Abstract
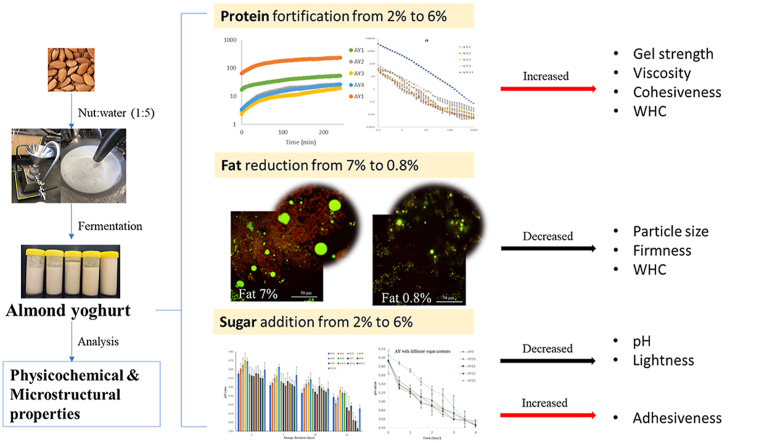
The influence of the protein, fat and sugar in almond milk on the formation of the acidic gel was investigated by determining their physicochemical and microstructural properties. The protein, fat and sugar in the almond milk were varied from 2% to 6%, 0.8%–7% and 0.6%–7%, respectively and fermented using Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophiles cultures to form a gel structure. Both protein and fat increased the gel strength, viscosity (stirred gel) and lightness of almond yoghurts as the concentration increased. The addition of protein content increased the cohesiveness (from 0.70 to 1.17), water holding capacity (from 28.75% to 52.22%) and D4,3 value of particle size (from 32.76 μm to 44.41 μm) of almond yoghurt. Fat reduction decreased the firmness (from 6.56 g to 4.69 g), D4,3 value (from 88.53 μm to 18.37 μm), and water holding capacity (from 48.96% to 27.66%) of almond yoghurt. With sugar addition, almond yoghurt showed increased adhesiveness, decreased lightness and a low pH, with no significant difference in firmness, particle size, and flow behaviour. The confocal images provided evidence that the fortified protein contents homogeneously entrapped fat globules resulting in a more stable gel network and increased fat content led to large fat globule formation resulting in a harder gel network, while the added sugar did not significantly affect the gel network. The results suggested that the protein fortification enhances the texture of almond yoghurt. The fat content of 7% with 3.5% protein showed poor consistency and gel strength of yoghurt. Sugar mainly contributed to bacterial metabolism during fermentation.
Keywords: Almond yoghurt, Protein, Fat, Sugar, Gel properties
Graphical abstract
Highlights
-
•
Protein or fat fortification gives almond yoghurt a rigid and stable gel structure.
-
•
Excess fat accounts for weak poor consistency and gel strength in almond yoghurt.
-
•
Sugar mainly contributes to bacterial metabolism during almond yoghurt fermentation.
-
•
The microstructure of almond yoghurt verified the effects of main composition variation.
1. Introduction
Almond-based products can meet the public interest as an alternative to animal-sourced foods, especially proteins, to address dairy allergy issues, and to meet the requirements of a vegan or calorie-reduced diet for specific patients with obesity, hypercholesterolemia or cardiovascular disease (Jeske et al., 2018; Sethi et al., 2016). Almond yoghurt with pro- and prebiotic functions has been developed as dairy substitutes and health-promoting products in the recent decade that have become more popular in the market (Grasso et al., 2020; Rinaldoni et al., 2012).
In general, almond yoghurt gels are produced through base milk standardisation, homogenisation, pasteurisation, fermentation and cooling steps (Jeske et al., 2018). During fermentation of almond milk, a weak gel structure is formed with improved organoleptic features (Rinaldoni et al., 2012). As a kind of emulsion-filled gel (Geremias-Andrade et al., 2016), the gel properties (e.g. appearance, viscosity, firmness) of yoghurt (dairy or plant-based) are typically influenced by changes to formulations (e.g. total solids, proteins, fats), heat and pressure treatment conditions prior to fermentation (Lee and Lucey, 2010; Lucey, 2002). Korzendorfer et al. (2018) found that the particle size in yoghurts usually is less than 100 μm, and it is highly dependent on the processing technique (e.g. homogenisation) and conditions (e.g. temperature), followed by the protein content and cultures, the yoghurt may have a grainy texture and poor consistency caused by excessive particle formation if the size is higher than 150 μm.
The commercial almond yoghurt contains 2.3% of protein, 7.9% of fat and 3.0% of carbohydrate (include 0.80% of sugar) (Grasso et al., 2020). In comparison, most commercial dairy yoghurts contain more than 2.7% of protein, 0.1–3.5% of fat, and 4–16% of sugar (Jørgensen et al., 2019; Miklavec et al., 2015). A total solid ranging between 10 and 15% in almond yoghurt could be comparable to the nutritional value of bovine yoghurt, but with excess fat and lower sugar contents (Martinez et al., 2008). Jørgensen et al. (2019) reviewed that protein-fortified acid gels tends to satisfy consumers weight wellness demand as intake calories from protein seems healthier than carbohydrate or fat.
In addition to the nutritional value, protein fortification also modifies the texture of yoghurt gels. It is already well-known that the texture and viscosity of acid-induced yoghurt gels can be modified by protein fortification; for example, with increased protein contents the firmness, viscosity and storage modulus of tigernut yoghurt increased (Kizzie-Hayford et al., 2016). In their study, Marafon et al. (2011) reported that protein fortification optimised the flow behaviour such as increased storage modulus, loss modulus and viscosity of dairy yoghurt.
In dairy yoghurt gels, the lactose content is considered as desirable nutrients for the growth of probiotics, affecting the flow behaviour and structure of yoghurt gels (Jørgensen et al., 2019). As no lactose content is present in almond, sugar needs to be added to almond milk as lactose substitutes to provide nutrients for starter culture growth and survival during fermentation. However, the concentration of sugar needs to be considered for almond yoghurt production, not only because it provides sweetness but also because the concentration of sugar affects the growth and survival of starter culture associated with the osmotic pressure.
Studies on almond yoghurt gels have mainly focused on sensory attributes and nutritional benefits such as pro- and prebiotic functions (Akin and Ozcan, 2017; Grasso et al., 2020; Jeske et al., 2018; Makinen et al., 2016) with limited information related to the textural performance of products. The balanced nutritional panel of almond yoghurt also need to be considered as reducing the calorie intake from sugar and fat has become the main concern for public health. Based on consumers’ health claim preference analysis, the public prefers yoghurts with low fat and sugars (Miklavec et al., 2015).
Therefore, this research evaluated how the major components protein, fat and sugar influence the gel properties (e.g. particle size, viscosity, gel strength) of almond yoghurt. The physicochemical characterisation results of almond yoghurts with composition variance will provide useful knowledge on the production of reduced-fat and sugar contents of almond yoghurt with considerable protein content comparable to dairy yoghurts.
2. Materials and methods
2.1. Materials
Australian grown almond nuts (21.4% protein, 43.8% total fat, 14.3% carbohydrate, 6.3% sugar content) were purchased from Royal Nut Company, Victoria. Almond protein concentrate (XAQA® Almond protein powder) with ≥90% protein, ≤ 5% moisture and ≤5% ash content was purchased from Xi'an Quanao Biotech Co., Ltd, China. A thermophilic freeze-dried culture (FD-DVS YC-X11, YoFlex®) which consists of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus were donated by Chr. Hansen Pty. Ltd (Australia).
2.2. Preparation of almond yoghurt
The raw almond nuts were soaked in distilled water overnight at 4 °C. The brown skin of the almond nut was not removed to ensure consumers benefit from the fibre. The soaked almond nuts were mixed with water and then processed by a PUC colloidal mill (rotary speed 3000 rpm, milling gap 0.04 mm, 30 min) to obtain the almond milk. Almond milk with a nut to water ratio of 1:5 (AY13 sample in Table 1) without any formulation adjustment was included as a control to compare with the samples with formulation adjustment as it contained total solids of 13.74%, very close to commercial dairy yoghurt with a total solid between 14 and 15% (Lee and Lucey, 2010). The constant protein (3.5%), fat (4%) and sugar (5%) content were chosen based on nutritional value consideration that is comparable with dairy yoghurt (Chalupa-Krebzdak et al., 2018; Vanga and Raghavan, 2018). The maximum fat (7%) and the minimum protein (2%) were used to imitate the commercial almond yoghurt (Grasso et al., 2020). The maximum sugar (7%) in formulations was selected according to Martinez et al. (2008), which reported that 7% sugar confers good flavour, and benefits the survival of the probiotic in almond yoghurt. Almond milk samples were obtained by adding almond protein isolate or sugar or water to form 13 formulations (Table 1).
Table 1.
Formulations of almond yoghurt (AY) made from almond milk (AM) with composition variance (in %) that was calculated from the composition of base almond milk (1:5) and almond protein concentrate (90%).
| Ingredients |
||||
|---|---|---|---|---|
| P x F x S |
P x F x S |
P x F x S |
P x F x S |
|
| AY1 = 2 × 4 x 5 | AY2 = 3.5 × 4 x 5 | AY3 = 4.5 × 4 x 5 | AY4 = 6 × 4 x 5 | |
| AM (1:5) | 50 | 50 | 50 | 50 |
| Sugar | 4.4 | 4.4 | 4.4 | 4.4 |
| Water | 45.61 | 44 | 42.88 | 41.22 |
| APC | 0 | 1.61 | 2.72 | 4.39 |
| P x F x S | P x F x S | P x F x S | P x F x S | |
| AY5 = 3.5 × 7 x 5 | AY6 = 3.5 × 5 x 5 | AY7 = 3.5 × 2.5 x 5 | AY8 = 3.5 × 0.8 x 5 | |
| AM (1:5) | 85.2 | 60 | 30 | 10 |
| Sugar | 3.97 | 4.27 | 4.64 | 4.88 |
| Water | 10.83 | 34.57 | 62.84 | 81.69 |
| APC | 0 | 1.15 | 2.52 | 3.43 |
| P x F x S | P x F x S | P x F x S | P x F x S | |
| AY9 = 3.5 × 4 x 0.6 | AY10 = 3.5 × 4 x 3 | AY11 = 3.5 × 4 x 5 | AY12 = 3.5 × 4 x 7 | |
| AM (1:5) | 50 | 50 | 50 | 50 |
| Sugar | 0 | 2.4 | 4.4 | 6.4 |
| Water | 48.39 | 46 | 44 | 42 |
| APC | 1.61 | 1.61 | 1.61 | 1.61 |
| P x F x S | ||||
| AY13 = 4 × 8.5 x 1.2 | ||||
| AM (1:5) | 100 | |||
APC = Almond protein concentrate (90%), AM (1:5) = almond milk milled with the nut to water ratio 1:5, AY13: Control almond yoghurt sample.
P = protein, F = fat, S = sugar (in %).
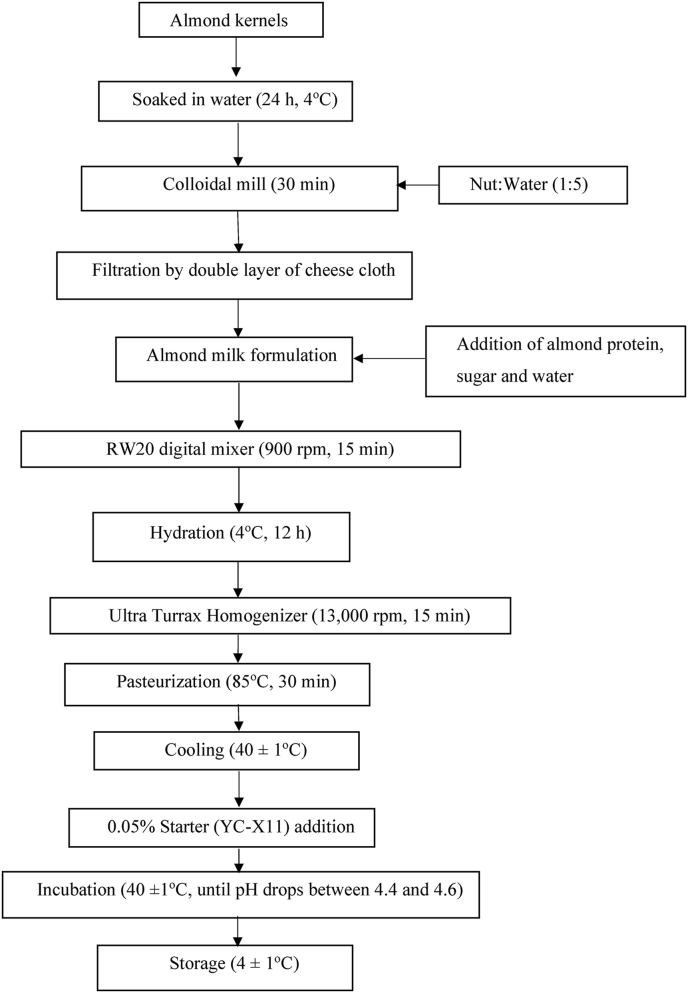
To ensure complete hydration of the added protein, all ingredients were mixed using the RW20 digital mixer (IKA®, Germany) at 900 rpm for 15 min and then kept refrigerated (4 °C) for 12 h as reported by Meletharayil et al. (2015) for milk protein concentrate powder. This was followed by homogenisation with a T 25 digital ULTRA-TURRAX® homogeniser (IKA®, Germany) at 13000 rpm for 15 min. The emulsion was then pasteurised at 85 °C for 30 min as described by Bernat et al. (2015) and allowed to cool down to 40 ± 1 °C, followed by inoculation with 0.05% (w/w) starters (CHR- HANSEN YC-X11). Fermentation was performed at 40 ± 1 °C and ended when the final pH was between 4.4 and 4.6 (Bernat et al., 2015) that lasted for approximately 4 h. The yoghurt samples were kept refrigerated (4 °C) for further analysis. The schematic base almond milk preparation and fermentation process is presented in Fig. 1, including milling of almond nuts, formulation, homogenisation, pasteurisation, fermentation and cold storage.
Fig. 1.
Flow diagram for the preparation of almond yoghurt.
2.3. Physicochemical analysis of almond yoghurt
2.3.1. Chemical analysis of products
2.3.1.1. Total solids
Total solids of each sample were determined by a method described by Lakshanasomya et al. (2011). Samples were weighed before and after drying in a vacuum oven (DZ-2BCII) at 70 °C, for 24 h. The equation (eq (1)) is listed below:
| (1) |
2.3.1.2. Protein contents
The Kjeldahl method was used for protein content determination. A semiautomatic Kjeldahl distiller device analysed total nitrogen with a corresponding digestion block. The conversion factor used for calculation was 5.18 (AOAC, 1995).
2.3.1.3. Fat contents
The Gerber method was selected for total fat content measurement as mentioned by Kundu et al. (2018). A mixture comprising 10 mL sulfuric acid (90%), 1 mL amyl alcohol and 10.75 mL sample was digested in a butyrometer, then centrifuged under 1500 rpm for 15 min at 55 °C, followed by heating for 15 min in a 65 °C water bath for accurate fat content reading.
2.3.1.4. Total sugars
The Phenol-Sulfuric acid method (Nielsen, 2017) was used to determine the total sugars (polysaccharides, oligosaccharides, simple sugars and their derivatives) in each sample. Sample with 1:1000 dilution and D-Glucose (standard) was read under 490 nm by the spectrophotometer after mixing with 1 mL 5% Phenol and 5 mL 98% Sulfuric acid.
2.3.1.5. pH value
According to reference methods AOAC (1995), pH value was determined by a digital pH meter during the acidification process and after 1, 7, 14- and 21-days storage at 4 °C. Buffer solutions with pH value 4 and 7 were used for pH meter calibration.
2.3.2. Water holding capacity (WHC)
The water holding capacity (WHC) of almond yoghurt was measured following the method described by Meletharayil et al. (2015) for dairy yoghurt gel, albeit at a higher speed. Almond milk (30 g) was weighed and fermented in a 70 mL centrifuge tube. After storage for 1 day at 4 °C, samples were centrifuged in a 5702 R centrifuge machine (Eppendorf, AU) at 2.5 1000 rpm for 15 min at 4 °C. The mass of pellet divided by the mass of almond gel was used to calculate the percentage of water holding capacity (WHC%).
2.3.3. Textural assessment
A texture profile analysis (TPA) device (TA-XT plus, Micro Stable System Co., UK) was used to perform the compression test. The cylindrical-shaped probe with a diameter of 10 mm was selected for measurement at a 5 g trigger force at 1 mm/s speed. The yoghurt sample was taken out from refrigerated and was left to equilibrate at room temperature for 10 min. Textural properties of almond yoghurt were analysed through force-time curves as described by Siefarth et al. (2014). Firmness is the maximum force in first compression, adhesiveness is the negative area of the curve after first compression and cohesiveness is the ratio obtained through second position compression area divided by the first positive compression area.
2.3.4. Particle size determination
Particle size determination of samples was performed by a laser light scattering Mastersizer (Hydro 2000, Malvern Scientific Instruments Ltd., Malvern, UK), the refractive index (RI) of almond yoghurt gel was measured by a digital hand-held refractometer (AR200, Leica, USA), the average value was 1.46, and the refractive index selected for dispersed water was 1.33 (Ng et al., 2018). The target laser obscuration ranged between 10% and 13% at 2000 rpm motor speed at room temperature. The particle size parameters extracted from the software include the volume-weighted mean (D4,3), the surface average diameter (D3,2), less than 10% of the gel particles d(0.1), 50% of the gel particles d(0.5), and less than 90% of the gel particles d(0.9).
2.3.5. Colour of almond yoghurt
The colour of each almond yoghurt was measured in triplicates using a CR-400 Chroma-meter (Konica Minolta, Japan) after fermentation with 24 h storage period at 4 °C. The CIE-LAB space system was selected for colour measurement on lightness (L*, 0–100), redness to greenness (a*, positive to negative values) and yellowness to blueness (b*, positive to negative values).
2.3.6. Flow behaviour and gel strength determination
A rheometer (Model AR-G2, TA Instruments Ltd, US) was utilised to measure flow properties of almond yoghurts. The sandblasted stainless-steel parallel plate with 40 mm diameter was used at 35 °C with a gap of 200 μm to obtain shear rate and shear stress value. The shear stress versus shear rate curve was performed between 0.1 and 1000 (s−1) within 5 s, sample as described by Ng et al. (2018). Oscillatory stress and strain values within the linear area report the consistent rheological properties of stirred almond-based gel (equilibrated at room temperature for 1 h and gently stirred before measurement to eliminate the effect of syneresis). Frequency ranged from 0.01 to 100 Hz with 1% strain. Parameter selected for gel strength determination was G’, which corresponds to the elastic features of almond yoghurt (set-gel). The dynamic oscillatory rheological measurement was conducted at the same gap and shear rate ranges to imitate the gelation (40 °C, 4 h), cooling (from 40 to 4 °C), post-acidification (4 °C, 1 h) and consumption (from 4 to 35 °C) conditions.
2.3.7. Confocal laser scanning microscopy (CLSM)
The microstructure of almond yoghurt was observed through a Zeiss LSM700 confocal laser scanning microscope (Carl-Zeiss-Promenade, Germany). Samples were stained and imaged, as described by Ningtyas et al. (2018). Briefly, fat and protein content were stained using equal proportions of Nile Red (0.02% mixed with PEG 200) and Rhodamine B (0.005% mixed with PEG 200). 488 and 555 nm lasers were selected for fat and protein content excitation, respectively. In the captured overlay images, fat, proteins and protein-wrapped fat globules are stained in green, red and yellow colours, respectively. The rest dark space represents moisture content.
2.3.8. Statistical analysis
All measurements were performed in triplicate. One-way analysis of variance (ANOVA) through the Tukey procedure was used to statistically evaluate the obtained data. The General linear model allows the p-value of the F-test computed in the ANOVA results; the information explains the interaction between treatments on the significance of variables. The Pearson procedure was used to determine the correlation between the data. All of the significant levels were set at p-values less than 0.05 by the XLSTAT software (Xlstat-sensory 2019, Addinsoft, France).
The influence of the interactions between protein*fat, protein*sugar, fat*sugar, and protein*fat*sugar on the tested physicochemical features, which include firmness, adhesiveness, WHC, apparent viscosity at 50s−1, L*, a*, b*, D4,3, D3,2, d(0.1), d(0.5) and d(0.9) values were analysed. There was no significant effect from the interactions (protein* sugar, fat*sugar, and protein*fat*sugar) on the tested physicochemical features of almond yoghurts. The interaction between protein and fat had a highly significant effect on firmness, adhesiveness, WHC, apparent viscosity, L*, and d(0.5) with p < 0.01 that is presented and discussed in the results section.
3. Results and discussion
3.1. Total solids and major components (protein, fat and sugars) of almond yoghurts
The protein, fat and sugar contents in the yoghurt formulations (Table 1) were measured and presented in (Table 2). The composition variation resulted in different total solids in the almond yoghurt samples (Table 2).The sample AY5 (7% fat) had the highest total solids (15.15%), while the sample AY9 (0.61% sugar) had the lowest total solids (7.83%) among all samples. The significant differences (p < 0.05) in total solids were expected as the samples of almond milk were prepared to vary the proteins, fat, sugar and other components in base almond milk formulation (Table 1) to study their effect on the almond gel formation. This has been previously observed in dairy yoghurts by Rinaldoni et al. (2012), who observed a variation in total solids of different dairy yoghurts mainly because of the raw milk standardisation.
Table 2.
Composition (protein, fat and sugar contents) and total solids of each almond yoghurt (AY) in %.
| Samples | Protein (%) | Fat (%) | Total sugars (%) | Total solids (%) |
|---|---|---|---|---|
| AY1 | 2.09 ± 0.03e | 4.24 ± 0.05d | 5.03 ± 0.15b | 10.21 ± 0.04e |
| AY2 | 3.56 ± 0.05d | 4.23 ± 0.05d | 5.07 ± 0.11b | 11.29 ± 0.65d |
| AY3 | 4.54 ± 0.05b | 4.24 ± 0.05d | 4.98 ± 0.13b | 12.41 ± 0.24c |
| AY4 | 5.93 ± 0.10a | 4.23 ± 0.05d | 5.00 ± 0.12b | 14.18 ± 0.10b |
| AY5 | 3.49 ± 0.05d | 7.21 ± 0.06b | 5.03 ± 0.11b | 15.15 ± 0.19a |
| AY6 | 3.50 ± 0.04d | 5.09 ± 0.03c | 5.04 ± 0.15b | 12.58 ± 0.32c |
| AY7 | 3.48 ± 0.03d | 2.55 ± 0.04e | 4.97 ± 0.15b | 10.79 ± 0.08de |
| AY8 | 3.51 ± 0.06d | 0.84 ± 0.03f | 4.99 ± 0.16b | 9.30 ± 0.24f |
| AY9 | 3.50 ± 0.04d | 4.24 ± 0.05d | 0.61 ± 0.06e | 7.83 ± 0.06g |
| AY10 | 3.46 ± 0.05d | 4.25 ± 0.06d | 2.97 ± 0.10c | 9.34 ± 0.06f |
| AY11 | 3.49 ± 0.03d | 4.24 ± 0.08d | 4.98 ± 0.14b | 12.90 ± 0.05c |
| AY12 | 3.50 ± 0.08d | 4.23 ± 0.06d | 7.03 ± 0.10a | 14.12 ± 0.15b |
| AY13 | 4.09 ± 0.03c | 8.46 ± 0.05a | 1.22 ± 0.07d | 13.74 ± 0.36b |
Values are expressed as means ± SD from triplicate measurements; values with different letters in the same columns show significant differences at 95% of confidence. AY13: Control almond yoghurt sample.
3.2. The change of pH value during fermentation and storage
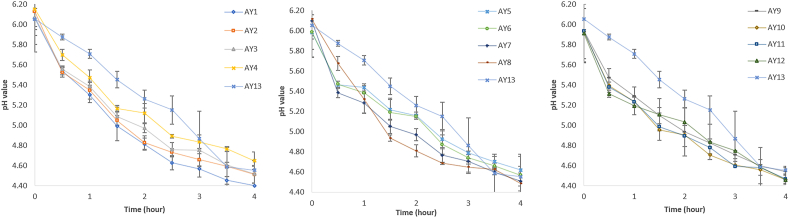
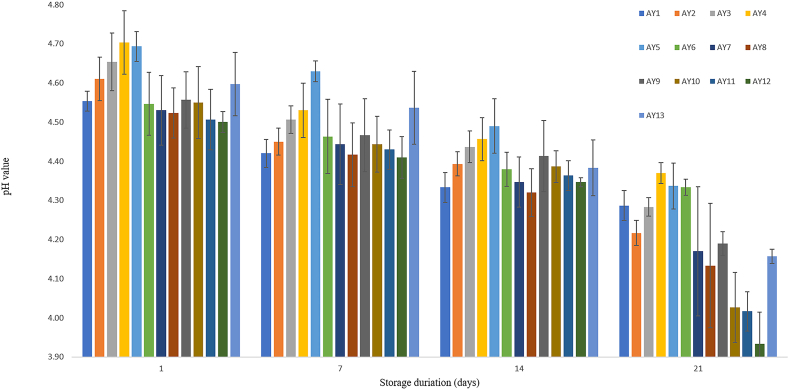
The changes in pH values during 4-h fermentation and 21 days of cold storage are presented in Fig. 2 and Fig. 3, respectively. The pH ranged between 4.4 and 4.6 for all the samples after 4 h of fermentation, suggesting coagulation was achieved and gel matrix formed as protein denatured at a pI of 4.5–5.5 (Dhakal et al., 2014; Jeske et al., 2018). The initial pH value of sample AY4 was higher than others, and it dropped slowly during fermentation, possibly due to the high protein content (6%). From Fig. 2, samples with more protein content displayed slower pH drop in the order AY4>AY3>AY2>AY1 that can be associated to the increased buffering capacity as the protein content increases. This result confirmed the finding by Jørgensen et al. (2019) in a protein-fortified dairy yoghurt (9.5% protein). They associated the high pH value to the high buffering capacity of added proteins. After the 4-h fermentation, the pH value of almond yoghurts continuously decreased throughout the cold storage period (21 days) for all tested samples suggesting viability of the lactic acid bacteria in the products throughout the storage period. The sample AY12 made from almond milk containing the highest sugar content (7%) had a significantly (p < 0.05) lower pH value (3.93) that may introduce unpleasant flavour and texture to yoghurt at the end of the storage period (day 21). A similar phenomenon was observed by Sodini et al. (2004) who reported that higher sugar content contributes towards continuous metabolism in the microorganism, producing more lactic acid that lowers the pH of yoghurt during storage.
Fig. 2.
Changes in pH value of almond yoghurts made from the different composition of almond milk during fermentation. Values are averages of triplicate measurements. Error bars represent the standard deviation of averages.
Fig. 3.
Changes in pH of almond yoghurt over 21 days storage at 4 °C. Values are averages of triplicate measurements. Error bars represent the standard deviation of averages.
3.3. Water holding capacity (WHC) of almond yoghurt
The water holding capacity (WHC) indicates an emulsion gel's ability to retain the serum that is crucial for the quality of a fermented product as it influences consumer acceptability (Bong and Moraru, 2014). From Table 2, the WHC of almond yoghurts ranged between 27.66% (AY8) and 52.22% (AY4). According to Pearson correlation results (Table 4), the WHC is highly correlated with the total solid (r2 = 0.81, p < 0.05). The higher WHC of samples AY4 (52.22%) and AY5 (48.96%) can be attributed to their higher total solids (14.18% and 15.15%, respectively) caused by the higher protein contents (6%) in AY4 and higher fat contents (7%) in AY5 compared with other samples. The sample AY3 has similar WHC (46.56%) like AY5 possibly due to the higher protein contents (4.5%).
Table 4.
Pearson correlation coefficients (r2) of almond yoghurts within physicochemical features.
| Variables | Protein | Fat | Total sugars | Total solids | Firmness | Adhesiveness | Cohesiveness | WHC | Viscosity at 50s−1 | La | aa | ba | D (4,3) | D (3,2) | d (0.1) | d (0.5) | d (0.9) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | 1.00 | ||||||||||||||||
| Fat | 0.10 | 1.00 | |||||||||||||||
| Total sugars | −0.03 | −0.36 | 1.00 | ||||||||||||||
| Total solids | 0.42 | 0.58a | 0.43 | 1.00 | |||||||||||||
| Firmness | 0.30 | 0.50 | 0.21 | 0.65a | 1.00 | ||||||||||||
| Adhesiveness | 0.51 | 0.53 | 0.26 | 0.80a | 0.48 | 1.00 | |||||||||||
| Cohesiveness | 0.53 | 0.51 | −0.15 | 0.47 | 0.32 | 0.55 | 1.00 | ||||||||||
| WHC | 0.75a | 0.56a | 0.13 | 0.81a | 0.75a | 0.76a | 0.55 | 1.00 | |||||||||
| Viscosity | 0.21 | 0.79a | −0.42 | 0.48 | 0.16 | 0.39 | 0.21 | 0.45 | 1.00 | ||||||||
| La | 0.40 | 0.73a | −0.42 | 0.34 | 0.55a | 0.34 | 0.59a | 0.68a | 0.46 | 1.00 | |||||||
| aa | −0.26 | 0.82a | −0.31 | 0.33 | 0.45 | 0.26 | 0.07 | 0.29 | 0.63a | 0.54 | 1.00 | ||||||
| ba | 0.95a | −0.03 | 0.08 | 0.39 | 0.36 | 0.41 | 0.58a | 0.68a | −0.01 | 0.31 | −0.35 | 1.00 | |||||
| (D4,3) | 0.18 | 0.90a | −0.21 | 0.67a | 0.70a | 0.54 | 0.32 | 0.69a | 0.79a | 0.67a | 0.80a | 0.06 | 1.00 | ||||
| (D3,2) | 0.24 | 0.87a | −0.14 | 0.69a | 0.63a | 0.54 | 0.31 | 0.74a | 0.80a | 0.72a | 0.74a | 0.09 | 0.96a | 1.00 | |||
| d (0.1) | 0.10 | 0.75a | −0.09 | 0.61a | 0.69a | 0.39 | 0.14 | 0.61a | 0.72a | 0.53 | 0.76a | 0.01 | 0.94a | 0.92a | 1.00 | ||
| d (0.5) | 0.18 | 0.87a | −0.13 | 0.67a | 0.79a | 0.52 | 0.37 | 0.70a | 0.67a | 0.67a | 0.78a | 0.11 | 0.96a | 0.91a | 0.93a | 1.00 | |
| d (0.9) | 0.18 | 0.87a | −0.24 | 0.64a | 0.65a | 0.53 | 0.26 | 0.66a | 0.80a | 0.64a | 0.78a | 0.03 | 0.98a | 0.94a | 0.91a | 0.89a | 1.00 |
Level of correlation: 0.9–1.00 very high; 0.7–0.9 high; 0.5–0.7, moderate; 0.3–0.5, low; 0–0.3: negligible correlation.
The fact that sample AY5 (3.5% protein and 7% fat), with the highest total solids (15.15%) has a lower WHC (48.96%) compared to AY4 (6% protein, 4% fat and 14.18% of total solids) with a WHC of 52.22% suggests that not just the total solids but the amount of protein has a significant contribution towards the WHC compared to fat. Similar influence of protein can be observed when comparing the total solids in samples AY5 (3.5% protein and 7% fat) and AY3 (4.5% protein, 4% fat) that were 12.41% and 15.15%, respectively. The significant role of protein can be confirmed from Table 4 where the concentration of protein (r2 = 0.75, p < 0.05) is more correlated with WHC than fat (r2 = 0.56, p < 0.05). The high protein content promotes gel network formation that can hold more water in the gel matrix as previously observed by Kizzie-Hayford et al. (2016) in tigernut yoghurt and Jørgensen et al. (2019) in dairy yoghurt with protein >5.6%.
Although less significant than protein, the role of fat in combination with protein is critical towards the WHC as observed from the statistical analysis where the interaction of protein and fat significantly (p < 0.01) influenced the WHC of almond yoghurt. As reviewed by Alzagtat and Alli (2002), the fat globules are surrounded by the proteins and interactions between protein and fat (mainly hydrophilic bonds and hydrophobic bonds) affect the arrangement of protein-fat matrix, and therefore determines the amount of water trapped in the gel network. Thus, the presence of fat droplets in the protein gels contributed to the changes of the gel structure and influence the WHC of the almond yoghurt.
3.4. Texture profile analysis (TPA) of almond yoghurt
The textural properties (firmness, cohesiveness and adhesiveness) of various almond yoghurt with different concentrations of protein, fat and sugar contents are presented in Table 3.
Table 3.
TPA (firmness, cohesiveness, and adhesiveness), WHC, viscosity, CIELAB space colour results and particle size values (in μm) of almond yoghurt with protein, fat and sugar concentration variance and a control sample AY13.
| Samples | Firmness (g) | Adhesiveness (g.s) | Cohesiveness | WHC (%) | Viscosity (Pa.s) at 50s−1 | L* | a* | b* | (D4,3) | (D3,2) | d (0.1) | d (0.5) | d (0.9) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | AY1 | 4.86 ± 0.06a | 65.33 ± 1.70ab | 0.70 ± 0.00a | 28.75 ± 0.79a | 0.03 ± 0.00a | 77.81 ± 0.08a | 0.55 ± 0.06c | 7.46 ± 0.24a | 32.76 ± 1.78a | 5.49 ± 0.27a | 1.62 ± 0.24a | 30.62 ± 1.46a | 60.91 ± 3.61a |
| AY2 | 5.13 ± 0.26ab | 71.52 ± 1.01ab | 1.01 ± 0.08b | 36.14 ± 0.33b | 0.02 ± 0.01a | 78.17 ± 0.36a | −0.76 ± 0.09a | 9.33 ± 0.13b | 39.13 ± 2.48b | 5.78 ± 0.73a | 2.15 ± 1.09a | 34.86 ± 3.68a | 76.29 ± 3.20b | |
| AY3 | 5.44 ± 0.26ab | 72.54 ± 1.90bc | 1.12 ± 0.05b | 46.56 ± 0.66c | 0.05 ± 0.01b | 81.23 ± 0.02b | −0.49 ± 0.06b | 11.23 ± 0.41c | 42.53 ± 2.45c | 5.97 ± 0.47b | 2.16 ± 0.64a | 36.98 ± 2.67b | 84.10 ± 5.25c | |
| AY4 | 5.54 ± 0.33b | 73.39 ± 0.26a | 1.17 ± 0.00b | 52.22 ± 0.37d | 0.11 ± 0.01c | 81.73 ± 0.25b | −0.61 ± 0.13ab | 13.07 ± 0.24d | 44.41 ± 2.69c | 6.39 ± 0.40b | 3.58 ± 1.12b | 40.81 ± 2.06c | 83.30 ± 5.49c | |
| AY13 | 5.00 ± 0.27ab | 73.70 ± 0.83c | 1.10 ± 0.22b | 43.20 ± 3.08c | 0.56 ± 0.00d | 81.61 ± 0.22b | 0.94 ± 0.13d | 9.56 ± 0.13b | 79.91 ± 2.32d | 8.00 ± 0.29c | 8.67 ± 0.64c | 57.15 ± 0.80d | 171.94 ± 5.63d | |
| Fat | AY5 | 6.56 ± 0.41b | 72.50 ± 2.40b | 1.02 ± 0.15a | 48.96 ± 2.58d | 0.18 ± 0.08b | 81.93 ± 0.37c | 1.22 ± 0.23d | 9.99 ± 0.32a | 88.53 ± 3.40e | 8.12 ± 0.23d | 13.11 ± 0.99d | 77.01 ± 2.51d | 175.82 ± 6.91d |
| AY6 | 5.13 ± 0.16a | 66.66 ± 7.85a | 1.00 ± 0.32a | 40.07 ± 1.61bc | 0.24 ± 0.04b | 80.41 ± 0.58c | 0.18 ± 0.02c | 9.69 ± 0.37a | 54.41 ± 5.33c | 7.39 ± 0.62c | 8.81 ± 3.70b | 49.66 ± 4.76b | 101.13 ± 10.01c | |
| AY7 | 5.03 ± 0.21a | 65.02 ± 0.74c | 0.80 ± 0.18a | 34.74 ± 1.52b | 0.02 ± 0.00a | 77.96 ± 0.87b | −0.57 ± 0.03b | 9.57 ± 0.60a | 31.86 ± 1.09b | 5.49 ± 0.26a | 2.07 ± 0.25a | 17.18 ± 0.40a | 85.50 ± 3.26b | |
| AY8 | 4.69 ± 0.18a | 63.77 ± 9.16d | 0.62 ± 0.14a | 27.66 ± 1.09a | 0.01 ± 0.00a | 71.41 ± 0.73a | −1.03 ± 0.07a | 9.85 ± 0.80a | 18.37 ± 0.70a | 3.97 ± 0.08b | 1.11 ± 0.02a | 16.98 ± 0.82a | 33.41 ± 1.54a | |
| AY13 | 5.00 ± 0.27a | 73.70 ± 0.83e | 1.10 ± 0.22a | 43.20 ± 3.08c | 0.56 ± 0.00c | 81.61 ± 0.22c | 0.94 ± 0.13d | 9.56 ± 0.13a | 79.91 ± 2.32d | 8.00 ± 0.29d | 8.67 ± 0.64c | 57.15 ± 0.80c | 171.94 ± 5.63d | |
| Sugar | AY9 | 4.86 ± 0.59a | 64.48 ± 5.77c | 1.12 ± 0.10a | 30.82 ± 0.48a | 0.02 ± 0.00a | 80.62 ± 0.07c | −0.07 ± 0.02b | 9.94 ± 0.09a | 32.52 ± 2.06a | 5.05 ± 0.23a | 1.35 ± 0.11a | 30.31 ± 1.50a | 61.50 ± 4.76a |
| AY10 | 5.13 ± 0.16a | 62.65 ± 1.58a | 1.07 ± 0.02a | 31.86 ± 0.86a | 0.01 ± 0.00a | 80.17 ± 0.43c | −0.19 ± 0.02b | 9.99 ± 0.20a | 32.17 ± 2.38a | 4.95 ± 0.23a | 1.29 ± 0.08a | 30.80 ± 2.12a | 59.85 ± 4.26a | |
| AY11 | 5.10 ± 0.27a | 65.30 ± 1.62b | 1.13 ± 0.05a | 34.51 ± 1.02ab | 0.06 ± 0.02b | 77.43 ± 0.32b | −0.86 ± 0.12a | 10.16 ± 0.97a | 34.75 ± 2.31a | 5.27 ± 0.41a | 1.47 ± 0.27a | 31.85 ± 1.97a | 62.22 ± 5.17a | |
| AY12 | 5.06 ± 0.12a | 75.40 ± 0.93d | 1.21 ± 0.20a | 36.88 ± 0.74b | 0.01 ± 0.00a | 75.75 ± 0.41a | −0.25 ± 0.02b | 10.19 ± 0.60a | 32.26 ± 1.67a | 5.10 ± 0.13a | 1.37 ± 0.07a | 29.25 ± 1.73a | 61.61 ± 5.33a | |
| AY13 | 5.00 ± 0.27a | 73.70 ± 0.83cd | 1.10 ± 0.22a | 43.20 ± 3.08c | 0.56 ± 0.00c | 81.61 ± 0.22d | 0.94 ± 0.13c | 9.56 ± 0.13a | 79.91 ± 2.32b | 8.00 ± 0.29a | 8.67 ± 0.64b | 57.15 ± 0.80b | 171.94 ± 5.63b | |
Values are expressed as means ± SD from 3 batches; significant differences at 95% of confidence among almond yoghurt (AY) was labelled with different letters in the same column within three group (Protein: effect of protein concentration, AY1-AY4 with AY13; Fat: effect of fat concentration, AY5-AY8 with AY13; Sugar: effect of sugar concentration, AY9-AY12 with AY13). AY13: Control almond yoghurt sample.
The firmness of sample AY5 (6.56 g) made from almond milk containing 7% of fat was the highest (p < 0.05) among all samples, which can be associated to the highest total solids (15.15%) in the formulation. In Table 4, the firmness shows a high positive correlation with the total solids value (r2 = 0.65, p < 0.05), WHC (r2 = 0.75, p < 0.05), and all particle size values include D4,3, D3,2, d(0.1), d(0.5) and d(0.9) with p < 0.05. This finding agrees with the results observed by Kaminarides et al. (2007), where yoghurt made from bovine milk with the highest total solids (17.00%) was the firmest. Further, for yoghurt with different protein concentrations (AY1-AY4), the increased firmness as shown in Table 3 were associated with the increased protein content. These results are in line with previous studies in tiger nut yoghurt by Kizzie-Hayford et al. (2016) and dairy yoghurt with fortified whey proteins by Sodini et al. (2002); the researcher describes the increased firmness due to the addition of proteins in associate with increased total solids. The increase in firmness may be due to higher protein and total solids content that contribute to the increase of cross-linkage of the gel network thus resulting in denser and firmer structure. Thus, both fat and protein contribute towards the firmness of the gel and as previously explained by Alzagtat and Alli (2002), the fine fat droplets interfered or modified the gelation property of protein in dispersions and therefore formed a hard gel with a smooth texture. The analysis of interactions also confirms this, associating the variability of the firmness values obtained for the various yoghurt samples to a significant (p < 0.01) interaction of fat and protein.
The adhesiveness represents the effort needed for jaw movement in the mouth due to stickiness (Siefarth et al., 2014). In Table 3, protein, fat and sugar increases (up to 6%, 7% and 7%, respectively), the adhesiveness value increases to 73.39 g s in AY4, 72.50 g s in AY5 and 75.40 g s in AY12. The obtained results can be explained by the highly positively correlation between adhesiveness and total solids (r2 = 0.80, p < 0.05) (Table 4). The total solids value is in association with the composition variation (Table 2), the different concentrations of protein, fat and sugar contents affects the strength of internal bonds and change in the three-dimensional gel structure (Siefarth et al., 2014).
The cohesiveness reflects the ability to form a uniform body in almond yoghurt. In Table 3, as the protein increased from 2% in AY1 to 6% in AY4, the cohesiveness value significantly (p < 0.05) increased from 0.70 to 1.17. In comparison, no significant difference was found in samples with fat contents variance (AY5-AY8) and with sugar contents variance (AY9-AY12). This result suggests that the interaction between protein and other components (fat and sugar) determines the strength of the three-dimensional network in almond yoghurt as reported previously by Kumar and Mishra (2003). Hence, it can be concluded that the textural properties of yoghurt are influenced mainly by the solid content (protein, fat, sugar) as well as their interactions in almond yoghurt matrix.
3.5. Particle size of almond yoghurt
The stability and texture of yoghurt depend mainly on the particle size (Sonne et al., 2014). The particle size parameters ((D4,3, D3,2, d(0.1), d(0.5) and d(0.9)) of the almond yoghurt gels as affected by protein, fat and sugar content are presented in Table 3. The Pearson correlation matrix (Table 4) shows that all of the particle size parameters were highly significant and positively (p < 0.05) correlated with the total solids, the concentration of fat, firmness, WHC, and viscosity properties of almond yoghurts.
From Table 3, as the protein content increased from 2% (AY1) to 6% (AM4), a significant increase (p < 0.05) in all tested particle size values was observed. D4,3 significantly increased from 32.76 μm in AY1 to 44.41 μm in AY4, and d(0.5) value increased from 30.62 μm in AY1 to 40.81 μm in AY4. It seems reasonable to speculate that the effect of protein on particle size depends upon protein concentration as the protein particles are involved in coagulation and gelation. This result is in agreement with the increased firmness in AY4 compared to AY1 (Table 3), as larger coagulated protein particles correspond to increased firmness (Jørgensen et al., 2019).
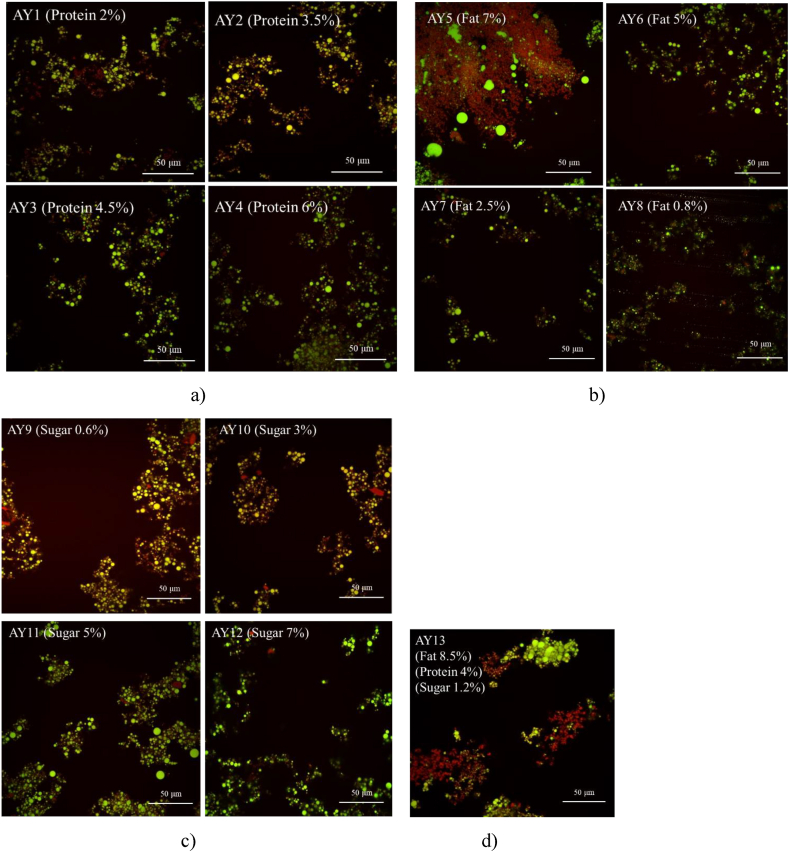
The values of D4,3, D3,2, d(0.1), d(0.5) and d(0.9) all significantly increased as the fat content increased from 0.8% in AY8 to 7% in AY5 (Table 3) with a significant positive correlation (p < 0.05) with the concentration of fat (Table 4). The large particle size that presented in high fat (7%) almond yoghurt (AY5) is probably due to the lack of sufficient surface-active substance (such as protein) to stabilise the fat droplets. According to Kalab (1985); Lee and Lucey (2010), the structure of emulsion (milk) is re-arranged and transferred into a yoghurt gel structure during fermentation, high concentration of fats in base milk allows fat globules to flocculate together and the newly exposed surface area are wrapped with proteins (surface-active substance) during the gelation process, thus resulting in large particles in the final yoghurt gels. This is further supported by the micrographs (Fig. 6) that shows large size/shape (irregular edge) fat droplets in the gel matrix of AY5 sample. The coalescence of fat droplets was also found by Chen et al. (2019) in a soy protein ice cream containing high fat. They associate the coalescence to the large molecular weight of the plant-sourced protein with restricted surface activity between the oil and water surface in base emulsion due to the slow diffusion rate and adsorption ability that affects the stability of fat droplets and causing partial coalescence of fat.
Fig. 6.
CLSM images of almond yoghurt with different protein, fat and sugar contents.
No significant difference in particle size was obtained in yoghurt samples with the addition of sugar (AY9, AY10, AY11, AY12) in Table 3. This may be associated with the dissolved state of the sugars, while protein and fat are present in a particulate state. However, sugar can assist in a uniform dispersion of protein particles in the aqueous phase (base milk), resulting in a good consistency of final almond yoghurt. Similar results have been reported by Dickinson and Merino (2002). They found that the particle size of an acid-induced protein-based gel did not change with sugar concentration <60% due to the dissolution of sugar in base milk without influencing the disruption of protein particles and fat droplets.
The control sample AY13 (4% protein, 8.5% fat and 1.2% sugar) shows a larger proportion of large particle size among all tested samples, except for AY5. That may be due to the high fat and protein contents in this sample. The significant effect of protein-fat interaction on the particle size of yoghurt gels is further confirmed by the statistically strong interaction (p < 0.01) obtained between protein and fat through the F-test computed in the ANOVA data. The large particles generated by high fat content could be minimised by regulating the protein content as fat covers the surface of the protein, the particle size in emulsions are influenced by the surface activity and other properties of protein fractions (Jayasundera et al., 2009).
3.6. Colour determination
The consideration of colour is vital for food production because it visually drives the acceptability, safety, ethics and sensory attributes of products by customers (Clydesdale, 1993). The CIELAB colour results of almond yoghurt with protein, fat and sugar concentration variation are presented in Table 3. The Pearson correlation results (Table 4) indicate that the L* and a* value positively correlated (p < 0.05) with the fat (%), while the b* values were significantly (p < 0.05) associated with the protein (%).
From statistical analysis, the interaction of protein and fat influences the lightness (L*) values of the almond yoghurt samples (p < 0.01). The L* significantly increased (p < 0.05) as the protein and fat content increased: from 77.81 to 81.73 as the protein content increased from 2% to 6%, and from 71.41 to 81.93 as the fat content increased from 0.8% to 7%. Further, the L* value positively correlated (r2 = 0.72, p < 0.05) with the particle size value (D3,2) (Table 4). The changes in lightness are related to the amount of protein and fat contents as the protein aggregation level and the particle size (e.g. protein particles, fat globules and fat and protein clusters) influence the light scattering of products, which is related to their ability to reflect light (Ferragut et al., 2015). This finding is supported by the particle size results in Table 3. For example, it is obvious that as the fat content reduced from 7% to 0.8%, the surface average diameter (D3,2) in AY5 and AY8 significantly dropped from 8.12 μm to 3.97 μm, indicating the lesser the surface area or lowers the number of particles exposed, the lesser the matter that can be scattered by light.
In terms of a* values, the control sample AY13 (8.5% fat, 4% protein and 1.2% sugar) and AY5 sample (7% fat, 5% sugar and 3.5% protein) showed significantly higher a* values of 0.94 and 1.22 respectively compared with other samples (Table 3). The r2 value of 0.82 (p < 0.05) in Table 4 further confirms this association between a* value and percentage of fat. Therefore, the higher redness values in samples AY5 and AY13 can be associated with the increased fat content as also previously reported by (Kaminarides et al., 2007).
The b* value highly correlated with the concentration of the protein (r2 = 0.95, p < 0.05) as shown in Table 4. The b* value significantly increased (p < 0.05) from 7.46 to 13.07 as the samples were fortified with protein up to 6% (Table 3). This increase in yellowness is related to the yellow colour of almond protein that increases as the protein content increases.
The colour results obtained in this study are different from that obtained by Bernat et al. (2015), who observed lower lightness and yellowness for the almond yoghurt produced in their research, possibly due to the variation in the formulation (e.g. proteins, fat, additives) and/or the variety of raw nuts used (Grasso et al., 2020).
3.7. Flow behaviour and viscosity
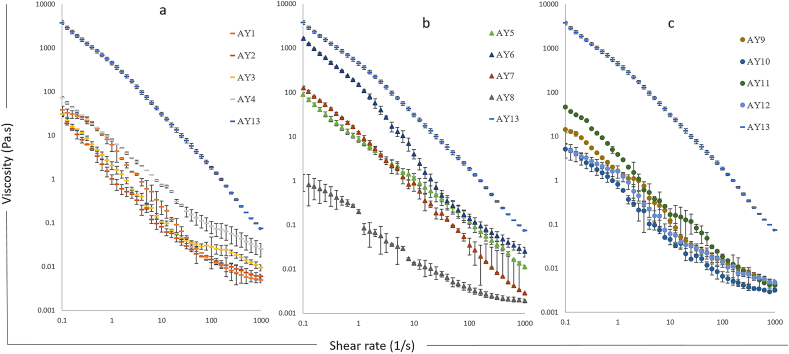
All the almond yoghurt samples showed shear thinning behaviour, and the viscosity decreased with an increased shear rate (Fig. 4). The apparent viscosity at a shear rate 50 s−1 of the various almond yoghurt samples are presented in Table 3.
Fig. 4.
Flow behaviour of almond yoghurt with different compositions. Values are averages of triplicate measurements. Error bars represent the standard deviation of average.
Fig. 4a presents the effect of protein concentrations on the flow behaviour of almond yoghurt. An increased in protein contents (2% for AY1 to 6% for AY4) resulted higher viscosity values. The apparent viscosity at a shear rate 50 s−1 significantly increased from 0.03 Pa s in AY1 to 0.11 Pa s in AY4 (Table 3), due to the stronger protein-protein interactions formed during fermentation. Moreover, the heat treatment during the preparation of almond yoghurt causes protein denaturation that resulted in an increased water-binding capacity (Devnani et al., 2020) thus producing higher viscosity yoghurt. Similar results were obtained by Kizzie-Hayford et al. (2016) who found that enriching base tigernut milk with whey protein (1–3%) improved the apparent viscosity of the final yoghurt. Marafon et al. (2011) also observed similar results, they associated the increased viscosity of yoghurt to increased total solids with protein fortification. Although the control sample AY13 contains less protein content (4%) than AY3 (4.5%) and AY4 (6%), it is significantly (p < 0.05) more viscous (0.56, 0.05 and 0.11 Pa s at 50 s−1, respectively) may be due to the high fat contents (8.5% in AY13, 4% in AY3 and AY4).
In Fig. 4b, as the fat content increased from 0.8% in AY8 to 5% in AY6, the viscosity significantly increased from 0.01 Pa s (AY8) to 0.24 Pa s (AY6) as observed at 50 s−1 in Table 3 with viscosity value positively correlated with the concentration of fats (r2 = 0.79, p < 0.05) in Table 4. The increase in viscosity with increased fat content was also observed by Geremias-Andrade et al. (2016), who reported that the viscosity of yoghurts is influenced by the concentration of fat and total solids in base milk, which is in line with the total solids (Table 2) and viscosity results (Fig. 4b) obtained in our study. The increased viscosity has a positive correlation (p < 0.05) with the particle size values (Tables 3 and 4), it can be inferred that more fat contents result in large particle formation that consequently affects the protein matrix in the gel network as more and large fat droplets entrapped in the three-dimensional protein matrix, and therefore increases the viscosity of fermented samples. However, for sample AY5 (7% fat) with fat content higher than 5%, the apparent viscosity at 50 s−1 (0.18 Pa s) was lower compared to AY6 (5%) although not significantly (p > 0.05). This probably is due to the high-fat content that leads to strong hydrophobic repulsion force among fat droplets, weakening the strength between protein to protein interactions, thereby leading towards a low viscosity gel (Gu et al., 2009).
In Fig. 4c and Table 3, an increase in sugar content from 0.6% in AY9 and 7% in AY12 did not significantly influence the viscosity of the almond yoghurt samples, possibly because sugar dissolves in base milk and did not affect the gel structure formation during fermentation like protein and fat (Dickinson and Merino, 2002).
For the apparent viscosity at 50 s−1, the interaction of protein and fat is the most influential factor (p < 0.01) among the explanatory model (data not shown). As mentioned previously, the gel network is affected by the fat droplets that coat the proteins, the interaction of protein and fat changes the gel matrix and thus affects the WHC, firmness, viscosity and gel strength. The particle size results discussed in section 3.5, and micrographs (Fig. 6) provide supporting evidence.
3.8. Gel properties
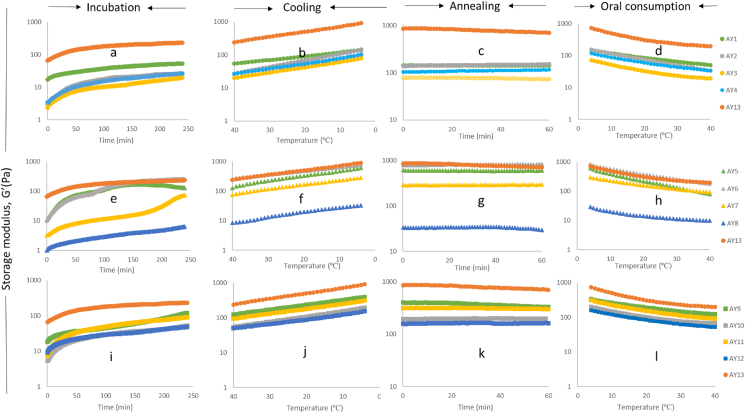
The measurement of elastic properties of acid gels is expressed as storage modulus (G’), reflecting the solid behaviour of gels. Fig. 5 presents the gelation behaviour of 13 yoghurt samples during incubation, cooling, annealing and the oral consumption stages.
Fig. 5.
Gelation behaviour of almond yoghurt with different compositions.
In the incubation stage (4-h at 40 °C), all samples showed a linear increase in G′ value as the concentration of solids (protein, fat and sugar) increased (Fig. 5a, e and i). This indicates extra protein-protein bonds were formed and protein matrix re-arranged in gel network during fermentation (Lee and Lucey, 2010). From Fig. 5a, increased protein contents up to 6% in AY4 resulted in higher G′ values. As described by Jørgensen et al. (2019), with increased protein contents in acid gels, more particles are present in the gel network that increased branching within the protein matrix, resulting in higher G′ value of the gels. The increased G′ with more fat contents (Fig. 5e) agrees with Sodini et al. (2004), who reported with increased fat contents, the connection between fat globules membrane and protein particles will be strengthened, thus showing strong gel strength in acid milk gels. The slight dropp in G’ for the sample AY5 at the end of the incubation period (Fig. 5e) could be due to the wall-slip effect that is possibly caused by the high-fat contents displaced from the protein matrix at the continually applied shear force and high temperature (40 °C) during measurement. This is in line with Gallegos and Franco (1999), who suggests the wall slip phenomenon may be associated with the increased temperature as large particle sizes are formed by the coalescence of fat droplets in food emulsions and gels. Plucinski et al. (1998) also observed the wall slip phenomenon in semi-solid material (mayonnaises), they ascribed it to the weak structure, as excess fluid may trim out by touch and may also correlate with the high volume of fat (may not be stabilised in the emulsion colloid matrix).
In the cooling stage (Fig. 5 b, f and j), all of the samples exhibit increased G′ as the temperature decreased from 40 °C to 4 °C. It was evident from Fig. 5f that variation in the fat concentration significantly changed the storage modulus of almond yoghurt compared to variation in protein concentration (Fig. 5b) and sugar concentration (Fig. 5j) as the temperature dropped. The results obtained in this study are supported by Rios et al. (2014), who summarised that the melting point and crystallisation point of fats are affected by the temperature that changes the textural characteristics (e.g. viscosity, firmness) of foods. In detail, fat globules become more rigid due to the change of fat crystallinity under lower temperature, allowing it to fit the porous in a protein matrix, thus reinforcing the gel strength in yoghurt gels (Sodini et al., 2004). The increased protein and fat contents increased the G′ of each almond yoghurt, mainly due to the increased total solids (Table 2). This result agrees with Marafon et al. (2011), where base milk standardised with more solid contents (e.g. protein, fibre) resulted in high G’ value in yoghurts and acid gels. The highly positive correlation (p < 0.05) between total solids and firmness, adhesiveness and WHC provides supporting evidence (Table 4).
During the 1-h annealing stage at 4 °C (Fig. 5c, g and k), all almond yoghurts showed relatively stable gel strength, indicating that the gel network is not affected significantly by the annealing stage. The last stage represents the almond yoghurt oral consumption process, from storage temperature at 4 °C to the yoghurt in-mouth temperature of 35 °C. As shown in Fig. 5 d, h and i, the G′ value of each almond yoghurt sample steadily decreased as the temperature increased. The G′ values showed an increasing trend with increased protein, fat and sugar contents mainly due to the increased total solids (Table 2), similar to the results obtained by Marafon et al. (2011). As is evident from Fig. 5h, the G’ value of AY5 (7% fat) dropped from over 500 Pa at 4 °C to around 100 Pa at 35 °C. This is due to the increased temperature that affects the hydrophobic interactions between protein-protein bindings and protein-fat connections thus weakening the structure of the gel network (Lucey, 2002).
3.9. Microstructure
The size and shape of the clusters (e.g. protein and fat aggregates, fat droplets, and protein particles) in the micrographs are clearly showing differences with compositional variation in almond yoghurt (Fig. 6).
In Fig. 6a, increased protein content from 2% in AY1 to 6% in AY4 tended to homogeneously entrap fat globules, while the size of fat globules did not change much. The protein fortification contributed to more compact protein-protein matrix formation and allowed a more homogeneous distribution of the fat globules within the protein matrix. In Fig. 6b, as the fat content decreased from 7% (AY5) to 0.8% (AY8), the size of the fat droplet significantly reduced and is trapped in the protein matrix more uniformly. Large fat droplets were observed in AY5. Fig. 6c shows that the increased sugar contents up to 7% no clear effect on the size of protein particles and fat globules were observed. Thus, the concentrations of suspended particles (e.g. protein and fat) has a significant impact on the microstructure of almond yoghurt, with higher protein and/or fat particles interacting and associating closely in the colloidal system and further strengthening the interaction between particles during fermentation (Sonne et al., 2014). This highlights the effects of protein and fat addition on the improvement of WHC, firmness, viscosity (Table 3), gelation behaviour (Fig. 5) and flow behaviour (Fig. 4) in almond yoghurts.
4. Conclusions
This research provides essential knowledge on how the major components (protein, fat, and sugar) of base almond milk determines the gel properties of the final almond yoghurt based on their physicochemical characteristics. The results revealed that an increase in proteins from 2% to 6% in base almond milk enhances the water holding capacity, firmness, cohesiveness, lightness, the flow and gelation behaviour of almond yoghurt. Increased fat contents in the range 0.8%–5% played a similar role on the gelling properties of almond yoghurt similar to increased protein contents as described above. However, increased fat contents up to 7% may have an adverse effect, where decreased viscosity, flow and gelation behaviour was observed compared to samples with lower fat content (5%, 2.5%). There was no significant difference observed in flow behaviour of almond yoghurt with sugar concentration variation. The improved adhesiveness, cohesiveness, and gel strength were mainly caused by the increased total solids that are in relation to the composition variance. The increased protein contents conferred a more stable pH during the 21 days storage period, while the increased sugar content resulted in a significant drop in pH.
The effects of composition (protein, fat and sugar) variation on the microstructure of almond yoghurts were observed by CLSM, where protein and fat addition resulted in a more compact gel structure, in accordance with the particle size, firmness, WHC, flow and gelation behaviour results. The physicochemical characterisation results of almond yoghurts with composition variance makes the production of reduced-fat and balanced-sugar contents of almond yoghurt with considerable proteins become possible. Further study in this area is being pursued to modify the gel structure of almond yoghurt with hydrolysed protein addition, and then evaluate the sensory attributes and monitor products’ shelf life.
Funding
Jia ZHAO acknowledges the award of a scholarship from the China Scholarship Council and the University of Queensland.
CRediT authorship contribution statement
Jia Zhao: Investigation, Data curation, Writing – original draft, Writing – review & editing. Bhesh Bhandari: Writing – review & editing. Claire Gaiani: Writing – review & editing. Sangeeta Prakash: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Akin Z., Ozcan T. Functional properties of fermented milk produced with plant proteins. LWT-Food Science and Technology. 2017;86:25–30. doi: 10.1016/j.lwt.2017.07.025. [DOI] [Google Scholar]
- Alzagtat A.A., Alli I. Protein-lipid interactions in food systems: a review. Int. J. Food Sci. Nutr. 2002;53(3):249–260. doi: 10.1080/09637480220132850. [DOI] [PubMed] [Google Scholar]
- AOAC . AOAC Intl. pv; Arlington, Va: 1995. Official Methods of Analysis of AOAC International. [Google Scholar]
- Bernat N., Chafer M., Chiralt A., Gonzalez-Martinez C. Development of a non-dairy probiotic fermented product based on almond milk and inulin. Food Sci. Technol. Int. 2015;21(6):440–453. doi: 10.1177/1082013214543705. [DOI] [PubMed] [Google Scholar]
- Bong D.D., Moraru C.I. Use of micellar casein concentrate for Greek-style yogurt manufacturing: effects on processing and product properties. J. Dairy Sci. 2014;97(3):1259–1269. doi: 10.3168/jds.2013-7488. [DOI] [PubMed] [Google Scholar]
- Chalupa-Krebzdak S., Long C.J., Bohrer B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018;87:84–92. doi: 10.1016/j.idairyj.2018.07.018. [DOI] [Google Scholar]
- Chen W., Liang G., Li X., He Z., Zeng M., Gao D., Qin F., Goff H.D., Chen J. Effects of soy proteins and hydrolysates on fat globule coalescence and meltdown properties of ice cream. Food Hydrocolloids. 2019;94:279–286. doi: 10.1016/j.foodhyd.2019.02.045. [DOI] [Google Scholar]
- Clydesdale F.M. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 1993;33(1):83–101. doi: 10.1080/10408399309527614. [DOI] [PubMed] [Google Scholar]
- Devnani B., Ong L., Kentish S., Gras S. Heat induced denaturation, aggregation and gelation of almond proteins in skim and full fat almond milk. Food Chem. 2020;325:126901. doi: 10.1016/j.foodchem.2020.126901. [DOI] [PubMed] [Google Scholar]
- Dhakal S., Liu C., Zhang Y., Roux K.H., Sathe S.K., Balasubramaniam V.M. Effect of high pressure processing on the immunoreactivity of almond milk. Food Res. Int. 2014;62:215–222. doi: 10.1016/j.foodres.2014.02.021. [DOI] [Google Scholar]
- Dickinson E., Merino L.M. Effect of sugars on the rheological properties of acid caseinate-stabilized emulsion gels. Food Hydrocolloids. 2002;16(4):321–331. doi: 10.1016/S0268-005X(01)00105-9. [DOI] [Google Scholar]
- Ferragut V., Valencia-Flores D.C., Perez-Gonzalez M., Gallardo J., Hernandez-Herrero M. Quality characteristics and shelf-life of ultra-high pressure homogenized (UHPH) almond beverage. Foods. 2015;4(2):159–172. doi: 10.3390/foods4020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos C., Franco J. Rheology of food, cosmetics and pharmaceuticals. Curr. Opin. Colloid Interface Sci. 1999;4(4):288–293. doi: 10.1016/S1359-0294(99)00003-5. [DOI] [Google Scholar]
- Geremias-Andrade I.M., Souki N., Moraes I.C.F., Pinho S.C. Rheology of emulsion-filled gels applied to the development of food materials. Gels. 2016;2(3) doi: 10.3390/gels2030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso N., Alonso-Miravalles L., O'Mahony J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods. 2020;9(3) doi: 10.3390/foods9030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Campbell L.J., Euston S.R. Effects of different oils on the properties of soy protein isolate emulsions and gels. Food Res. Int. 2009;42(8):925–932. doi: 10.1016/j.foodres.2009.04.015. [DOI] [Google Scholar]
- Jayasundera M., Adhikari B., Aldred P., Ghandi A. Surface modification of spray dried food and emulsion powders with surface-active proteins: a review. J. Food Eng. 2009;93(3):266–277. doi: 10.1016/j.jfoodeng.2009.01.036. [DOI] [Google Scholar]
- Jeske S., Zannini E., Arendt E.K. Past, present and future: the strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018;110:42–51. doi: 10.1016/j.foodres.2017.03.045. [DOI] [PubMed] [Google Scholar]
- Jørgensen C.E., Abrahamsen R.K., Rukke E.-O., Hoffmann T.K., Johansen A.-G., Skeie S.B. Processing of high-protein yoghurt – a review. Int. Dairy J. 2019;88:42–59. doi: 10.1016/j.idairyj.2018.08.002. [DOI] [Google Scholar]
- Kalab M. Microstructure of dairy foods. 2. Milk products based on fat. J. Dairy Sci. 1985;68(12):3234–3248. doi: 10.3168/jds.S0022-0302(85)81232-7. [DOI] [PubMed] [Google Scholar]
- Kaminarides S., Stamou P., Massouras T. Comparison of the characteristics of set type yoghurt made from ovine milk of different fat content. Int. J. Food Sci. Technol. 2007;42(9):1019–1028. doi: 10.1111/j.1365-2621.2006.01320.x. [DOI] [Google Scholar]
- Kizzie-Hayford N., Jaros D., Zahn S., Rohm H. Effects of protein enrichment on the microbiological, physicochemical and sensory properties of fermented tiger nut milk. LWT (Lebensm.-Wiss. & Technol.) 2016;74:319–324. doi: 10.1016/j.lwt.2016.07.067. [DOI] [Google Scholar]
- Korzendorfer A., Temme P., Schlucker E., Hinrichs J., Nobel S. Vibration-induced particle formation during yogurt fermentation-Effect of frequency and amplitude. J. Dairy Sci. 2018;101(5):3866–3877. doi: 10.3168/jds.2017-13905. [DOI] [PubMed] [Google Scholar]
- Kumar P., Mishra H. Effect of mango pulp and soymilk fortification on the texture profile of set yoghurt made from buffalo milk. J. Texture Stud. 2003;34(3):249–269. doi: 10.1111/j.1745-4603.2003.tb01060.x. [DOI] [Google Scholar]
- Kundu P., Dhankhar J., Sharma A. Development of non dairy milk alternative using soymilk and almond milk. Current Research in Nutrition and Food Science Journal. 2018;6(1):203–210. doi: 10.12944/crnfsj.6.1.23. [DOI] [Google Scholar]
- Lakshanasomya N., Danudol A., Ningnoi T. Method performance study for total solids and total fat in coconut milk and products. J. Food Compos. Anal. 2011;24(4–5):650–655. doi: 10.1016/j.jfca.2010.10.002. [DOI] [Google Scholar]
- Lee W., Lucey J. Formation and physical properties of yogurt. Asian-Australas. J. Anim. Sci. 2010;23(9):1127–1136. doi: 10.5713/ajas.2010.r.05. [DOI] [Google Scholar]
- Lucey J. Formation and physical properties of milk protein gels. J. Dairy Sci. 2002;85(2):281–294. doi: 10.3168/jds.S0022-0302(02)74078-2. [DOI] [PubMed] [Google Scholar]
- Makinen O.E., Wanhalinna V., Zannini E., Arendt E.K. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit. Rev. Food Sci. Nutr. 2016;56(3):339–349. doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- Marafon A.P., Sumi A., Alcântara M.R., Tamime A.Y., Nogueira de Oliveira M. Optimization of the rheological properties of probiotic yoghurts supplemented with milk proteins. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2011;44(2):511–519. doi: 10.1016/j.lwt.2010.09.005. [DOI] [Google Scholar]
- Martinez, G. P., Aracil, M. M., Vidagany, A. M., & Martinez, I. (2008). U.S. Patent Application No. 11/587,975.
- Meletharayil G.H., Patel H.A., Huppertz T. Rheological properties and microstructure of high protein acid gels prepared from reconstituted milk protein concentrate powders of different protein contents. Int. Dairy J. 2015;47:64–71. doi: 10.1016/j.idairyj.2015.02.005. [DOI] [Google Scholar]
- Miklavec K., Pravst I., Grunert K.G., Klopčič M., Pohar J. The influence of health claims and nutritional composition on consumers' yoghurt preferences. Food Qual. Prefer. 2015;43:26–33. doi: 10.1016/j.foodqual.2015.02.006. [DOI] [Google Scholar]
- Ng S.B.X., Nguyen P.T.M., Bhandari B., Prakash S. Influence of different functional ingredients on physical properties, rheology, tribology, and oral perceptions of no fat stirred yoghurt. J. Texture Stud. 2018;49(3):274–285. doi: 10.1111/jtxs.12307. [DOI] [PubMed] [Google Scholar]
- Nielsen S.S. Food Analysis Laboratory Manual. Springer International Publishing; 2017. Total carbohydrate by phenol-sulfuric acid method; pp. 137–141. [DOI] [Google Scholar]
- Ningtyas D.W., Bhandari B., Bansal N., Prakash S. Effect of homogenisation of cheese milk and high-shear mixing of the curd during cream cheese manufacture. International journal of dairy technology. 2018;71(2):417–431. doi: 10.1111/1471-0307.12482. [DOI] [Google Scholar]
- Plucinski J., Gupta R.K., Chakrabarti S. Wall slip of mayonnaises in viscometers. Rheol. Acta. 1998;37(3):256–269. doi: 10.1007/s003970050113. [DOI] [Google Scholar]
- Rinaldoni A.N., Campderrós M.E., Pérez Padilla A. Physico-chemical and sensory properties of yogurt from ultrafiltreted soy milk concentrate added with inulin. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2012;45(2):142–147. doi: 10.1016/j.lwt.2011.09.009. [DOI] [Google Scholar]
- Rios R.V., Pessanha M.D.F., Almeida P.F.d., Viana C.L., Lannes S.C.d.S. Application of fats in some food products. Food Sci. Technol. 2014;34(1):3–15. doi: 10.1590/S0101-20612014000100001. [DOI] [Google Scholar]
- Sethi S., Tyagi S.K., Anurag R.K. Plant-based milk alternatives an emerging segment of functional beverages: a review. J. Food Sci. Technol. 2016;53(9):3408–3423. doi: 10.1007/s13197-016-2328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefarth C., Tran T.B.T., Mittermaier P., Pfeiffer T., Buettner A. Effect of radio frequency heating on yoghurt, II: microstructure and texture. Foods. 2014;3(2):369–393. doi: 10.3390/foods3020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodini I., Lucas A., Oliveira M., Remeuf F., Corrieu G. Effect of milk base and starter culture on acidification, texture, and probiotic cell counts in fermented milk processing. J. Dairy Sci. 2002;85(10):2479–2488. doi: 10.3168/jds.S0022-0302(02)74330-0. [DOI] [PubMed] [Google Scholar]
- Sodini I., Remeuf F., Haddad S., Corrieu G. The relative effect of milk base, starter, and process on yogurt texture: a review. Crit. Rev. Food Sci. Nutr. 2004;44(2):113–137. doi: 10.1080/10408690490424793. [DOI] [PubMed] [Google Scholar]
- Sonne A., Busch-Stockfisch M., Weiss J., Hinrichs J. Improved mapping of in-mouth creaminess of semi-solid dairy products by combining rheology, particle size, and tribology data. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2014;59(1):342–347. doi: 10.1016/j.lwt.2014.05.047. [DOI] [Google Scholar]
- Vanga S.K., Raghavan V. How well do plant based alternatives fare nutritionally compared to cow's milk? J. Food Sci. Technol. 2018;55(1):10–20. doi: 10.1007/s13197-017-2915-y. [DOI] [PMC free article] [PubMed] [Google Scholar]