Abstract
Improving exercise capacity is a primary objective in COPD. Declines in exercise capacity result in reduced physical activity and health-related quality of life (HRQoL). Self-management interventions can teach patients skills and behaviours to manage their disease. Technology-mediated interventions have the potential to provide easily accessible support for disease self-management. We evaluated the effectiveness of a web-based self-management intervention, focused on physical activity promotion, on exercise capacity in COPD. This 6-month randomised controlled trial (NCT02099799) enrolled 153 persons with COPD at two US sites (VABoston, n=108; VABirmingham, n=45). Participants were allocated (1:1) to the web-based self-management intervention (physical activity promotion through personalised, progressive step-count goals, feedback, online COPD-related education and social support via an online community) or usual care. The primary outcome was exercise capacity (6-min walk distance (6 MWD)). Secondary outcomes included physical activity (daily steps per day), HRQoL (St. George's Respiratory Questionnaire Total Score), dyspnoea, COPD-related knowledge and social support. Change in step-count goals reflected intervention engagement. Participants' mean age was 69 (sd=7), and mean forced expiratory volume in 1 s % predicted was 61% (sd=21%). Change in 6MWD did not differ between groups. Intervention participants improved their mean daily step counts by 1312 more than those in the usual care group (p<0.001). Groups did not differ on other secondary outcomes. VABirmingham participants were significantly more engaged with the intervention, although site did not modify the effect of the intervention on 6MWD or secondary outcomes. The intervention did not improve exercise capacity but improved physical activity at 6 months. Additional intervention modifications are needed to optimise its COPD self-management capabilities.
Short abstract
A web-based self-management intervention improved physical activity but not exercise capacity. There is a need to develop and study accessible self-management interventions for COPD. https://bit.ly/3iT1yvU
Introduction
COPD is a leading cause of death in the US [1] and a major contributor to morbidity, mortality and resource utilisation worldwide [2, 3]. Patients with COPD are limited in their exercise capacity, which can result in deleterious downstream effects including reduced physical activity and health-related quality of life (HRQoL) [4]. With no cure, proactive self-management of COPD is critical to maintaining or improving health outcomes [5]. Ultimate goals of COPD self-management include optimising and preserving exercise capacity, reducing symptoms and functional impairments in daily life, and increasing emotional and social well-being, and HRQoL. Self-management interventions for COPD are often multicomponent, structured and personalised programmes with goals of motivating, engaging and supporting patients to adopt positive health behaviours (e.g. physical activity) and develop skills to better manage their disease [6, 7]. Technology-based platforms may offer a promising approach to promote disease self-management and bring interventions directly to patients [8, 9]. Such solutions are particularly relevant during the coronavirus disease 2019 pandemic when many in-person programmes are closed. Rigorous examination of how effective technology-based self-management interventions are in promoting key COPD outcomes, such as exercise capacity, is needed [10].
We designed a web-based self-management intervention focused on physical activity promotion for persons with COPD [11–13]. This multicomponent intervention, based on the Theory of Self-Regulation [14], promotes physical activity through individualised, progressive goal setting and iterative step-count feedback, delivers online COPD-related education and motivational messages, and provides social support via an online community forum. Our intervention focuses on promoting walking since persons with COPD who walk more have better HRQoL [11], and decreased risks of acute exacerbations [15] and mortality [16, 17].
We have previously demonstrated at a single site that use of our web-based physical activity self-management intervention for 3 [13] and 4 months [11] resulted in significant, clinically meaningful improvements in daily step counts [11, 13] and HRQoL [11]. In the current randomised controlled trial (RCT), we extend our previous work in two ways: 1) the duration of the intervention was increased to 6 months to assess if it could improve exercise capacity in addition to physical activity; and 2) we enrolled COPD participants at two geographically distinct study sites to assess response to the intervention in a heterogeneous cohort. Our primary hypothesis was that participants assigned to the intervention would demonstrate significant improvements in exercise capacity compared to controls at 6 months. Our secondary hypothesis was that participants in the intervention group would demonstrate significant improvements in other COPD self-management outcomes (HRQoL, dyspnoea, physical activity, disease education, social support and occurrence of acute exacerbations) compared to controls. Some of the results of this study have been previously reported in the form of an abstract [18].
Material and methods
See online supplementary material for description of the intervention.
Study participants
Participants were recruited from May 2015-February 2019 at two US sites. At VABoston (BOS), mailings were sent to COPD patients in outpatient pulmonary clinics. VABirmingham (BIR) patients were approached by their provider (J.A.C.) who referred interested participants to the research coordinator. The study was approved by the Institutional Review Boards (BOS #2791 and BIR #01534) and registered on ClinicalTrials.gov (NCT02099799). Participants provided written informed consent.
Baseline assessment and randomisation
At baseline, demographics, medical history, postbronchodilator mean forced expiratory volume in 1 s (FEV1) % predicted and outcomes were assessed [19]. Rural–urban commuting area codes (RUCA) classified participants’ rurality [20]. Participants wore a Fitbit Zip pedometer (fitbit, San Francisco, CA, USA) for 10 days with a sticker covering the display to prevent feedback. Participants who met inclusion criteria (table 1) and had ≥7 valid wear-days at baseline (>200 steps/day) [21] were randomised 1:1 to either the internet-mediated, pedometer-based physical activity intervention or usual care (control) for 6 months (figure 1). The study statistician (D.G.) generated the random allocation sequence.
TABLE 1.
Inclusion and exclusion criteria
| Inclusion | Exclusion |
| 1. 40 years of age | 1. COPD exacerbation in the previous month |
| 2. Diagnosis of COPD (defined by FEV1/FVC <0.70, or computed tomography evidence of emphysema, or prior documentation of FEV1/FVC <0.70 and clinical evidence of COPD (≥10 pack-years cigarette smoking history, dyspnoea or on bronchodilators)) | 2. Inability to ambulate with or without assistance |
| 3. Medical clearance from a healthcare provider to participate in an exercise programme | 3. Clinical signs of unstable cardiovascular disease or congestive heart failure |
| 4. Access to a computer with an internet connection, a USB port or Bluetooth capability, and Windows XP/Vista/7/8/10 or higher, or Mac OSX 10.5 or higher operating system, or willing to come to VA Medical Center to use study computers | 4. Hypoxaemia during 6MWT, i.e. oxygen saturation <85%, with or without using supplemental oxygen |
| 5. Pedometer with >90% accuracy compared to manual counts on a short clinic walk | 5. Inability to complete questionnaires |
| 6. Competent to provide informed consent | 6. Inability to collect at least 7 out of 10 days of baseline step counts |
| 7. Willingness to make return visits and be available by telephone for duration of study | 7. Participation in a pulmonary rehabilitation programme at time of screening or within the previous 3 months |
| 8. Participation in another exercise-related research study at time of screening | |
| 9. Plans to participate in an exercise-related research study in the next 12 months | |
| 10. Plans to enrol in a supervised exercise programme, such as pulmonary rehabilitation, in the next 6 months | |
| 11. Average daily baseline step counts ≥10 000 steps per day |
FEV1: mean forced expiratory volume in 1 s; FVC: forced vital capacity; 6MWT: 6-min walk test.
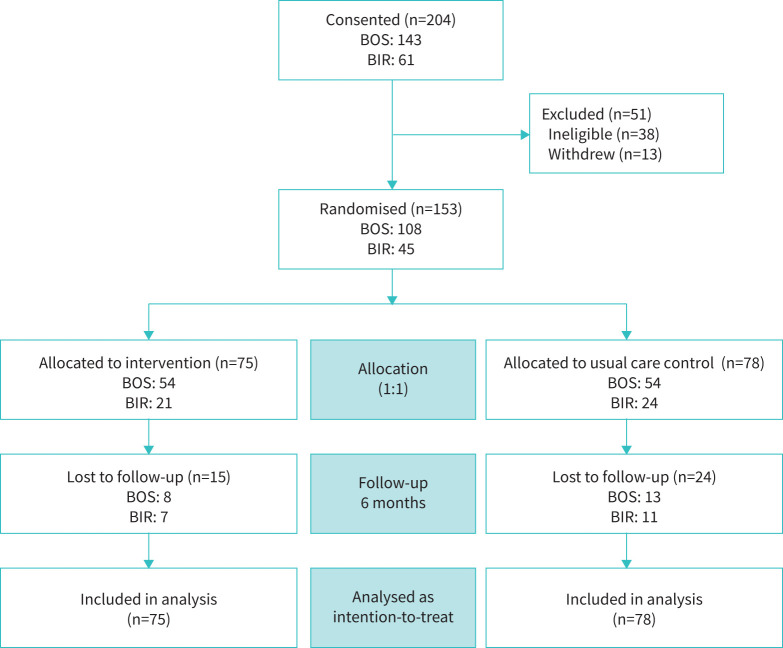
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. BOS: VABoston; BIR: VABirmingham.
Randomisation groups
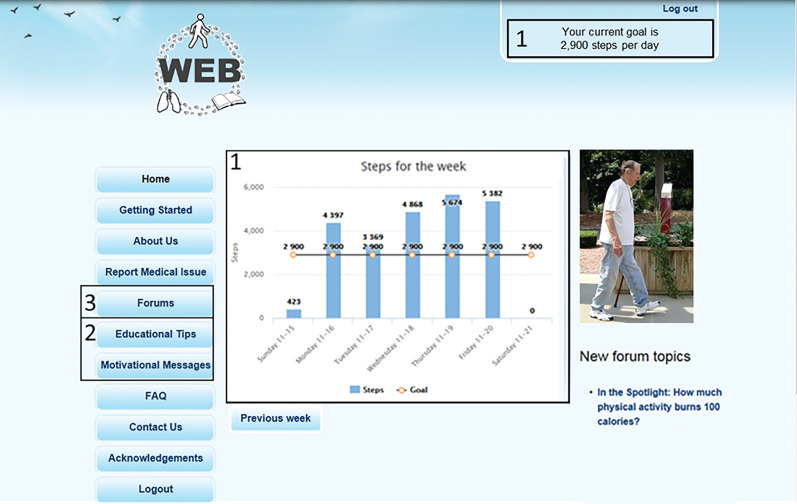
Participants in both the intervention and control groups received verbal encouragement to increase daily physical activity and an education booklet that contained COPD self-management information about aerobic endurance and strengthening exercises, an action plan for COPD acute exacerbations, how to resume exercise after an acute exacerbation, oxygen use during exercise, and smoking cessation [6]. The usual care control group did not have contact with the blinded study staff until the in-person outcome assessments 3 and 6 months following the baseline visit. Participants randomised to the intervention were mailed detailed instructions about the study website. The intervention group was instructed to wear the pedometer daily and received access to the content on the website. The intervention supports the cycle of self-regulation with four unique components to promote physical activity and COPD self-management: 1) objective walking assessment and feedback; 2) individualised step-count goals; 3) educational tips and motivational messages; and 4) an online community (figure 2) [12, 13]. Participants were asked to upload their step-count data at least weekly. Participants received weekly automated, personalised step-count goals that progressively increased if previous goals were achieved [13]. Follow-up, in-person assessments occurred at 3 and 6 months for both groups. At each follow-up visit, study staff reminded participants not to disclose randomisation assignment and that they should be working to increase their walking and exercise. The outcome assessor and statistician were blinded to randomisation assignment.
FIGURE 2.
Screenshot of intervention webpage. The intervention supports physical activity and self-management with four unique components: 1) objective walking assessment and feedback; 2) individualised step-count goals; 3) educational tips and motivational messages; and 4) an online community.
Outcome measures
All outcomes were assessed during in-person visits at baseline, 3 and 6 months; daily step counts were assessed over a 14-day period after 3- and 6-month visits. The primary outcome was change in exercise capacity from baseline to 6 months. This was assessed with participants’ 6-min walk distance (6 MWD), performed according to American Thoracic Society (ATS) guidelines (except no practice walk was performed in either randomisation group) [22]. Secondary outcomes included physical activity, HRQoL, dyspnoea, COPD knowledge, social support and acute exacerbations. At 3 and 6 months, physical activity (steps/day) was averaged over ≥4 valid pedometer wear-days within a 14-day period. The St. George's Respiratory Questionnaire Total Score (SGRQ-TS) [23] measured HRQoL, and the modified Medical Research Council (mMRC) assessed dyspnoea [24]. We evaluated COPD knowledge and social support with the Bristol COPD Knowledge Questionnaire (BCKQ) [25] and Medical Outcomes Study Social Support (MOS-SS) scale [26]. Self-reported acute exacerbations of COPD, with or without hospitalisation were assessed at the 3- and 6-month study visits. Participants were coded as either having experienced at least one exacerbation in the last 6 months or none. Other serious (defined as non-COPD acute exacerbation-related hospitalisations and/or deaths) or musculoskeletal (new or worsening leg, foot and/or back pain or discomfort) adverse events were also self-reported and tracked at 3 and 6 months to assess safety. Participants were coded as having experienced a serious adverse event or not, and as having experienced a musculoskeletal event or not.
Within the intervention group, engagement was assessed with change in weekly step-count goals at 6 months from baseline. Participants who met their goals received a higher goal the following week. Thus, a positive change in step-count goals indicated engagement. An intervention feedback questionnaire administered at 6 months also assessed engagement. Participants were asked if: 1) they would recommend the intervention to others with COPD; 2) the intervention helped them adhere to their walking for exercise; 3) they will continue to walk for exercise after the research study ends; 4) they looked at graphs of the step counts; 5) the motivational messages and educational tips were easy to understand; and 6) they learned helpful information using the online community forum. Participants could respond true, false or indicate that they did not use the component.
Sample size
Using the minimum clinically important difference in 6MWD of 54 m [27] and a sd of 100 m [27], we estimated that 110 participants (55/group) at 6 months would provide adequate power (β=80%) at an α=0.05.
Statistical analysis
Independent sample t-tests and Chi-square tests assessed between-group and between-site differences in baseline characteristics and adverse events. Chi-square tests assessed between-site differences in intervention feedback responses. In intention-to-treat analyses, generalised linear mixed-effects models (PROC MIXED, SAS v9.4, Cary, NC, USA) for repeated measures employing a first-order auto-regressive covariance matrix were constructed. Change in outcomes was predicted by randomisation group (intervention versus usual care), study visit (3 and 6 months), and the interaction between randomisation group and study visit. Models were adjusted for FEV1 % predicted, age, site and rurality. p-value <0.05 was considered statistically significant. Season of enrolment was initially included as a covariate but had no significant impact on the results and was therefore removed for a more parsimonious model.
Results
Of the 204 eligible participants, 153 participants were randomised to either the intervention (n=75) or control (n=78) group (figure 1). Those who were randomised (n=153) reported significantly higher SGRQ-TS (worse HRQoL) compared to those who were not randomised (n=51) (mean±sd SGRQ-TS = 38±18 versus 27±23, respectively, p=0.005). There were no other significant differences between those who were and were not randomised. A significantly larger proportion of participants who were lost to follow-up were rural (p=0.048), less educated (p=0.016) and enrolled at BIR (p=0.008) compared to those who completed the 6-month study.
There were no significant between-randomisation group differences in baseline characteristics or occurrence of severe or musculoskeletal adverse events during the study (p-values >0.05; table 2).
TABLE 2.
Characteristics by randomisation group and site
| Baseline characteristic | All# | Boston¶ | Birmingham+ | Between-site p-value | ||||
| Intervention | Control | Between-group p-value | Intervention | Control | Intervention | Control | ||
| Subjects n | 75 | 78 | 54 | 54 | 21 | 24 | ||
| Age years | 69.2±7.2 | 70.4±7.3 | 0.307 | 70.4±7.4 | 70.8±7.5 | 65.8±5.7 | 69.4±7.0 | 0.024 |
| Male, n (%) | 70 (93) | 72 (92) | 0.806 | 52 (96) | 51 (94) | 18 (86) | 21 (87.5) | 0.058 |
| White, n (%) | 69 (92) | 69 (89) | 0.462 | 51 (94) | 50 (93) | 18 (86) | 19 (79.2) | 0.032 |
| BOS, n (%) | 54 (72) | 54 (69) | 0.707 | – | – | – | – | – |
| Annual income <USD 30 000, n (%) | 32 (43) | 34 (44) | 0.908 | 18 (33) | 20 (37) | 14 (67) | 14 (58.3) | 0.002 |
| Greater than high school education, n (%) | 53 (71) | 63 (81) | 0.145 | 38 (70) | 46 (85) | 15(71) | 17 (71) | 0.380 |
| Rural, n (%) | 4 (5) | 11 (14) | 0.068 | 0 (0.00) | 4 (7) | 4 (19) | 7 (29) | <0.001 |
| FEV1 % predicted | 60.6±23.1 | 61.5±19.8 | 0.794 | 68.1±20.4 | 64.7±18.6 | 41.51±18.3 | 54.5±20.9 | <0.001 |
| Current oxygen use, n (%) | 19 (25) | 22 (28) | 0.689 | 8 (15) | 8 (15) | 11 (52) | 14 (58) | <0.001 |
| 6MWT | 360.8±92.0 | 357.2±103.5 | 0.822 | 381.7±90.9 | 382.6±106.3 | 307.0±72.4 | 300.1±70.1 | <0.001 |
| Daily step-count | 3176.6±2211.6 | 3210.2±2247.9 | 0.926 | 3385.0±2197.2 | 3516.0±2511.7 | 2640.8±2210.1 | 2522.1±1290.5 | 0.013 |
| SGRQ-TS | 40.0±15.3 | 38.0±17.8 | 0.474 | 36.3±14.4 | 32.2±14.7 | 49.4±13.6 | 53.4±16.3 | <0.001 |
| mMRC dyspnoea | 2.0±1.2 | 2.1(1.2 | 0.596 | 2.1±1.3 | 2.1±1.3 | 1.8±0.8 | 2.2±1.1 | 0.578 |
| BCKQ | 46.9±15.0 | 46.1±16.2 | 0.751 | 46.3±12.7 | 47.3±15.5 | 48.6±20.2 | 42.8±18.0 | 0.773 |
| MOS-SS | 76.8±23.8 | 69.4±27.7 | 0.081 | 74.5±25.5 | 70.6±29.6 | 83.0±17.5 | 65.9±22.1 | 0.702 |
| Adverse events (n=114) § | ||||||||
| Serious non-COPD adverse event, n (%) | 7 (12) | 9 (17) | 0.443 | 6 (13) | 9 (22) | 1 (7) | 0 (0) | 0.077 |
| Musculoskeletal adverse event, n (%) | 32 (53) | 23 (43) | 0.252 | 27 (59) | 23 (56) | 5 (36) | 0 (0) | 0.001 |
All values represent the mean±sd unless otherwise noted. BOS: Boston site; FEV1: mean forced expiratory volume in 1 s; 6 MWT: 6-minute walk test; SGRQ-TS: St. George's Respiratory Questionnaire – Total Score; mMRC: modified Medical Research Council; BCKQ: Bristol COPD Knowledge Questionnaire; MOS-SS: Medical Outcomes Study Social Support Survey. #: n=153. ¶: n=108. +: n=45. §: Number of participants who provided information about adverse events.
Primary outcome
There was no significant difference between randomisation groups in 6-month change in 6MWD (mean difference of −12 m; p=0.189). Both groups demonstrated statistically and clinically significant within-group changes in 6MWD at 6 months compared to baseline (table 3). The intervention group increased by 25 m (p=0.010) and the control group increased by 37 m (p<0.001).
TABLE 3.
Between-group differences in change in primary and secondary outcomes from baseline to 3 and 6 months (n=153)
| Intervention | Usual care control | |||||
| Mean | se | Mean | se | Mean difference | p-value# | |
| Primary outcome | ||||||
| Δ6MWD | ||||||
| 3 months | 23.86 | 9.58 | 27.58 | 9.51 | −3.72 | 0.686 |
| 6 months | 25.14 | 9.61 | 37.41 | 9.63 | −12.27 | 0.189 |
| Secondary outcomes | ||||||
| ΔDaily step-count | ||||||
| 3 months | 645.95 | 391.97 | −385.78 | 411.45 | 1031.73 | 0.005 |
| 6 months | 672.90 | 392.48 | −639.38 | 415.31 | 1312.28 | <0.001 |
| ΔSGRQ-TS | ||||||
| 3 months | −14.63 | 3.59 | −13.86 | 3.22 | −0.78 | 0.833 |
| 6 months | −13.05 | 3.59 | −15.13 | 3.22 | −28.18 | 0.572 |
| ΔmMRC dyspnoea | ||||||
| 3 months | −0.17 | 0.21 | 0.07 | 0.21 | −0.25 | 0.225 |
| 6 months | −0.06 | 0.21 | 0.00 | 0.21 | −0.05 | 0.799 |
| ΔBCKQ | ||||||
| 3 months | 1.44 | 1.81 | 0.76 | 1.66 | 0.69 | 0.692 |
| 6 months | 0.92 | 1.81 | 1.97 | 1.66 | −1.05 | 0.544 |
| ΔMOS-SS | ||||||
| 3 months | −4.93 | 4.24 | −1.01 | 4.30 | −3.92 | 0.279 |
| 6 months | 0.55 | 4.25 | 4.38 | 4.36 | −3.83 | 0.293 |
| Acute exacerbations | n | % | n | % | Difference | p-value¶ |
| Total | ||||||
| 3 months | 2 | 3 | 3 | 6 | −1 | 0.563 |
| 6 months | 7 | 12 | 5 | 9 | 2 | 0.676 |
| With hospitalisation | ||||||
| 3 months | 1 | 2 | 1 | 2 | 0 | 0.940 |
| 6 months | 1 | 2 | 3 | 6 | −2 | 0.260 |
Acute exacerbations are displayed as number and percentage of participants from study sample (n, %). Δ: change; 6MWD: 6-min walk distance (metres); SGRQ-TS: St. George's Respiratory Questionnaire Total Score; mMRC: modified Medical Research Council; BCKQ: Bristol COPD Knowledge Questionnaire; MOS-SS: Medical Outcomes Study Social Support survey. #: based on linear mixed-effect model adjusting for group, 3- and 6-month indicators, group×time interaction indicators, site, age, mean forced expiratory volume in 1 s % predicted and rurality. ¶: obtained from Chi-square test.
Secondary outcomes
The intervention group significantly increased physical activity by a mean daily step-count of 1312 steps/day (95% CI 600–2024, p<0.001) more than the control group at 6 months (table 3). The intervention group increased by 673 steps/day, compared to the control group who decreased by 639 steps/day from baseline to 6 months. Change in SGRQ-TS, mMRC dyspnoea score, BCKQ score, MOS-SS score or number of acute exacerbations (total and COPD-related hospitalisations) at 6 months did not significantly differ between randomisation groups at 6 months (table 3).
Site
Participants at the two study sites differed on several baseline measurements. Compared to BOS, BIR participants were significantly younger, had a higher proportion of non-white participants and those who currently used oxygen, made <USD 30 000 annually and were rural (p's<0.05; table 2). BIR participants also had lower FEV1 % predicted, lower 6MWD, lower daily step counts and higher SGRQ-TS (worse HRQoL) (p-values <0.05; table 2). Of the 114 (out of 153) participants who completed the 6-month visit, more BOS participants reported experiencing a musculoskeletal adverse event compared to BIR (59% versus 22%; p=0.001).
Because site was a significant predictor of 6-month change in 6MWD (p=0.006) and HRQoL (p= 0.005) in our a priori models, we conducted exploratory analyses to examine if site modified the effect of intervention on outcomes. Two exploratory models predicting outcomes at 6 months were examined, which included site, randomisation group, the interaction between site and randomisation group, and covariates (age, FEV1 % predicted and rurality). There were no significant interactions between site and randomisation group on any of the outcomes at 6 months.
Intervention engagement
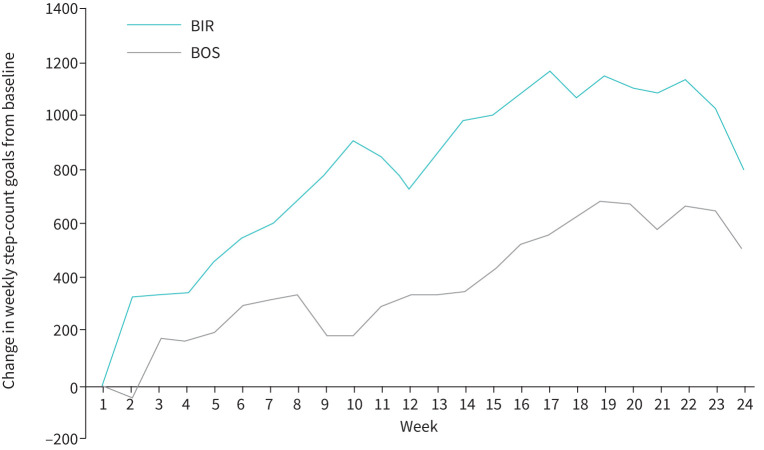
In those assigned to the intervention, BIR participants’ mean daily step-count goals increased by 437 more steps/day across the 6-month (24-week) study period compared to BOS (figure 3), although the difference between groups was not significant (p=0.239). Both sites showed significant increases in step-count goals across the 6-month study period, compared to baseline. For example, BOS participants increased their mean daily step-count goal by 505 steps/day (p=0.014), while BIR participants had an increase of 807 steps/day (p=0.021).
FIGURE 3.
Within the intervention group, change in step-count goal from baseline across the 24-week (6-month) study period by site. A higher change in step-count goal reflects higher engagement in the intervention. Values plotted on the y-axis represent the least squares means from a general linear mixed-effects model for repeated measures, employing a first-order auto-regressive covariance matrix, adjusted for age, mean forced expiratory volume in 1 s % predicted and rurality. Both sites demonstrated increased within-group change in weekly step-count goals from baseline to 24 weeks (BOS: p=0.014; BIR: p=0.021), with BIR goals consistently higher than BOS goals. BOS: VABoston; BIR: VABirmingham.
Of the 60 intervention participants (out of 75) who completed the 6-month visit, 44 (73%) responded to the intervention feedback questionnaire. The majority (n=42, 95%) indicated they would recommend the intervention to others with COPD, 33 (75%) thought the intervention helped them stick to their walking and 42 (95%) reported they would continue to exercise after the research study ended. Regarding engagement with intervention components, 38 (86%) looked at the step-count graphs. Twenty-four (55%) found the motivational and educational tips easy to understand, while 20 (45%) reported that they did not use this component. Eleven (25%) participants indicated they learned helpful information from the online forum, while 33 (75%) reported they did not use this forum.
Discussion
This dual-site RCT assessed the impact of a web-based self-management intervention on exercise capacity, physical activity, HRQoL, dyspnoea, disease education, social support and acute exacerbations. The intervention showed no significant change in the primary outcome, exercise capacity, despite having participants enrolled in the study for a duration of 6 months, which is longer than our prior work [11, 13]. Extending our previous work at a single site [11, 13], this dual-site study demonstrated significant and clinically meaningful increases in daily physical activity (1312 steps/day) in the intervention compared to the control group, independent of lung function and site. This change exceeds findings from both our prior studies (779 [11] to 804 [13] steps/day) and the minimum clinically important difference for daily step counts in COPD [32, 33]. There were no significant changes in HRQoL, dyspnoea, COPD knowledge, social support or acute exacerbations compared to controls. We hypothesised that use of the web-based intervention across 6 months would significantly improve exercise capacity, demonstrated by increased 6MWD. While we found strong evidence that our intervention improved physical activity, this improvement did not translate to improved exercise capacity. Previous research has shown weak correlations between exercise capacity and physical activity [30]. Exercise capacity, as measured by the episodic 6MWD, represents what the patient can do. Physical activity, integrated over time and in the home environment, represents what the patient does do, accounting for many factors (e.g. psychosocial, environmental) other than the underlying COPD that may impact physical activity [31].
Our results suggest that if technology-based self-management interventions are to improve exercise capacity, they will need to focus on both physical activity duration and intensity [32]. Our self-management intervention did not include an aerobic exercise training component as the goal was to promote walking, which most patients with COPD can do. The importance of exercise training can be seen in the PHYSACTO trial [33], which examined the effects of a self-management intervention coupled with placebo or tiotropium/olodaterol and with or without high intensity, in-person exercise training. The combination of a self-management intervention with pharmacological treatment and exercise training resulted in the greatest improvements in exercise capacity compared to self-management with placebo, or self-management with pharmacological treatment (and no exercise training) [33]. Similarly, a community-based exercise programme (including walking, cycling and strength exercises) coupled with a self-management programme (COPE-active) resulted in significant between-group differences in both exercise capacity and physical activity after 12 months [34]. An important difference between the current study and the PHYSACTO and COPE-active trials is that our self-management intervention, fully automated and remotely delivered, could be accessed by those who cannot attend in-person programmes.
Technology-mediated interventions vary in the level of automation and how self-guided they are (i.e. some may include more contact or guidance from research or clinical staff). Our technology-mediated intervention increased physical activity but not exercise capacity; this is in line with other technology-mediated trials which also used a more self-guided internet-mediated physical activity intervention [35]. In a contrasting example, Vorrink et al. [36] had participants who recently completed pulmonary rehabilitation use a smartphone app-mediated physical activity intervention for 12 months, with a physiotherapist-facing component that allowed for personalised text messaging. In that study, participants decreased physical activity but were able to maintain their post-rehabilitation exercise capacity levels [36]. Demeyer et al. [37] found increases in both physical activity and 6MWD in an intervention that included interviews with the investigator to discuss motivation, barriers, favourite activities and strategies to become more active. In addition, automatically updated goals could be changed by the research staff, and participants received text messages with activity proposals from the investigator, taking local weather into account. In our study, we had contact with the participant only when we called them weekly to give them their step-count goals and during the 3- and 6-month study visits. Taken together, it appears that technology-mediated interventions can be more widely accessible, but conventional in-person programmes, or a combination of both, may be more motivating and engaging given the accountability of in-person contact with healthcare professionals. Since one size does not fit all, it is important to develop and evaluate as many interventions as possible to give patients choices to support their exercise capacity, physical activity and COPD self-management. It is important to identify the type of programme that would allow for the greatest adherence and engagement for the unique circumstances of an individual patient.
Participants provided positive feedback about the intervention, indicating they would recommend it to others with COPD, that it helped them “stick” to their walking and that they would continue to walk after the study. Variable levels of engagement with the website as reported in the feedback questionnaire provide context for why we did not see significant changes in other secondary outcomes besides physical activity. Educational tips on COPD medications, symptom management, oxygen use, smoking cessation and acute exacerbations, as well as motivational messages, were provided on the website. The majority of participants looked at their step-count graphs, though almost half of participants indicated they did not look at the motivational and educational tips, and most participants did not look at the online forum. While technology-mediated interventions coupled with guidance from healthcare professionals may yield significant improvements, they likely require more staff labour, training and higher costs. Modification of our intervention with additional guidance or encouragement by research staff or clinical team members to increase use of its components may improve COPD self-management and associated outcomes.
While BIR participants (intervention and control groups) improved more than BOS participants in 6MWD and HRQoL, there was no statistical evidence that site modified the effect of the intervention on the specified outcomes. However, there was some evidence to suggest that for those assigned to the intervention, sites differed in their level of engagement with the physical activity promotion component. The differences observed in engagement by site may be explained by the notable differences in their sample characteristics and recruitment strategies. BIR participants appeared more limited by their disease (i.e. lower average 6MWD, FEV1 % predicted and daily step counts at baseline) compared to BOS and thus may have more potential for improvement. Additionally, BIR participants were recruited pragmatically by their usual provider, whereas BOS participants were recruited with a less personal approach through mailings by research staff. Thus, BIR patients may have been more motivated to increase their walking because of their personal connection with the provider recruiting for the research study. Recruitment at BIR may have been influenced by selection bias since participants who were more likely to be successful or engaged in the intervention may have been enrolled.
Using two sites, we demonstrate that our web-based self-management intervention can significantly improve physical activity at an urban site like BOS as well as a more rural site like BIR that enrolled participants with lower annual income and were more likely from rural areas. Along with greater risk for poor health status, acute exacerbations, hospitalisations and mortality, rural patients with COPD have limited access to in-person care and centre-based pulmonary rehabilitation programmes [38]. Thus, our intervention may be a “better than nothing” alternative to promote physical activity in a diverse group of patients with COPD. This trial compared our intervention to usual care. Future research would benefit from a comparative-effectiveness or inferiority trial to conventional pulmonary rehabilitation which is standard of care in promoting COPD self-management.
Limitations and future directions
Strengths include the randomised and blinded study design, dual-site approach, 6-month time frame, and examination of HRQoL, COPD knowledge, social support and acute exacerbations using validated questionnaires [39, 40]. Current guidelines for the 6MWT, published in 2014, recommend a practice walk 24 h before the actual test [41]. When we started this study in 2015, we chose not to include a practice walk to reduce participant burden and not require travel to the medical centre on six separate occasions (twice at baseline, 3 months and 6 months). Because we did not ask participants to perform a practice walk, there was a possibility that the within-group increase in 6MWD from baseline to 6 months in both groups could be attributable to a learning effect. However, it is known that this learning effect dissipates after 3 months [41]. We evaluated change in 6MWD after 6 months; therefore, any learning effect is likely to be even less. Additionally, although 6MWD consistently increased at the second walk test performed 2–5 days apart, Spencer et al. [42] demonstrated that changes in 6MWD at 3 months compared to baseline after pulmonary rehabilitation did not differ significantly from each other regardless of whether one chose the first, second or better 6MWD at each time point. Thus, our results examining change scores at 6 months are unlikely to be different had practice walks been performed. While the study expanded our past work with a more socioeconomically diverse sample, our cohort was self-selected and majority white male, limiting generalisability in some respects. The 6-month study period extends our prior work, and it is promising that significant improvements in daily step counts were maintained. Future work should extend the follow-up period beyond 6 months, focus on exercise intensity to optimise effects on exercise capacity and maximise engagement with other components of the intervention.
Conclusion
A web-based physical activity self-management intervention did not improve exercise capacity but improved physical activity in COPD over 6 months. Modifications are needed to optimise engagement with other components of the intervention to see changes in other health outcomes in COPD. It is important to develop and rigorously test novel, engaging and accessible self-management interventions that can reach a variety of patients with COPD.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00158-2021.SUPPLEMENT (281KB, pdf)
Acknowledgements
We thank the veterans for their participation in this research study, and the Data Safety and Monitoring Board (Huong Nguyen, Linda Nici and Elizabeth Klings).
Footnotes
This article has supplementary material available from openres.ersjournals.com
This study is registered at www.clinicaltrials.gov with identifier number NCT02099799. Data will not be shared outside the Dept of Veterans’ Affairs (VA), except as required under the Freedom of Information Act, since the dataset is comprised of secured VA data.
Conflict of interest: J.A. Cooper Jr has nothing to disclose.
Conflict of interest: R.L. Goldstein has nothing to disclose.
Conflict of interest: M. Polak has nothing to disclose.
Conflict of interest: P.N. Cruz Rivera has nothing to disclose.
Conflict of interest: D.R. Gagnon has nothing to disclose.
Conflict of interest: A. Samuelson has nothing to disclose.
Conflict of interest: S. Moore has nothing to disclose.
Conflict of interest: R. Kadri has nothing to disclose.
Conflict of interest: C.R. Richardson has nothing to disclose.
Conflict of interest: M.L. Moy has nothing to disclose.
Support statement: This work was supported by Merit Award Number I01 RX001150 from the US Dept of Veterans Affairs Rehabilitation Research and Development Service. S.A. Robinson is supported by the National Heart, Lung, and Blood Institute (K12HL138049). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Kochanek KD, Murphy SL, Xu J, et al. . Deaths: Final Data for 2017. Natl Vital Stat Rep 2019; 68: 1–77. [PubMed] [Google Scholar]
- 2.Celli BR, Decramer M, Wedzicha JA, et al. . An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191: e4–e27. doi: 10.1164/rccm.201501-0044ST [DOI] [PubMed] [Google Scholar]
- 3.Waschki B, Kirsten AM, Holz O, et al. . Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 295–306. doi: 10.1164/rccm.201501-0081OC [DOI] [PubMed] [Google Scholar]
- 4.Watz H, Pitta F, Rochester CL, et al. . An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44: 1521–1537. doi: 10.1183/09031936.00046814 [DOI] [PubMed] [Google Scholar]
- 5.Murphy LA, Harrington P, Taylor SJ, et al. . Clinical-effectiveness of self-management interventions in chronic obstructive pulmonary disease: an overview of reviews. Chron Respir Dis 2017; 14: 276–288. doi: 10.1177/1479972316687208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effing TW, Vercoulen JH, Bourbeau J, et al. . Definition of a COPD self-management intervention: international expert group consensus. Eur Respir J 2016; 48: 46–54. doi: 10.1183/13993003.00025-2016 [DOI] [PubMed] [Google Scholar]
- 7.Vogelmeier CF, Criner GJ, Martinez FJ, et al. . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 2017; 195: 557-582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 8.Robinson S, Troosters T, Moy M. Technology to enhance engagement in physical activity. In:Moy M, Blackstock F, Nici L, eds. The Art and Science of Enhancing Patient Engagement in Pulmonary Healthcare. Cham, Switzerland, Springer, 2020. [Google Scholar]
- 9.Ding H, Fatehi F, Maiorana A, et al. . Digital health for COPD care: the current state of play. J Thorac Dis 2019; 11: S2210–S2220. doi: 10.21037/jtd.2019.10.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spruit MA, Singh SJ, Garvey C, et al. . An Official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 11.Moy ML, Collins RJ, Martinez CH, et al. . An internet-mediated pedometer-based program improves health-related quality-of-life domains and daily step counts in COPD: a randomized controlled trial. Chest 2015; 148: 128–137. doi: 10.1378/chest.14-1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moy ML, Martinez CH, Kadri R, et al. . Long-term effects of an internet-mediated pedometer-based walking program for chronic obstructive pulmonary disease: randomized controlled trial. J Med Internet Res 2016; 18: e215. doi: 10.2196/jmir.5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan ES, Kantorowski A, Homsy D, et al. . Promoting physical activity in COPD: insights from a randomized trial of a web-based intervention and pedometer use. Respir Med 2017; 130: 102–110. doi: 10.1016/j.rmed.2017.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron LD, Leventhal H. The Self-Regulation of Health and Illness Behaviour. London, UK, Psychology Press, 2003. [Google Scholar]
- 15.Wan ES, Kantorowski A, Polak M, et al. . Long-term effects of web-based pedometer-mediated intervention on COPD exacerbations. Respir Med 2020; 162: 105878. doi: 10.1016/j.rmed.2020.105878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moy ML, Gould MK, Liu IA, et al. . Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res 2016; 2: 00062-02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaes AW, Garcia-Aymerich J, Marott JL, et al. . Changes in physical activity and all-cause mortality in COPD. Eur Respir J 2014; 44: 1199–1209. doi: 10.1183/09031936.00023214 [DOI] [PubMed] [Google Scholar]
- 18.Robinson S, Goldstein R, Cruz Rivera P, et al. Results from a Multi-Site Web-Based Physical Activity Intervention in COPD: Between Group and Site Differences. American Thoracic Society 2020 International Conference2020Virtual. [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the General US Population. Am J Respir Crit Care Med 1999; 159: 179–187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 20.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health 2005; 95: 1149–1155. doi: 10.2105/AJPH.2004.042432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart TL, Swartz AM, Cashin SE, et al. . How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Physical Activity 2011; 8: 62. doi: 10.1186/1479-5868-8-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ATS. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 23.Jones PW, Quirk FH, Baveystock CM, et al. . A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 24.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93: 580–586. doi: 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 25.White R, Walker P, Roberts S, et al. . Bristol COPD Knowledge Questionnaire (BCKQ): testing what we teach patients about COPD. Chron Respir Dis 2006; 3: 123–131. doi: 10.1191/1479972306cd117oa [DOI] [PubMed] [Google Scholar]
- 26.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991; 32: 705–714. doi: 10.1016/0277-9536(91)90150-B [DOI] [PubMed] [Google Scholar]
- 27.Redelmeier DA, Bayoumi AM, Goldstein RS, et al. . Interpreting small differences in functional status: the six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997; 155: 1278–1282. doi: 10.1164/ajrccm.155.4.9105067 [DOI] [PubMed] [Google Scholar]
- 28.Demeyer H, Burtin C, Hornikx M, et al. . The minimal important difference in physical activity in patients with COPD. PLoS One 2016; 11: e0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teylan M, Kantorowski A, Homsy D, et al. . Physical activity in COPD: minimal clinically important difference for medical events. Chron Respir Dis 2019; 16: 1479973118816424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moy ML, Danilack VA, Weston NA, et al. . Daily step counts in a US Cohort with COPD. Respir Med 2012; 106: 962–969. doi: 10.1016/j.rmed.2012.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koolen EH, van Hees HW, van Lummel RC, et al. . “Can do” versus “do do”: a novel concept to better understand physical functioning in patients with chronic obstructive pulmonary disease. J Clin Med 2019; 8: 340. doi: 10.3390/jcm8030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris NR, Walsh J, Adams L, et al. . Exercise training in COPD: what is it about Intensity? Respirology 2016; 21: 1185–1192. doi: 10.1111/resp.12864 [DOI] [PubMed] [Google Scholar]
- 33.Troosters T, Maltais F, Leidy N, et al. . Effect of bronchodilation, exercise training, and behavior modification on symptoms and physical activity in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2018; 198: 1021–1032. doi: 10.1164/rccm.201706-1288OC [DOI] [PubMed] [Google Scholar]
- 34.Effing T, Zielhuis G, Kerstjens H, et al. . Community based physiotherapeutic exercise in COPD self-management: a randomised controlled trial. Respir Med 2011; 105: 418–426. doi: 10.1016/j.rmed.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 35.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. . Long-term Efficacy and Effectiveness of a Behavioural and Community-based Exercise Intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J 2018; 52: 1800063. doi: 10.1183/13993003.00063-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vorrink SN, Kort HS, Troosters T, et al. . Efficacy of an mHealth intervention to stimulate physical activity in copd patients after pulmonary rehabilitation. Eur Respir J 2016; 48: 1019–1029. doi: 10.1183/13993003.00083-2016 [DOI] [PubMed] [Google Scholar]
- 37.Demeyer H, Louvaris Z, Frei A, et al. . Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax 2017; 72: 415–423. doi: 10.1136/thoraxjnl-2016-209026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson BE, Coultas DB, Suzuki S, et al. . Rural-urban disparities in quality of life among patients with COPD. J Rural Health 2013; 29: Suppl. 1, s62–s69. doi: 10.1111/jrh.12005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blackstock FC, Lareau SC, Nici L, et al. . Chronic Obstructive Pulmonary Disease Education in Pulmonary Rehabilitation. An Official American Thoracic Society/Thoracic Society of Australia and New Zealand/Canadian Thoracic Society/British Thoracic Society Workshop Report. Ann Am Thorac Soc 2018; 15: 769–784. doi: 10.1513/AnnalsATS.201804-253WS [DOI] [PubMed] [Google Scholar]
- 40.Reijnders T, Schuler M, Jelusic D, et al. . The impact of loneliness on outcomes of pulmonary rehabilitation in patients with COPD. COPD 2018; 15: 446–453. doi: 10.1080/15412555.2018.1471128 [DOI] [PubMed] [Google Scholar]
- 41.Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society Technical Standard: Field Walking Tests in Chronic Respiratory Disease. Eur Respir J 2014; 44: 1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 42.Spencer LM, Alison JA, McKeough ZJ. Six-minute walk test as an outcome measure: are two six-minute walk tests necessary immediately after pulmonary rehabilitation and at three-month follow-up? Am J Phys Med Rehabil 2008; 87: 224–228. doi: 10.1097/PHM.0b013e3181583e66 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00158-2021.SUPPLEMENT (281KB, pdf)