Highlights
-
•
X-irradiation increased cellular neutral amino acid uptake via LAT1.
-
•
JPH203 inhibited the radiation-induced increase in neutral amino acid uptake.
-
•
JPH203 significantly sensitized cancer cells to radiation.
-
•
JPH203 downregulated mTOR activity after irradiation.
-
•
JPH203 enhanced cellular senescence after irradiation.
Keywords: Cancer metabolism, JPH203, LAT1, Neutral amino acids, Radiation
Abbreviations: BCAA, branched-chain amino acid; EAA, glutathione; GSH, essential amino acid; LAT1, L-type amino acid transporter 1; mTOR, mammalian target of rapamycin
Abstract
L-type amino acid transporter 1 (LAT1) is important for transporting neutral amino acids into cells. LAT1 expression is correlated with cancer malignancy, suggesting that LAT1 is a promising target for cancer therapy. JPH203, a potential novel drug targeting LAT1, has been shown to suppress tumor growth in various cancer cell lines. However, a combination study of JPH203 and radiation therapy has not been reported. Here, we examined the effects of JPH203 on radiosensitivity after irradiation in A549 and MIA Paca-2 cells. We showed that X-irradiation increased cellular neutral amino acid uptake via LAT1 in both cell lines. JPH203 inhibited the radiation-induced increase in neutral amino acid uptake. We demonstrated that JPH203, at minimally toxic concentrations, significantly sensitized cancer cells to radiation. JPH203 significantly downregulated mTOR activity and enhanced cellular senescence post-irradiation without reducing ATP and GSH levels. These results indicate that LAT1 inhibition by JPH203 sensitizes cancer cells to radiation by enhancing cellular senescence via mTOR downregulation. Thus, JPH203 may be a potent anti-cancer drug in combination with radiation therapy.
1. Introduction
Cancer cells depend on aerobic glycolysis rather than oxidative phosphorylation for energy production, even under oxygenated conditions. This behavior has been referred to as Warburg effect [1]. Recently, it has been reported that most cancers retain mitochondrial function and promote oxidative phosphorylation for cancer survival [2]. In addition to glucose metabolism, essential amino acid (EAA) uptake is essential for cancer proliferation [3]. EAA uptake is higher in tumors in vivo than in normal tissues [4], [5], [6]. Subsequently, cancer cells rapidly die upon removal of a single EAA in vitro [7]. Among EAAs, metabolic reprogramming for branched-chain amino acids (BCAAs) has recently been highlighted in many types of human cancers [8], [9], [10]. BCAAs are considered important precursors of protein synthesis, nucleotide synthesis, and energy production in cancer proliferation [10]. BCAAs, namely valine, leucine, and isoleucine, may either be metabolized to produce energy through the TCA cycle or participate in intracellular signaling events, such as the mammalian target of rapamycin (mTOR) pathway to coordinate protein synthesis and cellular growth [11], [12], [13].
The L-type amino acid transporter family (LAT1–4) is important for transporting EAAs into cells. The expression level of LAT1 in cancer cells is higher than that in normal cells, and LAT1 has been considered crucial for cancer survival [14,15]. LAT1 imports various neutral EAAs (valine, leucine, isoleucine, phenylalanine, tryptophan, tyrosine, methionine, and histidine) into cells [16], [17], [18]. LAT1 expression levels are positively correlated with cancer malignancy, suggesting that LAT1 is a promising therapeutic target for cancer therapy. Recently, a selective LAT1 inhibitor, JPH203 [(S)-2-amino-3-(4-((5-amino-2-phenylbenzo [d] oxazol-7-yl) methoxy)-3, 5-dichlorophenyl) propanoic acid], was developed [19,20]. Currently, phase 1 and 2 trials of JPH203 are ongoing in patients with advanced solid tumors. Accumulating evidence has demonstrated that JPH203 successfully inhibits tumor growth in various cancer cell lines [19,[21], [22], [23]]. Among lung and pancreatic cancers, non-small cell lung cancer (NSCLC) and pancreatic ductal adenocarcinoma (PDAC) have been reported to have high levels of LAT1 expression [24,25]. Previous studies have shown that JPH203 successfully inhibited in vitro and in vivo tumor growth in the NSCLC cell lines A549 and MIA Paca-2 cells [26,27]. However, a combination study of JPH203 and radiation therapy has not been reported.
X-irradiation has been shown to increase glucose uptake, oxygen consumption rate, and cellular ATP levels [28,29]. Previous studies have reported that 2-deoxy-D-glucose enhances radiosensitivity in cancer cells [30,31]. These observations suggest that radiation activates glucose metabolism and mitochondrial energy production, and that glucose metabolism is important for cancer survival after irradiation. In addition to glucose metabolism, inhibition of glutamine metabolism enhances radiosensitivity in cancer cells [32], suggesting that amino acid metabolism is also crucial for cancer survival after irradiation. However, it is still unclear whether radiation influences neutral amino acid metabolism via LAT1. Here, we examined the effects of the LAT1 inhibitor JPH203 on neutral amino acid uptake after irradiation and radiosensitivity in A549 and MIA Paca-2 cells, which showed high levels of LAT1 expression [33,34]. This study showed that X-irradiation increased cellular neutral amino acid levels via LAT1, and that JPH203 sensitized cancer cells to radiation, resulting in increased cellular senescence via downregulation of mTOR signaling.
2. Materials and methods
2.1. Reagents
The LAT1 inhibitor JPH203 was kindly provided by J-Pharma (Kanagawa, Japan). The following antibodies were used: anti-phospho-p70 S6k, anti-p21 Waf1/Cip1 (12D1), HRP-conjugated secondary antibodies (Cell Signaling Technology, Beverly, MA, USA), anti-p70 S6k, and anti-actin (Santa Cruz Biotechnology, Dallas, TX, USA). The Western Lightning Plus-ECL chemiluminescence detection kit was purchased from PerkinElmer (Waltham, MA, USA). The cell ATP assay reagent was obtained from Toyo Ink (Tokyo, Japan).
2.2. Cell culture and X-irradiation
Human lung carcinoma (A549) and pancreatic cancer (MIA Paca-2) cells were obtained from RIKEN Cell Bank (Tsukuba, Japan) and JCRB Cell Bank (Tokyo, Japan), respectively. Cells were grown in RPMI1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) and DMEM medium (Thermo Fisher Scientific). Both media were supplemented with 10% FBS (KOHJIN BIO, Saitama, Japan). The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. X-irradiation was performed using a Shimadzu X-TITAN 225S X-ray generator (Shimazu, Kyoto, Japan) at 200 kV and 14 mA with a 1.0 mm aluminum filter.
2.3. Measurement of amino acid and GSH by LC-MS analysis
LC-MS analyses of amino acids in cancer cells were performed as previously described [35]. Cells were collected and lysed in 200 µL of 50 mM ammonium bicarbonate (pH 8.0) containing 20 mM N-ethylmaleimide (NEM; FUJIFILM Wako Pure Chemical, Osaka, Japan). The lysate was heated at 100 °C for 5 min to inactivate the metabolic enzymes and then further incubated for 10 min at room temperature. A 100 µL aliquot of the sample was mixed with 200 µL of methanol containing 5 µM N-methylmaleimide (NMM)-derivatized GSH as an internal standard and an additional 200 µL of chloroform. The mixture was thoroughly stirred and centrifuged at 12,000 × g for 15 min at 4 °C. The upper aqueous layer was filtered through 0.45 µm filters (Millex-LH, Millipore Co.). A 90 µL aliquot of the resulting filtrate was lyophilized, and the resulting residue was dissolved in 30 µL of Milli-Q water and subjected to LC-MS analysis. A Q Exactive Hybrid Quadruple-Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with a heated electrospray ionization source was operated in positive ionization mode. The Ultimate 3000 liquid chromatography system consisted of a WPS3000 TRS autosampler, a TCC-3000 RS column oven, and an HPG-3400RS quaternary pump (Dionex, Sunnyvale, CA, USA). A SeQuant® ZIC®-pHILIC column (2.1 × 150 mm, 5 µm particle size; Merck KGaA, Germany) was maintained at 30 °C. Mobile phase A was 20 mM ammonium bicarbonate (pH 9.8), and mobile phase B was 100% acetonitrile. System control, data acquisition, and quantitative analysis were performed using Xcalibur 2.2 software. Standard curves for amino acids and GSH-NEM showed linearity in the concentration ranges examined.
2.4. Western blotting
Cells were collected and lysed with lysis buffer (50 mM Tris-HCl, pH 7.5, 1% [v/v] Triton X-100, 5% [v/v] glycerol, 5 mM EDTA, and 150 mM NaCl). After centrifugation at 18,000 × g for 15 min at 4 °C, supernatants were collected using threefold-concentrated Laemmli's sample buffer (0.1875 M Tris-HCl, pH 6.8, 15% [v/v] β-mercaptoethanol, 6% [w/v] SDS 30% [v/v] glycerol, and 0.006% [w/v] bromophenol blue) was added to the supernatants, and the samples were boiled for 3 min. Proteins were separated by SDS–PAGE and transferred onto a nitrocellulose membrane (Advantec TOYO, Tokyo, Japan). The membrane was blocked with TBST (10 mM Tris-HCl, pH 7.4, 0.1 M NaCl, and 0.1% Tween-20) containing 5% (w/v) nonfat skim milk and probed with specific antibodies diluted with TBST containing 5% (w/v) nonfat skim milk or 5% (w/v) bovine serum albumin (BSA) overnight at 4 °C. After probing with HRP-conjugated secondary antibodies, the bound antibodies were detected with Western Lightning Plus-ECL. Image acquisition was performed with an image analyzer (ImageQuant LAS500, GE Healthcare), and image analysis was performed using ImageJ software.
2.5. Clonogenic survival assay
Cells were seeded in 6 cm dishes and cultured for 6 h. The cells were then X-irradiated, and the medium was replaced with fresh growth medium containing JPH203 (20 μM for MIA Paca-2 and 10 μM for A549 cells). After incubation for 14 d, they were fixed with methanol and stained with Giemsa solution. Colonies containing >50 cells were scored as surviving cells. Survival fractions were calculated with a correction for the plating efficiency of the non-irradiated controls.
2.6. Cellular ATP measurement
Total ATP was quantified using the Cell ATP Assay reagent (Toyo Ink). After X-irradiation with 5 Gy, the cells were collected at the indicated times. The cell suspension was mixed with luciferin-luciferase solution in a 96-well plate and incubated for 1 h at room temperature. Luminescence was then measured using a multilabel plate reader ARVO X3 (PerkinElmer).
2.7. Senescence-associated β-galactosidase (SA β-gal) staining
Cells were plated on 35 mm dishes and incubated overnight. After irradiation and subsequent incubation for the indicated times, the cells were stained with a senescence-associated β-galactosidase staining kit (#9860, Cell Signaling Technology) according to the manufacturer's instructions. Senescent cells were identified and counted using a light microscope.
2.8. Statistical analysis
All results are expressed as mean ± SD of at least three experiments. Comparisons between the two groups were performed using Student's t test. For multiple comparisons, the Tukey-Kramer test was used. The minimum level of significance was set at p < 0.05.
3. Result
3.1. LAT1 inhibition by JPH203 sensitized cancer cells to radiation
We first tested the cytotoxicity of JPH203 on two tumor cell lines, MIA Paca-2 and A549. Cells were treated with various concentrations of JPH203, and cell viability was assessed using a clonogenic survival assay. As shown in Fig. 1A and 1B, JPH203 reduced cell viability in a dose-dependent manner. The minimally toxic concentrations (approximately 10% reduction in clonogenic survival) of JPH203 (20 μM for MIA Paca-2 and 10 μM for A549 cells) were used for further investigations. To confirm the effect of JPH203 on LAT1 activity, we measured the content of cellular leucine, isoleucine, phenylalanine, tryptophan, and tyrosine before and after irradiation. These neutral amino acids are transported into cells via LAT1 [18]. Cells were irradiated with 5 Gy and treated with JPH203 immediately after irradiation. The cells were collected 1 or 2 d after irradiation and analyzed using LC-MS. The contents of cellular neutral amino acids were dramatically reduced by JPH203 treatment in both cell lines (Fig. 1C and 1D). Importantly, X-irradiation significantly increased the level of cellular neutral amino acids, excluding isoleucine and tryptophan, in MIA Paca-2 cells. Furthermore, JPH203 completely inhibited the radiation-induced increase in cellular neutral amino acids. This indicates that JPH203 could inhibit LAT1 activity and that MIA Paca-2 and A549 cells largely depend on LAT1 to uptake neutral amino acids. We then evaluated the effect of JPH203 on cell viability after X-irradiation using a clonogenic survival assay. Cells were treated with JPH203 immediately after irradiation at the indicated doses. As shown in Fig. 2A and 2B, X-irradiation decreased the viability of both tumor cell lines in a dose-dependent manner. JPH203 significantly enhanced radiation-induced clonogenic cell death at doses of 2.5 Gy or higher in both cell lines. These results indicate that MIA Paca-2 and A549 cells increase neutral amino acid uptake via LAT1 after irradiation and that the LAT1 inhibitor JPH203 enhances the radiosensitivity of these tumor cells.
Fig 1.
JPH203 inhibited uptake of neutral amino acids via LAT1
(A and B) Cytotoxicity of JPH203 was examined using a clonogenic assay. MIA Paca-2 and A549 cells were treated with JPH203 at the indicated concentrations for 13 d. The survival fractions with or without JPH203 were calculated with a correction for the plating efficiency of cells. Data are expressed as means ± SD from three experiments. (C and D) Cellular neutral amino acids were measured by LC-MS analysis. After X-irradiation with 5 Gy, MIA Paca-2 and A549 cells were cultured with or without JPH203 for 48 h and 24 h, respectively. Data are expressed as mean ± SD from three experiments. (Tukey-Kramer test).
Fig 2.
JPH203 enhanced radio-sensitivity in MIA Paca-2 and A549 cells
(A) The effects of JPH203 on survival fraction after X-irradiation. After X-irradiation at the indicated doses, MIA Paca-2 and A549 cells were cultured with or without JPH203 for 13 d. Colonies containing >50 cells were scored as surviving cells. Data are expressed as mean ± SD of three experiments. *p < 0.05 vs. Ctl (Student's t test).
3.2. JPH203 did not reduce cellular ATP and GSH after X-irradiation
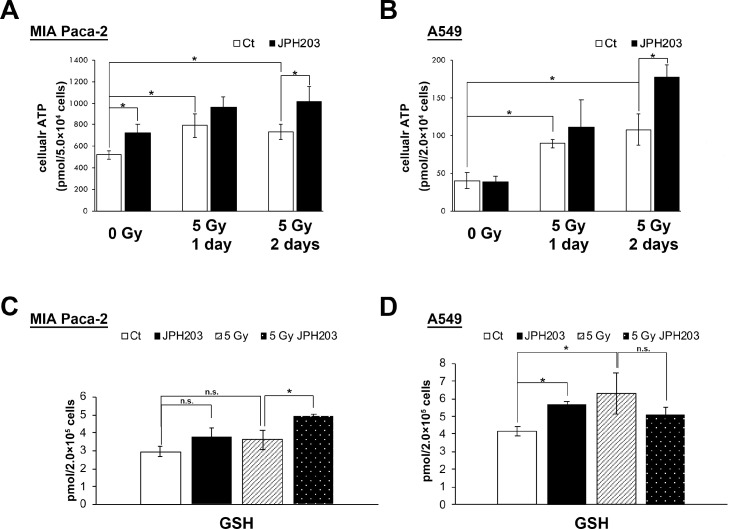
X-irradiation has been reported to increase ATP synthesis through oxidative phosphorylation [29]. BCAAs imported by LAT1 are considered important for energy production [12]. Sato et al. reported that LAT1 knockout decreased cellular ATP levels, and LAT1 overexpression increased it [36]. To examine the effect of JPH203 on ATP synthesis after irradiation, we measured the cellular ATP content after irradiation with or without JPH203 (Fig. 3A and 3B). Cellular ATP levels were higher in both cell lines at 1 d and 2 d after X-irradiation. Surprisingly, JPH203 increased cellular ATP content rather than decreasing it 2 d after irradiation.
Fig 3.
JPH203 did not decrease ATP and GSH synthesis after X-irradiation
(A and B) After X-irradiation with 5 Gy, cells were incubated with or without JPH203 for the indicated times. After incubation, the cells were collected and cellular ATP levels were measured. Data are expressed as mean ± SD of three experiments. *p < 0.05 (Tukey-Kramer test) (C and D) After X-irradiation at 5 Gy, MIA Paca-2 and A549 cells were cultured with or without JPH203 for 48 h and 24 h, respectively. Cellular GSH levels were measured by LC-MS analysis. Data are expressed as mean ± SD of three experiments. (Tukey-Kramer test).
BCAA metabolism is also considered to contribute to GSH synthesis [37]. It has been reported that inhibition of EAA metabolism by branched chain amino acid transaminase 1 (BCAT1) RNAi decreased cellular GSH levels [38]. To confirm the hypothesis that JPH203 sensitized cancer cells to radiation by GSH depletion, we measured cellular GSH levels after irradiation with or without JPH203 by LC-MS analysis (Fig. 3C and 3D). However, JPH203 increased or significantly increased cellular GSH levels before and after X-irradiation in MIA Paca-2 and A549 cells, respectively. These results strongly suggest that JPH203 does not sensitize cancer cells to radiation by ATP or GSH depletion.
3.3. JPH203 significantly attenuated mTOR activity after X-irradiation
LAT1-mediated leucine uptake interacts with cell growth, transcription, and translation through the mTOR signaling pathway [39,40]. Previous studies have shown that LAT1 inhibition by JPH203 suppresses tumor growth via mTOR downregulation [23,41]. To determine whether JPH203 affects mTOR activity after irradiation, we examined and quantified the phosphorylation status of p70-s6k, a downstream target of mTOR, after irradiation with or without JPH203. In MIA Paca-2 cells, p70-S6K phosphorylation was slightly decreased at 2 h post-irradiation and returned to the basal level, and JPH203 significantly enhanced the radiation-induced decrease in p70-S6K phosphorylation up to 8 h after irradiation (Fig. 4A). Along with this observation, X-irradiation decreased phospho-p70-S6K up to 16 h post-irradiation in A549 cells, and JPH203 significantly enhanced this attenuation up to 48 h after irradiation (Fig. 4B). These results suggest that JPH203 enhances the downregulation of mTOR after irradiation.
Fig 4.
JPH203 enhanced the downregulation of mTOR activity after X-irradiation
(A and B) The effects of JPH203 on p70-S6K phosphorylation status after X-irradiation. MIA Paca-2 and A549 cells were collected at the indicated times after X-irradiation with 5 Gy. Top, representative blots of phospho-p70-S6K and total p70-S6K. Bottom, time course analysis of phospho-p70-S6K. The intensities of phospho-p70-S6K bands were normalized to those of total p70-S6K bands. Data are expressed as mean ± SD of three experiments. *p < 0.05 (Student's t test).
3.4. JPH203 significantly enhanced cellular senescence after X-irradiation
The activity of mTOR is known to have a significant impact on cell division and tumor growth. We further examined whether mTOR inhibition by JPH203 affected cellular senescence. We evaluated the induction of p21, a cellular senescence inducer, after irradiation with or without JPH203 (Fig. 5A and 5B). Whereas X-irradiation alone induced p21 expression at 48 h after irradiation, JPH203 dramatically promoted p21 expression at 4 h post-irradiation in MIA Paca-2 cells. In A549 cells, X-irradiation induced p21 at 8 h post-irradiation, and JPH203 slightly promoted p21 induction at 2 to 4 h after irradiation. These observations suggest that JPH203 enhanced radiation-induced cellular senescence. To confirm this possibility, we performed an SA β-gal staining assay (Fig. 5C–F). The cells treated with X-irradiation and JPH203 displayed enlarged and flattened cell shapes, which are characteristic morphologies of cellular senescence. SA β-gal-positive cells were increased by JPH203 and X-irradiation (Fig. 5C and 5D). Quantitative analysis showed that JPH203 significantly increased the ratio of SA β-gal-positive cells 5 d after irradiation in comparison with X-irradiation alone in both cell lines (Fig. 5E and 5F). These results indicate that JPH203 significantly downregulates mTOR activity, enhancing cellular senescence after irradiation.
Fig 5.
JPH203 enhanced radiation-induced cellular senescence
(A and B) The effects of JPH203 on p21 expression after X-irradiation. MIA Paca-2 and A549 cells were collected at the indicated times after X-irradiation with 5 Gy. Representative blots of p21 and actin. (C-F) The effects of JPH203 on SA β-gal positive cells after X-irradiation. After X-irradiation, MIA Paca-2 and A549 cells were evaluated with SA β-gal staining assay. (C and D) Representative images of SA β-gal positive cells with or without JPH203 after X-irradiation. (E and F) Quantitative analysis of SA β-gal positive cells with or without JPH203 after X-irradiation. Data are expressed as mean ± SD of three experiments. *p < 0.05 (Student's t test).
4. Discussion
Here, we demonstrated that the LAT1 inhibitor JPH203 sensitized MIA Paca-2 and A549 cells to radiation by enhancing cellular senescence. As shown in Fig. 1C and 1D, JPH203 dramatically reduced cellular neutral amino acids levels, suggesting that JPH203 successfully inhibited LAT1 activity. This study is the first, to our knowledge to show that X-irradiation increases cellular neutral amino acid uptake via LAT1. Furthermore, we demonstrated that JPH203, at minimally toxic concentrations, significantly enhanced the cellular radiosensitivity of both cell lines. These results indicate that, these cancer cell lines utilize neutral amino acid metabolism for survival after irradiation and depend on LAT1 for the neutral amino acid uptake. This observation suggests that JPH203 may be a potent drug in combination with cancer radiotherapy for targeting cancers that rely on LAT1 for assimilating neutral amino acid.
To investigate how JPH203 sensitized cancer cells to radiation, we analyzed the involvements of ATP production, GSH synthesis, mTOR activity, and cellular senescence. In this study, we demonstrated that LAT1 inhibition by JPH203 dramatically suppressed mTOR activity after irradiation, while it increased cellular ATP and GSH content after X-irradiation rather than decreasing them (Figs. 3 and 4). BCAAs are metabolized into acetyl-CoA and succinyl-CoA by BCAT1/2 and the branched-chain alpha-keto acid dehydrogenase complex, and are utilized for energy production through the TCA cycle [37]. However, we did not observe a decrease in ATP levels following JPH203 treatment, suggesting that LAT1 inhibition may lead to decreased ATP consumption by mTOR downregulation rather than decreasing ATP synthesis by inhibiting neutral amino acid metabolism. Along with this study, previous reports have shown that the mTOR inhibitor rapamycin increased cellular ATP in mouse embryonic fibroblasts and C2C12 cells [42,43]. To precisely understand how neutral amino acid metabolism contributes to cancer survival, we need further analyses targeting neutral amino acid-metabolizing enzymes, such as BCAT1/2 and the branched-chain alpha-keto acid dehydrogenase complex.
In this study, JPH203 significantly decreased mTOR activity after irradiation (Fig. 4). Previous reports have also shown that JPH203 suppresses mTOR activity and tumor growth in in vitro and in vivo models [23,41,44]. LAT1 is known to import various neutral EAAs into cells. Among these amino acids, leucine and isoleucine have been reported to be essential for mTOR activity. Han et al., have shown that leucyl-tRNA synthetase activates mTORC1 in an intracellular leucine concentration-dependent manner [45]. In particular, JPH203 significantly decreased cellular leucine and isoleucine levels (Fig. 1C and 1D). These data suggest that the depletion of leucine and isoleucine by JPH203 leads to the downregulation of mTOR activity after X-irradiation. In addition, mTOR inhibitors have been reported to increase radiosensitivity in cancer cell lines [46,47], suggesting that mTOR activity is essential for cancer survival after irradiation. Thus, these data indicate that JPH203 sensitizes cancer cells to radiation via mTOR inhibition. In addition, JPH203 significantly increased p21 expression in MIA Paca-2 cells after irradiation, whereas p21 expression was almost unchanged in A549 cells after irradiation. In contrast, we demonstrated that JPH203 significantly enhanced cellular senescence in both cell lines after X-irradiation (Fig. 5). These observations might be due to the difference in the level of cellular senescence between MIA Paca-2 and A549 cell lines. Taken together, these results suggest that LAT1 inhibition reduces mTOR activity, resulting in an increase in cellular senescence after irradiation.
In summary, we showed that X-irradiation increased cellular neutral amino acid uptake via LAT1 in A549 and MIA Paca-2 cells, and that the LAT1 inhibitor JPH203, at minimally toxic concentrations, significantly sensitized the cancer cells to radiation. Our study also demonstrated that this radiosensitization effect was induced through the induction of cellular senescence by mTOR downregulation. A limitation of this study is that the experiments demonstrating that JPH203 sensitized A549 and MIA Paca-2 cells to radiation were only conducted on an in vitro model. Further studies are necessary to determine the effectiveness of this treatment combination in an in vivo model and on different types of cancers. Despite the current limitations, the study draws focus towards a new potent anti-cancer drug JPH203 that can used in combination with radiotherapy.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
Acknowledgments
We thank J-Pharma for providing JPH203.
Funding
This work was supported by Grant-in-Aids for Scientific Research from the Japan Society for the Promotion of Science (grant numbers 20K22830 (TB) and 21K14971 (TB)).
Author contribution statement
Tomoki Bo and Hironobu Yasui designed and performed the experiments, analyzed data and wrote the manuscript. Sho Kobayashi and Tsunekata Ito performed LC/MS analysis and analyzed the data. Osamu Inanami, Junichi Fuji and Osamu Nakajima discussed the data and revised the manuscript.
Contributor Information
Tomoki Bo, Email: bo.tomoki@med.id.yamagata-u.ac.jp.
Hironobu Yasui, Email: yassan@vetmed.hokudai.ac.jp.
Reference
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Zong W.X., Rabinowitz J.D., White E. Mitochondria and cancer. Mol. Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosios A.M., Hecht V.C., Danai L.V., Johnson M.O., Rathmell J.C., Steinhauser M.L., Manalis S.R., Vander Heiden M.G. Amino acids Rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell. 2016;36:540–549. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi W., Guan Q., Sun T., Cao Y., Zhang L., Guo Y. Improving detection sensitivity of amino acids in thyroid tissues by using phthalic acid as a mobile phase additive in hydrophilic interaction chromatography-electrospray ionization-tandem mass spectrometry. Anal. Chim. Acta. 2015;870:75–82. doi: 10.1016/j.aca.2015.02.048. [DOI] [PubMed] [Google Scholar]
- 5.Kirikae M., Diksic M., Yamamoto Y.L. Quantitative measurements of regional glucose utilization and rate of valine incorporation into proteins by double-tracer autoradiography in the rat brain tumor model. J. Cereb. Blood Flow Metab. 1989;9:87–95. doi: 10.1038/jcbfm.1989.12. [DOI] [PubMed] [Google Scholar]
- 6.Wang L.B., Shen J.G., Zhang S.Z., Ding K.F., Zheng S. Amino acid uptake in arterio-venous serum of normal and cancerous colon tissues. World J. Gastroenterol. 2004;10:1297–1300. doi: 10.3748/wjg.v10.i9.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122:501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 8.Sivanand S., Vander Heiden M.G. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell. 2020;37:147–156. doi: 10.1016/j.ccell.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. (Lond.) 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ananieva E.A., Wilkinson A.C. Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care. 2018;21:64–70. doi: 10.1097/MCO.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baracos V.E., Mackenzie M.L. Investigations of branched-chain amino acids and their metabolites in animal models of cancer. J. Nutr. 2006;136(Suppl):237S–242S. doi: 10.1093/jn/136.1.237S. [DOI] [PubMed] [Google Scholar]
- 12.Neinast M., Murashige D., Arany Z. Branched chain amino acids. Ann. Rev. Physiol. 2019;81:139–164. doi: 10.1146/annurev-physiol-020518-114455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nie C., He T., Zhang W., Zhang G., Ma X. Branched chain amino acids: beyond nutrition metabolism. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19040954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q., Holst J. L-type amino acid transport and cancer: targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015;5:1281–1294. [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Z., Cho H.T., Williams L., Zhu A., Liang K., Huang K., Wu H., Jiang C., Hong S., Crowe R., Goodman M.M., Shim H. Potential biomarker of L-type amino acid Transporter 1 in breast cancer progression. Nucl. Med. Mol. Imaging. 2011;45:93–102. doi: 10.1007/s13139-010-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanai Y., Segawa H., Miyamoto Ki, Uchino H., Takeda E., Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J. Biol. Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 17.Mastroberardino L., Spindler B., Pfeiffer R., Skelly P.J., Loffing J., Shoemaker C.B., Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 18.Meier C., Ristic Z., Klauser S., Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21:580–589. doi: 10.1093/emboj/21.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda K., Hosoda N., Endo H., Saito K., Tsujihara K., Yamamura M., Sakata T., Anzai N., Wempe M.F., Kanai Y., Endou H. l-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101:173–179. doi: 10.1111/j.1349-7006.2009.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohshima Y., Kaira K., Yamaguchi A., Oriuchi N., Tominaga H., Nagamori S., Kanai Y., Yokobori T., Miyazaki T., Asao T., Tsushima Y., Kuwano H., Ishioka N.S. Efficacy of system l amino acid transporter 1 inhibition as a therapeutic target in esophageal squamous cell carcinoma. Cancer Sci. 2016;107:1499–1505. doi: 10.1111/cas.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cormerais Y., Giuliano S., LeFloch R., Front B., Durivault J., Tambutté E., Massard P.A., de la Ballina L.R., Endou H., Wempe M.F., Palacin M., Parks S.K., Pouyssegur J. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016;76:4481–4492. doi: 10.1158/0008-5472.CAN-15-3376. [DOI] [PubMed] [Google Scholar]
- 22.Yun D.W., Lee S.A., Park M.G., Kim J.S., Yu S.K., Park M.R., Kim S.G., Oh J.S., Kim C.S., Kim H.J., Kim J.S., Chun H.S., Kanai Y., Endou H., Wempe M.F., Kim D.K. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J. Pharmacol. Sci. 2014;124:208–217. doi: 10.1254/jphs.13154fp. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto K., Sato F., Tamagawa S., Gunduz M., Onoda N., Uchino S., Muragaki Y., Hotomi M. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci. Rep. 2019;9:14616. doi: 10.1038/s41598-019-51144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi K., Ogata S., Nakanishi K., Ozeki Y., Hiroi S., Tominaga S., Aida S., Matsuo H., Sakata T., Kawai T. LAT1 expression in non-small-cell lung carcinomas: analyses by semiquantitative reverse transcription-PCR (237 cases) and immunohistochemistry (295 cases) Lung Cancer. 2010;68:58–65. doi: 10.1016/j.lungcan.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Altan B., Kaira K., Watanabe A., Kubo N., Bao P., Dolgormaa G., Bilguun E.O., Araki K., Kanai Y., Yokobori T., Oyama T., Nishiyama M., Kuwano H., Shirabe K. Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother. Pharmacol. 2018;81:141–153. doi: 10.1007/s00280-017-3477-4. [DOI] [PubMed] [Google Scholar]
- 26.Cormerais Y., Giuliano S., LeFloch R., Front B., Durivault J., Tambutté E., Massard P.A., de la Ballina L.R., Endou H., Wempe M.F., Palacin M., Parks S.K., Pouyssegur J. Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Res. 2016;76:4481–4492. doi: 10.1158/0008-5472.CAN-15-3376. [DOI] [PubMed] [Google Scholar]
- 27.Quan L., Ohgaki R., Hara S., Okuda S., Wei L., Okanishi H., Nagamori S., Endou H., Kanai Y. Amino acid transporter LAT1 in tumor-associated vascular endothelium promotes angiogenesis by regulating cell proliferation and VEGF-A-dependent mTORC1 activation. J Exp Clin Cancer Res. 2020;39:266. doi: 10.1186/s13046-020-01762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasui H., Yamamoto K., Suzuki M., Sakai Y., Bo T., Nagane M., Nishimura E., Yamamori T., Yamasaki T., Yamada K.I., Inanami O. Lipophilic triphenylphosphonium derivatives enhance radiation-induced cell killing via inhibition of mitochondrial energy metabolism in tumor cells. Cancer Lett. 2017;390:160–167. doi: 10.1016/j.canlet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto K., Ikenaka Y., Ichise T., Bo T., Ishizuka M., Yasui H., Hiraoka W., Yamamori T., Inanami O. Evaluation of mitochondrial redox status and energy metabolism of X-irradiated HeLa cells by LC/UV, LC/MS/MS and ESR. Free Radic. Res. 2018;52:648–660. doi: 10.1080/10715762.2018.1460472. [DOI] [PubMed] [Google Scholar]
- 30.Dwarakanath ‘B.S. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-D-glucose in vitro. J. Cancer Res. Ther. 2009;5(Suppl. 1):S27–S31. doi: 10.4103/0973-1482.55137. [DOI] [PubMed] [Google Scholar]
- 31.Lin X., Zhang F., Bradbury C.M., Kaushal A., Li L., Spitz D.R., Aft R.L., Gius D. 2-deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63:3413–3417. [PubMed] [Google Scholar]
- 32.Sappington D.R., Siegel E.R., Hiatt G., Desai A., Penney R.B., Jamshidi-Parsian A., Griffin R.J., Boysen G. Glutamine drives glutathione synthesis and contributes to radiation sensitivity of A549 and H460 lung cancer cell lines. Biochim. Biophys. Acta. 2016;1860:836–843. doi: 10.1016/j.bbagen.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai H., Kaira K., Oriuchi N., Shimizu K., Tominaga H., Yanagitani N., Sunaga N., Ishizuka T., Nagamori S., Promchan K., Nakajima T., Yamamoto N., Mori M., Kanai Y. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- 34.Hong S., Fang Z., Jung H.Y., Yoon J.H., Hong S.S., Maeng H.J. Synthesis of gemcitabine-threonine amide prodrug effective on pancreatic cancer cells with improved pharmacokinetic properties. Molecules. 2018;23 doi: 10.3390/molecules23102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi S, Tokairin Y, Miyakoshi T, Saito T, Nagaoka K, Ikeda Y. Quantitative analysis of γ-glutamylpeptides by liquid chromatography-mass spectrometry and application for γ-glutamyltransferase assays. Anal Biochem. 2019;578:13–22. doi: 10.1016/j.ab.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Sato M., Harada-Shoji N., Toyohara T., Soga T., Itoh M., Miyashita M., Tada H., Amari M., Anzai N., Furumoto S., Abe T., Suzuki T., Ishida T., Sasano H. L-type amino acid transporter 1 is associated with chemoresistance in breast cancer via the promotion of amino acid metabolism. Sci. Rep. 2021;11:589. doi: 10.1038/s41598-020-80668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng H., Wang Y., Luo W. Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene. 2020;39:6747–6756. doi: 10.1038/s41388-020-01480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y., Zhang J., Ren S., Sun D., Huang H.Y., Wang H., Jin Y., Li F., Zheng C., Yang L., Deng L., Jiang Z., Jiang T., Han X., Hou S., Guo C., Li F., Gao D., Qin J., Gao D., Chen L., Lin S.H., Wong K.K., Li C., Hu L., Zhou C., Ji H. Branched-chain amino acid metabolic reprogramming orchestrates drug resistance to EGFR tyrosine kinase inhibitors. Cell Rep. 2019;28:512–525. doi: 10.1016/j.celrep.2019.06.026. e6. [DOI] [PubMed] [Google Scholar]
- 39.Hayase S., Kumamoto K., Saito K., Kofunato Y., Sato Y., Okayama H., Miyamoto K., Ohki S., Takenoshita S. L-type amino acid transporter 1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol. Lett. 2017;14:7410–7416. doi: 10.3892/ol.2017.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cormerais Y., Massard P.A., Vucetic M., Giuliano S., Tambutté E., Durivault J., Vial V., Endou H., Wempe M.F., Parks S.K., Pouyssegur J. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5) J. Biol. Chem. 2018;293:2877–2887. doi: 10.1074/jbc.RA117.001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maimaiti M., Sakamoto S., Yamada Y., Sugiura M., Rii J., Takeuchi N., Imamura Y., Furihata T., Ando K., Higuchi K., Xu M., Sazuka T., Nakamura K., Kaneda A., Kanai Y., Kyprianou N., Ikehara Y., Anzai N., Ichikawa T. Expression of L-type amino acid transporter 1 as a molecular target for prognostic and therapeutic indicators in bladder carcinoma. Sci. Rep. 2020;10:1292. doi: 10.1038/s41598-020-58136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delic V., Noble K., Zivkovic S., Phan T.A., Reynes C., Zhang Y., Phillips O., Claybaker C., Ta Y., Dinh V.B., Cruz J., Prolla T.A., Bradshaw P.C. The effects of AICAR and rapamycin on mitochondrial function in immortalized mitochondrial DNA mutator murine embryonic fibroblasts. Biol. Open. 2018;7 doi: 10.1242/bio.033852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z., Xu H.N., Li S., Jr A.D., Chellappa K., Davis J.G., Guan Y., Frederick D.W., Chu W., Zhao H., Li L.Z., Baur J.A. Rapamycin maintains NAD+/NADH redox homeostasis in muscle cells. Aging (Albany, NY) 2020;12:17786–17799. doi: 10.18632/aging.103954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Häfliger P., Graff J., Rubin M., Stooss A., Dettmer M.S., Altmann K.H., Gertsch J., Charles R.P. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J Exp Clin Cancer Res. 2018;37:234. doi: 10.1186/s13046-018-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han J.M., Jeong S.J., Park M.C., Kim G., Kwon N.H., Kim H.K., Ha S.H., Ryu S.H., Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 46.Chang L., Graham P.H., Hao J., Ni J., Bucci J., Cozzi P.J., Kearsley J.H., Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao C., Subhawong T., Albert J.M., Kim K.W., Geng L., Sekhar K.R., Gi Y.J., Lu B. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]