Abstract
Rosins and turpentines present in pine resins have been widely used both industrially (coatings, electronics and paint) and pharmaceuticals (ointments). Among the main components of these matrices is abietic acid. This study focuses on the characterization and evaluation of the quality of rosins and turpentines in addition to the quantification of abietic acid in rosins. Rosin and spirit of turpentine were obtained separately through the distillation method from P patula and P. oocarpa resin, species grown in the Department of Cauca-Colombia. Resin-tapping was made using the traditional method (cup and gutter). Quality indicators were determined according to ASTM standards. Solubility tests and identification of functional groups were performed on the obtained rosin by ultraviolet-visible spectroscopy (UV-Vis), infrared (IR) and nuclear magnetic resonance (1H-NMR). The abietic acid present in the rosins was determined by high performance liquid chromatography (HPLC). According to their high acid value and low percentage of unsaponifiable matter, the extracted rosins are considered of medium-high quality. Quantification of abietic acid by HPLC showed 14.85 ± 0.24% and 16.09 ± 0.11% for P. patula and P. oocarpa rosin respectively.
Keywords: Pinus patula, Pinus oocarpa, HPLC, Abietic acid, Characterization
Pinus Patula; Pinus oocarpa; HPLC; Abietic acid; Characterization
1. Introduction
Polymers are part of today's way of life. The vast majority of polymeric products used today are derived from fossil fuels. Due to continuous consumption of oil reserves and the adverse environmental effects of several of its uses, new alternatives are being sought in renewable natural resources. Plant biomass is a source of polymers with important properties and applications, which has recently captured more and more interest due to its abundance, low cost and easy chemical modification [1, 2]. Pine resin is just one of the many NWFPs (Non-Wood Forest Products) derived from coniferous forests, especially the genus Pinus. Resin-tapping is a forest activity that aims to extract the oleoresin which flows from the pines during the year. This is done through pikes in selected trees [3, 4]. The technology used is the downward pike system (herringbone).
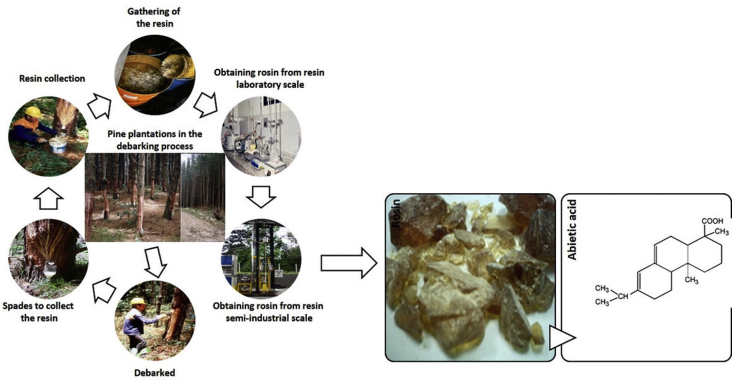
In Colombia and other countries, oleoresin is extracted from this variety of pine to extract rosin and turpentine [5, 6, 7]. Currently, resin is used in the manufacture of waxes, paints, soaps, adhesives, and pharmaceutical products, among others [8]. Industries include these products or the precursors to manufacture them from gum resin or its two main fractions: rosin and turpentine. Figure 1 shows the pine resin and rosin that can be obtained from the resin.
Figure 1.
Pine resin and abietic acid, major component of rosin. Own elaboration.
The process begins with the debarking in plantations of patula pine and oocarpa pine with ages greater than 16 years. Once the debarking has been carried out, the pikes or wounds are made by placing the resin collecting container. Later, the resin is collected from the pines in buckets which are transported to the collection center. With the resin obtained, it is processed on a laboratory scale to obtain rosin and turpentine, adjusting the physical variables. Finally, the process is carried out on a semi-industrial scale.
Turpentine intended for use as a solvent, fragrance and flavor compounds and other derivatives since its composition is mainly formed by α-pinene, β-pinene and δ-3-carene.
Rosin is obtained through the distillation process of pine resin. The most important uses of rosin are: manufacture of adhesives, printing ink, insulating materials for the electronics industry, synthetic rubber, chewing gum, soaps and detergents due to its mixture between abietic acid and isopimaric acid [6, 9, 10, 11, 12, 13]. Rosin remains as a non-volatile fraction after distillation for turpentine separation. It is a glassy, crystalline and brittle solid. Most of the rosin is used and chemically modified [14, 15]. It is mainly made up of 10–20% neutral compounds (Terpenes) and 80–90% resin acids [16], a mixture of isomeric organic acids from abietic acid (40–60%) [17, 18].
Abietic acid has a rigid tricyclic skeleton, possessing two double bonds and a carboxylic functional group. Today, rosin has been used in homopolymerization and copolymerization with fatty esters derivatives using the ADMET (Acyclic Diene Metathesis) methodology to obtain thermoplastic polymers [2, 19].
Modified rosins have also been obtained for different purposes by hydrogenation, polymerization and disproportionation [20]. Rosin extracts are used in the synthesis of Al2O3 nanoparticles, among other applications [21, 22].
The acid fraction of rosin is mainly made up of abietic, pimaric and labdanic acid, where each acid group contains numerous isomers [23]. These acids can be oxidized and they can undergo isomerization under high temperature [24]. The melting point of rosins is estimated between 70 °C and 85 °C. Thermal degradation of rosin is typically measured by thermogravimetry (TGA) starting at 215 °C and concluding at 295 °C [25]. Turpentine is a clear, flammable liquid with a pungent odor and sour to taste. The boiling point of turpentine starts at around 150 °C, being volatile terpenic hydrocarbons (C10H16) its main components, which can vary depending on the growth site, the tree species and the distillation process. Turpentine is mainly composed of α-pinene (65–75%) and β-pinene (20–26%), and small amounts of camphene, limonene, 3-carene and terpinolene.
Turpentine is usually used as a paint thinner and varnishes or as a cleaning agent. Derivatives are widely used in fragrances and vitamins [26]. Synthetic pine oil used in disinfectants, cleaning agents, and other pine-scented products is the main derivative of turpentine [27, 28, 29].
There have been reports of many different analytical techniques used for the characterization of rosins, however, the analysis of the resin acids are generally including GC, pyrolysis-GC-MS, CE, HPLC, UV-Vis spectroscopy, H-NMR and TLC [30, 31, 32].
In this study, rosin was obtained from Pinus patula and Pinus oocarpa species using the distillation method. Quality tests for the resin, rosin and turpentine were carried out. Physicochemical properties of the obtained products were determined and the characterization of the rosin was performed using spectroscopic techniques. Finally, the abietic acid content of these two species grown in the Department of Cauca was determined by high-resolution liquid chromatography.
2. Material and methods
Cajibio area has a humid mesophytic forest (semi-humid forest of the sub-Andean zone) with an altitude of 1840 m.a.s.l., latitude: 2° 31´ (north), longitude: 76° 35´ (west), a frost-free temperature between 15 and 20 °C, (maximum fluctuations) and light winds or storms. The Sotará area has an average altitude of 2726 m.a.s.l., latitude: 2° 15´ (north), longitude: 76° 36´ (west).
The studied plantations are industrial plantations to obtain pulp for paper with ages of 18 years, these plantations are not carried out any type of drilling or treatment before the study. The study was carried out two years before being cut down for use. In these plantations there are 1,111 trees planted per hectare planted.
The Pinus patula plantation is located in the village of Chiribio, la Catana, municipality of Sotará Cauca. This path belongs to the central mountain range which is made up of metamorphic rocks with a laminar structure and igneous rocks, product of the eruptions of the Sotará volcano. (CONIF, 1998). The relief is strongly inclined to strongly broken with slopes of 7, 12, 25, 50 and even 75%. They are medium to slightly acid soils, becoming less acidic with depth (5.6–6.4); with high and very high organic carbon contents in the first 100 cm, and very low in the total bases. In the case of the Pinus oocarpa plantation, it is located in the municipality of Cajibío Cauca. The relief presents a gentle slope between 7 and 38%. Acidic soils with influence from the Puracé volcano. (CONIF, 1998). Table 1 shows the main characteristics of the two plantations studied [33].
Table 1.
Main characteristics of Pinuspatula and Pinus oocarpa plantations.
| Plantation | ∗m.a.s.l. | Weather (°C) | Coordinates | Soil Acidity (pH) | Topography (%) | Precipitation/Year (mm) | Daylight (h/month) |
|---|---|---|---|---|---|---|---|
| Sotará | 2000–3000 | 12–18 | 2° 18’ .05.50″ N 76° 35′ 41.41″ O |
5,6–6,4 | 7–75 | 40,6–403,6 | 101,8–171,2 |
| Cajibío | 1700–1840 | 15–24 | 2° 31’ .18.28″ N 76° 35′ 56.69″ O |
5,3–5,5 | 7–38 | 1028,5–3085,5 | 240,0–300,0 |
∗m.a.s.l. Meters above sea level.
The resin-tapping method used was the cup and gutter method, in which shallow v-shaped cuts (scarifications) are conducted on the resin surface at chest level, which is previously prepared with a debarking ax. Scarifications were performed at intervals during the growing season, and their frequency varies according to weather conditions [29].
Spectroscopic characterization was carried out by using ultraviolet visible spectroscopy (UV-Vis), infrared (IR) and proton nuclear magnetic resonance (1H-NMR).
2.1. Samples
Collection of P. patula resin samples (18 years) was carried out in forest plantations located in the municipality of Sotará (Cauca-Colombia). P. oocarpa resin samples (18 years) were taken from plantations located in the municipality of Cajibio (Cauca-Colombia).
2000 patula pine trees and 2000 oocarpa pine trees were resined for 12 months. The pikes or wounds were made every three days for 12 months.
2.2. Resin distillation
The physical process of pine resin distillation was conducted to separate the turpentine from the rosin. In this case, turpentine is the volatile substance since its boiling point, at normal pressure is 156 °C, while the rosin boils at normal pressure in the range from 225 °C to 320 °C being it the heavy fraction. P. patula and P. oocarpa resin samples were placed in balloons attached through a condenser to a vacuum trap. The samples were distilled in a system where temperature was controlled to 60 °C during 3–4 h, then the temperature was increased to 320 °C. The distilled turpentine was separated from the solid rosin for characterization [29].
2.3. Determination of resins quality indicators
The quality of the resin, rosin and turpentine is mainly determined by the physicochemical variables such as acidity index, saponification index, unsaponifiable matter among others [34, 35, 36, 37, 38, 39]. The acidity index, saponification index, unsaponifiable matter, color, melting point and humidity were determined for the rosins. The acidity index, degree of esterification, soluble solids and density were determined for turpentine samples. The quality of rosin and turpentine obtained in this study was compared with the international standards ASTM (American Society for Testing and Materials) D1392 and the Bureau Indian Standards (IS 533: 1973) [29, 40].
2.4. Characterization of the obtained rosin
Quality tests for the resin, rosin and turpentine were applied. Physicochemical properties of the obtained products were determined and the characterization of the rosin was performed using spectroscopic techniques. Finally, the abietic acid content of these two species grown in the Department of Cauca was determined by using high-performance liquid chromatography (HPLC).
The melting point and the physical characteristics of the obtained rosins were determined. Solubility tests were applied in water, methanol, acetonitrile and hexane. Ultraviolet-visible spectra, infrared and proton nuclear magnetic resonance (1H-NMR) [14] were taken in both species of rosin.
2.5. HPLC analysis
A Waters 1515 brand liquid chromatograph and a μ-Bonda Pack C18 column at 60 °C protected by a C18 guard column were used. Acetonitrile-Water-Phosphoric Acid (ACN/H2O/H3PO4) was used as mobile phase, (60:40:0.1). An isocratic elution was performed at a flow rate of 1 mL/min and detection was carried out at 241 nm using a Waters brand UV Vis 2487 detector. All samples were filtered through 0.45 μm PALL GHP Acrodisc brand membrane filters. A 98% abietic acid standard (SIGMA) was used to determine abietic acid content in the Pinus patula and Pinus oocarpa rosin samples. Besides, the quantification on an external standard calibration curve [30] was performed.
3. Results and discussion
Turpentine and rosin percentage present in the resin samples was established through distillation process. A rosin percentage of 74.55±(0.02)% and 75.00±(0.03)%, and a turpentine percentage of 14.55±(0.02)% and 14.40±(0.03)% for P.patula and P. oocarpa resin respectively was obtained. These values are very close to those reported by other authors for species such as P. caribea with 70.0%–75.0% of rosin, and approximately 15.0% of turpentine [41]. Rosin extracted from Pinus pinaster distillation grown in Morocco has shown percentages between 64% and 70% [41]. Abietic acid is a by-product of the rearrangement of pimaric acid, neoabietic acid and palustric acid during the distillation process [42]. At high temperatures and through disproportion abietic acid is converted into dehydroabietic acid, dihydroabietic acid and tetrahydroabietic acid. When rosin is stored for long periods of time under specific conditions, the proportion of original resin acids of rosin, including abietic acid and dehydroabietic acid, tend to decrease due to oxidation forming hydroperoxides, epoxides or hydroxylated compounds [20]. High percentages of obtained rosin suggest its production potential. China, some Latin American countries and Portugal are major producers of rosin [21]. The United States, Finland, Switzerland and some countries of the Soviet Union are major producers of rosin obtained from the wood treatment process. Approximately, 1.1 million tons of rosin are produced annually, and most of it is modified to obtain different by-products [43]. Table 2 shows the quality indicators of Pinus patula and Pinus oocarpa resin, rosin and turpentine. These results show medium-high acidity index and saponification index for the Pinus patula and Pinus oocarpa resins, as well as a low content of unsaponifiable material. The acid values from 136.20 to 138.0 and from 134.0 to 137.35 for Pinus patula and Pinus oocarpa resin respectively indicate the good quality of the extracted resin and a high proportion of resin acids in its composition, compared to Pinus caribea var. caribea with acidic index between 140 and 145 [42]. The amount of unsaponifiable matter and impurities was low for the two analyzed species of resins. Moisture content was low, under 5.0% for the two resin samples.
Table 2.
Quality indicators of P. patula and P. oocarpa resin, rosin and turpentine.
|
Pinus Resin | ||||||
|---|---|---|---|---|---|---|
| Sample | Acidity index (mg KOH.g−1 sample) | Saponification index (mg KOH.g−1 sample) | Unsaponifiable matter (%) | Impurities % | Moisture % | |
| P.PAT | 134.39–140.0 | 136.20–138.0 | 22.33 | 0.43–1.13 | 3.23–4.07 | |
| P.OOC | 133.89–135.0 | 134.0–137.35 | 24.50 | 0.43–1.16 |
2.62–5.68 |
|
| Turpentine | ||||||
| Sample |
Acidity index (mg KOH.g−1 sample) |
Degree of esterification |
Refractive index | Boiling point °C |
Density g/mL |
|
| P.PAT | 0.40–0.46 | 0.30–0.33 | 1.484 | 154–157 | 0.852 | |
| P.OOC | 0.41–0.45 |
0.31–0.35 |
1.520 | 153–156 |
0.861 |
|
| Rosin | ||||||
| Sample |
Acidity index (mg KOH.g−1 sample) |
Saponification index (mg KOH.g−1 sample) |
Unsaponifiable matter (%) |
Color |
Melting point °C |
Moisture% |
| P.PAT | 155–158 | 153.7–160 | 4.8–7.6 | M | 76.7–78 | 0.89–1.15 |
| P.OOC | 155–158 | 158–160 | 5.0–7.7 | N | 76.5–78 | 0.75–1.15 |
P.PAT: Pinus patula; P.OOC: Pinus oocarpa.
The best quality rosins usually have an acid value in the range of 160–170 [34]. The Pinus patula and Pinus oocarpa rosins showed acidity index between 158 and 160 respectively. Oxidized material is present in rosin due to air exposure. Abietane-type resin acids with conjugated double bonds tend more to oxidation than dehydroabietic acids and pimaran-type acids. The percentage of unsaponifiable matter indicates the amount of non-acidic material, therefore, the lower this value, the higher quality of the rosin. The analyzed rosins showed unsaponifiable matter percentages between 4.8 and 7.7. Percentages of unsaponifiable matter above 10% may indicate low quality rosins [41]. There are no international standards for rosin, although ASTM describes standard tests, companies in the rosin industry have their own quality specifications, varying from company to company. This makes generalization of analytical data difficult. Pinus patula and Pinus oocarpa rosins presented M and N classification colors respectively. This classification make of them medium rosins according to the United States Department of Agriculture. Pinus caribea rosins presented X–WW classification colors which categorize them as clear-type rosins [41,35,36].
Rosin consists mainly of diterpenic acids representing 90% of the total weight and the remaining 10% consists of a mixture of esters, alcohols, aldehydes and hydrocarbons. The acidic fraction consists mainly of three groups of abietic, pimaric and labdanic acids [25]. Rosin is mainly used in the manufacture of adhesives, printing inks, electronics industry, synthetic rubber, rubbers, soaps and detergents. There are quality standards for turpentine established by ASTM D 13 92 and the Bureau Indian Standards (IS 533: 1973), which are based on the evaluation of the turpentine quality to be used as a solvent. Generally, the tested quality parameters are density, refractive index, evaporation and distillation residues, among others. Pinus patula and Pinus oocarpa turpentines presented acidity index between 0.40 and 0.46, and degree of esterification between 0.30 and 0.35. Turpentines obtained from Pinus caribea have presented degree of esterification between 0.27 to 0.36. The analyzed turpentines presented refractive indices between 1.48 and 1.52, boiling points between 153 and 154, and density between 0.85 and 0.86 g/mL. Turpentines obtained from Pinus caribea have presented acidity index of 1.46 and density of 0.86 g/mL [37,38,39].
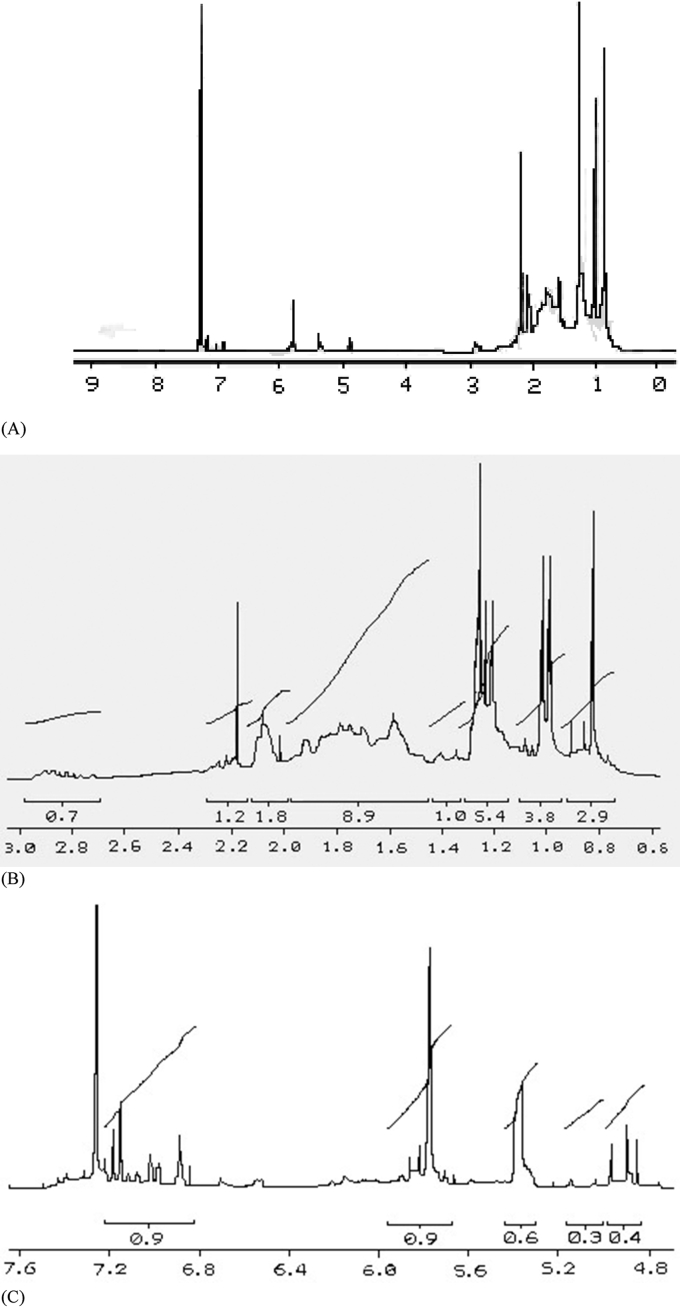
Rosin and turpentine obtained from Pinus patula and Pinus oocarpa resins meet the quality standards according to the ASTM (American Society for Testing and Materials) standards. Pinus patula and Pinus oocarpa rosins obtained from the distillation process showed solubility in methanol, acetonitrile and hexane but insolubility in water. Resin acids of the abiethane series that make up the rosins have a double bond system and an isopropyl group as a substituent in the third ring, and those of the pimaran series have a vinyl group and a methyl group in the same position [43]. The UV-Vis spectra of the Pinus patula and Pinus oocarpa rosins showed their most representative bands at 216 and 236 nm respectively. These absorption bands correspond to n-π∗ and π- π∗ transitions that show C=O and C=C double bonds. Figure 2 (A and B) shows ultraviolet (UV) spectra of the P. patula and P. oocarpa rosins. Infrared (IR) spectra for Pinus patula and Pinus oocarpa rosins (Figure 2, C and D) showed broad bands at 3500 cm−1, characteristics of tension vibrations of the –OH groups from the carboxylic acid groups of the rosins. They also showed a band similar to 1690 cm−1 of C=O from the carboxylic acids of the rosins, some bands at 1460 cm−1 to a C–H asymmetric deformation. Bands at 1240 cm−1 to C–O bonds, a band at 550.64 cm−1 to a C–O vibration, and a band at 829.33 cm−1 corresponding to –OH elongations of aromatic nucleus. Bands at 650 and 820 cm−1 that correspond to C–H bends of aromatic nucleus are observed.
Figure 2.
UV spectra (A and B) and IR (C and D) of P. patula and P. oocarpa rosins respectively.
There were not big differences between 1H-NMR spectra of Pinus patula and Pinus oocarpa rosins. 5 characteristic regions in the 1H-NMR spectrum (protonic) for Pinus patula and Pinus oocarpa rosins were differentiated: 1. 0.5–1ppm (2 signals, singlet and doublet-doublet, methyl group region, –CH3). 2. 1.0–1.4ppm (4 signals, singlets, methyl group region, –CH3). 3. 1.4–2.2ppm (signal groups with great multiplicity, methyl group region, –CH2). 4. 4.8–6.0ppm (3 signals, olefinic region, endo- and exocyclic bonds, typical for abietic acid, –C=C). 5. 6.8–7.4ppm (4 signals, 3 aromatic protons region, typical for dehydroabietic acid). Figure 3 (A, B and C) shows the proton NMR spectra for Pinus patula and Pinus oocarpa rosins respectively. Figures 3(B) and 3(C) are enlargements of the spectral zone between 0.6-3.0 ppm and 4.8–7.6 ppm respectively.
Figure 3.
1H-NMR spectra of Pinus patula (A) and Pinus oocarpa (B, C) rosins respectively.
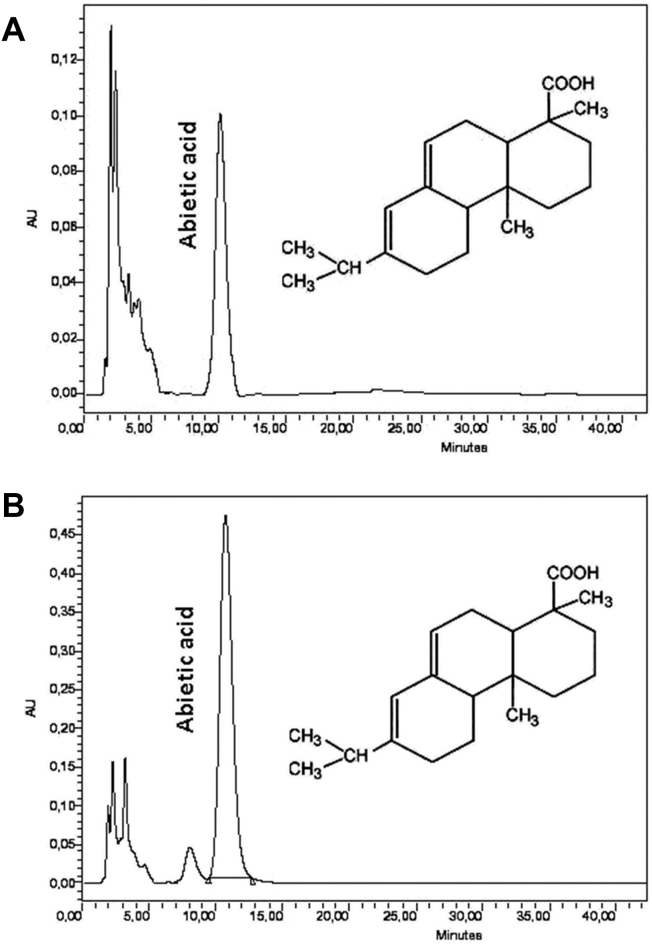
Pinus patula and Pinus oocarpa rosin samples were taken to perform their chromatographic analysis and determine the abietic acid content. Analysis of rosin samples by HPLC was conducted by using a C18 column (μ-Bondapak C18 150 × 3.9 mm), a mobile phase made up of acetonitrile-water-phosphoric acid (ACN/H2O/H3PO4), (60:40:0.1), run time of 20 min at a flow of 1.0 mL/min and a 60 °C temperature. The wavelength of the detector was at 241 nm [29].
Chromatographic method was validated by using the abietic acid standard to determine its content in Pinus patula and Pinus oocarpa rosins. Linearity of the method was good, showing a range of 10–100 ppm (r2 = 0.9980), a detection limit of 0.091 ppm, and a quantification limit of 0.304 ppm. The method presented good repeatability in the concentration range examined with values of % RSD (Relative Standard Deviation Percentages) between 0.335 and 1.128, not exceeding 5%, acceptance criterion for the abietic acid analysis. The method is reproducible because the % RSD values are below 5% (0.040–0.964%). The method is accurate presenting a 1.163 tobt, lower than the 2.31 ttab for eight (8) degrees of freedom and 95% confidence. Figure 4 (A and B) shows chromatograms of Pinus patula and Pinus oocarpa rosins respectively. The samples presented abietic acid as the major component.
Figure 4.
Liquid chromatograms of P. patula (A) and P. oocarpa (B) rosins respectively.
Chromatographic method was validated through optimal separation conditions of the abietic acid present in the rosin samples. Abietic acid presented a retention time of 12.20 ± 0.20 min, which allowed its identification and quantification in the analyzed samples.
C18 ODS columns allowed good separation between abietic acid and dehydroabietic acid using a mobile phase made up of acetonitrile-water for LC-MS [30]. A C18 column and a gradient water-acetonitrile mobile phase (40–100%) allowed the successful separation of levopimaric acids, palustric acid, abietic acid and dehydroabietic acid present in Pinus caribbaea using an ultraviolet detector [44]. High performance liquid chromatography (HPLC) has displayed better results in the separation of rosin components when compared to other techniques such as gas chromatography (GC), since it allows separation at room temperature preventing isomerization of resin acids [45]. In addition, the use of HPLC prevents rosin derivatization, chromatographic and extraction conditions are mild, and the recovery of its components is simple, when it is solubilized in organic solvents such as methanol, ethanol and acetonitrile. This is an advantage when conducting biological studies and determining its bioactive properties [20]. Techniques such as gas chromatography-mass spectrometry (GC-MS), capillary electrophoresis (CE) and thin-layer chromatography (TLC) [23] have also been implemented to separate rosin components. P. oocarpa rosin samples presented the highest percentage of abietic acid with 16.09 ± 0.11%, while P. patula rosin samples presented 14.85 ± 0.24% abietic acid [31]. The composition of the resin depends on genetic, environmental factors, fertilization, tree age, variations in genetic composition of founding plant material, environmental-related factors, tapping season, weather, among others [17, 18, 46]. An F test and a T test were taken to determine the existence of a statistical difference between the abietic acid contents of the two pine species. The F test yielded a value of 4.42, lower than the critical value of 19.0, and the t value indicated a value of -7.99, lower than the critical t of 2.77. Statistical tests indicated non-existence of significant differences in abietic acid content between Pinus patula and Pinus oocarpa species at 95% confidence. The abietic acid contents of P. patula and P. oocarpa rosins were lower than those of P. ellioti var. elliotti (19%), P. palustris (18%), P. ponderosa (22%), P. pinaster (26%) and P. caribea (19%) rosins [45, 48, 49]. Pinus merkussi rosins grown in Indonesia have presented methyl-dehydroabietic acid as the major component reaching percentages of 27–28% [46, 50].
4. Conclusions
Pinus patula and Pinus oocarpa resin components showed overall physicochemical parameters compatible with medium to high quality based on industry standards. Moreover, abietic acid, one of the main industrially relevant compounds derived from rosin, was well represented in this fraction of the resins of both species. Data indicate that pine forests of grown in the Department of Cauca, Colombia, represent a valuable source of pine chemicals.
With the development of this research, it was possible to find possible processes that would allow the use, with a good added value, of a potential raw material that in some regions, due to its poor disposal, constitutes a polluting waste.
Declarations
Author contribution statement
Rodrigo A. Sarria-Villa: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
José A. Gallo-Corredor: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ricardo Benítez-Benítez: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Minciencias, Cootraforc, Vicerrectoria de Investigaciones (VRI) 4423-08-12083 (Contract 450 -2002) and the Department of Chemistry of the University of Cauca (501100005682).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the University of Cauca, the Chemistry of Natural Products research group (QPN) and the Environmental Analytical Chemistry research group (GIQA).
References
- 1.Neis F.A., De Costa F., De Araújo A.T., Fett J.P., Fett-Neto A.G. Multiple industrial uses of non-wood pine products. Ind. Crop. Prod. 2019;130:248–258. [Google Scholar]
- 2.Llevot A., Grau E., Carlotti S., Grelier S., Cramail H. Dimerization of abietic acid for the design of renewable polymers by ADMET. Eur. Polym. J. 2015;67:409–417. [Google Scholar]
- 3.De Oliveira C., De Araújo A., De Lima J., De Costa F., Füller T. Resin tapping transcriptome in adult slash pine (Pinus elliottii var. elliottii) Ind. Crop. Prod. 2019;139:111545. [Google Scholar]
- 4.Soliño M., Yu T., Alía R., Auñón F., Bravo-Oviedo A., Chambel M.R. Resin-tapped pine forests in Spain: ecological diversity and economic valuation. Sci. Total Environ. 2018;625:1146–1155. doi: 10.1016/j.scitotenv.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Calderón M. 2011. Renacimiento en el trópico. [Google Scholar]
- 6.Osorio C., Cueva G., Gonzáles H. Caracterización de oleorresina de Pinus caribaea obtenido por el sistema de pica de corteza con ácido sulfúrico. Revista Forestal del Perú. 2016;31:58–68. [Google Scholar]
- 7.Wang H., Malcolm D., Fletcher A. Pinus caribaea in China: introduction, genetic resources and future prospects. For. Ecol. Manag. 1999;117:1–15. [Google Scholar]
- 8.Wang J., Lu Ch, Liu Y., Wang Ch, Chu F. Preparation and characterization of natural rosin stabilized nanoparticles via miniemulsion polymerization and their pressure-sensitive adhesive applications. Ind. Crop. Prod. 2018;124:244–253. [Google Scholar]
- 9.Bhomick P.C., Supong A., Baruah M., Pongener C., Sinha D. Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: isotherm, kinetic, thermodynamic and regeneration studies. Sustain. Chem. Pharm. 2018;10:41–49. [Google Scholar]
- 10.Neis F.A., de Costa F., de Almeida M.R., Colling L.C., de Oliveira C.F. Resin exudation profile, chemical composition, and secretory canal characterization in contrasting yield phenotypes of Pinus elliottii Engelm. Ind. Crop. Prod. 2019;132:76–83. [Google Scholar]
- 11.Casal A., Martín E., Gutiérrez L., Fonseca M., Navarro A., Valdés M., Spengler B., Guerra S. Oleorresina de pinos. Una nueva fuente para la obtención de aditivos químicos. Cienc. Ergo Sum. 2005;12(1):64–70. [Google Scholar]
- 12.Barnola L., Ceden A. Inter-population di!erences in the essential oils of Pinus caribaea needles. Biochem. Systemat. Ecol. 2000;28:923–931. doi: 10.1016/s0305-1978(00)00036-3. [DOI] [PubMed] [Google Scholar]
- 13.Coppen J., Hone G. Vol. 2. FAO; 1995. Gum naval stores: turpenti•n e and rosin from pine resin . Non-wood forest products. [Google Scholar]
- 14.Mirabedini S.M., Zareanshahraki F., Mannari V. Enhancing thermoplastic road-marking paints performance using sustainable rosin ester. Prog. Org. Coating. 2020;139:1–9. [Google Scholar]
- 15.Costa I.L., Alves A.R., Mulinari D.R. Surface treatment of Pinus elliottii Fiber and its application in composite materials for reinforcement of polyurethane. Proc. Eng. 2017;200:341–348. [Google Scholar]
- 16.Kim K.H., Daugaard T.J., Smith R., Wright M.M., Brown R.C. Recovery of resin acids from fast pyrolysis of pine. J. Anal. Appl. Pyrol. 2019;138:132–136. [Google Scholar]
- 17.De Oliveira C.F., Duz J.V., Kerber M.R., Wieczorek J., Galvan J.L. Resinosis of young slash pine (Pinus elliottii Engelm.) as a tool for resin stimulant paste development and high yield individual selection. Ind. Crop. Prod. 2019;135:179–187. [Google Scholar]
- 18.Ferreira A.G., Fior C.S., Gualtieri S.C. Oleoresin yield of Pinus elliottii Engelm seedlings. Braz. J. Plant Physiol. 2011;23:313–316. [Google Scholar]
- 19.Lu C., Yu J., Wang C., Wang J., Chu F. Fabrication of UV-absorbent cellulose-rosin based thermoplastic elastomer via “graft from” ATRP. Carbohydr. Polym. 2018;188:128–135. doi: 10.1016/j.carbpol.2018.01.062. [DOI] [PubMed] [Google Scholar]
- 20.Jia P., Ma Y., Feng G., Hu L., Zhou Y. High-value utilization of forest resources: dehydroabietic acid as a chemical platform for producing non-toxic and environment-friendly polymer materials. J. Clean. Prod. 2019;227:662–674. [Google Scholar]
- 21.Neis F., Costa F., Araújo A., Palma J., Fett-Neto A. Multiple industrial uses of non-wood pine products. Ind. Crop. Prod. 2019;130:248–258. [Google Scholar]
- 22.Berglund L.A., Burgert I. Bioinspired wood nanotechnology for functional materials. Adv. Mater. 2018;30:1–15. doi: 10.1002/adma.201704285. [DOI] [PubMed] [Google Scholar]
- 23.Sifontes Á., Gutierrez B., Mónaco A., Yanez A., Díaz Y. Preparation of functionalized porous nano-γ-Al2O3 powders employing colophony extract. Biotechnol. Rep. 2014;4:21–29. doi: 10.1016/j.btre.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauhan P., Chen H., Goswami S.R., Yan N. Improved mechanical properties of flexible bio-based polymeric materials derived from epoxy mono/di-abietic acid and soyabean oil. Ind. Crop. Prod. 2019;138:1–8. [Google Scholar]
- 25.Hasan A.M., El-Saeed A.M., Al-Shafey M.A., El-Sockary M.A., El-Ghazawy R.A. A preliminary study on liquid crystalline epoxy curatives from natural abietic acid. Egypt. J. Petrol. 2019;28:127–136. [Google Scholar]
- 26.Cabaret T., Gardere Y., Frances M., Leroyer L., Charrier B. Measuring interactions between rosin and turpentine during the drying process for a better understanding of exudation in maritime pine wood used as outdoor siding. Ind. Crop. Prod. 2019;130:325–331. [Google Scholar]
- 27.Zhu S., Xu S., Yi X., Wang J., Zhao Z. High value-added application of turpentine as a potential renewable source for the synthesis of heterocyclic Schiff base derivatives of cis-1,8-p-menthane-diamine serving as botanical herbicides. Ind. Crop. Prod. 2018;115:111–116. [Google Scholar]
- 28.Salvador V.T., Silva E.S., Gonçalves P.G., Cella R. Biomass transformation: hydration and isomerization reactions of turpentine oil using ion exchange resins as catalyst. Sustain. Chem. Pharm. 2020;15:1–23. [Google Scholar]
- 29.Gallo-Corredor J.A., Sarria-Villa R.A. Determinación de ácido abiético en colofonia extraída de la resina de Pinus patula presente en los bosques forestales caucanos empleando cromatografía líquida de alta resolución. Journal de Ciencia e Ingeniería. 2014;6:61–64. [Google Scholar]
- 30.Mitani K., Fujioka M., Uchida A., Kataoka H. Analysis of abietic acid and dehydroabietic acid in food samples by in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Chromatogr. A. 2007;1146:61–66. doi: 10.1016/j.chroma.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 31.Sadhra S., Gray C.N., Foulds I.S. High-performance liquid chromatography of unmodified rosin and its applications in contact dermatology. J. Chromatogr. B. 1997;700:101–110. doi: 10.1016/s0378-4347(97)00293-4. [DOI] [PubMed] [Google Scholar]
- 32.Wiyono B., Tachibana S., Tinambunan D. Chemical compositions of pine resin, rosin and turpenmik-tine oil from west java. Indones. J. Forest. Res. 2006;3:7–17. [Google Scholar]
- 33.Municipal de Cajibío Alcaldía. 2016. Plan de Desarrollo Territorial del Municipio de Cajibío.http://uvsalud.univalle.edu.co/pdf/procesos_de_interes/cauca/6._cajibio.pdf Retrieved from. [Google Scholar]
- 34.Sabyasachi M., Ray N., Rosin Kundu A. A renewable resource for polymers and polymer chemicals sukumar. Prog. Polym. Sci. 1989;14:297–338. [Google Scholar]
- 35.Chen G. Developments in the field of rosin chemistry and its implications in coatings. Prog. Org. Coating. 1992;20:139–167. [Google Scholar]
- 36.Corporación de fomento de la producción gerencia de desarrollo . 1987. Aplicación de estimulantes y rendimientos de oleorresina en dos rodales de pino radiate. Santiago. Chile. [Google Scholar]
- 37.Sukarno A. Physical Properties of Turpentine and Gum Rosin Pinus merkusii Jungh et de Vriese Tapped Oleoresin by Borehole Method. J. Exp. Life Sci. 2018;8(1):43–46. [Google Scholar]
- 38.Riveros J., Cueva G., Gonzáles E. Evaluación de la oleorresina de pino (Pinus oocarpa) en la zona de Oxapampa, Pasco, Perú. Revista Forestal del Perú. 2017;32 1:45–55. [Google Scholar]
- 39.Silvestre A., Gandini A. 2008. Rosin: Major Sources, Properties and Applications. Momers, Polymers and Composites from Renewable Resources. [Google Scholar]
- 40.Gallo-Corredor J.A., Sarria-Villa R.A. Obtenci´on de colofonia y trementina a partir de la resina de Pinus oocarpa extra´ıda de un bosque industrial en Cauca-Colombia. Journal de Ciencia e Ingeniería. 2014;6:65–69. [Google Scholar]
- 41.ANNUAL BOOK OF ASTM STANDARDS Paint-fatty oils an acids, solvents, micellaneous; aromatic hydrocarbons. Philadelphia (USA) Edit. Starff. 1991;6(3):82–85. p.150-152, p.385-386. [Google Scholar]
- 42.Pastor-Bustamante J.F. Estudio de la calidad de la resina del Pinus caribaea var. caribaea y sus componentes. Revista Chapingo Serie Ciencias Forestales y del Ambiente. 2001;7:159–162. [Google Scholar]
- 43.Zhang D., Zhou D., Wei X., Liang J., Chen X. Green catalytic conversion of hydrogenated rosin to glycerol esters using subcritical CO2 in water and the associated kinetics. J. Supercrit. Fluids. 2017;125:12–21. [Google Scholar]
- 44.Johna G., Nagarajan S., Vemula P.K., Silverman J.R., Pillai C.K. Natural monomers: a mine for functional and sustainable materials–Occurrence,chemical modification and polymerization. Prog. Polym. Sci. 2019;92:158–209. [Google Scholar]
- 45.Joye M.Jr, Resin Lawrence R. Acid composition of pine oleoresins. J. Chem. Eng. Data. 1967;12:279–282. [Google Scholar]
- 46.Neis F.A., de Costa F., Füller T.N., de Lima J.C., da Silva R.K. Biomass yield of resin in adult Pinus elliottii Engelm. Trees is differentially regulated by environmental factors and biochemical effectors. Ind. Crop. Prod. 2019;118:20–25. [Google Scholar]
- 48.Park J.Y., Lee Y.K., Lee D.S., Yoo J.E., Shin M.S. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017;203:279–287. doi: 10.1016/j.jep.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 49.Ghanmi M., Satrani B., Aafi A., Ismail M.R., Farah A. Évaluation de la qualité de la colophane du pin maritime (Pinus pinaster) et du pin d'Alep (Pinus halepensis) du Maroc. Acta Bot. Gall.: Bot. Lett. 2009;156:427–435. [Google Scholar]
- 50.Lai M., Zhang L., Lei L., Liu S., Jia T. Inheritance of resin yield and main resin components in Pinus elliottii Engelm. at three locations in southern China. Ind. Crop. Prod. 2020;144:112065. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.