized here as DSM-5 diagnoses of attention-deficit/hyperactivity disorder (ADHD) plus either oppositional defiant disorder (ODD), conduct disorder (CD), or unspecified disruptive behavior disorder. None of the participants were excluded due to gross in-scanner motion (mean relative root mean squared displacement > 0.2 mm) (Ciric et al., 2017). Demographics such as age, IQ, race, and parental education, in addition to lifetime psychotropic medication use, did not differ between boys and girls (Table 1). However, tanner staging differed between sexes, such that girls had more advanced pubertal development (Table 1). Additionally, all subjects identified their gender according to their biological sex.
Table 1.
Demographics, UPPS-P-C (child report), Trauma History, and Disorders.
| Boys (N = 43) | Girls (N = 43) | t | p | 95% CI | χ2 | p | |
|---|---|---|---|---|---|---|---|
| Age (M/SD) | 11.89 (0.48) | 11.97 (0.53) | -0.756 | 0.452 | -0.29–0.13 | ||
| IQ (M/SD) | 103.6 (12.53) | 101.9 (15.04) | 0.601 | 0.549 | −4.07–7.61 | ||
| Race (n/%) | |||||||
| Caucasian | 26 (60.5%) | 23 (53.5%) | – | – | – | 0.427 | 0.514 |
| African American | 10 (23.3%) | 16 (37.2%) | – | – | – | 1.985 | 0.159 |
| More than one race | 7 (16.3%) | 4 (9.3%) | – | – | – | 0.938 | 0.333 |
| Tanner Stage (M/SD) | 1.63 (0.81) | 3.17 (1.41) | −6.16 | 0.000 | −2.04 - (-1.04) | ||
| Parent’s education (M/SD) | 4.44 (2.74) | 5.19 (2.64) | −1.27 | 0.204 | −1.90 - 0.41 | ||
| Lifetime Psychotropic | |||||||
| Medication Use (n/%) | |||||||
| Yes | 21 (48.8%) | 19 (44.2%) | – | – | – | 0.187 | 0.665 |
| No | 22 (51.2%) | 24 (55.8%) | |||||
| UPPS-P-C Total Score (M/SD) | 101.93 (14.81) | 92.58 (16.05) | 2.80 | 0.006 | 2.72–15.97 | ||
| Lack of Premeditation (M/SD) | 18.16 (4.88) | 16.32 (3.76) | 1.95 | 0.054 | -0.03–3.70 | ||
| Negative Urgency (M/SD) | 21.04 (5.47) | 20.67 (5.47) | 0.31 | 0.75 | −1.97–2.71 | ||
| Sensation Seeking (M/SD) | 24.32 (4.42) | 21.04 (5.21) | 3.14 | 0.002 | 1.20–5.35 | ||
| Lack of Perseverance (M/SD) | 16.76 (3.12) | 15.23 (2.61) | 2.47 | 0.016 | -0.29–2.77 | ||
| Positive Urgency (M/SD) | 21.62 (5.66) | 19.30 (6.18) | 1.81 | 0.073 | -0.21–4.86 | ||
| CTQ Lifetime Number of Traumatic Events–Parent Report (M/SD) | 2.14 (1.35) | 2.07 (1.26) | 0.24 | 0.805 | -0.49–0.63 | ||
| CTQ Severity of Trauma–Parent Report (M/SD) | 9.79 (8.41) | 10.07 (7.34) | -0.16 | 0.870 | −3.66–3.10 | ||
| Parental Substance Use Disorder (n/%) | 29 (67.4%) | 23 (53.5%) | – | – | – | 1.751 | 0.186 |
| ADHD (n/%) | |||||||
| Inattentive | 13 (30.2%) | 26 (60.5%) | – | – | – | 7.929 | 0.005 |
| Hyperactive-Impulsive | 2 (4.7%) | 4 (9.3%) | – | – | – | 0.717 | 0.397 |
| Combined | 28 (65.1%) | 13 (30.2%) | – | – | – | 10.488 | 0.001 |
| Oppositional Defiant Disorder (ODD) (n/%) | 33 (76.7%) | 31 (72%) | – | – | – | 1.277 | 0.735 |
| Conduct Disorder (n/%) | 4 (9.3%) | 4 (9.3%) | – | – | – | 1.144 | 0.564 |
| Unspecified Disruptive Behavior (n/%) | 6 (14%) | 9 (21%) | – | – | – | 3.403 | 0.182 |
| Past Mood Disorders (n/%) | 5 (11.6%) | 10 (23.3%) | – | – | – | 2.184 | 0.139 |
| Anxiety Disorders (n/%) | 16 (37.2%) | 22 (51.2%) | – | -. | – | 2.025 | 0.155 |
CI: confidence interval; M/SD: Mean/Standard Deviation; CTQ: Childhood Traumatic Questionnaire; ADHD: Attention-Deficit/Hyperactivity Disorder. Bold fonts indicate differences were significant between sexes.
Exclusion criteria included: lifetime history of bipolar disorder; psychotic symptoms; autism spectrum disorders; substance use disorders (SUD); current major depressive disorder; current psychopharmacologic treatment (none within 2 weeks) other than psychostimulants (held the days of assessment and scanning (e.g., Dir et al., 2019, Hove et al., 2015, Martel et al., 2007, Swanson et al., 2011, Valera et al., 2005)); history of neurological problems; IQ < 80; active or debilitating medical conditions; an active maternal SUD during pregnancy; claustrophobia; pregnancy; MRI contraindications; use of recreational or prescription drugs; alcohol or nicotine (other than caffeine); left handedness; and any individuals who have siblings already enrolled and participating in the study.
2.2. Behavioral assessment
The Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS-PL) (Kaufman et al., 1997) modified for DSM-5, Childhood Traumatic Questionnaire (CTQ) (Hastings and Kelley, 1997), working memory tasks and Wechsler abbreviated scale of intelligence (WASI) (Axelrod, 2002) were administered. Regarding the details of the administration of the full K-SADS (screening plus triggered supplements), our master’s and doctoral level child mental health clinicians administrated a full psychiatric interview with each participant and parent (reporting on their child’s symptoms) to assess psychiatric and substance use disorders. Teacher report forms were requested to corroborate clinician impressions. Clinician ratings were reviewed by a consensus clinical team to confirm each administered K-SADS.
For the assessment of impulsivity, each child was asked at baseline to complete the UPPS-P-C (Zapolski et al., 2010), a modification of the UPPS-P Impulsive Behavior Scale (Lynam et al., 2006), which is a 40 item Likert scale self-report questionnaire written at a 4th grade reading level that assesses severity and distinguishes five facets of impulsivity: lack of premeditation, lack of perseverance, positive and negative urgency and sensation-seeking. Independent samples t-tests were performed to examine sex differences in the five facets of impulsivity.
2.3. Imaging data acquisition and analysis
Each subject completed a mock-scanning session (MoTrak software, PST) with real-time feedback and an incentive game for eliminating head motion. Any stimulant medications were held the morning of the scan, as is routine in ADHD imaging studies. All scans were performed on a research dedicated 3.0 Tesla Siemens Prisma MRI scanner with a 32-channel head coil. A multiband gradient-echo EPI sequence was used for resting-state BOLD fMRI data acquisition with the following parameters: TR/TE: 1200/29 ms, Flip Angle: 65°, FOV: 220×220 mm, Matrix: 88×88, Voxel size: 2.5×2.5×2.5 mm, 54 interleaved slices without gap. A total of 400 images were acquired from over 8 min scanning for each participant. Participants were instructed to lie still in the scanner, keeping their eyes open and looking at a cross fixation on the center of a screen. After the functional scans, high-resolution (1.0×1.0×1.2 mm) T1-weighted anatomic images were obtained using a standard 3D MPRAGE sequence for structural reference.
Image data processing and analyses were carried out with the Statistical Parametric Mapping software (SPM 12, Wellcome Department of Cognitive Neurology, UK), the CONN (https://www.nitrc.org/projects/conn), the DPABI V5.0 toolbox (http://rfmri.org/dpabi), and the BrainNet Viewer toolbox (https://www.nitrc.org/projects/bnv/) implemented in Matlab 2020a (Math Works, Natick, MA). Image processing included field mapping distortion correction, slice timing correction, realignment, smoothing using an isotropic Gaussian kernel with a full-width at half-maximum (FWHM) of 4 mm, co-registration, normalization to the standard Montreal Neurological Institute (MNI) space, detrending, ICA-AROMA with global signal regression, and band pass filtering at 0.01–0.08 Hz. We combined ICA-AROMA (Pruim et al., 2015a, Pruim et al., 2015b) with global signal regression, which has proved to be a good strategy to remove motion-related variance and to control for nuisance regressors such as physiologic noise for studies of FC in youth (Ciric et al., 2017).
The seed regions of ventral striatum (VS), medial prefrontal cortex (mPFC), middle frontal gyrus (MFG) and amygdala were derived both bilaterally and unilaterally from the Harvard-Oxford atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). We examined both bilateral and unilateral seeds to explore the laterality of sex-related FC results for the selected seeds as prior studies have reported sex differences in resting-state FC relating to hemispheric lateralization in healthy children and adults (Tian et al., 2011, Zuo et al., 2010). Mean BOLD time series from each seed region were extracted and correlated with time series of all other voxels within the brain to create whole-brain Pearson’s correlation coefficient maps. Fisher’s r-to-z transformation {z = 0.5 Ln [(1 + r)/(1 - r)]} was then applied on these maps to improve the normality of the correlation coefficients. A whole-brain voxel-wise group-level independent samples t-test was performed on these z-transformed correlation maps to compare the resting-state FC patterns across sexes for each seed, with tanner staging as a covariate. Boys and girls could have different impulsivity levels (measured by the UPPS-P-C total score), which may affect the estimation of sex differences in FC. Therefore, to test the effects of UPPS-P-C total score on the FC, we also performed the t-test described above controlling for UPPS-P-C total score. The threshold was set as the whole brain cluster-level family-wise error (FWE) corrected p < 0.05, with cluster size > 30 voxels. The brain regions showing significant sex differences in FC to each seed were then defined as clusters of interest. Independent samples t-tests were performed to examine sex differences in seed-cluster FC values. Pearson’s and Spearman’s correlation analyses and multiple comparisons correction for the correlation analyses were performed to examine the associations between FC values and scores of UPPS-P-C subscales for boys and girls respectively and jointly using SPSS Statistics for Windows (Version 22.0, IBM, Chicago, IL). To further testify the robustness of the correlation results, we estimated the significance of the Pearson’s correlation and the Spearman’s correlation respectively using permutation testing. Specifically, the permutation testing procedure included: i) the observed Pearson/Spearman’s correlation was computed between the connectivity values and the impulsivity ratings; ii) the impulsivity ratings were permuted relative to the connectivity values; iii) Pearson/Spearman’s correlation was then re-computed after each permutation; iv) i) and ii) were repeated 10,000 times to build a null distribution of Pearson/Spearman’s correlation for comparison with the observed Pearson/Spearman’s correlation.
In addition, we identified brain regions (in seed-based FC) associated with UPPS-P-C total score and with each subscale score for girls and boys respectively, and then compared seed-cluster FC between sexes. A whole-brain voxel-wise group-level multiple regression was performed on the z-transformed correlation maps for each seed and UPPS-P-C total score or each subscale score to identify brain regions associated with impulsivity ratings in girls and boys separately, with tanner staging as a covariate. The threshold was also set as the whole brain cluster-level FWE corrected p < 0.05, with cluster size threshold > 30 voxels. The brain regions showing significant associations with UPPS-P-C total and subscale scores in FC to each seed were then compared using independent samples t-tests to examine sex differences in FC values.
The Institutional Review Board of Indiana University approved this study, and researchers carried out the study in accordance with the Declaration of Helsinki. Parents of participants provided written informed consent, and youth provided assent before enrollment and were compensated for participating in the study.
3. Results
As shown in Table 1, all youth met DSM-5 criteria for externalizing disorders, operationalized here as ADHD and either ODD, CD, or DBD unspecified. There were no sex differences in reported trauma history, parental substance use disorder or past mood disorders of the children (including anxiety disorders). Regarding impulsivity (Table 1), sensation seeking (p < 0.01) and lack of perseverance (p < 0.05) were significantly more prominent in boys than girls, while no sex differences were found in the other scales (all p > 0.05).
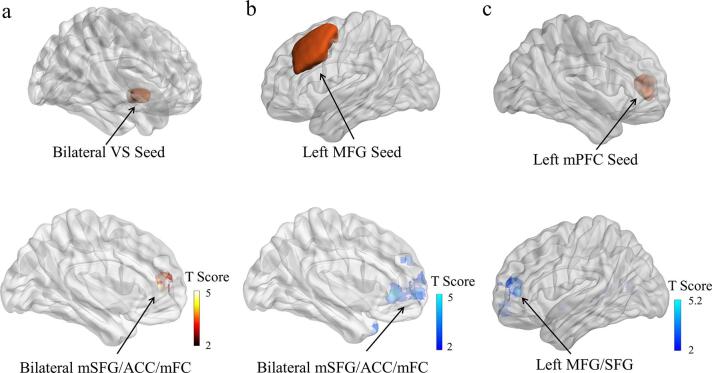
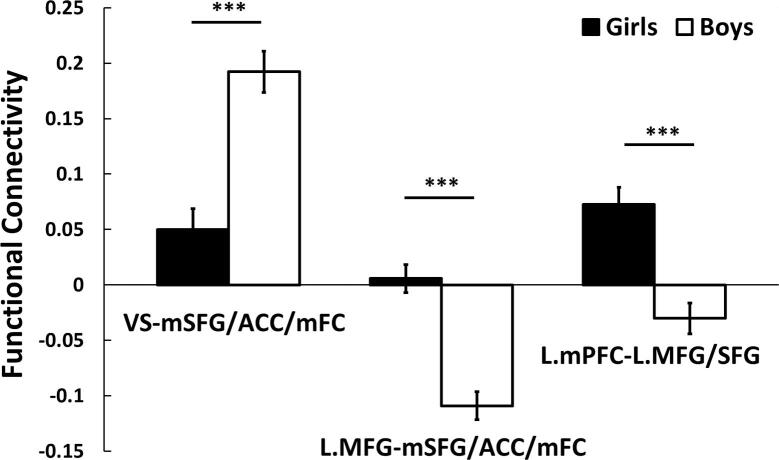
As shown in Fig. 1 and listed in Table 2, FC was significantly greater in boys than girls between bilateral VS seed and multiple regions within the ventromedial prefrontal cortex (vmPFC), including parts of bilateral medial superior frontal gyrus (mSFG), anterior cingulate cortex (ACC), and medial frontal cortex (mFC). In contrast, FC was significantly greater in girls than boys between left middle frontal gyrus (L.MFG) seed and vmPFC as well as between left medial prefrontal cortex (L.mPFC) seed and regions within the left lateral prefrontal cortex (L.lPFC), including parts of left middle frontal gyrus (L.MFG) and left superior frontal gyrus (L.SFG). From the seed-cluster FC analyses, the weaker connectivity between L.MFG and vmPFC and between L.mPFC and L.lPFC in boys from the whole-brain analysis was due to negative connectivity (anti-correlations), as compared to girls (Fig. 2). Analyses with UPPS-P-C total score as a covariate resulted in extremely similar findings. All of these brain regions survived whole brain cluster-level FWE corrected at p < 0.05. We did not find significant sex difference in FC when using the amygdala as seeds.
Fig. 1.
Seed-based functional connectivity differences between boys and girls. Significantly greater connectivity was found a) between bilateral ventral striatum (VS) seed and bilateral medial superior frontal gyrus (mSFG), anterior cingulate cortex (ACC), medial frontal cortex (mFC) in boys compared to girls; b) between left middle frontal gyrus (L.MFG) seed and bilateral mSFG, ACC, mFC and c) between left medial prefrontal cortex (L.mPFC) seed and left middle frontal gyrus (L.MFG), left superior frontal gyrus (L.SFG) in girls relative to boys. Images were displayed at a threshold of uncorrected p < 0.001, with cluster size > 30 voxels.
Table 2.
Brain regions showing significant sex differences in seed-based functional connectivity.
| Brain Regions | x, y, z | p value (FWE corrected) | Cluster Size | Peak T |
|---|---|---|---|---|
| Boys > Girls | ||||
| Seed: Bilateral Ventral Striatum | ||||
| Bilateral mSFG/ACC/mFC | −10, 56, −4 | < 0.05 | 484 | 3.83 |
| Girls > Boys | ||||
| Seed: Left Middle Frontal Gyrus | ||||
| Bilateral mSFG/ACC/mFC | −8, 36, −4 | < 0.001 | 1100 | 4.51 |
| Seed: Left Medial Prefrontal Cortex | ||||
| Left MFG/SFG | –22, 46, 12 | < 0.05 | 534 | 5.00 |
All clusters survived whole brain family-wise error (FWE) corrected p < 0.05.
mSFG: medial superior frontal gyrus; ACC: anterior cingulate cortex; mFC: medial frontal cortex; MFG: middle frontal gyrus.
Fig. 2.
Seed-cluster functional connectivity analysis for sex differences. Functional connectivity between bilateral VS seed and bilateral mSFG/ACC/mFC was greater in boys than girls. The connectivity between L.MFG seed and bilateral mSFG/ACC/mFC and between L.mPFC seed and L.MFG/SFG was negative (anti-correlations) in boys as compared to girls. Error bars represent standard errors of the mean. *** p < 0.001. VS: ventral striatum; mSFG: medial superior frontal gyrus; ACC: anterior cingulate cortex; mFC: medial frontal cortex; L.mPFC: left medial prefrontal cortex; L.MFG/SFG: left middle frontal gyrus/superior frontal gyrus.
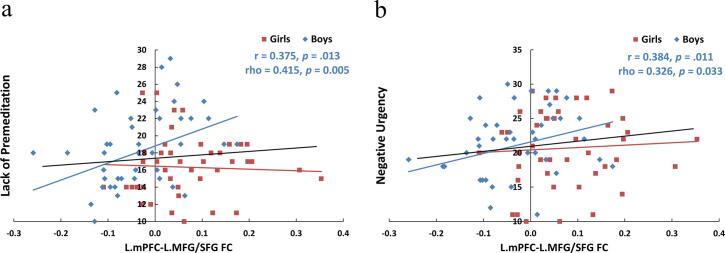
Only in boys, L.mPFC-L.lPFC connectivity correlated positively with lack of premeditation (Fig. 3a, Pearson’s r = 0.375, p (uncorrected) = 0.013; Spearman’s rho = 0.415, p (uncorrected) = 0.005; permutation test Pearson p = 0.006, Spearman p = 0.003, see Fig. S3a and Fig. S3c) and negative urgency (Fig. 3b, Pearson’s r = 0.384, p (uncorrected) = 0.011; Spearman’s rho = 0.326, p (uncorrected) = 0.033; permutation test Pearson p = 0.03, Spearman p = 0.015, see Fig. S3b and Fig. S3d).
Fig. 3.
The scatter plots showed the Pearson’s (r) and Spearman’s (rho) correlation between functional connectivity values and UPPS-P-C subscale scores. Only in boys, connectivity between L.mPFC and L.MFG/SFG positively correlated with lack of premeditation (3a) and negative urgency (3b). The p values were not corrected for multiple comparisons. L.mPFC: left medial prefrontal cortex; L.MFG/SFG: left middle frontal gyrus/superior frontal gyrus; FC: functional connectivity. Black lines: boys and girls pooling together; Blue dots/lines: boys; Red dots/lines: girls. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Findings from models derived from UPPS-P-C subscale scores were consistent with our findings from a priori region of interest (ROI) based group FC differences (with or without controlling for UPPS-P-C total score). Specifically, FC between a number of regions correlated with impulsivity scores between boys and girls (Table S1), but only FC between a L.mPFC seed and a L.MFG/SFG cluster differed between sexes (p < 0.05). This connectivity was positive in girls while negative (i.e., anti-correlated) in boys (Fig. S1). Using models derived from UPPS-P-C total score, we found sex-specific associations between UPPS-P-C total score and the FC involving different brain regions (Table S2), but none of the FC values differed between sexes (Fig. S2).
4. Discussion
In this study of sex differences with youth exhibiting externalizing disorders, behavioral and neuroimaging differences were observed. As hypothesized, we found significantly elevated impulsivity ratings in boys compared to girls, driven by greater sensation seeking and lack of perseverance. Higher sensation seeking in boys is consistent with previous children/adolescent studies (d'Acremont and Linden, 2005, Dekkers et al., 2019, Shulman et al., 2015), however, boys also reported higher scores for lack of perseverance in our study. d'Acremont and Van der Linden (2005) observed a higher level of negative urgency in girls, which we did not observe. This discrepancy may be due to the different ages of the participants (12–19 vs 11–12). In addition, d'Acremont and Van der Linden did not assess for the positive urgency facet of impulsivity captured in the UPPS-P-C.
In relation to imaging findings, we found sex differences in FC in three networks, while controlling for differences in pubertal development within psychiatrically and demographically comparable groups. Moreover, we found consistent results of FC differences between sexes via different approaches (e.g., group comparisons with or without UPPS-P-C total score as a covariate; impulsivity derived models that differ between sexes), which suggest the robustness of these findings. Of note, we found no group differences with amygdala seeds, unlike prior studies in youth with (Rosch et al., 2018) or without ADHD (Alarcón et al., 2015, Müller-Oehring et al., 2018). In addition, we didn’t observe hemisphere-related sex differences in FC, unlike prior studies in healthy participants (Tian et al., 2011, Zuo et al., 2010).
First, we found stronger averaged FC between VS and ventromedial prefrontal regions (i.e., mSFG, ACC and mFC) in boys. This finding partly supported our hypothesis that VS-prefrontal connectivity would differ between sexes. However, the direction of sex differences in frontostriatal connectivity in the current study differed from the only previous study on FC in youth with ADHD (Rosch et al., 2018). In Rosch’s study, girls with ADHD had stronger positive connectivity between striatum and vmPFC/ACC compared to boys, and stronger negative connectivity between striatum and dlPFC than boys. Frontostriatal circuitry is thought to underpin executive function and impulse control (Castellanos et al., 2006, Dickstein et al., 2006). Previous studies have demonstrated both hyperconnectivity (Costa Dias et al., 2013, Ma et al., 2016, Oldehinkel et al., 2016) and hypoconnectivity (Costa Dias et al., 2015, Rubia et al., 2009) of the frontostriatal network (i.e., between VS/nucleus accumbens (NAcc) and PFC) in children and adolescents with ADHD, indicating aberrant prefrontal top-down modulation over reward-related subcortical regions (Heatherton and Wagner, 2011). In our study, the stronger VS-prefrontal connectivity in boys rather than girls might be consistent with the idea that the increased signaling of VS/NAcc to the prefrontal cortex would lead to excessive approach and failure in estimating future consequences, and as a result, impulsivity (Nigg and Casey, 2005).
Second, the averaged FC between left mPFC and left lateral prefrontal regions (i.e., left MFG and SFG) was positive in girls while negative in boys. The mPFC is a core node of the default mode network (DMN), which is thought to be involved in internally-oriented attention (Di and Biswal, 2014, Gusnard et al., 2001). The MFG has been proposed to be a key region of the task-positive ventral attention network (VAN), which is thought to be implicated in externally-oriented attention (Corbetta and Shulman, 2002, Fox et al., 2009). During rested wakefulness in task-free settings, the task-negative DMN is usually engaged whereas the task-positive VAN is suppressed, demonstrating an anti-correlation (e.g., a functional decoupling; Fox and Raichle, 2007, Fox et al., 2005) between these two networks. The positive connectivity between these two networks in girls suggests that task-negative and task-positive networks may fail to remain functionally distinct from each other, which may lead to an altered allocation of cognitive resources to brain networks and poor modulation of attention processes in response to shifting cognitive demands (Clapp et al., 2011, Turner and Spreng, 2012). In contrast, the negative connectivity between these two networks in boys suggests relatively intact distinction between these two networks (Castellanos and Proal, 2012, Kelly et al., 2008, Kessler et al., 2014, Mills et al., 2018, Sonuga-Barke and Castellanos, 2007, Sripada et al., 2014). Though we did not observe sex differences in lack of premeditation and negative urgency ratings, weaker L.mPFC-L.lPFC connectivity correlated with lower levels of these two ratings respectively only in boys, indicating boys with more intact DMN-VAN distinction tended to be more forethoughtful and prudent. In contrast, no such relationships between L.mPFC-L.lPFC connectivity and lack of premeditation and negative urgency ratings were found in girls. One possible explanation is that though we did not observe sex differences in these two self-report measures, differences of L.mPFC-L.lPFC connectivity exist between boys and girls. Therefore, sex-specific differences of L.mPFC-L.lPFC connectivity contribute to sex-specific correlations between L.mPFC-L.lPFC connectivity and lack of premeditation and negative urgency ratings. Another possible explanation is that many factors can contribute to behavior, and FC is just one possible factor. Thus, the L.mPFC-L.lPFC connectivity cannot entirely reflect these two self-reported measures of impulsivity. It is also possible that the sample size in the current study is not large enough to detect sex differences in lack of premeditation and negative urgency ratings since lack of premeditation showed a trend of significance (p = 0.054). Thus, our findings highlight the sensitivity of FC measures to sex differences. Future studies are needed to clarify such possibilities.
Third, the averaged FC between left MFG and vmPFC was positive in girls while negative in boys. MFG, SFG, and ACC/mFC play important roles in executive function including cognitive and motor control (Dalley et al., 2011, Diamond, 2013). Specifically, the MFG is implicated in executive control (Dosenbach et al., 2007) and reward processing (Knutson et al., 2000). Our finding may suggest greater functional segregation within the prefrontal networks in boys (Arnsten, 2009).
Placing this work in the context of the only other similar study (Rosch et al., 2018), we note the inconsistent direction of sex differences in frontostriatal FC and the differing of the exact cortical regions functionally connected with the striatum. Rosch et al. found stronger positive striatum-vmPFC and striatum-ACC connectivity and stronger negative striatum-dlPFC connectivity in girls, while we reported stronger positive ventral striatal-ventromedial prefrontal connectivity in boys. In addition, Rosch et al. found sex differences in amygdala-vmPFC connectivity, while we did not observe differences in amygdala-related connectivity. In spite of varied data acquisition parameters and preprocessing pipelines, this discrepancy is possibly due to the different inclusion criteria for the participant sample. For example, conduct disorder was excluded in Rosch’s study. Moreover, the children recruited in Rosch’s study had a larger age range (e.g., 8–12 years), while the current study included only 11–12-year-olds. Another explanation may be the different methods used to detect group differences as Rosch and colleagues used group independent components analyses (ICA) to identify the intrinsic functional networks, whereas we defined seed regions based on the Harvard-Oxford atlas. We controlled for tanner staging while Rosch included head motion, age, and IQ as covariates. In addition, Rosch’s striatal seed was functionally defined to include the caudate and putamen rather than focusing on the much smaller area of ventral striatum, as used in our study. We know that distinct FC patterns may be observed across different regions of the striatum (Di Martino et al., 2008). Furthermore, Rosch et al. measured impulsive behavior (delay discounting) while we focused on self-reported estimates of impulsivity (UPPS-P-C scale). Therefore, we are likely measuring different facets of impulsivity given its multidimensional features. Although the direction was different, both groups found sex differences in frontostriatal connectivity in youth with externalizing disorders.
Several limitations warrant consideration when interpreting the present findings including our reliance on self-report measures and the cross-sectional design. Future work could couple self-report and behavioral estimates of impulsivity to gain a more complete picture of the degree to which boys and girls differ in different facets of impulsivity. Longitudinal studies are needed to understand the developmental trajectory of FC within the frontostriatal and the medial–lateral prefrontal functional networks in boys and girls with externalizing disorders. In addition, typically developing youth were not included in the current study. Therefore, our findings of sex differences in impulsivity and fronto-subcortical FC are specific to youth with externalizing disorders without knowledge about how these youth differ from typically developing samples.
5. Conclusion
To our knowledge, this is one of the first studies to examine sex-specific FC networks in youth with externalizing disorders. The study serves as a replication, but also characterizes a narrower age band and is the first study to focus on sex-differences in self-reported (versus lab measured) impulsivity, as well as examines connectivity associated with specific impulsivity subtypes. Findings suggested that a frontostriatal network and two medial–lateral prefrontal networks at rest differed between preadolescent boys and girls with externalizing psychopathology. Compared to girls, the prefrontal regions in boys were tightly connected to the VS but were less interconnected within the cortex. In other words, the prefrontostriatal network was more functionally integrated (e.g., positively correlated), whereas the prefrontal network was more functionally segregated (e.g., negatively correlated) in boys. In addition, boys exhibited negative L.mPFC-L.lPFC connectivity on average (versus positive in girls), indicating relatively intact distinction between the DMN and the VAN in boys than girls. Moreover, less impulsivity was related to more intact DMN-VAN distinction only in boys. The neurobiological differences between boys and girls with externalizing disorders that underlie sex differences in impulsivity suggested in the present study may inform novel sex-tailored interventions for SUD targeting high-risk youth.
CRediT authorship contribution statement
Ya Chai: Conceptualization, Methodology, Writing - original draft, Writing - review & editing. José R. Chimelis-Santiago: Writing - review & editing. Kristy A. Bixler: Writing - review & editing. Matthew Aalsma: Writing - review & editing. Meichen Yu: Methodology, Writing - review & editing. Leslie A. Hulvershorn: Conceptualization, Supervision, Methodology, Funding acquisition, Project administration, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments and Disclosures
This study was funded by the National Institute on Drug Abuse to LH (R01DA039764). The authors would like to thank Mario Dzemidzic, Tom Hummer and Michael Smoker for providing valuable support and suggestions and Jackson Richey for assistance with data collection. The authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102789.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. J. Pediatrics. 2009;154:I-s43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod B.N. Validity of the Wechsler abbreviated scale of intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002;9:17–23. doi: 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- Barkley-Levenson E.E., Van Leijenhorst L., Galvan A. Behavioral and neural correlates of loss aversion and risk avoidance in adolescents and adults. Dev. Cogn. Neurosci. 2013;3:72–83. doi: 10.1016/j.dcn.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Bennett M., Orr C., Icke I., Banaschewski T., Barker G.J., Bokde A.L.W., Bromberg U., Buchel C., Quinlan E.B., Desrivieres S., Flor H., Frouin V., Garavan H., Gowland P., Heinz A., Ittermann B., Martinot J.L., Nees F., Orfanos D.P., Paus T., Poustka L., Hohmann S., Frohner J.H., Smolka M.N., Walter H., Schumann G., Whelan R., Consortium I. Mapping adolescent reward anticipation, receipt, and prediction error during the monetary incentive delay task. Hum. Brain Mapp. 2019;40:262–283. doi: 10.1002/hbm.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn. Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J., Milham M.P., Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G.L., Ruparel K., Shinohara R.T., Elliott M.A., Eickhoff S.B., Davatzikos C., Gur R.C., Gur R.E., Bassett D.S., Satterthwaite T.D. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp W.C., Rubens M.T., Sabharwal J., Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc. Natl. Acad. Sci. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Costa Dias T.G., Iyer S.P., Carpenter S.D., Cary R.P., Wilson V.B., Mitchell S.H., Nigg J.T., Fair D.A. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Develop. Cogn. Neurosci. 2015;11:155–174. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dias T.G., Wilson V., Bathula D., Iyer S., Mills K., Thurlow B., Stevens C.A., Musser E.D., Carpenter S.D., Grayson D.S., Mitchell S.H., Nigg J.T., Fair D.A. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie J., Arnold P., Paterson A., Swanson J., Dupuis A., Li X., Shan J., Goodale T., Tam C., Strug L.J., Schachar R.J. Response inhibition and ADHD traits: correlates and heritability in a community sample. J. Abnorm. Child Psychol. 2013;41:497–507. doi: 10.1007/s10802-012-9693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C.P., Copping L.T., Campbell A. Sex differences in impulsivity: a meta-analysis. Psychol. Bull. 2011;137:97–130. doi: 10.1037/a0021591. [DOI] [PubMed] [Google Scholar]
- Cyders M.A. Impulsivity and the sexes: measurement and structural invariance of the UPPS-P impulsive behavior scale. Assessment. 2013;20:86–97. doi: 10.1177/1073191111428762. [DOI] [PubMed] [Google Scholar]
- d'Acremont M., Linden M.V.d. Adolescent impulsivity: findings from a community sample. J. Youth Adolesc. 2005;34:427–435. [Google Scholar]
- Dalley J.W., Everitt B.J., Robbins T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dekkers T.J., van Bergen N.R.J., Jansen B.R.J. An assessment of the psychometric properties of the brief sensation seeking scale for children. J. Pers. Assess. 2019;101:446–451. doi: 10.1080/00223891.2018.1468336. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Di X., Biswal B.B. Modulatory interactions between the default mode network and task positive networks in resting-state. PeerJ. 2014;2 doi: 10.7717/peerj.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J. Child Psychol. Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Gruber O. When desire collides with reason: functional interactions between anteroventral prefrontal cortex and nucleus accumbens underlie the human ability to resist impulsive desires. J. Neurosci. 2010;30:1488–1493. doi: 10.1523/JNEUROSCI.4690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E.K., Keil M., Obst K.U., Henseler I., Dechent P., Falkai P., Gruber O. A functional neuroimaging study assessing gender differences in the neural mechanisms underlying the ability to resist impulsive desires. Brain Res. 2012;1473:63–77. doi: 10.1016/j.brainres.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Dir A.L., Hummer T.A., Aalsma M.C., Hulvershorn L.A. Pubertal influences on neural activation during risky decision-making in youth with ADHD and disruptive behavior disorders. Develop. Cogn. Neurosci. 2019;36 doi: 10.1016/j.dcn.2019.100634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss M., Caudle K., Drysdale A.T., Johnston N.E., Cohen A.O., Somerville L.H., Galvan A., Tottenham N., Hare T.A., Casey B.J. Teens impulsively react rather than retreat from threat. Dev. Neurosci. 2014;36:220–227. doi: 10.1159/000357755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökçe S., Yusufoğlu C., Akin E., Ayaz M. Effect of gender differences on impulsivity in adolescents with attention-deficit/hyperactivity disorder. Anadolu Psikiyatri Dergisi. 2017;18:379–386. [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson R., Fine J.G. Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. J. Attention Disorders. 2012;16:190–198. doi: 10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- Hastings T.L., Kelley M.L. Development and validation of the Screen for Adolescent Violence Exposure (SAVE) J. Abnorm. Child Psychol. 1997;25:511–520. doi: 10.1023/a:1022641916705. [DOI] [PubMed] [Google Scholar]
- Heatherton T.F., Wagner D.D. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove M.J., Zeffiro T.A., Biederman J., Li Z., Schmahmann J., Valera E.M. Postural sway and regional cerebellar volume in adults with attention-deficit/hyperactivity disorder. NeuroImage: Clinical. 2015;8:422–428. doi: 10.1016/j.nicl.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.D., Weaver A.J., Ivic R., Imboden K. Developing a brief sensation seeking scale for children: establishing concurrent validity with video game use and rule-breaking behavior. Media Psychology. 2011;14:71–95. [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly A.M., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kessler D., Angstadt M., Welsh R.C., Sripada C. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J. Neurosci. 2014;34:16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Liu T., Xiao T., Shi J. Response inhibition, preattentive processing, and sex difference in young children: an event-related potential study. NeuroReport: For Rapid Communication of Neuroscience Research. 2013;24:126–130. doi: 10.1097/WNR.0b013e32835d846b. [DOI] [PubMed] [Google Scholar]
- Lynam D.R., Smith G.T., Whiteside S.P., Cyders M.A. Purdue University; West Lafayette, IN: 2006. The UPPS-P: Assessing five personality pathways to impulsive behavior (Technical Report) [Google Scholar]
- Ma I., van Holstein M., Mies G.W., Mennes M., Buitelaar J., Cools R., Cillessen A.H.N., Krebs R.M., Scheres A. Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2016;82:225–236. doi: 10.1016/j.cortex.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Martel M., Nikolas M., Nigg J.T. Executive function in adolescents with ADHD. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:1437–1444. doi: 10.1097/chi.0b013e31814cf953. [DOI] [PubMed] [Google Scholar]
- Martin C.A., Kelly T.H., Rayens M.K., Brogli B.R., Brenzel A., Smith W.J., Omar H.A. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Mills B.D., Miranda-Dominguez O., Mills K.L., Earl E., Cordova M., Painter J., Karalunas S.L., Nigg J.T., Fair D.A. ADHD and attentional control: impaired segregation of task positive and task negative brain networks. Netw. Neurosci. 2018;2:200–217. doi: 10.1162/netn_a_00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring E.M., Kwon D., Nagel B.J., Sullivan E.V., Chu W., Rohlfing T., Prouty D., Nichols B.N., Poline J.B., Tapert S.F., Brown S.A., Cummins K., Brumback T., Colrain I.M., Baker F.C., De Bellis M.D., Voyvodic J.T., Clark D.B., Pfefferbaum A., Pohl K.M. Influences of age, sex, and moderate alcohol drinking on the intrinsic functional architecture of adolescent brains. Cereb. Cortex. 2018;28:1049–1063. doi: 10.1093/cercor/bhx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas J.F., Martín-Pérez C., Petrova D., Verdejo-García A., Cano M., Sagripanti-Mazuquín O., Perandrés-Gómez A., López-Martín Á., Cordovilla-Guardia S., Megías A., Perales J.C., Vilar-López R. Sex differences in the association between impulsivity and driving under the influence of alcohol in young adults: the specific role of sensation seeking. Accid. Anal. Prev. 2019;124:174–179. doi: 10.1016/j.aap.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Nigg J.T., Casey B.J. An integrative theory of attention-deficit/ hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- O'Brien J.W., Dowell L.R., Mostofsky S.H., Denckla M.B., Mahone E.M. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archiv. Clin. Neuropsychol. 2010;25:656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M., Beckmann C.F., Franke B., Hartman C.A., Hoekstra P.J., Oosterlaan J., Heslenfeld D., Buitelaar J.K., Mennes M. Functional connectivity in cortico-subcortical brain networks underlying reward processing in attention-deficit/hyperactivity disorder. NeuroImage: Clinical. 2016;12:796–805. doi: 10.1016/j.nicl.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patros C.H.G., Sweeney K.L., Mahone E.M., Mostofsky S.H., Rosch K.S. Greater delay discounting among girls, but not boys, with ADHD correlates with cognitive control. Child Neuropsychol. 2018;24:1026–1046. doi: 10.1080/09297049.2017.1359525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., Buitelaar J.K., Beckmann C.F. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Rosch K.S., Mostofsky S.H. Increased delay discounting on a novel real-time task among girls, but not boys, with ADHD. J. Int. Neuropsychol. Soc. 2016;22:12–23. doi: 10.1017/S1355617715001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch K.S., Mostofsky S.H., Nebel M.B. ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J. Neurodevelopmental Disorders. 2018;10:34. doi: 10.1186/s11689-018-9254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Roalf D.R., Ruparel K., Erus G., Vandekar S., Gennatas E.D., Elliott M.A., Smith A., Hakonarson H., Verma R., Davatzikos C., Gur R.E., Gur R.C. Linked sex differences in cognition and functional connectivity in youth. Cereb. Cortex. 2015;25:2383–2394. doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman E.P., Harden K.P., Chein J.M., Steinberg L. Sex differences in the developmental trajectories of impulse control and sensation-seeking from early adolescence to early adulthood. J. Youth Adolesc. 2015;44:1–17. doi: 10.1007/s10964-014-0116-9. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E.J., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sripada C.S., Kessler D., Angstadt M. Lag in maturation of the brain's intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc. Natl. Acad. Sci. U S A. 2014;111:14259–14264. doi: 10.1073/pnas.1407787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Baler R.D., Volkow N.D. Understanding the effects of stimulant medications on cognition in individuals with attention-deficit hyperactivity disorder: a decade of progress. Neuropsychopharmacology. 2011;36:207–226. doi: 10.1038/npp.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Wang J., Yan C., He Y. Hemisphere- and gender-related differences in small-world brain networks: a resting-state functional MRI study. Neuroimage. 2011;54:191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Turner, G.R., Spreng, R.N., 2012. Executive functions and neurocognitive aging: Dissociable patterns of brain activity. Neurobiol. Aging 33, 826.e1–826.e13. [DOI] [PubMed]
- Valera E.M., Faraone S.V., Biederman J., Poldrack R.A., Seidman L.J. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:439–447. doi: 10.1016/j.biopsych.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Weafer J., de Wit H. Sex differences in impulsive action and impulsive choice. Addict. Behav. 2014;39:1573–1579. doi: 10.1016/j.addbeh.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R., Conrod P.J., Poline J.B., Lourdusamy A., Banaschewski T., Barker G.J., Bellgrove M.A., Büchel C., Byrne M., Cummins T.D., Fauth-Bühler M., Flor H., Gallinat J., Heinz A., Ittermann B., Mann K., Martinot J.L., Lalor E.C., Lathrop M., Loth E., Nees F., Paus T., Rietschel M., Smolka M.N., Spanagel R., Stephens D.N., Struve M., Thyreau B., Vollstaedt-Klein S., Robbins T.W., Schumann G., Garavan H. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Whiteside S.P., Lynam D.R. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality Individ. Differ. 2001;30:669–689. [Google Scholar]
- Zapolski T.C., Stairs A.M., Settles R.F., Combs J.L., Smith G.T. The measurement of dispositions to rash action in children. Assessment. 2010;17:116–125. doi: 10.1177/1073191109351372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Kelly C., Di Martino A., Mennes M., Margulies D.S., Bangaru S., Grzadzinski R., Evans A.C., Zang Y.F., Castellanos F.X., Milham M.P. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.