Highlights
-
•
MRPs were obtained by heating camellia seed meal hydrolysates, and different sugars.
-
•
The ratio of essential amino acids in R-MRPs was increased and the antioxidant activity was the highest.
-
•
MR could improve the flavor and antioxidant activity of camellia seed meal.
Keywords: Camellia seed meal, Maillard reaction, Maillard reaction products, Protein structure, Antioxidant
Abstract
In the present study, camellia seed meal Maillard reaction products (MRPs) were prepared using camellia seed meal protein as a raw material. The effects of MR on protein structure and volatile components of camellia seed meal were investigated by fluorescence, UV absorption, infrared spectroscopy, and gas chromatography-mass spectrometry. Not only the change of amino acid content in MRPs, but also the antioxidant capacity of MRPs and the antioxidant capacity after in vitro digestion were determined. Our result showed that the ratio of essential amino acids in R-MRPs was increased and the antioxidant activity was the highest. For the potential of MRPs as flavoring, our sensory evaluation results showed improved flavor and antioxidant activity of camellia seed meal after MR which can be used as flavoring agents at industrial level.
Nomenclature
- Abbreviations
The full name
- MR
Maillard reaction
- MRPs
Maillard reaction products
- UV
Ultraviolet
- HCS
Hydrolysates
- DS
Degree of substitution
- MW
Molecular weight
- ROS
Reactive oxide species
- G-MRPs
Glucose MRPs
- F-MRPs
Fructose MRPs
- R-MRPs
Ribose MRPs
- X-MRPs
Xylose MRPs
- FT-IR
Fourier transform infrared
- GC–MS
Gas chromatogram-mass spectrometry
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- ABTS
2,2′-Azinobis-(3-ethylbenzthiazoline-6-sulphonate)
- VC
Ascorbic Acid
1. Introduction
The species Camellia oleifera Abel belongs to genus Camellia which is native to East Asia. Camellia seeds have been utilized for more than 1000 years in China and camellia oil serves as a major vegetable cooking oil in South China. Camellia seed meal as a co-product obtained during the oil production, if directly discarded, may cause environmental pollution and economic losses and other challenges (Guo, Tong, Ren, Tu, & Li, 2018).
Camellia seed meal is enriched in protein, sugar, crude fiber, and minerals, etc. (Shi, Wu, Jin, & Wang, 2020). It consists of 15% crude protein, 40% sugar, 5% crude fat, 6% ash, 10% saponin, and 2% tannin (Guo, Guo, Wu, Lu, & Zhang, 2020). At present, camellia seed meal is mainly used in animal feed. In addition, because of its high protein content, camellia seed meal can also be used as an important protein reservoir (Guo, Tong, Ren, Tu, & Li, 2018). In one of recent studies, three novel peptides with anti-diabetic potential were separated from the hydrolysates of camellia seed meal (Shang et al.,2020).
In the process of food processing, the reaction rate of the corresponding MR is affected by the pH value of the reaction environment, the temperature of the reaction system and other factors (Nooshkam, Varidi, & Bashash, 2018). These proteins are broken down into diverse kinds of peptides and different amino acids during the MR, and as the MR progresses, they produce a meat-like taste (Hou et al., 2017). It has been reported that the Maillard peptide (1000–5000 Da) produced during the MR process can enhance umami, such as umami solution umami. In addition, studies have shown that appropriate addition of cysteine can control the browning of MRPs and boost its taste and continuity (Zhao et al., 2018). MR is a succession of reactions imparting food flavor and color during food processing and storage (Jaeger, Janositz, & Knorr, 2010). Different kinds of sugars and amino mixtures react differently, resulting in different structures and types of final products. As MR can improve the structural characteristics of proteins, the fluorescence spectrum, Ultraviolet absorption spectrum, and Fourier transform infrared (FT-IR) spectrum of MRPs were observed by relevant instruments (Meng et al., 2019). In addition, it has been shown that MRPs have remarkable antioxidant potential (Han et al., 2018). One previous study have shown that the antioxidant activity of MRPs which molecular weight is less than 1 kDa is about eight times to that of MRPs with molecular weight greater than 50 kDa (Xiong et al.,2020). Development of these nutritional and functional foods faces a huge challenge in demonstrating the efficacy of their biologically active ingredients in the body. It is well known that the gastrointestinal tract is the main oxidation site of the human body, and many proteolytic substances and their peptides have good antioxidant activity in vitro (Srigiridhar, Nair, Subramanian, & Singotamu, 2001). Many studies have found the effect of reactive oxide species (ROS) produced during digestion on digestive tract diseases (Carrier, Aghdassi, Platt, Cullen, & Allard, 2001). In the process of digestion, antioxidants will be exposed to iron, copper, hemoglobin, lipid peroxides, nitric oxide and aldehydes from food. Any antioxidant that enters the body may be influenced by these oxidation conditions (Sannaveerappa, Westlund, Sandberg, & Undeland, 2007). Therefore, it is necessary to study whether antioxidants can function normally in human gastrointestinal tract (Jiratanan & Liu, 2004). The method of simulating human digestive tract in vitro has been widely used because of its rapidity, safety and no ethical limitation. At present, there are few studies on camellia seed meal, and the antioxidant activity of MPRs of camellia seed meal has not been studied. Therefore, we studied the flavor characteristics and antioxidant capacity of MRPs produced by MR between diverse sugars and the enzymatic hydrolysates (HCS) of camellia seed meal.
In this study, we selected four kinds of monosaccharides (ribose, xyLose, glucose, fructose) and took advantage of a simple system composed of monosaccharides, HCS, and l-cysteine to authenticate the influence of different sugars on MRPs generated by MR. In this experiment, the browning intensity, degree of substitution (DS), molecular weight distribution (MW), free amino acids and volatile compounds of MRPs were measured by a design scheme. The structure and sensory performance of MRPs were compared. In addition, the antioxidant activity of the in vitro digested camellia seed meal hydrolysate was compared with that of the original camellia seed meal, and its components were analyzed.
2. Material and methods
2.1. Material and instruments
Camellia seed meal was obtained from Da Tuan Jie Edible Oil Co., Ltd. (Anhui, China). The alkaline protease (200,000 U/ g) and flavourzyme (23200 U/g) were purchased from Novozymes (Beijing, China). The anhydrous glucose, xylose, ribose, and fructose were procured from Shanghai McLean Biochemical Technology Co., Ltd. DPPH and ABTS reagents were procured from Shanghai Haoyang Biotechnology Co., Ltd. The instruments used were SCION SQ from Brock (Karlsruhe, Germany) and SYKAM S-433D amino acid analyzer (Beijing, China). The infrared analysis was performed using a Therme Fisher Company, Nicolet 6700 (Waltham, MA, USA).
2.2. Preparation of HCS and MRPs
The treatment of camellia seed meal used in the experiment was carried out according to the method of previous study (Guo, Tong, Ren, Tu, & Li, 2018). At a stable rate of 20 r/s and a substrate concentration of 20%, the camellia seed meal was stirred with n-hexane, which was changed every 24 h until the filtrate was clear and colorless. The residue was then filtered and collected, cleaned with distilled water, and dried in an oven. Degreased camellia seed meal was first crushed by grinding machine and then passed through 80 mesh sieve. The camellia seed meal should be blended with distilled water at a concentration of 5% (m/V), and then heated at the temperature of 90 °C for 30 min. When this mixture was cooled, pH was adjusted to 10.0. The mixture was hydrolyzed with an alkaline protease (1500 U/g) for 2.5 h at 50℃. After 2.5 h, as to pH of this solution would be calibrated to 6.5, and the mixture was hydrolyzed at 50℃ with flavor protease (300 U/g) for 3 h. The mixture was heated to 90 °C for 30 min. The enzymatic hydrolysate (top layer) and residue (bottom layer) of camellia seed meal were obtained by centrifugation at 6000 rpm for 30 min (Wei, Thakur, Liu, Zhang, & Wei, 2018). The supernatant was freeze-dried and stored at −20℃, which was used as the HCS for the experiment. 0.5 g of glucose, fructose, xylose, and ribose, 1 g freeze-dried protein, and 0.2 GL-cysteine were added and placed in 20 mL ampoules. After that, the mixture was put in an oil bath at 110 ℃ for 90 min followed by immediate cooling and centrifugation (Wei et al., 2019). Relevant supernate (MRPs) should be gathered and freeze-dried for further experiments (G-MRPs, glucose MRPs; F-MRPs, fructose MRPs; R-MRPs, ribose MRPs; X-MRPs, xylose MRPs).
2.3. Degree of substitution and browning intensity measurement
The relevant MRPs browning intensity can be seen by detecting the absorbance of the relevant samples at 294 nm (A294) and 420 nm (A420). Due to the heavy color of camelina seed powder, MRPs were diluted 20 times (at 294 nm) and 40 times (at 420 nm) with sterile water, respectively, and then absorbance was measured with a spectrophotometer (UV-2100, Unico Instruments LTD.; Shanghai, China). The changes of amino acids in the samples before and after MR were also measured (Karangwa et al., 2017). For this, 1 mL specimen before and after reaction would be added with 1 mL sodium dodecyl sulfate (0.1%). The first step was to take 0.4 mL of each related solution and mix it with 2 mL phosphate buffer (pH 8.2) and then with 1 mL 2,4, 6-trinitrobenzene sulfonic acid solution (0.1%). This properly treated compound should be heated at the temperature of 50℃ under dark condition for 1 h, and 2 mL 0.1 M sodium sulfite was added to cease the reaction. After the reaction product was cooled to room temperature, an appropriate amount of the reaction final solution was taken and the absorbance of the corresponding solution was tested several times at 340 nm. The blank control data used in this experiment was obtained by utilizing sterile water to replace the test sample. DS is calculated as follows:
A0 is the absorbance prior to this reaction take place. A is the absorbance of the reaction product. Relevant specimens were measured three times and mean values were obtained (Li et al., 2013).
2.4. Fluorescence spectra analysis
Relevant fluorescence spectra of MRPs was determined by fluorescence spectrophotometer (F97pro.Shanghai Leng Light Technology Co., LTD.; Shanghai, China). Firstly, test specimen should be diluted with phosphate buffer solution (0.01 mol/L, pH7.0) to the concentration of protein or enzymatic hydrolysate of 1 mg/mL, and then the parameters were set as follows: excitation wavelength of fluorescence spectrum was 347 nm, and the scanning emission spectrum range was 370–550 nm (Liu et al., 2019).
2.5. Ultraviolet absorption spectra analysis
The relevant MRPs samples were diluted to 1 mg/mL. Ultraviolet absorption spectra were then obtained by multiple measurements at 190–400 nm using an UV spectrophotometer (UV-2100, Unico Instrument Co., Ltd.; Shanghai, China) (Yu, Zhao, Hu, Zeng, & Bai, 2012).
2.6. FT-IR analysis
25 mg of sample and KBr 225 mg were mixed and pressed into thin slices respectively, and then scanned at full wavelength (4000–400 cm−1). KBr slice was used as blank to obtain the infrared spectrogram of the sample (Liu et al., 2019).
2.7. Free amino acid determination
Automatic amino analyzer (L-8900, Hitachi, Tokyo, Japan) was used to detect free amino acids in the corresponding MRPs. The freeze-dried specimens would be hydrolyzed with 6 mol/L HCl at 105 °C for 24 h. In order to precipitate proteins and/or peptides, 4% sulfonyl salicylic acid of corresponding volume was added in 0.1 g MRPs, followed by ultrasonic treatment for 30 min. After centrifugation at 9,000 r/min twice for 30 min, the contents of every type amino acid were measured by hold time and peak area of normal amino acid compound (Sigma-Aldrich Co., MO, USA) with a 0.22-μm small hole filtration membrane (Wei et al., 2018).
2.8. Gas chromatogram-mass spectrometry (GC–MS) analysis
5 mL of MRPs solution was placed at 55 °C for 40 min, and the fiber (75 μm, carbon oxygen /poly-dimethylsiloxane) was simultaneously extracted by solid-phase microextraction extraction fiber. 2 μL of 1, 2-dichlorobenzene (50 g/mL methyl alcohol) was considered as the internal standard. In order to ascertain volatile compounds (Song et al., 2013), gas chromatogra-mass spectrometry (Agilent GC–MS 7890A, Santa Clara, CA, USA) was employed in this study, and BR-5MS column (30 m × 0.25 mm × 0.25 μm) was used to separation of volatiles. The column temperature was installed as 45℃ (2 min), 45-100℃ (2℃/min), 100-150℃ (10℃/min), and 140-290℃ (20℃/min). The mass spectrometer detector had a scanning range of 35–450 m/z and a scanning rate of 4.5 times /s (Song et al., 2013).
2.9. Molecular weight distribution
The relevant MRPs samples were first diluted to 1 mg/mL. This MW was tested by Waters 2695 Alliance HPLC with TSK gel 2000 SWXL 7.8 I.D. X 300 mm (Tosoh, Tokyo, Japan). The mobile phase adopted in this experiment was composed of acetonitrile/water/trifluoroacetic acid with the volume ratio is 45/55/0.1. According to relevantstudy, the flow rate and injection volume used in this experiment were 0.5 mL/min and 10 mol/ L, respectively (Shang et al., 2020). Finally, the UV absorbance of the product was tested at 220 nm (Lan et al., 2010).
2.10. Antioxidant activity
2.10.1. DPPH free radical scavenging activity determination
Lyophilized camellia seed meal and MRPs obtained from enzymatic hydrolysis of camellia seed meal were dissolved in distilled water and prepared into 0.5 mg/mL, 1.0/ 1.5/2.0/2.5/3.0 mg/mL, respectively. 2.5 mL of 100 μmol/L DPPH methanol solution and 2 mL of the corresponding MRPS solution were mixed, shaken, and they were placed in a 37℃ under dark conditions for 40 min. The absorbance A1 was measured at 517 nm with double steamed water as the reference, and A2 was measured with deionized water instead of DPPH under the same conditions, and the camellia seed meal solution with the same concentration was used as the control (Wu, Chen, & Shiau, 2003).
2.10.2. ABTS+ radical scavenging ability
ABTS+ solution was prepared by mixing 7 mM ABTS+ with 2.45 mM potassium peroxodisulfate. The mixed solution was stored at 25℃ for 12 h before it came into effect. Before the experiment, the ABTS+ solution was diluted with 0.2 mM phosphate buffer (pH 7.4) to obtain the absorbance of the tested solution as 0.70 ± 0.02 at 734 nm. After the addition of 40 μL samples (2.0 mg/mL) to 4 mL ABTS+ solution, the compound was gently mixed and allowed to stand in the dark for 6 min. In this experiment, equal amount of distilled water was used to replace samples as blank control. Finally, the absorbance of the solution was tested at 734 nm. Before the formal experiment, the prepared 40 μL Trolox (0.05, 0.1, 0.25, 0.5, and 1 mM) was reacted with 4 mL ABTS+ diluent to prepare the standard curve. Then the ABTS+ radical scavenging capacity in digestive juice was calculated according to Trolox standard curve. In this experiment, Trolox equivalent antioxidant capacity (TEAC, mM) was used to represent the free radical scavenging capacity of corresponding MRPs (Wang, & Xiong, 2005).
2.10.3. Hydroxyl radical scavenging ability
Sample solutions of different concentrations were prepared. 1 mL sample solution was taken and mixed with 1 mL 6 mmol/L FeSO4, 1 mL 6 mmol/L H2O2, and 1 mL 6 mmol/L salicylic acid–ethanol solution. After 30 min in a water bath at 37℃, relevant absorbance value of this mixed solution at 510 nm was determined. VC was used as positive control. This calculation formula of hydroxyl radical scavenging capacity used in this experiment is shown below (Li, Jiang, Zhang, Mu, & Liu, 2008).
A0 -- Blank control;
A1 -- Light absorption value of relevant specimens;
A2 -- Sample without salicylic acid;
2.10.4. Lipid peroxidation inhibition assay
Enzymatic hydrolysate samples of camellia seed meal with various concentration (5.0, 10.0, 15.0, 20.0, 25.0, 30.0, and 35.0 mg/mL) were manufactured. Firstly, the prepared 200 mL solution was evenly blended with 1 g peanut oil, 5 mL solution containing thiobarbituric acid (15%, W/V), trichloroacetic acid (0.37%, W/V), and hydrochloric acid (1.8%, V/V). After mixing well, this mixture would be heated at the temperature of 90℃ for 6 h. When mixture was cooled, then centrifuged at a speed of 2000 rpm/min for 5 min and filtered twice to obtain the appropriate filtrate. The absorbance of the corresponding filtered filtrate was tested at 532 nm, and the average value was taken for multiple measurements. In this experiment, distilled water (Ablank) was used instead of the sample to prepare the blank control. The lower the sample value, the stronger the antioxidant capacity was reported in the previous study (Yang, & Stockwell, 2016).
2.11. In vitro digestion experiment
For in vitro pepsin-trypsin simulated gastrointestinal digestion, different MRPS (3% W/V) were dissolved and pH was maintained to 2.0 using 1 M HCL. Pepsin (4%m/ V) was added and incubated at 37℃ for 2 h. The pH of 0.9 mM NaHCO3 solution was adjusted to 5.3 and the pH of 1.0 M NaOH solution was adjusted to 7.5. Trypsin (4% m/ V) was added, mixed, and allowed to react at 37℃ for 2 h, and then after, the reaction was terminated. After boiling for 10 min, the mixture was centrifuged at 11,000 g for 15 min. The supernatant was freeze-dried and refrigerated at −20℃. To evaluate the changes of antioxidant activity of MRPs during simulated digestion, samples were taken at 0, 0.5, 1.0, 1.5, and 2.0 (pepsin), 2.5, 3.0, 3.5 and 4.0 h (trypsin), respectively during in vitro digestion. In vitro digestion experiment included DPPH free radical scavenging, ABTS+, and reduction force experiments. The first two methods were the same as mentioned in the above sections. The experimental method for reduction force determination was as follows. Firstly, the lyophilized digested product was dissolved in distilled water at a concentration of 2.0 mg/ mL, and the corresponding digested product was evenly mixed with 2.0 mL sodium phosphate buffer (0.2 mM, pH 6.6) and 2.0 mL 1% potassium ferricyanide (W/V) per serving. After the above mixture was bathed at 50℃ for 20 min, 10% trichloroacetic acid (2.0 mL) was added. At the end of the reaction, 2.0 mL supernatant of the final reactants was collected and mixed evenly with 2.0 mL distilled water and 0.4 mL 0.1% (W/V) FeCl3. The resulting mixture was allowed to stand at room temperature for 10 min. The absorbance of the final reaction product was determined at 700 nm. The blank control sample was replaced with equal amounts of distilled water (Ahmadi, Kadivar, & Shahedi, 2007).
2.12. Sensory analysis
The flavor evaluation of MRPs was carried out based on previous studies (Wei et al., 2018). Firstly, 0.5% MRPs solution was dissolved in a umami solution consisting of 1.0% (W/V) sodium glutamate and 0.5% (W/V) NaCl. Our sensory assessment group consists of 16 experienced persons (7 men and 9 women, aged 23 to 38). Our evaluation team debated the evaluation criteria in detail and evaluated eight related indicators, including meaty, umami, salty, caramel-like taste, bitterness, mouthfulness, continuity, and total acceptance.
2.13. Data analysis and measurement in triplicate
Data were analyzed through one-way ANOVA using SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL, USA). All the data were expressed as ± standard deviation. The correlation between different parameters was analyzed by PLSR of version 9.7 (CAMO ASA, Oslo, Norway).
3. Results and discussion
3.1. Analysis of browning intensity and ultra violet spectrum
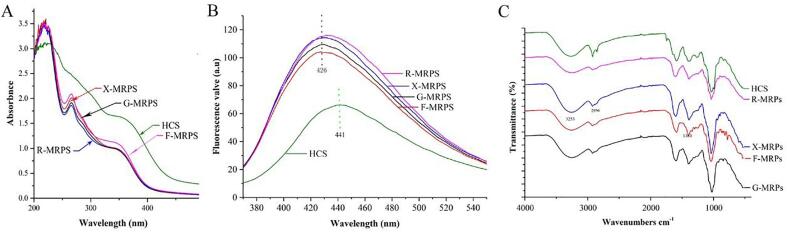
Browning intensity and UV absorption spectra in MRPs systems containing protein or peptide and reducing sugar are the significant features. For the index of browning intensity, the MRPs generated by disparate sugars were significantly diverse, as displayed in Table 1 (P > 0.05). Different MRPs were absorbed in the range of 190–800 nm, and their spectra were shown in Fig. 1A. Due to the presence of schiff base, the MRPs generated by diverse sugars had two absorption peaks within the limits of 190–300 nm. The maximum absorption peaks of R-MRPs were noticed at 219 and 278 nm. The difference in the location of the maximum absorption peak might be related to the type of sugar. Early studies have shown that the absorption peaks of MRPs generated by lysine and glucose were obtained approximately at 265 nm and 215 nm. When combined with xylose, the peak value of MRPs occurred at 300 nm and 215 nm, respectively (Yu, Zhao, Hu, Zeng, & Bai, 2012). The absorbance of different MRPs at 294 nm was greatly influenced by the type of sugar, which is consistent with the results of browning experiment (Fig. 1A).
Table 1.
Browning intensity and substitution degree of MRPs with disparate sugar.
| Samples | G-MRPs | F-MRPs | X-MRPs | R-MRPs |
|---|---|---|---|---|
| A249 | 0.806 ± 0.12c | 0.813 ± 0.29c | 0.843 ± 0.27b | 0.882 ± 0.27a |
| A420 | 0.757 ± 0.23c | 0.762 ± 0.59c | 0.824 ± 0.63b | 0.921 ± 0.46a |
| DS | 16.36 ± 0.46c | 15.75 ± 0.36c | 18.47 ± 0.85b | 21.24 ± 0.39a |
Annotation: Diverse letters within a row manifested significant difference (p < 0.05).
Fig. 1.
Physi-chimical analysis of the Maillard reaction products of Camellia seeds. (A) Ultraviolet (UV) absorption spectra within the wavelength range of 190–400 nm; (B) Fluorescence spectra of Maillard reaction products generated by different sugars; (C) Fourier transform infrared (FT-IR) spectra within the range of 4,000–400 cm−1.
3.2. DS and fluorescence spectra analysis
As shown in Table 1, compared with other MRPs, R-MRPs had the largest DS (21.24%) and the smallest R-MRPs (15.75%). MR generates substances with fluorescent characteristic under excitation wavelength of 340–370 nm and emission wavelength of 420–440 nm. The products formed by MR in the primary stage have no fluorescence, but before the formation of brown pigment in the advanced reaction stage, the substances generated in the early reaction phase can cross-link with adjacent protein or amino acid to produce polymers with fluorescence properties. At the excitation wavelength of 347 nm, the strongest fluorescence emission wavelength was in the range of 420–427 nm (Fig. 1B), which is consistent with the characteristics of Maillard producing fluorescent substances (Farroni, & Buera, 2012).
3.3. FT-IR analysis
The FT-IR spectra of HCS and MRPs generated by diverse sugars were displayed in Fig. 1C. Due to the chemical changes between HCS and sugars caused by MR, few functional groups disappeared and new functional groups were generated simultaneously. The depletion of the NH2 group had been reported, while new groups such as the Amadori complex (C O), Schiff base (C N), and pyrazine (c-n) were generated in the process of MR. The absorption peak of the hydroxyl group was in the range of 3200–3650 cm−1. When the hydroxyl group was intermolecular, it formed a polymer connected by hydrogen bonds, and the force constant K value was dropped. Therefore, the infrared absorption position moved to a lower wave number (3300 cm−1), with a broad and flat peak. Fig. 1C showed that there was a small peak with low intensity around 3000 cm−1, indicating the existence of unsaturated C—H bond, corresponding to the GC–MS analysis. Small spikes at 2820 cm−1 and 2720 cm−1 indicated the presence of an aldehyde group. A moderately strong peak between 1600 and 1670 cm−1 indicated a certain number of carbon–carbon double bonds, corresponding to GC–MS analysis (Carbonaro, & Nucara, 2010).
3.4. Amino acid analysis
The amino acid spectra of the enzymolysis products of camellia seed meal and the camellia seed meal were displayed in Table 2. After the MR, amino acid content in the experimental specimens was decreased. Flavor compound generation is caused by a reaction between protein and amino acid, and it is triggered by the binding of a reducing sugar carbonyl group to an amino group (Lu, Hao, Payne, & Ho, 2005). Thus, this explains the decrease in total amino acids after the MR. The essential amino acid content of MRPs obtained from camellia seed meal under optimized process was about 33% of the total amino acid content. Earlier study showed that the percentage of essential amino acids in the protein of diverse parts of Alaskan salmon and yellow-tailed king fish was 26%-36% of the total amino acids, which is considered as an abundant protein source. It can be seen that MRPs obtained from camellia seed meal under optimized process conditions has a promising future. MRPs of camellia seed meal is a suitable material for nutrient food or dietary essential amino acid supplement.
Table 2.
Free amino acid content in Hydrolysates and Maillard reaction products generated by disparate sugars.
| Amino acid | Content (mg/g) |
||||
|---|---|---|---|---|---|
| B1 | B2 | B3 | B4 | B0 | |
| Asp | 10.71 ± 0.08b | 8.31 ± 0.49c | 11.33 ± 0.12a | 8.71 ± 0.30c | 8.81 ± 1.05c |
| Thr | 15.12 ± 0.2bb | 13.66 ± 0.39c | 15.94 ± 0.04a | 14.82 ± 0.34b | 12.13 ± 2.03c |
| Ser | 5.56 ± 0.15a | 4.13 ± 0.76c | 6.86 ± 0.10a | 5.43 ± 0.15b | 4.62 ± 0.05c |
| Glu | 28.43 ± 0.56c | 21.65 ± 0.44e | 30.49 ± 0.02b | 26.17 ± 0.04d | 32.15 ± 0.07a |
| Gly | 6.64 ± 0.33b | 6.22 ± 0.13b | 9.33 ± 0.15a | 6.29 ± 0.04b | 6.42 ± 0.11b |
| Ala | 8.42 ± 0.16a | 6.78 ± 0.11c | 8.27 ± 0.05a | 7.51 ± 0.27b | 7.85 ± 0.10b |
| Cys | 84.85 ± 0.26a | 82.43 ± 0.05a | 65.98 ± 0.1c | 57.82 ± 0.73d | 77.93 ± 0.01b |
| Val | 17.45 ± 0.74c | 14.15 ± 0.06e | 19.24 ± 0.03a | 16.75 ± 0.82d | 18.31 ± 0.06b |
| Met | 5.53 ± 0.58c | 3.33 ± 0.10d | 8.91 ± 0.18b | 5.82 ± 0.32c | 18.29 ± 0.04a |
| Ile | 8.72 ± 0.31bc | 8.86 ± 0.06c | 11.97 ± 0.10a | 5.68 ± 0.59d | 10.13 ± 0.08b |
| Leu | 8.87 ± 0.25d | 6.54 ± 0.18e | 14.24 ± 0.17a | 10.53 ± 0.16c | 12.72 ± 0.17b |
| Tyr | 5.75 ± 0.07a | 4.48 ± 0.41b | 5.65 ± 0.14a | 5.52 ± 0.03a | 5.14 ± 0.98a |
| Phe | 6.65 ± 0.06b | 5.24 ± 0.68c | 9.35 ± 0.16a | 5.45 ± 0.01c | 4.98 ± 0.02c |
| Lys | 4.75 ± 0.17a | 4.64 ± 0.29a | 4.67 ± 0.14a | 3.52 ± 0.95b | 3.62 ± 0.09b |
| Nh3 | 5.56 ± 0.14b | 4.53 ± 0.37c | 6.56 ± 0.79a | 4.97 ± 0.29bc | 4.54 ± 1.26c |
| His | 4.55 ± 0.17a | 3.49 ± 0.45b | 3.93 ± 0.14a | 3.59 ± 0.16b | 3.15 ± 0.55b |
| Arg | 7.63 ± 0.43a | 5.17 ± 0.75c | 7.15 ± 0.06a | 6.17 ± 0.13b | 6.12 ± 0.76b |
| A1 | 67.09 ± 0.23b | 56.42 ± 0.12c | 79.65 ± 0.05a | 59.05 ± 0.12c | 80.18 ± 0.09a |
| A2 | 205.87 ± 0.24b | 203.61 ± 0.12b | 239.87 ± 0.04a | 168.29 ± 0.04c | 236.91 ± 0.04a |
| A1/A2 | 0.32 | 0.28 | 0.33 | 0.35 | 0.33 |
Note: Means within different letters were significantly (p < 0.05) different on the same line. B0, B1, B2, B3 and B4 respectively represent the enzymolysis products of camellia seed meal, G-MRPs, F-MRPs, X-MRPs and R-MRPs. Essential amino acid (A1), Total amino acid (A2).
3.5. GC–MS analysis
The content of furan in MRPs of ribose and xylose was higher than that of glucose and fructose. Furfural was also tested in the MRPs of these two sugars (Table 3). This is consistent with a previous research result (Wei et al., 2018), namely, furfural content was higher X-MRPs, while the content in G-MRPs and F-MRPs was relatively low. It is a necessary intermediate in some chemical systems and composed of pentose and generated by Amadori intermediate 1, 2-enolization. Sulfur-containing flavor compounds have great influence on meat and roast flavors. These compounds are produced by the reciprocity of carbonyl compound with sulfur-containing amino acid or by the thermal degradation of cysteine. In this study, upto 20 sulfur-containing compounds were found, including 3 thifurans, 3 thiazoles, 5 thiols, and 9 thiophene compounds. Of the four MRPs, X-MRPs had the highest sulfide content (4794.6 ng/g). Studies have shown that thiobenzene is produced by the reaction between carbohydrate and amino acid (Hou et al., 2017).
Table 3.
Volatile chemical compounds tested in Maillard reaction products generated by disparate sugars.
| Compounds | RI | Amouts (ng/g) |
|||
|---|---|---|---|---|---|
| R-MRPs | X-MRPs | G-MRPs | F-MRPs | ||
| Furans | 2238.7 ± 67.9b | 2374.1 ± 96.2b | 1788.4 ± 60.8c | 2512.2 ± 47.9a | |
| 2-methyl-furan | —— | ND | 134.7 ± 23.0 | 33.3 ± 18.4 | ND |
| 3-methyl-furan | —— | 90.6 ± 14.1 | 70.4 ± 17.2 | ND | ND |
| 2-ethyl-furan | 738 | ND | ND | 88.6 ± 8.6 | 68.4 ± 18.2 |
| Furfural | 833 | 1341.2 ± 94.8 | 1212.6 ± 151.8 | ND | ND |
| 2-acetyl furan | 928 | ND | ND | 618.7 ± 85.8 | 1034.3 ± 10.2 |
| 2-pentyl-furan | 1014 | 126.3 ± 19.8b | 134.5 ± 27.5b | 223.7 ± 35.6a | 209.3 ± 47.3a |
| trans-2-(2-Pentenyl) furan | 1021 | ND | 85.3 ± 9.4 | ND | 90.5 ± 12.5 |
| (E)-2-(1-pentenyl)-furan | 1074 | 98.8 ± 16.3 | ND | 78.7 ± 6.8 | ND |
| 4,7-dimethyl-Benzofuran | 1233 | 295.1 ± 17.2d | 472.7 ± 60.5b | 500.2 ± 53.4a | 377.6 ± 21.8c |
| (E)-2,2′-(1,2-ethenediyl) bis-furan | 1339 | 161.9 ± 1.5 | 128.2 ± 2.9 | ND | ND |
| 2(3H)-Furanone, dihydro-5-methyl-5-phenyl- | 1378 | 124.8 ± 12.7c | 135.7 ± 14.8c | 245.2 ± 26.3b | 732.1 ± 37.3a |
| Thiophenes | 894.1 ± 21.7d | 1274.8 ± 76.8c | 3299.6 ± 97.1a | 2363.3 ± 55.3b | |
| 3-methyl-thiophene | 804 | 21.2 ± 3.2c | 28.5 ± 4.5b | 23.7 ± 2.5c | 34.7 ± 9.2a |
| Thiophene, 2-ethyl- | 889 | ND | ND | 103.2 ± 17.3 | 85.6 ± 13.7 |
| Thiophene, 2,3-dimethyl- | 893 | ND | ND | 3. 7 ± 1.6 | ND |
| Thiophene, 2,5-dimethyl- | 895 | ND | ND | ND | 405.6 ± 23.6 |
| 2-Thiophenecarboxaldehyde | 1013 | 19.5 ± 2.9d | 112.8 ± 14.1c | 236.6 ± 24.7b | 312.8 ± 21.8a |
| Ethanone, 1-(3-thienyl)- | 1102 | ND | ND | 58.2 ± 11.4 | 118.5 ± 22.1 |
| 3-Methyl-2-thiophenecarboxaldehyde | 1134 | 490.1 ± 32.9c | 613.9 ± 61.8b | 1543.7 ± 147.2a | 647.3 ± 64.9b |
| Thiophene,2-(1,1-dimethylethoxy)-5-methyl_ | 1308 | 327.7 ± 42.5d | 519.6 ± 62.4c | 1392.4 ± 106.8a | 758.8 ± 83.9b |
| 5-Methylthiophen-3-ylamine | 1590 | 35.6 ± 9.5 | ND | ND | ND |
| Thiazoles | ND | 28.1 ± 6.2 | ND | ND | |
| 4(5H)-Thiazolone, 2-imino- | 984 | ND | 28.1 ± 5.4 | ND | ND |
| Thiols | 1712.2 ± 142.3b | 3497.4 ± 78.5a | 91.6 ± 20.4c | 41.6 ± 6.9d | |
| 2-Pentanethiol | 809 | ND | ND | 56.1 ± 5.4 | ND |
| 2-Methyl-3-furanthiol | 895 | 221.6 ± 15.3 | 121.2 ± 9.3 | ND | ND |
| 2-methyl-3-Pentanethiol | 906 | 42.3 ± 3.1b | 59.6 ± 9.5a | 22.6 ± 3.1c | 26.7 ± 2.5c |
| 2-Furfurylthiol | 921 | 1418.9 ± 27.4 | 3295.5 ± 34.9 | ND | ND |
| 2-Thiophenemethanethiol | 1074 | 29.4 ± 7.3a | 21.1 ± 4.3b | 12.9 ± 2.1c | 14.9 ± 5.1c |
| Sulfur substituted furans | 478.4 ± 27.3b | 676.9 ± 45.1a | 34,0.5 ± 5.9d | 171.3 ± 11.2c | |
| Furfuryl sulfide | 1452 | 185.3 ± 13.7a | 131.6 ± 19.1b | 34.5 ± 2.9c | 27.4 ± 3.2c |
| 2-[(methyldithio)methyl]-Furan | 1567 | 86.6 ± 7.1 | 197.9 ± 14.6 | ND | ND |
| Bis(2-furfuryl) disulfide | 1699 | 206.5 ± 17.8b | 347.4 ± 27.5a | ND | 143.9 ± 14.8c |
| Aliphatic sulfur compounds | |||||
| Diisopropyl sulfide | 912 | 21.2 ± 1.4b | 44.7 ± 3.9a | 15.2 ± 4.2c | 13.6 ± 3.2c |
| Nitrogen containing compounds | |||||
| Pyrazines | 473.4 ± 27.4a | 362.8 ± 13.2b | 258.2 ± 23.1c | 67.1 ± 11.3d | |
| 2-methyl pyrazine | 842 | 47.6 ± 3.6b | 42.9 ± 7.3c | 56.7 ± 6.4a | 47.8 ± 6.8b |
| Pyrazine,2-ethyl-3,5-dimethyl- | 937 | 425.8 ± 47.3a | 319.9 ± 24.5b | 201.5 ± 34.4c | 19.3 ± 4.3d |
| Pyridines and pyrimidines | 214.7 ± 21.2a | 211.8 ± 25.3a | 65.7 ± 18.2c | 83.7 ± 17.3b | |
| 4-Hydroxypyridine | 969 | ND | ND | 65.6 ± 10.5 | 83.3 ± 15.4 |
| 2,5-dimethoxy-pyrimidine | 1213 | 214.7 ± 10.3 | 211.4 ± 38.2 | ND | ND |
| Oxygen containing compounds | |||||
| Ketones | 521.9 ± 77.2d | 579.3 ± 36.7c | 703.3 ± 135.5b | 754.1 ± 49.3a | |
| Acetone | 797 | 14.6 ± 47.4d | 16.9 ± 6.4c | 21.4 ± 3.9b | 27.7 ± 4.1a |
| 2-Butanone | 875 | 47.4 ± 10.2d | 58.5 ± 14.8c | 74.1 ± 13.6a | 67.4 ± 12.9b |
| 2-Pentanone | 967 | 38.3 ± 5.9b | 45.9 ± 10.2a | 12.7 ± 3.8c | 16.4 ± 2.9c |
| 2-Heptanone | 911 | 27.4 ± 6.3c | 36.1 ± 6.4c | 289.5 ± 43.7b | 341.2 ± 62.2a |
| 4-Methoxy-2(1H)-quinolone | 1017 | 24.5 ± 5.4c | 32.2 ± 6.7c | 127.9 ± 24.7a | 91.2 ± 18.4b |
| 4-Hydroxy-2-methylacetophenone | 1292 | ND | ND | 62.5 ± 10.3 | 73.4 ± 13.5 |
| 4-Hydroxy-3-methylacetophenone | 1292 | 111.7 ± 23.6 | 105.9 ± 21.9 | ND | ND |
| Ethanone,1-(2-hydroxy-5-methylphenyl)- | 1292 | 72.2 ± 13.7 | 115.7 ± 34.7 | ND | ND |
| Ethanone,1-(2-hydroxy-4-methoxyphenyl)- | 1351 | 11.6 ± 3.4c | 15.7 ± 2.5b | ND | 24.8 ± 4.6a |
| 2(3H)-Furanone, dihydro-5-methyl-5-phenyl- | 1438 | 174.2 ± 37.1a | 152.4 ± 29.7b | 91.4 ± 10.4d | 112 ± 15.2c |
| Ethanone,1-(2-hydroxy-5-methoxyphenyl)- | 1457 | ND | ND | 23.8 ± 3.0 | ND |
| Alcohols | 969.2 ± 146.2d | 1063.7 ± 208.7c | 1320.8 ± 241.6b | 1456.8 ± 317.8a | |
| .alpha-Terpineol | 637 | 15.5 ± 3.2d | 208.9 ± 29.5b | 173.2 ± 30.2c | 204.6 ± 38.8a |
| 1-Butanol | 705 | 39.5 ± 15.3b | 34.2 ± 6.2c | 46.1 ± 9.5a | 42.5 ± 7.3a |
| Eucalyptol | 1051 | 116.7 ± 19.4c | 113.8 ± 20.5c | 282.2 ± 14.3b | 318.6 ± 45.9a |
| 1-Octanol | 1091 | 14.4 ± 3.8c | 11.5 ± 3.9c | 103.5 ± 19.5b | 183.4 ± 28.5a |
| (2,4,6-Trimethylcyclohexyl) methanol | 1156 | 107.2 ± 17.4c | 128.6 ± 20.1b | 92.1 ± 18.5c | 174.8 ± 30.2a |
| 4-(1-methylethenyl)-1-cyclohexene-1-methanol | 1372 | 491.4 ± 95.3a | 395.1 ± 63.1b | 371.8 ± 78.4b | 349.5 ± 10.4c |
| 3,7-dimethyl-1, 6-octadien-3-ol | 1438 | 184.5 ± 4.5b | 171.6 ± 12.5c | 251.9 ± 11.9a | 183.4 ± 19.4b |
| Esters | 47.5 ± 4.4c | 63.1 ± 3.6b | 67.7 ± 11.4b | 75.9 ± 14.8a | |
| Ethanethioic acid,S-(2-furanylmethyl) ester | 1422 | 47.5 ± 7.4 | 63.1 ± 11.7 | ND | ND |
| i-Propyl tricosanoate | 1692 | ND | ND | 19.2 ± 5.9 | 23.6 ± 4.9 |
| Methyl salicylate | 1210 | ND | ND | 48.5 ± 7.3 | 52.3 ± 9.3 |
| Aldehydes | 964.5 ± 132.5b | 1012.3 ± 203.4a | 690.2 ± 121.4c | 582.7 ± 105.5d | |
| Benzaldehyde | 975 | 609.9 ± 81.7b | 656.9 ± 66.2a | 415.9 ± 78.4c | 349.4 ± 46.3d |
| Nonanal | 1121 | 43.7 ± 6.74b | 58.3 ± 9.46a | 28.2 ± 5.8c | 31.6 ± 5.9c |
| Decanal | 1222 | 54.2 ± 12.4a | 26.7 ± 4.8c | 31.6 ± 9.6b | ND |
| Benzene,2,4-pentadiynyl- | 1254 | 127.7 ± 21.7a | 92.8 ± 12.4b | ND | 13.9 ± 7.8c |
| Benzaldehyde, 4-ethoxy- | 1249 | ND | ND | 21.3 ± 4.8 | 25.7 ± 5.8 |
| Benzaldehyde, 4-methoxy- | 1267 | 16.9 ± 4.8c | 9.5 ± 3.7d | 31.6 ± 7.3a | 28.2 ± 5.3b |
| Cinnamaldehyde, (E)- | 1282 | 43.7 ± 7.4c | 28.6 ± 4.9d | 62.2 ± 11.5a | 54.6 ± 11.3b |
| 2-Propenal, 3-phenyl- | 1284 | 56.1 ± 7.4a | 46.4 ± 9.4b | 15.8 ± 4.3c | 17.9 ± 4.7c |
| Hexadecanal,2-methyl- | 1905 | 12.3 ± 1.1d | 93.1 ± 7.5a | 83.6 ± 5.3b | 61.4 ± 1.8c |
| Carboxylic acids | |||||
| Benzoic acid | 1198 | 95.7 ± 14.5b | 92.9 ± 18.4b | 159.6 ± 29.4a | 42.8 ± 6.9c |
Note: ND indicated not detected; Means within different letters were significantly (p < 0.05) different on the same line.
The contents of thiophene substances in R-MRPs (893.4 ng/g), X-MRPs (1274.2 ng/g) and G-MRPs (3360.8 ng/g) were very high. 3-methyl-2-thiophenaldehyde was found in all the MRPs and early studies have shown that it leads to cooked meat aroma. 2-methyl-3-furanethiol and 2-furanthiol were detected in R-MRPs and X-MRPs. These two mercaptan compounds have a very lower odor threshold and considered to be the key odor for meat (Madruga et al., 2009). The content of thiofuran in MRPs produced by ribose was higher (P > 0.05). Some nitrogenous compounds are also generated in MR as revealed in the previous studies (Akiyama et al. 2008). Pyrazine usually has a roasted, nutty, and charred deli aroma (Lee, Chung, & Kim, 2012). R-MRPs and X-MRPs had higher pyrazine content (472.1 ng /g and 361.9 ng /g), respectively. In this study, 2-methylpyrazine was detected in MRPs of four sugars. A total of 28 oxygen-containing compounds were identified, including 11 ketones, 8 alcohols, and 9 aldehydes. Their levels in R-MRPs and X-MRPs were significantly higher than those in other sugars. Oxygen-containing compounds have a high odor threshold and have no effect on the sensory properties of MRPs (Su et al., 2011). The content of ketones in pentose MRPs was higher than that in other sugar MRPs. Among them, 2-butanone and 2-heptanone were the main ketones in R-MRPs and X-MRPs. The content of alcohol in X-MRPs (294.3 ng/g) was higher than other MRPs. Benzaldehyde was detected in all the MRPs which as previously mentioned can cause unpleasant odors (Table 3).
3.6. MW Distribution estimation
As displayed in supplemental Table 1, this molecular weight distribution of MRPs generated by diverse saccharides alter noteworthy (P > 0.05). 128 Da (43.48%), 128–500 Da (31.12) and 500–1000 Da (18.27%) is the main component of HCS. Different from HCS, the 128 Da was noteworthy decreased in MRPs. High molecular weight elements are generated by the cross-linking of peptide and sugar or their degradation products in MR (Lan et al., 2010). In all the MRPs, peptide levels were significantly increased between 500–1000 Da and 1000–3000 Da (P > 0.05). Another study found that 500–1000 Da peptides lead to the strongest bitter taste (Wei et al., 2019).
3.7. Antioxidant analysis
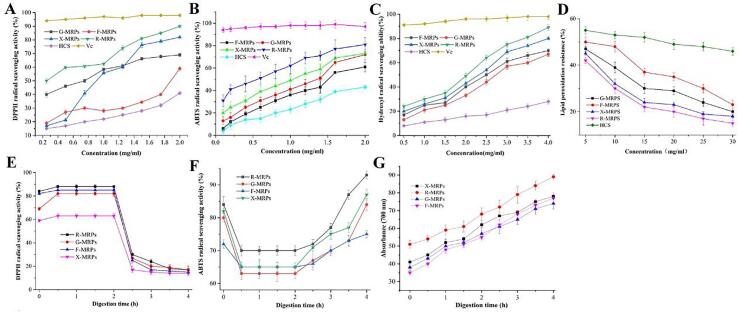
It was found that the antioxidant activity of camellia seed meal hydrolysate after MR was enhanced (Fig. 2). The MRPs are complex and consist of many substances with different molecular weights. Among them, the main antioxidant effect of substances like melanin related products is mainly in the advanced stage, but its antioxidant mechanism is still unclear, possibly with some short peptides containing antioxidant peptide structure like melanin products, so they have antioxidant properties. At the same time, the intermediate products of MR, heterocyclic compounds containing N and S, reductive ketones, and other substances generated during the reaction can provide hydrogen atoms, and also have certain reducing ability (Zeng et al., 2018).
Fig. 2.
Antioxidant activity of Maillard reaction products of Camellia seeds. (A) DPPH free radical scavenging activity of MRPs with disparate sugar concentration and camellia seed meal; (B) ABTS+ radical scavenging ability of MRPs with disparate sugar concentration and camellia seed meal; (C) Hydroxyl radical scavenging ability of MRPs with disparate sugar concentration and camellia seed meal; (D) Lipid peroxidation inhibition assay of MRPs with disparate sugar concentration and camellia seed meal. (E) Changes of DPPH radical scavenging activity in disparate sugar MRPS during continuous in vitro digestion; (F) Changes of ABTS free radical scavenging activity in disparate sugar MRPS during continuous in vitro digestion; (G) Reduction capacity measurement (P > 0.05) in disparate sugar MRPS during continuous in vitro digestion. Data with diverse letters in the same test were statistically significant (P > 0.05).
The MRPs of camellia seed meal have been proved to have good antioxidant index and nutritional value. The following is the digestion experiment analysis of MRPs as the reactants in vitro. For xylose, DPPH free radical scavenging activity in digestive juices can be seen in Fig. 2E, F, G. DPPH free radical scavenging activity was incremental after digestion by pepsin for 0.5 h (85 ± 1.2%) (P > 0.05). After being treated with pepsin, DPPH free radical scavenging activity showed no further significant change. This is because more hydrophobic amino acid residue side chain groups are exposed during pepsin digestion, which makes the peptides more susceptible to DPPH radicals and makes them easier to capture free radicals (Nie et al., 2019). After being treated with pepsin for 0.5 h, the related indexes were decreased by 60% (85 ± 1.2% to 25 ± 1.5%). DPPH scavenging activity of gastrointestinal digestion after 4.0 h was only 15 ± 1.9% (reduced by 70%). This is because MRPs are completely hydrolyzed during trypsin digestion, resulting in the production of short peptide (tripeptide and dipeptide) and amino acids. Enhancive polarity in gastrointestinal tract results makes it harder to produce chemical reaction with liposoluble DPPH free radical (Zhu, Chen, Tang, & Xiong, 2008). Because trypsin treatment reduced the free radical scavenging activity of MRPs. The experiment proved that the scavenging activity of ABTS+ free radicals in the digestion solution was strong, which showed a powerful scavenging activity on hydrosoluble ABTS+ free radicals (Fig. 2F). The consequence displayed that the antioxidant activity was enhanced by the production of some peptides after in vitro digestion. The tendency of ABTS+ in digestive juice was diverse from DPPH (You, Zhao, Regenstein, & Ren, 2010). This is due to the increased hydrophobicity of the resulting short peptide after treatment with pepsin, which makes it unlikely to produce chemical reaction with hydrosoluble ABTS+ free radicals (Wei et al., 2020). But, increased hydrophilic properties of digestive tract after trypsin treatment were beneficial to ABTS+ free radicals capture. Reduction capacity of the digestive system during reduction force measurements depends on the principle that the presence of antioxidants in the sample results in the reduction of the Fe3+/FeSCN complex to the Fe2+ form. For X-MRPS, as shown in Fig. 2G, the reduction force of the untreated sample was 0.41 ± 1.4. The level of pepsin was significantly increased after digestion for 1.0 h (P > 0.05) to 0.52 +/-1.9. After incubation with pepsin for 1 h, no significant change in reducing power was observed (P > 0.05). After trypsin digestion for 1.0 h, the reduction force was increased to 0.69 ± 1.1 (P > 0.05). Compared with non-digested liquid, the reduced capacity of the final digestive juice was increased by 90%. The variation tendency was analogous to ABTS. With the improvement of the reduction capacity of the digestive system, MRPs have a stronger antioxidant capacity after in vitro digestion which is consistent with a previous study stating that the reductive power of maize proteolytic substances was prodigiously improved after being treated with pepsin and trypsin (Zhu et al. 2008).
3.8. Sensory evaluation
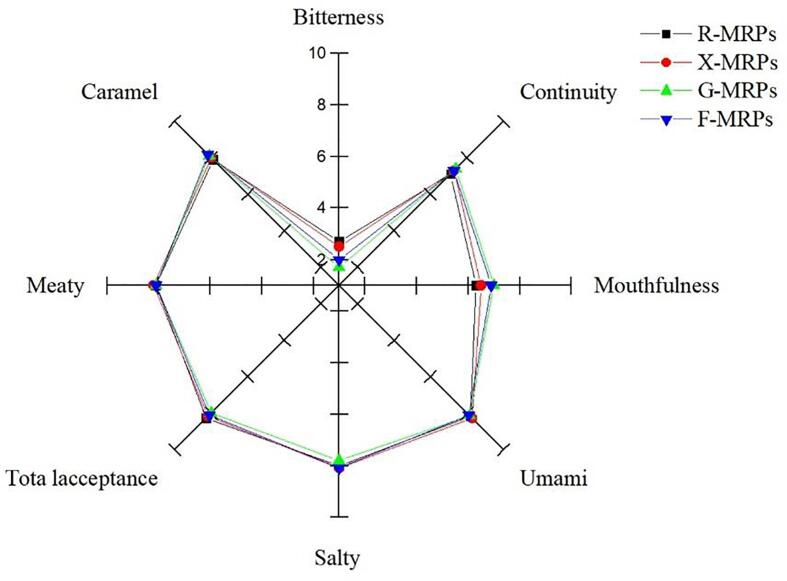
Sensory evaluation results showed that diverse sugars had greater effects on the sensory property of MRPs (Fig. 3). R-MRPs and X-MRPs were more common in meat and umami flavors than other MRPs. The sensory score of meat taste of umami soup was 8.2 ± 0.13 (R-MRPs) and 8.3 ± 0.22 (X-MRPs) . The taste of meat is due to the sulfide compound generated by cysteine in the MR (Lee, Jo, & Kim, 2010). There was no greater diversity between MRPs in taste and continuity strength, but continuity strength had influence on its taste. This may be resulted from 1000 to 5000 Da peptide generated by MRPs during the MR process, which can improve the flavor characteristics of the product (Ogasawara et al., 2006). In the MRPs generated by diverse sugars, there was no significant difference in saltiness. In terms of overall acceptance, R-MRPs and X-MRPs were superior to other MRPs. The enzymatic hydrolysis could improve the flavor characteristics of camellia seed meal protein. These results indicated that the sensory properties of MRPs protein were associated with the type of sugar.
Fig. 3.
Presentation of various changes in sensory evaluation as a function of sugar type.
4. Conclusion
Camellia seed meal generally refers to the by-product of industrial camellia oil extraction. Despite, its higher nutrition, its development intensity is not enough resulting in the waste of natural resources. The type of sugar has obvious influence on the structure and flavor characteristics of MRPs extracted by HCS. FT-IR analysis has shown the different structures of MRPs produced by adding different sugars. MW distribution revealed that 128–500 and 500–1000 Da accounted for the majority of MRPs. Compared with other MRPs, R-MRPs had higher sulfur compound and umami amino acid content, which led to meaty and umami taste. Antioxidant analysis showed that the modified camellia seed meal protein modified by MR could act as an appropriate antioxidant. In vitro digestion confirmed that treatment with pepsin and trypsin could increase the antioxidant properties of camellia seed meal protease hydrolysates. To conclude, MRPs improved by diverse saccharides could be regard as flavor enhancer and natural antioxidant. Furthermore, camellia seed meal could be employed as a significant ecological resource to obtain novel antioxidant compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the National Natural Science Foundation of Ningxia Province (2021AAC02019), the Youth talent cultivation project of North Minzu University (2021KYQD27), the Major Projects of Science and Technology in Anhui Province (201903a06020021, 202004a06020042, 202004a06020052, 201904a06020008), the National Natural Science Foundation of China (31850410476).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100127.
Contributor Information
Zhi-Jing Ni, Email: lovebear@vip.163.com.
Xiang Liu, Email: 2331466439@qq.com.
Bing Xia, Email: 823221734@qq.com.
Long-Teng Hu, Email: 2633113679@qq.com.
Kiran Thakur, Email: kumarikiran@hfut.edu.cn.
Zhao-Jun Wei, Email: zjwei@hfut.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahmadi F., Kadivar M., Shahedi M. Antioxidant activity of Kelussia odoratissima Mozaff in model and food systems. Food Chemistry. 2007;105(1):57–64. [Google Scholar]
- Carbonaro M., Nucara A. Secondary structure of food proteins by Fourier transform spectroscopy in the mid-infrared region. Amino Acids. 2010;38(3):679–690. doi: 10.1007/s00726-009-0274-3. [DOI] [PubMed] [Google Scholar]
- Carrier J., Aghdassi E., Platt I., Cullen J., Allard J.P. Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Alimentary Pharmacology and Therapeutics. 2001;15:1989–1999. doi: 10.1046/j.1365-2036.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- Farroni A., Buera M.D.P. Colour and surface fluorescence development and their relationship with Maillard reaction markers as influenced by structural changes during cornflakes production. Food Chemistry. 2012;135(3):1685–1691. doi: 10.1016/j.foodchem.2012.05.114. [DOI] [PubMed] [Google Scholar]
- Guo L., Guo Y., Wu P., Lu F., Zhu J., Ma H., Chen Y., Zhang T. Camellia oil lowering blood pressure in spontaneously hypertension rats. Journal of Functional Foods. 2020;70:103915. doi: 10.1016/j.jff.2020.103915. [DOI] [Google Scholar]
- Guo N., Tong T., Ren N., Tu Y., Li B. Saponins from seeds of genus camellia: Phytochemistry and bioactivity. Phytochemistry. 2018;149:42–55. doi: 10.1016/j.phytochem.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Hou L.i., Xie J., Zhao J., Zhao M., Fan M., Xiao Q.…Chen F. Roles of different initial maillard intermediates and pathways in meat flavor formation for cysteine-xylose-glycine model reaction systems. Food Chemistry. 2017;232:135–144. doi: 10.1016/j.foodchem.2017.03.133. [DOI] [PubMed] [Google Scholar]
- Jaeger H., Janositz A., Knorr D. The maillard reaction and its control during food processing. the potential of emerging technologies. Pathologie Biologie. 2010;58(3):207–213. doi: 10.1016/j.patbio.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Karangwa, E., Habimana, J. D., Yu, J. Y., Murekatete, N., Zhang, X. M., Masamba, K., … Muhoza, B. (2017). Sensory characteristics of Maillard reaction products obtained from sunflower protein hydrolysates and different sugar types. International Journal of Food Engineering, 13(3).
- Jiratanan T., Liu R.H. Antioxidant activity of processed table beets (Beta vulgaris var. Conditiva) and green beans (Phaseolus vulgaris L.) Journal of Agricultural and Food Chemistry. 2004;52:2659–2670. doi: 10.1021/jf034861d. [DOI] [PubMed] [Google Scholar]
- Lan X., Liu P., Xia S., Jia C., Mukunzi D., Zhang X.…Xiao Z. Temperature effect on the non-volatile compounds of Maillard reaction products derived from xylose-soybean peptide system: Further insights into thermal degradation and cross-linking. Food Chemistry. 2010;120(4):967–972. [Google Scholar]
- Lee S.E., Chung H., Kim Y.-S. Effects of enzymatic modification of wheat protein on the formation of pyrazines and other volatile components in the Maillard reaction. Food Chemistry. 2012;131(4):1248–1254. [Google Scholar]
- Lee S.M., Jo Y.-J., Kim Y.-S. Investigation of the aroma-active compounds formed in the Maillard reaction between glutathione and reducing sugars. Journal of Agricultural and Food Chemistry. 2010;58(5):3116–3124. doi: 10.1021/jf9043327. [DOI] [PubMed] [Google Scholar]
- Li Y., Jiang B., Zhang T., Mu W., Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate. Food Chemistry. 2008;106(2):444–450. [Google Scholar]
- Li Y., Zhong F., Ji W., Yokoyama W., Shoemaker C.F., Zhu S., Xia W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocolloids. 2013;30(1):53–60. [Google Scholar]
- Liu L.u., Li X., Du L., Zhang X., Yang W., Zhang H. Effect of ultrasound assisted heating on structure and antioxidant activity of whey protein peptide grafted with galactose. LWT-Food Science and Technology. 2019;109:130–136. [Google Scholar]
- Lu C.-Y., Hao Z., Payne R., Ho C.-T. Effects of water content on volatile generation and peptide degradation in the Maillard reaction of glycine, diglycine, and triglycine. Journal of Agricultural and Food Chemistry. 2005;53(16):6443–6447. doi: 10.1021/jf050534p. [DOI] [PubMed] [Google Scholar]
- Madruga M.S., Stephen Elmore J., Dodson A.T., Mottram D.S. Volatile flavour profile of goat meat extracted by three widely used techniques. Food Chemistry. 2009;115(3):1081–1087. [Google Scholar]
- Meng X., Li T., Song T., Chen C., Venkitasamy C., Pan Z., Zhang H. Solubility, structural properties, and immunomodulatory activities of rice dreg protein modified with sodium alginate under microwave heating. Food Science & Nutrition. 2019;7(8):2556–2564. doi: 10.1002/fsn3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie P., Wei Q.J., Gong J.T., Wei C.K., Thakur K., Hu F., Wei Z.J. Antioxidant attributes of maillard reaction products of chitosan derived from shrimp shell along with xylose, fructose and glucose. Current Topics in Nutraceutical Research. 2019;17(4):445–450. [Google Scholar]
- Nooshkam M., Varidi M., Bashash M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chemistry. 2018;275:644–660. doi: 10.1016/j.foodchem.2018.09.083. [DOI] [PubMed] [Google Scholar]
- Ogasawara M., Katsumata T., Egi M. Taste properties of Maillard-reaction products prepared from 1000 to 5000 Da peptide. Food Chemistry. 2006;99(3):600–604. [Google Scholar]
- Sannaveerappa T., Westlund S., Sandberg A.-S., Undeland I. Changes in the antioxidative property of herring (Clupea harengus) press juice during a simulated gastrointestinal digestion. Journal of Agricultural and Food Chemistry. 2007;55(26):10977–10985. doi: 10.1021/jf0721904. [DOI] [PubMed] [Google Scholar]
- Shang Y.F., Cao H., Wei C.K., Thakur K., Liao A.M., Huang J.H., Wei Z.J. Bio-utilization of peony seed meal for Flavoring production via Maillard reaction: Effects of sugar types on sensory and flavor of products. Journal of Food Processing and Preservation. 2020;44 [Google Scholar]
- Song N.a., Tan C., Huang M., Liu P., Eric K., Zhang X.…Jia C. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chemistry. 2013;136(1):144–151. doi: 10.1016/j.foodchem.2012.07.100. [DOI] [PubMed] [Google Scholar]
- Srigiridhar K., Nair K.M., Subramanian R., Singotamu L. Oral repletion of iron induces free radical mediated alterations in the gastrointestinal tract of rat. Molecular and Cellular Biochemistry. 2001;219:91–98. doi: 10.1023/a:1011023111048. [DOI] [PubMed] [Google Scholar]
- Su G., Zheng L., Cui C., Yang B., Ren J., Zhao M. Characterization of antioxidant activity and volatile compounds of Maillard reaction products derived from different peptide fractions of peanut hydrolysate. Food Research International. 2011;44(10):3250–3258. [Google Scholar]
- Wang L.L., Xiong Y.L. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. Journal of Agricultural and Food Chemistry. 2005;53(23):9186–9192. doi: 10.1021/jf051213g. [DOI] [PubMed] [Google Scholar]
- Wei C.K., Ni Z.J., Thakur K., Liao A.M., Huang J.H., Wei Z.J. Color and flavor of flaxseed protein hydrolysates Maillard reaction products: Effect of cysteine, initial pH, and thermal treatment. International Journal of Food Properties. 2019;22:84–99. [Google Scholar]
- Wei C.K., Ni Z.J., Thakur K., Liao A.M., Hu F., Huang J.H., Wei Z.J. Acute, genetic and sub-chronic toxicities of flaxseed derived Maillard reaction products. Food and Chemical Toxicology. 2019;131 doi: 10.1016/j.fct.2019.110580. [DOI] [PubMed] [Google Scholar]
- Wei C.K., Ni Z.J., Thakur K., Liao A.M., Huang J.H., Wei Z.J. Aromatic effects of immobilized enzymatic oxidation of chicken fat on flaxseed (Linum usitatissimum L.) derived Maillard reaction products. Food Chemistry. 2020;306 doi: 10.1016/j.foodchem.2019.125560. [DOI] [PubMed] [Google Scholar]
- Wei C.-K., Thakur K., Liu D.-H., Zhang J.-G., Wei Z.-J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chemistry. 2018;263:186–193. doi: 10.1016/j.foodchem.2018.04.120. [DOI] [PubMed] [Google Scholar]
- Wu H.-C., Chen H.-M., Shiau C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Research International. 2003;36(9–10):949–957. [Google Scholar]
- Xiong G.Y., Chen X., Zhang X.X., Miao Y., Zou Y., Wang D.Y., Xu W.M. Process optimization and the relationship between the reaction degree and the antioxidant activity of Maillard reaction products of chicken liver protein hydrolysates. Poultry Science. 2020;99(7):3733–3741. doi: 10.1016/j.psj.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L., Zhao M., Regenstein J.M., Ren J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chemistry. 2010;120(3):810–816. [Google Scholar]
- Yu X., Zhao M., Hu J., Zeng S., Bai X. Correspondence analysis of antioxidant activity and UV-Vis absorbance of Maillard reaction products as related to reactants. LWT-Food Science and Technology. 2012;46(1):1–9. [Google Scholar]
- Zeng Q., Cui Y.L., Su D.X., Bin T., He S., Yuan Y. Process optimization and anti-oxidative activity of peanut meal maillard reaction products. LWT-Food Science and Techonlogy. 2018;97:573–580. [Google Scholar]
- Zhao J., Wang T., Xie J., Xiao Q., Du W., Wang Y.…Wang S. Meat flavor generation from different composition patterns of initial Maillard stage intermediates formed in heated cysteine-xylose-glycine reaction systems. Food Chemistry. 2018;274:79–88. doi: 10.1016/j.foodchem.2018.08.096. [DOI] [PubMed] [Google Scholar]
- Zhu L., Chen J., Tang X., Xiong Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. Journal of Agricultural and Food Chemistry. 2008;56(8):2714–2721. doi: 10.1021/jf703697e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.