Summary
The hypothalamic magnocellular neuroendocrine cells (MNCs) project to the posterior pituitary (PPi), regulating reproduction and fluid homeostasis. It has been challenging to selectively label and manipulate MNCs, as they are intermingled with parvocellular neuroendocrine cells projecting to the median eminence. Here, we provide a step-by-step protocol for specifically targeting the MNCs by infusing retrograde viral tracers into the PPi. When combined with optogenetics, chemogenetics, and transgenic animals, this approach allows cell-type-specific manipulation of MNCs in multiple sites for functional dissection.
For complete details on the use and execution of this protocol, please refer to Zhang et al. (2021) and Tang et al. (2020).
Subject areas: Microscopy, Model organisms, Neuroscience
Graphical abstract

Highlights
-
•
A detailed protocol for injection and viral infusion into the PPi
-
•
Specific labeling of the hypothalamic MNCs
-
•
Retrograde tracing from the PPi prevents labeling of the PNCs
-
•
Optimized coordinates and volume for viral injection and infusion into male SD rats
The hypothalamic magnocellular neuroendocrine cells (MNCs) project to the posterior pituitary (PPi), regulating reproduction and fluid homeostasis. It has been challenging to selectively label and manipulate MNCs, as they are intermingled with parvocellular neuroendocrine cells projecting to the median eminence. Here, we provide a step-by-step protocol for specifically targeting the MNCs by infusing retrograde viral tracers into the PPi. When combined with optogenetics, chemogenetics, and transgenic animals, this approach allows cell-type-specific manipulation of MNCs in multiple sites for functional dissection.
Before you begin
This approach is mainly based on the well-established stereotaxic injection in neuroscience research to deliver drugs or viruses into targeted rodent brain areas (Cetin et al., 2006; Xiong and Gendelman, 2013). We adopted common stereotaxic injection and made several optimizations to specifically target the hypothalamic magnocellular neuroendocrine cells (MNCs), especially in rats, aiming to assist the neuroendocrine research. Although widely distributed in the hypothalamus and mixed up with the parvocellular neuroendocrine cells (PNCs) in the hypothalamic paraventricular nucleus (PVN), all MNCs share a unique feature of sending axons to the posterior pituitary (PPi). Therefore, infusion of the retrogradely-transported viral tracers (Tervo et al., 2016) into the PPi provides an excellent way to selectively label and target MNCs.
To perform PPi stereotaxic infusion, a stereotaxic instrument (RWD), a pressure microinjector (KdScientific), a 2.5 μL syringe (Hamilton), and surgical tools are essential requirements.
This protocol below is followed to label the ensemble of MNCs in adult Sprague-Dawley (SD) rats; however, we have also tested it in C57BL/6 mice with minor adjustments.
CRITICAL: The procedure follows the animal surgery guidelines, including minimizing the pain by appropriate anesthetization and preventing possible infection by surgical sterility and post-surgical application of antibiotics. To avoid possible infection and viral leakage, the surgery was operated under sterile conditions inside a biological safety cabinet (The Baker company) equipped in a negative-pressure room.
Instruments and preparation of surgical area
Timing: 3 h
-
1.
Sterilize surgical tools with a laboratory autoclave. Clean the surgical area with 75% ethanol wipes.
-
2.
Connect the Hamilton syringe (2.5 μL) to the microinjector and the microinfusion pump, assemble the stereotaxic instruments and the dental drill (Figure 1A).
CRITICAL: Place a rubber seal gasket at the root of the 33 G needle prior to attaching it to the microliter syringe, which helps guarantee the air impermeability. Adjust the needle to perfectly straight before use (Figure 1B).
Note: The impermeability of the syringe can be ensured by filling it up with paraffin liquid before viral loading. Check the impermeability by feeding the plunger forward and backward and observing a small paraffin drop appears and disappears at the needle tip with no air bubbles formed in the syringe.
-
3.
Turn on the heat mat and adjust to maintain the temperature around 37°C to warm the rat during surgery.
Figure 1.
Preparation of the stereotaxic injection instruments and clean the surgical area
(A) Core devices include a stereotaxic injection instrument, a microinfusion pump, a dental drill, a Hamilton syringe, and a cold light source. The coordinates calculator provides convenience during the measure of Bregma and Lambda, but is not essential. A disposable diaper is placed on the top of the heat mat. Surgical packs must be sterilized before use.
(B) The 33 G needle needs to be attached to the Hamilton syringe before being connected to the microinjector.
Reagents preparation
Timing: 10 min
-
4.
Dissolve 1.0 g pentobarbital in 100 mL sterilized 0.9% saline to make 1% pentobarbital solution, for anesthetization.
Note: The pentobarbital solution (1%) can be stored at room temperature (RT, 15°C–25°C) for up to 1 month. Sterilized 0.9% saline can be stored at 4°C for up to 3 months.
-
5.
Prepare iodophor disinfectant and penicillin-streptomycin solution (1:100 dilution in 0.9% saline from 100 × stock solution), for infection prevention during and after the surgery.
Note: The penicillin-streptomycin 100 × stock solution, 10,000 U/mL, can be stored at −20°C for up to 1 year.
-
6.
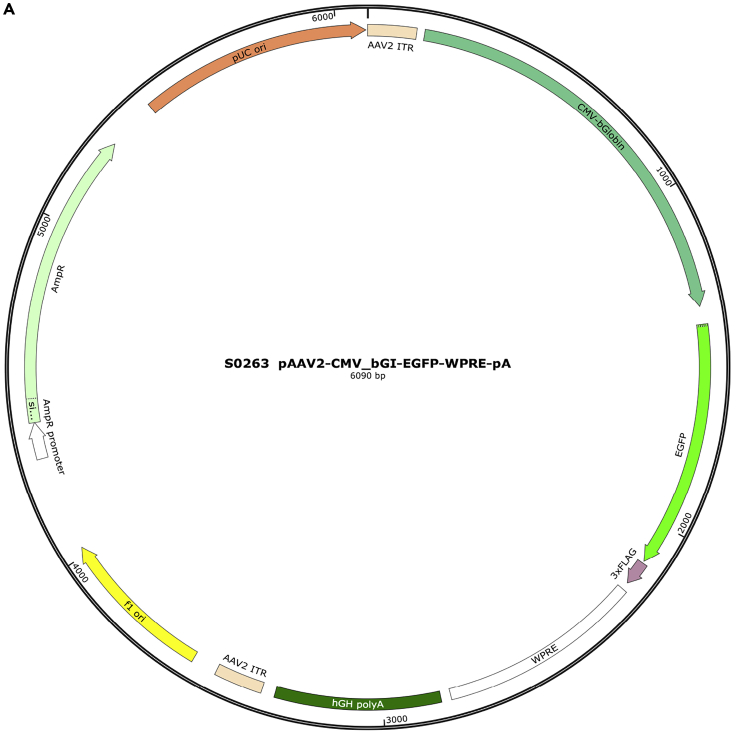
Take out an adeno-associated viral (AAVs, e.g., rAAV2/2-Retro-CMV_bGI-EGFP-WPRE-pA, hereafter referred to as AAV-Retro-GFP; for plasmid map, see Figure 2, Data S1, and materials availability) aliquot and put it on the ice right before viral loading. Commonly, it takes less than 1 min for the AAV (3 μL aliquot) stored at −80°C to thaw on ice. Avoid multiple freeze-thaw processes.
Alternatives: If necessary, dilute the virus with 5% Glycerol in PBS. The diluent can be stored at −80°C for up to 1 year.
Note: We chose the AAV-Retro-GFP to perform morphological analysis. There are commercially available retrogradely transported viral tools that are inserted with elements for different functional research, e.g., Channelrhodopsin (ChR2) for optogenetics, Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) for chemogenetics, and GCaMP for fiber photometry.
Figure 2.
Schematic illustration of the AAV-Retro-GFP plasmid map
For more details about the plasmid map, see Data S1 and Mendeley Data: https://doi.org/10.17632/xzb5hcymkw.3.
Animal preparation
Timing: 10 min
-
7.
Transfer the rat (male SD rat, aged 12 weeks, weighing about 260 g) to a fresh and clean cage to reduce the risk of infection.
-
8.
Weigh the rat and calculate the dosage of pentobarbital solution used for anesthetization.
CRITICAL: Rats were given drinking water containing 100 U/mL penicillin-streptomycin and 1 mg/mL Aspirin 1 day before surgery until at least 3 days after surgery to prevent potential infection and relieve pain.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| rAAV2/2-Retro-CMV_bGI-EGFP-WPRE-pA (1.28 E+13 vg/mL) | Taitool | Cat#S0263-2R |
| Chemicals, peptides, and recombinant proteins | ||
| 5% Glycerol in PBS | Taitool | Cat#6217280 |
| Iodophor disinfectant | LIRCON | N/A |
| Penicillin-streptomycin solution | Gibco | Cat#15140122 |
| Aspirin (Acetylsalicylic Acid) | MedChemExpress | Cat#HY-14654 |
| Deposited data | ||
| SnapGene file of the rAAV2/2-Retro-CMV_bGI-EGFP-WPRE-pA plasmid map | Taitool | Mendeley Data: https://doi.org/10.17632/xzb5hcymkw.3 |
| Experimental models: Organisms/strains | ||
| Rat: Sprague-Dawley (male, 12 weeks, 260 g) | Shanghai SLAC Laboratory Animal Co.,Ltd | SLAC Strain: Slac : SD |
| Other | ||
| Biological Safety Cabinet | The Baker Company | SG403A-HE-INT |
| Stereotaxic instruments (with coordinates calculator) | RWD | 68025 |
| LEGATO® 130 syringe pump | KD Scientific | 788130 |
| 2.5 μL Microliter syringe (62 RN) | Hamilton | 7632-01 |
| Small hub RN needle (33 G) | Hamilton | 7803-05 |
| Zoom stereomicroscope | Olympus | SZ61 |
| Dental micro motor handpiece drill | Saeshin | Strong 90 |
| Tungsten carbide burs | Meisinger | HM1 005 |
| Cold light source (LED fiber optic illuminator) | ARPK | S3000 |
| Disposable diaper (animal surgery pad) | RWD | 80099 |
| Animal surgery pack | RWD | SP0002-G |
| Medical silk braided suture | Shanghai Pudong Jinhuan Medical Products Co., Ltd | 5-0 |
| 3/8 needle | Huaiyin Medical Instruments Co., Ltd | HTJ-3-412 |
| Heat mat | Xinban | I53V9_1626097468013 |
Step-by-step method details
Induction of anesthesia and fixation of animal in stereotaxic instruments
Timing: 15 min
This step helps the transition from awake state to deep anesthesia in the rats, which makes it easy to fix the rat and reduce pain during the surgery.
-
1.
Intraperitoneally (i.p) inject appropriate volume of anesthetics (for adult male SD rats, 5–6 mL/kg body weight is recommended, e.g., 1.5 mL 1% pentobarbital for a 260 g male SD rat, aged 12 weeks) into the rat and put it back to the homecage, wait 5–10 min and check the responsiveness of the rat to nociceptive stimuli (e.g., a pinch of the paw) to make sure that it is in deep anesthesia state. See troubleshooting 1.
-
2.
Trim the fur on the head with scissors and disinfect the skin with three rounds application of iodophor disinfectant followed by 75% ethanol.
Note: The iodophor disinfectant is stable at RT for up to 1 year.
-
3.Place the rat on the stereotaxis instruments (Figure 3).
-
a.First, tuck the ear bars into the ear canals loosely but stably.
-
b.Second, gently move the ear bars toward the center symmetrically.
-
c.Third, fit the up incisors into the tooth bar and slightly tighten the nose clamp.
-
d.Finally, apply eye ointment to protect its eyes from strong illumination of the cold light source as well as to prevent eye dryness.
-
a.
CRITICAL: Move the ear bars gently to avoid any damages to the skull. The criteria for successful fixation are that the rat head is horizontal to the base plate and vertical to the ear bars, with free up-down rotation while no medial-lateral or front-back movements allowed.
Figure 3.
Fix the rat in the stereotaxic instruments
(A and B) The apparatus (A) and schematic (B) of the rat fixation using the tooth bar, ear bars, and nose clamp.
Craniotomy and determination of skull marks
Timing: 5 min
This step exposes skull marks, including the Bregma and the Lambda, which act as reference points to target the PPi.
-
4.
In order to expose the skull, cut the scalp from the middle of the eyes straight backwards to make a 10 mm incision.
CRITICAL: The scalp incision might lead to bleeding. When bleeding happens, press the wound with wet cotton for 1–2 min to stop bleeding. Otherwise, skull marks will hardly be visible and the rat can be weak as a result of blood loss. After the stanch, keep the skull moist with sterilized 0.9% saline throughout the surgery.
-
5.Clean the exposed skull with sterilized 0.9% saline-immersed cotton swabs, followed by dry cotton swabs to display the Bregma and the Lambda clearly.
-
a.The Bregma is the cross point of the sagittal suture and the coronal suture.
-
b.The Lambda is the midpoint of the lambdoid suture’s best-fit curve, usually the cross point of the sagittal suture and the extending tangent line of lambdoid suture.
-
a.
-
6.Measure the AP, ML, and DV coordinates of the Bregma and the Lambda with a dissecting microscope (Figures 4A and 4B). Adjust the tooth bar so that the AP axis and ML axis of the skull were both horizontal to the plate of the stereotaxic instruments.
-
a.The AP, ML, and DV refer to the anterior-posterior, medial-lateral, and dorsal-ventral axis, respectively.
-
b.The DV coordinates between the Bregma and the Lambda should not differ beyond 0.05 mm.
-
c.The DV coordinates 2 mm left and right from the center of midline between the Bregma and the Lambda should not differ beyond 0.02 mm.
-
a.
-
7.
Set the coordinates of the Bregma as the origin of coordinates (AP, 0 mm; ML, 0 mm; DV, 0 mm) (Figures 4B and 4C).
Figure 4.
Measure the Bregma/Lambda and determine the drilling point
(A and B) Under a dissecting microscope, the Bregma and Lambda can be clearly observed.
(C) Set the Bregma as the original point.
(D) Mark the drilling point at (AP, −5.80 mm; ML, +0.05 mm) on the skull exactly above the PPi.
(E and F) HM1 005 burs are suitable for drilling a bony hole in the SD rat skull.
Mark and open a bony hole
Timing: 5 min
This step determines the drilling point and removes the skull in the target area.
-
8.
Rotate the AP and ML modulators to position the needle tip right above the PPi, with the coordinates (AP, −5.80 mm; ML, +0.05 mm) (Figures 4B and 4D).
CRITICAL: Since the position of the pituitary can be changed during dissection, the PPi coordinates provided by the most commonly used brain atlas are not precise enough (Paxinos and Watson, 2006; Swanson, 2004). Based on the atlas, we modified and optimized the coordinates of the PPi specifically for SD rats weighing about 260 g, which is (AP, −5.80 mm; ML, 0 mm; DV, −10.20 mm). For those weighing 220–300 g, the coordinates need further adjustment. The AP and DV coordinates vary in the range of −5.40 – −6.00 mm and −9.50 – −10.50 mm, respectively. To reduce the risk of sagittal suture damage, we usually adjust the ML coordinate to +0.05 – +0.10 mm, which means the needle tip is 50–100 μm left to the skull midline. See troubleshooting 2 and 3.
Note: We recommend the PPi coordinate as (AP, −2.90 mm; ML, +0.05 mm; DV, −5.60 mm) in male mice aged 8 weeks, weighing 25 g.
Alternatives: One might prefer to move the syringe 50–100 μm right to the midline, which makes no difference regarding the labeling efficiency.
-
9.
Slowly rotate the DV modulator to lower down the syringe till the tip touches the surface of the skull and mark the point with a thin mark pen under the dissecting microscope.
-
10.
Uplift the DV modulator to leave space for drilling a bony hole.
-
11.
Carefully drill through the skull bone at the marked point with a dental drill (Figures 4E and 4F). Drill until the blood vessels beneath the skull are visible, but not to hurt the brain parenchyma.
CRITICAL: Do not apply downward pressure, the self-gravity of the drill is enough to thin the skull. Drill for some time and then suspend to clean the bony powder with a rubber suction bulb. Drill very slowly and stop immediately when feeling a hit pass. The skull is drilled through and vessels become clearly visible.
Viral loading and infusion into the PPi
Timing: 25 min
This step loads the AAVs to the syringe and infuses appropriate volume of AAVs into the PPi.
-
12.
Configure the withdraw mode of the microinjector to load 1200 nL AAVs at 1200 nL/min (Figure 5A).
CRITICAL: If the 2.5 μl Microliter Syringe (62 RN) has not been preset in the library of the LEGATO® 130 Syringe Pump, customize the syringe definition as 0.343 mm in diameter and 2.5 μL in volume. The final infusion volume is 1000 nL, we recommend that you load an extra 200 nL AAVs to prevent the paraffin liquid being infused into the PPi (see Alternatives below).
Note: The needle is of point style 3, with 20 mm in length, 0.21 mm in outer diameter, and 0.051 mm in inner diameter.
Alternatives: Paraffin liquid (500 nL) can be loaded before AAVs to enhance the impermeability of the syringe. The residual paraffin on the outer wall of the needle must be wiped before viral loading. Otherwise, paraffin will damage the brain tissue and contaminate the virus, resulting in decreased transfection efficiency.
-
13.
Reconfigure the withdraw mode to load an extra 20 nL air into the needle tip (Figure 5B).
CRITICAL: This step helps to maintain a small negative pressure that can prevent the outflow of the virus when the needle goes into the brain tissue.
-
14.
Clean the outer wall of the needle thoroughly with wet cotton swabs unidirectionally towards the needle tip to remove the residual virus. See troubleshooting 4.
CRITICAL: Given the deep location of the PPi, removing the residual virus is essential to avoid potential contamination along the needle track. It is important to wipe the outer wall unidirectionally in case the needle is accidentally dragged by swabs.
-
15.
Move the syringe back to the drilling point (AP, −5.80 mm; ML, +0.05 mm) so that the needle tip is close to the dura.
-
16.
Penetrate the dura and slowly lower the syringe until the needle reaches the PPi (AP, −5.80 mm; ML, +0.05 mm; DV, −10.20 mm).
-
17.
Infuse the virus at a low rate (70–100 nL/min, total 1020 nL including 20 nL air and 1000 nL AAVs) to the PPi. After infusion, maintain the syringe at the position for another 10 min to allow viral diffusion and to avoid viral backflow. See troubleshooting 5.
-
18.
Slowly uplift the syringe for 50 μm and maintain it for 3 min before full withdrawal.
-
19.
Uplift the syringe until the needle completely leaves the brain tissue and clean the needle tip after full withdrawal.
CRITICAL: Cleaning the needle immediately after withdrawal helps avoid potential blockage of the tip by blood clot.
-
20.
Wipe off the blood and suture the craniotomy after applying drops of antibiotics.
CRITICAL: During continuous operations of multiple rats, disinfection measures must be taken for each one. Sterilize the surgical area with 75% ethanol wipes and change the diaper for different rats. Clean all the surgical tools with three rounds application of iodophor disinfectant followed by 75% ethanol for every operation.
Note: A 5-0 medical silk braided suture and a 3/8 needle were used to close the skull skin.
Figure 5.
Configure the microinjection pump and load the virus
(A) Configure the pump to withdraw 1200 nL virus at 1200 nL/min.
(B) Reload 20 nL air into the tip to avoid viral outflow.
Perioperative care and viral expression
Timing: 4 weeks
This step ensures that the rat recovers from the surgery and full expression of the virus.
-
21.
Monitor the rat in an isolated cage for 24 h before sending it back for normal housing.
Note: The rats were housed in an individually ventilated caging (IVC) system with high efficiency-particulate air (HEPA) filter to avoid airborne contamination.
-
22.Perform post-operative monitoring of general conditions daily for 3–7 days, including the physiological and behavioral states.
-
a.Food and water intake are most important for the rats to recover. Make sure that the rats eat, drink and eliminate normally. Measure and record post-operative body weight.
-
b.Monitor general appearance, grooming, posture and locomotor activity. The rats should be active, alert, and social interacting with cagemates.
-
c.Carefully scrutinize those curling up frequently or behaving like depression, in case there is untreated pain or infection. Examine the rats for surgical sites swelling, inflammation and signs of surgical complications such as pain, paralysis, and seizures.
-
a.
-
23.
Withdraw the suture 7 days after surgery from the wounds.
-
24.
Allow the virus to express for 2–4 weeks.
Expected outcomes
Typically, it takes at least 2 weeks for the virus to be expressed in the soma. We recommend 4 weeks to allow full expression of AAV-Retro-GFP in the soma and fibers. The epifluorescence of the endogenous GFP is strong enough to visualize the signal, there is no need for immunohistochemical staining. After cryostat section, intense epifluorescence (e.g., GFP) can be observed in the nerve terminals within the PPi, fibers in the stalk, and somas in the PVN, supraoptic nucleus (SON), and other magnocellular neuroendocrine nuclei in the hypothalamus.
Previously, the stereotaxic injection has been reported as an approach to deliver genes into the rodent brain (Cetin et al., 2006). We adopted the protocol with specific adjustments to efficiently target the PPi in SD rats and labeled approximately 60% of MNC ensemble in various nuclei within the hypothalamus. Originally, we infused AAV-Retro-GFP to perform morphological analysis (Figure 6). It is suitable for functional manipulation if optogenetic or chemogenetic elements, e.g., ChR2, DREADDs, are carried by the virus and exclusively introduced into the MNCs via PPi infusion.
Figure 6.
Retrograde tracing from the PPi to label the hypothalamic MNCs
(A–C) Infusion of AAV-Retro-GFP into the PPi can retrogradely label the MNCs in the hypothalamus.
(D and E) The side view (D) and schematic (E) of the hypothalamo-neurohypophysial system reconstructed with whole brain imaging.
ACN, anterior commissural nucleus; AF, anterior perifornical nucleus; PVN, hypothalamic paraventricular nucleus; CiN, nucleus circularis; PoF, posterior perifornical nucleus; Nmfb, nucleus of the medial forebrain bundle; SON, supraoptic nucleus; RCN, retrochiasmatic nucleus; PPi, posterior pituitary; HYP, hypothalamus.
C, caudal; D, dorsal; M, medial. Scale bar, 500 μm.
Limitations
Since the PPi is located at the bottom of the brain and it takes a long distance for the needle to reach it, precautions should be taken against potential contaminations on route during needle insertion. One should scrutinize the regions adjacent to the needle track including the arcuate nucleus (ARC), in which GFP+ somas should not be found. Samples with possible needle track contamination must be excluded. Only those with no contamination can be used for subsequent analyses, which might increase the total usage amount of the animals.
The PPi coordinates above are specifically tested in male SD rats weighing 250–280 g. For rats beyond this weight range, coordinates must be adjusted. Due to the small size of the PPi, it is more challenging to perform the injection in mice, which needs more optimization.
Regarding the loss-of-function analysis, the label efficiency of 60% might not be high enough. Higher-titer viral preparation might help improve the efficiency, however, the viral toxicity is another concern.
Troubleshooting
Problem 1
The pentobarbital treatment does not fully anesthetize the rats (step 1).
Potential solution
During the maintenance of anesthesia, inhaled anesthetics such as the isoflurane are recommended, which provide rapid and reliable control of anesthesia depth and duration without reducing the survivability. Examine the respiratory rate and pattern carefully. Commonly, there should be a slow drop of respiratory and heart rate during appropriate anesthesia. When tachypnea and tachycardia occurred, apply paw pinch to check whether the anesthesia level is inadequate. If it happens, increase the oxygen flow rate or the isoflurane dose. Alternatively, in absence of an isoflurane vaporizer, one might i.p administrate additional 0.1 mL 1% pentobarbital solution to the rat. At the same time, monitor the state of the rats to avoid excessive depression of respiratory and cardiac functions due to over-anesthesia.
Problem 2
Epifluorescence is found in the intermediate lobe (IL) of the pituitary gland besides the PPi (step 8).
Potential solution
The epifluorescence in the IL might not be a major concern, if no retrogradely labeled neurons were seen in the ARC, considering that there are few neural fibers in the IL. When it happens, we recommend adjusting the DV coordinate up by about 200 μm. Since the thickness of the PPi and the IL is approximately 700 μm and 200 μm, on a midline sagittal section, respectively, lifting the syringe upwards by 200–500 μm is safe to maintain the needle tip in the PPi.
Problem 3
Blood is pouring when the needle is inserted into the brain (step 8).
Potential solution
The bleeding is probably due to the damage of the sagittal sinus underneath the midline skull, which can even lead to the death of the rat. Drilling a hole 50–100 μm lateral to the midline helps to reduce the risk of bleeding by avoiding damage to the sagittal sinus. The PPi can still be accurately targeted even if the hole is 200 μm lateral.
Problem 4
Retrogradely labeled cells are found outside the hypothalamus or even in the forebrain and the midbrain (step 14).
Potential solution
Different from Problem 2, the extrahypothalamic neurons that are labeled by the AAV-Retro-GFP probably originate from the viral leakage along the needle track. To target the PPi, the needle has to go through various nuclei including the periaqueductal gray (PAG), which is a hotspot innervated by multiple brain areas ranging from the forebrain to the midbrain. The ectopically labeled cells can be resulted from the contaminant adjacent to the needle track. To avoid this problem, the outer wall of the needle must be thoroughly cleaned after viral loading. Check the regions around the needle track carefully for every sample with or without extrahypothalamic cells being labeled.
Problem 5
The tracing efficiency is lower than 40% (step 17).
Potential solution
High titer virus, e.g., 1 × 1013 vg/mL, is recommended for rat injection. Avoid frequent freeze-thaw cycles. Do not make a dilution. Other factors include the PPi injection accuracy and the viral infusion volume. To achieve the expected efficiency (no less than 60%), minimal 700 nL virus needs to be infused into the PPi.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Bin Zhang (izid1257@hotmail.com).
Materials availability
This study did not generate new unique reagents. Details about the plasmid map of the rAAV2/2-Retro-CMV_bGI-EGFP-WPRE-pA can be accessed via the Mendeley Data: https://doi.org/10.17632/xzb5hcymkw.3.
Acknowledgments
This protocol has been developed with the help from Hongbin Yang and Lunhao Chen. We are thankful to Huifang Lou and Liya Zhu for laboratory animal management. We thank Dafeng Xu for providing the plasmid map. This work was supported by the National Key Research and Development Program of China (2016YFA0501000), National Natural Science Foundation of China (31671057, 81821091, 61721092, 81527901, 81827901, 92032000, and 31490592), Science and Technology Planning Project of Guangdong Province (2018B030331001 and 2019B030335001), Fundamental Research Funds for the Central Universities (2019FZA7009), Zhejiang Provincial Natural Science Foundation (LQ21C090003), and a China Postdoctoral Science Foundation grant (2019TQ0282).
Author contributions
This protocol has been developed by B.Z. and is routinely exerted by B.Z. and L.Q. The detailed procedure was written by Z.G. and B.Z. and was further checked by C.L.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2021.100787.
Contributor Information
Bin Zhang, Email: izid1257@hotmail.com.
Zhihua Gao, Email: zhihuagao@zju.edu.cn.
Supplemental information
Data and code availability
This study did not generate new unique data or code.
References
- Cetin A., Komai S., Eliava M., Seeburg P.H., Osten P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 2006;1:3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. Elsevier; 2006. The rat brain in stereotaxic coordinates: Hard cover edition. [Google Scholar]
- Swanson L. Gulf Professional Publishing; 2004. Brain maps: structure of the rat brain. [Google Scholar]
- Tang Y., Benusiglio D., Lefevre A., Hilfiger L., Althammer F., Bludau A., Hagiwara D., Baudon A., Darbon P., Schimmer J. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 2020;23:1125–1137. doi: 10.1038/s41593-020-0674-y. [DOI] [PubMed] [Google Scholar]
- Tervo D.G., Hwang B.Y., Viswanathan S., Gaj T., Lavzin M., Ritola K.D., Lindo S., Michael S., Kuleshova E., Ojala D. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H., Gendelman H.E. Springer; 2013. Current laboratory methods in neuroscience research. [Google Scholar]
- Zhang B., Qiu L., Xiao W., Ni H., Chen L., Wang F., Mai W., Wu J., Bao A., Hu H. Reconstruction of the hypothalamo-neurohypophysial system and functional dissection of magnocellular oxytocin neurons in the brain. Neuron. 2021;109:331–346.e7. doi: 10.1016/j.neuron.2020.10.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate new unique data or code.