Highlights
-
•
The results of our study strongly indicate that blocking GRP/GRP-R signaling by targeting GRP-R is sufficient to inhibit ARVs expression. In addition, the combination of blocking GRP/GRP-R signaling (targeting ARVs) and anti-androgens (targeting AR-FL) is a potential new therapeutic approach for treatment of CRPC and therapy-induced tNEPC.
Keywords: GRP/GRP-R, NF-kappa B, Androgen receptor variants, Castration-resistant prostate cancer, neuroendocrine prostate cancer
Abstract
Clinical management of castration-resistant prostate cancer (CRPC) resulting from androgen deprivation therapy (ADT) remains challenging. Many studies indicate that androgen receptor splice variants (ARVs) play a critical role in the development of CRPC, including resistance to the new generation of inhibitors of androgen receptor (AR) action. ARVs are constitutively active and lack the ligand-binding domain (LBD), thereby allowing prostate cancer (PC) to maintain AR activity despite therapies that target the AR (full-length AR; AR-FL). Previously, we have reported that long-term ADT increases the neuroendocrine (NE) hormone – Gastrin Releasing Peptide (GRP) and its receptor (GRP-R) expression in PC cells. Further, we demonstrated that activation of GRP/GRP-R signaling increases ARVs expression by activating NF-κB signaling, thereby promoting cancer progression to CRPC. Most importantly, as a cell surface protein, GRP-R is easily targeted by drugs to block GRP/GRP-R signaling. In this study, we tested if blocking GRP/GRP-R signaling by targeting GRP-R using GRP-R antagonist is sufficient to control CRPC progression. Our studies show that blocking GRP/GRP-R signaling by targeting GRP-R using RC-3095, a selective GRP-R antagonist, efficiently inhibits NF-κB activity and ARVs (AR-V7) expression in CRPC and therapy-induced NEPC (tNEPC) cells. In addition, blocking of GRP/GRP-R signaling by targeting GRP-R can sensitize CRPC cells to anti-androgen treatment (such as MDV3100). Further, preclinical animal studies indicate combination of GRP-R antagonist (targeting ARVs) with anti-androgen (targeting AR-FL) is sufficient to inhibit CRPC and tNEPC tumor growth.
Significance.
Antiandrogen treatment of PC cells induces NED and reprograms cancer cells to colonize and grow in the bone microenvironment. Blocking of GRP/GRP-R signaling can sensitize CRPC cells to anti-androgen treatment by decreasing ARVs expression. Combination of ADT with blocking of GRP/GRP-R signaling is a potential new approach to control CRPC tumor growth.
Alt-text: Unlabelled box
Introduction
If prostate cancer (PC) remains localized, therapy such as prostatectomy or radiation therapy can cure the patient. Since PC is an androgen-dependent disease, androgen deprivation therapy (ADT) is the standard of care for initial systemic treatment of metastatic and recurrent PC [1], [2], [3]. ADT, in the majority of PC patients, results in initial regression of disease and a dramatic decrease in serum PSA. Despite the success of androgen blockade, including new generation of anti-androgens, nearly all patients eventually progress to castration-resistant prostate cancer (CRPC). Many studies have demonstrated that failure to the new generation of anti-androgens, such as abiraterone acetate or enzalutamide, appears to be mediated through the induction of AR splice variants (ARVs) [4], [5], [6], [7], [8]. ARVs, which lack the ligand-binding domain (LBD), are constitutively active in the absence of ligand, thereby allowing CRPC to maintain AR activity. Therefore, the traditional androgen-ablation therapy [9], such as luteinizing hormone-releasing hormone analogs that block the production of testicular androgens and/or LBD targeted AR blockers alone cannot inhibit ARVs activation to control advanced PC.
Recently, neuroendocrine differentiation (NED) has become increasingly recognized as a mechanism that allows transdifferentiation of PC cells to escape ADT, including resistant to new generation of anti-androgen therapies [10], [11], [12], [13], [14], [15]. Although a de novo clinical presentation of small cell NE carcinoma of the prostate is rare, a subset of patients previously diagnosed with prostate adenocarcinoma may develop neuroendocrine features in later stages of CRPC progression as a result of treatment resistance. Despite sharing clinical, histologic, and some molecular features with other NE carcinomas, including small cell lung cancer, castration-resistant neuroendocrine prostate cancer (CRPC-NE) is clonally derived from prostate adenocarcinoma. A clinical study shows that NED is positive in up to 52% of PC patients with bone metastasis [16]. Most recently, Aggarwal and colleagues have reported that prostatic metastatic bone cancer is made up of about 10–15% NEPC (small cell carcinoma) which is histologically similar to NE prostate cancer (NEPC) metastasis to the lung and liver [17]. Regardless NEPC develops from primary NE cells or prostatic adenocarcinoma cells that transdifferentiate to therapy-induced NEPC (tNEPC), the tumors express NE markers [11,18,19], NEPC/NED has a more aggressive clinical behavior, an unfavorable prognosis, and is a non-curable disease [20]. Therefore, developing a new treatment for CRPC and NEPC/NED is a critical challenge.
Previously, we have demonstrated that activation of GRP/GRP-R signaling increases NF-κB activity and ARVs expression thereby contributing to progression to CRPC [21]. These findings strongly indicate that GRP/GRP-R signaling is a potential target to control CRPC. GRP is a 27-amino acid neuropeptide that is the mammalian homologue of the linear tetradecapeptide bombesin (BN) originally isolated from the skin of frogs. It shares homology with BN at the amidated C-terminal sequence in the final 7 amino acids [22,23]. The GRP-R is the receptor to which GRP and BN bind with a high affinity [22,23]. Most important, our studies show that GRP-R expression is increased in both of human prostate adenocarcinoma and NEPC [21]. As a cell surface protein, GRP-R is easily targeted by drugs to block GRP/GRP-R signaling.
In this study, we tested if blocking of GRP/GRP-R signaling by targeting GRP-R using GRP-R antagonist is sufficient to control CRPC and anti-androgen induced bone growing NEPC/NED. We demonstrated that blocking of GRP/GRP-R signaling by targeting GRP-R can sensitize CRPC cells to anti-androgen treatment by decreasing ARVs expression in PC cells. Further, combination of GRP-R antagonist (targeting ARVs) with anti-androgen (targeting AR-FL) is sufficient to inhibit tumor growth in CRPC and therapy-induced (t)NEPC mouse xenograft models.
Materials and methods
Cell culture and materials
The human prostate carcinoma cell line LNCaP and 22RV1 were obtained from the ATCC (Manassas, VA). LNCaP-MDV cells were generated by treating LNCaP cells with MDV3100 (10−5M) for more than 3 months (named LNCaP-MDV) and maintained in the regular culture medium with MDV3100 (10−5M) for further studies. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in the air and were tested for contamination within the past 6 months using a Mycoplasma Detection Kit (Southern Biotech). Cell lines were routinely cultured in RPMI 1640 (Gibco-BRL) medium containing 5% fetal calf serum (FBS) (Hyclone), 0.1% Insulin-Transferrin-Selenium (ITS) and 0.1% Glutamine (Gibco-BRL). The following reagents were purchased for in vitro and in vivo experiments: RC3095 (a selective GRP-R antagonist; Sigma-Aldrich) and MDV3100 (an AR antagonist; Sigma).
Reverse transcription and real-time PCR
Total RNAs from experimental cells were extracted using Trizol (Gibco-BRL), and residual genomic DNA was removed by DNaseI (Invitrogen) treatment. The RNAs were reverse transcribed using random primers and Superscript II (Gibco-BRL) according to the manufacturer's protocol. The primers used to amplify wild-type AR (AR-FL) were 5′-TTCGAATGAACTACATCAAGGAACTCGATCG-3′ (forward), 5′-TTGGGCACTTGCACAGAGAT-3′ (reverse); primers of AR-V7 were 5’-CCATCTTGTCGTCTTCGGAAATGTTATGAAGC-3’ (forward), 5’- TTTGAATGAGGCAAGTCAGCCTTTCT-3’ (reverse) [5]; primers of Neuron-Specific Enolase (NSE) were 5’-GAACTATCCTGTGGTCTCC-3’ (forward), 5’- CGACATTGGCTGTGAACTTG-3’ (reverse); primers of Synaptophysin were 5’-TCAGTTCCGGGTGGTCAAG-3’ (forward), 5’- AAGACCCATTGCAGCACCTT-3’ (reverse); primers of Chromogranin A were 5’-TCCAAGGCGCCAAGGA-3’ (forward), 5’- CATCTTCAAAACCGCTGTGTTTC-3’ (reverse); primers of GAPDH were 5’-CCATGGAGAAGGCTGGGG-3’ (forward), 5’- CAAAGTTGTCATGGATGACC-3’ (reverse). Real-time qPCR reactions were carried out in a 20μl volume using a 96-well plate format and fluorescence was detected utilizing the Bio-Rad I-Cycler IQ Real-time detection system. Gene expression was normalized to GAPDH (housekeeping gene) by the 2−ΔΔCt method [24]. The values plotted represent the mean of at least three individual samples ± SD.
Western blot analysis
Whole cell lysate was extracted from experimental cells. A 20μg aliquot of each protein sample was separated on a 4 to 12% Tris-glycine gradient gel (NOVEXTM), and then transferred to nitrocellulose membranes (Schleicher & Schuell, Germany). The membranes were blocked with 5% skim milk in TBS-T (Trypsin buffered saline, 1% Tween-20) buffer. The AR (N20, Santa Cruz), AR-V7 (Precision), Synaptophysin (Abcam) antibodies were added, and the blots were incubated o/n in 4 C°. After washing three times for 10 min each in TBS-T, incubation was performed for 1 h with the secondary horseradish-peroxidase-conjugated anti-rabbit/anti-mouse antibody. β-actin was used as the loading control. The signals were developed by an ECL detection system (Amersham Biosciences, Amersham, USA).
Transient transfection assay
The NGL vector [a NF-κB responsive reporter vector which has Luciferase and Green Fluorescent Protein (GFP) reporter genes] [25] was used to measure NF-κB activity and the ARR2PB-Luc vector (an AR responsive reporter vector) [26] was used to measure AR activity in the PC cells by transient transfection experiments. Cells were plated at an initial density of 2.5 × 104/well in 24-well tissue culture plates. After 24 h, the cells were transfected with NGL/ARR2PB-Luc vectors using Lipofectamine (Invitrogen) for four hours according to the manufacturer's protocol. Luciferase activity was determined using the Promega Corp luciferase assay system 24 h after treatment with RC3095 and/or MDV3100. The transfection efficiency was determined by co-transfecting pRL-CMV containing the Renilla luciferase reporter gene (Promega). The values plotted represent the mean of at least three individual samples ± SD.
Proliferation assay
Experimental cells were plated in a 96-well plate (1 × 104/well). After 24 h, the cells were treated with or without different combination of drugs (MDV3100 or RC3095) in different concentration. MTT assay was performed at 48 h after the cells had been treated with referenced drugs. All of the measurements were carried out in triplicate.
CRPC xenograft mouse model
All animal studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Vanderbilt Institutional Animal Care & Use Committee (Permit Number: M1600233). 1) CRPC xenograft mouse model was generated by injection of 22RV1 cells (1 × 106 cells) subcutaneously into the right flank of 6 -to 7 weeks old male athymic nude mice (BALB/c strain). After the primary tumors reached 3 to4 mm diameter (2 to3 weeks), the mice were treated with RC3095 (20ug/day, sc) alone or in combination with castration or MDV3100 (10mg/kg/day, gavage) to mimic ADT for two weeks. Tumor volume was measured weekly and calculated by the formula: Volume = π/6 × W × H × L (mm3). At 2 weeks after treatment, the xenograft tissues were harvested and fixed in 10% buffered formalin and paraffin embedded for histologic and immunohistochemical analyses. 2) tNEPC bone growth mouse model, was generated by intratibial injection of LNCaP-MDV cells (1 × 105 cells) into the 6 and 7 weeks old male athymic nude mice (BALB/c strain). Tumor formation was monitored using small animal X-ray radiograph imaging (Faxitron LX-60; Lincolnshire). After bone tumor formation (about 4 weeks after grafting), the mice were treated with RC3095 (20ug/day, sc) alone or in combination with castration or MDV3100 (10 mg/kg/day, gavage) to mimic ADT for two weeks. At 2 weeks after treatment, harvested tibiae were fixed in 10% neutral buffered-formalin solution for 24 h and decalcified in 0.5 mol/L EDTA in Ca2+- and Mg2+-free Dulbecco's PBS for one week before embedding in paraffin for histologic and immunohistochemical analyses. Each group had at least five mice. The results are reported as the mean percent ± SD.
Immunohistochemistry
Paraffin-embedded tissue sections were stained immunohistochemically with antibodies against Ki67 (clone TEC-3, DAKO), AR (N-20, Santa Cruz) and AR-V7 (Precision). The primary antibody was incubated at the appropriate concentration (Ki67, 1:1000; AR, 1:1000; AR-V7, 1:200) for one hour at room temperature. The secondary antibody was incubated for 60 min. Slides were rinsed extensively in tap water, counterstained with Mayer's hematoxylin and mounted. For quantitation of the cell proliferation, the cells were counted as positive for Ki67 when nuclear immunoreactivity was observed. Each tissue section was counted manually in three different areas to assess the Ki67 positive cells index. The data were then presented as number of Ki67 positive cells (%). Each group had at least five mice. The results are reported as the mean percent ± SD.
Serum PSA measurements
Mouse plasma (22RV1 group) was obtained from the caudal vein before scarified at 2 weeks after treatment for measurement of PSA levels using a Quantikine human PSA immunoassay kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's instructions. The values plotted represent the mean of each in triplicated individual samples ± SD.
Statistical analysis
Where appropriate, experimental groups were compared using two-sample t-test, with significance defined as p <0.05.
Results
Generating therapy-induced bone-growing NEPC cell line
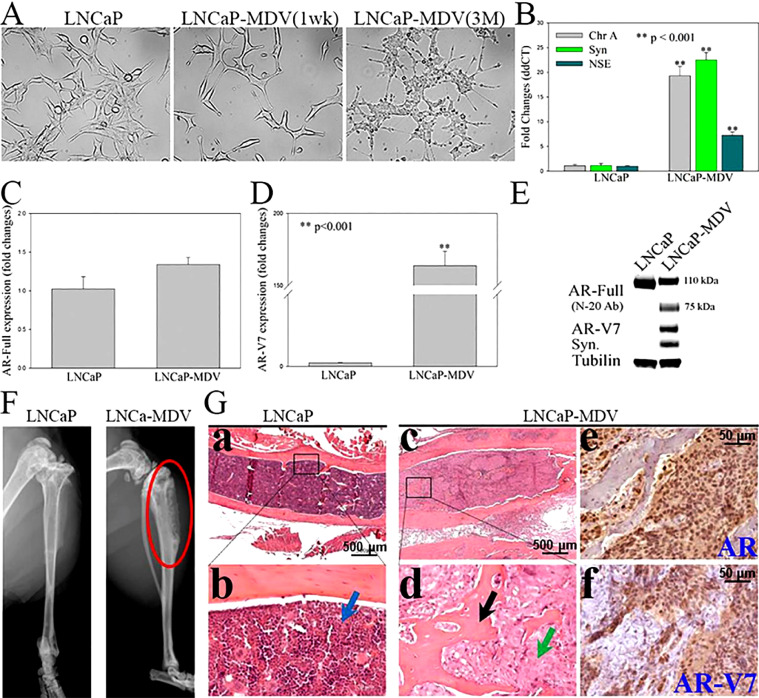
Increasing evidence indicates NED plays a critical role in PC cells escape of ADT, including resistant to a new generation of anti-androgen therapies [10–14]. To test if GRP/GRP-R targeted therapy is sufficient to inhibit growth of castration-resistant prostate adenocarcinoma and NED PC cells, first, we generated an ADT-resistant PC cell by treating androgen dependent LNCaP PC cells with MDV3100 for more than 3 months (named LNCaP-MDV). Our studies show that long-term ADT with anti-androgens induces PC cell transdifferentiation to NEPC, as demonstrated by morphology, gene expression profiles (Fig. 1). Morphologically, LNCaP-MDV cells developed many long cytoplasmic processes, with secondary and tertiary neurotic-like branching, well beyond 3 to 5 times the length of the cell body (Fig. 1A). Relative to the parental cells, LNCaP-MDV had increased NE markers (chromogranin A, synaptophysin and NSE) expression (Fig. 1B). Further, although full-length AR (AR-FL) expression is slightly decreased (at protein level; Fig. 1E), AR variants (AR-V7) expression is significantly increased (Fig. 1D, E). These results indicate long-term treatment with anti-androgens induces NED in PC cells.
Fig. 1.
Long-term ADT reprogrammed PC cells to enable cancer cells to grow in the bone microenvironment. (A) Morphological changes in LNCaP and those treated with MDV3100 (LNCaP-MDV) when observed by light microscopy. (B, C and D) NE markers (Chromogranin A, Synaptophysin and Neuron Specific Enolase), full-length AR (AR-Full) and AR-V7 expression were determined by qPCR. (E) AR-Full (SC, N-20 antibody), AR-V7 and Synaptophysin (Syn) expression were determined by WB. (F & G) LNCaP and LNCaP-MDV (Gc to f) were grafted into the mouse bones by intratibial injection. Tumor formation (red circle) was determined (8 weeks after grafting) by small animal X-ray radiograph imaging (F), H&E and IHC staining of AR, and AR-V7 (Ga,b: LNCaP negative; Gc to f: LNCaP-MDV). Arrow in Gb: bone marrow (blue); in Gd: new bone (black), tumor cells (green). Statistical significance was determined by student's t-test. ** p <0.001.
In order to test if therapy-induced NED allows cancer cells to grow in the bone microenvironment, LNCaP-MDV, the therapy-induced NEPC (tNEPC) cells were inoculated into the bone of nude mice by intratibial injection. Small animal X-ray radiograph imaging was performed to monitor bone lesion development. Mice were sacrificed for histological analysis at 8 weeks post-inoculation. It is well known that LNCaP cells are difficult to grow in murine bone following intratibial or intrafemural injection [27]. As expected, no growth (0/5) was detected by either X-ray radiographic imaging or histological analysis for control LNCaP cells grafted into the bone after 8 weeks (Fig. 1F left panel and G a, b). Most surprisingly, long-term ADT enabled LNCaP-MDV cells to colonize and grow in the bone by intratibial injection (Fig. 1F right panel, G c, d, e and f). X-ray radiograph imaging and histological analysis show that LNCaP-MDV cells formatted osteoblastic and osteoclastic mixed tumors in the bone environment (Fig. 1F and G c, d). These results indicate that long-term ADT can changes the characteristics of LNCaP to LNCaP-MDV cells that enables a non-bone-growing PC cell to progress to a bone-growing PC cell. Additionally, the LNCaP-MDV cell line is the first model of tNEPC that grows in the bone.
Blocking of GRP/GRP-R signaling efficiently inhibits NF-κB activity and ARVs (AR-V7) expression in PC cells
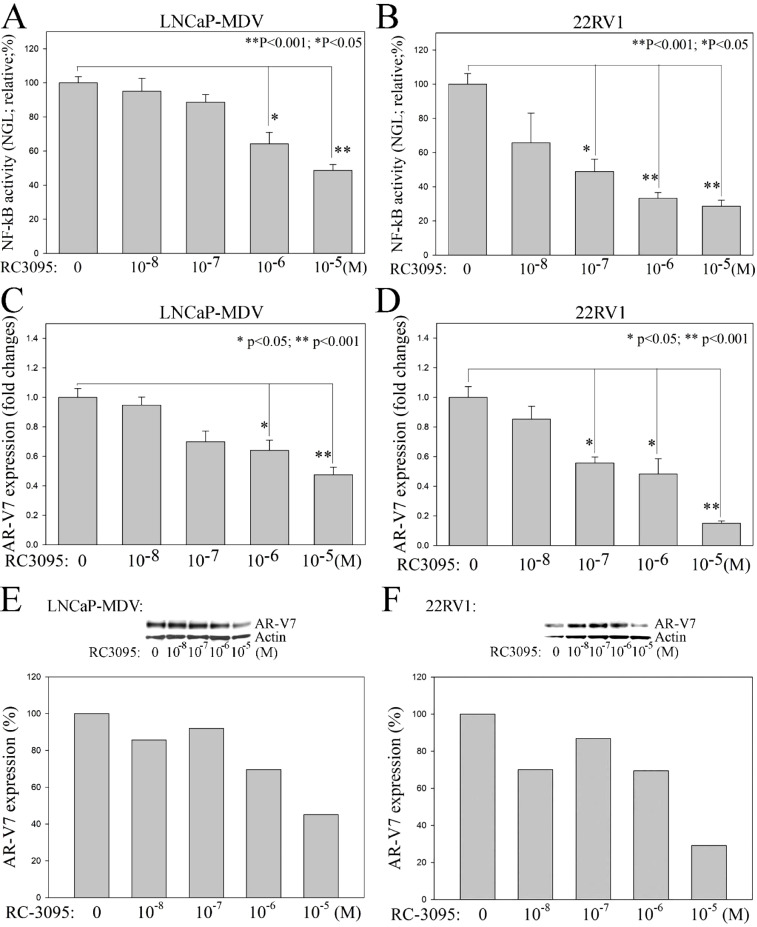
Previously, we demonstrated that axis of ADT → GRP/GRP-R → NF-κB → ARVs is an important mechanism that contributes PC progression to CRPC [21]. In addition, we have demonstrated that GRP-R expression is high in 99% of the PC patients, including NEPC [21]. Most importantly, as a cell surface protein, GRP-R is easily targeted by drugs. As a selective GRP-R antagonist, RC-3095 has been shown to have anti-inflammatory properties in many types of murine models [[28], [29], [30]]. In order to determine if blocking of GRP/GRP-R signaling efficiently inhibits NF-κB activity and ARVs expression thereby control CRPC progression, 22RV1, an androgen-independent AR-FL and AR-V7 positive prostate adenocarcinoma cell, and LNCaP-MDV, a therapy-induced tNEPC cells were treated with RC-3095. NF-κB activity in PC cells was measured using the NGL reporter that is a NF-κB responsive vector which has Luciferase and Green Fluorescent Protein (GFP) reporter genes [25]. The results show that blocking GRP/GRP-R signaling using RC3095 is sufficient to inhibit NF-κB activity in both of prostate adenocarcinoma (22RV1) and tNEPC (LNCaP-MDV) cells (Fig. 2A and B). In addition, blocking of GRP/GRP-R signaling efficiently inhibits ARVs (AR-V7) expression in these PC cells (Fig. 2 C –F).
Fig. 2.
Blocking of GRP/GRP-R signaling efficiently inhibits NF-κB activity and ARVs (AR-V7) expression in PC cells. GRP-R antagonist (RC3095) was used to block GRP/GRP-R signaling in 22RV1 and LNCaP-MDV cells. A and B) NF-κB activity in PC cells was measured using the NGL reporter. C and D) ARVs (AR-V7) expression was determined by qPCR. E and F) ARVs (AR-V7) expression was further confirmed by Western blot analysis. Blot signals were quantified using ImageJ program. Results were normalized by actin signals. Statistical significance was determined by student's t-test. * p <0.05; ** p <0.001.
Blocking of GRP/GRP-R signaling increases anti-androgen sensitivity in CRPC cells
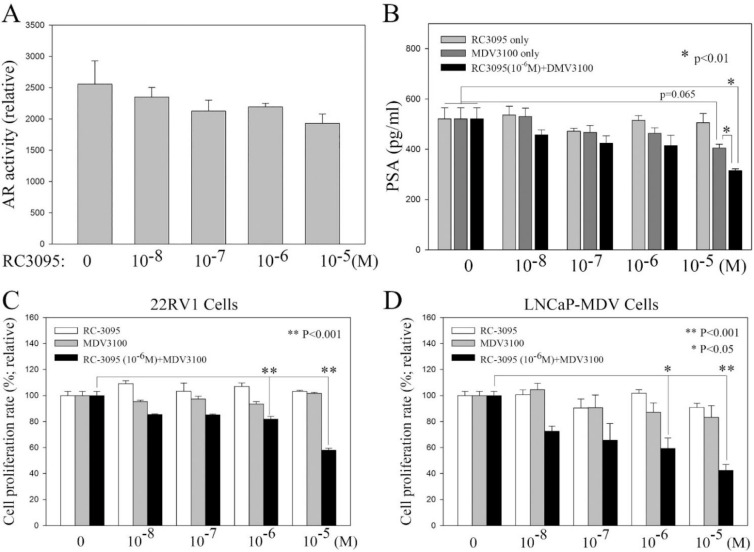
It is known that both wild-type AR-FL and ARVs regulate AR target genes and contributes to PC cells survival and progression. Our previous study indicated that GRP/GRP-R signaling contributes CRPC progression through increasing ARVs expression [21]. Blocking GRP/GRP-R signaling alone may inhibit ARVs expression (Fig. 2C to F) but may not be sufficient to block both wild-type AR-FL and ARVs activity. However, anti-androgens (such as Bicalutamide and MDV3100) will block AR-FL activity efficiently in PC cells. Therefore, it is possible that blocking ARVs expression by blocking GRP/GRP-R signaling may reverse anti-androgen insensitive CRPC cells to become anti-androgen sensitive PC cells. To investigate if blocking of GRP/GRP-R signaling increases anti-androgen sensitivity in CRPC cells, first, we investigate if blocking of GRP/GRPR signaling is sufficient to block AR activity in CRPC cells. 22RV1 cells were treated with RC3095, MDV3100 or RC3095+MDV3100. AR activity was measured using ARR2PB-Luc vector, AR responsive reporter vector [26]. The results show that although RC3095 efficiently inhibits AR-V7 expression (Fig. 2D and F), RC3095 alone treatment failed to inhibit AR activity and PSA expression significantly (Fig. 3A and B). Also, as expected, MDV3100 alone treatment failed to inhibit PSA expression significantly in 22RV1 cells (Fig. 3B). However, when RC3095 [10−6M; 10−6M is the lowest sufficient concentration of RC3095, which can significantly inhibit expression of AR-V7 in both of 22RV1 and LNCaP-MDV cells (Fig. 2C–F)] was present, MDV3100 efficiently inhibited PSA expression and AR activity in 22RV1 cells (Fig. 3B). These results indicate that although RC3095 is sufficient to inhibit AR-V7 expression efficiently (Fig. 2D and F), it cannot inhibit AR-FL activity efficiently; and, MDV3100 may inhibit AR-FL activity but it cannot block AR-V7 activity. To block AR activity efficiently in 22RV1 cells, both of RC3095 (10−6M; blocks AR-V7 expression/activity) and MDV3100 (10−5M; blocks AR-FL activity) are needed (Fig. 3B). To further confirm this observation, 22RV1 and LNCaP-MDV tNEPC cells were treated with RC3095 alone or in combination of RC3095 with MDV3100. Our studies show that RC3095 or MDV3100 alone had no significant effect on the growth rate of 22RV1 and LNCaP-MDV cells (Fig. 3C and D). However, when the cells were treated with anti-androgen (MDV3100; ≥10−6M) plus GRP-R antagonist (RC3095; 10−6M), the growth rate of the cells was significantly inhibited (Fig. 3C and D). These results indicate that blocking of GRP/GRP-R signaling is sufficient to decrease AR-V7 expression thereby increasing responsiveness of CRPC and tNEPC to the anti-androgen treatment.
Fig. 3.
Blocking of GRP/GRP-R signaling increases anti-androgen sensitivity in CRPC cells. A and B) 22RV1 cells were treated with RC3095, MDV3100 or RC3095+MDV3100. AR activity was measured using ARR2PB-Luc vector (A). PSA expression was measured by ELISA assay (B). C and D) 22RV1 (C) and LNCaP-MDV (D) cells were treated with RC-3095, MDV3100 or RC3095+MDV3100. MTT assay was performed at 48 h after the cells had been treated with referenced drugs. Statistical significance was determined by student's t-test. * p <0.05; ** p <0.001.
Blocking of GRP/GRP-R signaling in combination with ADT is sufficient to control CRPC tumor growth in vivo
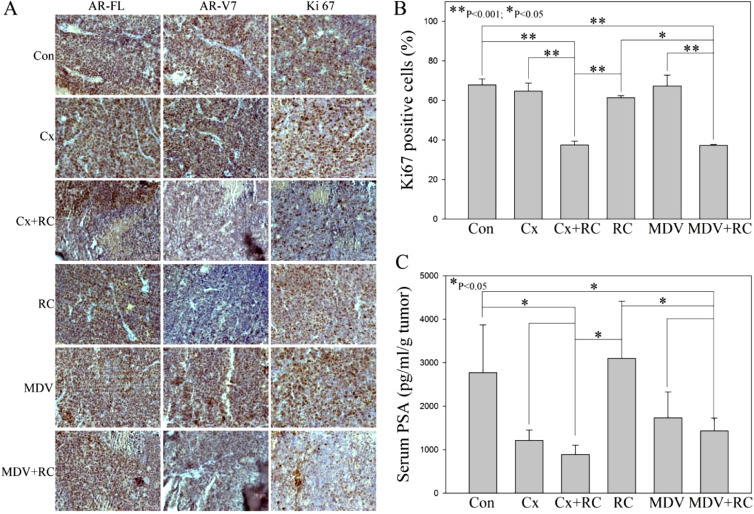
In order to test if blocking of GRP/GRP-R signaling alone or in combination with anti-androgens is sufficient to control CRPC tumor growth in vivo, 22RV1 cells were placed into the right flank of 6 to 7 weeks old male athymic nude mice by subcutaneous (s.c.) injection. After the primary tumor size reaches 3 to 4 mm in diameter (about 2 to 3 weeks), the mice were treated with RC3095 (20ug/day, sc) alone or in combination with ADT for two weeks. Castration or MDV3100 (10 mg/kg/day, gavage) was used to mimic ADT. The results show that after treatment with either RC-3095, MDV3100 or castration alone, tumors continued to grow and there was no significant difference in the tumor size compared to the control group (treated with vehicle). However, when the mice were treated with RC3095 combined with castration or MDV3100, the tumor growth was significantly inhibited (Fig. 4A and B). Immunohistochemical (IHC) staining shows that blocking of GRP/GRP-R signaling (RC3095 treatment) efficiently inhibits AR-V7 expression (Fig. 5A) and the number of the cancer cells stained by Ki67 (proliferation marker) in the tumor from mice treated with RC3095 plus ADT was significantly lower than that the mice treated with RC3095, MDV3100 or castration alone (Fig. 5A and B). In addition, when the mice were treated with RC3095 combined with castration or MDV3100, the serum PSA levels were significantly decreased (Fig. 5C). These results strongly support that blocking of GRP/GRP-R signaling in combination with ADT is sufficient to control CRPC tumor growth.
Fig. 4.
Blocking of GRP/GRP-R signaling in combination with ADT is sufficient to control CRPC tumor growth in vivo. 22RV1 cells were placed into the right flank of male athymic nude mice by subcutaneous (s.c.) injection. After the primary tumor formation (about 2-3 weeks), the mice were treated with RC3095 (20ug/day, sc Inj) alone or in combination with ADT for two weeks. Castration (Cx) or MDV3100 (10mg/kg/day, gavage) was used to mimic ADT. Control group mice (Con) were treated with vehicle only. (A) Tumor volume was measured weekly and calculated by the formula: Volume = π/6 × W × H × L (mm3). (B) is showing the tumor volumes at the end point. Results are expressed as the mean percentage change in tumor volume; bars, ± SD. Statistical significance was determined by student's t-test. * p <0.05; ** p <0.001.
Fig. 5.
RC3095 efficiently inhibits AR-V7 expression and blocking of GRP/GRP-R signaling in combination with ADT is sufficient to inhibit PSA expression in vivo. A) IHC staining of AR-FL (N-20), AR-V7 and Ki67 were performed. B) Each tissue section was counted manually in three different areas to assess the Ki67 positive cells index. The data were then presented as number of Ki67 positive cells (%). C) Serum PSA levels were measured by ELISA assay. Results are expressed as the means ± SD. Statistical significance was determined by student's t-test. * p <0.05; ** p <0.001.
Blocking of GRP/GRP-R signaling in combination with ADT efficiently inhibits tNEPC tumor growth in the bone
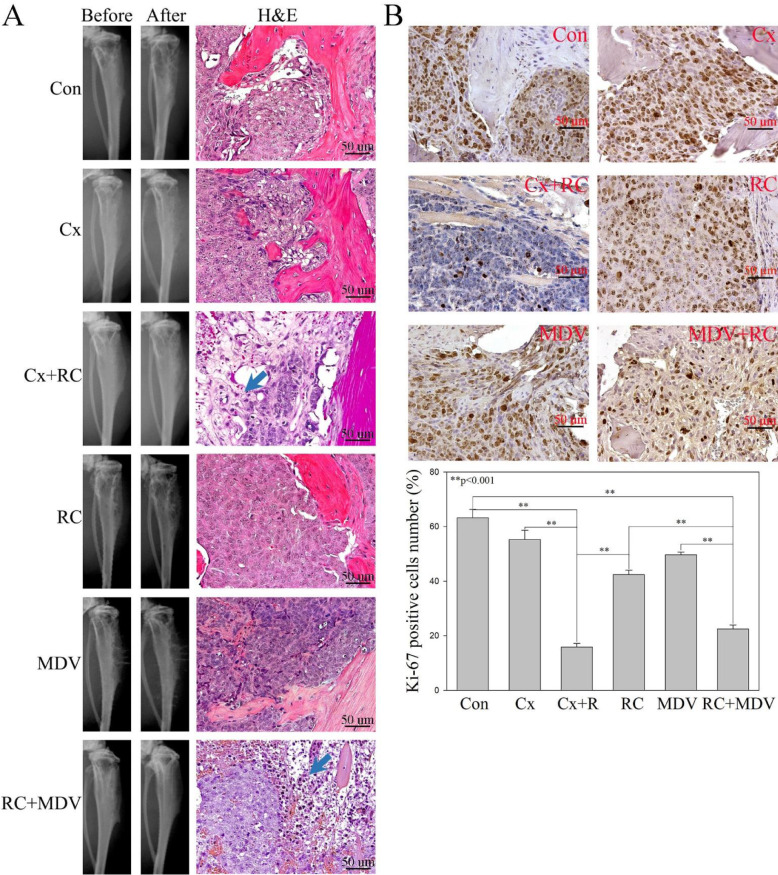
Unlike other types of cancer, patients with advanced PC develop osseous metastasis and the initial metastasis of PC is almost strictly limited to bone which is often the only site of spread even in late disease [31,32]. A clinical study shows that NED is positive in up to 52% of patients with bone metastasis [16] and up to 15% of the CRPC bone metastasis are tNEPC [17]. To test if blocking of GRP/GRP-R signaling or in combination with anti-androgens is sufficient to control tNEPC tumor growth in the bone, LNCaP-MDV cells were placed into the right flank of 6to 7 week old male athymic nude mice bone by direct intratibial injection. Tumor formation and growth were monitored by a small animal x-ray radiograph once a week. After bone tumor formation (6 weeks after grafting), the mice were treated with RC3095 (20ug/day, sc) alone or in combination with ADT for two weeks. Although conventional x-ray radiograph is an easy and convincing method to monitor tumor growth and detect bone lesion, it was difficult to quantitate the tumor size or bone lesions for assessment of treatment response (Fig. 6A). However, a significant increase in necrosis was observed in the combination treatment (RC3095+Castration or RC3095+MDV3100) groups by histological analysis (Fig. 6A). In addition, the number of the cancer cells stained by Ki67 in the tumor from mice treated with RC3095 plus ADT was significantly lower than that the mice treated with RC3095, MDV3100 or castration alone (Fig. 6B).
Fig. 6.
Blocking of GRP/GRP-R signaling in combination with ADT efficiently inhibits tNEPC tumor growth in the bone. LNCaP-MDV cells were placed into the right flank of male athymic nude mice bone by direct intratibial injection. Tumor formation and growth were monitored by a small animal x-ray radiograph once a week. After bone tumor formation (6 weeks after grafting), the mice were treated with RC3095 alone or in combination with ADT for two weeks. A) Tumor formation was confirmed by small animal X-ray radiograph imaging and H&E staining. Blue arrow indicates tumor necrosis area. B) IHC staining of Ki67 was performed. Each tissue section was counted manually in three different areas to assess the Ki67 positive cells index. The data were then presented as percentage of Ki67 positive cells in grafted PC tumor cells. Results are expressed as the means ± SD. Statistical significance was determined by student's t-test. ** p <0.001.
Taken together, these results strongly indicate that blocking of GRP/GRP-R signaling in combination with ADT is a potential new approach to control CRPC tumor growth, including ADT induced tNEPC.
Discussion
Numerous studies indicate that ARVs play a critical role in the development of CRPC, including the resistance to the new generation of inhibitors of AR action [4–8]. Previously, we have reported that increased NE peptides, such as BN and GRP, contribute to CRPC through the activation of NF-κB signaling [33,34]. In addition, we demonstrated that activation of NF-κB signaling increases ARVs expression in PC cells, thereby promoting progression to CRPC [35]. However, how NF-κB signaling is activated after ADT is unclear. Further, although NF-κB signaling is a promising target in advanced CRPC, it has been difficult to find drugs that block the oncogenic activity of NF-κB without interfering with its physiological roles and leading to highly toxic side effects. In this study, we successfully conducted the following research: 1) Blocking GRP/GRP-R signaling using GRP-R antagonist (RC3095) efficiently inhibits NF-κB activity and decreases AR-V7 expression. 2) Blocking of GRP/GRP-R signaling by targeting GRP-R can sensitize CRPC cells to antiandrogen treatment. 3) Blocking of GRP/GRP-R signaling (targeting ARVs) plus anti-androgen (targeting AR-FL) efficiently inhibits CRPC, including tNEPC, tumor growth both in vitro and in vivo. Importantly, based upon these findings, GRP/GRP-R is a potential target to control growth of CRPC and tNEPC.
GRP-R belongs to a family of G-coupled protein receptors, and the GRP binds selectively to the GRP-R [22,23]. BN-analogues have been developed that bind the GRP-R and are now being used for clinical studies [[36], [37], [38]]. Many studies have shown that GRP-R is expressed at very low levels in normal prostate glands but is increased in 45-100% of human PC [39,40]. Consistent with these findings, our previous studies shown that up to 90% of human PC are positive for GRP-R [21]. Most important, GRP-R expression is increased in AR negative NEPC tumors [21]. Therefore, GRP-R is a sufficient target for both of AR positive adenocarcinoma and AR negative NEPC. Recently, several new clinical imaging studies by targeting GRP-R, using a radiolabeled BN-analogue, successfully detected primary, recurrent and metastatic lesions of PC, and displayed good tumor delineation in a subset of patients with recurrent PC, including lymph node and bone metastatic lesions in patients with PC [41,42]. Taken together, these findings strongly indicate that GRP-R is a promising target for a theragnostic approach in both AR positive CRPC and AR negative NEPC.
Potent GRP-R antagonists were developed by Coy and co-workers by modifying the BN peptide backbone, substituting the amide bond with a pseudopeptide bond [43]. Subsequently, it was demonstrated that [Leu13-ψ-CH2NH-Leu14] BN derivatives can inhibit BN-stimulated growth of Swiss 3T3 cells and of various cancer cell lines [44,45]. The first clinical study using the BN antagonist [D-Tpi6-Leu13-ψ-CH2NH-Leu14] BN, also known as RC-3095 [44] was conducted by Schwartsmann et al in 25 patients with advanced solid malignancies, including 6 PC patients [36]. No toxicity was observed after administration of this drug but, unfortunately, no significant response to the treatment was reported either. Based upon our findings, we expect PC would have a unique response to GRP/GRP-R activation owing to a close functional interaction between GRP-R and AR signaling through elevating ARVs expression. Therefore, the effects of GRP-R antagonist monotherapy may be sufficient to decrease ARVs expression but may not be sufficient to totally block both AR-FL and ARVs levels and activity, thereby control PC progression.
In summary, the results of our study strongly indicate that blocking of GRP/GRP-R signaling by targeting GRP-R is sufficient to inhibit ARVs expression thereby control CRPC tumor progression and the combination therapy of blocking of GRP/GRP-R signaling (targeting ARVs) with anti-androgens (targeting AR-FL) is sufficient to inhibit CRPC including tNEPC tumor growth. As a specific antagonist of GRP-R, RC-3095 successfully inhibited CRPC tumor growth by reducing ARVs expression in our study, including AR-V7 positive tNEPC tumors. However, whether RC3095 may have an AR-independent effect to control AR negative NEPC tumors needs further study.
CRediT authorship contribution statement
Thomas C. Case: Methodology, Data curation. Alyssa Merkel: Methodology, Data curation. Marisol Ramirez-Solano: Data curation. Qi Liu: Data curation, Writing – review & editing. Julie A. Sterling: Conceptualization, Methodology, Visualization, Data curation, Investigation, Writing – review & editing. Renjie Jin: Conceptualization, Methodology, Visualization, Data curation, Investigation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Grant Support: This study was supported to R. Jin by the Ferring Innovation Grant from Ferring Research Institute and the W. L. Bray Endowment.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101213.
Appendix. Supplementary materials
References
- 1.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 2.Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr. Relat. Cancer. 2010;17(4):R305–R315. doi: 10.1677/ERC-10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu-Yao GL, Albertsen PC, Moore DF. Fifteen-year survival outcomes following primary androgen-deprivation therapy for localized prostate cancer. JAMA Intern. Med. 2014;174(9):1460–1467. doi: 10.1001/jamainternmed.2014.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu R, Dunn TA, Wei S. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Z, Yang X, Sun F. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson PA, Chen YF, Balbas MD. Inaugural Article: Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl. Acad. Sci. USA. 2010;107(39):16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Lu C, Wang H. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rove KO, Crawford ED. Traditional androgen ablation approaches to advanced prostate cancer: new insights. Can. J. Urol. 2014;21(2 Supp 1):14–21. [PubMed] [Google Scholar]
- 10.Mosquera JM, Beltran H, Park K. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 2013;15(1):1–10. doi: 10.1593/neo.121550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terry S, Beltran H. The many faces of neuroendocrine differentiation in prostate cancer progression. Front. Oncol. 2014;4:60. doi: 10.3389/fonc.2014.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beltran H, Tomlins S, Aparicio A. Aggressive variants of castration-resistant prostate cancer. Clin. Cancer Res. 2014;20(11):2846–2850. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltran H, Prandi D, Mosquera JM. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 2016;22(3):298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beltran H, Jendrisak A, Landers M. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin. Cancer Res. 2016;22(6):1510–1519. doi: 10.1158/1078-0432.CCR-15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou M, Toivanen R, Mitrofanova A. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017;7(7):736–749. doi: 10.1158/2159-8290.CD-16-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aprikian AG, Cordon-Cardo C, Fair WR. Neuroendocrine differentiation in metastatic prostatic adenocarcinoma. J. Urol. 1994;151(4):914–919. doi: 10.1016/s0022-5347(17)35121-2. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal R, Huang J, Alumkal JJ. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J. Clin. Oncol. 2018 doi: 10.1200/JCO.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr. Relat. Cancer. 2007;14(3):531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 19.Marcu M, Radu E, Sajin M. Neuroendocrine differentiation in prostate adenocarcinoma biopsies and its correlation to histological grading. Curr. Health Sci. J. 2010;36(1):37–42. [PMC free article] [PubMed] [Google Scholar]
- 20.Conteduca V, Aieta M, Amadori D, De Giorgi U. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Crit. Rev. Oncol. Hematol. 2014;92(1):11–24. doi: 10.1016/j.critrevonc.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Qiao J, Grabowska MM, Forestier-Roman IS. Activation of GRP/GRP-R signaling contributes to castration-resistant prostate cancer progression. Oncotarget. 2016;7(38):61955–61969. doi: 10.18632/oncotarget.11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erspamer V, Erspamer GF, Inselvin M. Some pharmacological actions of Alytesin and Bombesin. J. Pharm. Pharmacol. 1970;22(11):875. doi: 10.1111/j.2042-7158.1970.tb08465.x. [DOI] [PubMed] [Google Scholar]
- 23.Ischia J, Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide: different forms, different functions. Biofactors. 2009;35(1):69–75. doi: 10.1002/biof.10. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Everhart MB, Han W, Sherrill TP. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J. Immunol. 2006;176(8):4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JF, Gao N, DeGraff DJ. Characterization of cis-elements of the probasin promoter necessary for prostate-specific probasin gene expression. Prostate. 2010;70(9):934–951. doi: 10.1002/pros.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhau HE, Li CL, Chung LW. Establishment of human prostate carcinoma skeletal metastasis models. Cancer. 2000;88(12Suppl):2995–3001. doi: 10.1002/1097-0142(20000615)88:12+<2995::aid-cncr15>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira PG, Brenol CV, Edelweiss MI. Effects of an antagonist of the Bombesin/gastrin-releasing peptide receptor on complete Freund's adjuvant-induced arthritis in rats. Peptides. 2008;29(10):1726–1731. doi: 10.1016/j.peptides.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 29.Kahan Z, Sun B, Schally AV. Inhibition of growth of MDA-MB-468 estrogen-independent human breast carcinoma by Bombesin/gastrin-releasing peptide antagonists RC-3095 and RC-3940-II. Cancer. 2000;88(6):1384–1392. doi: 10.1002/(sici)1097-0142(20000315)88:6<1384::aid-cncr16>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 30.Czepielewski RS, Porto BN, Rizzo LB. Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils. Proc. Natl. Acad. Sci. U.S.A. 2012;109(2):547–552. doi: 10.1073/pnas.1110996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubendorf L, Schopfer A, Wagner U. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum. Pathol. 2000;31(5):578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 32.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 33.Jin RJ, Wang Y, Masumori N. NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res. 2004;64(15):5489–5495. doi: 10.1158/0008-5472.CAN-03-3117. [DOI] [PubMed] [Google Scholar]
- 34.Jin RJ, Lho Y, Connelly L. The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res. 2008;68(16):6762–6769. doi: 10.1158/0008-5472.CAN-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin RJ, Yi Y, Yull F. NFkappaB gene signature predicts prostate cancer progression. Cancer Res. 2014;74(10):2763–2772. doi: 10.1158/0008-5472.CAN-13-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartsmann G, DiLeone LP, Horowitz M. A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Invest. New Drugs. 2006;24(5):403–412. doi: 10.1007/s10637-006-6886-5. [DOI] [PubMed] [Google Scholar]
- 37.Van de Wiele C, Dumont F, Vanden Broecke R. Technetium-99m RP527, a GRP analogue for visualisation of GRP receptor-expressing malignancies: a feasibility study. Eur. J. Nucl. Med. 2000;27(11):1694–1699. doi: 10.1007/s002590000355. [DOI] [PubMed] [Google Scholar]
- 38.Varvarigou A, Bouziotis P, Zikos C, Scopinaro F, De Vincentis G. Gastrin-releasing peptide (GRP) analogues for cancer imaging. Cancer Biother. Radiopharm. 2004;19(2):219–229. doi: 10.1089/108497804323072002. [DOI] [PubMed] [Google Scholar]
- 39.Ischia J, Patel O, Bolton D, Shulkes A, Baldwin GS. Expression and function of gastrin-releasing peptide (GRP) in normal and cancerous urological tissues. BJU Int. 2014;113(Suppl 2):40–47. doi: 10.1111/bju.12594. [DOI] [PubMed] [Google Scholar]
- 40.Korner M, Waser B, Rehmann R, Reubi JC. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate. 2014;74(2):217–224. doi: 10.1002/pros.22743. [DOI] [PubMed] [Google Scholar]
- 41.Sah BR, Burger IA, Schibli R. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J. Nucl. Med. 2015;56(3):372–378. doi: 10.2967/jnumed.114.147116. [DOI] [PubMed] [Google Scholar]
- 42.Minamimoto R, Sonni I, Hancock S. Prospective evaluation of (68)Ga-RM2 PET/MRI in patients with biochemical recurrence of prostate cancer and negative findings on conventional imaging. J. Nucl. Med. 2018;59(5):803–808. doi: 10.2967/jnumed.117.197624. [DOI] [PubMed] [Google Scholar]
- 43.Coy DH, Heinz-Erian P, Jiang NY. Probing peptide backbone function in bombesin. A reduced peptide bond analogue with potent and specific receptor antagonist activity. J. Biol. Chem. 1988;263(11):5056–5060. [PubMed] [Google Scholar]
- 44.Schally AV, Comaru-Schally AM, Nagy A. Hypothalamic hormones and cancer. Front. Neuroendocrinol. 2001;22(4):248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 45.Hohla F, Schally AV. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle. 2010;9(9):1738–1741. doi: 10.4161/cc.9.9.11347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.