Abstract
Locally available organic inputs to soil, solely or in combination with inorganic fertilizers, are used to reverse declining soil fertility and improve soil organic matter content (SOM) in smallholder farms of most Sub-Saharan Africa (SSA) countries. Soil organic matter characterization can indicate soil organic input, carbon (C) sequestration potential, or even an authentication tool for soil C dynamics in C stocks accounting. This study determined the effects of the long-term application of selected integrated soil fertility management (ISFM) technologies on SOM functional group composition and maize yields. The study was carried out on an ongoing long-term soil fertility field experiment established in 2004 in Mbeere South sub-county, the drier part of upper Eastern Kenya. The experimental design was a randomized complete block design. The ISFM treatments were 60 kg ha−1 nitrogen (N) from goat manure (GM60); 30 kg ha−1 inorganic N fertilizer (IF30); 60 kg ha−1 inorganic N fertilizer (IF60); GM30+IF30; 90 kg ha−1 inorganic N fertilizer (IF90); 60 kg ha−1 N from lantana (Lantana camara) (LC60); LC30+IF30; 60 kg ha−1 N from mucuna beans (Mucuna pruriens) (MP60); MP30+IF30; 60 kg ha−1 N from Mexican sunflower (Tithonia diversifolia) (TD60); TD30+IF30, and a control with no inputs. The C compositions of ground soil samples and organic amendments were analyzed using 13C solid-state NMR. The GM60, GM30+IF30, LC60, and TD60 treatments had much higher Alkyl and O-Alkyl C SOM functional groups than the control and other treatments. The average soil C for the control was 7.47 mg kg−1 and ranged from 5.03 to 7.37, 9.57 to 18.77, and 7.03–14.50 mg kg−1 for inorganic fertilizers, organic fertilizers, and organic + inorganic fertilizers, respectively. The mean grain yield for the control was 0.56 Mg ha−1 and ranged from 1.51 to 1.99, 1.94 to 4.16, and 2.98–4.60 Mg ha−1 for inorganic fertilizers, organic fertilizers, and organic + inorganic fertilizers, respectively. The results showed that a long-term application of sole organic fertilizers or combined with inorganic fertilizers increases maize yield and soil C sequestration potential. The increase was attributed to high Alkyl and O-Alkyl C SOM functional groups. Hence, knowing the C fraction content of organic inputs is vital in determining the best-fit management technologies for ameliorating soil fertility and sustaining and/or improving crop yields.
Keywords: Grain yield, Carbon sequestration, Organic amendments, Soil carbon fractions, Soil fertility
Grain yield; Carbon sequestration; Organic amendments; Soil carbon fractions; Soil fertility.
1. Introduction
Maintenance of soil fertility is critical to sustaining food security under the prevailing climate variability and increasing population. Conversely, deteriorating soil fertility reduces crop yields and increases the threat to food insecurity. Continuous cultivation and low soil replenishment are the leading causes of declining soil fertility (Shisanya et al., 2009). The result being the development of integrated soil fertility management (ISFM) technologies that employ a judicious application of organic and inorganic nutrients to ameliorate soil fertility and boost or sustain crop productivity. In most semiarid environments and Sub-Saharan Africa's (SSA) smallholder farming systems, where soil fertility is declining together with soil organic matter (Badalucco et al., 2010), locally accessible organic inputs have been used to ameliorate soil fertility and boost soil organic matter content (SOM) (Kiboi et al., 2018).
Improving SOM content and raising soil nutrients' bioavailability for improved soil quality requires good management of applied organic inputs (Kiboi et al., 2019). Maintaining a high SOM status is desirable in the long term due to its multiple beneficial effects attributable to its structure, such as soil physical and water holding capacity and good biological properties (von Lützow et al., 2002; Laudicina et al., 2012). Besides its dependence on edaphic and environmental factors, the quantity and quality of SOM in agricultural soils can vary due to agriculture-related management practices such as application of organic inputs (Martyniuk et al., 2019). Therefore, depending on chemical composition of SOM (Li et al., 2015), biomass input levels, micro- and bioclimatic change (Zomer et al., 2017), and management, soils can act as both C sources and sinks.
The physico-chemical environment of the soil and the chemical structure of its organic C controls the biological stability of SOM (Schöning et al., 2005). It, in turn, influences the organic nutrients mineralization of (into plant-available forms) and sequestered soil C amounts. Chemical recalcitrance can explain the formation of passive or long-residence-time of SOM fractions in the soil (Eusterhues et al., 2003). Soil organic carbon (SOC), a measurable component of SOM, is composed of inorganic and organic components (Wang et al., 2012; Were et al., 2015) and is controlled by organic C and degradation rates. Its dynamics can denote the balance between C input and C output (Breulmann et al., 2010) and influence crop productivity. In light of predicted climate change, the ability of soil to retain C and thus to act as a sink or a source for increasing anthropogenic CO2 concentrations remains largely unknown (Trumbore, 2009; Solomon et al., 2012). Therefore, SOM characterization can indicate soil organic input, carbon (C) sequestration potential, or even an authentication tool for soil C dynamics in C stocks accounting (Leifeld and Ko, 2005; Laudicina et al., 2015). for different agricultural production systems.
The 13C cross-polarization magic angle spinning (CPMAS) nuclear magnetic resonance (NMR) spectroscopy can be applied in structural characterization of SOM for the interpretation of changes induced by the different management practices (Berns and Conte, 2011; Knicker, 2011; Panettieri et al., 2013). This is because most SOM-constituting compounds have poor solubility, making NMR spectroscopy an appropriate option for an in-depth description.
The NMR spectroscopy operation principle is based on the application of a magnetic field to nuclei and determining the amount of energy required to put the nuclei in resonance (Freitas et al., 2012). The NMR spectrum provides peaks/signals that help determine the structure of C fractions in soil samples (Martínez-Richa and Silvestri, 2017). The number of peaks in the spectrum equals the number/type of hydrogen or other atoms in a molecule (Freitas et al., 2016). The 13C NMR spectra of soil samples are assigned to dominant C forms, including carboxyl, aromatic, o-alkyl, alkyl C, phenolic, and methoxyl C. Solid-state CPMAS 13C NMR can offer an in-depth understanding of C composition (Normand et al., 2017). Based on chemical peak shifts, it detects the carbon functional groups with varying molecular composition and microbial utilization in SOM (Knicker, 2011). Hence, 13C NMR spectroscopy can be applied in soil C pools changes and trajectories evaluation at various stages of soil fertility and landuse management changes.
This study aimed to quantify SOM's composition and stability dynamics as influenced by organic and inorganic soil inputs. Specifically, SOM in a long-term soil fertility experiment testing selected ISFM technologies was evaluated. The tested techniques were comprised of four organic inputs – lantana (Lantana camara), mucuna beans (Mucuna pruriens), Mexican sunflower (Tithonia diversifolia), and goat manure – solely applied or combined with different levels of inorganic nitrogen (N) fertilizers. The long-term field experiment in Mbeere South sub-county, the drier part of the Central Highlands of Kenya, is still running after 17 years. The specific objectives were to (i) determine the long-term effects of organic and inorganic soil fertilization on the SOC functional groups composition and (ii) establish the relationship between the SOC functional groups composition and maize (Zea mays) grain yields.
2. Materials and methods
2.1. Study area
The study was conducted at Machang' a (00° 47′ 26.81″ S; 37° 39′ 45.34″ E) secondary school, Mbeere South Sub-County, Embu County. The predominant soils were sandy-clay-loam, Nitro-rhodic Ferralsols (FAO, 1991; Ngetich et al., 2014). The soils were typically shallow (about 100 mm deep), with low fertility and limited SOM content (about 1% total organic C, as per Mucheru-Muna et al., 2010). On average, typical 0–15 cm topsoil had a pH of 6.4, about 0.1% total N, 1% total organic C (TOC), 12 mg kg−1 bicarbonate extractable P, 1.49 cmolc kg−1 exchangeable cation exchange capacity (ECEC) (in cmolc kg−1), 0.35, 1.0, and 1.49 cmolc kg−1 of exchangeable K, Ca, Mg, respectively (Mucheru-Muna et al., 2010). Hence, according to Micheni et al. (2004) and Jaetzold et al. (2006), the soil requires protection from water erosion and intensive continuous fertilization every season. Before establishing the experiment, the site was used as grazing land for livestock.
The site experiences a bimodal pattern, i.e., long rains (LR) season lasting from March and ending in June and short rains (SR) season starting from October and ending in December. The annual rainfall amounts range from 800 to 900 mm. However, the rainfall is erratic and unreliable.
The site is a typical marginal region with limited agricultural potential in the Lower Midland Agro-ecological Zone 4 (LM4). Lower Midland 4 is a cotton (Gossypium hirsutum) and livestock-millet (Pennisetum glaucum and Eleusine coracana) zone characterized by a short cropping season (Jaetzold et al., 2006). The site is within a typical sub-humid agro-climatic conditions, with relatively low agricultural production potential. Maize, cowpeas, pigeon peas (Cajanus cajan), and common beans (Phaseolus vulgaris) are the dominant crops grown by most housholds in the region (Ngetich et al., 2014).
2.2. Experimental layout, treatments, and management
The experimental design was a randomized complete block design replicated thrice. The plot sizes were 6 m by 4.5 m. The organic sources were Lantana camara (LC), Mucuna pruriens (MP), Tithonia diversifolia (TD) and Goat Manure (GM). Table 1 shows the details of the treatments.
Table 1.
Experimental treatments and amounts of N supplied by the different treatments.
| Treatments | Abbreviation | N from biomass (kg N ha−1) | N from inorganic fertilizer (kg N ha−1) |
|---|---|---|---|
| Control | Ctrl | 0 | 0 |
| Goat Manure | GM60 | 60 | 0 |
| Inorganic fertilizer (30 kg ha−1 N) | IF30 | 0 | 30 |
| Inorganic fertilizer (60 kg ha−1 N) | IF60 | 0 | 60 |
| Goat Manure + Fertilizer (30 kg ha−1 N) | GM+IF30 | 30 | 30 |
| Inorganic fertilizer (90 kg ha−1 N) | IF90 | 0 | 90 |
| Lantana camara | LC60 | 60 | 0 |
| Lantana camara + Inorganic Fertilizer (30 ha−1 N) | LC30+IF30 | 30 | 30 |
| Mucuna pruriens | MP60 | 60 | 0 |
| Mucuna pruriens + Inorganic Fertilizer (30 kg ha−1 N) | MP30+IF30 | 30 | 30 |
| Tithonia diversifolia | TD60 | 60 | 0 |
| Tithonia diversifolia + Inorganic Fertilizer (30 kg ha−1 N) | TD30+IF30 | 30 | 30 |
The test crop was maize (Zea mays L, var. DH04), and it was planted at a spacing of 0.9 m between the rows and 0.6 m within the rows. During sowing, three maize seeds were planted per hill. Immediately after emergence, the third seedling was thinned out to remain with two seedlings per hill. Both organic and inorganic fertilizer application rates were based on the recommended 60 kg N ha−1 (FURP, 1987). The nutrient content of both fertilizers were from laboratory analyses of the input samples (Table 2). The organic materials (TD and LC) were harvested from the established nearby bulking plots (biomass transfer).
Table 2.
Nutrient composition (% N, P, Ca, Mg and K) of organic soil inputs used in the experiment.
| Treatment | N | P | Ca | Mg | K |
|---|---|---|---|---|---|
| GM | 2.0 | 0.7 | 4.3 | 1.2 | 4.2 |
| TD | 3.0 | 0.2 | 2.2 | 0.6 | 2.9 |
| LC | 1.5 | 0.1 | 1.1 | 0.4 | 0.8 |
| MP | 2.4 | 0.1 | 1.2 | 0.2 | 0.7 |
Where: LC - Lantana camara; GM is Goat Manure, MP - Mucuna pruriens; TD - Tithonia diversifolia.
At the onset of each season, the organic inputs were collected (from the hedgerows and those planted on the soil conservation structures/terraces). They were then dried under a shade, chopped, and required amounts per plot weighed. During land preparation, the organic inputs were spread and incorporated into the soil to a depth of 15 cm. Calcium ammonium nitrate (CAN), the source of inorganic N, was split-applied as a top-dresser at the rate of a third (of the target inorganic N amount as per Table 1) four weeks after planting and two-thirds six weeks after planting. Due to the low P content of organic inputs, triple superphosphate (TSP) fertilizer was blanket applied in all plots, taking into account the average residual soil available P, to attain 60 kg P ha−1, the recommended rate. Standard agronomic practices for maize production were implemented during crop growth and development.
Maize was harvested at maturity. Before harvesting the plots, the edge effects were accounted for by excluding the first and the last maize plants in each row, and the two guard rows per plot, resulting in a net plot of 21 m2. After harvesting, maize cobs were air-dried (at about 27–30 °C) for about a month. Once dry hand-shelling, weighing, and grain moisture content determination (Dickey-john MiniGAC® moisture meter with a moisture range of 5–45% with a ±°0.02% precision) (http://www.dickey-john.com/product/mini-gac/) were done. To standardize the yields, grain weight correction was done by considering the determined weight against the measured moisture content to standard 12.5% moisture. The grain weight was then presented on Mg per hectare basis.
Soil sampling was done at the end of the short rains growing season 2017 (February 2018). Before sampling, the surface was cleared of obvious plant debris and other obvious organic material. Seven disturbed soil sub-samples from each experimental plot were taken at a depth of 0–15 cm using a stainless Edelman auger, composited, resampled, air-dried, and split into two portions per sample. For NMR analysis, one portion of about 50 g was put into 60 ml plastic vials, labeled, and shipped to the National High Magnetic Field Laboratory, USA. For the C analysis, the second portion of about 60 g was put into plastic bags, labeled, and shipped to the National Agriculture Laboratories, Kenya.
2.3. Lab analysis
Soil samples and organic amendments were air-dried to constant weight, ground using an automatic grinding machine, and passed through a 100-mesh sieve prior to analysis. Ground soil and organic amendments samples were analyzed using magic angle spinning (MAS) 13C ssNMR, on a Bruker 4.0 mm double resonance MAS NMR probe equipped 300 MHz NMR spectrometer (Bruker DRX300 - https://einsteinmed.org/research/shared-facilities/nmr/bruker-drx300/). Before spinning to 9.5 kHz ± 3 Hz at RT using a Bruker pneumatic MAS control unit, samples were packed into 4.0 mm zirconia rotors with Kel-F drive caps. Through cross-polarization, i.e., A 4.0 μs 1H π/2 pulse followed by a 1H spin-lock field of 45 kHz for 1.0 ms contact time, and the 13C RF field ramped from 35 to 50 kHz, all 13C signals were enhanced. Under the irradiation of the SPINAL64 decoupling sequence, and with a 1H RF amplitude of 62.5 kHz, 13C signals were recorded (Fung et al., 2000). The signals were accumulated using 10,000 and 50,000 scans, with recycle delays of 2s depending on the samples. Based on assignments from Knicker (2011), the spectral regions were integrated to determine the contribution of each C functional group in the sample: alkyl (0–45 ppm), methoxyl (45–60 ppm), O-alkyl (60–110 ppm), aromatic (110–140 ppm), phenolic (140–160 ppm), and carboxyl (160–220 ppm). The functional group's %C was converted to g functional group per kg sample using soil C values of the soil samples, after which the 13C chemical shifts were referenced to the carbonyl C of glycine at 176.4 ppm. The soil C was determined using the modified Walkley, and Black method and soil N using Kjeldahl method (Ryan et al., 2001).
2.4. Statistical analysis
Data were analyzed using SAS 9.4 (SAS Institute, 2004). Soil carbon, Nitrogen, and grain yields were subjected to analysis of variance to establish the effects across the treatments. The mean separation was done using the least significant difference (LSD) at p = 0.05. The relationship of grain yields against soil N or soil C was evaluated by subjecting the data to bivariate Pearson Correlation to produce a correlation coefficient.
3. Results and discussion
3.1. Carbon fractions of the organic inputs
The total C of MP, GM, LC and TD were 40.53 g kg−1, 40.13 g kg−1, 39.8 g kg−1, 36.52 g kg−1, respectively (Figure 1). The O- alkyl C fraction was comparatively the highest across the organic inputs, ranging from 19.14 g kg−1 in TD to 25.83 g kg−1 in LC. It was evident that alkyl was the second-highest fraction. The Alkyl C fraction was highest in GM (10.35 g kg−1) and lowest in MP (5.03 g kg−1). Aromatic C and methoxyl C fractions did not vary much across the organic inputs. Compared to the other soil organic inputs, GM had the lowest carboxyl C and phenolic C content (0.71 g kg−1 and 0.46 g kg−1, respectively) compared to the other inputs.
Figure 1.
Carbon fractions composition of the organic inputs used in the experiment. LC is Lantana camara; GM is Goat Manure, MP is Mucuna pruriens; and TD is Tithonia diversifolia.
3.2. Soil organic carbon fractions
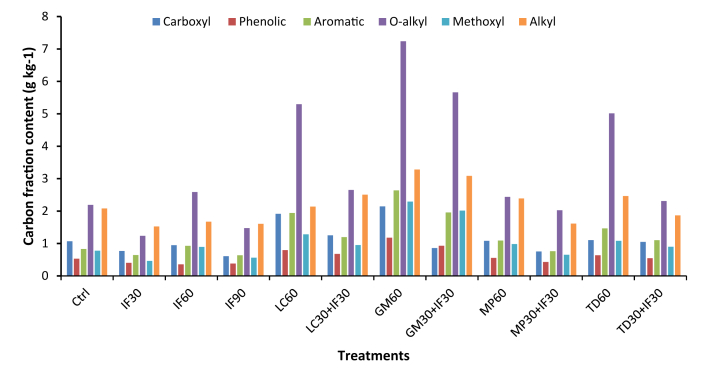
With respect to the relative abundance of C functional groups, based on 13C NMR spectra of the different soil input treatments, a declining trend was observed from soils treated with only organic amendments, followed by soils with organic + inorganic amendments, and then soils with only inorganic amendments (Figure 2). The reported high C content under organic amendments was consistent with Goyal et al. (1999) Carbon functional groups of the soil treated with sole organic inputs was in the order of O-alkyl C>alkyl C>methoxyl>carboxyl>aromatic C content, with GM treatment having the highest of these fractions. The C fractions in the inorganic fertilizer-based treatments were closely identical to the control (Figure 2).
Figure 2.
Total amount of each soil C fraction (Carboxyl, Phenolic, Aromatic, O-alkyl, Methoxyl and Alkyl) in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
3.2.1. O-alkyl C
O-alkyl C was the dominant C fraction among the different functional groups under different treatments (Figure 2). Compared to the control, GM60 treatment had significantly (p < 0.001) the highest content (231%) followed by GM30+IF30 (159%), then sole LC60 (142%) followed by TD60 (129%) (Figure 3). Compared to the control, slight differences were observed in the remaining treatments ranging from -44% in IF30 to 21% in LC30+IF30. The O-alkyl C content in the sole inorganic fertilizer treatments, were generally low, with IF30 and IF90 having significantly (p < 0.001) lower O-alkyl C (about -44% and -33%, respectively) than that of the control. IF60, LC30+IF30, MP30+IF30, TD30+IF30 and MP60 treatments were not significantly different from the control.
Figure 3.
Total amount of O-alkyl C fraction in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
O-alkyl C is composed of methoxyl C (lignin) and carbohydrate C (cellulose and hemicellulose) components (Wang et al., 2013; Yu et al., 2015; Li et al., 2017; He et al., 2018; Guan et al., 2018). Based on the C fraction composition of the organic inputs (Table 1), it is evident that the amounts of O-alkyl C across the four organic inputs were almost equal. Contrariwise, the soil residual O-alkyl C at the end of the season showed significant variation across the sole organic inputs and their combinations with the inorganic inputs. GM60 treatment showed strikingly high amounts of O-alkyl C, depicting a potential for high contribution to SOM. The high decrease of the O-alkyl in the three plant-based residues suggests that a larger portion of the constituent is the cellulose and hemicellulose, which are easily biodegraded by microorganisms (Schöning et al., 2005; Solomon et al., 2010).
Organic + inorganic inputs applied to the soil that showed no relative difference of O-alkyl C content relative to the untreated control could be due to the positive effect on the organic input mineralization rated of the N from the inorganic fertilizers. It can indicate that the integration of inorganic and organic inputs facilitates a faster decomposition of the original inputs throughout the season (Gram et al., 2020).
3.2.2. Alkyl C
The GM60 treatment had significantly (p < 0.001) the highest Alkyl C content (58%) followed by GM+IF30 (48%) treatment, then LC30+IF30 (20%) and TD60 (19%), compared to the control (Figure 4). Except for MP60, all the other treatments (LC60, IF60, IF90, MP30+IF30, TD30+IF30, IF30) had significantly lower Alkyl C content compared to the control.
Figure 4.
Total amount of Alkyl C fraction in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
Alkyl C is a recalcitrant C; that is, it is more stable, hydrophobic (Carrington et al., 2012; Chen et al., 2013; Habte et al., 2013; Yu et al., 2015), and an aliphatic hydrocarbon with strong chemical structure bonds that are more resistant to degradation (Zhang et al., 2019). Singh and Rengel (2007) associate high recalcitrant organic C content in the soil with alkyl C. The accumulation of alkyl C content, derived from lignin and polyphenol components of the plant residues, occurs at the onset of decomposition of the plants. In the case of this study, the organic amendments supplied lesser proportions of alkyl C, relative to O-alkyl C, suggesting that during decomposition of the plant residues, the stable alkyl C is left intact while the carbohydrate C (O-alkyl C) undergoes decomposition. In addition, degradation of labile O-alkyl results in accumulation of alkyl C and aromatic C (Quideau et al., 2001). However, the presence of oxygen enhances degradation of aromatic C (Fuchs et al., 2011). The findings of Kögel-Knabner (2002) corroborates this and further underscores that the lignin component minimizes the decomposition of the plant residues and increases the likelihood of the organic inputs to contribute to soil C stocks.
3.2.3. Aromatic C
Compared with control, GM60, LC60, GM30+IF30, LC30+IF30, TD60, and TD30+IF30 treatments had significantly (p < 0.001) higher aromatic C contents (Figure 5). The aromatic C content in soils treated with MP60, IF60, and MP30+IF30 treatments were not significantly (p < 0.001) different from the control while those of IF30 (by -22%) and IF90 (by -23%) treatments were significantly lower.
Figure 5.
Total amount of Alkyl C fraction in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
The presence of Aromatic C is indicative of the dominance of the stable and recalcitrant C fraction in the organic inputs (Fuchs et al., 2011). The results indicate an increase in aromatic C under the treatments composed of organic inputs and showed a positive relationship with the high O-alkyl C trends. Panettieri et al. (2014) reported similar results, attributing the high aromatic C content to the incorporation of high amounts of crop residues due to minimum tillage effects. Aromatic C is derived from lignin and tannin (Nogueirol et al., 2014), which undergoes microbial degradation. On degradation, the lignin-derived aromatic C contributes to a humic fraction of SOM (humification), which is core to soil fertility (Fuchs et al., 2011). Abakumov et al. (2018) suggest that humification processes supplemented by organic inputs application boosts SOM.
Conversely, the application of sole inorganic inputs decreases aromatic C, underscoring the potentially adverse effects of inorganic fertilizers on SOM content in agricultural lands. Furthermore, the observed results accentuate the importance of the applied organic inputs in increasing aromatic C, and by extension, enhancing SOM content. The relatively high resistance of aromatic C to microbial decomposition (Eldridge et al., 2017) shows that GM60, GM30+IF30, LC60, TD60, and MP60 treatments have a high potential of promoting soil C sequestration.
3.2.4. Methoxyl C
Methoxyl C content was significantly (p < 0.001) the highest in GM30+IF30, GM60, LC60, LC30+IF30, MP60 and TD60 compared to the control (Figure 6). IF30 had the lowest (p < 0.001) amounts compared to the control. There was no significant difference (p < 0.001) between the control and IF60, TD30+IF30, MP30+IF30 and IF90.
Figure 6.
Total amount of Alkyl C fraction in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
Methoxyl C is mostly associated with the lignin and phenolic part of O-alkyl C (Wang et al., 2013; Yu et al., 2015). It is considered relatively resistant to microbial degradation, thus suggesting a significant contribution towards the SOM content in the soil. The high contents of methoxyl C in the GM60 and GM30+IF30 indicate high lignin and polyphenols in the constituent dietary composition of the GM60. GM60, being goat manure from drier areas of the Central Highlands of Kenya, their diets are majorly acacia and herbaceous plants common in marginal lands. The high Methoxyl C in GM60 treatment can also be attributed to the process the goat manure undergoes, from production to application as soil input. Part of the process is the decomposition, meaning which might have some impact on its stability. This opinion is based on the organic input C fractions analysis shown in Table 1, which indicated almost equal amounts of Methoxyl C across LC60, MP60, GM60, and TD60. Hence, GM60 has a high potential of sequestering C compared to the other organic inputs.
3.2.5. Carboxyl C
Carboxyl C content was significantly (p < 0.001) highest in GM60 and LC60 treatments by 106% and 74%, respectively, compared to control (Figure 7). Carboxyl C content LC30+IF30, MP60, TD60, IF60 and TD30+IF30 treatments were not significantly (p < 0.001) different from the control. On the other hand, carboxyl C content in GM30+IF30, IF30, MP30+IF30 an IF90 treatments were significantly lower compared to the control.
Figure 7.
Total amount of Alkyl C fraction in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
Carboxyl C, an aliphatic acid of plant and microbial origins (Yu et al., 2015), was relatively abundant under the GM60, although they were very low in the input characterization (Figure 1). Carboxyl C is an organic input constituent, and it is also microbially generated. Carboxyl-rich compounds are oxidation products of plant-derived biomolecules, such as lignin and associated phenolic substances (Kramer et al., 2012). Although highly oxidized lignin polyphenols, tannins, and other recalcitrant plant-derived compounds are partly solubilized and mobilized by peroxidase and ligninase enzymes in the soil, the resulting carboxyl-rich ring structures are more resistant to microbial biodegradation (Kalbitz et al., 2006). Carboxyl C is considered an important pathway for DOM production and potential for organic matter accumulation in soil (Kramer et al., 2012). The high amounts of carboxyl C under the GM60 treatment indicate the high potential of the GM60 treatment to contribute significantly towards SOM enrichment over time, hence soil C sequestration. Besides the SOM enrichment, Carboxyl C is responsible for the negative charge of soil organic matter (Anda et al., 2013), which relates to in cation exchange capacity (CEC) of soil (Schnitzer and Desjardins, 1965), hence soil fertility potential. Although the carboxyl C for the other three treatments was also high, it is worth noting that, compared to the amounts of carboxyl C in the organic characterization, the observed results show a general decline in the amounts, unlike in the GM60 treatment. In contrast, treatments with organic + inorganic inputs and sole inorganic inputs led to decreased carboxyl C content, which might be detrimental to soil C stocks.
3.2.6. Phenolic C
The GM60 treatment had significantly highest Phenolic C contents followed GM30+IF30, LC30+IF30, LC60, TD60 and MP60 treatments compared to control (Figure 8). Phenolic C contents in the TD30+IF30 MP30+IF30 IF30 and IF90 treatments were not any different (p < 0.001) relative to control. However, phenolic C contents, IF60 was significantly (p < 0.001) lower from the control.
Figure 8.
Total amount of Alkyl C fraction in each treatment. Ctrl is the Control; GM60 is Goat Manure (60 kg ha−1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 is Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
Phenolic C is a less humified organic material in SOM, as it contains an abundance of diester P and amide N (Wissing et al., 2013). Generally, phenolic C was significantly the lowest C fraction in the soil, indicating, but then, based on Table 1, the difference between the phenolic C in the inputs and the residual at the end of the season was small. Phenols originate from recalcitrant plant litter compounds (Rumpel et al., 2004); hence its degradation is slower than the degradation of other C fractions (Min et al., 2015). Therefore, the observed high phenolic contents in the GM60 treatment indicate its high potential to contribute to SOM. This observation is supported by Yu et al. (2015), who observed that as a result of lignin recalcitrance of organic inputs, there was an accumulation of phenolic C in the soil. Pane et al. (2013) also reported that high phenolic C content reflects the lack of microbial degradation due to the recalcitrant characteristic of the organic inputs. Further, according to Ng et al. (2014), phenolic compounds correlate with the antioxidant capacity of soils that neutralize free radicals and protect organic matter from oxidation.
3.3. The effects of treatments on soil nitrogen, soil carbon, and grain yields
The average grain yield ranged from 0.56 Mg ha−1 in the control to 4.60 Mg ha−1 in the MP30+IF30 treatment (Table 3). The IF30, IF60, IF90, and LC60 treatments had low grain yields within the range of the control treatment. The LC30+IF30 and LC60 treatments had an average effect on the grain yields. At the same time, the combination of organic and inorganic amendments, i.e., fertilizer LC30+IF30, TD30+IF30, GM30+IF30, and MP30+IF30 (2.98 Mg ha−1, 3.30 Mg ha−1, 3.36 Mg ha−1, and 4.6 Mg ha−1, respectively) and sole application of MP60 and GM60 (4.03 Mg ha−1 and 4.16 Mg ha−1) produced higher grain yield compared with control.
Table 3.
Treatment effect on soil Nitrogen (g kg−1), carbon (g kg−1), and maize grain yields (Mg ha−1).
| Treatment | Nitrogen (g kg−1) | Carbon (g kg−1) | Grain Yield (Mg ha−1) |
|---|---|---|---|
| Control | 0.73 ± 0.03ef∗ | 7.47 ± 0.50de | 0.56 ± 0.045f |
| IF30 | 0.53 ± 0.12ef | 5.03 ± 1.14e | 1.89 ± 0.338def |
| IF60 | 0.80 ± 0.06def | 7.37 ± 0.61de | 1.51 ± 0.171ef |
| IF90 | 0.50 ± 0.06f | 5.27 ± 0.63e | 1.99 ± 0.457cdef |
| LC60 | 1.37 ± 0.07bc | 13.37 ± 0.71bc | 1.94 ± 0.091cdef |
| LC30+IF30 | 0.97 ± 0.03cde | 9.23 ± 0.38cde | 2.98 ± 0.366bcd |
| GM60 | 1.83 ± 0.12a | 18.77 ± 1.11a | 4.16 ± 0.208ab |
| GM30+IF30 | 1.47 ± 0.12ab | 14.50 ± 1.50a | 3.36 ± 0.191abc |
| MP60 | 0.97 ± 0.03cde | 9.57 ± 0.41bcde | 4.03 ± 0.516ab |
| MP30+IF30 | 0.70 ± 0.15ef | 7.03 ± 1.78de | 4.60 ± 0.151a |
| TD60 | 1.23 ± 0.09bcd | 11.77 ± 1.52bcd | 2.23 ± 0.103cde |
| TD30+IF30 | 0.80 ± 0.06def | 7.77 ± 0.57de | 3.30 ± 0.345abcd |
| P value | <0.0001 | <0.0001 | <0.0001 |
Mean with same superscript letters indicate no significant difference between treatments. Ctrl is the Control; GM60 is Goat Manure (60 kg ha-1 N), IF30 is Inorganic fertilizer (30 kg ha−1 N), IF60 is Inorganic fertilizer (60 kg ha−1 N); GM30+IF30 is the Goat Manure + Fertilizer (at a rate of 30 kg ha−1 N each); IF90 is Inorganic fertilizer (90 kg ha−1 N); LC60 is Lantana camara (60 kg ha−1 N); LC30+IF30 is Lantana camara + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); MP60 - Mucuna pruriens (60 kg ha−1 N); MP30+IF30 is the Mucuna pruriens + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each); TD60 is Tithonia diversifolia; and TD30+IF30 is Tithonia diversifolia + Inorganic Fertilizer (at a rate of 30 kg ha−1 N each).
The GM60 and GM30+IF30 treatments had the highest soil N, while IF30, IF60, IF90, MP30+IF30, and TD30+IF30 treatments had the lowest. The LC60, LC30+IF30, MP60, and TD60 treatments had moderate amounts of soil N (Table 3). There was a positive correlation between grain yields and soil N content (Figure 9). Concerning grain yields, MP60 and MP30+IF30 treatments had the highest grain yields. The high soil N in the GM60 and GM30+IF30 treatments are indicative of the potential of these treatments to build up soil N over time.
Figure 9.
Correlation of grain yields to soil carbon and soil nitrogen content. (a) grain yield versus soil carbon and (b) grain yield versus soil nitrogen.
The effects of the treatments on the soil C content followed almost the same trend as the soil N with GM60 and GM30+IF30 treatment having, strikingly, the highest soil C levels (Table 3). Except for the sole MP60 treatment, the soil C content increased in organic-based treatments, i.e., TD60, LC60, and GM60, compared to the control. Apart from for GM30+IF30 treatment, combined organic and inorganic inputs resulted in slight changes in soil C contents. The amount of C was much lower for these treatments than the organic inputs when applied solely. Sole inorganic fertilizer-based treatments had the lowest soil C content, close to that of the control.
Based on the observed results, GM60 and GM30+IF30 treatments emerged superior in terms of enhancing grain yields and soil N and C. Coincidentally, the two treatments had high O-alkyl and alkyl C fraction, most likely attributable to the nature of goat manure. This is not only indicative of the potential dual benefits the treatments have both in terms of soil C sequestration and enhancing crop productivity but also the synergetic influence of N and C on crop yields. Also, based on the chemical composition, except for N content, goat manure had superior amounts of P, Ca Mg, and K. based on the law of the minimum, as applied in soil fertility and plant nutrition, it implies that GM related treatments present a more nutrient balanced soil fertility inputs compared to the other inputs. This agrees with the observation by Awodun et al. (2007) that manure improves soil nutrient availability, nutrient status and enhances crop growth and yields. The SOC storage in agricultural systems is a balance between carbon losses and C additions (from crops residues and organic inputs) (Thelen et al., 2010), resulting in increased soil fertility and high yield linked to improved physical properties of the soil (Stroosnijder, 2009; Nayak et al., 2012). The application of organic amendments is regularly used to improve the SOM levels and increase atmospheric CO2 sequestration potential in soils (Yu et al., 2015).
Besides the goat manure-related treatments, MP30+IF30 treatment registered the highest grain yields. The high grain yield was probably due to a lower C:N ratio compared to other treatments. The additional inorganic N in this treatment created a N (mineralization) surplus, which allowed for decomposition, N uptake, and significantly increased yield (Shang et al., 2014). It points towards the novelty of combining the inorganic and organic amendment, commonly referred to as integrated nutrient management (Schuman et al., 2002). Contrariwise, the treatment effect on soil C content was detrimental, probably due to the observed low Alkyl and O-alkyl fraction present in MP60, making it less recalcitrant and prone to exhaustion within a season of application. The low recalcitrance has a direct implication on the SOC status in that, to sustain SOM, there will be a need for continuous addition of mucuna. Integration of chemical fertilizers into farming systems through a combination of inorganic fertilizer and organics such as farmyard manure or crop residue, or green manure improves the SOC (Kirkby et al., 2011; Nayak et al., 2012; Kirkby et al., 2013).
The observed negative effects of the sole inorganic related treatments on yields and soil N and C were attributed to the lack of organic inputs. Nitrogen is highly mobile, and with limited SOM, it is prone to losses through leaching, runoff, and volatilization (Wissing et al., 2013). Previous studies that evaluate Fertilizer N management have shown similar results that varying amounts of N fertilizer can produce significantly high levels of soil mineral N, leading to soil degradation (Owens et al., 1994). A significant portion of the applied N is removed during harvest. The remaining N may be stored in soils in the form of organic matter, while some might be lost through different pathways, such as N denitrification, volatilization, and leaching. The lower C content under inorganic inputs compromised the N storage ability of soil. Given the prevailing rainfed conditions, leaching is inevitable. As a result, this creates N deficiency and makes these treatments unsustainable in the long term.
4. Conclusion
The contribution of sole organic, or combined with inorganic fertilizers to SOM and soil fertility, is essential, especially in sub-Saharan Africa's tropical smallholder farming systems. This study demonstrates the effects of organic and inorganic fertilizers, and their combination on maize yields and soil N and C. High SOM under the GM60 GM60, GM30+IF30, LC60, and TD60 treatments were linked to the high Alkyl and O-alkyl C fractions. This points towards a high C sequestration potential of these treatments, besides having an immediate beneficial impact on crop productivity. Besides the sole organic inputs, the results imply that long-term application of organic inputs combined with inorganic fertilizers can have a dual effect, i.e., improved soil physicochemical properties and crop productivity. This was demonstrated by the GM+IF30, a treatment where significant soil N and C built-up was observed, besides enhanced grain yields. Hence, the conclusion was that: GM60, with its high Alkyl and O-alkyl fractions, can significantly influence SOM and crop productivity; the dominance of alkyl and O-alkyl C fractions in an organic input directly affected its SOC recalcitrance; hence SOM content and built-up potential; goat manure contained adequate amounts of nutrients to meet plant requirements for optimal growth. As a result, the manure retained more N, thus increasing its fertilizing potency; a combination of organic and inorganic inputs can have the desired dual effect of simultaneously improving crop productivity (economic and social benefits) and soil C sequestration (environmental benefit). Finally, the knowledge of the C fraction content of organic soil inputs is vital in the soil input characterization and development of soil fertility ameliorating technologies.
Declarations
Author contribution statement
Ndung'u, M.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ngatia, L.W.: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Onwonga, R.N.: Analyzed and interpreted the data; Wrote the paper.
Mucheru-Muna, M.W.: Conceived and designed the experiments.
Fu R.: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Moriasi D.N.: Analyzed and interpreted the data.
Ngetich, K.F.: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Science Foundation United States Cooperative Agreement No. DMR-1644779 and the State of Florida.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abakumov E.V., Rodina O.A., Eskov A.K. Humification and humic acid composition of suspended soil in oligotrophous Environments in South Vietnam. Appl. Environ. Soil Sci. 2018;2018 [Google Scholar]
- Anda M., Shamshuddin J., Fauziah C.I. Increasing negative charge and nutrient contents of a highly weathered soil using basalt and rice husk to promote cocoa growth under field conditions. Soil Tillage Res. 2013;132:1–11. [Google Scholar]
- Awodun M.A., Omonijo L.I., Ojeniyi S.O. Effect of goat dung and NPK fertilizer on soil and leaf nutrient content, growth and yield of pepper. Int. J. Soil Sci. 2007;2:142–147. [Google Scholar]
- Badalucco L., Rao M., Colombo C., Palumbo G., Laudicina V.A., Gianfreda L. Reversing agriculture from intensive to sustainable improves soil quality in a semiarid South Italian soil. Biol. Fertil. Soils. 2010;46(5):481–489. [Google Scholar]
- Berns A.E., Conte P. Effect of ramp size and sample spinning speed on CPMAS 13C NMR spectra of soil organic matter. Org. Geochem. 2011;42:926–935. [Google Scholar]
- Breulmann M., Schulz E., Words K. Response of soil Carbon pools to plant diversity in semi-natural grasslands of different land-use history. World Congr. Soil Sci. Soil Solut. a Chang. World. 2010:83–86. [Google Scholar]
- Carrington E.M., Hernes P.J., Dyda R.Y., Plante A.F., Six J. Biochemical changes across a carbon saturation gradient: lignin, cutin, and suberin decomposition and stabilization in fractionated carbon pools. Soil Biol. Biochem. 2012;47:179–190. [Google Scholar]
- Chen J.S., Chung T.L., Tian G., Chiu C.Y. Characterization of soil organic matter in perhumid natural cypress forest: comparison of humification in different particle-size fractions. Bot. Stud. 2013;54:56–60. doi: 10.1186/1999-3110-54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge S.M., Chen C., Xu Z., Chan K.Y., Boyd S.E., Collins D., Meszaros I. Plant available N supply and recalcitrant C from organic soil amendments applied to a clay loam soil have correlations with amendment chemical composition. Geoderma. 2017;294:50–62. [Google Scholar]
- Eusterhues K., Rumpel C., Kleber M., Ko I. Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Org. Geochem. 2003;34:1591–1600. [Google Scholar]
- FAO . Food and Agriculture Organization of the United Nations; Rome: 1991. World Soil Resources: an Explanatory Note on the FAO World Soil Resources Map at 1:25,000,000 Scale. [Google Scholar]
- Fertilizer Use Recommendation Project (FURP) Vol. 24. National Agricultural Research Laboratories; Embu District, Nairobi, Kenya: 1987. (Description of First Priority Trial Site in the Various Districts. Fertilizer Use Recommendation Project (FURP)). [Google Scholar]
- Freitas J.C., Cunha A.G., Emmerich F.G. Solid-state nuclear magnetic resonance (NMR) methods applied to the study of carbon materials. Combinator. Probab. Comput. 2012:85–170. [Google Scholar]
- Freitas J.C.C., Cipriano D.F., Zucolotto C.G., Cunha A.F., Emmerich F.G. Solid-state 13C NMR spectroscopy applied to the study of carbon blacks and carbon deposits obtained by plasma pyrolysis of natural gas. J. Spectrosc. 2016:1–16. ID 1543273. [Google Scholar]
- Fuchs G., Boll M., Heider J. Microbial degradation of aromatic compounds — from one strategy to four. Nat. Rev. Microbiol. 2011;9:803–816. doi: 10.1038/nrmicro2652. [DOI] [PubMed] [Google Scholar]
- Fung B.M., Khitrin A.K., Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2000;142:97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- Goyal S., Chander K., Mundra M.C., Kapoor K.K. Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol. Fertil. Soils. 1999;29:196–200. [Google Scholar]
- Gram G., Roobroeck D., Pypers P., Six J., Merckx R., Vanlauwe B. Combining organic and mineral fertilizers as a climate-smart integrated soil fertility management practice in sub-Saharan Africa: a meta-analysis. PloS One. 2020;15 doi: 10.1371/journal.pone.0239552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan S., An N., Zong N., He Y., Shi P., Zhang J., He N. Climate warming impacts on soil organic carbon fractions and aggregate stability in a Tibetan alpine meadow. Soil Biol. Biochem. 2018;116:224–236. [Google Scholar]
- Habte M., Gebrekidan H., Haile W. Decomposition and nutrient release of selected green manure species at different stages of growth on Alisols at Areka, southern Ethiopia. Int. J. Nat. Sci. Res. Conscientia Beam. 2013;1:30–42. [Google Scholar]
- He Y.T., He X.H., Xu M.G., Zhang W.J., Yang X.Y., Huang S.M. Long-term fertilization increases soil organic carbon and alters its chemical composition in three wheat-maize cropping sites across central and south China. Soil Tillage Res. 2018;177:79–87. [Google Scholar]
- Jaetzold R., Schmidt H., Hornet Z.B., Shisanya C.A. second ed. Vol. 11. C Ministry of Agriculture/GTZ; Nairobi, Kenya, Eastern Province: 2006. (Farm Management Handbook of Kenya. Natural Conditions and Farm Information). [Google Scholar]
- Kalbitz K., Kaiser K., Bargholz J., Dardenne P. Lignin degradation controls the production of dissolved organic matter in decomposing foliar litter. Eur. J. Soil Sci. 2006;57:504–516. [Google Scholar]
- Kiboi M.N., Ngetich K.F., Mugendi D.N., Muriuki A., Adamtey N., Fliessbach A. Microbial biomass and acid phosphomonoesterase activity in soils of the Central Highlands of Kenya. Geoderma Reg. 2018 [Google Scholar]
- Kiboi M.N., Ngetich K.F., Fliessbach A., Muriuki A., Mugendi D.N. Soil fertility inputs and tillage influence on maize crop performance and soil water content in the Central Highlands of Kenya. Agric. Water Manag. 2019;217:316–331. [Google Scholar]
- Kirkby C., Kirkegaard J., Richardson A.E., Wade L., Blanchard C., Batten G. Stable soil organic matter: a comparison of C:N:P:S ratios in Australian and other world soils. Geoderma. 2011;163(3-4):197–208. [Google Scholar]
- Kirkby C.A., Richardson A.E., Wade L.J., Batten G.D., Blanchard C., Kirkegaard J.A. Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol. Biochem. 2013;60:77–86. [Google Scholar]
- Knicker H. Solid state CPMAS 13C and 15N NMR spectroscopy in organic geochemistry and how spin dynamics can either aggravate or improve spectra interpretation. Org. Geochem. 2011;42:867–890. [Google Scholar]
- Kögel-Knabner I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002;34:139–162. [Google Scholar]
- Kramer M.G., Sanderman J., Chadwick O.A. Long-term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Global Change Biol. 2012;18:2594–2605. [Google Scholar]
- Laudicina V., Dennis P.G., Palazzolo E., Badalucco L. In: Environmental Protection Strategies for Sustainable Development. Strategies for Sustainability. Malik A., Grohmann E., editors. Springer; Dordrecht: 2012. Key biochemical attributes to assess soil ecosystem sustainability. [Google Scholar]
- Laudicina V.A., Novara A., Barbera V., Egli M., Badalucco L. Long-term tillage and cropping system effects on chemical and biochemical characteristics of soil organic matter in a Mediterranean semiarid environment. Land Degrad. Dev. 2015;26(1):45–53. [Google Scholar]
- Leifeld J., Ko I. Soil organic matter fractions as early indicators for carbon stock changes under different land-use ? Geoderma. 2005;124:143–155. [Google Scholar]
- Li Z., Zhao B., Wang Q., Cao X., Zhang J. Differences in chemical composition of soil organic carbon resulting from long-term fertilization strategies. PloS One. 2015;10 doi: 10.1371/journal.pone.0124359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Cao Z., Chang J., Zhang Y., Zhu G., Zong N., He Y., Zhang J., He N. Elevational gradient affect functional fractions of soil organic carbon and aggregates stability in a Tibetan alpine meadow. Catena. 2017;156:139–148. [Google Scholar]
- Martínez-Richa A., Silvestri R.L. IntechOpen; 2017. Developments in Solid-State NMR Spectroscopy of Polymer Systems, Spectroscopic Analyses - Developments and Applications, Eram Sharmin and Fahmina Zafar. [Google Scholar]
- Martyniuk S., Pikuła D., Kozieł M. Soil properties and productivity in two long-term crop rotations differing with respect to organic matter management on an Albic Luvisol. Sci. Rep. 2019;9:1878. doi: 10.1038/s41598-018-37087-4. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheni A.N., Kihanda F.M., Warren G.P., Probert M.E. In: Modeling Nutrient Management in Tropical Cropping Systems. Delve R.J., Probert M.E., editors. Australian Center for International Agricultural Research (ACIAR) No 114; Canberra: 2004. Testing the APSIM model with experiment data from the long term manure experiment at Machang'a (Embu), Kenya; pp. 110–117. [Google Scholar]
- Min K., Freeman C., Kang H., Choi S. 2015. The Regulation by Phenolic Compounds of Soil Organic Matter Dynamics under a Changing Environment 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucheru-Muna M., Pypers P., Mugendi D., Kung J., Mugwe J., Merckx R., Vanlauwe B. Field Crops Research A staggered maize – legume intercrop arrangement robustly increases crop yields and economic returns in the highlands of Central Kenya. Field Crop. Res. 2010;115:132–139. [Google Scholar]
- Nayak A.K., Gangwar B., Shukla A.K., Mazumdar S.P., Kumar Anjani, Raja R., Kumar Anil, Kumar V., Rai P.K., Mohan U. Long-term effect of different integrated nutrient management on soil organic carbon and its fractions and sustainability of rice-wheat system in Indo Gangetic Plains of India. Field Crop. Res. 2012;127:129–139. [Google Scholar]
- Ng E.L., Patti A.F., Rose M.T., Schefe C.R., Wilkinson K., Smernik R.J., Cavagnaro T.R. Does the chemical nature of soil carbon drive the structure and functioning of soil microbial communities? Soil Biol. Biochem. 2014;70:54–61. [Google Scholar]
- Ngetich K.F., Diels J., Shisanya C.A., Mugwe J.N., Mucheru-Muna M., Mugendi D.N. Effects of selected soil and water conservation techniques on runoff, sediment yield and maize productivity under sub-humid and semiarid conditions in Kenya. Catena. 2014;121:288–296. [Google Scholar]
- Nogueirol R.C., Cerri C.E.P., Silva W.T.L. da, Alleoni L.R.F. Effect of no-tillage and amendments on carbon lability in tropical soils. Soil Tillage Res. 2014;143:67–76. [Google Scholar]
- Normand A.E., Smith A.N., Clark M.W., Long J.R., Reddy K.R. Chemical composition of soil organic matter in a subarctic peatland: influence of shifting vegetation communities. Soil Sci. Soc. Am. J. 2017;81:41–49. [Google Scholar]
- Owens L.B., Edwards W.M., Vankeuren R.W. Groundwater nitrate levels under fertilized grass and grass-legume pastures. J. Environ. Qual. 1994;23:752e758. [Google Scholar]
- Pane C., Piccolo A., Spaccini R., Celano G., Villecco D., Zaccardelli M. Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl. Soil Ecol. 2013;65:43–51. [Google Scholar]
- Panettieri M., Knicker H., Berns A.E., Murillo J.M., Madejón E. Moldboard plowing effects on soil aggregation and soil organic matter quality assessed by 13 C CPMAS NMR and biochemical analyses. Agric. Ecosyst. Environ. 2013;177:48–57. [Google Scholar]
- Panettieri M., Knicker H., Murillo J.M., Madejón E., Hatcher P.G. Soil organic matter degradation in an agricultural chronosequence under different tillage regimes evaluated by organic matter pools, enzymatic activities and CPMAS13C NMR. Soil Biol. Biochem. 2014;78:170–181. [Google Scholar]
- Quideau S., Chadwick O., Benesi A., Graham R., Anderson M. A direct link between forest vegetation type and soil organic matter composition. Geoderma. 2001;104:41–60. [Google Scholar]
- Rumpel C., Eusterhues K., Ko I. Location and chemical composition of stabilized organic carbon in topsoil and subsoil horizons of two acid forest soils. Soil Biol. Biochem. 2004;36:177–190. [Google Scholar]
- Ryan J., George E., Rashid A. second ed. 2001. Soil and Plant Analysis Laboratory Manual. Jointly Published by International Center for Agricultural Research in the Dry Areas (ICARDA) and the National Agricultural Research Centre (NARC) pp. 46–48. [Google Scholar]
- SAS Institute Inc . SAS/STAT; Cary, NC, USA: 2004. p. 5121. [Google Scholar]
- Schnitzer M., Desjardins J.G. Carboxyl and phenolic hydroxyl groups in some organic soils and their relation to the degree of humification. Can. J. Soil Sci. 1965;45:257–264. [Google Scholar]
- Schöning I., Knicker H., Kögel-Knabner I. Intimate association between O/N-alkyl carbon and iron oxides in clay fractions of forest soils. Organic Geochem. 2005;36:1378–1390. [Google Scholar]
- Schuman G.E., Janzen H.H., Herrick J.E. Soil carbon dynamics and potential carbon sequestration by rangelands. Environ. Pollut. 2002;116:391–396. doi: 10.1016/s0269-7491(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Shang Z.H., Cao J.J., Guo R.Y., Long R.J., Deng B. The response of soil organic carbon and nitrogen 10 years after returning cultivated alpine steppe to grassland by abandonment or reseeding. Catena. 2014;119:28–35. [Google Scholar]
- Shisanya C., Mucheru M., Mugendi D., Kung'u J. Effect of organic and inor- ganic nutrient sources on soil mineral nitrogen and maize yields in central highlands of Kenya. Soil Tillage Res. 2009;103:239–246. [Google Scholar]
- Singh B., Rengel Z. In: Nutrient Cycling in Terrestrial Ecosystems. Marschner P., Rengel Z., editors. Springer; 2007. The role of crop residues in improving soil fertility; pp. 183–214. [Google Scholar]
- Solomon D., Lehmann J., Kinyangi J., Amelung W., Lone I., Pell A.N., Riha S., Ngoze S., Verchot L., Mbugua D. Long-term impacts of anthropogenic perturbations on dynamics and speciation of organic carbon in tropical forest and subtropical grassland ecosystems. Global Change Biol. 2010;13:511–530. [Google Scholar]
- Solomon D., Lehmann J., Harden J., Wang J., Kinyangi J., Heymann K., Karunakaran C., Lu Y., Wirick S., Jacobsen C. Micro- and nano-environments of carbon sequestration: multi-element STXM-NEXAFS spectromicroscopy assessment of microbial carbon and mineral associations. Chem. Geol. 2012;329:53–73. [Google Scholar]
- Stroosnijder L. Modifying land management in order to improve efficiency of rainwater use in the African highlands. Soil Tillage Res. 2009;103:247–256. [Google Scholar]
- Thelen K.D., Fronning B.E., Kravchenko A., Min D.H., Robertson G.P. Integrating livestock manure with a corn-soybean bioenergy cropping system improves short-term carbon sequestration rates and net global warming potential. Biomass Bioenergy. 2010;34:960–966. [Google Scholar]
- Trumbore S. Radiocarbon and soil carbon dynamics. Annu. Rev. Earth Planet Sci. 2009;37:47–66. [Google Scholar]
- von Lützow M., Leifeld J., Kainz M., Kögel-Knabner I., Munch J.C. Indications for soil organic matter quality in soils under different management. Geoderma. 2002;105:243–258. [Google Scholar]
- Wang Q.J., Zhang L., Zhang J.C., Shen Q.R., Ran W., Huang Q.W. Effects of compost on the chemical composition of SOM in density and aggregate fractions from rice-wheat cropping systems as shown by solid-state 13C-NMR spectroscopy. J. Plant Nutr. Soil Sci. 2012;175:920–930. [Google Scholar]
- Wang H., Liu S., Wang J., Shi Z., Lu L., Guo W., Jia H., Cai D. Dynamics and speciation of organic carbon during decomposition of leaf litter and fine roots in four subtropical plantations of China. For. Ecol. Manage. 2013;300:43–52. [Google Scholar]
- Were K., Bui D.T., Dick Ø.B., Singh B.R. A comparative assessment of support vector regression, artificial neural networks, and random forests for predicting and mapping soil organic carbon stocks across an Afromontane landscape. Ecol. Indicat. 2015;52:394–403. [Google Scholar]
- Wissing L., Kolbl A., Hausler W., Schad P., Cao Z.-H., Gel-Knabner I.K. Management-induced organic carbon accumulation in paddy soils : the role of organo-mineral associations. Soil Tillage Res. 2013;126:60–71. [Google Scholar]
- Yu H., Ding W., Chen Z., Zhang H., Luo J. Accumulation of organic C components in soil and aggregates. Nat. Publ. Gr. 2015:1–12. doi: 10.1038/srep13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chi F., Wei D., Zhou B., Cai S., Li Y., Kuang E. Impacts of long-term fertilization on the molecular structure of humic acid and organic carbon content in soil aggregates in Black soil. Sci. Rep. 2019:1–7. doi: 10.1038/s41598-019-48406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer R.J., Bossio D.A., Sommer R., Verchot L.V. Global sequestration potential of increased organic carbon in cropland soils. Sci. Rep. 2017:1–8. doi: 10.1038/s41598-017-15794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.