Abstract
Pesticides entering our body, either directly or indirectly, are known to increase the risk of developing neurodegenerative disorders. The pesticide-induced animal models of Parkinson's disease and Alzheimer's disease recapitulates many of the pathologies seen in human patients and have become popular models for studying disease biology. However, the specific effect of pesticides at the cellular and molecular levels is yet to be fully established. Here we investigated the cellular effect of three commonly used pesticides: DEET, fipronil and maneb. Specifically, we looked at the effect of these pesticides in the formation of stress granules and the concomitant translational arrest in a neuronal cell line. Stress granules represent an ensemble of non-translating mRNAs and appear in cells under physiological stress. Growing evidence indicates that chronic stress may covert the transient stress granules into amyloids and may thus induce neurodegeneration. We demonstrate here that all three pesticides tested induce stress granules and translation arrest through the inactivation of the eukaryotic initiation factor, eIF2α. We also show that oxidative stress could be one of the major intermediary factors in the pesticide-induced stress granule formation and that it is a reversible process. Our results suggest that prolonged pesticide exposure may result in long-lived stress granules, thus compromising the neuronal stress response pathway and leading to neurodegeneration.
Keywords: Translation arrest, Neurodegeneration, Neurotoxicity, Homeostasis, Neuronal survival
Highlights
-
•
Pesticides exposure increases the risk of developing neurodegenerative disorders.
-
•
Cellular effect of three common pesticides (DEET, fipronil and maneb) were tested.
-
•
All three induce stress granules and translation arrest via inactivation of eIF2α.
-
•
Compromised stress response may result in pesticide-induced neurodegeneration.
1. Introduction
Pesticide is a generic word that refers to various groups of compounds that act on insects, fungi and herbs in the agricultural sector and the common disinfectants used in household applications. These compounds may vary in their physicochemical properties, but most of them are associated with health and environmental issues [1]. Residual pesticides in the environment may also each the human system via water, vegetables, meat, and other dietary products [2,3]. The pesticides, thus entering the human body, may significantly affect physiology leading to abnormalities in the gastrointestinal, respiratory, endocrine, and/or reproductive systems, among others [[4], [5], [6]]. Intriguingly, pesticides are known to have a long-term impact on the neurological functions of humans. For example, gestational exposures to pesticides increase the risk of neurodevelopmental anomalies in humans [7]. Exposure to the residual amount of pesticides, such as maneb and paraquat, was found to increase the risk of developing Parkinson's disease [[8], [9], [10]]. Similar reports exist for Alzheimer's disease as well [[11], [12], [13]]. Indeed pesticide-induced animal models of Parkinson's disease and Alzheimer's disease recapitulate many of the pathologies seen in human patients and have become popular models to study [12,14]. However, the specific effect of pesticides at the cellular and molecular levels is yet to be fully established.
Despite the homeostatic mechanisms, cells in multicellular organisms, such as mammals, often experience fluctuations in the physicochemical components of their internal environment. To confer protection against such changes and to promote survival, eukaryotic cells have evolved a variety of cellular stress mechanisms, collectively called the stress response pathways [15]. One such response pathways are the formation of cytoplasmic stress granules (SGs), induced by a variety of stressors, such as heat shock and oxidative stress, to inhibit the translation process [16]. The SGs represent a transient ensemble of proteins bound to the mRNAs that are stalled from translation, and their formation is regulated by signalling pathways [16]. These structures, appearing as aggregated proteins during the stress, disassemble upon recovery, and the stalled mRNAs resume their translation [16,17]. Thus, SGs are thought to confer transient protection against rapid changes in the cellular milieu and that the SGs are a reliable readout of physiological stress that the cell is experiencing [17]. While SGs are seldom seen in physiologically normal cells, growing evidence suggests that chronic stress can result in persistent SGs and converting these structures into amyloids may lead to neurodegeneration [17,18]. Indeed, stable SGs are seen in the neurons affected with Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD) [19,20]. Thus, SGs could potentially be involved in the aetiology of pesticide-induced diseases like Alzheimer's and Parkinson's diseases. Here we investigated the effect of three commonly used pesticides (DEET, fipronil and maneb) for their ability to induce SGs and concomitant translation arrest in neuronal cells. We demonstrate that each of these chemicals induces SGs via the inactivation of eIF2α, and that pesticide-induced oxidative stress could be one of the contributor factors for SGs formation.
2. Materials and methods
2.1. Reagents and antibodies
The following antibodies were used: anti-γ-tubulin (Cat #: T5326; IB, 1:10000; Sigma-Aldrich Pvt Ltd, India), anti-G3BP1 (Cat #: ab56574; IC, 1:500; Abcam), anti-TIAR (Cat #: D32D3; IC, 1:100), anti-eIF2ɑ (Cat #: L57A5; IB, 1:1000), and anti-P-eIF2ɑ (Cat #: 119A11; IB, 1:1000) (all from Cell Signalling Technology Inc, USA). The secondary antibodies were from Jackson ImmunoResearch Inc, USA. All fine chemicals, including the pesticides (DEET [Cat #: D100951], fipronil [Cat #: 16785], maneb [Cat #: 45554]) used were procured form Sigma-Aldrich Pvt Ltd, India.
2.2. Cell culture, cell death assay, immunocytochemistry, and fluorescence in situ hybridization (FISH)
Neuro2A cells were purchased from the National Centre for Cell Science (Pune, India) and were grown at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum and 1% antibiotic solution (Sigma-Aldrich India Pvt Ltd). Cells grown on gelatin-coated glass coverslips were processed for immunofluorescence staining as described [21,22]. Briefly, for immunostaining, cells were fixed with formaldehyde (4%), permeabilized with Triton X-100 (0.05%), incubated with blocking buffer (0.5% fish gelatin and 0.5% equine serum) followed by incubation with the primary and secondary antibodies as detailed as described previously [21,22]. For the RNA FISH, fixed cells were denatured at 73 °C in a denaturation buffer (70% formamide, 2 × SSC, 50 mM sodium phosphate, pH 7.0), and the cells were dehydrated in graded alcohol series. Biotinylated probe (1 μM) was denatured and hybridized overnight at 42 °C as described previously [23]. Fluorescence images were captured using a fluorescence microscope (Axio observer 2.0, Carl Zeiss) with Apotome module, and images were processed with Zen blue software using 40x oil objective. MTT assay, to measure cell death, was carried out essentially as described previously [21].
2.3. Immunoblotting
Cell lysis, sample preparation immunoblotting methods were essentially as described earlier [21,22]. Briefly, protein samples were size separated on 8% SDS-PAGE and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% skimmed milk powder and incubated with primary and secondary antibodies. Immunoreactive bands were visualized using a chemiluminescence detection kit (SuperSignal West PICO, Thermo Scientific). Digital images of the blot were acquired, and the signal intensity on the bands was calculated using the Image Lab software (Bio-Rad, India).
2.4. Free radical measurement and TBARS assay
The concentration of free radicals was measured using 2′,7’ – dichlorofluorescin diacetate (DCFDA) as described earlier [22]. Briefly, cells were treated with 10 μM DCFDA for 1hr, thereafter the pesticide (desired concentration) was added to the medium and incubated for 1 h. The medium was then washed off with 1x phosphate-buffered saline (PBS), and the fluorescence was measured using Spectramax M3 Multi-Mode Microplate Reader (Molecular Devices, USA). The thiobarbituric acid reactive substance (TBARS) assay was carried out as previously described [24]. Briefly, cells treated with the pesticide (1 h) were harvested 1xPBS, lysed and 200 μl of the lysate was mixed with 1.5 ml of trichloroacetic acid and 1.5 ml of thiobarbituric Acid (TBA) and 200 μl of 8% sodium dodecyl sulfate (SDS). This mixture was put in a boiling water bath for 45 min, followed by incubation on ice for 10 min. Adducts formed were extracted into 3 ml of butanol. The upper layer thus extracted was read at 532 nm on Spectramax M3 Multi-Mode Microplate Reader (Molecular Devices, USA) and quantified as malonaldehyde equivalents.
2.5. Statistical analysis
Cells containing two or more SGs were considered as SG-positive cells, and a minimum of 100 cells per coverslip were counted for each experimental set. Each experiment was performed in triplicates, and the average of each experiment was calculated and plotted. Standard deviations for the observed values were calculated, and statistical significance was analysed with two-tailed unpaired t-test (*/#P < 0.05, **/##P < 0.01, ***/###P < 0.001, ****/####P < 0.0001) using the GraphPad software.
3. Results
3.1. Higher concentrations of DEET, fipronil and maneb induce the transient assembly of stress granules in Neuro2A cells
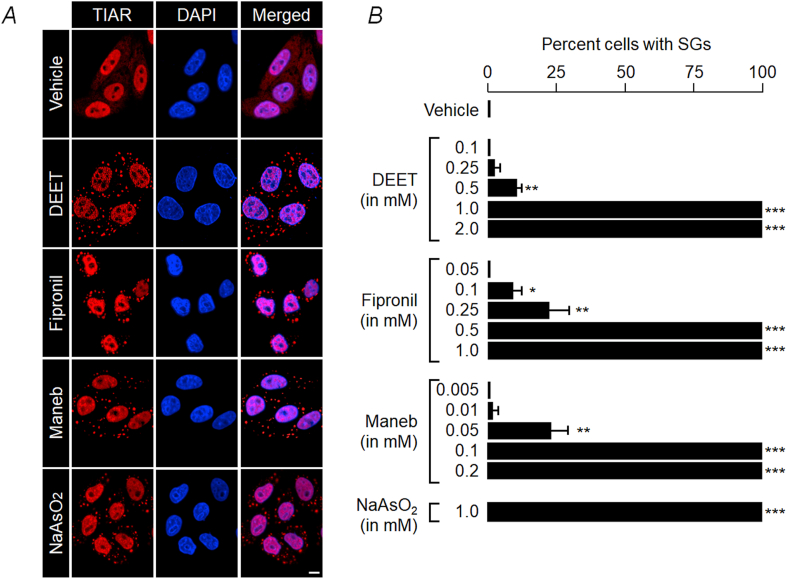
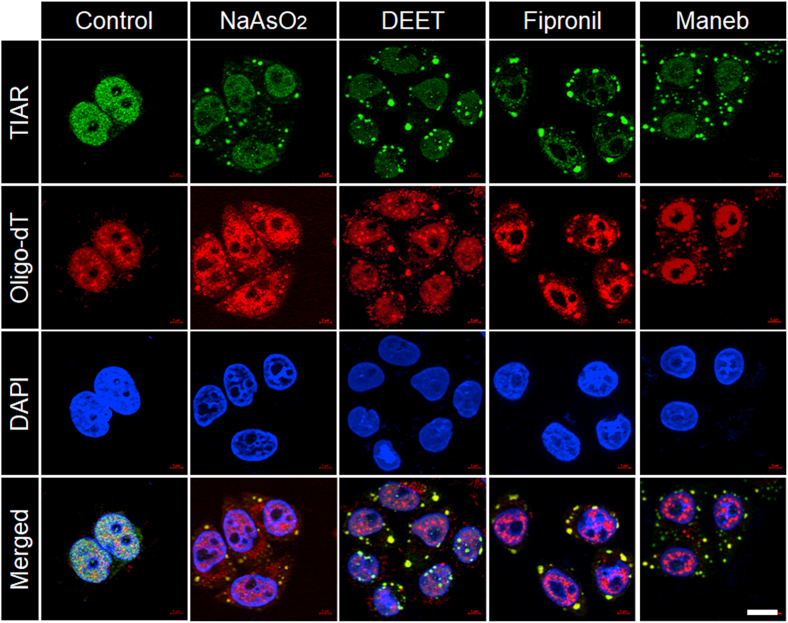
To test our hypothesis that higher concentrations of pesticides and fungicides might induce transient translational arrest in the neurons, we have selected three commonly used pesticides: (i) N, N-Diethyl-meta-toluamide (DEET), one of the most common ingredients in insect repellents and is known to be neurotoxic [25]; (ii) fipronil, a broad-spectrum insecticide; and (iii) maneb, a commonly used fungicide and widely used in agricultural produce. Administration of fipronil and maneb is known to cause to induce Parkinson disease phenotype in rodent models [26,27] and is known to be a risk factor for Parkinson's disease in humans [8,9]. For our cell biology assays, we have tested varying concentrations of these chemicals (DEET: 0.1, 0.25, 0.5, 1.0 and 2.0 mM; fipronil: 0.05, 0.1, 0.25, 0.5 and 1 mM; and maneb: 0.005, 0.01, 0.05, 0.1 and 0.2 mM) in the cultured neuroblastoma cell line Neuro2A (30 min for DEET and 1 h for fipronil and maneb) and looked for their ability to induce stress granule (SGs) using an antibody against TIA-1 related protein (TIAR), an established marker for SGs [28]. As shown in Fig. 1, all three chemicals induced TIAR-positive cytoplasmic SGs in the treated cells. The size and distribution of these SGs were similar to those induced by sodium arsenite, an established inducer of SGs (Fig. 1). Each of these pesticides showed a minimum threshold level for inducing the SGs in 100% cells (Fig. 1), and intriguingly, the threshold concentrations exhibited <20% cell death in an MTT assay (Supplementary Fig. S1). Therefore, for all subsequent experiments, the minimum concentration of the chemical that induced SGs in nearly 100% of the cells was used (DEET: 1.0 mM; fipronil: 0.5 mM; maneb: 0.1 mM). To further confirm that the TIAR-positive cytoplasmic induced by the three pesticides are indeed SGs, we used fluorescence in situ hybridization (FISH) with oligo-dT probes to colocalize the poly(A)-containing mRNAs with the TIAR-positive SGs. As shown in Fig. 2, the oligo signals showed complete co-localization with the TIAR-positive cytoplasmic granules in cells treated with each of the three pesticides. The pattern was identical to that of the positive control (sodium arsenite). Similarly, another established marker for SGs, the G3BP1, also showed induction of SGs upon the treatment with the pesticides (Supplementary Fig. S2).
Fig. 1.
Pesticides induce stress granules in Neuro2A cells: (A) Representative confocal immunofluorescence images showing TIAR-positive cytoplasmic stress granules in Neuro2A cells treated (for 30 min) with DEET (1 mM), fipronil (0.5 mM) or maneb (0.1 mM) for 1 h, as indicated. Treatment with the vehicle and sodium arsenite (NaAsO2; 1 mM) served as a negative and positive control, respectively. Nuclei were stained with DAPI (scale bar, 10 μm). (B) Bar diagram showing the percentage of cells showing TIAR-positive stress granules upon treatment with the indicated chemical and its concentration. Data shown are mean ± s.d (100 cells per set; N = 3). *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed, unpaired Student's t-test).
Fig. 2.
Immuno-FISH images showing double positivity for the poly(A)-containing mRNAs with the TIAR-positive stress granules induced by the pesticides in Neuro2A cells: Representative confocal immunofluorescence images showing TIAR-positive cytoplasmic stress granules colocalizing with the signals for the oligo-dT probes for each of the chemicals used, as indicated. Nuclei were stained with DAPI (scale bar, 10 μm).
3.2. Assembly and disassembly of pesticide-induced SGs
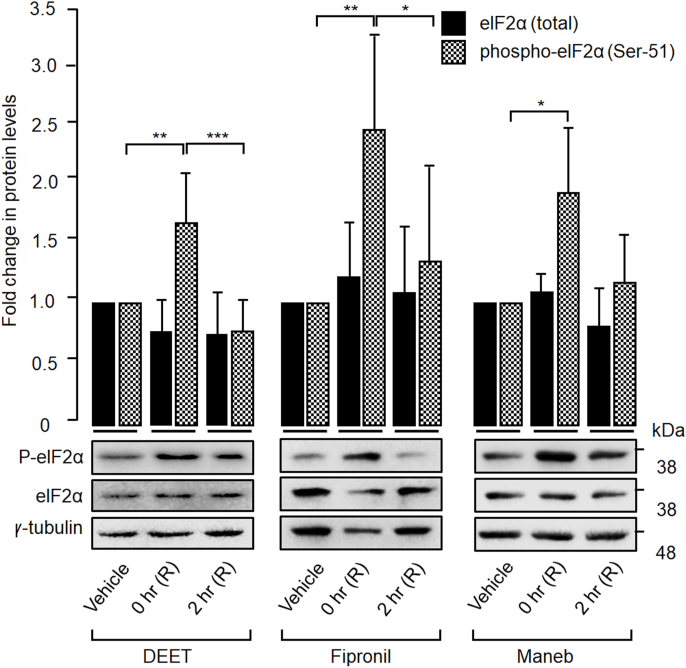
SGs are known to be in a dynamic equilibrium with actively translating polysomes [16,29]. To check if the SGs induced by the three pesticides do show such a property, Neuro2A cells were double treated with one of the three pesticides and cycloheximide (0.5 μg/ml) for 30 min, and the TIAR-positive SGs were visualized. Cycloheximide is known as a translation elongation inhibitor, and hence its addition would prevent stress granule assembly [30]. As expected, cycloheximide treatment led to the loss of SGs in cells treated with DEET, fipronil or maneb, suggesting that TIAR-positive cytoplasmic granules induced by the three pesticides are bona fide SGs (Supplementary Fig. S3). SGs are transient structures that disassemble during stress recovery. To test if the SGs induced by DEET, fipronil or maneb represent such transient structures, cells treated with the pesticides were restored to normal medium, and the disassembly of the SGs was followed. As shown in Supplementary Figs. S4–S6, there was a significant reduction in the number of cells with SGs during 2–4 h of recovery, thus confirming the transient nature of the SGs induced by the pesticides. One of the mediators of the translation arrest of mRNA sequestered on the SGs is the eukaryotic initiation factor-2 (eIF2α) [31]. During translation, the eIF2α helps in the recruitment of initiator tRNA-Met to the translation initiation codon of the mRNA. However, under stress conditions, the phosphorylation of Ser-51 residue of elF2α renders it inactive and thus, translation is arrested in the mRNA-tRNA-Met complex sequestered to the stress granules [15,31]. To check if the phosphorylation of elF2α is required for the formation of SGs induced by the pesticides, we measured the relative levels of phospho-elF2α by immunoblotting in the lysates of cells treated with the chemicals. As shown in Fig. 3, a significant increase in the phospho-elF2α levels was observed in the cells treated with each of the chemicals. However, the phospho-elF2α levels were restored within 2 h during the recovery phase (Fig. 3), suggesting the resumption of translation after chemical washout. Taken together, these data suggest that DEET, Fipronil or maneb induce SGs via the canonical pathway and that it is a reversible process.
Fig. 3.
Pesticide treatment increases the phospho-elF2α levels in Neuro2A cells: Representative immunoblots (lower panel) showing the relative levels of total and phosphorylated (Ser-51) forms of elF2α in cells treated with indicated chemicals, and the cells were arrested at 0 or 2 h of recovery (R). Vehicle treated cells served as control and tubulin as the loading control. The bar diagram (upper panel) total and phosphorylated (Ser-51) forms of elF2α as measured by densitometric quantification of signal intensities in the immunoblots. Signal intensities were normalized for tubulin, and the values obtained for the vehicle-treated group was considered as 1. All data show mean ± s.d. (n = 3). *P < 0.05 (two-tailed, unpaired Student's t-test).
3.3. DEET, fipronil and maneb induce oxidative stress in neuronal cells
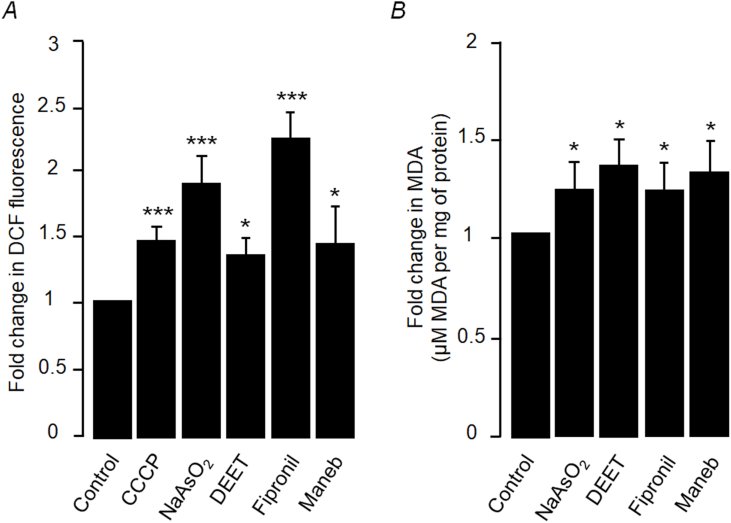
Oxidative stress is one of the factors that induce the formation of SGs and the transient translational arrest via the phosphorylation of elF2α [31,32]. Indeed, sodium arsenite, the most widely used chemical for the inductions of SGs, is known to activate oxidative stress and the SGs via elF2α [33,34]. Further, antioxidant supplementation is known to inhibit sodium arsenite-induced SG formation [35]. Therefore, we were curious to check whether the addition of DEET, fipronil or maneb leads to oxidative stress in the Neuro2a cells or not. For this, we measured the reactive oxygen species (ROS) level by treating the live cells with 2′,7′-dichlorofluorescein diacetate (DCFDA). The non-fluorescent DCFDA is oxidized by ROS into a fluorescent dye (2′,7′-dichlorofluorescein; DCF), and hence the fluorescence generated is directly proportional to the cellular ROS levels [36]. As shown in Fig. 4A, each of the pesticides tested led to a significant increase in the fluorescence as compared to the control, suggesting an increase in the ROS levels. We have also used the thiobarbituric acid reactive substances (TBARS) assay to measures the concentration of free radicals levels, a readout of oxidative stress [37]. As shown in Fig. 4B, there was a significant increase in the level of malondialdehyde, a readout oxidative stress, in the cells treated with the pesticides. Taken together, these results confirm that DEET, fipronil and maneb induce oxidative stress in neuronal cells.
Fig. 4.
Pesticide-induced oxidative stress in Neuro2A cells: (A) Bar diagram showing a relative difference in the DCF fluorescence, as a readout of ROS levels, in cells treated with the chemicals as indicated. The value obtained for the untreated cells (control) was considered as 1. CCCP and sodium arsenite (NaAsO2) served as positive controls. (B) Bar diagram showing the levels of TBARS in cells treated with the indicated chemicals. For both A and B, the values obtained in vehicle-treated group was considered as 1. Each bar represents mean ± s.d. (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed, unpaired Student's t-test).
4. Discussion
Using cell biological assays, we have demonstrated that three of the common pesticide that we tested induce SGs and translation arrests in a neuronal cell line and that this effect could most likely be due to the oxidative stress that they generate. Of these three pesticides, fipronil and maneb are known to induce Parkinson's disease in animal models [26,27]. Similarly, exposure to pesticides also results in a neurodegenerative condition in humans [[8], [9], [10], [11], [12], [13]]. Our results suggest that chronic exposure to pesticides may have a long-term impact on neuronal translation, eventually leading to cell death.
Cells, especially the nondividing cells such as neurons, experience a variety of stress during their lifetime, and they activate a variety of response pathways to survive during the stress [15]. Most of such stress conditions disrupt cellular proteostasis and promote protein misfolding. Cells respond to such conditions by activating a range of cellular pathways to fix the physiological imbalances and promote cell survival. The formation of SGs is one such cell survival pathway wherein cells transiently stall translation to minimize a load of unfolded proteins in the cellular milieu [[16], [17], [18]]. In this regard, our findings that all three pesticides tested induce SGs offer novel insight into the pathomechanisms through which pesticides may cause neurodegeneration. While the concentration of the pesticides that we have used in the study could be way too high as compared to the concentration that is seen in disease subjects [[9], [10], [11], [12], [13]], it should be noted here that our in vitro exposure was limited to 1 h while patients may have exposure spanning months and years, and secondly, our assay system used only one pesticide while in nature, the population may be exposed to multiple pesticides/toxins albeit each one at a lower level. Thus, longer exposure and the additive effect of multiple toxins could have a profound effect on neurons and may alter their translation machinery. In this regard, it is intriguing to note that while SGs represent a transient short-term cytoprotective mechanism, chronic stress is known to compromise the function of the SGs and result in neuronal death [38]. Alternatively, prolonged pesticide exposure may result in long-lived SGs may eventually transform into insoluble fibre aggregates, thus compromising the stress response pathway and triggering the cell death pathway [17,18]. Clearly, further work is required to understand the specific effect of pesticides in neurodegeneration. Our findings provide possible evidence for a role for SGs in pesticide-induced neurodegeneration.
Funding
This work was supported by the Young Scientist Grant from the Council of Science & Technology, Government of Uttar Pradesh, to PB, and the Tata Innovation Fellowship from the Department of Biotechnology, Government of India [Grant Number BT/HRD/35/01/01/2017], to SG.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2021.101110.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nicolopoulou-Stamati P., Maipas S., Kotampasi C., et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nougadère A., Sirot V., Kadar A., et al. Total diet study on pesticide residues in France: levels in food as consumed and chronic dietary risk to consumers. Environ. Int. 2012;45:135–150. doi: 10.1016/j.envint.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Blaznik U., Yngve A., Eržen I., Hlastan Ribič C. Consumption of fruits and vegetables and probabilistic assessment of the cumulative acute exposure to organophosphorus and carbamate pesticides of schoolchildren in Slovenia. Publ. Health Nutr. 2016;19(3):557–563. doi: 10.1017/S1368980015001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization & United Nations Environment Programme . World Health Organization; 1990. Public Health Impact of Pesticides Used in Agriculture.https://apps.who.int/iris/handle/10665/39772 [Google Scholar]
- 5.Mostafalou S., Abdollahi M. Pesticides and human chronic diseases: evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013;268(2):157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Nicolopoulou-Stamati P., Maipas S., Kotampasi C., et al. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W., Carmichael S.L., Roberts E.M., et al. Residential agricultural pesticide exposures and risk of neural tube defects and orofacial clefts among offspring in the San Joaquin Valley of California. Am. J. Epidemiol. 2014;179(6):740–748. doi: 10.1093/aje/kwt324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costello S., Cockburn M., Bronstein J., Zhang X., Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am. J. Epidemiol. 2009;169(8):919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A., Costello S., Cockburn M., et al. Parkinson's disease risk from ambient exposure to pesticides. Eur. J. Epidemiol. 2011;26(7):547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwer M., Huss A., van der Mark M., et al. Environmental exposure to pesticides and the risk of Parkinson's disease in The Netherlands. Environ. Int. 2017;107:100–110. doi: 10.1016/j.envint.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Yan D., Zhang Y., Liu L., Yan H. Pesticide exposure and risk of Alzheimer's disease: a systematic review and meta-analysis. Sci. Rep. 2016;6:32222. doi: 10.1038/srep32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang B.L. Neuropathological mechanisms associated with pesticides in Alzheimer's disease. Toxics. 2020;8(2):21. doi: 10.3390/toxics8020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syeda T., Cannon J.R. Environmental exposures and the etiopathogenesis of Alzheimer's disease: the potential role of BACE1 as a critical neurotoxic target. J. Biochem. Mol. Toxicol. 2021;35(4) doi: 10.1002/jbt.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Gamal M., Salama M., Collins-Praino L.E., et al. Neurotoxin-induced rodent models of Parkinson's disease: benefits and drawbacks. Neurotox. Res. 2021;39(3):897–923. doi: 10.1007/s12640-021-00356-8. [DOI] [PubMed] [Google Scholar]
- 15.Goenka A., Parihar R., Ganesh S. In: Asea A.A.A., Kaur P., editors. vol. 15. Springer; 2018. Heat shock-induced transcriptional and translational arrest in mammalian cells; pp. 267–280. (Heat Shock Proteins and Stress). [Google Scholar]
- 16.Wolozin B., Ivanov P P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019;20(11):649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Advani V.M., Ivanov P. Stress granule subtypes: an emerging link to neurodegeneration. Cell. Mol. Life Sci. 2020;77(23):4827–4845. doi: 10.1007/s00018-020-03565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F., Li J., Fan S., et al. Targeting stress granules: a novel therapeutic strategy for human diseases. Pharmacol. Res. 2020;161:105143. doi: 10.1016/j.phrs.2020.105143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shukla S., Parker R. Hypo- and hyper-assembly diseases of RNA-protein complexes. Trends Mol. Med. 2016;22(7):615–628. doi: 10.1016/j.molmed.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderweyde T., Apicco D.J., Youmans-Kidder K., et al. Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity. Cell Rep. 2016;15(7):1455–1466. doi: 10.1016/j.celrep.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upadhyay M., Bhadauriya P., Ganesh S. Heat shock modulates the subcellular localization, stability, and activity of HIPK2. Biochem. Biophys. Res. Commun. 2016;472(4):580–584. doi: 10.1016/j.bbrc.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal S., Ganesh S. Perinuclear mitochondrial clustering, increased ROS levels, and HIF1 are required for the activation of HSF1 by heat stress. J. Cell Sci. 2020;133(13):jcs245589. doi: 10.1242/jcs.245589. [DOI] [PubMed] [Google Scholar]
- 23.Goenka A., Sengupta S., Pandey R., et al. Human satellite-III non-coding RNAs modulate heat-shock-induced transcriptional repression. J. Cell Sci. 2016;129(19):3541–3552. doi: 10.1242/jcs.189803. [DOI] [PubMed] [Google Scholar]
- 24.Park J.H., Park Y.S., Lee J.B., et al. Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. J. Appl. Toxicol. 2016;36(1):10–23. doi: 10.1002/jat.3136. [DOI] [PubMed] [Google Scholar]
- 25.Swale D.R., Sun B., Tong F., Bloomquist J.R. Neurotoxicity and mode of action of N, N-diethyl-meta-toluamide (DEET) PloS One. 2014;9(8) doi: 10.1371/journal.pone.0103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tinakoua A., Bouabid S., Faggiani E., et al. The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat. Neuroscience. 2015;311:118–129. doi: 10.1016/j.neuroscience.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Park J.H., Park Y.S., Koh H.C. Progressive loss of nigrostriatal dopaminergic neurons induced by inflammatory responses to fipronil. Toxicol. Lett. 2016;258:36–45. doi: 10.1016/j.toxlet.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Kedersha N.L., Gupta M., Li W W., et al. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 1999;147(7):1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler J.R., Matheny T., Jain S., Abrisch R., Parker R. Distinct stages in stress granule assembly and disassembly. Elife. 2016;5 doi: 10.7554/eLife.18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann S., Cherkasova V., Bankhead P., et al. Translation suppression promotes stress granule formation and cell survival in response to cold shock. Mol. Biol. Cell. 2012;23(19):3786–3800. doi: 10.1091/mbc.E12-04-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kedersha N.L., Gupta M., et al. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 1999;147(7):1431–1442. doi: 10.1083/jcb.147.7.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian X.J., Gallouzi I.E. Oxidative stress increases the number of stress granules in senescent cells and triggers a rapid decrease in p21waf1/cip1 translation. J. Biol. Chem. 2009;284(13):8877–8887. doi: 10.1074/jbc.M806372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu L., Han A.P., Chen J.J. Translation initiation control by heme-regulated eukaryotic initiation factor 2alpha kinase in erythroid cells under cytoplasmic stresses. Mol. Cell Biol. 2001;21(23):7971–7980. doi: 10.1128/MCB.21.23.7971-7980.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwen E., Kedersha N., Song B., et al. Heme-regulated inhibitor kinase-mediated phosphorylation of eukaryotic translation initiation factor 2 inhibits translation, induces stress granule formation, and mediates survival upon arsenite exposure. J. Biol. Chem. 2005;280(17):16925–16933. doi: 10.1074/jbc.M412882200. [DOI] [PubMed] [Google Scholar]
- 35.Basu M., Courtney S.C., Brinton M.A. Arsenite-induced stress granule formation is inhibited by elevated levels of reduced glutathione in West Nile virus-infected cells. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hempel S.L., Buettner G.R., O'Malley Y.Q., et al. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2',7'-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 1999;27(1–2):146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 37.Aguilar Diaz De Leon J., Borges C.R. Evaluation of oxidative stress in biological samples using the thiobarbituric acid reactive substances assay. JoVE. 2020:159. doi: 10.3791/61122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shelkovnikova T.A., Dimasi P., Kukharsky M.S., et al. Chronically stressed or stress-preconditioned neurons fail to maintain stress granule assembly. Cell Death Dis. 2017;8(5) doi: 10.1038/cddis.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.